Abstract
Background
Sleep disturbance in bipolar disorder can be both a risk factor and symptom of mood episodes. However, the associations among sleep and clinical characteristics, function, and quality of life in bipolar disorder have not been fully investigated.
Methods
The prevalence of sleep disturbance, duration, and variability, as well as their associations with mood, function, and quality of life, was determined from 2,024 bipolar patients enrolled in the National Institute of Mental Health Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD).
Results
Analyses indicated that 32% of patients were classified as short sleepers, 38% normal sleepers, and 23% long sleepers. Overall, short sleepers demonstrated greater mood elevation, earlier age at onset, and longer illness duration compared to both normal and long sleepers. Both short and long sleepers had greater depressive symptoms, poorer life functioning, and quality of life compared to normal sleepers.
Discussion
Short sleep duration in bipolar disorder was associated with a more severe symptom presentation, whereas both short and long sleep duration are associated with poorer function and quality of life compared to normal sleep duration. Sleep disturbance could be a trait marker of bipolar disorder, though longitudinal assessments are warranted to assess potential causal relations and the longer-term implications of sleep disturbance in bipolar disorder
Keywords: Bipolar disorder, sleep, STEP-BD
1. Introduction
Bipolar disorder is a severe, recurrent, and often chronic psychiatric illness associated with significant functional impairment, morbidity, and mortality (Coryell et al., 2003) and up to 30% of bipolar patients attempt suicide (Yuan-Who and Dilsaver, 1996). Despite advances in pharmacological and psychological treatments (Angst et al., 2002; Craighead et al., 2002; Ketter, 2005), the risks of relapse and inter-episode dysfunction remain high. Thus, it is important to enhance our understanding of precipitates of mood episodes and mechanisms that may contribute to inter-episode dysfunction (Johnson, 2005).
Several lines of evidence highlight the importance of sleep disturbance in bipolar disorder. First, sleep disturbance is a core symptom of bipolar disorder and is exhibited across mood phases. During manic episodes, for example, there is a reduced need for sleep, whereas episodes of depression can involve either insomnia or hypersomnia. Second, sleep disturbances often persist despite treatment, and up to 70% of euthymic bipolar patients exhibit clinically significant sleep disturbance (Harvey et al., 2005). Furthermore, compared to healthy individuals, euthymic bipolar patients exhibit longer sleep duration and greater sleep variability (Millar et al., 2004). Third, sleep disturbance is the most common prodrome of manic episodes and not uncommonly occurs prior to the onset of depressive episodes (Jackson et al., 2003). Fourth, alterations in sleep patterns may precipitate mood episodes (Leibenluft and Suppes, 1999; Bauer et al., 2006). For example, experimentally induced sleep deprivation is associated with reductions in depressive symptoms and the onset of hypomania or mania (Benedetti et al., 2007; Colombo et al., 1999; Feldman-Naim et al., 1997; Francesco et al., 2007; Kasper and Wehr, 1992; Wehr et al., 1987; Wirz-Justice et al., 2005; Wirz-Justice & Van den Hoofdakker, 1999; Wu and Bunney, 1990).
Our goal in this paper is to use data gathered as part of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) (Sachs et al., 2003) to determine the prevalence of disordered sleep in bipolar disorder. Our primary aim was to conduct a detailed descriptive study of two important sleep functioning parameters (duration, variability) and their relations with clinical status, function, and quality of life in patients with bipolar disorder. Given evidence that sleep deprivation has adverse consequences on performance (Pilcher and Huffcut, 1996) and mood (e.g., Wehr et al., 1989; although see Benedetti et al., 2007), we hypothesized that patients who were short sleepers (SS) would exhibit greater overall symptom severity, poorer function, and poorer quality of life relative to patients classified as normal sleepers (NS). We also hypothesized that, given literature documenting the adverse consequences of hypersomnia on functioning (Dauvilliers and Buguet, 2005), patients classified as long sleepers (LS) would exhibit poorer function and poorer quality of life relative to NS. Third, given that sleep disturbance is evident during euthymia (Harvey et al., 2005; Millar et al., 2004), we examined differences in sleep duration and variability within euthymic bipolar patients. We also explored differences in sleep duration and variability according to bipolar subtype.
2. Method
Procedures
All participants were patients with bipolar disorder recruited as part of the STEP-BD protocol. Upon study entry, participants were provided with a complete description of the study and informed consent was obtained. Next, clinician-and patient-rated measures of sleep functioning, clinical diagnosis and course, symptom severity, function, and life satisfaction were assessed. If a patient was unable to complete a self-report measure at study entry, he or she completed it at home and returned it within six weeks and the return date was documented. The institutional review boards of all 16 sites approved the STEP-BD protocol.
