Abstract
Animal conflicts are influenced by social experience such that a previous winning experience increases the probability of winning the next agonistic interaction, whereas a previous losing experience has the opposite effect. Since androgens respond to social interactions, increasing in winners and decreasing in losers, we hypothesized that socially induced transient changes in androgen levels could be a causal mediator of winner/loser effects. To test this hypothesis, we staged fights between dyads of size-matched males of the Mozambique tilapia (Oreochromis mossambicus). After the first contest, winners were treated with the anti-androgen cyproterone acetate and losers were supplemented with 11-ketotestosterone. Two hours after the end of the first fight, two contests were staged simultaneously between the winner of the first fight and a naive male and between the loser of first fight and another naive male. The majority (88%) of control winners also won the second interaction, whereas the majority of control losers (87%) lost their second fight, thus confirming the presence of winner/loser effects in this species. As predicted, the success of anti-androgen-treated winners in the second fight decreased significantly to chance levels (44%), but the success of androgenized losers (19%) did not show a significant increase. In summary, the treatment with anti-androgen blocks the winner effect, whereas androgen administration fails to reverse the loser effect, suggesting an involvement of androgens on the winner but not on the loser effect.
Keywords: social experience, winner effect, androgens, testosterone, aggression
1. Introduction
Animal conflicts are influenced by social experience such that a previous winning experience increases the probability of winning a subsequent interaction against a different opponent, whereas a previous losing experience has the opposite effect (Dugatkin 1997; Hsu & Wolf 1999). These winner and loser effects are widespread across different animal taxa, with the magnitude of loser effects being, in general, higher than that of winner effects and frequently lasting longer (Hsu et al. 2006; Rutte et al. 2006). Thus, together with other fighting asymmetries, such as physical body size or prior residence, winner/loser effects influence the establishment of dominance hierarchies and shape emerging social structures.
Despite their ubiquity, the mechanisms underlying winner and loser effects are still poorly understood. At the ultimate level, two hypotheses have been proposed to explain the adaptive value of winner/loser effects: (i) the social-cue hypothesis, according to which winning and losing experiences leave traces in the individuals that are detected by subsequent opponents therefore affecting their fighting decisions, and (ii) the self-assessment hypothesis, which postulates that by winning or losing a fight individuals gain information about their fighting ability in relation to the population (Rutte et al. 2006). At the proximate level, experience effects on aggressive behaviour have been explained either by learning or by status (winning/loser)-dependent changes in internal state which would, in turn, affect the outcome of subsequent contests (Hsu et al. 2006). A variety of physiological mechanisms can contribute to this phenomenon, including changes induced by social experience in brain neuromodulators and hormonal responses to social interactions (Winberg & Nilsson 1993; Huber & Delago 1998; Oyegbile & Marler 2005).
One of the most studied hormonal responses so far in relation to social challenge has been testosterone. John Wingfield and co-workers have proposed the ‘challenge hypothesis’ as a conceptual framework for the androgen responsiveness to social interactions, according to which the magnitude of the response is a function of the social system, namely the degree of paternal care and the regime of intrasexual competition, and serves to adjust the expression of subsequent aggressive behaviour after an initial aggressive encounter (Wingfield et al. 1990; Hirschenhauser & Oliveira 2006). Since the hormonal response to a social challenge occurs after an interaction with a current intruder, it has been proposed that its adaptive value should be related to maintaining a heightened aggressive motivation in an environment rich in potential social challenges (Wingfield et al. 1990; Wingfield 2005). Several studies have documented such a role for experience-dependent rises in androgen levels in different vertebrate taxa. For example, in free-living song sparrows, an increase in testosterone levels elicited by a staged territorial encounter enhanced subsequent territorial behaviour for 24 hours after removal of the stimulus (Wingfield 1994; Wingfield & Hahn 1994), and in fish, bystanders of agonistic encounters between conspecific males, although not directly involved in the interaction, increased their androgen levels and are primed for increased aggression in subsequent encounters with naive opponents (Oliveira et al. 2001; Clotfelter & Paolino 2003). These effects of prior experience-dependent androgen levels on aggressive motivation suggest a possible physiological mechanism for winner/loser effects based on transient changes in androgen levels modulated by previous social interactions (Oyegbile & Marler 2005). Previous studies conducted with California mice (Peromyscus californicus) have found an association between prior winning experience and both an increased likelihood of winning future fights and increased testosterone levels (Oyegbile & Marler 2005; see §4 for further details on the ‘winning hypothesis’ in California mice). Although these data suggest a role for testosterone as a reinforcer of aggressive behaviour in winners of previous encounters, an experimental manipulation of circulating androgen levels and its implications for subsequent aggressive behaviour in winners and losers is still missing in order to demonstrate a causal link between experience-dependent changes in androgens and winner/loser effects. This manipulation also has the added value of allowing winners and losers different social experiences without experiencing the changes in androgen levels that are usually associated with them.
Here we tested the role of androgens on winner/loser effects in male cichlid fish (Oreochromis mossambicus), by treating winners and losers of a first aggressive encounter, respectively, with an anti-androgen (cyproterone acetate, CA) or with 11-ketotestosterone (KT) and observing their aggressive behaviour in a second encounter. Our predictions were that if androgens mediated both winner and loser effects, then anti-androgen (CA) administration to winners should block the winner effect, whereas androgen (KT) administration to losers should reverse the loser effect.
