Abstract
Mammalian societies in which females dominate males are rare, and the factors favouring the evolution of female dominance have yet to be clearly identified. We propose a new hypothesis for the evolution of female dominance and test its predictions with empirical data from the spotted hyena (Crocuta crocuta), a well-studied species characterized by female dominance. We suggest that constraints imposed by the development of a feeding apparatus specialized for bone cracking, in combination with the intensive feeding competition characteristic of spotted hyenas, led to the evolution of female dominance. Specifically, we propose that protracted development of the feeding apparatus in young hyenas led to selection for increased aggressiveness in females as a compensatory mechanism for mothers to secure food access for their young after weaning. Our analyses yielded results consistent with this hypothesis. Morphological and behavioural measurements indicate that skull development is indeed protracted in this species; spotted hyenas do not achieve adult skull size or feeding performance capabilities until after sexual maturity. The period between weaning and completed skull development is particularly challenging, as indicated by high mortality. Finally, maternal presence between weaning and full skull maturity, as well as the relative ability of females to aggressively displace conspecifics from food, are important determinants of offspring survival.
Keywords: development, female dominance, life history, mammals, skull, spotted hyena
1. Introduction
In most mammals, sexual dimorphisms in body size, weaponry and aggressive behaviour occur in association with contest competition among males for access to females or for control of the resources required by females (Darwin 1871; Short & Balaban 1994). As males are usually larger, better armed and more aggressive than females, they seldom have difficulty achieving social dominance over females, and males easily win contests for resources needed by both sexes. Examples of male social dominance abound among ungulates, primates and carnivores. Along with those of several lemurs and two mole rat species (Sherman et al. 1991; Kappeler 1993), the societies of spotted hyenas (Crocuta crocuta) represent rare exceptions to the typical mammalian pattern of male dominance. Adult female spotted hyenas are more aggressive than males (Szykman et al. 2003) and socially dominant to them in virtually all social contexts (Kruuk 1972; Tilson & Hamilton 1984; Frank 1986). Despite great interest in this unusual pattern of ‘sex-role-reversed’ behaviour, there is not yet a satisfactory functional explanation for the evolution of female dominance in any mammalian species.
Spotted hyenas are gregarious carnivores that live in large social groups, called clans, structured by linear dominance hierarchies. They feed primarily on fresh ungulate carcasses, which are rich, but highly ephemeral, food sources. Feeding priority is determined by dominance rank within the clan (Tilson & Hamilton 1984; Frank 1986); however, a single carcass is often too big to be monopolized by one individual. As multiple hyenas feed simultaneously, feeding competition intensifies and an entire carcass may be consumed within minutes (Kruuk 1972; Frank 1986). Therefore, the ability to feed rapidly is a critical determinant of the quantity of food that an individual can ingest.
Frank (1986) hypothesized that the highly competitive feeding environment typical of Crocuta clans selects for large, aggressive females who are better able to secure food resources for themselves and their dependent young. This hypothesis, hereafter called the ‘feeding competition’ hypothesis, predicts that feeding competition in Crocuta should be more intense than in species that do not exhibit female dominance. However, this prediction is not well supported, as other gregarious carnivores such as wolves (Canis lupus) and wild dogs (Lycaon pictus)—which, like Crocuta, feed on medium- to large-bodied ungulates—appear to experience similarly intense feeding competition. Despite substantial intra- and interspecific variations in social group size, the size of typical feeding groups is similar across these species, and adults of all three species feed at similarly rapid rates (see table S1 in the electronic supplementary material for data and references). Although each of these large carnivores experiences intense feeding competition, female dominance has evolved only in Crocuta, suggesting that feeding competition alone is not sufficient to explain the evolution of this unusual trait. Whereas we agree with Frank (1986) that the intensity of feeding competition has been critically important in the evolution of female dominance, we suggest that female dominance was favoured in spotted hyenas, but not in other gregarious carnivores, because this is the only species in which a highly competitive feeding environment is coupled with constrained development of a skull specialized for durophagy.
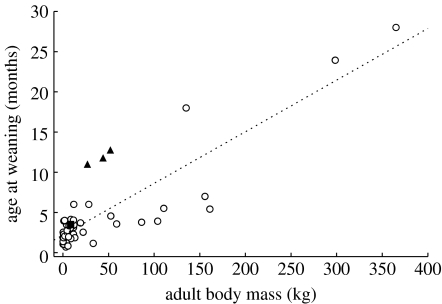
Unlike wolves or wild dogs, adult Crocuta are capable of cracking open large bones. Similar to those of their carrion-feeding ancestors and closest extant relatives, the skulls and teeth of Crocuta are highly specialized for durophagy (Werdelin & Solounias 1991; Koepfli et al. 2006). Cranio-dental adaptations of the bone-cracking hyenas include robust premolars with specialized crack-resistant enamel, a vaulted forehead, a large sagittal crest and massive zygomatic arches; these last two skull features provide attachment sites for powerful jaw muscles (Werdelin 1989; Werdelin & Solounias 1991; Joeckel 1998). This morphology allows spotted hyenas to exert bite forces great enough to crack open the bones of ungulates as large as zebras and giraffes. The ontogenetic development of this very robust feeding apparatus appears to be a slow and prolonged process (Binder & Van Valkenburgh 2000). Indeed, all the bone-cracking hyenas (spotted hyenas; striped hyenas, Hyaena hyaena; and brown hyenas Parahyaena brunnea) exhibit delayed weaning relative to most other carnivores (figure 1). However, as striped hyenas and brown hyenas feed solitarily (Kruuk 1976; Mills 1990), protracted skull development should not present the same challenge to their young as it does to those of spotted hyenas.
