Abstract
Exposure to stress alters the behavioral and neurochemical effects of drugs of abuse. However, it is unknown if chronic stress can affect the serotonergic depletions induced by the psychostimulant drug 3,4-methylenedioxymethamphetamine (MDMA). Rats were exposed to 10 days of chronic unpredictable stress (CUS) which resulted in the predicted elevation of basal plasma corticosterone concentrations. On the 11th day, rats received 4 challenge doses of MDMA (5 mg/kg every 2 h, i.p.) or saline. Five days later, rats were killed and 5-HT and dopamine content were measured in the striatum, hippocampus, and frontal cortex. MDMA produced greater depletions of 5-HT in all 3 brain regions of rats pre-exposed to CUS compared to rats not exposed to CUS. CUS-exposed rats also had an augmented acute hyperthermic response but a similar increase in plasma corticosterone after challenge injections of MDMA compared with non-stressed rats similarly challenged with MDMA. Moreover, CUS-exposed rats exhibited an MDMA-induced depletion of striatal dopamine that was absent in non-stressed rats that received MDMA. To investigate the role of corticosterone in these effects, the corticosterone synthesis inhibitor, metyrapone (50 mg/kg, i.p.), was administered prior to each stressor on each of the 10 days of CUS. Metyrapone blocked the chronic stress-induced elevation in basal plasma corticosterone, prevented the enhancement of MDMA-induced hyperthermia, and blocked the enhanced depletions of 5-HT and dopamine in CUS-exposed rats, but had no effect on the acute MDMA-induced increases in plasma corticosterone. These findings suggest that CUS alone can increase the basal level of corticosterone that in turn, plays an important role in enhancing the sensitivity of both 5-HT and dopamine terminals to the hyperthermic and monoamine depleting effects of MDMA without altering the acute corticosterone response to an MDMA challenge.
Keywords: metyrapone, hyperthermia, serotonin, striatum, hippocampus, frontal cortex
Stressful life events are associated with drug addiction in humans (Rhoads, 1983; Najavits et al., 1998; Koob and LeMoal, 2001) and repeated exposure to stress in rodents alters the behavioral and neurochemical responses to abused drugs. Prior exposure to stressors augments amphetamine- and cocaine-induced locomotor activity (Herman et al., 1984; DeRoche et al., 1995; Haile et al., 2001), the acquisition of psychostimulant drug self-administration (Piazza et al., 1990; Haney et al., 1995; Piazza and LeMoal, 1998), and the reinstatement of drug-seeking behavior in extinguished animals (Erb et al., 1996; Shalev et al., 2003), evidence indicative of a cross-sensitization between stress and the behavioral effects of psychostimulant drugs. However, little is known about the possible long-term consequences of the interaction between chronic stress and psychostimulants on monoamine depletions.
Stress results in activation of the hypothalamic-pituitary-adrenal axis and the subsequent release of glucocorticoids from the adrenal cortex (Munck et al., 1984). Chronic exposure to stress leads to loss of negative feedback inhibition of the corticosterone (CORT) response resulting in elevated circulating levels of CORT (Herman et al., 1995). These persistent elevations in CORT can result in increased susceptibility to depression, disease, and cognitive deficits (McEwen and Sapolsky, 1995; McEwen, 1998; Holsboer, 2000), as well as dendritic atrophy in the hippocampus and cortex of rodents (Woolley et al., 1990; Magarinos and McEwen, 1995a, b; Cook and Wellman, 2004). The relatively selective vulnerability of the hippocampus and cortex to the damaging effects of stress may be due to the high density of glucocorticoid receptors in these regions (Reul and de Kloet, 1985). Moreover, administration of CORT to rodents potentiates hippocampal neuronal damage in response to neurotoxins such as kainic acid and 3-acetylpyridine (Sapolsky, 1985a, b). Recently, our laboratory has demonstrated that prior exposure to chronic unpredictable stress (CUS) results in the enhancement of methamphetamine-induced dopamine (DA) and serotonin (5-HT) depletions in the striatum of rats (Matuszewich and Yamamoto, 2004; Tata et al., 2007). Taken together, these studies suggest that chronic stress-like elevations in CORT can enhance the vulnerability of the brain to the monoamine depleting effects of psychostimulants.
The psychostimulant MDMA is a widely abused “club drug” and member of the amphetamine family of compounds. High doses of MDMA result in selective long-term decreases in 5-HT concentrations in striatum, hippocampus, and frontal cortex of rodents and non-human primates (Schmidt, 1987; Commins et al., 1987; Ricaurte, 1988). Damage to 5-HT terminals of the striatum, hippocampus, and cortex induced by MDMA is also evidenced by decreases in the 5-HT metabolite, 5-hydroxytryptamine, tryptophan hydroxylase activity, and in the density of 5-HT reuptake sites (Stone et al., 1987; Schmidt and Taylor, 1987; Battaglia et al., 1987). These alterations have been shown to endure for as long as 52 weeks after exposure to MDMA (Sabol et al., 1996). The most severe and long-lasting depletions occur in the hippocampus and frontal cortex (Sabol et al, 1996), two areas that are also highly vulnerable to chronic stress (Magarinos and McEwen, 1995a, b; Cook and Wellman, 2004).
Several factors mediate MDMA-induced 5-HT depletions in naïve, non-stressed rats. Hyperthermia induced by high doses of MDMA is a known contributor to long-term 5-HT terminal damage. Prevention of elevations in core body temperature during MDMA administration prevents 5-HT depletions, while increasing ambient temperature during MDMA administration enhances the depletions in 5-HT concentrations (Broening et al., 1995; Malberg and Seiden, 1998). Our laboratory demonstrated that prior exposure to CUS enhances methamphetamine-induced hyperthermia and that prevention of the enhanced hyperthermia eliminates the ability of CUS to exacerbate the long term depletions of striatal DA and 5-HT produced by methamphetamine (Tata et al., 2007), suggesting an important role for hyperthermia in the ability of CUS to enhance monoamine depletions induced by this psychostimulant. In contrast, the relationship between hyperthermia, CUS, and MDMA is not known. Although MDMA has the ability to induce CORT secretion (Nash et al., 1988) and stress-induced CORT can cause neuronal atrophy in the hippocampus and cortex (Magarinos and McEwen, 1995b; Cook and Wellman, 2004), the role of MDMA-induced CORT in long-term 5-HT depletions has been controversial (Johnson et al., 1989, but also see Aquirre et al., 1997; Fernandez et al., 2002). Moreover, it is unknown if chronic stress will influence CORT or body temperature to potentiate MDMA-induced 5-HT depletions in the brain.
The current study examines the impact of CUS on changes in body temperature as well as 5-HT and DA tissue content in the striatum, hippocampus, and frontal cortex produced by MDMA. The role of CORT in mediating these effects is also investigated. It is predicted that the hippocampus and frontal cortex may be particularly vulnerable to the combination of CUS and MDMA due to the sensitivity of these brain regions to both MDMA and chronic stress.
EXPERIMENTAL PROCEDURES
Animals and stress exposure
Male Sprague-Dawley rats (175–250 g) were purchased from Harlan (Indianapolis, IN USA). Rats were group housed in clear plastic containers (45 × 24 × 20 cm) with food and water available ad libitum, in a temperature (21–23°C) controlled environment on a 12-h light/dark cycle (lights on 07:00 h and off 19:00 h). All procedures were carried out in accordance with the Boston University Institutional Animal Care and Use Committee and within the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Stressed rats were exposed to stressors that varied by day and time, two per day, for 10 days. The chronic unpredictable stress model varies the type, time, and exposure length of each stressor to avoid adaptation to the stressors (Herman et al., 1995). This model better mimics unexpected stressful life events encountered by humans. The following schedule was used: day 1: 10:00h 50 min cold room (4°C) and 13:00 h 60 min restraint; day 2: 11:00 h 60 min cage rotation and 18:00 h lights on overnight; day 3: 10:00 h 3 hr lights off and 15:00 h 3 min swim stress (23°C); day 4: 11:00 h 50 min restraint and 17:00 h food and water deprivation overnight; day 5: 12:00 h 15 min cold room isolation (4°C) and 12:30 h isolation housing overnight; day 6: 10:00 h 4 min swim stress (23°C) and 18:00 h lights on overnight; day 7: 9:00 h 2 h lights off and 18:00 h food and water deprivation overnight; day 8: 10:00 h 30 min restraint and 15:00 h 40 min cage rotation; day 9: 11:00 h 3 min swim stress (23°C) and 18:00 h lights on overnight; day 10: 10:00 h 3 h lights off and 13:00 h 20 min cage rotation. Non-stressed rats were transported daily to the area where stressors were administered but were not exposed to the stressors.
