Abstract
In many taxa, the left and right testes often differ in size. The compensation hypothesis states that one testis of the pair serves as a ‘back-up’ for any reduced function in the other and provides a mechanism to explain intraspecific variation in degree and direction of gonad asymmetry. Although testis asymmetry is common in birds, evidence for natural testis compensation is unknown. Using a novel quantitative approach that can be applied to any bilateral organ or structure, we show that testis compensation occurs naturally in birds and can be complete when one testis fails to develop. Owing to a recurrent risk of testis impairment and an evolutionary trade-off between natural and sexual selections acting on the arrangement of internal organs in species with abdominal and/or seasonal testes, compensation adds an important, but neglected, dimension to measures of male reproductive investment.
Keywords: testis size, reproductive investment, bilateral symmetry, evolutionary trade-off
1. Introduction
(a) Interspecific variation in testis asymmetry
Testes asymmetry is widespread in nature, and it is particularly common and often pronounced in birds (e.g. Newton 1896; Romer 1971; Lake 1981). One of the major unresolved issues in vertebrate zoology is why the two testes of a given male often differ in size and/or shape. Avian gonads are located internally and in most species show marked seasonal changes in size, following an annual cycle of growth and regression (Lofts & Murton 1973; reviewed in Briskie & Montgomerie 2007). A common situation in avian taxa is that the testis on one side, often the left, develops to be larger than the other (Lake 1981; Briskie & Montgomerie 2007). Several hypotheses have been proposed to explain the left-biased testis asymmetry, but very few explain right-biased or lack of asymmetry in certain species. One exception (Newton 1896; Witschi 1935) is what we refer to as the packaging hypothesis (PH), a hypothesis that states that asymmetry in male gonads might reflect space constraints within the male body cavity; some organs, such as the liver and the gizzard, are positioned asymmetrically and may restrict the space available for the growth of each testis. Differences in the degree and/or direction of asymmetry across species might therefore reflect interspecific differences in the relative (external or internal) selection pressures on body cavity arrangement. Comparative studies are needed to investigate the relative role of the PH or other potential evolutionary explanations for interspecific differences in testis asymmetry.
(b) Intraspecific variation in testis asymmetry
The degree and/or direction of testis asymmetry also varies within a species. For example, in some species where directional asymmetry in testis size is the norm, the extent of this asymmetry is greater in older birds (e.g. Birkhead et al. 1997; Merilä & Sheldon 1999; Graves 2004). The concept of compensatory organ growth in bilaterally symmetric internal organs, for which there is good evidence in the medical literature (e.g. Stocum 2006), could provide an adaptive mechanism for the observed intraspecific variation in testis asymmetry. The compensation hypothesis (hereafter referred to as CH) states that one testis may grow more than it would be expected in order to compensate for a reduced function in the other gonad. Taken together, the packaging and compensation hypotheses suggest an evolutionary trade-off between natural and sexual selection pressures: intense post-copulatory sexual selection for larger testes that need to be accommodated within an already space-restricted body cavity. Assuming a link between morphological (i.e. size) and physiological (e.g. sperm production; de Reviers & Williams 1984; Møller 1988, 1989; Tuttle & Pruett-Jones 2004; but see Schärer & Vizoso 2007) compensation, deviations in the degree and/or direction of asymmetry in testis size from the species-specific pattern might therefore reflect individual adaptation. In addition, these deviations might be costly in terms of gametic traits and consequently individual post-copulatory reproductive success (e.g. Lifjeld et al. 2007; Urbach et al. 2007).
