Abstract
High triglycerides (TG) and low high density lipoprotein cholesterol (HDL-C) are important cardiovascular risk factors in women. The prognostic utility of the TG/HDL-C ratio, a marker for insulin resistance and small dense low density lipoprotein particles, is unknown among high risk women.
Methods
We studied 544 women without prior myocardial infarction or coronary revascularization, referred for clinically indicated coronary angiography and enrolled in the Women’s Ischemia Syndrome Evaluation (WISE). Fasting lipid profiles and detailed demographic and clinical data were obtained at baseline. Multi-variate Cox-proportional hazards models for all cause mortality and cardiovascular events (death, myocardial infarction, heart failure, stroke) over a median follow-up of 6 years were constructed using log TG/HDL-C ratio as a predictor variable and accounting for traditional cardiovascular risk factors.
Results
Mean age was 57±11 years, 84% were white, 55% hypertensive, 20% diabetic, 50% current or prior smokers. TG/HDL-C ranged from 0.3 to 18.4 (median 2.2, first quartile 0.35 to <1.4, fourth quartile 3.66–18.4). Deaths (n=33) and CV events (n=83) increased across TG/HDL-C quartiles (both p<0.05 for trend). TG/HDL-C was a strong independent predictor of mortality in models adjusted for age, race, smoking, hypertension, diabetes, and angiographic coronary disease severity (HR 1.95, 95% CI 1.05, 3.64, p=0.04). For cardiovascular events, the multivariate HR was 1.54 (95% CI 1.05, 2.22, p=0.03) when adjusted for demographic and clinical variables, but became non-significant when angiographic results were included.
Conclusion
Among women with suspected ischemia, the TG/HDL-C ratio is a powerful independent predictor of all cause mortality and cardiovascular events.
Coronary heart disease (CHD) is the most common cause of death among women. [1] Dyslipidemia is an important risk factor for the development of CHD and treatment approaches to decrease low density lipoprotein (LDL) cholesterol levels have reduced cardiovascular events.[2] While the role of low high density lipoprotein cholesterol (HDL-C) in CHD development has been widely accepted, the role of hypertriglyceridemia remains controversial. Recent analyses demonstrate that hypertriglyceridemia is an independent predictor of CHD and may be a stronger risk factor among women than men.[3,4] Atherogenic dyslipidemia, the joint occurrence of high triglycerides (TG) and low HDL-C in association with elevated apoprotein B and small dense LDL particles, is an important component of the metabolic syndrome and strongly predictive of CHD.[5–8] The ratio of TG/HDL-C has been proposed as an easily obtainable atherogenic marker.[9] A high TG/HDL-C ratio correlates with LDL phenotype B, small HDL particles, and insulin resistance.[10–12] Data on the prognostic utility of the TG/HDL-C ratio are limited. Gaziano et al. were the first to report in a case control study that this ratio strongly predicted risk of myocardial infarction.[13] Others have linked a high TG/HDL-C ratio to coronary atherosclerosis [14,15], impaired heart rate recovery after exercise [16], CHD incidence [17], and CHD, cardiovascular and all cause death.[15,16,18] These studies enrolled predominantly healthy, younger individuals [16,18], only males [17], or did not report gender-specific risk ratios.[15,16,18] None of the outcomes studies evaluated the prognostic utility of the TG/HDL-C ratio in the context of angiographic coronary artery disease severity.
The purpose of the current study was to determine whether the TG/HDL-C ratio predicts cardiovascular events and total mortality among women undergoing coronary angiography for suspected myocardial ischemia.
Methods
Study Population
The WISE study is an NHLBI-sponsored four-center prospective cohort study designed to improve the diagnostic reliability of cardiovascular testing in the evaluation of ischemic heart disease in women. Between 1996 and 2000, 936 women (out of 7,603 screened and 1,903 found eligible) 18 years or older presenting to study sites for clinically indicated coronary angiography to evaluate suspected myocardial ischemia were enrolled. Major exclusion criteria were comorbidity that would compromise follow-up, pregnancy, contraindications to provocative diagnostic testing, cardiomyopathy, New York Heart Association Class IV heart failure, recent myocardial infarction or coronary revascularization, and significant valvular or congenital heart disease. Each site’s institutional review board approved the study, all participants provided written informed consent, and all data were monitored by an independent data and safety monitoring committee. Full details of the protocol and design of the WISE study have been previously published.[19]
Of the 936 WISE women, 655 had no prior history of myocardial infarction or revascularization. Blood sample collection was added to the protocol several months into the WISE study. TG and HDL-C were available in 585 women. Of these, 567 (97%) had follow-up information. We excluded 13 women who were missing information on race, smoking, blood pressure, or diabetes. The present analysis thus includes 554 women without prior myocardial infarction or coronary revascularization at baseline who had baseline lipid data, information on all the covariates in the model, and for whom we have follow-up information.
