Abstract
Body size variation across the Metazoa is immense, encompassing 17 orders of magnitude in biovolume. Factors driving this extreme diversification in size and the consequences of size variation for biological processes remain poorly resolved. Species diversity is invoked as both a predictor and a result of size variation, and theory predicts a strong correlation between the two. However, evidence has been presented both supporting and contradicting such a relationship. Here, we use a new comprehensive dataset for maximum and minimum body sizes across all metazoan phyla to show that species diversity is strongly correlated with minimum size, maximum size and consequently intra-phylum variation. Similar patterns are also observed within birds and mammals. The observations point to several fundamental linkages between species diversification and body size variation through the evolution of animal life.
Keywords: Metazoa, body size, biodiversity, niche, passive evolution, directed evolution
1. Introduction
Early work by Hutchinson & MacArthur (1959) and May (1988) proposed a relationship between body size and species diversity, implying that size-biased processes are paramount to the radiation of life. For example, elevated species richness in small-bodied groups might reflect a greater amount of usable space (Hutchinson & MacArthur 1959; Kozlowski & Gawelczyk 2002). Body size may affect range size (Gaston & Blackburn 1996), population size (Damuth 1981) and a variety of other ecological and life-history traits (Peters 1983), linking richness and size through size-biased extinction and speciation (Stanley 1973; Maurer et al. 1992; Kozlowski & Gawelczyk 2002). Hard boundaries on body size due to anatomical and physiological constraints (Hanken & Wake 1993; Chapelle & Peck 1999; McClain & Rex 2001; McClain et al. 2006; Makarieva et al. 2008) may prevent some clades from becoming speciose. Alternatively, a flexible bauplan that permits unimpeded exploration of size extremes and novel niches could promote clade diversification.
Conversely, a strong relationship between size variation and richness (ceteris paribus) is also expected if body size variation is a consequence of a passive diffusion process in a radiating clade (Trammer 2002, 2005), such that maximum size is positively and minimum size is negatively correlated with diversity. A passive diffusion model of body size evolution was invoked by Stanley (1973) to explain Cope's rule, the tendency for clades to evolve larger body size over time. This model of evolution suggests both a time component (size variation increases over the temporal duration of clade; Jablonski 1997) and a diversity component (reflecting a relationship between size variance and speciation events; Trammer 2002). Evidence for Cope's rule is mixed, but studies do indicate an increase in body size variation in vertebrates (Gillman 2007) and molluscs (Jablonski 1997) over time. Previous tests for a correlation between richness and body size provide support both for (based on maximum size; Trammer 2002) and against (based on median size; Orme et al. 2002) a relationship.
Given the differences between previous approaches, and their often-limited taxonomic scope, it remains unclear whether a relationship exists between biodiversity and body size variation across the Metazoa. Here, we test whether richness is significantly correlated with maximum body size, minimum body size and overall body size range among clades. After conducting an extensive literature survey and consulting with taxonomic experts, we compiled a comprehensive dataset on the largest and smallest known species for 26 metazoan phyla (of potentially 34). Size is quantified as biovolume, a measure of the space the organism occupies in three dimensions, based on linear measurements and approximations of organismal shape. For most phyla, the size range is well characterized (e.g. Mollusca), and for others it is reasonable to assume that discovery of new species would not appreciably increase size range in logarithmic space. For species richness we used estimates based on current knowledge, undoubtedly representing underestimates of global intra-phylum diversity. However, these estimates do provide an accurate assessment of ordinal ranking based on richness among phyla (e.g. Arthropoda>Porifera>Tardigrada) that is essential for our analyses. We also examined body size–richness relationships in two well-studied classes of vertebrates—mammals and birds—using published body size databases. In all analyses, phylogenetically independent contrasts were used to control for the influence of shared evolutionary history. We find that species diversity is strongly correlated with minimum size, maximum size and consequently intra-phylum size variation across Metazoa and within two vertebrate classes.
2. Material and methods
(a) Dataset
We constructed a database of the largest and smallest species, by biovolume, for 26 metazoan phyla and three subphyla (see appendix 1 in the electronic supplementary material). Eight phyla were not included (Placozoa, Monoblastozoa, Rhombozoa, Orthonectida, Kinorhyncha, Entoprocta, Cycliophora and Echiura) because insufficient data existed to quantify biovolume or determine the largest and smallest species. One phylum, the Monoblastozoa with one species, was excluded also because of questionable accuracy as an erected phylum or a genuine species. The three subphyla for the Chordata were included because the three clades represent fundamentally different body plans and because of the availability of data. The largest and smallest species were determined by consultation with experts, taxonomic monographs and comprehensive literature searches. Linear measurements (length, width, height and diameter) were taken from published records. In the few cases where insufficient data existed in the published literature to accurately assess size, we gathered estimates from specialists in the group. Linear measurements were converted to biovolume through formulae for shapes approximating the organismal shape (see appendix 1 in the electronic supplementary material for the formulae used). In some cases, additional linear measurements not available in the literature were required for the estimation of biovolume. These additional measurements were estimated from published photos and illustrations.