(c) The compensation hypothesis in birds
To date, evidence for testis compensation in birds comes from two quite different approaches: (i) unilateral castrations and (ii) correlations between absolute testis asymmetry and male quality. The first mention in the literature of the CH in birds is a study by Benoît (1925), who performed unilateral castrations in a small sample of domestic fowl (Gallus domesticus) to investigate whether the remaining testis showed hypertrophy, as had previously been found in mammals (Ribbert 1890). In a subsequent and more comprehensive study, Domm & Juhn (1927) defined compensatory hypertrophy as whenever the ‘loss [of one testis of the pair] results in stimulating the growth of the surviving member to an extent that tends to restore a normal quantitative balance between the total gonad tissue and the bird’ (Domm & Juhn 1927, p. 460). In other words, compensation occurs when the single retained testis grows to the size normally achieved by two gonads combined. Domm & Juhn's (1927) work showed that compensation can be complete, and it occurs in all ages and irrespective of side. Owing to the extreme nature of their experiment (surgical removal of one testis), the relevance of both Benoît's (1925) and Domm & Juhn's (1927) studies to unmanipulated birds is unknown. Also, the complete ablation of one testis might not mimic the situation in the wild and it remains largely unknown whether, and to what extent, males with a single testis, instead of the usual pair, occur in nature (cf. Riddle 1918, 1925). The only well-documented example of naturally occurring single gonad is the pattern within some coucal species (Centropus spp.) where the left testis is absent or severely atrophied in all males (e.g. Rand 1933; Ligon 1997; Goymann & Wingfield 2004). However note that the definition of compensation used in the current study (see §2) precludes scenarios of extreme asymmetry when the latter is a species-wide phenomenon, as in coucals, and instead reflects deviations from what is the normal (a)symmetry pattern for a given species.
The concept of testis compensation was used more recently in a study by Møller (1994): in two passerine species, males with larger secondary sexually selected traits, and hence assumed to be of higher quality, had more asymmetric testes than other males. This result was explained with reference to the CH, whereby it was suggested that the more symmetric testes of low-quality individuals constituted evidence for the (successful) compensatory ‘back-up’ function of the right testis, which was the smaller of the pair in both species studied (Møller 1994). By documenting within-species variation in testis asymmetry, this study provided the first qualitative and indirect evidence of natural testis compensation in wild populations. Although Møller's (1994) objective was not to test the CH, several subsequent studies examined the association between male quality and testis asymmetry, and sometimes interpreted it with reference to the CH (see table S1 in the electronic supplementary material). However, this correlational approach provides only a very indirect and limited test of the CH for it relies on at least five assumptions. First, it can be used only in taxa that show directional testis asymmetry and for which data on male quality are available, since the relationship between these two variables provides the basis for the test. Second, this analysis fails to control for differences between males in total testes size: it does not distinguish between males with two equally large or two equally small testes (i.e. high-symmetry cases). Moreover, absolute testis asymmetry is further confounded by seasonality, and males with more symmetric testes might simply be at an earlier growth or later regressive stage of the cycle. The use of relative asymmetry (e.g. Birkhead et al. 1997, 1998), however, eliminates this potential confounding factor, as there is no relationship between relative asymmetry and combined testis size during the testis cycle (see figure S1 in the electronic supplementary material). Third, Møller's (1994) interpretation of the CH assumes that the left testis of the pair (as the larger) has the major functional role and that the right testis (as the smaller) serves as back-up. Although difficult to test, two indirect methods have been used to check this assumption: (i) if the smaller testis is a constant proportion of the larger, the smaller testis cannot serve as a compensatory back-up (Birkhead et al. 1998) and (ii) the testis with the back-up function will be more variable in size (Kimball et al. 1997). Note that method (i) can be used only in species with directionally asymmetric testes. Fourth, it is implicitly assumed that only low-quality males show testis compensation. Fifth, the correlational method can ultimately provide only a qualitative assessment of the CH. Considering these various assumptions, it is not surprising, therefore, that the existing evidence for natural compensation in testis size in wild birds is unclear (summarized in table S1 of the electronic supplementary material).
In short, neither (i) experimental unilateral castration nor (ii) the correlational method can show conclusively whether, and to what extent, testis compensation occurs naturally in birds. We have developed a novel approach that allows the quantification of the incidence and extent of testis compensation ability within and between species, using intraspecific naturally occurring variation in testis size. This method does so independently of the species's pattern of testis (a)symmetry or side, and does not require data on male quality. Its major assumption is that the functionally optimal test(i/e)s size for a given species is the mean size of the population, provided individuals are all sampled at the peak breeding stage (see Calhim & Birkhead 2007). Selective forces would select for compensation when one of the testis falls bellow these thresholds. Since the relationship between testes size and daily sperm production in birds is close to 1 : 1 (e.g. de Reviers & Williams 1984; Møller 1988; but see Lüpold et al. 2009), it is not unreasonable to assume, as we have done here, that under natural and/or sexual selection, the optimal testes size for a given species corresponds to the mean value. Nevertheless, the proposed model (see §2) can be adapted to use different threshold values, if future studies suggest otherwise.
