Abstract
To combat disease, most fungus-growing ants (Attini) use antibiotics from mutualistic bacteria (Pseudonocardia) that are cultured on the ants' exoskeletons and chemical cocktails from exocrine glands, especially the metapleural glands (MG). Previous work has hypothesized that (i) Pseudonocardia antibiotics are narrow-spectrum and control a fungus (Escovopsis) that parasitizes the ants' fungal symbiont, and (ii) MG secretions have broad-spectrum activity and protect ants and brood. We assessed the relative importance of these lines of defence, and their activity spectra, by scoring abundance of visible Pseudonocardia for nine species from five genera and measuring rates of MG grooming after challenging ants with disease agents of differing virulence. Atta and Sericomyrmex have lost or greatly reduced the abundance of visible bacteria. When challenged with diverse disease agents, including Escovopsis, they significantly increased MG grooming rates and expanded the range of targets. By contrast, species of Acromyrmex and Trachymyrmex maintain abundant Pseudonocardia. When challenged, these species had lower MG grooming rates, targeted primarily to brood. More elaborate MG defences and reduced reliance on mutualistic Pseudonocardia are correlated with larger colony size among attine genera, raising questions about the efficacy of managing disease in large societies with chemical cocktails versus bacterial antimicrobial metabolites.
Keywords: Attini, mutualism, Pseudonocardia, metapleural gland, public health, social complexity
1. Introduction
Disease agents are important selective forces on social evolution (Hamilton 1982; Schmid-Hempel 1998; Fefferman et al. 2007; Stow et al. 2007). Pathogens can determine upper limits of host group size, because increasing density of individuals results in elevated contact rates that facilitate high rates of pathogen transmission (Hamilton 1982; Schmid-Hempel 1998; Boomsma et al. 2005), potentially leading to epidemics within genetically homogeneous groups (Schmid-Hempel 1998; Hughes et al. 2002). Social insects use diverse methods to prevent and combat disease agents, such as grooming or other hygienic behaviours and the use of antimicrobial compounds from glandular secretions or external sources (e.g. bacteria; Oi & Pereira 1993; Currie et al. 1999a; Mueller et al. 2005). The evolution of larger colony sizes places greater demands on ‘public health’ adaptations (e.g. Fefferman et al. 2007; Stow et al. 2007), but trade-offs associated with different disease management strategies are not well understood.
The fungus-growing ants (Attini) provide an opportunity to explore the evolutionary relationships between changes in hygienic strategies and social complexity (Mueller et al. 2005). This tribe includes 12 genera and over 210 species (Schultz & Brady 2008), among which colony size varies by six orders of magnitude (Weber 1972). Colony size is partly associated with queen mating frequency, such that gynes from basal and transitional genera mate once, while those from derived leafcutter genera mate multiply, so that genetic heterogeneity is greater in colonies with larger numbers of workers (Villesen et al. 2002), which is advantageous in disease management (Hughes & Boomsma 2006; Mattila & Seeley 2007).
The attine ants have an obligate and ancient (approx. 50 million years BP; Mueller 2002; Schultz & Brady 2008) mutualism with basidiomycete fungi, which are cultivated as a food source and defended from competitors and pathogens. They also have an ancient association with actinomycete Pseudonocardia bacteria (Currie et al. 1999b, 2006), which ants of most genera culture in specialized structures on their cuticle. Available evidence indicates that Pseudonocardia metabolites are narrow-spectrum antimicrobials active against Escovopsis, a potentially virulent specialized fungus that attacks the ants' mutualistic fungus (Currie et al. 1999b, 2003a, 2006). In addition to Escovopsis, an array of generalist pathogens may attack both the fungal symbiont and the ants (Currie et al. 1999b; Jaccoud et al. 1999; Currie & Stuart 2001; Hughes & Boomsma 2004). Exocrine gland secretions, especially from the metapleural glands (MG), are broad-spectrum antimicrobials (do Nascimento et al. 1996; Bot et al. 2002; Fernández-Marín et al. 2006). In short, ants reduce the pathogen load within nests by (i) monitoring contaminant levels (Fernández-Marín et al. 2006), (ii) grooming their bodies and those of adult and immature nest-mates (Bailey 1920; Weber 1972; Quinlan & Cherrett 1977), (iii) actively regulating antimicrobials from exocrine glands (Fernández-Marín et al. 2006), (iv) weeding the garden (Bass & Cherrett 1994; Currie et al. 2006), and (v) deploying antibiotics from Pseudonocardia (Currie et al. 1999a, 2003b; Little et al. 2006).
In the present paper, we examine the relative use of Pseudonocardia metabolites and MG secretions in disease management strategies, and associated trade-offs among derived and basal attine genera. We explore the evolutionary relationships between large-scale farming and the relative investment in hygienic strategies involving direct chemical and behavioural control of pathogens versus bacteria-derived antibiotics.