Baseline evaluation
Detailed data on demographics, cardiovascular risk factors, symptoms, medical and reproductive history, and medication use were obtained at baseline. Height, weight, waist circumference, and blood pressure were measured and body mass index (BMI) was calculated. Blood samples were obtained after an overnight fast. Coronary angiograms were analyzed at the WISE Angiographic Core Laboratory at Brown University by investigators blinded to all clinical and outcome data, using previously published quantitative analysis methods.[20] We used the coronary artery disease severity score as the measure for coronary artery disease.[20] Using presence or absence of coronary artery disease (i.e., lesion >50%) in the modeling yielded similar results (data not shown).
Laboratory Methodology
Fasting blood samples for determination of lipoproteins were analyzed at the Lipid Core Laboratory at the Cedars Sinai Medical Center which is enrolled in the Centers for Disease Control and Prevention lipid standardization program. Fasting total plasma cholesterol, TG, and HDL-C were determined by enzymatic assays as previously published.[21] LDL cholesterol was calculated using the Friedewald formula and is thus only available in women without hypertriglyceridemia.[22] The coefficients of variation for total cholesterol, HDL-C, and TG were 1.80%, 1.23%, and 3.93%, respectively. Lipid Core Laboratory results were not made available to the treating physicians or the WISE investigators. Treating physicians were free to determine cholesterol levels in their patients and treat as medically indicated.
Follow-up procedures
After enrollment, care was provided by each patient’s referring physician in accordance with local standards of care. Follow-up telephone interviews were conducted at 6 weeks, 1 year, and annually thereafter by an experienced nurse or physician who completed a scripted interview which assessed major adverse cardiovascular events or hospitalizations. In the event of death, a death certificate was obtained. For the current study, we evaluated all cause mortality and a combined endpoint of “any cardiovascular event” which included death or hospitalization for congestive heart failure, stroke, or myocardial infarction.
Statistical analysis
All statistical analyses were performed at the Data Coordinating Center at the University of Pittsburgh. For descriptive purposes, we compared means (standard deviations) or percentages, as appropriate, of demographic characteristics, cardiovascular risk factors, lipids and lipoproteins, medication use, angiographic coronary artery disease severity measures and clinical outcomes across TG/HDL-C quartiles. To calculate trend statistics, we used the Mantel-Haenszel test for categorical data, and the Jonckheere-Terpstra method for continuous data.[23]
Since the distributions of TG/HDL-C and angiographic coronary artery disease severity score were skewed, these variables were log-transformed prior to modeling. To determine whether the TG/HDL-C ratio related to severity of coronary artery disease, we performed multivariable linear regression with the log of the angiographic coronary artery disease severity score as the outcomes variable and the log of the TG/HDL-C ratio as the predictor variable, adjusting for demographic and clinical participant characteristics. Covariates considered for the multivariable model included age, race, history of hypertension, systolic blood pressure (per 10 mmHg increment), history of smoking, body mass index, waist circumference, menopausal status, and history of diabetes.
The associations between the log of the TG/HDL-C ratio and cardivascular events and death, respectively, were modeled using Cox proportional hazards models. The basic models adjusted for age and race. We then sequentially added history of smoking (a stronger predictor in this cohort than current smoking), systolic blood pressure (per 10 mmHg increment; a stronger predictor in this cohort than a history of hypertension), and history of diabetes to determine whether the relationship between TG/HDL-C and clinical outcomes was independent of these covariates. Waist circumference was only available in 486 women (88%), since some women did not allow this measurement to be taken. Waist circumference was not predictive of outcome in these models and did not affect the relationship between log TG/HDL-C and outcomes. We also considered use of lipid-lowering medications, but use was not predictive of death or cardiovascular events and did not affect the relationship between log TG/HDL-C and outcomes. Waist circumference and use of lipid-lowering medications were thus not included in the models. The coronary artery severity score (the strongest predictor among the angiographic coronary artery disease severity measures) was then added to the models. The proportional hazards assumption of invariant hazard ratios during follow-up was tested and found to be met. All analyses were conducted using SAS software, version 9 (Cary, NC), and all tests for statistical significance were 2-tailed. The authors are solely responsible for study design and conduct, all study analyses, and the drafting and editing of the paper and its final contents.