Body mass data for mammals and birds were obtained from published sources (Dunning 1992; Smith et al. 2003). For both groups, we used taxonomic families as the unit of analysis, taking the maximum and minimum body sizes and species richness of each family.
(b) Phylogenetically independent contrasts
To control for the influence of shared evolutionary history on diversity and body size, we performed phylogenetic independent contrast analyses. Because many alternative topologies for the relationships among the metazoan phyla have been proposed, we repeated our analysis on two different phylum-level phylogenies (fig. 1 in Orme et al. 2002 and fig. 1 in Dunn et al. 2008). We used a recently published supertree of all mammals (Bininda-Emonds et al. 2007) to assemble a phylogeny of mammalian families for analysis. Similarly, we took advantage of the ‘Early Bird’ project's recent phylogeny of birds (Hackett et al. 2008) to estimate the relationships between avian families. For all trees, a constant arbitrary length was assigned to each branch. Contrasts were calculated using the PDAP package (Garland et al. 1993) implemented in Mesquite v. 1.01 (Maddison & Maddison 2008). Contrasts were standardized and positivized on the x-axis following the methods of Garland et al. (1992), and linear regression through the origin was performed. Phylogenetically correct regressions were visualized in the original data space following the methods of Garland & Ives (2000).
3. Results and discussion
Biovolume (mm3) ranges 17 orders of magnitude among the modern metazoans, from the blue whale (Balaenoptera musculus, 1.9×1011 mm3) to small rotifers, nematodes, polychaetes, gastrotrichs and copepods (7.8×10−6 to 5.1×10−5 mm3; see appendix 1 in the electronic supplementary material). Size variation among the polyphyletic invertebrates is a substantial 15 orders of magnitude, ranging from the giant squid (Architeuthis dux, 5.9×109 mm3) to the aforementioned minuscule invertebrates.
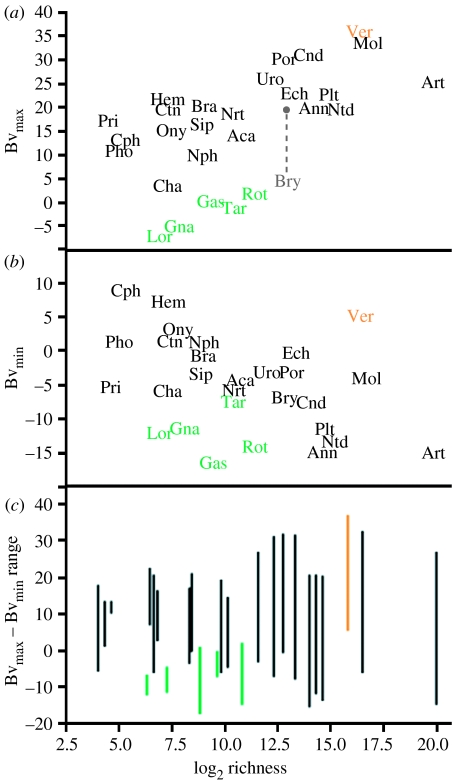
Even a cursory viewing of the dataset suggests a qualitative relationship between species richness and biovolume range among metazoan phyla/subphyla (see appendix 1 in the electronic supplementary material). Both quantitative, phylogenetically corrected and uncorrected tests yield significant correlations between richness and both maximum (positive correlation) and minimum (negative correlation) biovolume (tables 1 and 2; figure 1). Richness is correlated more strongly with maximum rather than minimum biovolume among the Metazoa (tables 1 and 2). Total biovolume range is the strongest correlate of richness, suggesting that although the correlation with minimum biovolume is weaker, it remains important (tables 1 and 2; figures 1c and 2). Surprisingly, minimum and maximum biovolume (Bvmin and Bvmax, respectively) among all phyla and subphyla are not significantly correlated with each other (non-corrected: r=0.33, p=0.1432; corrected: r=0.11, p=0.2222). These results also clearly show that there is no relationship, or at best only a weak relationship, between mean or median body size values and richness (Orme et al. 2002).
Table 1.