Drugs and drug administration
2-methyl-1,2-di-3-pyridyl-1-propanone (metyrapone) was purchased from Sigma-Aldrich (St. Louis, MI). Metyrapone inhibits corticosteroid biosynthesis by binding to 11β-hydroxylase, the enzyme that converts 11-deoxycorticosterone to corticosterone in the adrenal glands (Sonino, 1982). Metyrapone (50 mg/kg) was dissolved in 10% ethanol and was administered intraperitoneally (i.p.) at a volume of 1 ml/kg, 15 min. prior to each stressor on each of the 10 days of CUS. Control rats were injected with 10% ethanol (1 ml/kg, i.p.). +MDMA hydrochloride was provided by the National Institute on Drug Abuse (Rockville, MD, USA). The day after the last stressor (day 11), 5 mg/kg MDMA, dissolved in 0.9% NaCl, or 0.9% NaCl was injected i.p. either once (5 mg/kg × 1) or once every 2 h for a total of 4 injections (5 mg/kg; q 2 h × 4) at volumes of 1 mg/kg. This lower repeated dosing regimen of MDMA was selected for its less severe effects on 5-HT tissue content depletions as determined in preliminary experiments.
Measurement of plasma corticosterone
Rats were killed by rapid decapitation either the morning after (08:00 h), approximately 18 h after the last stressor, or 1 h after (09:00 h) a single injection of MDMA (5 mg/kg, i.p. × 1) or saline administered the morning after (08:00 h) the last stressor (day 11). Trunk blood was collected into microcentrifuge tubes on ice, centrifuged at 800 × g for 14 min at 4°C, and the plasma was collected and centrifuged further at 800 × g for 7 min at 4°C. Plasma was stored at −80°C until analysis. Plasma CORT was analyzed using a commercially available corticosterone EIA (Diagnostic Systems Laboratories, Webster, TX).
Temperature measurement
On the day of the experiments in which rats received repeated MDMA (5 mg/kg, i.p.; q 2 h × 4) or saline treatment (day 11), body temperature was measured using a rectal probe digital thermometer (Thermalert TH-8 monitor, Physitemp Instruments, Inc., Clinton, New Jersey). Temperature measurements were recorded prior to the first injection of MDMA or saline and 1 h after each injection by holding each rat at the base of the tail and inserting a probe (RET-2) 4.6 cm past the rectum into the colon for 6–8 s until rectal temperature was maintained for 3 s.
High-performance liquid chromatography for tissue 5-HT and DA content
Rats that received repeated MDMA (5 mg/kg, i.p.; q 2 h × 4) or saline treatment were killed by rapid decapitation 5 days after treatment. The brains were quickly removed and whole striata, hippocampi, and frontal cortices were dissected and frozen on dry ice. The tissue was stored at −80°C for later analysis of 5-HT and DA tissue content. Tissues were sonicated in cold 0.25 M perchloric acid and centrifuged at 14,000 × g for 20 min at 4°C. The supernatant was analyzed with high-performance liquid chromatography with electrochemical detection (HPLC-EC). Separation of biogenic amines from their metabolites was achieved with a 3 μm particle size reverse phase C-18 column (100 × 2.0 mm, Phenomenex, Torrance, CA) and a mobile phase consisting of 32 mM citric acid, 54.3 mM sodium acetate, 0.074 mM EDTA, 0.22 mM octyl sodium sulfate, and 3% methanol (pH 3.1). Compounds were detected with an LC-4B amperometric detector (Bioanalytical Systems, West Lafayette, IN) with a 6 mm glassy carbon working electrode maintained at a potential of +0.6 V relative to an Ag/AgCl reference electrode. Data were collected using EZChrom Elite software (Agilent Technologies, Santa Clara, CA). The pellet was dissolved in 1 N NaOH and protein content was determined using a Bradford assay (Bio-Rad, Hercules, CA). Data is presented as pg/μg protein.
Statistical analyses
Basal plasma CORT concentrations obtained the day after the last stressor were analyzed using a two-way analysis of variance (ANOVA; pretreatment × stress; Vehicle/No Stress, Vehicle/Stress, Metyrapone/No Stress, Metyrapone/Stress). Plasma CORT levels obtained 1 h after administration of a single injection of MDMA or saline were analyzed using a one-way ANOVA. Rectal temperature measurements were analyzed using a two-way repeated-measures ANOVA with treatment as the between-subjects factor and time as the repeated-measures factor. A two-way ANOVA was used for the analysis of 5-HT and DA tissue content (stress × drug; No Stress/Saline, Stress/Saline, No Stress/MDMA, Stress/MDMA). The regional differences in 5-HT depletions induced by MDMA alone or by the combination of stress and MDMA were analyzed by one-way ANOVAs (No Stress/Saline vs. No Stress/MDMA and No Stress/MDMA vs. Stress/MDMA). A three-way ANOVA was used for the analysis of 5-HT and DA content for the experiments determining the role of stress-induced CORT (pretreatment × stress × drug; Vehicle/No Stress/Saline, Vehicle No Stress/MDMA, Vehicle/Stress/Saline, Vehicle Stress/MDMA, Metyrapone/No Stress/Saline, Metyrapone No Stress/MDMA, Metyrapone/Stress/Saline, Metyrapone Stress/MDMA). Further analysis within the MDMA-treated groups was analyzed with a two-way ANOVA. Significant interactions for ANOVAs were further analyzed with Tukey’s post-hoc comparisons. Statistics were determined using Sigma Plot 11.0. Statistical significance was set at p<0.05 for all tests.
RESULTS
Effects of CUS and metyrapone on basal plasma CORT levels
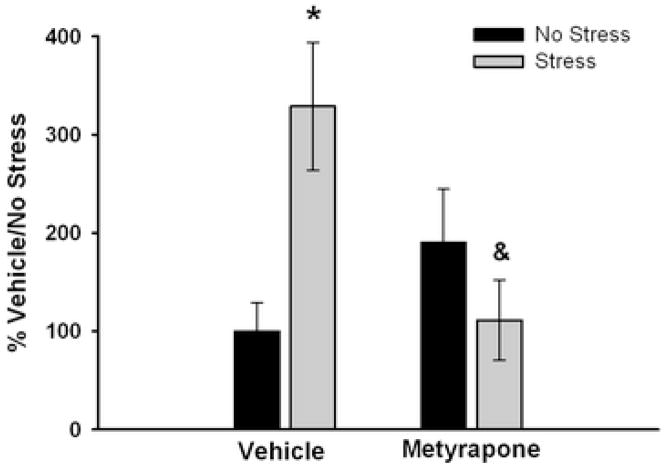
Figure 1 illustrates basal plasma CORT levels measured the day after 10 days of CUS or no stress in rats pretreated with vehicle (10% EtOH, 1 ml/kg, i.p.) or metyrapone (50 mg/kg, i.p.) 15 min prior to each stressor. There was no main effect of the pretreatment condition (F1,34 = 1.596, p>0.05) or the stress condition (F1,34 = 2.205, p>0.05), however there was a significant pretreatment × stress interaction (F1,34 = 9.348, p<0.005). Post hoc comparisons indicate a significant enhancement in basal plasma CORT in chronically stressed rats relative to non-stressed rats (p<0.01). Metyrapone pretreatment during CUS significantly blocked the enhanced basal plasma CORT levels (p<0.01). Metyrapone had no effect on basal plasma CORT when administered to non-stressed rats (p>0.05).
Figure 1.
Basal plasma corticosterone levels the morning after 10 days of CUS. Non-stressed and stressed rats were pretreated i.p. with metyrapone (50 mg/kg) or vehicle (10% EtOH) 15 min prior to each stressor. * p<0.01 compared to Vehicle No Stress; & p<0.01 compared to Metyrapone No Stress. Values are expressed as means ± SEM; n = 7–10/group.
Effects of CUS and metyrapone on plasma CORT levels in response to MDMA or saline
Table 1 illustrates that plasma CORT levels are significantly increased an hour after a single injection of MDMA (5 mg/kg, i.p. × 1) as compared to saline injected rats. There was an overall effect of MDMA as compared to saline (F5,50 = 6.512, p<0.001) on plasma CORT levels. All groups of rats receiving MDMA, regardless of pretreatment, demonstrated significant increases in plasma CORT levels (p<0.05). There was no statistical difference in MDMA-induced plasma CORT levels between stressed and non-stressed rats (p>0.05). There also was no impact of pretreatment (vehicle vs. metyrapone) on MDMA-induced plasma CORT levels (p>0.05).