2. Material and methods
(a) Relative abundance of Pseudonocardia
Nests from nine species representing five genera were collected in Soberanía National Park, Panama: Atta colombica (n=10 colonies); Atta sexdens (n=5); Atta cephalotes (n=7); Acromyrmex octospinosus (n=10); Trachymyrmex zeteki (n=9); Trachymyrmex cf. cornetzi (n=10); Sericomyrmex sp. 1 (n=4); Sericomyrmex amabilis (n=10); and Cyphomyrmex longiscapus (n=6). Ant colonies were transferred to plastic containers where they were maintained using standard laboratory methods (Weber 1972). Using a stereomicroscope, we scored 25–150 worker ants per colony for the presence of a ‘conspicuous white bloom’ on the propleural plates or other thoracic areas, which is the standard criterion for the presence of Pseudonocardia (Currie et al. 1999b, 2006; Poulsen et al. 2003b). To measure relative abundance at the colony level, we used the proportion of workers with visible actinomycetes.
(b) MG grooming following fungal inoculation with Penicillium
From each colony, we established sub-colonies in transparent plastic boxes (7.4×7.4×3.1 cm) that each contained 40 workers, 1 g of fungus garden (except for C. longiscapus, for which we used 0.5 g), six larvae and six pupae (Fernández-Marín et al. 2006). We used two pieces of parafilm (each approx. 5×5 mm2) to transfer conidia to each garden. On each piece, we placed an approximately 3 mm2 piece of pure culture (potato dextrose agar, PDA media; see below) of Penicillium sp. 1 with conidia, and each sub-colony thus received a total of 1.5–2.3×107 dry conidia. To transfer the dry conidia, each piece of parafilm was held with sterile forceps and gently brushed over the fungus garden and brood. During the next hour, we recorded MG grooming by all workers observed in a 3.5 cm diameter field of view of a stereomicroscope (7×). We recorded MG grooming for 1 hour as a baseline measure prior to inoculations, but first we manipulated the garden with two parafilm pieces that lacked conidia to control for physical changes to the garden. MG grooming is defined as a worker ant partially extending its legs to raise the body from the substrate, and then flexing the foreleg at the femorotibial joint to bring the posterior surface of the metatarsus into contact with the opening of the paired MG.
(c) MG grooming after infection with Metarhizium and Escovopsis
To ascertain MG grooming rates when challenged by a fungal pathogen (Escovopsis) and an entomopathogen (Metarhizium anisopliae), we used A. colombica, Ac. octospinosus, S. amabilis and T. zeteki. From each of 10 nests per species, we established two sub-colonies containing 20 workers and 1 g of fungus garden. Atta and Acromyrmex have polymorphic workers, so we used workers with head widths between 1.3 and 1.5 mm and the same body colour (and therefore approximately the same age; Poulsen et al. 2002, 2003b). We used a two-factorial treatment, where we added three larvae and three pupae to one replicate sub-colony, and left the other without brood. Five sub-colonies per species were inoculated with approximately 1.5×106 dry conidia of M. anisopliae, and five were inoculated with approximately 1.5×106 dry conidia of an appropriate Escovopsis strain (see below). Inoculations for both pathogens were done with the same procedure as for Penicillium. For each sub-colony, we recorded MG grooming during 90 min after inoculation. We also noted the targets that workers contacted with their legs immediately after each bout of MG grooming (i.e. garden, brood, nest-mate workers, a worker's own body).
We used the following pure cultures: a contaminant weed, Penicillium sp. 1, isolated from the cuticle of a queen of A. colombica; M. anisopliae strain Ma275 obtained from the University of Copenhagen, isolated from beetles; and Escovopsis strains isolated from the corresponding ant host species. Dry conidia were applied because inoculation by suspension with water plus detergent, as usually used (Currie et al. 2003b; Hughes & Boomsma 2004), induces lower MG grooming rates. All fungal cultures were grown and maintained in Petri dishes with PDA (19.5 g per 0.5 l of distilled water) without antibiotics.
Mature colony sizes were taken from the following sources: C. longiscapus (Mueller & Wcislo 1998); T. zeteki and T. cf. cornetzi (H. Fernández-Marín, E. B. Gomez, J. J. Boomsma, D. Nash & W. T. Wcislo 2008, unpublished data); Sericomyrmex sp. 1 and S. amabilis (Murakami et al. 2000; H. Fernández-Marín 2004–2006, unpublished data); A. colombica (Lewis 1975; Fowler et al. 1986); A. sexdens and A. cephalotes (Weber 1972); and Ac. octospinosus (Lewis 1975).
(d) Statistical analyses and voucher specimens
Rates of MG grooming before and after Penicillium infection were analysed using general linear models (GLMs) with Poisson errors (JMP 7.02, SAS Inc., Cary, NC, USA), using the number of grooming events per hour as the dependent variable. Comparisons of actinomycete cover among species were made using GLM with binomial errors, with the number of actinomycete-coated workers from each colony as the dependent variable and the total number of workers examined in each colony as the binomial denominator. The relationship between MG grooming rate after infection and relative abundance of actinomycetes was assessed using Spearman's rank correlation. Where colony size was used as a predictor variable, it was normalized by logarithmic transformation. The phylogenetically independent relationship between MG grooming rate after infection and relative abundance of actinomycetes was assessed using linear correlation through the origin (Garland et al. 1992) between phylogenetically independent contrasts for both variables, generated using the PDAP v. 1.14 module (Midford et al. 2008) of the program Mesquite v. 2.5 (Maddison & Maddison 2008), based on the phylogenetic tree presented in figure 1; branch lengths were estimated from Schultz & Brady (2008).