Non-phylogenetically corrected regressions between richness (R) and maximum body size, minimum body size and body size range. (Statistics are given for Pearson's r (correlation coefficient), reduced major axis (RMA) regression and ordinary least-squares (OLS) regression. Size is measured as biovolume (mm3) in metazoan phyla and mass (g) in birds and mammals. Bird and mammal analyses were conducted after the removal of monotypic families. Italic font indicates non-significant relationships.)
| n | Pearson r | RMA slope | OLS slope | r2 | p-value | |
|---|---|---|---|---|---|---|
| R versus max size | ||||||
| metazoan phyla | 27 | 0.53 | 2.86 | 0.71 | 0.28 | 0.0036 |
| bird families | 98 | 0.10 | −1.22 | −0.09 | 0.01 | 0.3372 |
| mammal families | 105 | −0.17 | −2.76 | −0.37 | 0.03 | 0.1649 |
| R versus min size | ||||||
| metazoan phyla | 27 | −0.40 | −1.77 | −0.71 | 0.16 | 0.0335 |
| bird families | 98 | −0.56 | −1.29 | −0.72 | 0.31 | <0.0001 |
| mammal families | 105 | −0.46 | −2.93 | −1.35 | 0.21 | <0.0001 |
| R versus size-range | ||||||
| metazoan phyla | 27 | 0.80 | 2.80 | 2.23 | 0.63 | <0.0001 |
| bird families | 98 | 0.20 | 1.48 | 0.28 | 0.04 | 0.0164 |
| mammal families | 105 | −0.14 | −2.79 | −0.11 | 0.02 | 0.6881 |
Table 2.
Phylogenetically corrected regressions between richness (R) and maximum body size, minimum body size and body size range. (Statistics follow table 1.)
| n | Pearson's r | RMA slope | OLS slope | r2 | p-value | |
|---|---|---|---|---|---|---|
| R versus max size | ||||||
| metazoan phyla | ||||||
| Orme tree | 27 | 0.52 | 2.77 | 1.47 | 0.28 | 0.003 |
| Dunn tree | 27 | 0.64 | 2.52 | 1.62 | 0.41 | <0.001 |
| bird families | 98 | 0.36 | 0.87 | 0.32 | 0.13 | <0.001 |
| mammal families | 105 | 0.13 | 1.48 | 0.20 | 0.02 | 0.160 |
| R versus min size | ||||||
| metazoan phyla | ||||||
| Orme tree | 27 | −0.51 | −1.95 | −1.01 | 0.26 | 0.004 |
| Dunn tree | 27 | −0.55 | −1.87 | −1.03 | 0.31 | 0.002 |
| bird families | 98 | −0.61 | −0.90 | −0.55 | 0.37 | <0.001 |
| mammal families | 105 | −0.49 | −1.63 | −0.81 | 0.25 | <0.001 |
| R versus size range | ||||||
| metazoan phyla | ||||||
| Orme tree | 27 | 0.74 | 3.35 | 2.47 | 0.55 | <0.001 |
| Dunn tree | 27 | 0.79 | 3.33 | 2.65 | 0.63 | <0.001 |
| bird families | 98 | 0.90 | 0.96 | 0.87 | 0.81 | <0.001 |
| mammal families | 105 | 0.80 | 1.25 | 1.01 | 0.65 | <0.001 |
Figure 1.
The relationship between body size and species richness among metazoan phyla and subphyla. (a) Log2 biovolume of the largest species (Bvmax) of a taxon versus its log2 richness. (b) Log2 biovolume of the smallest species (Bvmin) of a taxon versus its log2 richness. (c) Range between the largest and smallest sized species (indicated by lines) versus log2 richness. Increased richness among metazoan phyla is correlated with an increase in maximum size, a decrease in minimum size and an increase in overall body size range. A group of outliers (green) represents phyla that occupy physically space-limited habitats. Vertebrates (Ver, orange) also have a greater minimum size than expected from their richness alone. Bryozoa (Bry, grey) is shown with both the largest autozoid size and the largest colony size (connected with grey line). Aca, Acanthocephala; Ann, Annelida; Art, Arthropoda; Bra, Brachipoda; Bry, Bryozoa; Cha, Chaetognatha; Cnd, Cnidaria; Cph, Cephalochordata; Ctn, Ctenophora; Ech, Echinodermata; Gas, Gastrotricha; Gna, Gnathostomulida; Hem, Hemichordata; Lor, Loricifera; Mol, Mollusca; Nph, Nematomorpha; Nrt, Nemertea; Ntd, Nematoda; Ony, Onychophora; Pho, Phoronida; Plt, Platyhelminthes; Por, Porifera; Pri, Priapula; Rot, Rotifera; Sip, Sipuncula; Tar, Tardigrada; Uro, Urochordata; Ver, Vertebrata.
Figure 2.