Table 1.
Effect of chronic unpredictable stress and metyrapone on corticosterone levels 1 h after a single injection of MDMA or saline
| Pretreatment Condition | Stress Condition | Drug Condition | Corticosterone (ng/ml ± SEM) |
|---|---|---|---|
| Vehicle | No Stress | Saline | 85.3 ± 24.5 |
| Vehicle | Stress | Saline | 125.3 ± 25.6 |
| Vehicle | No Stress | MDMA | 464.7 ± 89.2* |
| Vehicle | Stress | MDMA | 381 ± 63.3* |
| Metyrapone | No Stress | MDMA | 501 ± 91.9* |
| Metyrapone | Stress | MDMA | 431.8 ± 58.1* |
p<0.05 when compared to vehicle pretreated non-stressed rats. n = 6–11 per treatment group.
Effects of CUS and MDMA on body temperature
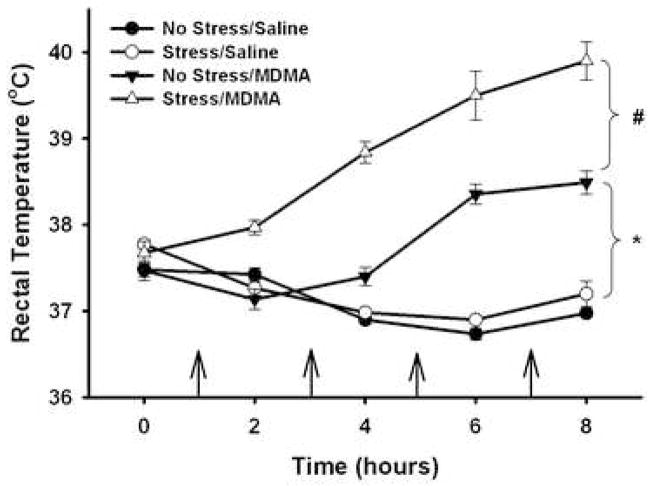
Figure 2 illustrates rectal temperature measurements made prior to administration of MDMA or saline and 1 h after each MDMA (5 mg/kg, i.p.; q 2 h × 4) or saline injection to both non-stressed and stressed rats. MDMA significantly increased temperatures over time compared to saline treatment as revealed by significant main effects of MDMA (F3,164=105.661, p<0.001) and time (F4,164=11.624, p<0.001) and a significant MDMA × time interaction (F12,164=22.262, p<0.001). Post hoc comparisons showed that MDMA administration in non-stressed rats caused a significant hyperthermic response relative to non-stressed, saline-treated rats (No Stress/Saline vs. No Stress/MDMA, p<0.001). Prior exposure to CUS enhanced the MDMA-induced hyperthermic response compared with non-stressed rats similarly treated with MDMA (No Stress/MDMA vs. Stress/MDMA, p<0.001).
Figure 2.
Effect of MDMA (5 mg/kg, i.p.; q 2 h × 4) or saline on body temperature of stressed and non-stressed rats. Body temperatures (°C) were measured prior to and 1 h after each MDMA injection. * p<0.01 compared to No Stress/Saline; # p<0.01 compared to No Stress/MDMA. Arrows indicate each injection of MDMA of saline. Values are expressed as means ± SEM; n = 6–11/group.
Effects of CUS and MDMA on 5-HT and DA concentrations in striatum, hippocampus, and frontal cortex
Table 2 illustrates the 5-HT and DA concentrations in the striatum, hippocampus, and frontal cortex 5 days after MDMA (5 mg/kg, i.p.; q 2 h × 4) or saline injections to stressed and non-stressed rats. In the striatum there was a main effect of stress (F1,32 = 8.813, p<0.01) and MDMA (F1,32 = 64.197, p<0.001), and a stress × MDMA interaction (F1,32 = 9.858, p<0.001) on 5-HT concentrations. Post hoc comparisons revealed that 5-HT tissue content was significantly depleted after MDMA compared to saline (30.7% depletion; p<0.01), and prior exposure to CUS enhanced this depletion (p<0.001). There was a 55.5% greater 5-HT depletion in stressed than non-stressed rats similarly treated with MDMA. There was no main effect of stress (F1,32 = 3.695, p=0.064), but there was a main effect of MDMA (F1,32 = 8.609, p<0.01) and a stress × MDMA interaction (F1,32 = 9.756, p<0.01) on striatal DA concentrations. Post hoc comparisons revealed that in non-stressed rats, MDMA had no effect on DA content compared to saline rats (p>0.05), but interestingly, MDMA treatment significantly depleted DA in chronically stressed rats (p<0.001).
Table 2.
Effect of chronic unpredictable stress and MDMA on 5-HT and DA tissue content
| Brain Region | Stress Condition | Drug Condition | 5-HT content (pg/μg protein ± SEM) | DA content (pg/μg protein ± SEM) |
|---|---|---|---|---|
| Striatum | No Stress | Saline | 5.11 ± 0.53 | 134.8 ± 5.06 |
| Stress | Saline | 5.16 ± 0.19 | 144.9 ± 6.33 | |
| No Stress | MDMA | 3.5 ± 0.25* | 136.4 ± 4.69 | |
| Stress | MDMA | 1.6 ± 0.27*# | 94.2 ± 11.09*# | |
| Hippocampus | No Stress | Saline | 4.4 ± 0.28 | |
| Stress | Saline | 4.4 ± 0.30 | ||
| No Stress | MDMA | 2.3 ± 0.23* | ||
| Stress | MDMA | 1.0 ± 0.15*# | ||
| Frontal Cortex | No Stress | Saline | 3.9 ± 0.13 | 0.66 ± 0.09 |
| Stress | Saline | 3.9 ± 0.23 | 0.79 ± 0.09 | |
| No Stress | MDMA | 2.8 ± 0.30* | 0.67 ± 0.03 | |
| Stress | MDMA | 1.6 ± 0.27*# | 0.57 ± 0.04 |
p<0.05 compared to No Stress/Saline,
p<0.01 compared to No Stress/MDMA
n = 6–11 per group.
In the hippocampus there was a main effect of stress (F1,32 = 6.568, p<0.05) and MDMA (F1,32 = 128.201, p<0.001), and a stress × MDMA interaction (F1,32 = 7.636, p<0.01) on 5-HT concentrations. Post hoc comparisons revealed that 5-HT tissue content was significantly depleted after MDMA compared to saline (47.4% depletion; p<0.001), and prior exposure to CUS enhanced this depletion (p<0.001). There was a 56% greater 5-HT depletion in stressed than non-stressed rats similarly treated with MDMA.
In the frontal cortex there was no main effect of stress (F1,31 = 3.397, p=0.076), but there was a main effect of MDMA (F1,31 = 34.274, p<0.001) and a stress × MDMA interaction (F1,31 = 4.69, p<0.05) on 5-HT concentrations. Post hoc comparisons showed that 5-HT tissue content was significantly depleted after MDMA compared to saline (28.7% depletion; p<0.05), and prior exposure to CUS enhanced this depletion (p<0.01). There was a 44% greater 5-HT depletion in stressed than non-stressed rats similarly treated with MDMA. There was no significant effect of stress (F1,31 = 0.0448, p>0.05), MDMA (F1,31 = 2.178, p>0.05), or a stress × MDMA interaction (F1,31 = 3.085, p>0.05) on DA concentrations in the frontal cortex.
There were no significant differences in the level of 5-HT depletions between brain regions after MDMA to non-stressed rats compared to saline (F2,32 = 2.801, p>0.05) or the level of enhanced MDMA-induced depletions in chronically-stressed rats compared to non-stressed rats (F2,29 = 0.731, p>0.05).