Participants
Participants were 2,024 individuals with bipolar disorder drawn from the STEP-BD study for which data on sleep functioning parameters (described below) was available. Eligibility in STEP-BD required that participants be at least 15 years of age and meet criteria for bipolar I disorder, bipolar II disorder, bipolar disorder not otherwise specified (NOS), schizoaffective disorder, or cyclothymia. STEP-BD exclusion criteria included an inability to obtain informed consent or comply with study requirements. In the present study, only patients who met lifetime criteria for bipolar disorder types I, II, or NOS were included.
Diagnostic Evaluation
All participants met DSM-IV-TR criteria for diagnoses of BP I, BP II, or BP NOS according to the Affective Disorder Evaluation (ADE; Sachs et al., 2003). The ADE is a semi-structured interview adapted from the mood and psychosis modules of the Structured Clinical Interview for DSM-IV, Patient Version (First et al., 1996) and also includes age at onset, number of prior mood episodes, treatment response, family history, and mental status. The ADE is regarded as a valid and reliable diagnostic instrument (Simon et al., 2004).
Clinical Status
Clinical status was measured using the Clinical Monitoring Form (CMF; Sachs et al., 2002). We used the CMF to divide patients into two symptomatic (syndromal elevation, syndromal depression), two subthreshold (subsyndromal elevation, subsyndromal depression) and one asymptomatic (euthymic) clinical status category based on the number of threshold depressive and mood elevation symptoms. This also allowed us to obtain information regarding subthreshold symptoms relevant to sleep functioning in bipolar disorder (Akiskal et al., 1997).
Current Symptom Severity
The Young Mania Rating Scale (YMRS; Young et al., 1978) and Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979) are clinician-rated scales to assess mania and depression, respectively. The YMRS is an 11-item instrument with scores ranging from 0 to 60, with higher scores corresponding to increased mania. The MADRS is a 10-item measure, with scores ranging from 0 to 60 with higher numbers corresponding to increased depression. Severity of irritability and anxiety during the previous 10 days were derived from the CMF using a single item ranging from 0 to 4, with higher scores indicating greater severity.
Sleep Functioning Parameters
The sleep parameters were derived from the CMF administered at study entry and included the maximum and minimum hours of sleep obtained per night in past week. We used these two variables as a basis to calculate (1) average total sleep time (TST) in the past week by averaging across the maximum and minimum sleep variables and (2) sleep variability calculated as the maximum minus the minimum sleep variable. If a participant was missing a score for either maximum or minimum hours slept in the past week they were excluded from analyses.
We further used TST to divide the sample into three sleep duration groups -- short sleepers (SS), normal sleepers (NS), and long sleepers (LS). Inclusion criteria for the SS was an average < 6 h sleep per night in the past week, NS an average of 6.5 – 8.5 h sleep per night in the past week, and LS an average of ≥ 9 h of sleep per night in the past week. Several studies have used the cutoff of < 6 h for SS (Aeschbach et al., 1996; Edinger et al., 2000; Hartman et al., 1971; Kaneita et al., 2007; Monk et al., 2001). Our cutoff of ≥ 9 h for the LS was based on prior work using this cutoff (Aeschbach et al., 1996; Hartman et al., 1971; Kaneita et al., 2007). We also adopted the 6.5 – 8.5 h range for NS based on prior cutoffs (Edinger et al., 2000; Monk et al., 2001).
Function and Quality of Life
The Global Assessment of Functioning Scale (GAF; Axis V; DSM-IV) was used to assess global functioning in the past month. The GAF assesses overall psychological, social, and occupational functioning on a scale ranging from 1 (lowest level of functioning) to 100 (highest level of functioning).
Life functioning was assessed with the Range of Impaired Functioning Tool (LIFE-RIFT; Leon et al., 2000). The LIFE-RIFT is a 9-item semi-structured, clinician-rated measure of impairment in life functioning across four life domains, including work, interpersonal relationships, recreation, and global satisfaction. Individual items are scored from 1 (no impairment) to 5 (severe impairment). Scores on each of the four domains are summed to yield an overall score ranging from 4 to 20.
Perceived life satisfaction was measured with the Quality of Life Enjoyment and Satisfaction-Short Form (Q-LES-Q; Endicott et al., 1993). The Q-LES-Q is a 14-item self-report measure that assesses satisfaction across several distinct life domains, including health, work, finances, and interpersonal relationships. The total score ranges from 14 to 70, with higher scores indicating greater life satisfaction. The Q-LES-Q also includes an overall life satisfaction item with a score ranging from 1 (very poor) to 5 (very good).