Figure 1.
Age at weaning in relation to adult body mass for 59 species of terrestrial Carnivora, including the bone-cracking hyaenids (triangles) and the sole non-bone-cracking hyaenid (i.e. the aardwolf; square). Data and references are presented in the electronic supplementary material, table S2. In addition to the bone-cracking hyenas, several species of bears also wean relatively late for their body size.
(a) The developmental constraint hypothesis
We suggest that female dominance represents an extreme form of parental behaviour permitting sustained access to food by youngsters who are handicapped during competitive feeding by their poorly developed skulls and jaw musculature. Hereafter, we refer to this hypothesis as the ‘developmental constraint’ hypothesis. By developmental constraint, we mean ‘a mechanism or process that limits the evolutionary response of a character or set of characters to external selection acting during a focal life stage’ (Schwenk & Wagner 2004, p. 400). Here, the rate of development of a skull specialized for cracking bone appears limited in its response to the external selection pressure of feeding competition during the focal life stage between weaning and the age at which the skull is fully mature. Crocuta cubs typically nurse until 12–18 months of age (Kruuk 1972; Hofer & East 1995; Holekamp et al. 1996) and reach sexual maturity at 24 months of age (Matthews 1939; Glickman et al. 1992). However, among captive hyenas, skull growth is incomplete at weaning, and performance maxima during bone consumption are not achieved until after puberty (Binder & Van Valkenburgh 2000).
The developmental constraint hypothesis generates a number of testable predictions regarding skull development and life-history patterns in Crocuta. Here, we first present data on the ontogeny of skull morphology and feeding performance from free-living Crocuta, to determine when development of the feeding apparatus is complete. If our new hypothesis is correct, then constrained skull development in Crocuta should affect not only the ability to generate large bite forces necessary for cracking bone, but also the speed at which hyenas can ingest even soft foods such as muscle or viscera. Furthermore, since female social rank accurately predicts how effectively mothers can maintain access to food for their cubs (Frank 1986; Henschel & Skinner 1987), we expect that lower-ranking cubs will be weaned at later stages of skull development than high-ranking cubs. This follows from the expectation that the lower the social rank of the mother, the less able she should be to compensate with aggression for constrained morphological development in her offspring. Low-ranking females are known to wean their cubs at later ages than high-ranking females (Frank et al. 1995a; Holekamp et al. 1996; Hofer & East 2003), so here we examine the relationship between weaning age and skull development.
We also test several predictions regarding patterns of mortality that follow from our hypothesis. First, if protracted development of the feeding apparatus represents an important force shaping the evolution of female dominance, then weaning should be a particularly challenging life-history event for juvenile hyenas. Here, we examine mortality rates in Crocuta after weaning and compare them with mortality during the lactation interval and during the periods following two other life-history events that are potentially dangerous: den independence (White 2005) and first parturition (Frank et al. 1995b). Permanent departure from the den (den independence), at 8–12 months of age, is likely to be challenging because it exposes cubs to unfamiliar environments and new dangers. First parturition is potentially dangerous for young female Crocuta because the birth canal is unusually long and passes through the elongated clitoris (‘pseudopenis’), which must tear at first parturition to allow passage of the foetus. Consequently, dystocia (difficult parturition) may be both common and fatal to primiparous female Crocuta (Frank & Glickman 1994; Frank et al. 1995b).
Both hypotheses predict that offspring of high-ranking females should survive better than offspring of low-ranking females. Furthermore, the developmental constraint hypothesis posits that the advantages conferred by female dominance should be particularly evident after weaning. Therefore, we compare survivorship after weaning between the offspring of high- and low-ranking females.
Finally, not only do female spotted hyenas routinely help their nursing offspring to access food resources (Frank 1986; Holekamp & Smale 1990; Engh et al. 2000), but they also aid their reproductively mature offspring during feeding competition with conspecifics (Kruuk 1972), an observation not predicted by the feeding competition hypothesis. The developmental constraint hypothesis further predicts that maternal presence should increase the probability of survival after weaning, but not once the development of the feeding apparatus is complete.
In this study, we use morphological, behavioural and demographic data collected during a long-term study of known individuals to test these predictions of the developmental constraint hypothesis as an explanation for the evolution of female dominance in Crocuta. This work represents a first step in elucidating the suite of selective pressures leading to the unusual behavioural trait of female dominance.
2. Material and methods
We tested the developmental constraint hypothesis using data collected between July 1988 and June 2007 from members of one large Crocuta clan in the Masai Mara National Reserve, Kenya. Individual hyenas were identified by their unique spots, and sex was determined based on penile morphology (Frank et al. 1990). Hyenas were observed daily between 05.30 and 09.00 and between 17.00 and 20.00. Agonistic interactions, recorded using all-occurrence sampling (Altmann 1974), were used to determine social ranks of adult females (see Engh et al. 2000). Adult females were assigned both a relative rank and rank category, depending on the analysis performed. A female's relative rank was the proportion of adult females over which she was dominant, with the highest ranking female having a rank of 1 and the lowest a rank of 0. When sample size was sufficient, three rank categories (high, mid and low) were designated by dividing the hierarchy into equal thirds, but when sample size was smaller, rank was categorized as either high or low. Maternal rank was assigned as the rank held by a cub's mother when that cub was born.