2. Material and methods
(a) Model system
Oreochromis mossambicus is an African lek-breeding cichlid. Males form dense reproductive aggregations during the breeding season. Within these aggregations, males engage in frequent agonistic interactions for the establishment of breeding territories to which they attract females (Bruton & Boltt 1975). Therefore, a male's reproductive fitness is largely influenced by his success in aggressive interactions and ability to maintain social status (Oliveira et al. 1996). Contest outcome is influenced by the relative fighting ability of the opponents, by resource value, and by previous fighting experience, namely prior victories and defeats (Baerends & Baerends Van Roon 1950; Turner 1994; L. A. Carneiro & R. F. Oliveira 2002, unpublished data).
(b) Subjects
Subjects were from a population bred at our laboratory, which is derived from fish caught at the Incomati River (Mozambique). Stock animals are kept in 400 l tanks at 24±1°C with a photoperiod of 12 L : 12 D and a sex ratio of approximately 1 : 1. A 7 cm layer of sand was placed in each tank to serve as substrate since this is a critical resource for the expression of social behaviour in this species (Galhardo et al. 2008). Fish were fed daily, except for the observation days, with commercial food flakes. All individuals were individually tagged with a small intraperitoneal magnetic transponder (size=2.2×11.5 mm; Trovan ID100, Trovan, Germany). In this study, males ranged from 15.0 to 19.5 cm in total length (36.1–94.0 g body weight). Animal care and use protocols were approved by the national authorities (Direcção Geral de Veterinária, Portugal).
(c) Experimental procedures
(i) Experiment 1: detection of winner/loser effects
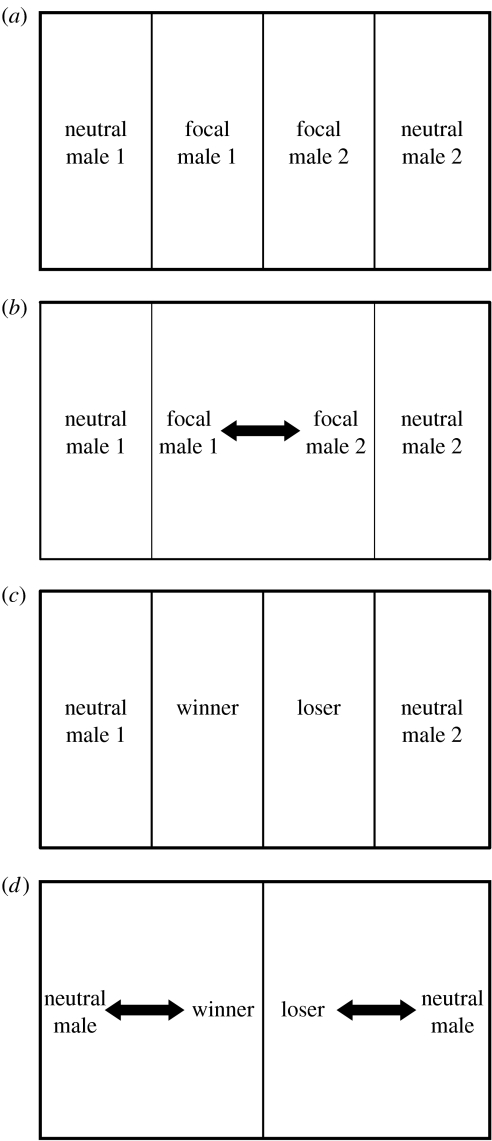
To test for the existence of winner/loser effects, sets of four males matched for size were used (n=16 male sets). Each set of males was placed in a 400 l tank (200×50×40 cm) with four compartments separated by opaque partitions (one socially isolated male per compartment of 50×50×40 cm; see figure 1a). The focal males of each male set were placed in the central compartments and the neutral males in the end compartments. The general maintenance conditions (i.e. temperature, photoperiod, substrate) in the experimental tanks were the same as those described above for the stock tanks. Males were socially isolated in each compartment for 8 days to control for possible effects of previous experience. On the day of the experiment, a first interaction was staged at 09.00 (when androgen levels reach their daily peak in this species, Oliveira et al. 2001) by removing the opaque divider between the central compartments (figure 1b). The interaction between the two focal males was observed using continuous recording (Martin & Bateson 2007). The outcome of the interaction was assessed using the criteria proposed by Earley et al. (2000). After the establishment of an asymmetry in the interaction, winners kept displaying attack behaviours such as chases and bites towards the opponent, and losers adopted submissive behaviours and retreated when approached. The interactions were observed for another period of 20 min after the asymmetry in the interaction (attack/submission) was detected to ensure that no reversals in the dominance–subordinance status occurred. Therefore, the total duration of each interaction was equal to the time it took for the attack–submission asymmetry to emerge together with 20 min. It was also noted whether fights were resolved with or without escalation into symmetric overt aggression (i.e. mouth-fighting behaviour). The fights were then stopped by replacing the opaque partition dividing the central compartments (figure 1c). In order to serve as controls for experiment 2, both winners and losers were injected with an isotonic solution (vehicle used in the treatments, see below) between the first and second contests.
Figure 1.
Experimental procedure to assess the presence of winner/loser effects in tilapia. (a) Four fish matched for size are placed in individual compartments separated by removable opaque partitions (social isolation); (b) after a period of social isolation, the central divider is removed and a fight between the two fish placed in the central compartments—the focal fish—is promoted (first interaction); (c) after assessing the winner and loser, the divider is put back in place and the winner and the loser of this first fight are back in their initial compartments (interval between the first and second interactions); (d) 2 hours after the end of the first fight, the lateral dividers are removed and two simultaneous fights are promoted between the winner of the previous fight and a neutral male and between the loser of the previous fight and another neutral male (second interactions).