Results
Baseline characteristics
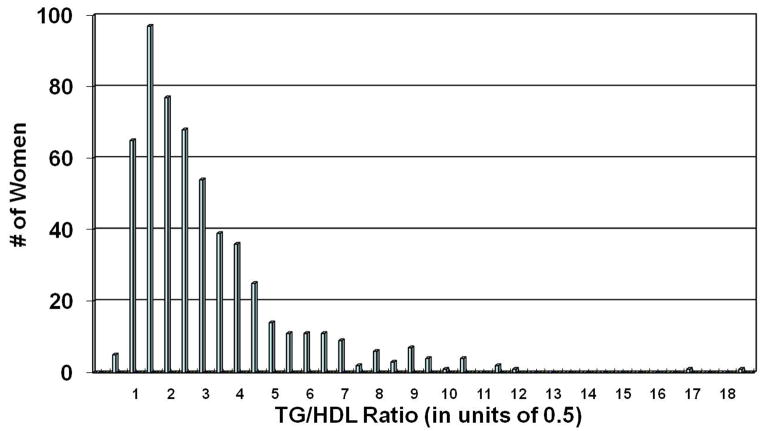
The average age was 57±11 years, 16% of women were African American, 55% had a history of hypertension, 20% were diabetic, and 50% had a history of smoking. The mean TG/HDL-C ratio with TG and HDL-C expressed in mg/dL (corresponding values expressed in mmol/L in parentheses) was 2.9±2.2 (1.3±0.96), median 2.2 (0.96), range 0.3–18.4 (0.13–8.0) (Figure 1).
Figure 1.
Distribution of the TG/HDL-C ratio in the study population
The distribution is highly skewed. All modeling was thus performed utilizing the log TG/HDL-C ratio.
Table I summarizes baseline characteristics by TG/HDL-C quartile. Age was similar across quartiles. Women with higher TG/HDL-C ratios were less likely to be African American. Smoking rates varied by group, but there was no consistent trend with increasing TG/HDL-C ratio. As expected, women with higher TG/HDL-C ratios were more likely to have other components of the metabolic syndrome, fulfill criteria for the metabolic syndrome [24], and have diabetes. Use of medications known to affect cardiovascular outcomes (aspirin, lipid-lowering drugs, beta blockers, angiotensin converting enzyme-inhibitors or angiotensin receptor blockers, and hormone replacement therapy), did not differ across TG/HDL-C strata. Women with higher TG/HDL-C ratios were more likely to have obstructive angiographic coronary artery disease and to have more severe coronary artery disease.
Table I.
Baseline Characteristics by TG/HDL-C* Quartile
| Characteristic | All n= 554 | Quartile 1 0.35–<1.4 n=138 | Quartile 2 1.4–<2.2 n=138 | Quartile 3 2.2–<3.66 n=138 | Quartile 4 3.66–18.4 n=140 | p (Trend) |
|---|---|---|---|---|---|---|
| Age (years) | 554 | 56.4±10.9 | 56.6±11.6 | 57.8±10.9 | 56.8±11.2 | 0.56 |
| African American (%) | 554 | 24 | 20 | 13 | 9 | 0.0001 |
| History of smoking (%) | 554 | 54 | 43 | 46 | 59 | 0.34 |
| History of hypertension (%) | 552 | 49 | 50 | 61 | 59 | 0.02 |
| History of dyslipidemia (%) | 514 | 43 | 35 | 47 | 60 | 0.0009 |
| Body mass index (mg/kg/m2) | 548 | 28.6±6.6 | 29.2±6.8 | 30.9±7.1 | 30.1±5.8 | 0.001 |
| Waist circumference (cm) | 486 | 87.6±18.3 | 90.2±16.8 | 96.5±17.8 | 97.0±15.7 | <.0001 |
| (inches) | 34.5±7.2 | 35.5±6.6 | 38.0±7.0 | 38.2±6.2 | ||
| Metabolic syndrome (%) | 549 | 12 | 20 | 50 | 85 | <.0001 |
| Diabetes (%) | 554 | 20 | 12 | 18 | 30 | 0.01 |
| Postmenopausal (%) | 550 | 73 | 67 | 73 | 71 | 0.99 |