The aim of this paper is to test the CH in two species that differ in both testis asymmetry patterns and post-copulatory sexual selection (e.g. Birkhead et al. 1990; Dallimer & Jones 2007): the red-billed quelea (Quelea quelea) and the zebra finch (Taeniopygia guttata).
2. Material and methods
(a) Assessing compensation
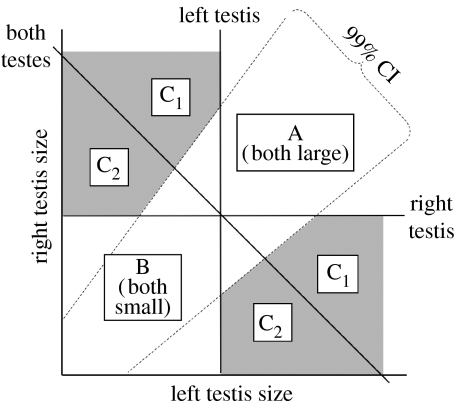
All else being equal, compensation is expected whenever one testis of the pair shows hypotrophy; in other words, when that testis is smaller than the population average size for that side, or in the most extreme case, missing altogether. If the other testis is in turn larger than expected for its respective side (based on population average), then compensation has occurred. We defined compensation as complete if the combined size of the two gonads was equal to or greater than the population average combined testes size (sensu Domm & Juhn 1927); otherwise, compensation was only partial. The method used to assess whether, and to what extent, compensation occurred comprises a graphical analysis of a plot of right testis size against left testis size (figure 1). Males in area A have each testis larger than the population average size for the respective side. By contrast, those in area B have both testes smaller than the population average. Cases of testis compensation occur in the shaded area C (C1 and C2): one testis of the pair is smaller and the other is larger than the population average for each given side. The upper and lower shaded areas refer to compensation achieved by the right or by the left gonad, respectively. Areas C1 and C2 to complete (i.e. combined size above the diagonal line corresponding to the population median combined size) and partial (i.e. combined size is below the latter line) compensation, respectively. The dashed lines refer to the 99 per cent confidence levels for the relationship between the left and right testis sizes (using standard major axis regression; Sokal & Rohlf 1981) and are used to differentiate considerable deviations from this relationship, which defines the normal variability in testes size within a species. Compensation incidence corresponds to the ratio of individuals in area C (showing compensation) expressed as a proportion of those individuals that ought to have shown compensation (all except those in area A). In addition, we can speculate which testis had the greater compensatory potential and conversely which side had the major functional role. The latter can be assessed as the difference between the mean deviation from the relationship (i.e. |residuals|) within the upper and lower C areas, where compensation is achieved through the right or the left testis, respectively. According to Møller's (1994) prediction, the left testis has the major role, thus we should expect lower deviations and/or fewer cases in the lower compared with the upper C area. Bootstrap regression techniques were used to test the observed against a null scatter distribution of data points for each species (see the electronic supplementary material for full description and results of these analyses). Observed distributions were not random (see table S2 and figure S2 in the electronic supplementary material). All statistical analyses were conducted using R v. 2.3.1 (R Development Core Team 2006).
Figure 1.
Diagrammatic representation of the graphical approach used for determining the natural incidence and extent of testis compensation in wild birds. In a plot of right testis size against left testis size, males in area A have each testis larger than the population median size for that given side. By contrast, those in area B have both testes smaller than the population median. Any data point in the shaded area C indicates compensation: one testis is smaller, but the other is larger than the population median for the respective side. Upper and lower areas C refer to compensation achieved by the right or left gonad, respectively. For males showing compensation, if the combined size of both testes is equal or greater than the population median combined size, compensation is considered complete (area C1); otherwise compensation is partial (area C2). The dashed lines refer to the 99% confidence levels for the relationship between the left and right testis sizes and are used to differentiate considerable deviations from this relationship for a more conservative quantification of compensation incidence.