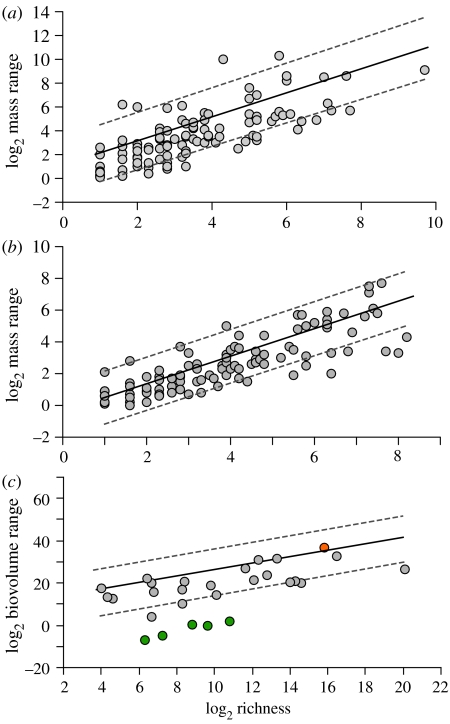
Log2 body size range between the largest and smallest species versus log2 richness for (a) mammalian families (y=1.01x+1.14, r2=0.65, p<0.0001), (b) avian families (y=0.87x+0.37, r2=0.81, p<0.0001) and (c) metazoan phyla (y=1.52+11.16, r2=0.55, p<0.0001). Outliers (green) represent phyla that occupy physically space-limited habitats. The vertebrates are indicated in orange. Phylogenetically correct regression lines (ordinary least-square) with confidence intervals are plotted in the original data space following the methods of Garland & Ives (2000). As similar results were obtained from the two metazoan phylogenies, only results from the Orme tree are shown. Overall, body size range correlates strongly with richness among groups both at higher and lower levels of taxonomic organization.
In families of mammals and birds, relationships between richness and body size are stronger after phylogenetic correction, although minimum mass for both groups and size range for birds are significant in the raw data (table 1). The importance of phylogeny here is to be expected, given the greater phylogenetic relatedness and greater non-independence among avian and mammalian families compared with the metazoan phyla. When phylogenetic relatedness is accounted for, regressions show strong relationships between species richness and both minimum and maximum body mass (g) among taxonomic families (table 2). The slopes of these relationships are shallower than across metazoan phyla, and minimum size is correlated more strongly with richness than maximum size (tables 1 and 2). Mass range is the strongest correlate of richness among avian and mammalian families (table 2; figure 2). In contrast to metazoans, maximum and minimum sizes are significantly correlated in both the corrected (birds: r=0.41, p<0.001; mammals: r=0.68, p<0.001) and non-corrected data (birds: r=0.88, p<0.001; mammals: r=0.92, p<0.001).
(a) Body size and biodiversity metrics
Three potentially confounding factors could influence the general results here. First is the use of biovolume for metazoan phyla, dictated by practicality and availability of measurements, as opposed to mass. The large range of biovolume values and log transformation minimize this impact. Use of biovolume also does not account for differences in actual organic material weights. For example, the largest cnidarian, Cyanea arctica, and poriferan, Aphrocallistes vastus, occupy approximately the same biovolume as the giant squid, A. dux, but they do not have similar organic material weights. For medusae, ash-free dry weight is less than 2 per cent of total weight (Lucas 1994) and for poriferans between 9.3 and 12.1 per cent (Ricciardi & Bourget 1998). By contrast, ash-free dry weight is 13.6–29.2% of wet weight in cephalopods (Ricciardi & Bourget 1998). This implies that the actual size range of cnidarians and poriferans is smaller than quantified here. However, a reduction in their size range would strengthen the correlation between richness and maximum size.
The second factor that may affect our interpretation is that estimates of species richness reflect only our current knowledge and represent an underestimate of the actual richness within a phylum. For example, the size range of Priapulida (figure 1c) is far greater than expected from its 16 currently described species, indicating that unknown biodiversity may increase richness estimates for this group by at least an order of magnitude. However, a Spearman's rank-order test also produces strong, significant relationships (Bvmax: ρ=0.68, p=0.0004; Bvmin: ρ=−0.73, p<0.0001), implying that revised estimates of richness would have to drastically alter the ordinal ranking of phyla to affect our results. Furthermore, change in the sign of the slope would require both gross overestimates of species-rich groups and gross underestimates of species-poor groups.
Third, the discovery of new species may increase the range of body sizes in the least studied groups. For some phyla, the size range is reasonably characterized (e.g. Mollusca) and for others it may be unwarranted to assume that an undiscovered giant exists that is substantially larger than any known species. Nevertheless, for this to influence the results, the effect would need to occur primarily in species-poor taxa and the new species would need to be at least twice the size of the largest or half of the smallest known species.
(b) Linkage between diversification and morphospace exploration
Evolutionary radiation in morphospace often coincides with increases in taxonomic richness in the initial diversification of a clade (Foote 1993). In many cases, morphological extremes are reached early in a clade's history with subsequent diversification simply filling in the previously defined morphospace (Foote 1997). By contrast, body size range appears to continuously increase through time, a trend documented in a variety of taxa (Alroy 1998; Trammer 2005; Hunt & Roy 2006; Novack-Gottshall & Lanier 2008) and across all life (Payne et al. 2009). Punctuating these gradual increases in size are sudden jumps in maximum size such as the K–T boundary for mammals (Alroy 1998) or at ca 1.9 Ga and 0.6–0.45 Ga during the evolution of life (Payne et al. 2009). Our results suggest that continued expansion of body size range over time parallels diversification across the Metazoa. This linkage not only occurs at broad phylogenetic scales but appears to apply equally for individual taxa such as mammals, birds, bivalves, trilobites, cetaceans and crinoids (Trammer 2005).