| Lipid Measures | ||||||
| Total cholesterol (mmol/L) | 554 | 4.78±0.96 | 4.86±1.03 | 5.20±1.22 | 5.38±1.27 | <.0001 |
| (mg/dL) | 185±37 | 188±40 | 201±47 | 208±49 | ||
| HDL-C (mmol/L) | 554 | 1.66±0.34 | 1.42±0.26 | 1.34±0.26 | 1.16±0.23 | <.0001 |
| (mg/dL) | 64±13 | 55±10 | 52±10 | 45±9 | ||
| LDL-C (mmol/L) | 491 | 2.79±0.83 | 2.95±0.96 | 3.05±1.11 | 3.00±1.14 | 0.12 |
| (mg/dL) | 108±32 | 114±37 | 118±43 | 116±44 | ||
| No LDL-C (%)* | 554 | 0 | 0 | 0 | 45 | <.0001 |
| Triglycerides (mmol/L) | 554 | 0.70±0.20 | 1.08±0.20 | 1.67±0.33 | 2.97±1.16 | <.0001 |
| (mg/dL) | 62±18 | 96±18 | 148±29 | 263±103 | ||
| Non-HDL-C (mmol/L) | 554 | 3.10±0.83 | 3.44±0.96 | 3.83±1.14 | 4.22±1.24 | <.0001 |
| (mg/dL) | 120±32 | 133±37 | 148±44 | 163±48 | ||
| TG/HDL-C | 554 | 1.0±0.2 | 1.8±0.2 | 2.8±0.4 | 5.9±2.4 | - |
| Coronary artery disease Measures | ||||||
| Coronary artery disease Severity Score | 554 | 9.9±10.6 | 11.0±11.6 | 11.8±12.5 | 12.9±12.1 | 0.004 |
| Coronary artery disease (50% or greater stenosis) (%) | 554 | 20 | 24 | 22 | 35 | 0.006 |
| Coronary artery disease (70% stenosis) (%) | 554 | 9 | 12 | 12 | 17 | 0.04 |
| Medications | ||||||
| Aspirin (%) | 552 | 50 | 52 | 58 | 48 | 0.91 |
| Statins (%) | 554 | 17 | 14 | 17 | 24 | 0.14 |
| Other lipid lowering drugs (%) | 554 | 3 | 2 | 2 | 6 | 0.12 |
| Any lipid lowering drug (%) | 554 | 20 | 17 | 20 | 28 | 0.053 |
| ACE-I or ARB (%) | 553 | 26 | 20 | 22 | 26 | 0.95 |
| Beta Blockers (%) | 553 | 29 | 32 | 28 | 36 | 0.31 |
| Calcium antagonists (%) | 554 | 21 | 19 | 23 | 21 | 0.82 |
| Diuretics (%) | 554 | 20 | 25 | 29 | 28 | 0.06 |
| Vasodilators (%) | 553 | 6 | 6 | 12 | 6 | 0.50 |
| Any antihypertensive drug (%) | 554 | 38 | 39 | 48 | 45 | 0.10 |
| Current postmenopausal hormone therapy (%) | 547 | 40 | 41 | 45 | 38 | 0.84 |
| Outcomes | ||||||
| Cardiovascular events (%) | 554 | 11.6 | 11.6 | 15.2 | 21.4 | 0.01 |
| All cause mortality (%) | 554 | 3.6 | 3.6 | 7.2 | 9.3 | 0.02 |
The ratio of TG/HDL-C is expressed with TG and HDL-C in mg/dL. The conversion for cholesterol is 1 mg/dL = 0.02586 mmol/L. The conversion for TG is 1 mg/dL = 0.01129 mmol/L. To convert to a TG/HDL-C ratio that reflects TG and HDL-C measurements in mmol/L, please multiply the above ratios by 0.4366.
LDL cholesterol was calculated by the Friedewald formula and is thus not available in women with hypertriglyceridemia; CRP was only measured in a subset of women.