(a) Morphological measurements and feeding performance
We used head circumference as a proxy for skull size as it includes both muscles and bones of the feeding apparatus. Hindfoot length was selected as a postcranial measure of body size that was not expected to exhibit protracted development. Head circumference and hindfoot length were recorded from 1990 to 2007 during routine immobilizations (Van Horn et al. 2003) of 214 hyenas ranging in age from 1.5 to 199 months. For animals anaesthetized more than once per lifetime, a single set of measurements was randomly selected to represent that individual. To examine skull development at weaning, data were analysed from 37 hyenas darted within one month of weaning. Weaning age was determined based on weaning conflicts and cessation of nursing as described by Holekamp et al. (1996); these data were available only from 1988 to 2000. Juveniles typically wean at 12–14 months of age in this population, but weaning age is highly variable.
Ontogenetic changes in feeding performance on soft foods were quantified using a standardized test of feeding speed (Tanner 2007). Between 1989 and 1996, 86 known-age hyenas were presented with 30 g dog biscuits, and ingestion time was measured with a stopwatch. A single ingestion time was randomly selected for individuals participating in more than one feeding trial.
(b) Determination of births, deaths and other life-history events
Most adult females in the study clan wore radio collars such that they could be relocated daily to monitor their reproductive status. Natal and communal den sites, where Crocuta cubs reside, were visited regularly to monitor births and cub development. Cubs born between 1988 and 2003 were included in analyses of survival. When cubs were first observed above ground, their ages were estimated to within ±7 days (as in Holekamp et al. 1996). In this population, most cubs become independent of the communal den at eight to nine months of age; a cub was considered independent of the den when it was found more than 200 m from the den on at least four consecutive occasions (Boydston et al. 2005). Reproductive maturity in spotted hyenas is achieved by 24 months of age in both sexes (Matthews 1939). Parous females were distinguished from nulliparous females by the permanent scarring of the pseudopenis that occurs at first parturition (Frank & Glickman 1994). In this population, males disperse from their natal clan after 2 years of age, but females are philopatric (Frank et al. 1995a). Therefore, disappearances of females were attributed to death. Once males reach 2 years of age, it is often difficult to distinguish between death and dispersal. Because we were interested in survival beyond 2 years of age, only females were used in these analyses.
(c) Data analysis
To determine the ages at which head circumference, hindfoot length and biscuit ingestion time reached adult values, we used nonlinear growth models to calculate the age at which each variable reached its asymptotic value. A number of different growth models were assessed for their fit to the data using Akaike's information criterion (AIC). For head circumference, the monomolecular model (following Gaillard et al. 1997) was used, as it had the highest AIC weight. The formula used was , where x(t) is the measurement of interest at time t; A is the asymptotic adult value; K is the rate of approach to adult value; and t0 is the age at which growth begins (Gaillard et al. 1997; Zelditch et al. 2003). For hindfoot length and ingestion time, the best-fitting model was the Gompertz growth model (as parametrized by Fiorello & German 1997): x(t)=A e−ke−bt, where k is the initial growth rate and b is the decay of the growth rate (Fiorello & German 1997; Zelditch et al. 2003). We designated 95 per cent of the asymptotic value as age at maturity, and present the percentage of mature size or feeding performance achieved at each of five ages: 0, 8, 13, 24 and 36 months, based on the predicted values at each age from the fitted growth curve. The evaluation of growth models, and estimation of parameters, was done using GrowChoice from the Integrated Morphometrics Programs series (Sheets, Canisius College, Buffalo, NY).
Survivorship curves were created using the Kaplan–Meier method, with individuals still alive at the end of the study period included as right-censored data points. To examine the overall effect of maternal rank on survival, we used a Cox proportional hazard model, which included relative rank as a continuous variable. Litter size and maternal parity were also included in the model. Litter size has been shown to influence survival in Crocuta during the first 2 years of life, with individuals from twin litters surviving better than singletons (Wahaj et al. 2007). We included maternal parity as a factor because breeding experience has been shown to influence offspring survival in other large mammals (Hastings & Testa 1998; McMahon & Bradshaw 2004; Robbins et al. 2006) and because primiparous Crocuta may be particularly ill-equipped to rear young. Specifically, female Crocuta first reproduce as early as 2.5 years of age in this population (Holekamp et al. 1996), when their own feeding and hunting capabilities are not yet fully developed. To evaluate cub survival during the 14 months after weaning, young females were categorized based on maternal rank or maternal presence, and survivorship curves were compared using Cox's F-test. We further examined the effect of maternal presence on survival after full skull maturity was achieved by comparing survivorship curves of females from 36 to 50 months of age; Gehan's Wilcoxon signed-rank test was used for analysis because there were many censored data points. Statistica v. 6.1 (StatSoft 2002) was used for all statistical analyses, unless otherwise noted. Mean values are presented ±s.e. Differences between groups were considered significant when p<0.05.