The increase in size range within a clade appears to follow in lock-step with increases in the number of species. One mechanism for this concordance is simple morphological diffusion during the radiation of a clade, where increases and decreases in size are equally likely to occur. This diffusive mechanism is invoked to explain Cope's rule and other patterns of body size evolution as an increase in body size variance rather than persistent directional selection (Jablonski 1997). Our observations are consistent with three main expectations of passive diffusion: both maximum and minimum body sizes show strong relationships with richness; body size range increases with increasing clade richness; and the slopes of maximum and minimum body sizes with richness mirror each other. However, in a purely diffusive model, a correlation between maximum and minimum sizes might be expected, a result not seen among Metazoa. This discrepancy might be explained by a limit to either maximum or minimum size (e.g. reflecting barrier, McKinney 1990; Kozlowski & Gawelczyk 2002) in some clades, while body size range continues to expand away from the barrier in the other direction. Teasing apart passive versus directional trends may be difficult, as patterns resembling passive diffusion may be produced by the interactions of a multitude of context and scale-dependent effects involved in dividing niche space among body sizes (Jablonski 1997).
The second possible mechanism behind our observations is that body size represents an important dimension of the niche, and substantial increase in the number of species requires expansion of the size morphospace. This implies that competitive displacement has to some extent limited the number of species that can be ‘packed’ between size extremes within a clade. The concept that differences in body size promote niche differentiation is well established (Hutchinson 1959; Grant 1968; Schoener 1970; Wilson 1975). For example, body size relates to food partitioning, space division and trophic level (Schoener 1968; Kerr & Dickie 2001; Marchinko et al. 2004; Layman et al. 2005), all important in separating species in niche space. Thus, a flexible bauplan and the greater body size range that results may allow for greater niche differentiation in some phyla. For example, over 10 orders of magnitude in biovolume exist between the smallest and the largest species in eight phyla (Nematoda, Annelida, Platyhelminthes, Arthropoda, Cnidaria, Porifera, Mollusca and Chordata; figure 1a–c), coinciding with the eight most speciose phyla among the metazoans (5000–137 000 species). This immense size variation parallels an equally expansive intra-phylum range in niche space (e.g. free living, parasitism, sessile, mobile, scavenging, predatory, filter and deposit feeding). Indeed, six of these phyla have successfully radiated into all three major biomes (i.e. freshwater, terrestrial and marine) on the Earth.
(c) Limits to size extremes and evolutionary novelty
Conspicuous outliers (clades outside the 95% confidence intervals of the relationship) do exist in the overall relationship between species richness and maximum and minimum biovolume. Gastrotricha, Tardigrada, Rotifera, Loricifera and Gnathostomulida, groups restricted to water films and interstitial spaces, are outliers in the relationship between maximum size and richness (phyla coloured green in figure 1a), and have smaller maximum sizes than expected for their richness. Interstitial habitats severely constrain organismal size through physical space limitation as individuals are unable to shift sediment particles and are confined to pore spaces (Schwinghamer 1981). Size bins adjacent to interstitial sizes in biomass spectra are well known to correspond to low biomass and potential fitness troughs (Warwick & Clarke 1984; Kerr & Dickie 2001). Experimental evidence indicates that interstitial nematodes readily obtain much larger sizes when grown in a less confining medium (Anderson & Coleman 1977). Rotifers found in space-limited habitats (e.g. water films on mosses, damp soil, interstitial spaces) are also much smaller (45–57 μm in length) than pelagic forms (R. Shiel, personal communication, 2005).
Among metazoan phyla, vertebrates are a distinctive outlier in that they have a larger minimum size than expected from their diversity (figure 1b). Minimum size in endothermic vertebrates is thought to be tightly constrained by both environmental factors and metabolic demands (Tracy 1977). Miniaturization of vertebrates, including the smallest known fish, Paedocypris progenetica, is often a result of developmental truncation (Hanken & Wake 1993). Within vertebrates, eight outlying families of birds have smaller body size ranges than expected given their diversity (figure 2). In these groups (seven passerine families and the hummingbirds), size constraints related to the physiology of flight may limit maximum size. Similarly, in mammals, several families of bats, fossorial rodents and arboreal primates also show smaller body size ranges than expected, indicating possible body size constraints associated with those lifestyles. Within arthropods, it is also clear that the subphylum Hexapoda, containing insects, has appreciably larger species richness compared with the subphylum Crustacea, despite the considerably larger size range in the latter. This probably reflects both the extraordinary niche diversification of insects, possibly unrelated to body size, and the relative underestimation of total crustacean diversity, especially among smaller body sizes.