3. Results
Demographic and Clinical Characteristics Across Entire Sample
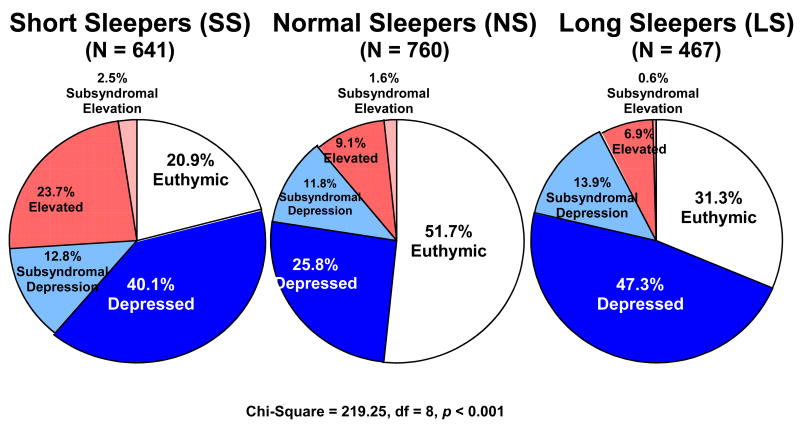
As evident in Table 1, approximately half (55.9%) of the sample was female, the majority was Caucasian, and the mean age was 38.0 (SD = 13.1) years. Mean age at onset for bipolar illness was 16.8 years (SD = 8.8). In the past year, 26.9% of participants exhibited a rapid cycling course. With respect to clinical status, the majority of participants were syndromally depressed (36.1%) or euthymic (36.0%), followed by subsyndromal depression (13.9%), syndromal elevation (6.9%) and subsyndromal elevation (0.6%).
Table 1.
Demographic and Clinical Characteristics of Short Sleepers (SS), Normal Sleepers (NS), Long Sleepers (LS), and Total Sample
| Characteristic | df | Statistic | p | SS (N = 641) | NS (N = 760) | LS (N = 467) | Total Sample (N = 2,024) |
|---|---|---|---|---|---|---|---|
| Ethnicity (% Caucasian) | 2 | χ2 = 8.96 | <.05a,b | 75.9% | 82.5% | 84.4% | 80.6% |
| Female | 2 | χ2 = 1.48 | .48 | 55.5% | 54.5% | 59.1% | 55.9% |
| Age at Study Entry | 2 | F = 4.12 | <.05a | 38.33 (12.51) | 38.86 (13.49) | 36.00 (12.99) | 38.00 (13.07) |
| Age at Onset | 2 | F = 17.35 | <.001a,c | 15.61 (8.19) | 18.20 (9.52) | 15.96 (8.03) | 16.76.(8.80) |
| Illness Duration (years) | 2 | F = 3.83 | <.05a,b | 23.13 (12.73) | 20.87 (13.06) | 20.62 (13.66) | 21.59 (13.12) |
| Rapid Cycling Past Year | 2 | χ2 = 12.59 | <.01a,b,c | 30.4% | 22.4% | 29.6% | 26.9% |
| Clinical Status | 8 | χ2 = 219.25 | <.001 | ||||
| Syndromal Elevation | 23.7%a,b | 9.1%a | 6.9%b | 13.5% | |||
| Syndromal Depression | 40.1%a | 25.8%a,c | 47.3%c | 36.1% | |||
| Euthymic | 20.9%a,b | 51.7%a,c | 31.3%b,c | 36.0% | |||
| Subsyndromal Elevation | 2.5% | 1.6% | 0.6% | 1.7% | |||
| Subsyndromal Depression | 12.8% | 11.8% | 13.9% | 12.7% |
Note: Mean values are displayed with standard deviations in parentheses where applicable.
p <.002 for Bonferroni-adjusted comparison of SS and NS.
p <.002 for Bonferroni-adjusted comparison of SS and LS.
p <.002 for Bonferroni-adjusted comparison of NS and LS.