Abbreviations: ACE = angiotensin converting enzymes; ARB = angiotensin receptor blocker; coronary artery disease = coronary artery disease; HDL-C = high density lipoprotein cholesterol; HRT= postmenopausal hormone replacement therapy; LDL-C = low density lipoprotein cholesterol; TG = triglycerides
In univariate linear regression modeling, log TG/HDL-C was significantly associated with the log angiographic severity score (p = 0.004). This relationship remained significant after adjustment for age, body mass index, and history of diabetes (beta for log TG/HDL-C in the final model: 0.11, p = 0.02); race, history of smoking, and waist circumference were not associated with the angiographic severity score.
Clinical Outcomes
Mean follow-up time for surviving women was 5.3+/−2.5 years (median 6.0 years, interquartile range 3.7–7.0 years). There were 33 deaths and 83 cardiovascular events. Both outcomes were significantly more common in women with higher TG/HDL-C ratios (p for trend 0.01 for cardiovascular events, 0.02 for death) (Table I).
Cardiovascular events
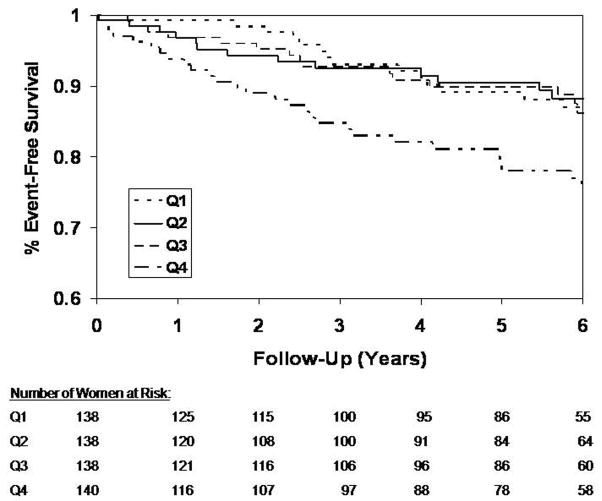
Kaplan Meier curves for freedom from cardiovascular events are shown in Figure 2 for quartiles of the TG/HDL-C ratio. At 6 years, almost 1 in 4 women in the highest quartile suffered an event during follow-up, approximately twice the rate of cardiovascular events of women with lower TG/HDL-C ratios. Results of the sequential modeling of cardiovascular events are shown in Table II. The log TG/HDL-C was a powerful predictor of cardiovascular events independent of age, race, smoking, and systolic blood pressure. Addition of diabetes attenuated the association between log TG/HDL-C and cardiovascular events, but did not abolish it. Log TG/HDL-C was no longer predictive of cardiovascular events, when angiographic coronary artery disease severity was added to the model.
Figure 2.
Kaplan-Meier curves for freedom from cardiovascular events by TG/HDL-C quartile Quartile 1 (Q1) through Q4 correspond to the quartiles of TG/HDL-C as shown in Table I (Q1: 0.35-<1.4, Q2: 1.4-<2.2, Q3: 2.2-<3.66, Q4: 3.66–18.4). Excess risk of cardiovascular events is limited to individuals in Q4 of the TG/HDL-C distribution.
Table II.
Modeling of Cardiovascular Events
| Predictor | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Log TG/HDL | 1.88 (1.29–2.74) | 0.001 | 1.82 (1.25–2.63) | 0.002 | 1.53 (1.05–2.22) | 0.03 | 1.41 (0.96–2.07) | 0.08 |
| Age | 1.03 (1.01–1.05) | 0.009 | 1.02 (1.002–1.04) | 0.03 | 1.02 (1.00–1.04) | 0.051 | 1.01 (0.99–1.04) | 0.26 |
| White Race | 0.39 (0.23–0.65) | 0.0003 | 0.48 (0.28–0.81) | 0.006 | 0.59 (0.34–1.001) | 0.050 | 0.60 (0.35–1.02) | 0.06 |
| History of Smoking | - | - | 2.37 (1.49–3.76) | 0.0002 | 2.87 (1.79–4.61) | <.0001 | 2.84 (1.77–4.55) | <.0001 |
| Systolic Blood Pressure (per 10 mmHg) | - | - | 1.17 (1.06–1.29) | 0.001 | 1.13 (1.02–1.26) | 0.02 | 1.13 (1.02–1.25) | 0.02 |
| Diabetes | - | - | - | - | 2.81 (1.74–4.53) | <.0001 | 2.47 (1.51–4.03) | 0.0003 |
| Log CAD Severity Score | - | - | - | - | - | - | 1.40 (1.03–1.90) | 0.03 |
| N | 554 | 554 | 554 | 554 | ||||
| # of Events | 83 | 83 | 83 | 83 | ||||
CAD = coronary artery disease; CI = confidence interval; HR = hazard ratio; N = sample size; TG/HDL = triglyceride to HDL ratio
Mortality
Results of the sequential modeling of all cause mortality are shown in Table III. Log TG/HDL-C was a strong and independent predictor of all cause mortality after adjustment for age, race, smoking, systolic blood pressure and diabetes and remained predictive even when the coronary artery disease severity score was added to the model.
