Abstract
Bioorthogonal tetrazine cycloadditions have been applied to live cell labeling. Tetrazines react irreversibly with the strained dienophile norbornene forming dihydropyrazine products and dinitrogen. The reaction is high yielding, selective, and fast in aqueous media. Her2/neu receptors on live human breast cancer cells were targeted with a monoclonal antibody modified with a norbornene. Tetrazines conjugated to a near-infrared fluorochrome selectively and rapidly label the pretargeted antibody in the presence of serum. These findings indicate that this chemistry is suitable for in vitro labeling experiments, and suggests that it may prove a useful strategy for in vivo pretargeted imaging under numerous modalities.
Bioorthogonal reactions for coupling materials in the presence of complex biological milieu are of great interest in biology and medicine. Such reactions have become key components in a variety of applications including protein engineering (1, 2), immunoassay development (3), and cell surface modification (4,5). To date, only a few bioorthogonal reactions have been reported, the most popular being the Staudinger ligation and the [3 + 2] cycloaddition “click” reaction between azides and alkynes (6, 7). Here, we report on the use of [4 + 2] Diels–Alder cycloadditions between a tetrazine and olefin as an alternative bioorthogonal reaction. The reaction is extremely selective, high yielding, and proceeds rapidly in aqueous media. The reaction partners show excellent stability in biological media and are simple to synthesize. The utility of this reaction is demonstrated by the specific labeling of Her2/neu receptors on breast cancer cells.
Recently, there has been tremendous interest in the use of the click reaction for biological labeling. The typical click reaction involves copper(I)-catalyzed coupling of an azide and terminal alkyne to generate a stable triazole (7). Until recently, the necessity of the copper catalyst precluded the use of this reaction in biological systems due to concerns regarding toxicity. Bertozzi and others have elegantly solved this problem by developing several new ring-strained cyclooctyne derivatives that do not require a catalyst (4, 8, 9). However, many of these derivatives have poor water solubility or require complex multistep synthesis and are not readily obtainable in large quantities. Despite these shortcomings, the cycloaddition reaction between azides and ring-strained cyclooctynes has been employed for imaging of cells (4) and zebra fish embryos (10).
Our search for alternative rapid, selective, and chemically accessible coupling reactions that do not require a catalyst led us to investigate the [4 + 2] Diels–Alder cycloaddition. Not only is the Diels–Alder reaction compatible with aqueous environments, but the second-order rate constants for this reaction are known to be enhanced up to several hundred-fold in aqueous media in comparison to organic solvents (11, 12). Many Diels–Alder reactions are reversible (13) and therefore may not be suitable for biological labeling. The inverse electron demand Diels–Alder cycloaddition of olefins with tetrazines, however, results in irreversible coupling, giving dihydropyridazine products (Scheme 1). During this reaction, dinitrogen is released in a retro Diels–Alder step (14). A variety of tetrazines (15) and dienophiles including cyclic and linear alkenes or alkynes (16) have been studied in this reaction. Selection of the appropriate reaction partners allows for tuning of the coupling rate by several orders of magnitude (15, 16).
Scheme 1.
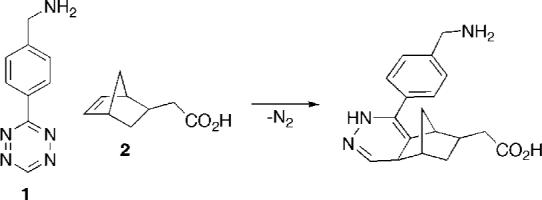
To probe the feasibility of the tetrazine–dienophile reaction as a tool for biological labeling, a modified norbornene (2) was selected as a model dienophile. Norbornenes offer an excellent balance between facile strain-promoted reactivity with tetrazines and overall chemical stability. Furthermore, a selection of norbornenes with additional conjugation handles are commercially available. In contrast, few tetrazines containing additional reactive groups have been reported. One potential starting point, dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate, has been investigated extensively, but is not stable in aqueous or protic media (17). Other amine-modified tetrazines such as 1,2,4,5-tetrazine-3,6-diamine (18) or 3,6-bis-(4-aminophenyl)-1,2,4,5-tetrazine (19) have been described, but have poor reactivity with dienophiles. In this communication, we detail the synthesis of a stable benzylamine-modified tetrazine with excellent dienophile reactivity and investigate its use in the bioorthogonal pretargeting of live cells.