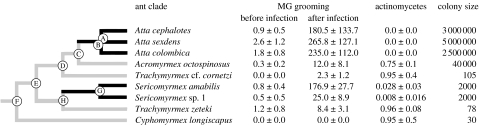
Figure 1.
Mapping traits onto the attine phylogenetic tree (from Schultz & Brady 2008) shows the independent recurrence of a reduction in visibly cultured Pseudonocardia bacteria, which is associated with increased MG grooming rates (black lines). Mapped traits are mean rates of MG grooming following exposure to conidia of Penicillium sp. 1, proportion of workers with visible bacteria and estimated colony sizes. Data are presented as mean±s.d., except estimated colony size. Letters of nodes in the phylogeny correspond to those in the inset of figure 2.
Comparisons among species of baseline and post-exposure rates of MG grooming for ant species with (Acromyrmex and Trachymyrmex) and without visible actinomycetes (Atta and Sericomyrmex) following exposure to Escovopsis and Metarhizium were examined using logistic regression. Since worker ants either groomed at a high rate or hardly groomed at all, each colony was classified as grooming (more than 10 grooming events in 90 min; mean=123.6) or not (less than five grooming events; mean=0.529), based on a k-means mixture analysis (JMP 7.02). Ant colony was nested within species and pathogen treatment to take into account differences in grooming behaviour between colonies. To compare targets following MG grooming, we used only treatments with brood, and employed a GLM and multinomial logistic regression analyses. Where appropriate, all GLMs were corrected for overdispersion in the data. Voucher specimens are deposited in the dry reference collection, Smithsonian Tropical Research Institute, Panama.
3. Results
A high percentage of Acromyrmex, Trachymyrmex and Cyphomyrmex workers had a conspicuous white bloom of Pseudonocardia on their exoskeletons, while workers of Atta lacked it (figures 1 and 2). Sericomyrmex had a few (less than 2.5%) workers with fine white lines of bacteria on the head or thorax, but no bloom. The proportion of workers with Pseudonocardia differed significantly between species (GLM with binomial errors: likelihood ratio, LR, Χ82=1812.56, p<0.0001), with 27 per cent of the variation between species explained by differences in average colony size, with a smaller proportion of workers from species with large average colony size having visible Pseudonocardia (LR Χ12=60.83, p<0.0001).
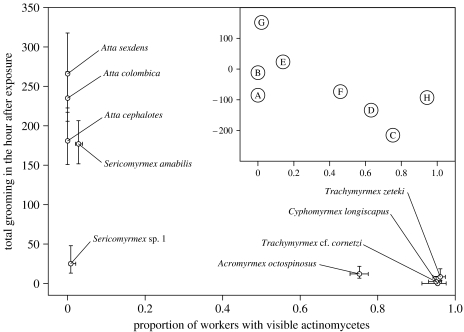
Figure 2.
MG grooming rates by attine workers (mean±s.e., based on log-transformed data) following exposure to Penicillium, compared with the proportion of workers with visible Pseudonocardia bacteria (mean±s.e., based on logit-transformed data). The inset graph shows the relationship between the positivized phylogenetically independent contrasts for the two variables, based on the phylogenetic tree presented in figure 1, with each data point labelled with the node letter from the phylogeny.
Prior to inoculation with Penicillium sp. 1, the ant species differed somewhat in baseline rates of MG grooming (GLM with Poisson errors: LR Χ82=23.92, p=0.0024), but grooming rates were universally low; the maximum recorded number of grooming movements was six in 1 h. After the inoculation, there was a significant increase in MG grooming rates, the magnitude of which differed significantly between species (LR Χ82=218.7, p<0.0001; figure 2), with the greatest increase in species without visible Pseudonocardia. This led to a negative correlation between species-specific MG grooming rates after infection and relative abundance of Pseudonocardia (Spearman's ρ=−0.763, p=0.017; figures 1 and 2), which was also present when the effects of phylogenetic relatedness were removed (R=−0.687, p=0.047; figure 2 inset). Workers in Atta and S. amabilis sharply increased MG grooming frequency; the increase for Sericomyrmex sp. 1 was less pronounced, while Ac. octospinosus, T. zeteki, T. cf. cornetzi and C. longiscapus workers hardly increased grooming rates (figures 1 and 2).