(b) Study species
(i) Red-billed quelea
The red-billed quelea is an agricultural pest weaver common across sub-Saharan Africa (Bruggers & Elliott 1989; Mundy & Jarvis 1989). The birds used were captured under licence as part of a control programme in a private farm in South Africa, using standard walk-in wire traps placed next to cereal fields. Birds were humanely killed and dissected immediately afterwards. We obtained linear dimensions (length and width) for the left and right testes and used the formula for volume of an ellipsoid to calculate an index of testis volume (e.g. Møller 1991). Testis volume was then converted to mass using a species-specific testis density constant (Calhim & Birkhead 2007), obtained from a subset of testes (n=28 males) for which we had both mass and linear dimensions (testis density (mean±s.d.)=1.086±0.096 g cm−3). These testes, which had been previously fixed in 10 per cent formalin solution, were weighed (to the nearest 0.001 g) using a high-precision Mettler AT261 digital balance. Tarsus length was recorded in the field in order to obtain body-size-controlled testis size (i.e. gonadosomatic index, GSI=ratio between test(i/e)s mass and tarsus length). All males were collected within a period of 4 days, at a time when breeding had started (all nests found contained partial clutches) at a known colony near the farm. Red-billed quelea are itinerant opportunistic breeders, with short duration and highly synchronous colonial breeding bouts (Jones 1989). Therefore, we can assume that sampling took place very close to the peak of this species's testis cycle (see Calhim & Birkhead 2007). However, we excluded six individuals that were clearly not in breeding condition since we were unable to obtain any sperm from the seminal glomera and both their testes were smaller than 0.084 g (equivalent to a left and right GSI=0.46, cf. figure 2a).
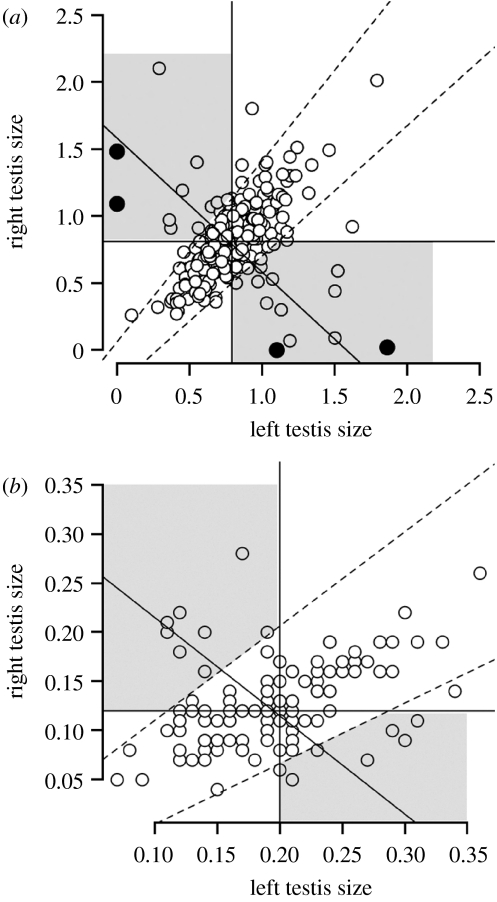
Figure 2.
Relationship between the size of the right and left testes (as percentage of body mass or GSI) coded as described in figure 1: (a) red-billed quelea (n=232) and (b) zebra finch (n=118). Solid lines refer to population median sizes for the left, right and combined testes. Dashed lines represent the 99% confidence levels for the relationship between the right and left testis sizes. Filled circles distinguish the n=4 males with a single testis.
(ii) Zebra finch
Testis size data were obtained from a population of domesticated birds from an outbred colony established at the University of Sheffield in 1985. All birds were sexually mature and known to be in breeding condition at the time when they were humanely killed; in fact, zebra finches tend to be in constant readiness to breed in captivity and in the wild (Farner & Serventy 1960; Sossinka 1982). Data were collected as part of an ongoing genetic study of the natural variation in sperm traits in the species (see Birkhead et al. 2005), and only individuals producing sperm were included (n=118). Both testes were removed and weighed fresh using a high-precision (to the nearest 0.001 g) Mettler AT261 digital balance. GSI for this species corresponds to the ratio between test(i/e)s mass and body mass at the time of death.