3. Results
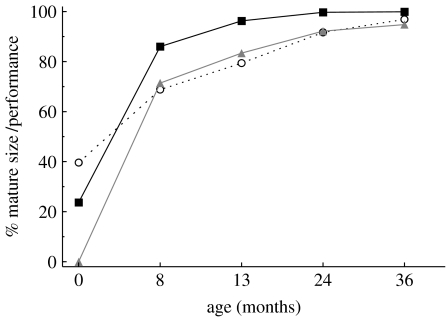
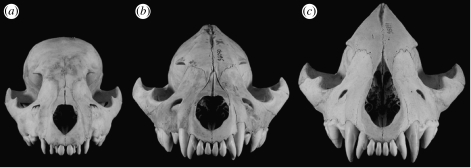
Head circumference reached maturity at 29 months of age (figure 2). Thus, the skulls of spotted hyenas have not yet reached adult size by either weaning or sexual maturity. Ontogenetic change in feeding speed followed a pattern similar to that observed for head circumference; hyenas achieved adult performance levels at 34 months of age, well after weaning and sexual maturity. By contrast, hindfoot length reached adult size at 12 months of age, a month before mean weaning age. Head circumference at weaning was positively correlated with age at weaning (r=0.695, p<0.0001), indicating that lower-ranking females wean their cubs at later stages of skull development (figure 3).
Figure 2.
Percentage of mature adult size achieved for head circumference (circles) and hindfoot length (squares), and percentage of adult performance achieved for feeding speed (triangles), at the ages of key life-history events: 0 (birth), 8 (den independence), 13 (weaning), 24 (sexual maturity) and 36 months. The percentages were calculated based on the values obtained from the growth curve for each trait at each age.
Figure 3.
Skulls of three known-age Crocuta in frontal view illustrating variation in skull development at weaning ((a) 7.53 months, (b) 14.4 months and (c) 22.2 months). On average, cubs wean at 13 months of age, but age at weaning ranges from 7.5 to 24 months. High-ranking cubs tend to wean when they are closer to the developmental stage shown in (a), whereas low-ranking cubs tend to wean closer to the stage shown in (c). Note that the nasal bones are missing from skull (c).
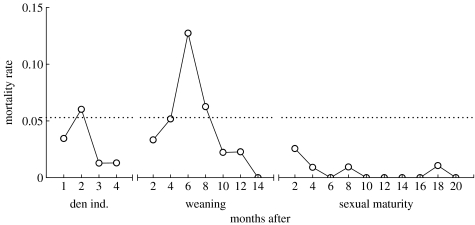
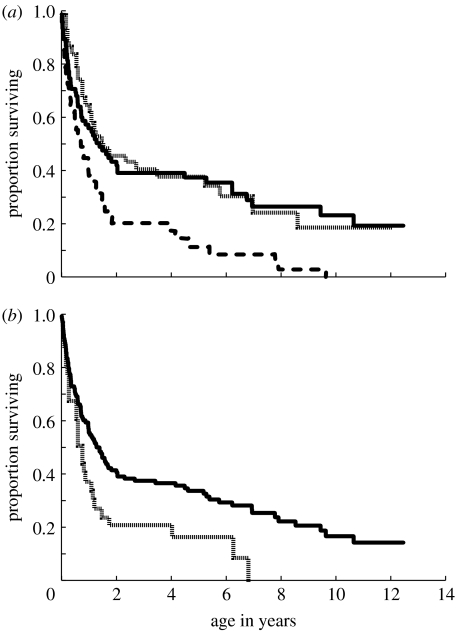
Monthly mortality rates increased after weaning, and were higher after weaning (maximum 0.13) than after den independence (maximum 0.06) or sexual maturity (maximum 0.03; figure 4). Mean monthly mortality for the first 12 months of life, when offspring are still nursing, was 0.053±0.008 (indicated by horizontal line on figure 4; range 0.011–0.093). We observed an increase in mortality associated with den independence, but, surprisingly, not with first parturition. Mortality could be monitored only for four months after den independence because juveniles then often began to wean. As it is difficult to detect conception in this species, mortality rates were estimated for the entire interval between sexual maturity and 44 months of age, which encompasses the ages when most females first reproduce (Holekamp et al. 1996). The mortality rate during this interval probably overestimates mortality associated with first parturition as it includes all mortalities, even those due to causes other than dystocia.
Figure 4.
Monthly mortality rates for females during the months immediately after den independence (n=85), weaning (n=60) and sexual maturity (n=59). The x-axis indicates the number of months following each event. For any individual, time zero is the date on which that individual left the den permanently, weaned or reached 24 months of age (i.e. sexual maturity), respectively. Fates after den independence could be followed only for four months before juveniles began to wean. The dotted line indicates the mean monthly mortality rate during the first 12 months of life, when most juveniles are still nursing.
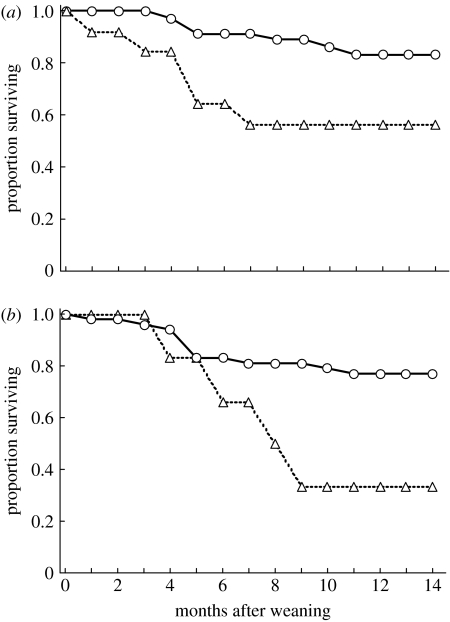
Female survival was influenced by maternal rank (Cox proportional hazard: whole model Χ2=15.13, n=164 females, p=0.002; maternal rank, p=0.005). Daughters of high- and mid-ranking females experienced higher survival rates than did their low-ranking counterparts (figure 5a). Maternal parity also influenced survival (p=0.004; figure 5b), but litter size did not (p=0.21). Cubs born to multiparous females survived better than those born to primiparous females. Furthermore, when we looked specifically at survival after weaning, maternal rank was important. The daughters of high-ranking females were more likely to survive after weaning than were daughters of low-ranking females (figure 6a; Cox's F12,22=3.29, n=60 females, p=0.008).