Demographic and Clinical Characteristics among Sleep Duration Groups
As evident in Table 1, 641 participants (31.7 %) were classified as short sleepers (SS), 760 participants (37.5 %) as normal sleepers (NS), and 467 participants (23.1 %) as long sleepers (LS). A total of 156 (7.0%) participants did not meet criteria for any of the three sleep duration groups (i.e., either had missing data or did not fit cutoffs for any of the sleep duration groups) and were retained in analyses based on the entire sample but excluded from analyses involving SS, NS and LS groups. The three sleep duration groups were similar with respect to gender and differed significantly in ethnicity, age at study entry, age at illness onset, illness duration, rapid cycling and clinical status. Specifically, although the three groups were predominantly Caucasian, the SS group was comprised of fewer Caucasian people (75.9%) than both the NS (82.5%) and LS (84.4%). Also, SS were younger than LS. SS and LS had an earlier age of onset compared to NS, though SS and LS did not differ from each other. SS also had a longer illness duration relative to both NS and LS. NS and LS did not differ from one another in illness duration. SS had a higher percentage of rapid cyclers in the past year relative to both NS and LS. LS had a higher percentage of rapid cyclers relative to NS.
Figure 1 illustrates the differences in clinical status across the three sleep duration groups. Planned follow-up analyses examined group differences among individual clinical status categories with a Bonferroni correction (adjusted p-value =.003). Results suggested that SS were the most symptomatic, followed by LS. SS tended to have a greater frequency of syndromal elevation compared to both LS and NS. Both SS and LS were more frequently syndromally depressed than NS, though SS and LS did significantly differ. NS were more frequently euthymic compared to both SS and LS. However, SS were less frequently euthymic than LS. No significant differences were obtained for subsdyndromal depression or subsyndromal elevation.
Figure 1.
Sleep Duration and Mood State at Entry to STEP-BD.
With respect to bipolar subtype, the majority of patients were diagnosed as BP I (62.4%), followed by BP II (25.8%) and BP NOS (7.9%). TST was significantly different among the three bipolar subtypes, F(2, 2,018) = 3.36, p < .05. Specifically, TST was higher in BP I (M = 7.38, SD = 2.59) and BP II (M = 7.31, SD = 2.30) than BP NOS (M = 7.06, SD = 2.27) subtypes. There were no significant differences for sleep variability, F(2, 2,023) = 0.14, p = 0.87, minimum hours of sleep, F(2, 2,023) = 2.77, p = 0.06, maximum hours of sleep, F(2, 2,023) = 2.23, p = 0.11, or sleep duration group, χ2 =5.67, p = .23.
Sleep Functioning Parameters among Sleep Duration Groups
Means for the sleep functioning parameters are presented in Table 2. Across the entire sample, average TST was 7.28 h (SD = 2.48), and sleep variability was 2.78 h (SD = 3.02), minimum sleep was 5.90 h (SD = 2.68), and maximum sleep in the past week was 8.66 h (SD = 3.12). As expected given the inclusion criteria for our sleep duration groups, the three groups differed significantly in TST, minimum sleep, and maximum sleep. Furthermore, LS displayed significantly higher sleep variability, relative to both the SS and NS. When age and ethnicity were included as covariates, all results remained the same.
Table 2.
Sleep Functioning Parameters across Short Sleepers (SS), Normal Sleepers (NS), Long Sleepers (LS), and Across the Total Sample
| Characteristic | df | Statistic | p | SS (n = 641) | NS (n = 760) | LS (n = 467) | Total Sample (n = 2,024) |
|---|---|---|---|---|---|---|---|
| Total Sleep Time (TST) | 2 | F = 3,456.95 | <.001a,b,c | 4.64 (1.11) | 7.24 (0.54) | 10.59 (1.83) | 7.28 (2.48) |
| Minimum Sleep | 2 | F=1,041.77 | <.001a,b,c | 3.45 (1.52) | 6.00 (1.49) | 8.63 (2.68) | 5.90 (2.68) |
| Maximum Sleep | 2 | F=1,636.64 | <.001a,b,c | 5.83 (1.57) | 8.49 (1.52) | 12.56 (2.81) | 8.66 (3.12) |
| Sleep Variability | 2 | F=44.77 | <.001b,c | 2.39 (2.13) | 2.50 (2.79) | 3.95 (4.07) | 2.78 (3.02) |
Note: Values are displayed as hours of sleep. Means are displayed with standard deviations in parentheses.
p <.05 for pairwise comparison of SS and NS.
p <.05 for pairwise comparison of SS and LS.
p <.05 for pairwise comparison of NS and LS.