Two hours after fish were placed in the experimental tank, the opaque partitions that separated each central compartment from its respective end compartment were simultaneously removed and two simultaneous interactions were promoted, between the winner of the first encounter (WC) and a neutral male (N) and between the loser of the first fight (LC) and a neutral male (figure 1d). One of these interactions was observed directly and the other was recorded using an 8 mm video camera (Sony) for later analysis. The same criteria as described above were used to assess the winners/losers of these second interactions.
(ii) Experiment 2: effects of androgens on winner/loser effects
To test the involvement of androgens on the effects of prior fighting experience, the winners were treated with the anti-androgen CA and the losers with KT between the end of the first experiment and the start of the second experiment. If androgens mediated the experiential effects, then anti-androgen-treated winners should lose their contest advantage and losers given KT should have an enhanced fighting ability. Thus, we used the same procedure as described above, but the focal males were given an intraperitoneal injection immediately after the end of the first interaction and were placed back into their compartments. The winners (WCA) were injected with the anti-androgen CA (1 mg g−1 body weight) and the losers (LKT) with 11-KT (2 μg g−1 body weight), considered the most active androgen in male teleosts (Kime 1993; Borg 1994). The CA dosage used was based on the work of Kramer et al. (1969), which showed that it was sufficient to inhibit nest building and aggressive behaviour in O. mossambicus without interfering in courtship behaviour. The dosage of KT used was based on published work in other laboratories and on the validation described below. The vehicle solution was peanut oil (10%) that was then diluted in a Ringer solution for freshwater fish (trout balanced salt solution, TBSS).
(iii) Potential confounds on fight outcome: I. Effects of body size
Since relative size is a major determinant of the outcome of agonistic interactions (Hsu et al. 2006), its possible effect on the outcome of the fights on both experiments was controlled for a priori by matching the size (standard length) of the four individuals within each replicate, and a posteriori by checking for possible differences in body size between winners and losers of each type of interaction (see below). Usually, it is considered that a difference in body size between opponents larger than 20 per cent establishes an asymmetry in relative fighting ability which will affect the outcome of the encounter irrespective of prior experience (Beaugrand et al. 1996). The within-replicate coefficient of variation varied between 1.3 and 7.4 per cent (median=3.7%; upper quartile =4.8%; lower quartile =2.6%) for standard length. Furthermore, there were no differences in standard length between winners and losers in any of the three types of staged encounters: first encounter winner–loser (Wilcoxon matched pairs signed-rank test: n=32, z=1.24, n.s.); second encounter winner–neutral (Wilcoxon matched pairs signed-rank test: n=32, z=0.16, n.s.); and second encounter loser–neutral (Wilcoxon matched pairs signed-rank test: n=32, z=1.58, n.s.). The average (±s.e.m.) standard length for the four groups was as follows: winners=16.6±0.2 cm; losers=16.8±0.2 cm; neutrals against winners=16.7±0.2 cm; and neutrals against losers=16.5±0.2 cm. In summary, body size, as assessed from standard length, is not a predictor of conflict outcome in this study.
(iv) Potential confounds on fight outcome: II. Effects of pheromones
Chemical communication has been described for this species and it is actively used in male–male interactions. Males store urine that contains a sterol-like pheromone and control urine release by a sphincter located in the urogenital papillae (Barata et al. 2008). In staged fights, resident males release urine pulses towards intruders that abstain from releasing urine as a sign of subordination (Barata et al. 2007). The urine of dominant males has higher olfactory potency than that of subordinates (Barata et al. 2008). Thus, it is possible that during the first fight, the focal males signalled to each other using urine, and that since the opaque partitions cannot block the flow of chemicals from one compartment to another, this information became available to the neutral males that were used as stimuli in the second fights. However, since there were 2 hours between the end of the first contest and the start of the second fight, it is expected, according to previous studies on the dynamics of diffusion of fluorescent dyes in experimental tanks, that any chemicals released during the first fight became uniformly distributed across compartments by the time of the second fight (Barata et al. 2007). Therefore, although neutral males may have chemical cues indicating that a previous fight occurred in the tank, no information on the identity of previous winners and previous losers is expected to be available to them. Therefore, we do not expect a differential response of neutral males towards previous winners versus previous losers based on passive chemical eavesdropping of the first contest.
(v) Terminology of fish used in the experiments
Focal males, winners and losers from the first interaction in both experiments; control males, non-treated winners and losers from experiment 1; neutral males, males that were used as stimuli to fight against previous winners and previous losers in the second fights of both experiments.
(d) Validation of hormonal treatments
In order to test the efficiency of the hormonal treatments, an independent group of males was used. These males were also isolated for 7 days, after which they were injected either with CA (1 mg g−1 body weight, n=7), KT (0.2 μg g−1 body weight, n=7), KT (2 μg g−1 body weight, n=7) or with TBSS (control, n=7), and their androgen levels (i.e. KT) were measured using a non-invasive method that allows for sequential sampling of small animals. This technique consists of assaying the steroids released by the fish into the holding water where they were kept (Scott & Ellis 2007; Scott et al. 2008). For this purpose, treated fish were placed (at 09.00) in a small tank (46×36×40 cm) filled with 1 l of water and the water was replaced at constant time intervals (i.e. every 2 hours at 11.00, 13.00, 15.00 and 17.00). Thus, we have four sequential samples of the same individual at 2 hour intervals after the injection.