3. Results
(a) Red-billed quelea
There was no significant difference between the left and right testis sizes, as a percentage of body size or GSI (combined GSI=1.58, left GSI=0.79, right GSI=0.81; Wilcoxon signed-rank test V=11054, p=0.0719). There was a positive association between the sizes of the left and right testes (Pearson r=0.30, d.f.=225, p<0.0001), with a slope not significantly different from unity (type II regression, forced through the origin: p=0.243). Therefore, males had on average equal-sized testes. Of the total n=232 males, 160 (68%) have at least one testis smaller than the median for the population, and therefore would be under selection for compensation. Using our criteria for compensation, 27 out of 160 (17%) males showed testis compensation, with compensation being complete in 15 (9%) males. Compensation was observed in both left (n=15) and right (n=12) gonads, with no difference in degree (Kolmogorov–Smirnov test, D=0.27, p=0.71). We also found evidence for naturally occurring extreme asymmetry (figure 2a): four individuals (approx. 2%, 4/232) had only a single testis (two males with only the right testis, the other two with only the left), and in two of these cases the single gonad compensated fully, or nearly so, for its missing partner. Left testis are 4 per cent less variable in size across males (coefficient of variation, CV, in mass: left=34%, right=38%).
(b) Zebra finch
The left and right testes are positively correlated (Pearson r=0.40, p<0.0001), but the left testis was significantly larger than the right (combined GSI=0.31, left GSI=0.19, right GSI=0.12, Wilcoxon signed-rank test, V=6553.5, p<0.0001, type II regression intercept=−0.21), confirming the previously reported left-biased testis asymmetry in this species (Birkhead et al. 1998). Of the total n=118 males, 82 (70%) have at least one testis smaller than the median for the population, and therefore would be under selection for compensation. Using our criteria for compensation, 14 out of 82 (17%) males showed testis compensation, of which nine (11%) achieved complete compensation. Compensation occurred in both left (n=6) and right (n=8) gonads, with no difference in degree (Kolmogorov–Smirnov test, D=0.5, p=0.30). In contrast to the results from the red-billed quelea, we found no males with a single testis in the present study (figure 2b), although a few cases of extreme asymmetry have previously been noted during the 20 years that this population has been studied (T. R. Birkhead 1985–2009, personal observation). Intermale variance in size of the right testis is 5 per cent higher than of the left (CV in mass: left=31%, right=36%).
4. Discussion
Our study provides the first evidence of naturally occurring compensation in testis size in birds. In almost 20 per cent of males with at least one underdeveloped testis the other testis was larger than expected. We found no compelling evidence for the right testis to have the major role in compensation, as had been suggested by Møller (1994; see also Kimball et al. 1997), since it shows only marginally higher intermale size variation than the left testis. Differences in the degree or incidence of compensation across species could be driven by various selection pressures and/or costs associated with compensation ability in each taxon. For instance, physiological costs of compensatory deviations might be larger under strong directional testis asymmetry. This could result in quantitatively reduced compensation in species with very asymmetric testes. Also, selection for compensatory ability is likely to be stronger in species already under selective pressure for larger testes; for instance, those taxa under intense sperm competition (e.g. promiscuous mating systems) or under high risk of sperm depletion (e.g. polygynous or lek mating systems). As a consequence, one might expect a higher incidence of compensation in these species than monogamous ones. Interpreting interspecific patterns requires more data than we had available. Nevertheless, incidence and degree of compensation was similar in the two species studied, despite differing levels of promiscuity.
(a) Evolutionary explanations
Many birds show marked seasonal changes in testis size (Lofts & Murton 1973), so the potential for possible testis developmental problems, for either internal or external reasons, reoccurs every breeding season. The physiological ability to adaptively compensate for ‘developmental accidents’ is likely to be important and be therefore subject to considerable (annual) selection. Our finding that testis compensation occurs in the zebra finch, despite no marked annual testis cycle (Farner & Serventy 1960; Sossinka 1982), suggests that the adaptive value of testis compensation is high irrespective of breeding seasonality. The strength of selection for compensation is best illustrated by the existence of males that naturally have only one testis. Although the frequency of such individuals was low in red-billed quelea (2%, 4/229) and even lower in the zebra finch (see above), data from other passerines suggest that this extreme scenario might be more frequent than previously thought (e.g. 7% (2/29) in the yellow-headed blackbird, Xanthocephalus xanthocephalus; S. Lüpold 2007, personal observation). In all these cases, the single testis was larger than expected for its side, regardless of whether it achieved full compensation. The physiological costs associated with having a single but much larger testis in a species where two gonads are expected is unknown. Histological analyses of hyper- and hypotrophic testis could prove fruitful. The fact that these cases are not uncommon, however, is in itself a strong indication of the selective pressure for adaptive compensatory potential of the avian testis.