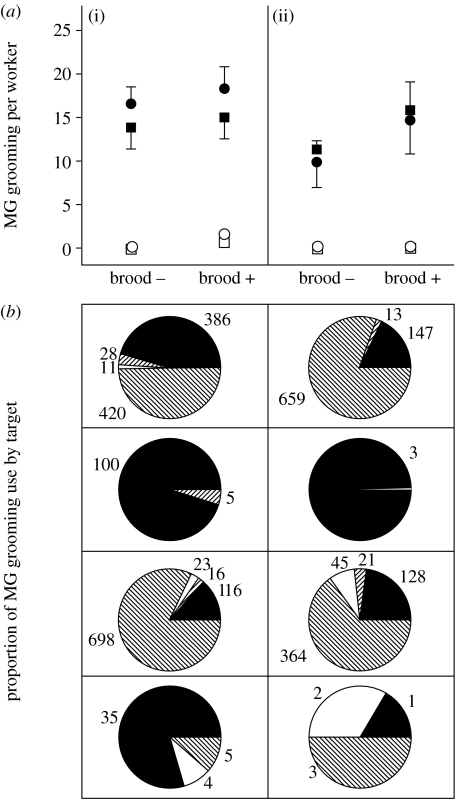
Prior to challenging with the ant pathogen M. anisopliae and the garden pathogen Escovopsis spp., MG grooming rates of all species were universally low (less than five events in 60 min), so all colonies fell into our ‘did not groom’ category. However, after challenging we found significant differences among species in MG grooming rates, comparing taxa with visible Pseudonocardia (Ac. octospinosus and T. zeteki) versus those without them (A. colombica and S. amabilis; logistic regression, LR Χ32=96.5, p=<0.0001; figure 3a). Planned comparisons showed that ants from sister genera differed in MG grooming frequency when infected with pathogens (A. colombica versus Ac. octospinosus: LR Χ12=44.68, p<0.0001; S. amabilis versus T. zeteki: LR Χ12=61.08, p<0.0001). Likewise, there were significant differences in MG grooming rates depending on the substrate (the presence or absence of brood; LR Χ12=15.36, p<0.0001; figure 3a). There were no significant differences in MG grooming rates, comparing the fungal pathogen Escovopsis and the entomopathogen Metarhizium (LR Χ12=0.0018, p=0.966; figure 3a). A decrease in Pseudonocardia abundance was associated with an expanded range of targets following MG grooming in Atta and Sericomyrmex (figure 3b). Trachymyrmex also have multiple targets, but the frequencies are very low in comparison with genera that lack abundant Pseudonocardia (figure 3b). Brood are primary targets of MG grooming in Ac. octospinosus. Comparisons among targets showed significant differences among species (LR Χ32=89.91, p<0.0001). There were no significant differences between pathogens (LR Χ12=0.007, p=0.9), but there was a significant interaction between species and pathogens (LR Χ32=125.79, p<0.0001; figure 3b).
Figure 3.
(a) MG grooming rates (mean±s.e.) in attine ants after infection with (i) Metarhizium anisopliae and (ii) Escovopsis in sub-colonies with (brood +) and without (brood −) brood: filled circles, Atta colombica; open circles, Acromyrmex octospinosus; filled squares, Sericomyrmex amabilis; open squares, Trachymyrmex zeteki. Prior to infection, all MG grooming rates for all species were less than 0.5. (b) Proportion of different targets (brood: filled; fungus garden: left-hatched; fellow workers: right-hatched; self: open) that are contacted after MG grooming, with the summed total of MG events.
4. Discussion
(a) Trade-offs in social control of pathogens
Sanitary behaviour in ants includes grooming to collect debris that are gathered in the form of waste pellets in the infrabuccal cavity and then removed (Bailey 1920; Fernández-Marín et al. 2006; Little et al. 2006). Most ants complement these behaviours by deploying antimicrobial exocrine products, particularly those from the MG (Hölldobler & Wilson 1990; Fernández-Marín et al. 2006). Increased MG grooming is associated with increased pellet production when ants are challenged with entomopathogenic fungi (Fernández-Marín et al. 2006). This novel behaviour may represent an evolutionary innovation that was necessary because MG secretions, at least in Acromyrmex, inhibit both the ants' fungal symbiont (Bot et al. 2002) and Pseudonocardia bacteria (Poulsen et al. 2003a), which otherwise provide fungus-growing ants with an additional source of antimicrobial compounds (Currie et al. 1999b, 2006). Much attention has focused on how Pseudonocardia antibiotics control Escovopsis, but our results suggest that Escovopsis is also targeted by MG products, consistent with the demonstration that MG compounds from Acromyrmex reduce germination of Escovopsis (Bot et al. 2002). Preliminary results also indicate that germination of Escovopsis conidia within infrabuccal pellets is inhibited by Atta and Sericomyrmex workers to the same degree (more than 80%; H. Fernández-Marín 2008, unpublished data) as in taxa with abundant Pseudonocardia (Little et al. 2006). In taxa with abundant actinomycetes, MG products are targeted primarily to brood, whereas Atta and Sericomyrmex expand the range of targets to include the fungal gardens and fellow workers, in addition to the brood.
The negative association between frequency of induced MG grooming and relative abundance of Pseudonocardia implies that Pseudonocardia may be effective against generalist pathogens, or that the ants use another exocrine source against them. The former possibility is unlikely, as isolates of bacteria from Ac. octospinosus have no detectable inhibitory effects against an array of generalist fungal pathogens (Currie et al. 1999b), although the generality of this result for all attine-associated Pseudonocardia has recently been questioned (Mueller et al. 2008). The latter possibility has not been thoroughly investigated, but mandibular gland secretions may have antiseptic activity (North et al. 1997).
(b) Is there decreasing reliance on an ancient bacterial mutualism in favour of MG chemical control?