Sleep Duration Groups and Associations with Symptom Severity
As evident in Table 3, SS exhibited higher mania severity scores on the YMRS relative to both LS and NS. LS and NS did not differ from each other. SS exhibited higher symptoms of depression on the MADRS compared to both LS and NS, whereas LS exhibited higher depressive symptoms than NS. SS were significantly higher on severity of anxiety from the CMF compared to both NS and LS. NS and LS did not significantly differ from one another on anxiety severity. While SS scored higher on irritability from the CMF than NS, SS did not differ significantly from LS. Results did not change when age and ethnicity were included as covariates, with the exception of irritability that no longer significantly differed among the sleep duration groups, F(1, 718) = 1.15, p = 0.31.
Table 3.
Symptom Severity, Functional Status, and Life Satisfaction across Short Sleepers (SS), Normal Sleepers (NS), Long Sleepers (LS) and the Total Sample.
| Characteristic | df | Statistic | p | SS (N = 641) | NS (N = 760) | LS (N = 467) | Total Sample (N = 2,024) |
|---|---|---|---|---|---|---|---|
| Symptom Severity | |||||||
| YMRS | 2 | F = 54.76 | <.001a,b | 9.87 (7.49) | 6.43 (6.52) | 5.91 (6.07) | 7.46 (6.97) |
| MADRS | 2 | F =59.69 | <.001a,c | 20.99 (10.72) | 14.46 (10.48) | 17.93 (10.45) | 17.53 (10.92) |
| CMF Anxiety Severity | 2 | F = 19.56 | <.001a,b | 2.36 (0.90) | 2.01 (0.87) | 2.08 (0.88) | 2.16 (0.90) |
| CMF Irritability Severity | 2 | F = 3.16 | <.05a | 2.22 (0.92) | 2.08 (0.90) | 2.11 (0.84) | 2.14 (0.90) |
| Functioning | |||||||
| GAF | 2 | F = 27.95 | <.001a,c | 55.78 (13.84) | 61.05 (13.75) | 56.68 (14.78) | 58.15 (14.25) |
| LIFE-RIFT | |||||||
| Work | 2 | F = 12.91 | <.001a,c | 3.41 (1.52) | 3.02 (1.41) | 3.35 (1.44) | 3.24 (1.47) |
| Relationships | 2 | F = 12.72 | <.001a,b,c | 4.03 (1.84) | 3.53 (1.74) | 3.63 (1.78) | 3.72 (1.80) |
| Recreation | 2 | F = 25.99 | <.001a,c | 3.05 (1.41) | 2.54 (1.29) | 3.01 (1.39) | 2.82 (1.38) |
| Satisfaction | 2 | F = 26.50 | <.001a,c | 3.21 (1.08) | 2.83 (1.10) | 3.24 (1.08) | 3.06 (1.11) |
| Total Score | 2 | F = 32.74 | <.001a,c | 13.70 (4.11) | 11.92 (3.87) | 13.23 (4.06) | 12.85 (4.08) |
| Quality of Life | |||||||
| Q-LES-Q | |||||||
| Overall Satisfaction | 2 | F = 27.87 | <.001a,c | 35.57 (12.83) | 41.84 (11.62) | 37.41 (13.44) | 38.52 (12.82) |
| Total Score | 2 | F = 17.10 | <.001a,c | 2.54 (1.13) | 3.00 (1.23) | 2.59 (1.40) | 2.74 (1.26) |
Note: YMRS = Young Mania Rating Scale; MADRS = Montgomery-Asberg Depression Rating Scale; GAF = Global Assessment of Functioning; LIFE-RIFT = Range of Impaired Functioning Tool; Q-LES-Q = Quality of Life Enjoyment and Satisfaction-Short Form. For both the YMRS and MADRS, p-values reflect group differences after individual sleep items were removed from the total scale score. Means are displayed with standard deviations in parentheses.
p <.05 for pairwise comparison of SS and NS.
p <.05 for pairwise comparison of SS and LS.
p <.05 for pairwise comparison of NS and LS
Given the construct overlap between our sleep disturbance groups and individual sleep items on measures of mania (YMRS Item #4) and depression (MADRS Item #4), we re-ran the above analyses with these individual sleep items removed. Results regarding sleep disturbance and mania symptom severity did not significantly change. Removing the sleep item from the MADRS, however, revealed that SS no longer significantly differed from LS in depression symptoms (p = 0.13).