Effects of CUS and metyrapone on MDMA-induced hyperthermia
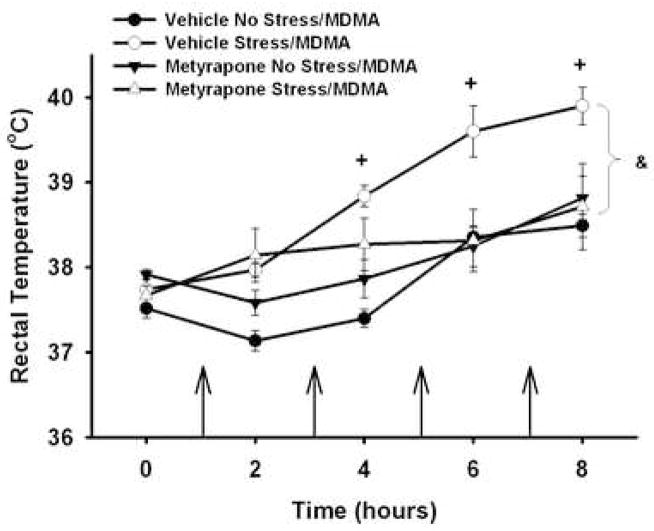
Rectal temperatures were measured prior to administration of MDMA or saline and 1 h after each MDMA (5 mg/kg, i.p.; q 2 h × 4) or saline injection to both non-stressed and stressed rats that had been pretreated with vehicle (10% EtOH, 1 ml/kg, i.p.) or metyrapone (50 mg/kg, i.p.) 15 min prior to each stressor. There was no difference in baseline temperatures between vehicle (37.65±0.06°C) and metyrapone (37.78±0.27°C; t(58) = 0.24) pretreated groups. Temperatures in response to saline for all manipulations (Vehicle No Stress/Saline, Vehicle Stress/Saline, Metyrapone No Stress/Saline, Metyrapone Stress/Saline) did not change over time or differ between groups (data not shown). For clarity, only the data obtained from rats treated with MDMA are depicted. Figure 3 illustrates the analysis of rectal temperatures for all rats treated with MDMA and shows a significant main effect of treatment (F3,169 = 9.991, p<0.001) and time (F4,169 = 10.25, p<0.001), and treatment × time interaction (F12,169 = 1.042, p<0.001). Post hoc comparisons indicate that baseline temperatures did not differ among groups (p>0.05). MDMA administration to both non-stressed and stressed rats resulted in increases in rectal temperatures over time (p<0.01). Rats exposed to CUS showed overall greater rectal temperatures during MDMA injections compared with non-stressed rats similarly treated with MDMA (Vehicle Stress/MDMA vs. Vehicle No Stress/MDMA; p<0.001). Administration of metyrapone to stressed, MDMA-treated rats significantly attenuated the stress-induced enhancement in hyperthermia (Metyrapone Stress/MDMA vs. Vehicle Stress/MDMA, p<0.05). There were no overall differences between metyrapone pretreated stressed and non-stressed rats (p>0.05) or between non-stressed rats that were pretreated with either vehicle or metyrapone (p>0.05).
Figure 3.
Effect of MDMA (5 mg/kg, i.p.; q 2 h × 4) on body temperature of stressed and non-stressed rats pretreated i.p. with metyrapone (50 mg/kg) or vehicle (10% EtOH). Body temperatures (°C) were measured prior to and 1 h after each MDMA injection. + p<0.01 compared to Vehicle No Stress/MDMA; & p<0.05 significantly different overall between Vehicle Stress/MDMA and all other groups. Arrows indicate each injection of MDMA. Values are expressed as means ± SEM; n = 6–11/group.
Effects of CUS, metyrapone, and MDMA on 5-HT and DA concentrations in the striatum, hippocampus, and frontal cortex
5-HT and DA tissue concentrations were measured in the striatum, hippocampus, and frontal cortex 5 days after MDMA (5 mg/kg, i.p.; q 2 h × 4) or saline administration in both non-stressed and stressed rats that had been pretreated with vehicle (10% EtOH, 1 ml/kg, i.p.) or metyrapone (50 mg/kg, i.p.) 15 min prior to each stressor.
Striatum
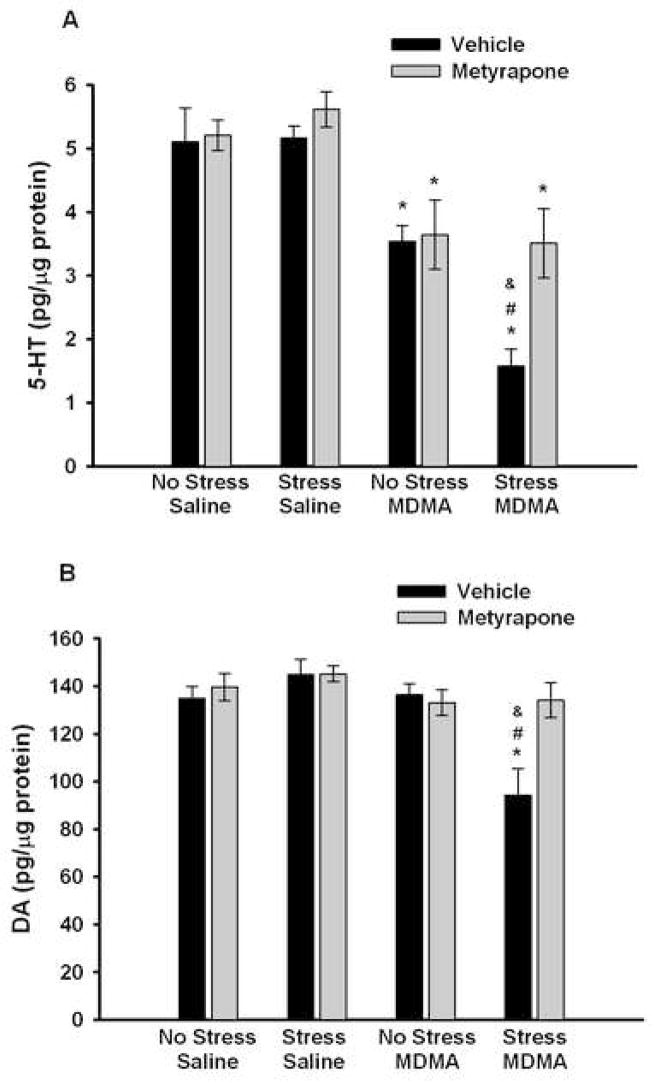
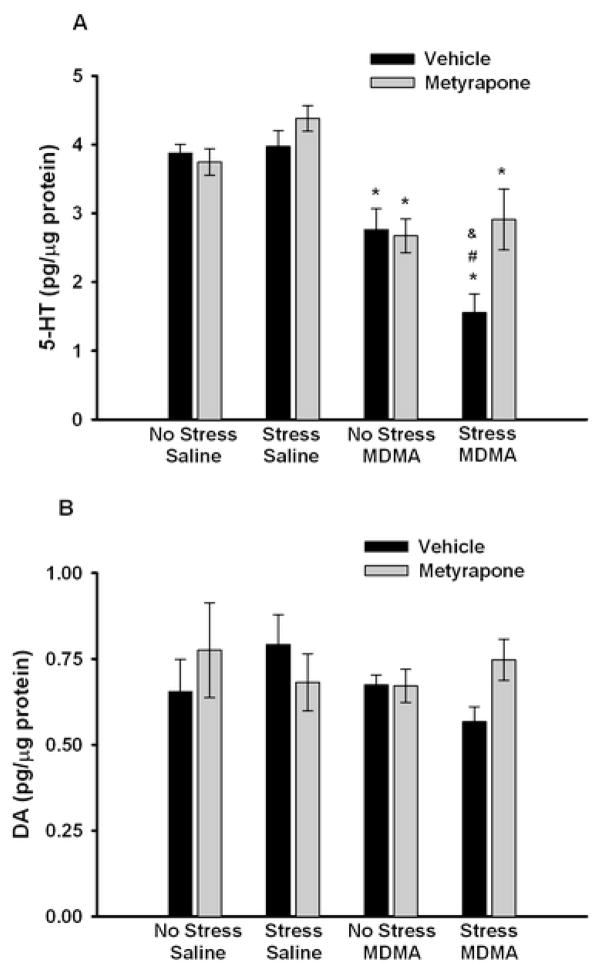
Analysis of striatal 5-HT tissue concentrations (Fig. 4A) revealed that MDMA caused a significant depletion of 5-HT compared to saline controls as noted by a significant main effect of MDMA (F1,57 = 69.399, p<0.001). The post hoc comparison showed that chronically stressed rats treated with MDMA had enhanced depletions of striatal 5-HT content as compared to non-stressed, MDMA-treated rats (p<0.001). While the pretreatment × stress × drug interaction (F1,57 = 1.941, p=0.17) did not reach significance, the significant main effect of metyrapone (F1,57 = 5.973, p<0.05), and the two-way interactions between metyrapone and stress within the MDMA treated groups(F1,57 = 4.257, p<0.05) and between stress and MDMA within the metyrapone treated rats (F1,57 = 5.85, p<0.05) demonstrate that pretreatment with metyrapone blocked the enhanced MDMA-induced depletion of 5-HT in CUS-exposed rats. Stress itself did not significantly impact 5-HT concentrations as noted by lack of a main effect of stress compared to non-stressed rats (F1,57 = 2.373, p=0.13). Further analysis of MDMA-treated rats using a two-way ANOVA with a Tukey’s post hoc comparison confirmed that metyrapone administration during CUS significantly blocked the enhanced 5-HT depletions after MDMA (Vehicle Stress/MDMA vs. Metyrapone Stress/MDMA, p<0.001). Analysis of striatal DA tissue concentrations (Fig. 4B) revealed that MDMA caused a significant depletion of DA in chronically stressed rats that in turn, was blocked by metyrapone administration during CUS as noted by a main effect of MDMA (F1,57 = 9.522, p<0.01), a significant stress × MDMA interaction within the metyrapone groups (F1,57 = 6.901, p<0.05), and a significant pretreatment × stress × drug interaction (F1,57 = 4.887, p<0.05). Post hoc comparison confirmed that MDMA treatment significantly decreased striatal DA concentrations in rats that had been chronically stressed compared to non-stressed rats (p<0.001). Stress itself did not significantly change DA concentrations as noted by lack of a main effect of stress compared to non-stressed rats (F1,57 = 1.401, p=0.24). Further analysis of the MDMA-treated rats using a two-way ANOVA with a Tukey’s post hoc comparison confirmed that metyrapone administration during CUS significantly blocked the DA depletions induced by the combination of CUS and MDMA (Vehicle Stress/MDMA vs. Metyrapone Stress/MDMA, p<0.01).