The water to fill sample containers always originated from a large ‘pool water tank’ that did not hold any fish. To exclude contamination, sample containers and all materials used were always washed with methanol and distilled water prior to sampling. Each water sample was filtered using filter paper and passed through a solid phase chromatography cartridge (Merck LiChrolut RP-18, 500 mg), which had been previously activated with 5 ml methanol followed by 5 ml distilled water. The lipophilic compounds of the sample adsorbed by the column were then eluted with ethanol. From each sample, the free steroid fraction and the corresponding glucuronides and sulphates were extracted following Scott & Canario (1992) and Oliveira et al. (1996). The measures presented here are the sum of all three fractions (free, sulphates and glucuronides), which is the total amount of hormone contained in each sample corrected for body mass and sampling volume (pg−1 g−1 h−1), measured by radioimmunoassays using the antibody kindly donated by Dr David Kime (University of Sheffield, UK). The details of the antibody cross-reactivities are given elsewhere (Kime & Manning 1982). To control for handling stress induced by the procedure, cortisol levels were also assayed using an antiserum produced in rabbits by Dr Patricia Ingleton (University of Sheffield, UK) against cortisol-3-carboxymethyloxime conjugated to bovine serum albumin (Sigma). Cross-reactions of the antiserum in relation to cortisol were 54 per cent for 11-desoxycortisol, 10 per cent for cortisone, 16 per cent for 17,21-dihydroxy-5β-pregnan-3,11,20-trione, 5 per cent for 11β,17,21-trihydroxy-5β-pregnan-3,20-dione, 0.05 per cent for 11β-hydroxytestosterone and less than 0.001 per cent for testosterone. Intra- and inter-assay coefficients of variation for the KT assay were 8 and 11 per cent, respectively. Cortisol levels were assayed in a single assay and its coefficient of variation was 9 per cent.
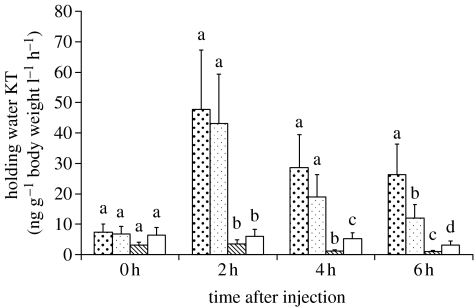
Baseline levels (0 hour) were not significantly different among treatments (figure 2) and did not show a significant temporal variation (repeated-measures ANOVA: F3,15=2.33, p=0.11). Both dosages of KT treatment promoted an increase in KT levels with a peak at 2 hours after injection (repeated-measures ANOVA: high KT treatment: F3,15=6.87, p<0.01; low KT treatment: F3,18=8.3, p<0.01; figure 2). In the high-dose treatment, KT levels at 2 hours were significantly higher than that at 0 hour, and levels at 4 and 6 hours were intermediate without being significantly different from 0 hour or from 2 hours (figure 2). In the low-dose KT treatment, levels at 2 hours were significantly higher than those at the other three sampling points (i.e. 0, 4 and 6 hours), indicating a more discrete peak in this group (figure 2). Therefore, it was decided to use the high-dose KT treatment in the behavioural experiments and to use an interval between the two contests of 2 hours in order to have the treated animals in the time window when their KT levels reach a post-treatment maximum. It should be noted that the KT levels induced by the treatment are within the physiological range reported for this species (Hirschenhauser et al. 2004).
Figure 2.
Validation of the hormonal treatments. Levels of 11-KT in fish subjected to different intraperitoneal injection treatments: high dose (2 μg g−1 body weight) of KT (large dot bars); low dose (0.2 μg g−1 body weight) of KT (small dot bars); CA (1 mg g−1 body weight) (hatched bars); and saline solution (control, white bars). Different letters indicate significantly different groups (p<0.05) within each sampling point.
The anti-androgenic effects of CA are due to its action as an antagonist of androgen receptors in androgen-receptive tissues (Namer 1988; Schroder 1993). Therefore, it would not be expected that the CA treatment could be validated by monitoring androgen levels. However, since CA structurally resembles testosterone, it also has anti-gonadotrophic action by inhibiting positive feedback at the level of the hypothalamus and/or pituitary, thus potentially decreasing circulating androgen levels (Namer 1988; Singh & Joy 1998; Sharpe et al. 2004). Indeed, in our study, the treatment with the anti-androgen CA also induced a significant temporal variation in KT levels (repeated-measures ANOVA: F3,15=5.44, p<0.01), inducing a significant decrease in comparison with baseline levels at 4 and 6 hours post-injection (figure 2). Although androgen levels responded to CA treatment only 4 hours post-injection, its effects on androgen-mediated traits are due to its action as an androgen receptor antagonist and therefore it was also decided to use the 2 hour interval between fights in the CA-treated group.
It could be argued that the handling stress due to administration of the injections could raise cortisol levels, which would in turn interfere with subsequent behaviour on the second fights. To control for this effect, we also assayed cortisol in the control group (i.e. injected with saline) and found no significant differences between the different sampling points (2, 4 and 6 hours) and the baseline (0 hour) (repeated-measures ANOVA: F3,18=0.58, p=0.63).
(e) Statistical analysis
Differences between proportions of second fights won versus lost by previous winners, as well as the proportions of initiative to start the interaction, and the proportion of escalated fights between groups, were tested using the difference between two proportion tests of the software Statistica (StatSoft 2007). Differences in fight durations between different types of interactions were assessed using Friedman ANOVA followed by post hoc comparisons. The temporal variation (i.e. time after injection: 0 versus 2 versus 4 versus 6 hours) of hormonal data was analysed with a repeated-measures ANOVA design, and the differences among different treatments at each sampling point with Tukey HSD post hoc tests. We have used a significance value of p=0.05 and two-tailed tests. All statistical tests were run on the statistical software package Statistica v. 8.0 (StatSoft 2007).