Table III.
Modeling of All-Cause Mortality
| Predictor | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Log TG/HDL | 2.90 (1.59–5.28) | 0.0005 | 2.79 (1.54–5.07) | 0.0007 | 2.21 (1.21–4.06) | 0.01 | 1.95 (1.05–3.64) | 0.04 |
| Age | 1.04 (1.01–1.07) | 0.02 | 1.04 (1.004–1.07) | 0.03 | 1.04 (1.003–1.08) | 0.03 | 1.02 (0.99–1.06) | 0.21 |
| White Race | 0.23 (0.11–0.50) | 0.0002 | 0.28 (0.13–0.61) | 0.001 | 0.35 (0.16–0.77) | 0.009 | 0.37 (0.17–0.80) | 0.01 |
| History of Smoking | - | - | 2.48 (1.17–5.25) | 0.02 | 2.96 (1.38–6.35) | 0.005 | 2.95 (1.37–6.34) | 0.006 |
| Systolic Blood Pressure (per 10 mmHg) | - | - | 1.17 (1.003–1.37 | 0.04 | 1.12 (0.94–1.32) | 0.19 | 1.11 (0.95–1.30) | 0.19 |
| Diabetes | - | - | - | - | 2.94 (1.38–6.24) | 0.005 | 2.54 (1.19–5.43) | 0.02 |
| Log CAD Severity Score | - | - | - | - | - | - | 1.64 (1.02–2.62) | 0.04 |
| N | 554 | 554 | 554 | 554 | ||||
| # of Events | 33 | 33 | 33 | 33 | ||||
CAD = coronary artery disease; CI = confidence interval; HR = hazard ratio; N = sample size; TG/HDL = triglyceride to HDL ratio
Other Lipid Predictors
In exploratory analyses, non-HDL-C, the total cholesterol/HDL-C ratio, and total cholesterol did not predict cardiovascular events or mortality in this cohort. When added to our final models together with TG/HDL-C, these lipids did not affect the relationship between TG/HDL-C and cardiovascular events or mortality. HDL-C correlated significantly with angiographic coronary artery disease severity, but did not predict cardiovascular events or mortality when modeled as a continuous variable. Low HDL-C, defined as HDL-C below 50 mg/dL (1.3 mmol/L), was predictive of cardiovascular events, but not mortality. Log TG did not relate to angiographic disease severity, but predicted both cardiovascular events and death during follow-up.
Discussion
To our knowledge, this is the first study among high risk women to show that the TG/HDL-C ratio is a powerful predictor of total mortality independent of important prognostic variables including age, race, smoking, hypertension, diabetes, and severity of coronary artery disease. We also found a strong relationship between the TG/HDL-C ratio and severity of coronary artery disease as well as subsequent cardiovascular events among these women with suspected myocardial ischemia.