Figure 5.
Survivorship curves for daughters born (a) to high-ranking (solid curve), mid-ranking (hatched curve) and low-ranking (dashed curve) mothers, and (b) to primiparous (hatched curve) and multiparous (solid curve) mothers. Cases in which the proportion of individuals surviving does not reach zero are owing to individuals still alive at the end of the study (i.e. right-censored data points).
Figure 6.
Proportion of females surviving after weaning (a) based on social rank (n=35 high ranking, circles; n=25 low ranking, triangles) and (b) when mother was present (n=53; circles) or absent (n=6; triangles). Time zero is assigned for each individual as its date of weaning.
Survival after weaning was greater for juvenile females whose mothers were present in the population than for those whose mothers died within six months of weaning (figure 6b; Cox's F24,8=3.68, n=59 females, p=0.031). In all cases considered in this analysis, mothers were still alive at the time of weaning. Thus, even though cubs stop relying on maternal milk as a food resource when they are weaned, they clearly continue to rely on maternal aid for survival. This effect did not persist once full adult maturity was achieved, as maternal presence had no effect on survival after 36 months of age (Gehan's Wilcoxon signed-rank test=−0.94, n=40 females, p=0.35).
4. Discussion
Our results demonstrate that maternal support can strongly influence offspring survival beyond weaning in the spotted hyena, a species in which protracted development of a specialized feeding apparatus occurs in an environment characterized by intensive feeding competition. Our analyses of ontogenetic change in head circumference and feeding speed indicate that young adult hyenas suffer a handicap relative to older hyenas, and that this handicap may affect their ability to compete with conspecifics for food. Whereas the skull of Crocuta does not reach maturity until well after puberty, the skulls in most other carnivores that have been studied in this regard reach maturity at or before puberty (spotted seal, Phoca largha: Mizuno & Ohtaishi 2002; sea otter, Enhydra lutris: Hattori et al. 2003; female polar bear, Ursus maritimus: Bechshøft et al. 2008; coyote, Canis latrans: S. LaCroix, B. L. Lundrigan, M. L. Zelditch, J. A. Shivak & K. E. Holekamp 2009, unpublished data; but exceptions include brown bear, Ursus arctos: Ohdachi et al. 1992; and male polar bear: Bechshøft et al. 2008). As predicted by the developmental constraint hypothesis, weaning among spotted hyenas was particularly challenging relative to other potentially dangerous life-history events. Maternal support, especially the ability of mothers to aggressively displace conspecifics from food (i.e. social rank), exerts a powerful influence on offspring survival during the difficult period after weaning. The cubs of high-ranking females survive better than the cubs of low-ranking females, despite the fact that high-ranking females tend to wean their cubs when cubs' skulls are relatively poorly developed. This suggests that maternal aggression can indeed successfully compensate for a poorly developed feeding apparatus, and that improved survival of high-ranking offspring is not attributable to the production of better quality offspring by high-ranking females. Overall, these findings are strongly consistent with the hypothesis that female dominance has evolved in Crocuta as a result not only of intensive feeding competition, but also of constrained development of the feeding apparatus.
Consistent with both developmental constraint and feeding competition hypotheses, we found that social rank had a significant effect on female survivorship overall. Prolonged development of the feeding apparatus may contribute to the reduced survivorship observed among daughters of primiparous females, if these mothers have not yet themselves achieved adult feeding or hunting capabilities. Alternatively, primiparous females may simply lack important experience in rearing young.
Although female dominance appears to have evolved as a mechanism to compensate for the handicap experienced by young Crocuta during competitive feeding after weaning, protracted development of the feeding apparatus nevertheless continues to pose a formidable challenge to young hyenas. This is particularly evident among low-ranking hyenas, whose post-weaning survivorship is poor despite their relatively advanced age and skull development at weaning.
In contrast to weaning, first parturition was not a period of high mortality for females in this study. This result is surprising in the light of previous work suggesting that female Crocuta experience unusually high rates of mortality when they first give birth (Frank et al. 1995b). Frequent observations of dystocia among primiparous females in captivity (Frank & Glickman 1994) led Frank et al. (1995b) to suggest that genital masculinization in female Crocuta is costly due to increased risk of mortality to both mother and cub(s) at parturition. However, we found no evidence of increased maternal mortality associated with first parturition. In fact, mean monthly mortality for females between 2.5 and 3.5 years of age, when most females first reproduce, was 0.0033±0.0022, considerably lower than that during surrounding age intervals. Our findings still leave open the possibility that female genital masculinization involves other potential costs such as increased risk of foetal mortality at parturition and difficulty associated with conceiving young or maintaining pregnancies.
Weaning is undoubtedly challenging for youngsters in most species of mammalian carnivores. We suggest, however, that weaning may be particularly difficult for young Crocuta who are handicapped during intensive feeding competition by the immaturity of their feeding apparatus. Although available data are sparse, they fail to indicate increased mortality after weaning in species other than Crocuta. Mortality rates around weaning have been examined in lions (Panthera leo), cheetahs (Acinonyx jubatus) and southern elephant seals (Mirounga leonina); in all three species, mortality rates are lower after than before weaning (Packer et al. 1988; Caro 1994; Pistorius et al. 2001). Although very preliminary, these comparisons are consistent with the idea that weaning may be particularly challenging for Crocuta.