3. Results
(a) Experiment 1: effect of prior experience on fight outcome
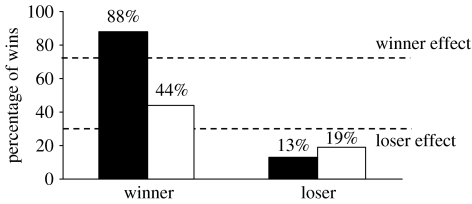
The majority (14 out of 16=88%) of the winners of the first interaction also won the second interaction, but only 13 per cent (2 out of 16) of the males that lost the first contest won the second fight, suggesting the presence of both winner and loser effects in this species (figure 3). Winners increased their initiative to start a fight from the first to the second encounter (62.5–93.8%, p<0.05), while a similar trend was observed for losers but it was not significant (37.5–56.3%, p=0.29). Winners also started significantly more second encounters against neutral conspecifics than losers (93.8% versus 56.3%, p<0.05). Fight duration and escalation of fights did not differ between the first and second interactions (table 1).
Figure 3.
Winner/loser effects in tilapia as assessed by the percentage of victories in the second fights of previous winners and losers. The treated winners were injected with a high dose of KT and the treated losers were injected with CA 2 hours before the second fight. The dashed lines indicate the cut-off values to consider the occurrence of winner/loser effects according to Bégin et al. (1996) (black bars, control; white bars, treatment).
Table 1.
Comparison of fight duration (min) and proportion of escalated fights in different types of interactions: WCLC, first interaction between control males; WCN, second interaction of control males between previous winner and naive individual; LCN, second interaction of control males between previous loser and naive individual; WTLT, first interaction between males of the treated groups; WTN, second interaction of previous winner treated with CA and naive individual; LTN, second interaction of previous loser treated with KT and naive individual. (Different letters indicate significant differences between types of interaction within each treatment. For fight duration, differences between groups were assessed using a non-parametric Friedman ANOVA followed by post hoc Dunn tests. For percentage of escalated fights, differences between groups were estimated using the difference between two proportions test of the software Statistica.)
| type of interaction | n | fight duration (min) | percentage of escalated fights |
|---|---|---|---|
| control groups | |||
| WCLC | 16 | 45.9±11.1 a | 43.75 a |
| WCN | 16 | 38.3±8.9 a | 25 a |
| LCN | 16 | 40.8±9.9 a | 37.5 a |
| treated groups | |||
| WTLT | 16 | 27.6±4.2 a | 62.5 a |
| WTN | 16 | 31.8±5.6 a | 31.25 ab |
| LTN | 16 | 20.6±0.7 b | 12.5 b |
(b) Experiment 2: effect of androgens on winner and loser effects
Seven out of 16 (44%) CA-treated winners also won the second fight, representing a significant decrease in the success of winners in second fights (figure 3). Three out of 16 (19%) KT-treated losers won the second fight, which did not represent a significant increase in the success of losers in second fights (figure 3).
The initiative to start the second fight was lower both in treated winners (62.5% versus 93.8%, p<0.05) and in treated losers (18.8–56.3%, p<0.05) when compared with the second fight of control winners and control losers, respectively. The duration of treated loser's second fights (LKT–N) was significantly shorter when compared with both the duration of the first interaction (WAC–LKT) and with the duration of the second interaction of treated winners (WCA–N; table 1). Also, the proportion of escalated fights was lower in second interactions involving treated losers (LKT–N) than in first interactions (WCA–LKT; table 1). The proportion of escalated fights in interactions involving treated winners did not differ significantly between the first (WCA–LKT) and second (WCA–N; table 1) fights. Finally, when comparing fight duration and proportion of escalated fights between the same type of interactions among control and treated animals (i.e. WC–LC versus WCA–LKT, WC–N versus WCA–N, and LC–N versus LKT–N), the only difference found was a shorter duration of treated loser's second fights when compared with control loser's second fights (LKT–N versus LC–N, Mann–Whitney U-test, p<0.05; table 1).
4. Discussion
Here we show that both winner and loser effects are present in O. mossambicus and that androgens are implicated in the winner but not in the loser effect.
The majority of the winners of the first interaction won the second interaction, while a minority of the males that lost the first contest won the second fight, indicating the presence of both winner and loser effects in this species. To further assess the behavioural mechanisms responsible for these effects, we analysed the relationship between the outcome of the first encounter and the initiative to start the second interaction. Winners, but not losers, increased their initiative to start a fight from the first to the second encounter, so that winners started significantly more second encounters against neutral conspecifics than losers, indicating a priming effect of social experience on aggressive motivation in winners but not in losers. Persistence of aggressive behaviour, as measured by fight duration and escalation of fights, did not differ between the first and second interactions, suggesting that persistence of aggressive behaviour was not affected by the outcome of a previous interaction. Together these data suggest that experience-elicited changes in the initiative to start an aggressive interaction play a key role in winner/loser effects in this species.