Sleep Duration Groups and Associations with Function and Quality of Life
On measures of function, both SS and LS generally exhibited poorer function than NS. On the GAF, SS and LS exhibited lower scores in global functioning compared to NS. SS and LS did not significantly differ from one another on the GAF. On the LIFE-RIFT, SS and LS again exhibited greater impairment than NS on the total LIFE-RIFT score and the work, recreation and satisfaction LIFE-RIFT domains. SS and LS did not differ significantly from one another on these measures. On the relationship domain, SS exhibited higher impairment than NS and LS. After controlling for age and ethnicity, NS and LS no longer significantly differed from one another in relationship impairment. With respect to quality of life, SS and LS had poorer life satisfaction compared to NS, as indicated by both the overall and total score on the Q-LES-Q. SS and LS did not significantly differ on the Q-LES-Q. Controlling for age and ethnicity did not significantly change the results.
We further examined sleep functioning within the euthymic patients to understand sleep disturbance during the inter-episode period. For euthymic patients (n = 750), average TST was 7.53 (SD = 1.73), maximum hours of sleep was 8.57 (SD = 2.04), minimum hours of sleep was 6.49 (SD = 2.01), and sleep variability was 2.10 (SD = 2.11). The proportion of euthymic patients classified as SS (34.6%), NS (38.0), and LS (27.4%) paralleled that across the entire sample. Paired samples t-tests indicated that a higher frequency of euthymic patients were classified as NS (38.0%) relative to both SS (34.6%) and LS (27.4%) (ps < .001). For euthymic patients, the three sleep duration groups did not significantly differ with respect to life satisfaction or total Q-LES-Q scores (ps > .50). For function, the three sleep duration groups did not differ on the GAF (p = .17) but differed on the LIFE-RIFT total score, F(2, 213) = 3.65, p < .05. Follow-up pairwise comparisons indicated that the SS had greater life impairment than NS (p < .05) but that none of the other sleep duration groups significantly differed.
4. Discussion
The first hypothesis tested was that the SS group would exhibit greater symptom severity, poorer function, and lower quality of life relative to the NS group. Indeed, SS exhibited greater symptoms of mania, depression, and anxiety compared to NS. This finding is consistent with prior research associating short sleep durations with increased mania or hypomania (Bauer et al., 2006; Colombo et al., 1999; Kasper et al., 1992; Wehr et al., 1987). Interestingly, irritability was not associated with our sleep functioning parameters. This non-replication of previous results (Billiard et al., 1994) may be attributable to the fact that we did not examine close temporal associations between sleep and irritability the subsequent day. Rather, we examined more general associations between sleep and mood averaged across a 7 to 10 day period. With respect to function, the finding that SS overall scored lower on measures of global impairment and life functioning compared to NS is consistent with more general findings associating short sleep duration with impairment across several domains of life functioning, including mental health status (Kaneita et al., 2007) and physical health (Kronholm et al., 2006). This also advances the emerging literature implicating the importance of sleep duration in prospective outcomes in bipolar disorder (Perlman et al., 2006). SS also reported poorer quality of life than NS on total life impairment and across all domains of functioning (i.e., work, relationships, recreation, and satisfaction). These findings are consistent with literature demonstrating a relation between restricted sleep duration and decreased quality of life (Groeger et al., 2004). In sum, results suggest that short sleep duration is associated with a particularly pernicious symptom course, functional impairment, and decreased quality of life in bipolar disorder. However, it will be important to tease apart the extent to which sleep, independent of current symptom severity associated with short sleep duration, influences life impairment and functioning. Future research is warranted to examine if short sleep duration represents a unique bipolar phenotype.
The second hypothesis tested was that LS would exhibit poorer function and poorer quality of life compared to NS. Indeed, LS was more impaired than NS across all domains of function. Although minimal research has examined functional impairment in people with long sleep durations, our findings are consistent with evidence suggesting that hypersomnia is associated with impairment in daily activities (Gooneratne et al., 2003) and work performance (Dauvilliers et al., 2005).
Third, we examined the extent to which sleep disturbance present during the inter-episode phase (euthymia) of bipolar disorder. Findings suggesting that over half (62.0%) of euthymic patients exhibited unusually short or long sleep durations are consistent with prior research (Harvey et al., 2005; Millar et al., 2004) indicating significant sleep impairment even during asymptomatic phases. However, associations between sleep disturbance and life satisfaction were no longer apparent for euthymic patients while that between disturbance and impaired life functioning still remained greatest for SS. It may be the case that the impact of sleep disturbance on life satisfaction and more global functioning are state-dependent, and thus not apparent during euthymia. Furthermore, sleep disturbance may continue to disrupt life functioning in a stable manner independent of symptom levels. Results in the present study also suggested the importance of current mood in influencing sleep patterns. For example, SS were more often syndromally elevated (i.e., manic) than LS or NS; similarly, SS and LS were more likely to have depressive symptoms than NS. Thus, it will be important to clarify whether sleep disturbance represents a trait-like marker of bipolar disorder or develops as a consequence of recurrent episodes (e.g., Kapczinski et al., 2008). It will also be important for longitudinal data to more fully address whether sleep disturbance, and associated impairment, is a stable, trait feature of bipolar disorder or a transient symptom that varies across mood states.