It also remains to be seen whether maternal support following weaning is of unique importance in Crocuta relative to other taxa. Whereas the young of other carnivore species may rely on their mothers' hunting skills for food after they are weaned, young Crocuta seem unusually heavily handicapped because they lack not only the hunting skills needed to capture prey (Holekamp et al. 1997), but also the necessary feeding capabilities to ingest food as quickly as adults once a kill has been made. The wild hyenas studied here were handicapped until 34 months of age, even during ingestion of a dog biscuit made of nothing more challenging than compressed cereal. Thus, although Binder & Van Valkenburgh (2000) earlier showed an effect of an immature feeding apparatus on bone consumption, our work clearly demonstrates this effect during ingestion of soft foods as well. It therefore appears that protracted skull development affects the individual's ability to consume all parts of a carcass. The next step in testing the developmental constraint hypothesis will be to compare carnivore species that vary with respect to both durophagy and intensity of feeding competition. In these species, it will be informative to evaluate patterns of development in the feeding apparatus, as well as patterns of mortality and effects of parental support. For example, this hypothesis predicts that skull development should be completed earlier in the life histories of carnivores feeding on soft diets than in those of durophagous species. When effects of feeding competition are controlled, this hypothesis also predicts that parental support should be more extensive in relatively durophagous species than in their close relatives that consume softer diets.
The developmental constraint hypothesis may also provide a useful framework for investigating the evolution of female dominance in other mammals, notably lemurs. Most hypotheses forwarded to explain the evolution of female dominance in lemurs have focused on competition for food resources resulting from harsh, seasonal fluctuations in food availability as a key selective pressure for the evolution of this trait (reviewed in Radespiel & Zimmermann 2001; but see Dunham 2008). However, as in the case of spotted hyenas, food competition alone is inadequate to explain female dominance because lemurs do not appear to experience uniquely intense competition (Dunham 2008 and references therein). We are not aware of any studies examining developmental patterns that might have selected for dominant females as a compensatory mechanism in lemurs. Thus, we suggest that detailed examination of developmental patterns in lemurs might be a profitable avenue for future research. If forces similar to those proposed for spotted hyenas shaped the evolution of female dominance in lemur species, then we would expect to find (i) that the age at which juveniles reach adult foraging competence is delayed relative to lemur species without female dominance, (ii) some critical form of assistance provided by mothers and (iii) strong effects of maternal aid on survival after weaning.
The rarity of female-dominated societies among mammals suggests that highly unusual conditions must prevail in order for this ‘role-reversed’ trait to evolve. Our findings suggest that female dominance in spotted hyenas was favoured by the coupling of protracted development of a specialized feeding apparatus with intensive direct feeding competition. We hope that future comparative work involving a large array of extant carnivores will allow us to determine whether this coupling has occurred uniquely in spotted hyenas, as predicted by the developmental constraint hypothesis.
Acknowledgments
The work presented here was described in Animal Research Protocol no. 07/08-099-00, approved most recently on 4 June 2008 by the Institutional Animal Care and Use Committee at Michigan State University.
We thank the Kenyan Ministry of Education, Science and Technology for permission to conduct this research, and Kenya Wildlife Service, Narok County Council and the Senior Warden of the Masai Mara National Reserve for assistance. We are deeply grateful to all those who contributed to the long-term data collection, and to L. Smale for her valuable contributions to all phases of this research. This paper was improved by the helpful suggestions of two anonymous reviewers. This work was supported by National Science Foundation (NSF) grants IBN0343381, IOB0618022 and IOS0819437 to K.E.H. and B.L.L., and graduate research fellowships from NSF and Michigan State University to H.E.W.