However, the association between the success of males on consecutive agonistic encounters can also be explained by variation of intrinsic fighting ability which makes some males consistently win and others consistently lose (Chase et al. 1994; Bégin et al. 1996; Hsu et al. 2006). To control for variation in intrinsic fighting ability, opponents were matched for body size, and variation in terms of internal state was minimized by using only territorial males in the experiment and by isolating them for 7 days before the start of the experiment. Nevertheless, one cannot rule out the existence of other uncontrolled sources of inter-individual variation in intrinsic fighting ability. Bégin et al. (1996) developed a model to estimate the dominance probability in encounters that use either winners or losers of a previous size-matched encounter, and demonstrated formally that with this self-selecting procedure, winners/losers of a first encounter have a probability of 0.67 of having higher/lower intrinsic fighting ability than neutral opponents in a second fight. Therefore, the null hypothesis against which to test the effects of prior experience is not the equiprobability of winning/losing the second encounter, but having prior winners winning at least two-thirds of subsequent interactions against a size-matched naive opponent (to demonstrate a winner effect), and reversely having prior losers winning less than one-third of second fights against size-matched neutral opponents (to demonstrate the loser effect). The results obtained in this study match these criteria and therefore both the winner and loser effects are established in this species even when controlling for the effects of intrinsic fighting experience. Interestingly, although the success of winners in the second contest was mainly due to an increased initiative to start the fight and not to the persistence of aggressive behaviour, neither initiative nor persistence can explain per se the effect of the treatments, suggesting that the winner effect cannot be reduced simply to a lower latency to engage in aggressive encounters but that it has a dynamical component which emerges during the fight. This indicates that winner/loser effects can be caused both by motivational changes in the subject and by the recognition of its heightened aggressive state in opponents.
Next, we tested the involvement of androgens in the effects of prior fighting experience. The rationale for these manipulations emerged from the challenge hypothesis, according to which circulating levels of testosterone are associated with the expression of aggressive behaviour in periods of social instability, such as when a territorial male is challenged by an intruder (Wingfield et al. 1990). Since in many species androgens respond differentially to the outcome of social interactions, increasing in winners and decreasing in losers (Hirschenhauser & Oliveira 2006), it has been hypothesized that socially induced transient changes in androgen levels could be the causal mediators of winner/loser effects (Oyegbile & Marler 2005). To date, few experimental studies have tried to assess the causal link between experience-dependent short-term increases in testosterone and the winner effect, and from these most have been performed with male California mice (P. californicus). In this species, it has been shown that winning aggressive encounters reduced the attack latency in future encounters, but that this effect was not present in castrated males (Trainor & Marler 2001). Moreover, repeated winning experiences increased both testosterone levels and the probability of winning a subsequent encounter independent of intrinsic fighting ability (Oyegbile & Marler 2005), and exogenous administration of testosterone following an aggressive encounter promoted an increase in aggressive behaviours in a subsequent fight (Trainor et al. 2004). Together, these data suggest a role for testosterone as a reinforcer of aggressive behaviour in winners of previous encounters, but for the establishment of a causal link, a direct manipulation of androgen levels in winners and losers, and its effect on subsequent fighting ability is still necessary.
Here we tested this hypothesis by treating winners with the anti-androgen CA and losers with the main fish androgen, KT. Thus, we expected to block the androgen surge in winners and to compensate for the decrease in androgen levels in losers of social interactions that have been previously described in this species (Oliveira et al. 1996; Hirschenhauser et al. 2004). This way winners and losers have experienced different social outcomes without having the associated changes in circulating androgen levels. If prior experience effects are androgen dependent, then chemically castrated winners should lose their contest advantage and losers given KT should have an enhanced fighting ability in ulterior fights. As predicted, the success of anti-androgen-treated winners in the second fight decreased significantly below the cut-off value used to identify a winner effect (44% versus 67%). However, the success of androgenized losers in the second fights (19%) was still within the levels compatible with the presence of a loser effect (less than 33%) (figure 1).
In a tentative step to understand the behavioural mechanisms involved in winner/loser affected by androgens, the effects of hormonal treatments on the initiative to start a fight and the persistence of aggressive behaviour were also assessed. Since the initiative to start the second fight was lower in treated winners and treated losers, when compared with the second fight of control winners and control losers, respectively, the winner effect cannot be reduced simply to experience-dependent variations in the readiness to engage in aggressive encounters, but has a dynamic component that emerges during the fight. The fact that the duration of the second fights of treated losers was significantly shorter than both that of their first and second interactions of treated winners suggests that the KT treatment may have a negative impact on the persistence of aggressive behaviour in losers. This is further supported by the lower proportion of escalated fights in second interactions involving treated losers than that in their first interactions. The proportion of escalated fights in interactions involving treated winners was not significantly different between their first and second contests, suggesting that the effect of androgens on the winner effect is not achieved by changes in persistence of aggressive behaviour in winners.
Together, the data presented here suggest that androgens may be playing a role as physiological mediators of the winner but not of the loser effect. Thus, although functionally related, the winner and loser effects may rely on different causal mechanisms. This idea is consistent with a recent meta-analysis across different taxa, which revealed an asymmetry in the magnitude of winner/loser effects. While previous winners double the chances of winning a subsequent fight, previous losers have a five times less chance of winning (Rutte et al. 2006). Moreover, loser effects last longer in time and can even be present in the absence of winner effects (Hsu et al. 2006; Rutte et al. 2006). The short duration of the winner effect is compatible with transient changes in androgens induced by previous social experience, while the longer duration of the loser effect suggests a more permanent change at the brain level, possibly mediated by central neuromodulators such as serotonin.
Acknowledgements
Animal care and use protocols were approved by the national authorities (Direcção Geral de Veterinária, Portugal).