Currie et al. (2006, p. 81) reported that Pseudonocardia bacteria are ‘associated with all attine-ant species examined’ and ‘occur on specific locations on the cuticle of a given ant species’. This contradicts the electronic supplementary materials of Currie et al. (2006), which stated that Atta and Sericomyrmex have ‘no filamentous bacteria or morphological structures’ on the exoskeleton. Our results are consistent with the latter observation. These taxa are derived from ancestors that possessed these structures, which indicates that they were evolutionarily lost. Recent molecular studies have failed to detect Pseudonocardia in Atta spp., including those species in our study (Mueller et al. 2008), which is inconsistent with in vitro evidence that isolates of actinomycetes from Atta are active against Escovopsis (Currie et al. 1999b). In vivo evidence is lacking, and nothing is known of the dosage-dependent efficacy of bacteria cultured from different ant taxa. Trace quantities of Pseudonocardia in some taxa may have retained specific disease control functions, and a recent study has shown that both Atta and Acromyrmex can transfer actinomycetes to the fungus garden during treatment of leaf fragments by workers (Mangone & Currie 2007). Such transfers occurred in only 10–25 per cent of the trials for both genera, however, so they are unlikely to play a primary role in defence against pathogens.
A loss or drastic reduction in visible bacteria, and an increased reliance on chemical control of pathogens by fungus-growing ants, may be related to three factors.
Health care costs. Using both bacterial and MG antimicrobials may account for up to 40 per cent of the basal metabolism of Acromyrmex leafcutting ants, with approximately equal costs for each defence (Poulsen et al. 2002, 2003a,b). The evolution of novel chemical compounds from MG secretions with broad-spectrum activity would be selected if they improve sanitation, reduce hygienic costs or both. We lack detailed comparative information on the diversity and function of MG secretions and other exocrine glands (see Schildknecht & Koob 1971; do Nascimento et al. 1996; Ortius-Lechner et al. 2000) that could test such hypotheses (but see North et al. 1997).
Minimizing resistance. The best public health strategy to deploy narrow-spectrum antibiotics is to apply a strong dose during an infection to kill a specific pathogen, but otherwise not use the medication (Levy 1998). Broad-spectrum antibiotics, by contrast, create unfavourable habitat for pathogen growth and most applications are general hygiene sanitation measures, which normally do not create resistance problems (Hoffken 2000; Bergstrom & Feldgarden 2007). Intriguingly, these rules of thumb of proper antibiotic use seem to be reversed in attine ants. The active use of MG secretions appears to be regulated for minimal dosage and maximal efficiency (Fernández-Marín et al. 2006), which may indicate that either some unknown MG compounds have narrow-spectrum antibiotic functions, or MG secretions are too costly to be used in a more profligate preventive way. A putative increased reliance on MG secretions in Atta and Sericomyrmex implies that these genera should have larger MGs relative to respective sister genera, but gland size is only slightly larger in Atta and Sericomyrmex (Hughes et al. 2008), suggesting that quality of secretions rather than quantity has been decisive. The use of actinomycete-derived antibiotics appears to be less precisely regulated, although it is possible that production of metabolites could be upregulated when needed. Although the abundance of bacteria may change in response to increasing disease challenge (Currie et al. 2003b), this continual presence hardly appears to be a fine-tuned mechanism and may help to explain why resistant strains of pathogens may occasionally appear (Little et al. 2006).
Antagonistic interactions in the fungus-growing ant mutualism. A black yeast is also associated with at least some attine ants (Little & Currie 2008). This yeast may antagonize the ants' mutualistic bacteria, which would inhibit their ability to control Escovopsis (Little & Currie 2008). This antagonism may have necessitated new hygienic strategies, in particular when black yeast infections are chronic, as would be expected in colonies with large numbers of workers.
(c) The evolution of public health strategies and social organization
Our results suggest that there has been evolutionary divergence in the use of antibiotic sources, which parallels changes in the social structure of attines. Large-scale farming of a genetically homogeneous crop by group members who are genetically related poses special problems for disease management and public health strategies. We speculate that the domestication of Pseudonocardia was a major evolutionary advance at the origin of attine fungiculture ca 50 Myr ago, but that later developments towards complex agricultural societies required additional mechanisms for disease control, including an increased reliance on chemical and behavioural defences. This was accompanied by a dramatic transition in the mating system of queens, from exclusively single mating to obligate multiple mating in the highly derived Acromyrmex and Atta leafcutters, leading to a substantial increase in genetic diversity among workers (Villesen et al. 2002; Mattila & Seeley 2007) with at least some documented advantages for resistance to disease (Hughes & Boomsma 2006). There are substantial differences in sizes of mature colonies when comparing those taxa with abundant actinomycetes versus elevated rates of MG use. Colony size is an important factor in ant life history, including defensive strategies against pathogens (Hughes et al. 2002). Limited data suggest that MG grooming rates and relative abundance of actinomycetes do not change as colonies mature. Within species, variation in MG grooming rates and relative abundance of actinomycetes are not associated with variation in colony size in four species of Trachymyrmex (H. Fernández-Marín, G. Bruner, E. Gomez, D. R. Nash, J. J. Boomsma & W. T. Wcislo 2008, unpublished data). In A. colombica, workers from three- to six-month old colonies have similar patterns of MG use to those of workers from colonies older than 24 months (H. Fernández-Marín, unpublished data), and overall tasks do not change with the age of colonies (Augustin & Lopes-Santos 2008). The decay and collapse of mutualistic relationships are not uncommon (see discussion in Mueller et al. 2005; Sachs & Simms 2006; Kost et al. 2007) and raise questions about compensatory changes that may be associated with the evolution of large colonies and social complexity across the fungus-growing ants.