Despite size constraints on either maximum size (interstitial groups) or minimum size (vertebrates), both groups continue to expand their size range in the direction opposite the constraint. Gastrotrichs, rotifers, gnathostomulids and loriciferans all contain species much smaller than predicted by their richness alone (figure 1b). In other clades, key innovations in niche space were required. Groups such as nematodes, with many interstitial members, evolved fundamentally different niches such as parasitism to expand their size range. For example, the largest species is Placentonema gigantissima, a 6–9 m long parasite occurring in the placentas of sperm whales. Buffered environmental conditions, a continuous food source and relaxation of mechanical requirements of locomotion (Kirchner et al. 1980) have allowed parasitic forms to reach much larger sizes than their free-living relatives in nematodes (Kirchner et al. 1980), amphipods (Poulin & Hamilton 1995) and copepods (Poulin 1995). The size range for vertebrates has greatly expanded through the evolution of the baleen whales, such as the blue whale, B. musculus, perhaps a maximum size limit set by either bioenergetics or life-history constraints (Dobson & Headrick 1995; Corkeron & Connor 1999). The evolution of colonial forms was also important in increasing size range. In Bryozoa, colonial forms greatly expand the size range from either the smallest autozoid (nine orders of magnitude) or the smallest colony (seven orders of magnitude) to the largest colony (figure 1a,c), and may explain its relatively heightened richness. Increases in colony size coincide with increases in colony individuation, dispersal ability, resistance to physical stress, homeostasis and division of labour, but the extent to which large colony size may be favoured by natural selection remains unknown (McShea & Venit 2002).
4. Conclusions
Despite the distinctive selection pressures probably affecting individual phyla, we demonstrate a remarkably consistent relationship between richness and body size minimum, maximum and range across metazoan phyla and two vertebrate classes. These relationships also remain when hypotheses of phylogeny are used for independent contrasts. The patterns are consistent with the predictions of passive diffusion of body size during evolution. From an alternative perspective, we provide preliminary evidence that linkages between body size variation and niche diversity may underlie these relationships. Limits to body size within phyla appear to have been met with either evolutionary innovation in organismal complexity or radiation into fundamentally different lifestyles. Further research with an emphasis on the fossil record will be required to distinguish between these two alternative scenarios.
Acknowledgments
We wish to thank Jonathan Payne, Seth Finnegan, Steve Haddock, Kenneth Smith, James Barry, James Brown, Jeff Nekola and two reviewers, who provided constructive reviews of the manuscript. We are additionally grateful for the input of the many taxonomic specialists who made this work possible. Research was funded by a postdoctoral fellowship from the Monterey Bay Aquarium Research Institute to C.R.M. This manuscript and the ideas within were greatly shaped by the authors' participation in the National Evolutionary Synthesis Center Working Group ‘Phanerozoic Body Size Trends in Time Space: Macroevolution and Macroecology’.
Supplementary Material
Number of species, smallest size species and largest sized species for metazoan phyla
References
- Alroy J. Cope's rule and the dynamics of body mass evolution in North American fossil mammals. Science. 1998;280:731–734. doi: 10.1126/science.280.5364.731. doi:10.1126/science.280.5364.731 [DOI] [PubMed] [Google Scholar]
- Anderson R.V., Coleman D.C. The use of glass microbeads in ecological experiments with bacteriophagic nematodes. J. Nematol. 1977;9:319–433. [PMC free article] [PubMed] [Google Scholar]
- Bininda-Emonds O.R.P., et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. doi:10.1038/nature05634 [DOI] [PubMed] [Google Scholar]
- Chapelle G., Peck L.S. Polar gigantism dictated by oxygen availability. Nature. 1999;399:114–115. doi:10.1038/20099 [Google Scholar]
- Corkeron P.J., Connor R.C. Why do baleen whales migrate? Mar. Mamm. Sci. 1999;15:1228–1245. doi:10.1111/j.1748-7692.1999.tb00887.x [Google Scholar]
- Damuth J. Population density and body size in mammals. Nature. 1981;290:699–700. doi:10.1038/290699a0 [Google Scholar]