(b) Male quality, revisited
Møller's (1994) study used the CH as an a posteriori interpretation for a positive relationship between male quality and degree of absolute testis asymmetry: low-quality males had more similar-sized testes because the normally larger left testis failed to develop properly in these individuals, and the smaller right testis (the back-up gonad) grew more than expected for its side to compensate. Our approach to the CH allows a more apposite test of the intraspecific relationship between male quality and testis compensation, since it allows a quantitative assessment of degree of compensation irrespective of species (a)symmetry. Unfortunately, we do not have reliable male quality data to conduct such analyses with the current sample. Nonetheless, we might predict that male quality measures should follow hierarchically with respect to the areas demarcated in figure 1 as A>C1>C2>B, provided the following four assumptions are met: (i) only males in reproductive condition are included in the analysis, (ii) the male quality traits used provide a reliable measure of reproductive fitness, (iii) individual reproductive fitness is strongly associated with relative testis size (e.g. no strong trade-off between sperm number and quality), and (iv) no confounds of alternative mating strategies. For instance, a male showing testis compensatory growth might be expected to be of higher quality than another male that did not, when both males have the same, but still lower than average, combined testes size; without reference to the CH, these two individuals would be considered of equally low quality. Furthermore, the use of the (vertical axis) residual values from the (type II) regression would allow an assessment of an individual's degree of compensation.
(c) Final conclusions
Using a novel quantitative approach that can be applied to any bilateral organ or structure, we have shown that testis compensation occurs naturally in birds and that it can be complete when one testis fails to develop. Selection for the ability to compensate is likely to be widespread and adaptive in any taxa where (i) space constraints create an evolutionary trade-off between natural and sexual selection forces on internal body arrangement and (ii) there is a recurrent risk of impaired testis growth due to seasonal cycles of gonad growth and regression. Compensation ability therefore adds an important, but so far neglected, dimension to a key issue in intraspecific sexual selection studies: the use of testes size as a measure of male reproductive investment. Since testis compensation is achieved through deviations from a species's naturally selected testis (a)symmetry pattern, and since these deviations are likely to be physiologically costly, whether and the degree to which it is achieved may therefore reflect male quality. By combining overall testes size with compensation ability, our study provides a framework that allows for a more thorough assessment of male reproductive investment.
Acknowledgments
This research was conducted with ethical approval from the Department of Tourism, Environmental & Economic Affairs, South Africa, and the Home Office, United Kingdom.
We thank M. Stander, A. Stander and R. Visagie for providing logistic and practical help in the field. J. Dale, S. Immler, S. Lüpold, R. Montgomerie and R. Snook provided their valuable comments on the manuscript. Research was conducted under licence from the government of South Africa (HK/P1/07081/001 and HK/P1/07425/001). S.C. was funded by the Portuguese Science and Technology Foundation (FCT Portugal; SFRH/BD/10040/2003). T.R.B. was funded by the Leverhulme Trust.