Acknowledgments
We thank A. Aguilar, A. Portugal and E. Gomez for their assistance; P. Bayman, N. Biani, C. Currie, A. Herre, E. Leigh and S. Tierney for their comments on the manuscript; and the Autoridad Nacional del Ambiente for permits. H.F.-M. was supported by the Secretaria Nacional de Ciencia, Tecnologia e Innovacion (República de Panamá) and Smithsonian Institution Predoctoral and Postdoctoral Fellowships; STRI provided supplemental support to H.F.-M., and general research support to W.T.W. J.J.B. and D.R.N. were supported by a grant from the Danish National Research Foundation, which provided supplemental support to H.F.-M.
References
- Augustin J.O., Lopes-Santos J.F. Behavior of early generations of Atta sexdens (Hymenoptera: Formicidae) workers during preparation of leaf substrate for symbiont fungus gardens. Sociobiology. 2008;51:265–281. [Google Scholar]
- Bailey I.W. Some relations between ants and fungi. Ecology. 1920;1:174–189. doi:10.2307/1929134 [Google Scholar]
- Bass M., Cherrett J.M. The role of leaf-cutting ant workers (Hymenoptera: Formicidae) in fungus garden maintenance. Ecol. Entomol. 1994;19:215–220. doi:10.1111/j.1365-2311.1994.tb00412.x [Google Scholar]
- Bergstrom, C. T. & Feldgarden, M. 2007 The ecology and evolution of antibiotic-resistant bacteria. In Evolution in health and disease (eds S. Stearns & J. Koella), pp. 125–137, 2nd edn. Oxford, UK: Oxford University Press.
- Boomsma J.J., Schmid-Hempel P., Hughes W.O.H. Life histories and parasite pressure across the major groups of social insects. In: Fellowes M.D.E., Holloway G.J., Rolff J., editors. Insect evolutionary ecology. CABI Publishing; Wallingford, UK: 2005. pp. 139–175. [Google Scholar]
- Bot A.N.M., Ortius-Lechner D., Finster K., Maile R., Boomsma J.J. Variable sensitivity of fungi and bacteria to compounds produced by the metapleural glands of leaf-cutting ants. Insect. Soc. 2002;49:363–370. doi:10.1007/PL00012660 [Google Scholar]
- Currie C.R., Stuart A.E. Weeding and grooming of pathogens in agriculture by ants. Proc. R. Soc. B. 2001;268:1033–1039. doi: 10.1098/rspb.2001.1605. doi:10.1098/rspb.2001.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie C.R., Mueller U.G., Malloch D. The agricultural pathology of ant fungus gardens. Proc. Natl Acad. Sci. USA. 1999a;96:7998–8002. doi: 10.1073/pnas.96.14.7998. doi:10.1073/pnas.96.14.7998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie C.R., Scott J.A., Summerbell C.R., Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999b;398:701–704. doi:10.1038/19519 [Google Scholar]
- Currie C.R., Wong B., Stuart A.E., Schultz T.R., Rehner S.A., Mueller U.G., Sung G.H., Spatafora J.W., Straus N.A. Ancient tripartite coevolution in the attine ant–microbe symbiosis. Science. 2003a;299:386–388. doi: 10.1126/science.1078155. doi:10.1126/science.1078155 [DOI] [PubMed] [Google Scholar]
- Currie C.R., Bot A.N.M., Boomsma J.J. Experimental evidence of a tripartite mutualism: bacteria protect fungus gardens from specialized parasites. Oikos. 2003b;101:91–102. doi:10.1034/j.1600-0706.2003.12036.x [Google Scholar]
- Currie C.R., Poulsen M., Mendenhall J., Boomsma J.J., Billen J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science. 2006;311:81–83. doi: 10.1126/science.1119744. doi:10.1126/science.1119744 [DOI] [PubMed] [Google Scholar]
- do Nascimento R.R., Schoeters E., Morgan E.D., Billen J., Stradling D.J. Chemistry of metapleural gland secretions of three attine ants, Atta sexdens rubropilosa, Atta cephalotes, and Acromyrmex octospinosus (Hymenoptera: Formicidae) J. Chem. Ecol. 1996;22:987–1000. doi: 10.1007/BF02029949. doi:10.1007/BF02029949 [DOI] [PubMed] [Google Scholar]
- Fefferman N.H., Traniello J.F.A., Rosengaus R.B., Calleri D.V. Evolution of disease prevention and resistance in social insects: modeling the survival consequences of immunity, hygienic behavior and colony organization. Behav. Ecol. Sociobiol. 2007;61:565–577. doi:10.1007/s00265-006-0285-y [Google Scholar]