Figure 4.
Effect of metyrapone (50 mg/kg) or vehicle (10% EtOH) during CUS on (A) 5-HT and (B) dopamine (DA) concentrations in the striatum 5 days after administration of MDMA (5 mg/kg, i.p.; q 2 h × 4) or saline. * p<0.01 compared to saline controls, # p<0.01 compared to Vehicle No Stress/MDMA; & p<0.01 compared to Metyrapone Stress/MDMA. Values are expressed as means ± SEM; n = 6–11/group.
Hippocampus
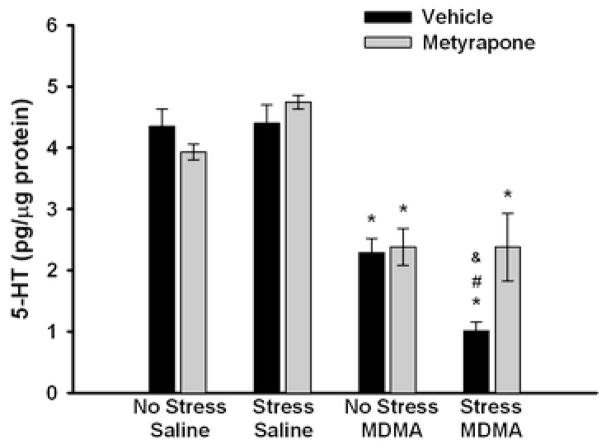
Analysis of hippocampal 5-HT tissue concentrations (Fig. 5) revealed that MDMA caused a significant depletion of 5-HT compared with saline controls as noted by a significant main effect of MDMA (F1,56 = 123.058, p<0.001). The post hoc comparison showed that chronically stressed rats administered MDMA had enhanced depletions of hippocampal 5-HT content as compared to non-stressed MDMA-treated rats (p<0.001). While the pretreatment × stress × drug interaction (F1,56 = 0.421, p>0.05) was not significant, the significant main effect of metyrapone(F1,56 = 4.009, p=0.05), and the two-way interactions between metyrapone and stress within the MDMA-treated groups (F1,56 = 5.962, p<0.05) and between stress and MDMA within the metyrapone-treated groups (F1,56 = 9.824, p<0.01) demonstrate that pretreatment with metyrapone blocked the enhanced MDMA-induced depletion in 5-HT in CUS-exposed rats. Stress itself did not significantly change 5-HT concentrations as noted by lack of a main effect of stress compared to non-stressed rats (F1,56 = 0.0004, p>0.05). Further analysis of the MDMA-treated rats using a two-way ANOVA with a Tukey’s post hoc comparison confirmed that metyrapone administration during CUS significantly blocked the enhanced 5-HT depletions after MDMA (Vehicle Stress/MDMA vs. Metyrapone Stress/MDMA, p<0.01).
Figure 5.
Effect of metyrapone (50 mg/kg) or vehicle (10% EtOH) during CUS on 5-HT concentrations in the hippocampus 5 days after administration of MDMA (5 mg/kg, i.p.; q 2 h × 4) saline. * p<0.01 compared to saline controls, # p<0.01 compared to Vehicle No Stress/MDMA; & p<0.01 compared to Metyrapone Stress/MDMA. Values are expressed as means ± SEM; n = 6–11/group.
Frontal Cortex
Analysis of frontal cortex 5-HT tissue concentrations (Fig. 6A) showed that MDMA caused a significant depletion of 5-HT compared with saline controls as noted by a significant main effect of MDMA (F1,57 = 47.762, p<0.001). The post hoc comparison revealed that chronically stressed, MDMA-treated rats had enhanced depletions of frontal cortex 5-HT content as compared to non-stressed, MDMA-treated rats (p<0.001). While the pretreatment × stress × drug interaction (F1,57 = 0.746, p>0.05) was not significant, the significant main effect of metyrapone (F1,57 = 3.577, p=0.05), and the two-way interaction between metyrapone and stress within the MDMA-treated groups (F1,57 = 6.356, p<0.05) demonstrate that the enhanced MDMA-induced depletion of 5-HT in CUS-exposed rats was blocked by pretreatment with metyrapone. Stress itself did not significantly alter 5-HT concentrations as noted by lack of a main effect of stress compared to non-stressed rats (F1,57 = 0.231, p>0.05). Further analysis of MDMA-treated rats using a two-way ANOVA with a Tukey’s post hoc comparison confirmed that metyrapone administration during CUS significantly blocked the enhanced 5-HT depletions after MDMA (Vehicle Stress/MDMA vs. Metyrapone Stress/MDMA, p<0.01). There were no significant main effects of metyrapone (F1,57 = 0.722, p>0.05), stress (F1,57 = 0.003, p>0.05), or MDMA (F1,57 = 1.223, p>0.05) or a pretreatment × stress × drug interaction (F1,57 = 3.53, p>0.05) on frontal cortex DA tissue concentrations (Fig. 6B).
Figure 6.
Effect of metyrapone (50 mg/kg) or vehicle (10% EtOH) during CUS on (A) 5-HT and (B) dopamine (DA) concentrations in the frontal cortex 5 days after administration of MDMA (5 mg/kg, i.p.; q 2 h × 4) or saline. * p<0.01 compared to saline controls, # p<0.01 compared to Vehicle No Stress/MDMA; & p<0.01 compared to Metyrapone Stress/MDMA. Values are expressed as means ± SEM; n = 6–11/group.
DISCUSSION
Ten days of unpredictable stress augmented the acute hyperthermic and long-term monoamine depleting effects of repeated MDMA administration. Although the acute CORT production was not enhanced, the hyperthermic effects and the long-term decreases in 5-HT tissue concentrations in the striatum, hippocampus, and frontal cortex produced by MDMA administration were potentiated by prior exposure to CUS. MDMA administration to naïve rats did not result in long-term decreases in DA tissue concentrations; however, MDMA administration to rats with prior exposure to CUS synergized to deplete striatal DA. Prevention of CUS-induced increases in CORT with metyrapone attenuated the enhancements in MDMA-induced hyperthermia as well as depletions in 5-HT and DA tissue concentrations. These findings suggest that the potentiation of both MDMA-induced hyperthermia and monoamine depletions by prior exposure to CUS was dependent on stress-induced elevated basal concentrations of CORT but independent of MDMA-induced increases in CORT.
Thirty days of CUS have been shown to increase basal concentrations of CORT in plasma as a result of the loss of negative feedback on the HPA axis (Herman et al., 1995). We have found that ten days of CUS also increased basal concentrations of CORT in plasma. Moreover, CORT synthesis inhibition by metyrapone during exposure to CUS blocked this enhancement (Fig. 1). This suggests that the elevated concentrations of CORT over the ten days of exposure determine the enhancement of MDMA-induced monoamine depletions by CUS. Metyrapone administration to non-stressed rats produced a slight but non-significant increase in basal plasma CORT (Fig. 1). While metyrapone has no effect on basal CORT when acutely administered to naïve rats (Calvo et al., 1998), there may be a compensatory response when administered chronically, although the effects of chronic metyrapone administration to naïve rats has not been investigated. In contrast to the findings with basal plasma CORT, an augmentation of an increase in CORT by the acute administration of MDMA was not observed in chronically-stressed rats (Table 1), indicating that the elevation of basal concentrations of CORT produced by CUS is a more important determinant of the enhanced monoamine depletions to MDMA.