- Dobson G.P., Headrick J.P. Bioenergtics scaling: metabolic design and body-size constraints in mammals. Proc. Natl Acad. Sci. USA. 1995;92:7317–7321. doi: 10.1073/pnas.92.16.7317. doi:10.1073/pnas.92.16.7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C.W., et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–749. doi: 10.1038/nature06614. doi:10.1038/nature06614 [DOI] [PubMed] [Google Scholar]
- Dunning J.B. CRC Press; Orlando, FL: 1992. Handbook of avian body weights. [Google Scholar]
- Foote M. Discordance and concordance between morphological and taxonomic diversity. Paleobiology. 1993;19:185–204. [Google Scholar]
- Foote M. The evolution of morphological diversity. Annu. Rev. Ecol. Syst. 1997;28:129–152. doi:10.1146/annurev.ecolsys.28.1.129 [Google Scholar]
- Garland T.J., Ives A.R. Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative models. Am. Nat. 2000;155:346–364. doi: 10.1086/303327. doi:10.1086/303327 [DOI] [PubMed] [Google Scholar]
- Garland T.J., Harvey P.H., Ives A.R. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 1992;41:18–32. doi:10.2307/2992503 [Google Scholar]
- Garland T.J., Dickerman A.W., Janis C.M., Jones J.A. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 1993;42:265–292. doi:10.2307/2992464 [Google Scholar]
- Gaston K.J., Blackburn T.M. Range size–body size relationships: evidence of scale dependence. Oikos. 1996;1996:479–485. doi:10.2307/3545889 [Google Scholar]
- Gillman M.P. Evolutionary dynamics of vertebrate body mass range. Evolution. 2007;61:685–693. doi: 10.1111/j.1558-5646.2007.00060.x. doi:10.1111/j.1558-5646.2007.00060.x [DOI] [PubMed] [Google Scholar]
- Grant P.R. Bill size, body size and the ecological adaptations of bird species to competitive situations on islands. Syst. Zool. 1968;17:319–333. doi:10.2307/2412010 [PubMed] [Google Scholar]
- Hackett S.J., et al. A phylogenomic study of birds reveals their evolutionary history. Science. 2008;27:1763–1768. doi: 10.1126/science.1157704. doi:10.1126/science.1157704 [DOI] [PubMed] [Google Scholar]
- Hanken J., Wake D.B. Miniaturization of body size: organismal consequences and evolutionary significance. Annu. Rev. Ecol. Syst. 1993;24:501–519. doi:10.1146/annurev.es.24.110193.002441 [Google Scholar]
- Hunt G., Roy K. Climate change, body size evolution, and Cope's rule in deep-sea ostracods. Proc. Natl Acad. Sci. USA. 2006;103:1347–1352. doi: 10.1073/pnas.0510550103. doi:10.1073/pnas.0510550103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson G.E. Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 1959;93:145–159. doi:10.1086/282070 [Google Scholar]
- Hutchinson G.E., MacArthur R.H. A theoretical ecological model of size distributions among species of animals. Am. Nat. 1959;93:117–125. doi:10.1086/282063 [Google Scholar]
- Jablonski D. Body-size evolution in Cretaceous molluscs and the status of Cope's rule. Nature. 1997;385:250–252. doi:10.1038/385250a0 [Google Scholar]
- Kerr S.R., Dickie L.M. Columbia University Press; New York, NY: 2001. The biomass spectrum. [Google Scholar]
- Kirchner T.B., Anderson R.V., Ingham R. Natural selection and the distribution of nematode sizes. Ecology. 1980;61:232–237. doi:10.2307/1935179 [Google Scholar]
- Kozlowski J., Gawelczyk A.T. Why are species' body size distributions usually skewed to the right? Funct. Ecol. 2002;16:419–432. doi:10.1046/j.1365-2435.2002.00646.x [Google Scholar]
- Layman C., Winemiller K.D.A., Jepsen D. Body size and trophic position in a diverse tropical food web. Ecology. 2005;86:2530–2535. doi:10.1890/04-1098 [Google Scholar]
- Lucas C.H. Biochemical composition of Aurelia aurita in relation to age and sexual maturity. J. Exp. Mar. Biol. Ecol. 1994;183:179–192. doi:10.1016/0022-0981(94)90086-8 [Google Scholar]
- Maddison, W. P. & Maddison, D. R. 2008 Mesquite: a modular system for evolutionary analysis. Version 2.5. (http://mesquiteproject.org)
- Makarieva A.M., Gorshkov V.G., Li B.-L., Chown S.L., Reich P.B., Gavrilov V.M. Mean mass-specific metabolic rates are strikingly similar across life's major domains: evidence for life's metabolic optimum. Proc. Natl Acad. Sci. USA. 2008;105:16 994–16 999. doi: 10.1073/pnas.0802148105. doi:10.1073/pnas.0802148105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchinko K.B., Nishizaki M.T., Burns K.C. Community-wide character displacement in barnacles: a new perspective for past observations. Ecol. Lett. 2004;7:114–120. doi:10.1046/j.1461-0248.2003.00557.x [Google Scholar]