- Fernández-Marín H., Zimmerman J.K., Rehner S.A., Wcislo W.T. Active use of metapleural glands by ants in controlling fungal infection. Proc. R. Soc. B. 2006;273:1689–1695. doi: 10.1098/rspb.2006.3492. doi:10.1098/rspb.2006.3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler G.H., Pereira-da-Silva V., Forti L.C., Saes N.B. Population dynamics of leaf-cutting ants: a brief review. In: Lofgren C.S., Vander Meer R.K., editors. Fire ants and leaf cutting ants: biology and management. Westview Press; Boulder, CO: 1986. pp. 123–145. [Google Scholar]
- Garland T., Jr, Harvey P.H., Ives A.R. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 1992;41:18–32. doi:10.2307/2992503 [Google Scholar]
- Hamilton W.D. Pathogens as causes of genetic diversity in their host populations. In: Anderson R.M., May R.M., editors. Population biology of infectious diseases. Springer; Berlin, Germany: 1982. pp. 269–296. [Google Scholar]
- Hoffken G. Is the use of narrow-spectrum antibiotics too narrow-minded in the treatment of severe infection? Clin. Microbiol. Infect. 2000;6:7–10. doi: 10.1046/j.1469-0691.2000.00003.x. doi:10.1046/j.1469-0691.2000.00003.x [DOI] [PubMed] [Google Scholar]
- Hölldobler B., Wilson E.O. Belknap Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Hughes W.O.H., Boomsma J.J. Let your enemy do the work: within-host interactions between two fungal parasites of leaf-cutting ants. Proc. R. Soc. B. 2004;271(Suppl. S3):S104–S106. doi: 10.1098/rsbl.2003.0115. doi:10.1098/rsbl.2003.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W.O.H., Boomsma J.J. Does genetic diversity hinder parasite evolution in social insect colonies? J. Evol. Biol. 2006;19:132–143. doi: 10.1111/j.1420-9101.2005.00979.x. doi:10.1111/j.1420-9101.2005.00979.x [DOI] [PubMed] [Google Scholar]
- Hughes W.O.H., Eilenberg J., Boomsma J.J. Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc. R. Soc. B. 2002;269:1811–1819. doi: 10.1098/rspb.2002.2113. doi:10.1098/rspb.2002.2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W.O.H., Pagliarini R., Madsen H.B., Dijkstra M.B., Boomsma J.J. Investment in disease defense across the fungus-growing ants indicates an abrupt transition in disease pressure. Evolution. 2008;62:1252–1257. doi: 10.1111/j.1558-5646.2008.00347.x. doi:10.1111/j.1558-5646.2008.00347.x [DOI] [PubMed] [Google Scholar]
- Jaccoud D.B., Hughes W.H.O., Jackson C.W. The epizootiology of a Metarhizium infection in mini-nests of the leaf-cutting ant Atta sexdens rubripilosa. Entomol. Exp. Appl. 1999;93:51–61. doi:10.1023/A:1003830625680 [Google Scholar]
- Kost C., Lakatos T., Böttcher I., Arendholz W.R., Redenbach M., Wirth R. Non-specific association between filamentous bacteria and fungus-growing ants. Naturwiss. 2007;94:821–828. doi: 10.1007/s00114-007-0262-y. doi:10.1007/s00114-007-0262-y [DOI] [PubMed] [Google Scholar]
- Levy S.B. The challenge of antibiotic resistance. Sci. Am. Mag. 1998;278:46–53. doi: 10.1038/scientificamerican0398-46. [DOI] [PubMed] [Google Scholar]
- Lewis T. Colony size, density and distribution of the leaf-cutting ant, Acromyrmex octospinosus (Reich) in cultivated fields. Trans. R. Entomol. Soc. Lond. 1975;127:51–64. [Google Scholar]
- Little A.E.F., Currie C.R. Black yeast symbionts compromise the efficiency of antibiotic defenses in fungus-growing ants. Ecology. 2008;89:1216–1222. doi: 10.1890/07-0815.1. doi:10.1890/07-0815.1 [DOI] [PubMed] [Google Scholar]
- Little A.E.F., Murakami T., Mueller U.G., Currie C.R. Defending against parasites: fungus-growing ants combine specialized behaviours and microbial symbionts to protect their fungus garden. Biol. Lett. 2006;2:12–16. doi: 10.1098/rsbl.2005.0371. doi:10.1098/rsbl.2005.0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison, W. P. & Maddison, D. R. 2008. Mesquite: a modular system for evolutionary analysis, version 2.5. See http://mesquiteproject.org
- Mangone D.M., Currie C.R. Garden substrate preparation behaviours in fungus-growing ants. Can. Entomol. 2007;139:841–849. [Google Scholar]
- Mattila H.R., Seeley T.D. Genetic diversity in honey bee colonies enhances productivity and fitness. Science. 2007;317:362–364. doi: 10.1126/science.1143046. doi:10.1126/science.1143046 [DOI] [PubMed] [Google Scholar]
- Midford, P. E., Garland, T. Jr, & Maddison, W. P. 2008. PDAP package of Mesquite, version 1.14. See http://mesquiteproject.org/pdap_mesquite/