We thank Tânia Oliveira and Elsa Couto for hormone analysis, Katharina Hirschenhauser for helping with the hormonal validation experiments and for fruitful discussions on this topic, and three anonymous reviewers for suggestions that improvedthe quality of the final manuscript. This study was funded by the grants REEQ/608/BIO/2005 and PTDC/PSI/71811/2006 from Fundação para a Ciência e a Tecnologia (FCT), the European Commission FEDER Programme and the FCT Plurianual Programme (R&D unit MAR-LVT-Lisboa-331).
References
- Baerends G.P., Baerends Van Roon J.M. An introduction to the study of the ethology of cichlid fishes. Behaviour. 1950;((Suppl. 1)):1–242. [Google Scholar]
- Barata E.N., Hubbard P.C., Almeida O.G., Miranda A., Canário A.V.M. Male urine signals social rank in the Mozambique tilapia (Oreochromis mossambicus) BMC Biol. 2007;5:e54. doi: 10.1186/1741-7007-5-54. doi:10.1186/1741-7007-5-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barata E.N., Fine J.M., Hubbard P.C., Almeida O.G., Frade P., Sorensen P.W., Canário A.V. A sterol-like odorant in the urine of Mozambique tilapia males likely signals social dominance to females. J. Chem. Ecol. 2008;34:438–449. doi: 10.1007/s10886-008-9458-7. doi:10.1007/s10886-008-9458-7 [DOI] [PubMed] [Google Scholar]
- Beaugrand J.P., Payette D., Goulet C. Conflict outcome in male green swordtail fish dyads (Xiphophorus helleri): interaction of body size, prior dominance/subordination experience, and prior residency. Behaviour. 1996;133:303–319. doi:10.1163/156853996X00161 [Google Scholar]
- Bégin J., Beaugrand J.P., Zayan R. Selecting dominants and subordinates at conflict outcome can confound the effects of prior dominance or subordination experience. Behav. Proc. 1996;36:219–226. doi: 10.1016/0376-6357(95)00031-3. doi:10.1016/0376-6357(95)00031-3 [DOI] [PubMed] [Google Scholar]
- Borg B. Androgens in teleost fish. Comp. Biochem. Physiol. C. 1994;109:219–245. doi:10.1016/0305-0491(94)90005-1 [Google Scholar]
- Bruton M.N., Boltt R.E. Aspects of the biology of Tilapia mossambica Peters (Pisces: Cichlidae) in a natural freshwater lake (Lake Sibaya, South Africa) J. Fish Biol. 1975;7:423–446. doi:10.1111/j.1095-8649.1975.tb04618.x [Google Scholar]
- Chase I.D., Bartolomeo C., Dugatkin L.A. Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim. Behav. 1994;48:393–400. doi:10.1006/anbe.1994.1253 [Google Scholar]
- Clotfelter E.D., Paolino A.D. Bystanders to contests between conspecifics are primed for increased aggression in male fighting fish. Anim. Behav. 2003;66:343–347. doi:10.1006/anbe.2003.2227 [Google Scholar]
- Dugatkin L.A. Winner effects, loser effects, assessment strategies and the structure of dominance hierarchies. Behav. Ecol. 1997;8:583–587. doi:10.1093/beheco/8.6.583 [Google Scholar]
- Earley R.L., Hsu Y., Wolf L.L. The use of standard aggression testing methods to predict combat behaviour and contest outcome in Rivulus marmoratus dyads (Teleostei: Cyprinodontidae) Ethology. 2000;106:743–761. doi:10.1046/j.1439-0310.2000.00586.x [Google Scholar]
- Galhardo L., Correia J., Oliveira R.F. The effect of substrate availability on behavioural and physiological indicators of welfare in the African cichlid (Oreochromis mossambicus) Anim. Welfare. 2008;17:239–254. [Google Scholar]
- Hirschenhauser K., Oliveira R.F. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 2006;71:265–277. doi:10.1016/j.anbehav.2005.04.014 [Google Scholar]
- Hirschenhauser K., Taborsky M., Oliveira T., Canario A.V.M., Oliveira R.F. A test of the ‘challenge hypothesis’ in cichlid fish: simulated partner and territory intruder experiments. Anim. Behav. 2004;68:741–750. doi:10.1016/j.anbehav.2003.12.015 [Google Scholar]
- Hsu Y., Wolf L.L. The winner and loser effect: integrating multiple experiences. Anim. Behav. 1999;57:903–910. doi: 10.1006/anbe.1998.1049. doi:10.1006/anbe.1998.1049 [DOI] [PubMed] [Google Scholar]
- Hsu Y., Earley R.L., Wolf L.L. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 2006;81:33–74. doi: 10.1017/S146479310500686X. doi:10.1017/S146479310500686X [DOI] [PubMed] [Google Scholar]
- Huber R., Delago A. Serotonin alters decisions to withdraw in fighting crayfish, Astacus astacus: the motivational concept revisited. J. Comp. Physiol. A. 1998;182:573–583. doi:10.1007/s003590050204 [Google Scholar]
- Kime D., Manning N.J. Seasonal patterns of free and conjugated androgens in the brown trout Salmo trutta. Gen. Comp. Endocrinol. 1982;48:222–231. doi: 10.1016/0016-6480(82)90020-x. doi:10.1016/0016-6480(82)90020-X [DOI] [PubMed] [Google Scholar]
- Kime D.E. ‘Classical’ and ‘non-classical’ reproductive steroids in fish. Rev. Fish Biol. Fish. 1993;3:160–180. doi:10.1007/BF00045230 [Google Scholar]