Correlates of high TG and low HDL-C were similar in WISE as reported by others.[7–9,11–13,16] High TG and low HDL-C characterize the dyslipidemia of metabolic syndrome.[5]. In WISE, 85% of women in the highest quartile of the TG/HDL-C ratio met Adult Treatment Panel III criteria for metabolic syndrome [24], the majority of women were obese and hypertensive, and 30% carried a diagnosis of diabetes. As expected, women with high TG and low HDL-C were less likely to be African American, an ethnic group in whom TG/HDL-C is a less reliable indicator of insulin resistance.[25] In the general population, women tend to have lower TG and higher HDL-C levels than their male counterparts and, since 1976, both TG levels and HDL-C levels have increased modestly among women.[26] TG/HDL-C quartiles in WISE women, in contrast, were similar to those reported among the predominantly male (78%) participants from the Lipid Research Clinics Prevalence study, indicative of a highly abnormal lipoprotein pattern despite pharmacologic lipid-lowering therapy in 21% of WISE women.[16]
Previous reports have shown that high TG/HDL-C ratios correlate independently with presence of angiographic coronary artery disease (defined as stenosis >50%) among men and women even after adjustment for traditional risk factors, including diabetes.[14,15] In the current analysis, we were able to reproduce this finding in an all-female cohort and extend the observation to demonstrate that the TG/HDL-C ratio was also associated with coronary artery disease severity as expressed by a modified Gensini score.[17]
The relationship between TG/HDL-C and clinical outcomes has been assessed in a large meta-analysis of Asian-Pacific cohorts [18] and in several smaller population studies from Europe and the US.[13,15,16,17] The strongest association was reported in the case control study by Gaziano and colleagues with a 16-fold increase in risk of myocardial infarction in the highest compared to the lowest quartile of the TG/HDL-C distribution.[13] Subsequent cohort studies showed more modest effect sizes (adjusted hazard ratios between 1.25 and 4), but demonstrated that the TG/HDL-C ratio was independently predictive of incident CHD, CHD and cardiovascular death, and total mortality.[15–18] However none of these studies took into account severity of angiographic coronary artery disease, most of the cohorts were established many years ago preceding contemporary pharmacologic and revascularization measures, and gender-specific hazard ratios were not reported.
WISE represents a contemporary cohort of women under evaluation for suspected myocardial ischemia and whose coronary anatomy is known. As in prior studies, we found that the TG/HDL-C ratio was independently predictive of cardiovascular events and all cause mortality, with an approximately 2-fold increase in 6 year event rates in the highest compared to the lowest quartile of the TG/HDL-C distribution. TG/HDL-C remained predictive of cardiovascular events even after adjustment for demographic variables and traditional coronary risk factors including diabetes. As the Kaplan Meier curves show (Figure 2), this increased risk seems to be confined to the highest quartile of the TG/HDL-C distribution, women with a TG/HDL-C ratio of 3.66 or higher (1.67 or higher, when TG and HDL-C are expressed in mmol/L). Further adjustment for coronary artery disease severity attenuated the hazard ratio for TG/HDL-C, suggesting that some of the risk associated with high TG/HDL-C might be explained by its covariation with disease severity. This attenuation may also indicate coronary artery disease to be the more proximal of the two variables to adverse events suggesting a possible causal pathway. Such a pathway would assume the TG/HDL-C ratio to be relatively consistent over time. While lipid levels do track over time, we cannot automatically assume that the women had similar levels throughout their lifetime.
In contrast, the TG/HDL-C ratio remained independently predictive of all cause mortality in this cohort, even after adjustment for traditional risk factors and the coronary artery disease severity score. In the absence of cause of death information and given our study design, we cannot asses the pathophysiologic mechanism(s) that underlie this strong relationship between the TG/HDL-C ratio and subsequent all cause mortality, but our data suggest that women with high TG/HDL-C ratios should be considered at high risk of death and should be closely followed clinically, even in the absence of obstructive coronary artery disease.
Limitations
Our study has several limitations. LDL-C was calculated by the Friedewald formula and was thus not available in hypertriglyceridemic women. Comparative analyses of the prognostic value of LDL-C and the TG/HDL-C ratio were therefore not feasible, nor could we simultaneously model the impact of LDL-C and TG/HDL-C on severity of coronary artery disease, cardiovascular events, or total mortality. Apolipoproteins were not measured in the WISE study. We were thus unable to compare the prognostic utility of the TG/HDL-C ratio with that of the apoprotein B/A ratio. Our ability to model mortality was limited by the small number of deaths –lack of statistical significance for some covariates may thus reflect low power rather than lack of prognostic value. Women enrolled in the WISE study represent a highly selected population of women who presented for clinically indicated angiography. It is unknown whether our findings extend to women without a history of cardiovascular events in the general population.
Conclusion
Among high risk women under evaluation for myocardial ischemia, the TG/HDL-C ratio is a powerful independent predictor of cardiovascular events and all cause mortality. Clinical trials targeting the abnormal TG/HDL-C ratio in such women appear to be warranted.
Acknowledgments
Funding Sources:
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania, and The Edythe Broad Endowment for Women’s Heart Research, Los Angeles, California.