Supplementary Material
Testes asymmetry and seasonality
Literature review
Boostrap analyses results
References used in Supplementary Materials 1–3
References
- Benoît M.J. Sur l'hypertrophie compensatrice aprés castration unilaterale, chez le coq domestique. CR Acad. Sci. 1925;180:1690–1692. [Google Scholar]
- Birkhead T.R., Burke T., Zann R., Hunter F.M., Krupa A.P. Extra-pair paternity and intrabrood parasitism in wild zebra finches Taeniopygia gutata revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 1990;27:315–324. doi:10.1007/BF00164002 [Google Scholar]
- Birkhead T.R., Buchanan K.L., Devoogd T.J., Pellatt E.J., Szèkely T., Catchpole C.K. Song, sperm quality and testes asymmetry in the sedge warbler. Anim. Behav. 1997;53:965–971. doi:10.1006/anbe.1996.0423 [Google Scholar]
- Birkhead T.R., Fletcher F., Pellatt E.J. Testes asymmetry, condition and sexual selection in birds: an experimental test. Proc. R. Soc. B. 1998;265:1185–1189. doi:10.1098/rspb.1998.0417 [Google Scholar]
- Birkhead T.R., Pellatt E.J., Brekke P., Yeates R., Castillo-Juarez H. Genetic effects on sperm design in the zebra finch. Nature. 2005;434:383–387. doi: 10.1038/nature03374. doi:10.1038/nature03374 [DOI] [PubMed] [Google Scholar]
- Briskie J.V., Montgomerie R. Testis size, sperm size and sperm competition. In: Jamieson B.G.M., editor. Reproductive biology and phylogeny of birds. Part A: phylogeny, morphology, hormones, fertilization. Science Publishers; Enfield, NH: 2007. pp. 513–551. [Google Scholar]
- Bruggers R.L., Elliott C.C. Oxford University Press; Oxford, UK: 1989. Quelea quelea, Africa's bird pest. [Google Scholar]
- Calhim S., Birkhead T.R. Testes size in birds: assumptions, errors and estimates. Behav. Ecol. 2007;18:271–275. doi:10.1093/beheco/arl076 [Google Scholar]
- Dallimer M., Jones P.J. An estimation of the rate of reproductive cheating in the red-billed quelea Quelea quelea. Ostrich. 2007;78:637–639. doi:10.2989/OSTRICH.2007.78.3.11.322 [Google Scholar]
- de Reviers M., Williams J.B. Testis development and production of spermatozoa in the cockerel (Gallus domesticus) In: Cunningham F.J., Lake P.E., Hewitt D., editors. Reproductive biology of poultry. British Poultry Science Ltd; Harlow, UK: 1984. pp. 183–202. [Google Scholar]
- Domm L.V., Juhn M. Compensatory hypertrophy of the testes in brown leghorns. Biol. Bull. 1927;52:458–473. doi:10.2307/1536907 [Google Scholar]
- Farner D.S., Serventy D.L. The timing of reproduction in birds in the arid regions of Australia. Anat. Rec. 1960;137:354. [Google Scholar]
- Goymann W., Wingfield J.C. Competing females and caring males. Sex steroids in African black coucals, Centropus grillii. Anim. Behav. 2004;68:733–740. doi:10.1016/j.anbehav.2003.12.012 [Google Scholar]
- Graves G.R. Testicular volume and asymmetry are age-dependent in black-throated blue warblers (Dendroica caerulescens) Auk. 2004;121:473–485. doi:10.1642/0004-8038(2004)121[0473:TVAAAA]2.0.CO;2 [Google Scholar]
- Jones P.J. The breeding cycle of the Quelea quelea and factors initiating breeding. In: Mundy N.I., Jarvis M.F.J., editors. Africa's feathered locust. Baobab Books; Harare, Zimbabwe: 1989. pp. 36–49. [Google Scholar]
- Kimball R.T., Ligon J.D., Merola-Zwartjes M. Testicular asymmetry and secondary sexual characters in red jungle fowl. Auk. 1997;114:221–228. [Google Scholar]
- Lake P.E. Male genital organs. In: King A.S., McLelland J., editors. Form and function in birds. vol. 2. Academic Press; London, UK: 1981. pp. 1–61. [Google Scholar]
- Lifjeld J.T., Laskemoen T., Fossoy F., Johnsen A., Kleven O. Functional infertility among territorial males in two passerine species, the willow warbler Phylloscopus trochilus and the bluethroat Luscinia svecica. J. Avian Biol. 2007;38:267–272. [Google Scholar]