Chronic CORT administration and chronic stress produce dendritic atrophy in the hippocampus and cortex (Wooley et al., 1990; Magarinos and McEwen, 1995a; Cook and Wellman, 2004). This effect is mediated by stress-induced CORT because inhibition of CORT synthesis blocks the atrophy associated with stress (Magarinos and McEwen, 1995b). However, Magarinos and McEwen (1995a) also found that 21 days, but not 14 days, of unpredictable stressors caused dendritic atrophy, suggesting that there is a threshold of CORT exposure necessary to produce this damage. We found that ten days of CUS alone did not produce long-term depletions of 5-HT or DA content in any of the brain regions examined (Table 2), thus indicating that the ability of CUS to enhance MDMA-induced monoamine depletions was not due to a pre-existing decrease in 5-HT or DA tissue concentrations. Therefore, other stress-related events may be responsible for an increase in the sensitivity of terminals to a subsequent exposure to MDMA.
Glucocorticoids have been shown to produce an energy-compromised and pro-oxidant environment, which may increase vulnerability to a subsequent insult. Chronic stress or chronic CORT exposure leads to decreased mitochondrial electron transport chain activity and glutathione levels (Madrigal et al., 2001), increased nitric oxide production (Olivenza et al., 2000), and decreased endogenous antioxidants enzymes copper/zinc superoxide dismutase and glutathione peroxidase activity (McIntosh et al., 1998). MDMA itself reduces endogenous antioxidants (Shankaran et al., 2001), increases hydroxyl radical production (Gudelsky and Yamamoto, 1994; Colado et al., 1997), inhibits mitochondrial function (Burrows et al., 2000), and causes ATP depletions (Darvesh and Gudelsky, 2005), suggesting that these pro-oxidant effects could be additive with the effects of chronic stress. This is supported by findings in neuronal culture which demonstrate that glucocorticoid application leads to accelerated hypoxia-induced ATP loss (Lawrence and Sapolsky, 1994) and increased adriamycin-induced reactive oxygen species accumulation and toxicity (McIntosh and Sapolsky, 1996). Furthermore, in vivo CORT administration decreases the capacity of neurons to survive co-incident insults by other neurotoxins such as kainic acid, and the ATP synthesis inhibitor 3-acetylpyridine (Sapolsky, 1985).
As CORT plays a role in stress-induced neuronal vulnerability and CUS enhances circulating CORT, we examined if stress enhances MDMA-induced monoamine depletions by augmenting MDMA-induced CORT concentrations. Acute MDMA administration leads to increases in plasma CORT (Nash et al., 1988; Table 1); however its role in MDMA-induced 5-HT depletions has been controversial. While Johnson et al. (1989) found that adrenalectomy attenuated MDMA-induced decreases in hippocampal tryptophan hydroxylase activity; others have shown that adrenalectomy did not block MDMA-induced depletions in 5-HT tissue concentrations in striatum, hippocampus, or frontal cortex (Aquirre et al., 1997; Fernandez et al., 2002). We found that MDMA significantly increased plasma CORT; however the increase was not augmented by prior exposure to CUS (Table 1) despite the finding that CUS augmented MDMA-induced monoamine depletions (Table 2). Metyrapone administration to chronically stress rats was also unable to alter MDMA-induced CORT (Table 1) despite the finding that it was able to prevent enhanced monoamine depletions such that 5-HT and DA concentrations approximated those of non-stressed rats treated with MDMA (Figs. 4, 5, and 6). These findings suggest that the enhanced monoamine depletions were not the result of a potentiation of MDMA-induced CORT but depend on the CUS-induced enhancement of basal CORT.
Several mechanisms contribute to MDMA-induced monoamine depletions of which hyperthermia may play an integral role in the augmented depletions in chronically stressed rats. MDMA-induced hyperthermia is correlated with MDMA-induced 5-HT depletions (Broening et al., 1995; Malberg and Seiden, 1998). In the present study, MDMA-induced increases in body temperature, along with 5-HT depletions, were enhanced in chronically stressed rats (Table 2 and Fig. 2). The enhanced hyperthermia (Fig. 3), as well as enhanced 5-HT and DA depletions, were prevented by the CORT synthesis inhibitor, metyrapone, administered prior to each stressor during the ten days of CUS (Figs. 4, 5, and 6). Recent findings from our laboratory demonstrated that preventing the enhancement of methamphetamine-induced hyperthermia by CUS blocks the enhancement of methamphetamine-induced depletions of DA and 5-HT produced by CUS (Tata et al., 2007). Therefore, enhanced hyperthermia can explain the exaggerated depletions of both 5-HT and DA observed 5 days after MDMA administration.
Stress-induced CORT likely mediates this ability of CUS to enhance MDMA-induced hyperthermia as metyrapone prevented both the augmentation of basal CORT and MDMA-induced hyperthermia. The interaction between the effects of CORT and 5-HT2A receptors may mediate this effect. Hypothalamic 5HT2A receptors contribute to temperature regulation in rats (Sheard and Aghajanian, 1967; Salmi and Ahlenius, 1998). Rats subjected to 16 days of chronic unpredictable stress have an increased 5-HT2A receptor density (Ossowska et al., 2001). Findings from our laboratory demonstrate that ten days of CUS results in a greater increase in rectal temperature to the systemic administration of the 5-HT2A/C receptor agonist ±1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane (Matuszewich and Yamamoto, 2003). Therefore, the enhancement of MDMA-induced hyperthermia after CUS may be mediated by a chronic stress-induced and CORT-dependent increase in 5-HT2A receptor density or function. Hypothalamic 5-HT2 receptors are also responsible for CORT regulation (Koenig et al., 1987), although we found enhanced MDMA-induced hyperthermia without a potentiation of CORT. This may be the result of a differential regulation of hyperthermia and CORT by 5HT2A receptors in subregions of the hypothalamus. It has been demonstrated that the 5-HT2 receptors in the preoptic area of the hypothalamus are responsible for temperature regulation (Lin et al., 1998) whereas 5-HT2 receptors in the paraventricular nucleus of the hypothalamus regulate CORT (Zhang et al., 2002). Future studies in our laboratory will directly investigate the expression of 5-HT2A receptors after CUS in these subregions of the hypothalamus.
An interesting finding of the current study is that prior exposure to CUS caused an MDMA-induced depletion of DA in the striatum (Table 2, Fig. 4B). Although long-term depletions of DA after MDMA have been reported in mice only (Logan et al., 1988; Granado et al., 2008), this is the first report of such a DA depletion in rats, a species that typically responds with a selective loss of 5-HT terminals after MDMA in a manner similar to that observed in non-human primates and humans (Stone et al., 1986; Ricaurte et al., 1988; Steele et al., 1994; Reneman et al., 2001). Based on the ability of chronic stress to create a pro-oxidant state (Madrigal et al., 2001; Olivenza et al., 2000; McIntosh et al., 1998), an enhanced neuronal sensitivity to oxidative stressors (Lawrence and Sapolsky, 1994; McIntosh and Sapolsky, 1996; Sapolsky, 1985), along with the particular vulnerability of DA neurons to oxidative stress (Gibb et al., 1989; Gotz et al., 1990, 1994) and excitotoxicity (Sonsalla et al.; 1989; 1998), it is reasonable to expect that regions with both DA and 5-HT terminals could be damaged by the serial exposure to CUS and MDMA. While this appears to be the case for the striatum (Table 2, Fig. 4B), no depletions of DA were observed in the frontal cortex (Table 2, Fig. 6B), although there does appear to be a slight trend, it does not reach significance. Terminal degeneration can occur via oxidative stress, which can be produced by DA-derived reactive oxygen species and quinones (Cohen, 1987). Consequently, the cortex may be less vulnerable to the detrimental effects of DA-associated free radicals because it contains lower concentrations of DA than the striatum (Mechanick et al., 1987, current study). In fact, methamphetamine produces relatively less of an increase in extracellular DA (Kashihara et al., 1991), a reduced level of oxidative stress (Gluck et al., 2001), as well as minimal DAergic damage (Ricaurte et al., 1980;Stephans et al., 1998) in the frontal cortex compared to the striatum, suggesting that DA neurons in the frontal cortex may be relatively resistant to the effects of DA-derived oxidative stress.