- Kramer B., Molenda W., Fiedler K. Behavioural effect of the antiandrogen cyproterone acetate (Schering) in Tilapia mossambica and Lepomis gibbosus. Gen. Comp. Endocrinol. 1969;13:515. [Google Scholar]
- Martin P., Bateson P. 3rd edn. Cambridge University Press; Cambridge, UK: 2007. Measuring behaviour: an introductory guide. [Google Scholar]
- Namer M. Clinical application of antiandrogens. J. Steroid Biochem. 1988;31:719–729. doi: 10.1016/0022-4731(88)90023-4. doi:10.1016/0022-4731(88)90023-4 [DOI] [PubMed] [Google Scholar]
- Oliveira R.F., Almada V.C., Canario A.V.M. Social modulation of sex steroid concentrations in the urine of male cichlid fish Oreochromis mossambicus. Horm. Behav. 1996;30:2–12. doi: 10.1006/hbeh.1996.0002. doi:10.1006/hbeh.1996.0002 [DOI] [PubMed] [Google Scholar]
- Oliveira R.F., Lopes M., Carneiro L.A., Canario A.V.M. Watching fights raises fish hormone levels. Nature. 2001;409:475. doi: 10.1038/35054128. doi:10.1038/35054128 [DOI] [PubMed] [Google Scholar]
- Oyegbile T.O., Marler C.A. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 2005;48:259–267. doi: 10.1016/j.yhbeh.2005.04.007. doi:10.1016/j.yhbeh.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Rutte C., Taborsky M., Brinkhof M.W.G. What sets the odds of winning and losing? Trends Ecol. Evol. 2006;21:16–21. doi: 10.1016/j.tree.2005.10.014. doi:10.1016/j.tree.2005.10.014 [DOI] [PubMed] [Google Scholar]
- Schroder F. Cyproterone acetate mechanism of action and clinical effectiveness in prostate cancer treatment. Cancer. 1993;72(Suppl.):3810–3815. doi: 10.1002/1097-0142(19931215)72:12+<3810::aid-cncr2820721710>3.0.co;2-o. doi:10.1002/1097-0142(19931215)72:12+<3810::AID-CNCR2820721710>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- Scott A.P., Canario A.V.M. 17α,20β-dihydroxy-4-pregnen-3-one 20-sulphate: a major new metabolite of the teleost oocyte maturation-inducing steroid. Gen. Comp. Endocrinol. 1992;85:91–100. doi: 10.1016/0016-6480(92)90176-k. doi:10.1016/0016-6480(92)90176-K [DOI] [PubMed] [Google Scholar]
- Scott A.P., Ellis T. Measurement of fish steroids in water: a review. Gen. Comp. Endocrinol. 2007;153:392–400. doi: 10.1016/j.ygcen.2006.11.006. doi:10.1016/j.ygcen.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Scott A.P., et al. Non-invasive measurement of steroids in fish-holding water: important considerations when applying the procedure to behaviour studies. Behaviour. 2008;145:1307–1328. doi:10.1163/156853908785765854 [Google Scholar]
- Sharpe R.L., MacLatchy D.L., Courtenay S.C., Van Der Kraak G.J. Effects of a model androgen (methyl testosterone) and a model anti-androgen (cyproterone acetate) on reproductive endocrine endpoints in a short-term adult mummichog (Fundulus heteroclitus) bioassay. Aquat. Toxicol. 2004;67:203–215. doi: 10.1016/j.aquatox.2004.01.009. doi:10.1016/j.aquatox.2004.01.009 [DOI] [PubMed] [Google Scholar]
- Singh M.S., Joy K.P. Effects of administration of cyproterone acetate on seminal vesicle and testicular activity, and serum testosterone and estradiol-17beta levels in the catfish, Clarias batrachus. Acta Biol. 1998;49:143–154. [Google Scholar]
- StatSoft, Inc. 2007 Statistica (data analysis software system), v. 8.0. See www.statsoft.com
- Trainor B.C., Marler C.A. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Horm. Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. doi:10.1006/hbeh.2001.1652 [DOI] [PubMed] [Google Scholar]
- Trainor B.C., Bird I.M., Marler C.A. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm. Behav. 2004;45:115–121. doi: 10.1016/j.yhbeh.2003.09.006. doi:10.1016/j.yhbeh.2003.09.006 [DOI] [PubMed] [Google Scholar]
- Turner G.F. The fighting tactics of male mouthbrooding cichlids: the effects of size and residency. Anim. Behav. 1994;47:655–662. doi:10.1006/anbe.1994.1089 [Google Scholar]
- Winberg S., Nilsson G.E. Roles of brain monoamine neurotransmitters in agonistic behaviour and stress reactions, with articular reference to fish. Comp. Biochem. Physiol. 1993;106C:597–614. [Google Scholar]
- Wingfield J.C. Regulation of territorial behavior in the sedentary song sparrow, Melospiza melodia morphna. Horm. Behav. 1994;28:1–15. doi: 10.1006/hbeh.1994.1001. doi:10.1006/hbeh.1994.1001 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C. A continuing saga: the role of testosterone in aggression. Horm. Behav. 2005;48:253–255. doi: 10.1016/j.yhbeh.2005.05.009. doi:10.1016/j.yhbeh.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C., Hahn T.P. Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim. Behav. 1994;47:77–89. doi:10.1006/anbe.1994.1009 [Google Scholar]
- Wingfield J.C., Hegner R.E., Dufty A.M., Jr, Ball G.F. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 1990;136:829–846. doi:10.1086/285134 [Google Scholar]