Footnotes
Disclosures:
Bittner: Research Support: NIH/Abbot (AIM HIGH Trial); Pfizer (TNT, ILLUMINATE Trials); Atherogenics (ARISE Trial); Merck (Merck Protocol 112); CV Therapeutics: (Angina in Women Study); Roche Laboratories (dal-Outcomes Study); Consultant: Pfizer, CV Therapeutics
Bairey-Merz: Research Support: None. Consultant: Novartis, CMP Media, Rodale Press, Adventist Health; Bayer; Remillard and Associates; Navigant; Hunt and Associates; Pfizer. Lectures: Bayer; CV Therapeutics. Stock Ownership: Boston Scientific; Eli Lilly; Medtronic; Johnson&Johnson; Teva Pharmaceuticals; ATS Medical
Johnson, Zineh, Rogers, Vido, Marroquin, Sopko: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart Disease and Stroke Statistics - 2008 Update. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Bittner V. Perspectives on dyslipidemia and coronary heart disease in women. J Am Coll Cardiol. 2005;46:1628–35. doi: 10.1016/j.jacc.2005.05.089. [DOI] [PubMed] [Google Scholar]
- 3.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–9. [PubMed] [Google Scholar]
- 4.Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the Risk of Coronary Heart Disease. 10 158 Incident Cases Among 262 525 Participants in 29 Western Prospective Studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and Management of the Metabolic Syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 6.Assmann G, Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience). Prospective Cardiovascular Munster Study. Am J Cardiol. 1992;70:733–7. doi: 10.1016/0002-9149(92)90550-i. [DOI] [PubMed] [Google Scholar]
- 7.Manninen V, Tenkanen L, Koskinen P, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study: implications for treatment. Circulation. 1992;85:37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 8.Ballantyne CM, Olsson AG, Cook TJ, et al. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046–3051. doi: 10.1161/hc5001.100624. [DOI] [PubMed] [Google Scholar]
- 9.Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FERHDL) Clinical Biochemistry. 2001;34:583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 10.Hanak V, Munoz J, Teague J, et al. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94:219–222. doi: 10.1016/j.amjcard.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin T, Reaven T, Abbasi F, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 12.Jiaa L, Longb S, Fua M, et al. Relationship between total cholesterol/high-density lipoprotein cholesterol ratio, triglyceride/high-density lipoprotein cholesterol ratio, and high-density lipoprotein subclasses. Metabolism. 2006;55:1141–8. doi: 10.1016/j.metabol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Gaziano JM, Hennekens CH, O’Donnell CJ, et al. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–2525. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 14.Frohlich J, Dobiasova M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49:1873–80. doi: 10.1373/clinchem.2003.022558. [DOI] [PubMed] [Google Scholar]
- 15.Drexel H, Aczel S, Marte T, et al. Is atherosclerosis in diabetes and impaired fasting glucose driven by elevated LDL cholesterol or by decreased HDL cholesterol? Diabetes Care. 2005;28:108–114. doi: 10.2337/diacare.28.1.101. [DOI] [PubMed] [Google Scholar]
- 16.Shishehbor MH, Hoogwerf BJ, Lauer MS. Association of triglyceride–to–HDL cholesterol ratio with heart rate recovery. Diabetes Care. 2004;27:936–941. doi: 10.2337/diacare.27.4.936. [DOI] [PubMed] [Google Scholar]
- 17.Jeppesen J, Hein HO, Suadicani P, et al. Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Intern Med. 2001;161:361–6. doi: 10.1001/archinte.161.3.361. [DOI] [PubMed] [Google Scholar]
- 18.Asia Pacific Cohort Studies Collaboration. A Comparison of Lipid Variables as Predictors of Cardiovascular Disease in the Asia Pacific Region. Ann Epidemiol. 2005;15:405–413. doi: 10.1016/j.annepidem.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol, design, methodology, and pilot phase report. J Am Coll Cardiol. 1999;33:1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 20.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia syndrome Evaluation (WISE) study angiographic core laboratory. Am J Cardiol. 2001;87:937–941. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 21.Lipid Research Clinics Program. The Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. Bethesda, MD: National Institutes of Health; 1974. DHEW Publication No. 75–628. [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York, NY: John Wiley & Sons; 1973. [Google Scholar]
- 24.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 25.Sumner AE, Finley KB, Genovese DJ, et al. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med. 2005;165:1395–1400. doi: 10.1001/archinte.165.12.1395. [DOI] [PubMed] [Google Scholar]
- 26.Caroll MD, Lacher DA, Sorlie PD, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294:1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]