- Maurer B.A., Brown J.H., Rusler R.D. The micro and macro in body size evolution. Evolution. 1992;46:939–953. doi: 10.1111/j.1558-5646.1992.tb00611.x. doi:10.2307/2409748 [DOI] [PubMed] [Google Scholar]
- May R.M. How many species are there on earth? Science. 1988;241:1441–1449. doi: 10.1126/science.241.4872.1441. doi:10.1126/science.241.4872.1441 [DOI] [PubMed] [Google Scholar]
- McClain C.R., Rex M.A. The relationship between dissolved oxygen concentration and maximum size in deep-sea turrid gastropods: an application of quantile regression. Mar. Biol. 2001;139:681–685. doi:10.1007/s002270100617 [Google Scholar]
- McClain C.R., Boyer A., Rosenberg G. The island rule and the evolution of body size in the deep sea. J. Biogeogr. 2006;33:1578–1584. doi:10.1111/j.1365-2699.2006.01545.x [Google Scholar]
- McKinney M.L. Trends in body-size evolution. In: McNamara K.J., editor. Evolutionary trends. University of Arizona Press.; Tucson, AZ: 1990. pp. 75–118. [Google Scholar]
- McShea D.W., Venit E.P. Testing for bias in evolution of coloniality: a demonstration in cyclostome bryozoans. Paleobiology. 2002;28:308–327. doi:10.1666/0094-8373(2002)028<0308:TFBITE>2.0.CO;2 [Google Scholar]
- Novack-Gottshall P., Lanier M. Scale-dependence of Cope's rule in body size evolution of Paleozoic brachiopods. Proc. Natl Acad. Sci. USA. 2008;105:5430–5434. doi: 10.1073/pnas.0709645105. doi:10.1073/pnas.0709645105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme C.D.L., Quicke D.L.J., Cook J.M., Purvis A. Body size does not predict species richness among the metazoan phyla. J. Evol. Biol. 2002;15:235–247. doi:10.1046/j.1420-9101.2002.00379.x [Google Scholar]
- Payne J.L., et al. Two-phase increase in maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. Proc. Natl Acad. Sci. USA. 2009;106:16 994–16 999. doi: 10.1073/pnas.0806314106. doi:10.1073/pnas.0806314106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R.H. The ecological implications of body size. Cambridge University Press; Cambridge, UK: 1983. [Google Scholar]
- Poulin R. Clutch size and egg size in free-living and parasitic copepods—a comparative analysis. Evolution. 1995;49:325–336. doi: 10.1111/j.1558-5646.1995.tb02245.x. doi:10.2307/2410343 [DOI] [PubMed] [Google Scholar]
- Poulin R., Hamilton W.J. Ecological determinants of body size and clutch size in amphipods: a comparative approach. Funct. Ecol. 1995;9:364–370. doi:10.2307/2389998 [Google Scholar]
- Ricciardi A., Bourget E. Weight-to-weight conversion factors for marine benthic macroinvertebrates. Mar. Ecol. Prog. Ser. 1998;163:245–251. doi:10.3354/meps163245 [Google Scholar]
- Schoener T.W. The Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology. 1968;49:704–726. doi:10.2307/1935534 [Google Scholar]
- Schoener T.W. Size patterns in W. Indian Anolis lizards. II. Correlations with the sizes of particular sympatric species-displacement and convergence. Am. Nat. 1970;104:155–174. doi:10.1086/282647 [Google Scholar]
- Schwinghamer P. Characteristic size distributions of integral benthic communities. Can. J. Fish. Aquat. Sci. 1981;38:1255–1263. [Google Scholar]
- Smith F.A., Lyons S.K., Jones K.E., Kaufman D.M., Dayon T., Marquet P.A., Brown J.H., Haskell J.P. Body mass of Late Quaternary mammals. Ecology. 2003;84:3403. doi:10.1890/02-9003 [Google Scholar]
- Stanley S.M. An explanation for Cope's rule. Evolution. 1973;27:1–26. doi: 10.1111/j.1558-5646.1973.tb05912.x. doi:10.2307/2407115 [DOI] [PubMed] [Google Scholar]
- Tracy C.R. Minimum size of mammalian homeotherms: role of the thermal environment. Science. 1977;198:1034–1035. doi: 10.1126/science.929184. doi:10.1126/science.929184 [DOI] [PubMed] [Google Scholar]
- Trammer J. Power formula for Cope's rule. Evol. Ecol. Res. 2002;4:147–153. [Google Scholar]
- Trammer J. Maximum body size in a radiating clade as a function of time. Evolution. 2005;59:941–947. doi:10.1554/04-254 [PubMed] [Google Scholar]
- Warwick R.M., Clarke R.H. Species size distributions in marine benthic communities. Oecologia. 1984;61:32–41. doi: 10.1007/BF00379085. doi:10.1007/BF00379085 [DOI] [PubMed] [Google Scholar]
- Wilson D.S. The adequacy of body size as a niche difference. Am. Nat. 1975;109:769–784. doi:10.1086/283042 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of species, smallest size species and largest sized species for metazoan phyla