- Ligon J.D. A single functional testis as a unique proximate mechanism promoting sex-role reversal in coucals. Auk. 1997;114:800–801. [Google Scholar]
- Lofts B., Murton R.K. Reproduction in birds. In: Farner D.S., King J.R., Parkes K.C., editors. Avian biology III. Academic Press; London, UK: 1973. pp. 1–107. [Google Scholar]
- Lüpold S., Linz G.M., Rivers J.W., Westneat D.F., Birkhead T.R. Sperm competition selects beyond relative testes size in birds. Evolution. 2009;63:391–402. doi: 10.1111/j.1558-5646.2008.00571.x. doi:10.1111/j.1558-5646.2008.00571.x [DOI] [PubMed] [Google Scholar]
- Merilä J., Sheldon B.C. Testis size variation in the greenfinch Carduelis chloris: relevance for some recent models of sexual selection. Behav. Ecol. Sociobiol. 1999;45:115–123. doi:10.1007/s002650050545 [Google Scholar]
- Møller A.P. Testes size, ejaculate quality and sperm competition in birds. Biol. J. Linn. Soc. 1988;33:273–283. doi:10.1111/j.1095-8312.1988.tb00812.x [Google Scholar]
- Møller A.P. Ejaculate quality, testes size and sperm production in mammals. Funct. Ecol. 1989;3:91–96. doi:10.2307/2389679 [Google Scholar]
- Møller A.P. Sperm competition, sperm depletion, paternal care, and relative testis size in birds. Am. Nat. 1991;137:882–906. doi:10.1086/285199 [Google Scholar]
- Møller A.P. Directional selection on directional asymmetry: testes size and secondary sexual characters in birds. Proc. R. Soc. B. 1994;258:147–151. doi:10.1098/rspb.1994.0155 [Google Scholar]
- Mundy N.I., Jarvis M.F.J. Baobab Books; Harare, Zimbabwe: 1989. Africa's feathered locust. [Google Scholar]
- Newton A. Adam and Charles Black; London, UK: 1896. A dictionary of birds. [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language and environment for statistical computing. [Google Scholar]
- Rand A.L. Testicular asymmetry in the Madagascar coucal. Auk. 1933;50:219–220. [Google Scholar]
- Ribbert H. Über die kompensatorische Hypertrophie der Geschlechtsdrüsen. Virchows Arch. 1890;120:247–272. doi:10.1007/BF01929284 [Google Scholar]
- Riddle O. Further observations on the relative size and form of the right and left testis of pigeons in health and disease as influenced by hybridity. Anat. Rec. 1918;14:283–334. doi:10.1002/ar.1090140503 [Google Scholar]
- Riddle O. Birds without gonads: their origin, behaviour, and bearing on the theory of the internal secretion. J. Exp. Biol. 1925;2:211–246. [Google Scholar]
- Romer A.S. Saunders; Philadelphia, PA: 1971. The vertebrate body. [Google Scholar]
- Schärer L., Vizoso D.B. Phenotypic plasticity in sperm production rate: there's more to it than testis size. Evol. Ecol. 2007;21:295–306. doi:10.1007/s10682-006-9101-4 [Google Scholar]
- Sokal R.R., Rohlf F.J. W.H. Freeman & Company; New York, NY: 1981. Biometry. [Google Scholar]
- Sossinka R. Domestication in birds. In: Farner D.S., King A.S., Parkes K.C., editors. Avian biology VI. Academic Press; London, UK: 1982. pp. 373–403. [Google Scholar]
- Stocum D.L. Academic Press; San Diego, CA: 2006. Regenerative biology and medicine. [Google Scholar]
- Tuttle E.M., Pruett-Jones S. Estimates of extreme sperm production: morphological and experimental evidence from reproductively promiscuous fairy-wrens (Malurus) Anim. Behav. 2004;68:541–550. doi:10.1016/j.anbehav.2003.09.014 [Google Scholar]
- Urbach D., Bittner D., Lenz T.L., Bernet D., Whali T., Wedekind C. Sperm velocity in an Alpine whitefish: effects of age, size, condition, fluctuating asymmetry and gonad abnormalities. J. Fish Biol. 2007;71:672–683. doi:10.1111/j.1095-8649.2007.01537.x [Google Scholar]
- Witschi E. Origin of asymmetry in the reproductive system of birds. Am. J. Anat. 1935;56:119–141. doi:10.1002/aja.1000560107 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Testes asymmetry and seasonality
Literature review
Boostrap analyses results
References used in Supplementary Materials 1–3