While prior exposure to CUS did not produce a MDMA-induced DA depletion in the frontal cortex, 5-HT depletions were enhanced, suggesting that while DA terminals in this region may be refractory to the effects of serial exposure to CUS and MDMA, 5-HT terminals remain vulnerable. In the striatum, MDMA increases DA release and causes DA-dependent 5-HT depletions (Yamamoto and Spanos, 1988; Shankaran et al., 1999); however the impact of DA-derived oxidative stress on MDMA-induced 5-HT depletions in the cortex is unknown. Based on the previous findings using methamphetamine and the current study on MDMA, a CUS-induced increase in DA-derived oxidative stress may not be the sole cause of enhanced 5-HT depletions in the frontal cortex. However, it remains to be seen if DA innervation can be predictive of 5-HT terminal vulnerability. Studies determining the impact of CUS on MDMA-induced DA release and free radical production are warranted.
We predicted that the hippocampus and frontal cortex may be more susceptible to serial exposure of CUS and MDMA based on the high density of glucocorticoid receptors (Reul and de Kloet, 1985), their vulnerability to chronic stress (Magarinos and McEwen, 1995; Cook and Wellman, 2004), as well as the more pronounced and long-lasting 5-HT depletions produced by MDMA in these regions (Sabol et al., 1996). However, both regions and the striatum appeared equally susceptible to MDMA-induced enhancements in 5-HT depletions after prior exposure to CUS (Table 2, Figs. 4A, 5, and 6A). Rats exposed to the combination of CUS and MDMA exhibited a greater than 80% depletion in tissue 5-HT content in all brain regions studied. The inability to detect regional differences may be due to a maximal depletion of 5-HT produced by the combined effects of CUS and MDMA. In fact, very high repeated doses of MDMA (40 mg/kg, i.p., every 12 h for 4 days) resulted in no more than an 80% loss of tissue 5-HT in multiple brain regions (Commins et al., 1987). Lower doses of MDMA may reveal regional differences in 5-HT depletions after CUS and MDMA. Nonetheless, the current results demonstrate that prior exposure to CUS does enhance MDMA-induced depletions of 5-HT in multiple brain regions including the striatum, hippocampus, and frontal cortex.
It is well known that chronic stress facilitates behavioral responses to psychostimulants, such as producing enhancements in locomotor activity, self-administration, and reinstatement of drug-seeking behavior (Marinelli and Piazza, 2002). The present study extends the influence of chronic stress on the effects of drugs of abuse and demonstrates for the first time that prior exposure to CUS also enhances the long-term monoamine depleting effects of the highly abused psychostimulant MDMA in the rat. While the precise mechanisms by which chronic stress mediates the increase in MDMA-induced monoamine depletions remain unknown, our studies demonstrate that chronic stress-induced increases in circulating CORT and the subsequent enhancement in MDMA-induced hyperthermia play a role in enhanced 5-HT depletions. Moreover, the current findings provide evidence that the interaction between chronic stress and MDMA is more pervasive and can also affect DA terminals. Given the role of 5-HT in mood, anxiety, sleep, appetite, and cognition and based on the co-morbidity of stress and drug abuse (Koob and LeMoal, 2001), these findings highlight the importance of examining MDMA abuse and its long-term consequences within the context of stressful life situations.
Acknowledgments
This work was supported by National Institutes of Health grant DA016866.
Abbreviations
- ANOVA
analysis of variance
- DA
dopamine
- 5-HT
serotonin
- CORT
corticosterone
- CUS
chronic unpredictable stress
- HPCL-EC
high performance liquid chromatography with electrochemical detection
- MDMA
3,4-methylenedioxymethamphetamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre N, Frechilla D, Garcia-Osta A, Lasheras B, Del Rio J. Differential regulation by methylenedioxymethamphetamine of 5-hydroxytryptamine1A receptor density and mRNA expression in rat hippocampus, frontal cortex, and brainstem: the role of corticosteroids. J Neurochem. 1997;68:1099–1105. doi: 10.1046/j.1471-4159.1997.68031099.x. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Yeh SY, O’Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]-paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- Broening HW, Bowyer JF, Slikker W., Jr Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (±)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- Burrows KB, Gudelsky G, Yamamoto BK. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur J Pharm. 2000;398:11–18. doi: 10.1016/s0014-2999(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Calvo N, Martijena ID, Molina VA, Volosin M. Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Res. 1998;800:227–235. doi: 10.1016/s0006-8993(98)00515-0. [DOI] [PubMed] [Google Scholar]
- Cohen G. Monoamine oxidase, hydrogen peroxide, and Parkinson’s disease. Adv Neurol. 1987;45:119–125. [PubMed] [Google Scholar]
- Colado MI, O’Shea E, Granados R, Murray TK, Green AR. In vivo evidence for free radical involvement in the degeneration of rat brain 5-HT following administration of MDMA (‘ecstasy’) and p-chloroamphetamine but not the degeneration following fenfluramine. Br J Pharmacol. 1997;121:889–900. doi: 10.1038/sj.bjp.0701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins DL, Yosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS. Biochemical and histological evidence that methylenedioxymethamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther. 1987;241:338–345. [PubMed] [Google Scholar]
- Cook MI, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Gudelsky GA. Evidence for a role of energy dysregulation in the MDMA-induced depletion of brain 5-HT. Brain Res. 2005;1056:168–175. doi: 10.1016/j.brainres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- DeRoche V, Marinelli M, Maccari S, LeMoal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids: I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharm. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Aguerre S, Mormede P, Chaouloff F. Influences of the corticotrophic axis and sympathetic activity on neurochemical consequences of 3,4-methylenedioxymethamphetamine (MDMA) administration in Fischer 344 rats. Eur J Neurosci. 2002;16:607–618. doi: 10.1046/j.1460-9568.2002.02110.x. [DOI] [PubMed] [Google Scholar]
- Gibb JW, Johnson M, Hanson GE. Neurochemical basis of neurotoxicity. Neurotoxicology. 1990;11:317–321. [PubMed] [Google Scholar]
- Gluck MR, Moy LY, Jayatilleke E, Hogan KA, Manzino L, Sonsalla PK. Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Neurochem. 2001;79:152–160. doi: 10.1046/j.1471-4159.2001.00549.x. [DOI] [PubMed] [Google Scholar]
- Gotz ME, Freyberger A, Riederer P. Oxidative stress: a role in the pathogenesis of Parkinson’s disease. J Neural Transm Suppl. 1990;29:241–249. doi: 10.1007/978-3-7091-9050-0_23. [DOI] [PubMed] [Google Scholar]
- Gotz ME, Kunig F, Riederer P, Youdim MBH. Oxidative stress: free radical production in neural degeneration. Pharmacol Ther. 1994;63:37–122. doi: 10.1016/0163-7258(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Granado N, O’Shea E, Bove J, Vila M, Colado MI, Moratalla R. Persistent MDMA-induced dopaminergic neurotoxicity in the striatum and substantia nigra of mice. J Neurochem. 2008;107:1102–1112. doi: 10.1111/j.1471-4159.2008.05705.x. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. MDMA increases the extracellular concentration of 2,3-dihydrobenzoic acid in the striatum: evidence for increased hydroxyl radical formation. Abs Soc Neurosci. 1994;20:1026. [Google Scholar]
- Haile CN, GrandPre T, Kotsen TA. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharm. 2001;154:213–220. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccar S, LeMoal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Herman JP, Sinus L, LeMoal M. Repeated stress increases locomotor response to amphetamine. Psychopharm. 1984;84:431–435. doi: 10.1007/BF00555227. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendo. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The stress hormone system is back on the map. Curr Psychiatry Rep. 2000;2:454–456. doi: 10.1007/s11920-000-0001-y. [DOI] [PubMed] [Google Scholar]
- Johnson M, Stone DM, Bush LG, Hanson GR, Gibb JW. Glucocorticoids and 3,4-methylenedioxymethamphetamine (MDMA)-induced neurotoxicity. Eur J Pharm. 1989;161:181–188. doi: 10.1016/0014-2999(89)90841-8. [DOI] [PubMed] [Google Scholar]