- Mueller U.G. Ant versus fungus versus mutualism: ant–cultivar conflict and the deconstruction of the attine ant–fungus symbiosis. Am. Nat. 2002;160:S67–S98. doi: 10.1086/342084. doi:10.1086/342084 [DOI] [PubMed] [Google Scholar]
- Mueller U.G., Wcislo W.T. Nesting biology of the fungus-growing ant Cyphomyrmex longiscapus weber (Attini Formicidae) Insect. Soc. 1998;45:181–189. doi:10.1007/s000400050078 [Google Scholar]
- Mueller U.G., Gerardo N.M., Aanen D.K., Six D.L., Schultz T.R. The evolution of agriculture in insects. Ann. Rev. Ecol. Evol. Syst. 2005;36:563–595. doi:10.1146/annurev.ecolsys.36.102003.152626 [Google Scholar]
- Mueller U.G., Dash D., Rabeling C., Rodrigues A. Coevolution between attine ants and actinomycete bacteria: a reevaluation. Evolution. 2008;62:2894–2912. doi: 10.1111/j.1558-5646.2008.00501.x. doi:10.1111/j.1558-5646.2008.00501.x [DOI] [PubMed] [Google Scholar]
- Murakami T., Higashi S., Windsor D. Mating frequency, colony size, polyethism and sex ratio in fungus-growing ants (Attini) Behav. Ecol. 2000;48:276–284. doi:10.1007/s002650000243 [Google Scholar]
- North R.D., Jackson C.W., Howse P.E. Evolutionary aspects of ant–fungus interactions in leaf-cutting ants. Trends Ecol. Evol. 1997;12:386–389. doi: 10.1016/s0169-5347(97)87381-8. doi:10.1016/S0169-5347(97)87381-8 [DOI] [PubMed] [Google Scholar]
- Oi D.H., Pereira R.M. Ant behavior and microbial pathogens (Hymenoptera: Formicidae) Florida Entomol. 1993;76:63–74. doi:10.2307/3496014 [Google Scholar]
- Ortius-Lechner D., Maile R., Morgan E.D., Boomsma J.J. Metapleural gland secretion of the leafcutter ant Acromyrmex octospinosus: new compounds and their functional significance. J. Chem. Ecol. 2000;26:1667–1683. doi:10.1023/A:1005543030518 [Google Scholar]
- Poulsen M., Bot A.N.M., Nielsen M.G., Boomsma J.J. Experimental evidence for the cost and hygienic significance of the antibiotic metapleural gland secretions in leaf-cutting ants. Behav. Ecol. Sociobiol. 2002;52:151–157. doi:10.1007/s00265-002-0489-8 [Google Scholar]
- Poulsen M., Bot A.N.M., Boomsma J.J. The effect of metapleural gland secretion on the growth of a mutualistic bacterium on the cuticle of leaf-cutting ants. Naturwiss. 2003a;90:406–409. doi: 10.1007/s00114-003-0450-3. doi:10.1007/s00114-003-0450-3 [DOI] [PubMed] [Google Scholar]
- Poulsen M., Bot A.N.M., Currie C.R., Nielsen M.G., Boomsma J.J. Within-colony transmission and the cost of a mutualistic bacterium in the leaf-cutting ant Acromyrmex octospinosus. Funct. Ecol. 2003b;17:260–269. doi:10.1046/j.1365-2435.2003.00726.x [Google Scholar]
- Quinlan R.J., Cherrett J.M. The role of substrate preparation in the symbiosis between the leaf-cutting ant Acromyrmex octospinosus Reich, and its food fungus. Ecol. Entomol. 1977;2:161–170. doi:10.1111/j.1365-2311.1977.tb00877.x [Google Scholar]
- Sachs J.L., Simms E.L. Pathways to mutualism breakdown. Trends Ecol. Evol. 2006;21:585–592. doi: 10.1016/j.tree.2006.06.018. doi:10.1016/j.tree.2006.06.018 [DOI] [PubMed] [Google Scholar]
- Schildknecht H., Koob K. Myrmicacin, the first insect herbicide. Angew. Chem. Int. Ed. 1971;2:124–125. doi: 10.1002/anie.197101241. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Princeton University Press; Princeton, NJ: 1998. Parasites in social insects. [Google Scholar]
- Schultz T.R., Brady S.G. Major evolutionary transitions in ant agriculture. Proc. Natl Acad. Sci. USA. 2008;105:5435–5440. doi: 10.1073/pnas.0711024105. doi:10.1073/pnas.0711024105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow A., Briscoe D., Gillings M., Holley M., Smith S., Leys R., Silberbauer T., Turnbull C., Beattie A. Antimicrobial defenses increase with sociality in bees. Biol. Lett. 2007;3:422–424. doi: 10.1098/rsbl.2007.0178. doi:10.1098/rsbl.2007.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villesen P., Murakami T., Schultz T.R., Boomsma J.J. Identifying the transition between single and multiple mating of queens in fungus-growing ants. Proc. R. Soc. B. 2002;269:1541–1548. doi: 10.1098/rspb.2002.2044. doi:10.1098/rspb.2002.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber N.A. American Philosophical Society; Philadelphia, PA: 1972. Gardening ants, the attines. [Google Scholar]