Supplementary Material
Indicators of intensity of feeding competition among large carnivores that feed in groups
Body mass and weaning age of carnivore species
References
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. doi:10.1163/156853974X00534 [DOI] [PubMed] [Google Scholar]
- Bechshøft T.Ø., Sonne C., Rigét F.F., Wiig Ø., Dietz R. Differences in growth, size and sexual dimorphism in skulls of East Greenland and Svalbard polar bears (Ursis maritimus) Polar Biol. 2008;31:945–958. doi:10.1007/s00300-008-0435-y [Google Scholar]
- Binder W.J., Van Valkenburgh B. Development of bite strength and feeding behaviour in juvenile spotted hyenas (Crocuta crocuta) J. Zool. 2000;252:273–283. doi:10.1111/j.1469-7998.2000.tb00622.x [Google Scholar]
- Boydston E.E., Kapheim K.M., Van Horn R.C., Smale L., Holekamp K.E. Sexually dimorphic patterns of space use throughout ontogeny in the spotted hyena (Crocuta crocuta) J. Zool. 2005;267:271–281. doi:10.1017/S0952836905007478 [Google Scholar]
- Caro T.M. University of Chicago Press; Chicago, IL: 1994. Cheetahs of the Serengeti plains. [Google Scholar]
- Darwin C. John Murray; London, UK: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Dunham A.E. Battle of the sexes: cost asymmetry explains female dominance in lemurs. Anim. Behav. 2008;76:1435–1439. doi:10.1016/j.anbehav.2008.06.018 [Google Scholar]
- Engh A.L., Esch K., Smale L., Holekamp K.E. Mechanisms of maternal rank ‘inheritance’ in the spotted hyaena, Crocuta crocuta. Anim. Behav. 2000;60:323–332. doi: 10.1006/anbe.2000.1502. doi:10.1006/anbe.2000.1502 [DOI] [PubMed] [Google Scholar]
- Fiorello C.V., German R.Z. Heterochrony within species: craniofacial growth in giant, standard, and dwarf rabbits. Evolution. 1997;51:250–261. doi: 10.1111/j.1558-5646.1997.tb02406.x. doi:10.2307/2410978 [DOI] [PubMed] [Google Scholar]
- Frank L.G. Social organization of the spotted hyaena (Crocuta crocuta). II. Dominance and reproduction. Anim. Behav. 1986;34:1510–1527. doi:10.1016/S0003-3472(86)80221-4 [Google Scholar]
- Frank L.G., Glickman S.E. Giving birth through a penile clitoris: parturition and dystocia in the spotted hyaena (Crocuta crocuta) J. Zool. 1994;234:659–665. [Google Scholar]
- Frank L.G., Glickman S.E., Powch I. Sexual dimorphism in the spotted hyaena (Crocuta crocuta) J. Zool. 1990;221:308–313. [Google Scholar]
- Frank L.G., Holekamp K.E., Smale L. Dominance, demography, and reproductive success of female spotted hyenas. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: dynamics, management, and conservation of an ecosystem. University of Chicago Press; Chicago, IL: 1995a. pp. 364–384. [Google Scholar]
- Frank L.G., Weldele M.L., Glickman S.E. Masculinization costs in hyaenas. Nature. 1995b;377:584–585. doi: 10.1038/377584b0. doi:10.1038/377584b0 [DOI] [PubMed] [Google Scholar]
- Gaillard J.M., Pontier D., Allainé D., Loison A., Herve J.C., Heizmann A. Variation in growth form and precocity at birth in eutherian mammals. Proc. R. Soc. B. 1997;264:859–868. doi: 10.1098/rspb.1997.0120. doi:10.1098/rspb.1997.0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman S.E., Frank L.G., Pavgi S., Licht P. Hormonal correlates of ‘masculinization’ in female spotted hyaenas (Crocuta crocuta). 1. Infancy to sexual maturity. J. Reprod. Fert. 1992;95:451–462. doi: 10.1530/jrf.0.0950451. [DOI] [PubMed] [Google Scholar]
- Hastings K.K., Testa J.W. Maternal and birth colony effects on survival of Weddell seal offspring from McMurdo Sound, Antarctica. J. Anim. Ecol. 1998;67:722–740. doi:10.1046/j.1365-2656.1998.00242.x [Google Scholar]
- Hattori K., Burdin A.M., Suzuki M., Ohtaishi N. Age-related change and allometry of skull and canine of sea otters, Enhydra lutris. J. Vet. Med. Sci. 2003;65:439–447. doi: 10.1292/jvms.65.439. doi:10.1292/jvms.65.439 [DOI] [PubMed] [Google Scholar]
- Henschel J.R., Skinner J.D. Social relationships and dispersal patterns in a clan of spotted hyaenas Crocuta crocuta in the Kruger National Park. S. Afr. J. Zool. 1987;22:18–24. [Google Scholar]
- Hofer H., East M.L. Population dynamics, population size, and the commuting system in Serengeti spotted hyenas. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: dynamics, management, and conservation of an ecosystem. University of Chicago Press; Chicago, IL: 1995. pp. 332–363. [Google Scholar]
- Hofer H., East M.L. Behavioral processes and costs of co-existence in female spotted hyenas: a life history perspective. Evol. Ecol. 2003;17:315–331. doi:10.1023/A:1027352517231 [Google Scholar]
- Holekamp K.E., Smale L. Provisioning and food sharing by lactating spotted hyenas, Crocuta crocuta (Mammalia: Hyaenidae) Ethology. 1990;86:191–202. [Google Scholar]
- Holekamp K.E., Smale L., Szykman M. Rank and reproduction in the female spotted hyaena. J. Reprod. Fert. 1996;108:229–237. doi: 10.1530/jrf.0.1080229. [DOI] [PubMed] [Google Scholar]
- Holekamp K.E., Smale L., Berg R., Cooper S.M. Hunting rates and hunting success in the spotted hyena (Crocuta crocuta) J. Zool. 1997;242:1–15. [Google Scholar]
- Joeckel R.M. Unique frontal sinuses in fossil and living Hyaenidae (Mammalia, Carnivora): description and interpretation. J. Vertebr. Paleontol. 1998;18:627–639. [Google Scholar]