- Kashihara K, Hamamura T, Okumura K, Otsuki S. Methamphetamine-induced dopamine release in the medial frontal cortex of freely moving rats. Jpn J Psychiatry Neurol. 1991;45:677–680. doi: 10.1111/j.1440-1819.1991.tb01190.x. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Gudelsky GA, Meltzer HY. Simulation of corticosterone and beta-endorphin secretion in the rat by selective 5-HT receptor subtype activation. Eur J Pharmacol. 1987;137:1–8. doi: 10.1016/0014-2999(87)90175-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharm. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Sapolsky RM. Glucocorticoids accelerate ATP loss following metabolic insults in cultured hippocampal neurons. Brain Res. 1994;646:303–306. doi: 10.1016/0006-8993(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Logan BJ, Laverty R, Sanderson WD, Yee YB. Differences between rats and mice in MDMA (methylenedioxymethamphetamine) neurotoxicity. Eur J Pharmacol. 1988;152:227–234. doi: 10.1016/0014-2999(88)90717-0. [DOI] [PubMed] [Google Scholar]
- Lin MT, Tsay HJ, Su WH, Chueh FY. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am J Physiol. 1998;274:R1260–1267. doi: 10.1152/ajpregu.1998.274.5.R1260. [DOI] [PubMed] [Google Scholar]
- Madrigal JLM, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharm. 2001;24:420–429. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparisons of stressors. Neurosci. 1995a;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neurosci. 1995b;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress, and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Long-lasting effects of chronic stress on DOI-induced hyperthermia. Psychopharm. 2003;169:169–175. doi: 10.1007/s00213-003-1498-7. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neurosci. 2004;124:637–646. doi: 10.1016/j.neuroscience.2003.12.007. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Ann NY Acad Sci. 1998;840:33–41. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McIntosh JL, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exper Neurol. 1996;141:201–206. doi: 10.1006/exnr.1996.0154. [DOI] [PubMed] [Google Scholar]
- McIntosh JL, Hong KE, Sapolsky RM. Glucocorticoids may alter antioxidant enzyme capacity in the brain: baseline studies. Brain Res. 1998;791:209–214. doi: 10.1016/s0006-8993(98)00115-2. [DOI] [PubMed] [Google Scholar]
- Mechanick JI, Cohen-Becker IR, Gregerson KA, Selmanoff M. Distribution of 3,4-dihydroxyphenylacetic acid (DOPAC) and 3,4-dihydroxyphenylglycol (DOPEG) in microdissected brain structures and the pituitary gland: metabolite changes in the median eminence in response to hyperprolactinemia and suckling. J Neural Transm. 1987;68:197–215. doi: 10.1007/BF02098498. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Gastfriend DR, Barber JP, Reif S, Muenz LR, Blaine J, Frank A, Crits-Christoph P, Thase M, Weiss RD. Cocaine dependence with and without PTSD among subjects in the National Institute of Drug Abuse Collaborative Cocaine Treatment Study. Am J Psychiatry. 1998;155:214–219. doi: 10.1176/ajp.155.2.214. [DOI] [PubMed] [Google Scholar]
- Nash JF, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988;245:873–879. [PubMed] [Google Scholar]
- Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Fernandez AP, Rodrigo J, Bosca L, Leza JC. Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J Neurochem. 2000;74:785–791. doi: 10.1046/j.1471-4159.2000.740785.x. [DOI] [PubMed] [Google Scholar]
- Ossowska G, Nowak G, Kata R, Klenk-Majewska, Danilczuk Z, Zebrowska-Lupina I. Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm. 2001;108:311–319. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Piazza PV, LeMoal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Reneman L, Booij J, de Bruin K, Reitsma JB, de Wolff FA, Boudewijn Gunning W, den Heeten GJ, van den Brink W. Effects of dose, sex and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet. 2001;358:1864–1869. doi: 10.1016/S0140-6736(01)06888-X. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems of corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rhoads DL. A longitudinal study of life stress and social support among drug abusers. Int J Addict. 1983;18:195–222. doi: 10.3109/10826088309027352. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, Langston JW. (±)3,4-methylenedioxymethamphetamine selectively damages central serotonergic neurons in non-human primates. J Am Med Assoc. 1988;206:51–55. [PubMed] [Google Scholar]
- Sabol KE, Lew R, Richards JB, Vosmer GL, Seiden LS. Methylenedioxymethamphetamine-induced serotonin deficits are followed by partial recovery over a 52-week period. Part I: synaptosomal uptake and tissue concentrations. J Pharmacol Exp Ther. 1996;276:846–854. [PubMed] [Google Scholar]
- Salmi P, Ahlenius S. Evidence for functional interactions between 5-HT1A and 5-HT2A receptors in rat thermoregulatory mechanisms. Pharm Toxicol. 1998;82:122–127. doi: 10.1111/j.1600-0773.1998.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. A mechanism for glucocorticoid toxicity in the hippocampus: increased neuronal vulnerability to metabolic insults. J Neurosci. 1985a;5:1228–1232. doi: 10.1523/JNEUROSCI.05-05-01228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoid toxicity in the hippocampus: temporal aspects of neuronal vulnerability. Brain Res. 1985b;359:300–305. doi: 10.1016/0006-8993(85)91440-4. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, MDMA. J Pharmacol Exp Ther. 1987;240:1–7. [PubMed] [Google Scholar]
- Schmidt CJ, Taylor VL. Depression of rat brain tryptophan hydroxylase activity following the acute administration of methylenedioxymethamphetamine. Biochem Pharmacol. 1987;36:4095–5102. doi: 10.1016/0006-2952(87)90566-1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharm. 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Yamamoto BK, Gudelsky GA. Mazindol attenuates the 3,4-methylenedioxymethamphetamine-induced formation of hydroxyl radicals and long-term depletion of serotonin in the striatum. J Neurochem. 1999;72:2516–2522. doi: 10.1046/j.1471-4159.1999.0722516.x. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Yamamoto BK, Gudelsky GA. Ascorbic acid prevents 3,4-methylenedioxymethamphetamine (MDMA)-induced hydroxyl radical formation and the behavioral and neurochemical consequences of the depletion of brain 5-HT. Synapse. 2001;40:55–64. doi: 10.1002/1098-2396(200104)40:1<55::AID-SYN1026>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sheard MH, Aghajanian GK. Neural release of brain serotonin and body temperature. Nature. 1967;216:495–496. doi: 10.1038/216495a0. [DOI] [PubMed] [Google Scholar]
- Sonino N. Inhibition of adrenal steroid biosynthesis by metyrapone. In: Agarwal MK, editor. Hormone antagonists. Berlin: Walter de Gruyter & Co; 1982. pp. 419–429. [Google Scholar]
- Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Albers DS, Zeevalk GD. Role of glutamate in neurodegeneration of dopamine neurons in several models of parkinsonism. Amino Acids. 1998;14:69–74. doi: 10.1007/BF01345245. [DOI] [PubMed] [Google Scholar]
- Steele TD, McCann UD, Ricaurte GA. 3,4-metyylenedioxymethamphetamine (MDMA, “Ecstasy”): pharmacology and toxicology in animals and humans. Addiction. 1994;89:539–551. doi: 10.1111/j.1360-0443.1994.tb03330.x. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Effect of repeated methamphetamine administrations on dopamine and glutamate efflux in rat prefrontal cortex. Brain Res. 1995;700:99–106. doi: 10.1016/0006-8993(95)00938-m. [DOI] [PubMed] [Google Scholar]
- Stone DM, Stahl DC, Hanson GR, Gibb JW. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur J Pharmacol. 1986;128:41–48. doi: 10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- Stone DM, Merchant KM, Hanson GR, Gibb JW. Immediate and long-term effects of 3,4-methylenedioxymethamphetamine on serotonin pathways in brain of rat. Neuropharm. 1987;26:1677–1683. doi: 10.1016/0028-3908(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Tata DA, Raudensky J, Yamamoto BK. Augmentation of methamphetamine-induced toxicity in rat striatum by unpredictable stress: contribution of enhanced hyperthermia. Eur J Neurosci. 2007;26:739–748. doi: 10.1111/j.1460-9568.2007.05688.x. [DOI] [PubMed] [Google Scholar]
- Woolley CE, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Spanos LJ. The acute effects of methylenedioxymethamphetamine on dopamine release in the awake-behaving rat. Eur J Pharmacol. 1988;148:195–203. doi: 10.1016/0014-2999(88)90564-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Damjanoska KJ, Carrasco GA, Dudas B, D’Souza DN, Tetxlaff J, Garcia F, Hanley NR, Scripathirathan K, Peterson BR, Gray TS, Battaglia G, Muma NA, Van de Kar LD. Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (-)DOI. J Neurosci. 2002;22:9635–9642. doi: 10.1523/JNEUROSCI.22-21-09635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]