We also examined differences in sleep duration and variability according to bipolar subtype. Results suggested there were no significant differences according to bipolar subtype for sleep variability, maximum sleep duration or minimum sleep duration. The finding that BP I and BP NOS, had significantly higher TST than BP II, however, may reflect differences in comorbid disorder rates. For example, given that BP II is associated with higher rates compared to BP I of comorbid anxiety disorders that are associated with shorter sleep durations (Papadimitriou and Linkowski, 2005). Thus, comorbid anxiety disorders may influence TST in BP II.
5. Limitations
These results should be interpreted within the confines of several limitations. First, the cross-sectional study design precludes conclusions about causality. Future studies with longitudinal designs are warranted in order to carefully assess the potential causal relationships. Indeed, it may be the case that both short and long sleep duration are important predictors of recurrence in bipolar disorder. Second, although the available sleep-related variables from the CMF enabled us to derive estimates of sleep duration and variability, it was not possible to make formal diagnoses of insomnia or hypersomnia. Future research should determine if the present findings could be replicated when formal insomnia and hypersomnia diagnoses are made. Third, we recognize that our sleep variables represent sleep averaged over the past 10 days and are unlikely to reflect the full complexity and variability in sleep over this time period. Future research should endeavor to use daily sleep diaries and objective assessments of sleep functioning (e.g., polysomnography). Fourth, the potentially confounding effects of medications need to be considered. Indeed research indicates that frequently prescribed medications for bipolar disorder, in addition to sleep-enhancing medications, may influence sleep architecture (DeMartinis and Winokur, 2007). This may be particularly true for the LS group whereby the sedating effects of medications may have contributed to extended sleep duration. Future studies should thus aim to assess the influence of pharmacotherapy on sleep duration and variability.
6. Conclusion
The present study suggests that restricted or extended sleep schedules may be associated with a pernicious symptom course in bipolar disorder. Moreover, the present findings contribute to an emerging literature suggesting that sleep may be a mechanism contributing to clinical and functional impairment in bipolar disorder.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aeschbach D, Cajochen C, Landolt H, Borbely AA. Homeostatic sleep regulation in habitual short sleepers and long sleepers. Am J Physiol. 1996;270:R41–R53. doi: 10.1152/ajpregu.1996.270.1.R41. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Judd LL, Gillin JC, Lemmi H. Subthreshold depressions: clinical and polysomnographic validation of dysthymic, residual, and masked forms. J Affect Disord. 1997;45(12):53–63. doi: 10.1016/s0165-0327(97)00059-1. [DOI] [PubMed] [Google Scholar]
- Angst F, Stausen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34 to 38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8(2):160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Benedetti M, Barbini B, Colombo C, Smeraldi E. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11(6):509–522. doi: 10.1016/j.smrv.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Fresi F, Maccioni P, Smeraldi E. Behavioural sensitization to repeated sleep deprivation in a mice model of mania. Behav Brain Res. 2008;187(2):221–227. doi: 10.1016/j.bbr.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Billiard M, Dolenc L, Aldaz C, Ondze B, Besset A. Hypersomnia associated with mood disorders: a new perspective. J Psychosom Res. 1994;38(Supp 1):41–7. doi: 10.1016/0022-3999(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Res. 1999;86:267–270. doi: 10.1016/s0165-1781(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Coryell W, Scheftner W, Keller M, Endicott J, Maser J, Klerman GL. The enduring psychological consequences of mania and depression. Am J Psychiatry. 1993;150:720–727. doi: 10.1176/ajp.150.5.720. [DOI] [PubMed] [Google Scholar]
- Craighead WE, Miklowitz DJ, Frank E, Vajk FC. Psychosocial treatments for bipolar disorder. In: Nathan PE, Gorman JM, editors. Treatments that work. Oxford University Press; New York, NY: 2002. pp. 263–275. [Google Scholar]