- Kappeler P.M. Female dominance in primates and other mammals. Perspect. Ethol. 1993;10:143–158. [Google Scholar]
- Koepfli K.-P., Jenks S.M., Eizirik E., Zahirpour T., Valkenburgh B.V., Wayne R.K. Molecular systematics of the Hyaenidae: relationships of a relictual lineage resolved by a molecular supermatrix. Mol. Phylogenet. Evol. 2006;38:603–620. doi: 10.1016/j.ympev.2005.10.017. doi:10.1016/j.ympev.2005.10.017 [DOI] [PubMed] [Google Scholar]
- Kruuk H. University of Chicago Press; Chicago, IL: 1972. The spotted hyena: a study of predation and social behavior. [Google Scholar]
- Kruuk H. Feeding and social behaviour of the striped hyaena (Hyaena vulgaris Desmarest) East Afr. Wildl. J. 1976;14:91–111. [Google Scholar]
- Matthews H.L. Reproduction in the spotted hyaena, Crocuta crocuta (Erxleben) Phil. Trans. R. Soc. B. 1939;230:1–78. doi:10.1098/rstb.1939.0004 [Google Scholar]
- McMahon C.R., Bradshaw C.J.A. Harem choice and breeding experience of female southern elephant seals influence offspring survival. Behav. Ecol. Sociobiol. 2004;55:349–362. doi:10.1007/s00265-003-0721-1 [Google Scholar]
- Mills M.G.L. Unwin Hyman; London, UK: 1990. Kalahari hyaenas: comparative behavioral ecology of two species. [Google Scholar]
- Mizuno A.W., Ohtaishi N. Cranial features of the spotted seal, Phoca largha, in the Nemuro Strait, considering age effects. J. Vet. Med. Sci. 2002;64:137–144. doi: 10.1292/jvms.64.137. doi:10.1292/jvms.64.137 [DOI] [PubMed] [Google Scholar]
- Ohdachi S., Aoi T., Mano T., Tsubota T. Growth, sexual dimorphism, and geographical variation of skull dimensions of the brown bear Ursus arctos in Hokkaido. J. Mammal. Soc. Jpn. 1992;17:27–47. [Google Scholar]
- Packer C., Herbst L., Pusey A.E., Bygott J.D., Hanby J.P., Cairns S.J., Mulder M.B. Reproductive success of lions. In: Clutton-Brock T.H., editor. Reproductive success. University of Chicago Press; Chicago, IL: 1988. pp. 363–383. [Google Scholar]
- Pistorius P.A., Bester M.N., Kirkman S.P., Taylor F.E. Pup mortality in southern elephant seals at Marion Island. Polar Biol. 2001;24:828–831. doi:10.1007/s003000100285 [Google Scholar]
- Radespiel U., Zimmermann E. Female dominance in captive gray mouse lemurs (Microcebus murinus) Am. J. Primatol. 2001;54:181–192. doi: 10.1002/ajp.1029. doi:10.1002/ajp.1029 [DOI] [PubMed] [Google Scholar]
- Robbins A.M., Robbins M.M., Gerald-Steklis N., Steklis H.D. Age-related patterns of reproductive success among female mountain gorillas. Am. J. Phys. Anthropol. 2006;131:511–521. doi: 10.1002/ajpa.20474. doi:10.1002/ajpa.20474 [DOI] [PubMed] [Google Scholar]
- Schwenk K., Wagner G.P. The relativism of constraints on phenotypic evolution. In: Pigliucci M., Preston K., editors. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Oxford University Press; Oxford, UK: 2004. pp. 390–408. [Google Scholar]
- Sherman P.W., Jarvis J.U.M., Alexander R.D. Princeton University Press; Princeton, NJ: 1991. The biology of the naked mole rat. [Google Scholar]
- Short R.V., Balaban E. Cambridge University Press; Cambridge, UK: 1994. The differences between the sexes. [Google Scholar]
- StatSoft. StatSoft; Tulsa, OK: 2002. Statistica (data analysis software system) [Google Scholar]
- Szykman M., Engh A.L., Van Horn R.C., Boydston E.E., Scribner K.T., Holekamp K.E. Rare male aggression directed toward females in a female-dominated society: baiting behavior in the spotted hyena. Aggress. Behav. 2003;29:457–474. doi:10.1002/ab.10065 [Google Scholar]
- Tanner, J. B. 2007 Behavioral and morphological development in a female-dominated species, the spotted hyena, Crocuta crocuta PhD dissertation, Michigan State University, East Lansing, MI.
- Tilson R.T., Hamilton W.J.I. Social dominance and feeding patterns of spotted hyaenas. Anim. Behav. 1984;32:715–724. doi:10.1016/S0003-3472(84)80147-5 [Google Scholar]
- Van Horn R.C., McElhinney T.L., Holekamp K.E. Age estimation and dispersal in the spotted hyena (Crocuta crocuta) J. Mammal. 2003;84:1019–1030. doi:10.1644/BBa-023 [Google Scholar]
- Wahaj S.A., Place N.J., Weldele M.L., Glickman S.E., Holekamp K.E. Siblicide in the spotted hyena: analysis with ultrasonic examination of wild and captive individuals. Behav. Ecol. 2007;18:974–984. doi:10.1093/beheco/arm076 [Google Scholar]
- Werdelin L. Constraint and adaptation in the bone-cracking canid Osteoborus (Mammalia: Canidae) Paleobiology. 1989;15:387–401. [Google Scholar]
- Werdelin L., Solounias N. The Hyaenidae: taxonomy, systematics and evolution. Fossils Strata. 1991;30:1–104. [Google Scholar]
- White P.A. Maternal rank is not correlated with cub survival in the spotted hyena, Crocuta crocuta. Behav. Ecol. 2005;16:606–613. doi:10.1093/beheco/ari033 [Google Scholar]
- Zelditch M.L., Lundrigan B.L., Sheets H.D., Garland T., Jr Do precocial mammals develop at a faster rate? A comparison of rates of skull development in Sigmodon fulviventer and Mus musculus domesticus. J. Evol. Biol. 2003;16:708–720. doi: 10.1046/j.1420-9101.2003.00568.x. doi:10.1046/j.1420-9101.2003.00568.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Indicators of intensity of feeding competition among large carnivores that feed in groups
Body mass and weaning age of carnivore species