- Dauvilliers Y, Buguet A. Hypersomnia. Dialogues Clin Neurosci. 2005;7:347–356. doi: 10.31887/DCNS.2005.7.4/ydauvilliers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartinis NA, Winokur A. Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol Disord DrugTargets. 2007;6(1):17–29. doi: 10.2174/187152707779940835. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Fins AI, Michael GD, Sullivan RJ, Bastian LA, Marsh GR, Dailey D, Hope TV, Young M, Shaw E, Vasilas D. Insomnia and the eye of the beholder: Are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol. 2000;68(4):586–593. [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- Feldman-Naim S, Turner EH, Leibenluft E. Diurnal variation in the direction of mood switches in patients with rapid-cycling bipolar disorder. J Clin Psychiatry. 1997;58:79–84. doi: 10.4088/jcp.v58n0205. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Biometrics Research; New York State Psychiatric Institute, NY: 1996. [Google Scholar]
- Gooneratne NS, Weaver TE, Cater JF, Pack FM, Arner HM, Greenberg AS, Pack AI. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51:642–649. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- Groeger JA, Zijlstra FR, Dijk DJ. Sleep quantity, sleep difficulties, and their perceived consequences in a representative sample of some 2000 British adults. J Sleep Res. 2000;13(4):359–371. doi: 10.1111/j.1365-2869.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- Hartman E, Baekland F, Zwiling G, Hoy P. Sleep need: how much sleep and what kind? Am J Psychiatry. 1971;127:41–48. doi: 10.1176/ajp.127.8.1001. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J Affect Disord. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit. Clin Psychol Rev. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneita Y, Ohida T, Osaki Y, Tanihata T, Minowa M, Suzuki K, Wada K, Kanda H, Hayashi K. Association between mental health status and sleep status among adolescents in Japan: A nationwide cross-sectional survey. J Clin Psychiatry. 2007;68:1426–1435. doi: 10.4088/jcp.v68n0916. [DOI] [PubMed] [Google Scholar]
- Kasper S, Wehr TA. The role of sleep and wakefulness in the genesis of depression and mania. Encephale. 1992;18:45–50. [PubMed] [Google Scholar]
- Ketter TA. Advances in the Treatment of Bipolar Disorder. American Psychiatric Publishing, Inc; Washington, DC: 2005. [Google Scholar]
- Kronholm E, Harma M, Hublin C, Aro AR, Partonen T. Self-reported sleep duration in Finnish general population. J Sleep Res. 2006;15(3):276–290. doi: 10.1111/j.1365-2869.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Suppes T. Treating bipolar illness: Focus on treatment algorithms and management of the sleep-wake cycle. Am J Psychiatry. 1999;156:1976–1981. doi: 10.1176/ajp.156.12.1976. [DOI] [PubMed] [Google Scholar]
- Leon AC, Solomon DA, Mueller TI, Endicott J, Posternak M, Judd LL, Schettler P, Akiskal HS, Keller MB. A brief assessment of psychosocial function of subjects with bipolar I disorder: the LIFE-RIFT (Longitudinal Interval Follow-up Evaluation-Range of Impaired Function Tool) J Nerv Ment Dis. 2000;188:805–812. doi: 10.1097/00005053-200012000-00003. [DOI] [PubMed] [Google Scholar]
- Millar A, Epsie CA, Scott J. The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. J Affect Disord. 2004;80:145–153. doi: 10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysee DJ, Welsh DK, Kennedy KS, Rose LR. A sleep diary and questionnaire study of naturally short sleepers. J Sleep Res. 2001;10:173–179. doi: 10.1046/j.1365-2869.2001.00254.x. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatry. 2005;17:229–236. doi: 10.1080/09540260500104524. [DOI] [PubMed] [Google Scholar]
- Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disord. 2006;8(3):271–274. doi: 10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Huffcut AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19:318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Guille C, McMurrich SL. A clinical monitoring form for mood disorders. Bipolar Disord. 2002;4(5):323–327. doi: 10.1034/j.1399-5618.2002.01195.x. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese JR, Kupfer D, Rosenbaum JF. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- Simon NM, Otto MW, Wisniewski SR, Fossey M, Sagduyu K, Frank E, Sachs GS, Nierenberg AA, Thase ME, Pollack MH. Anxiety disorder comorbidity in bipolar disorder patients: Data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2004;16:2222–2229. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. Am J Psychiatry. 1987;144:201–204. doi: 10.1176/ajp.144.2.201. correction 144; 542. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Berger M, Lam RW, Martiny K, Terman M, Wu JC. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939–944. doi: 10.1017/s003329170500437x. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: What do we know, where do we go? Biol Psychiatry. 1999;46(4):445–453. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yuan-Who C, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biol Psychiatry. 1996;39(10):896–899. doi: 10.1016/0006-3223(95)00295-2. [DOI] [PubMed] [Google Scholar]