The tetrazine, 3-(4-benzylamino)-1,2,4,5-tetrazine (1), is prepared by reaction of 4-(aminomethyl)benzonitrile with formamidine acetate and anhydrous hydrazine in the presence of elemental sulfur. The initial dihydrotetrazine product is oxidized to the tetrazine by treatment with sodium nitrite in acetic acid in 20% overall yield. The primary amine can be modified via standard amide-coupling procedures to prepare the near-infrared (NIR) fluorophore-modified conjugate, tetrazine-VT680 (3).
Tetrazine 1, shows excellent stability in PBS buffer with little or no observed decomposition after prolonged standing at room temperature (data not shown). The stability of 1 in biological media is also very good, with only 15% decomposition observed after 15 h in fetal bovine serum (FBS) at 20.0 °C. Since both tetrazine 1 and norbornene 2 are asymmetric compounds, the Diels–Alder cycloaddition of these two components generates multiple isomeric dihydropyridazine products (Scheme S1 and Figure S5, Supporting Information) Analysis of the reaction products by LCMS indicates a clean reaction with >93% conversion to the dihydropyridazines. Tetrazine 1 reacts smoothly and rapidly with norbornene 2 in aqueous buffer and FBS with second-order rate constants of 1.9 and 1.6 M−1 s−1, respectively. The use of more reactive tetrazines and highly strained dienophiles such as cycloctynes will likely increase coupling rate constants by several orders of magnitude, and we are currently exploring these strategies (20).
Labeling of specific functional groups on live cells has emerged as a powerful application of bioorthogonal couplings. To demonstrate this, the tetrazine-based cycloaddition reaction was used to label antibodies bound to Her2/neu receptors on live SKBR3 cancer cells. Such strategies are of interest, because although antibodies show great specificity for antigens, their long blood half-lives result in high background signals when directly labeled with imaging agents or cytotoxins. Pretargeting offers the opportunity for un- or nonspecifically bound antibody to clear before chasing with a smaller, readily excreted molecule that selectively couples to the remaining antibody, drastically reducing background (21). Previous pretargeting strategies primarily have relied on the interaction between biotin and avidins to achieve specificity (22). Although these approaches have shown promise in the clinic, they suffer from problems such as immunogenicity and steric crowding resulting from the >50 kDa size of the avidins. A small molecule based pretargeting strategy may circumvent these problems.
To demonstrate the utility of the tetrazine–dienophile reaction for live-cell labeling, we used the FDA-approved monoclonal antibody trastuzumab (Herceptin) which binds to Her2/neu growth factor receptors (Figure 1) (23). Trastuzimab was simultaneously labeled with norbornene and tetramethyl rhodamine using standard coupling conditions. Human breast cancer SKBR3 cells, which overexpress Her2/neu, were incubated with 200 nM antibody, and then treated with 50 μM 3 in 10% FBS/HBSS solution for 30 min at 37 °C. After washing, the cells were imaged using both rhodamine and NIR fluorescence channels (Figure 2A,B). Significant labeling, which colocalizes, was observed (Figure 2C). Cells incubated with a control antibody, which contained rhodamine but not norbornene, showed no NIR labeling after exposure to 3 (Figure 2D and E). These experiments demonstrate the specificity of the tetrazine imaging agent for norbornene-modified antibody in the presence of live cells and serum. The reaction is rapid even with micromolar concentrations of the labeling agent and importantly in the presence of serum. These findings clearly indicate that this chemistry is suitable for in vitro experiments and suggests that it may prove a useful strategy for in vivo imaging under numerous modalities.
Figure 1.
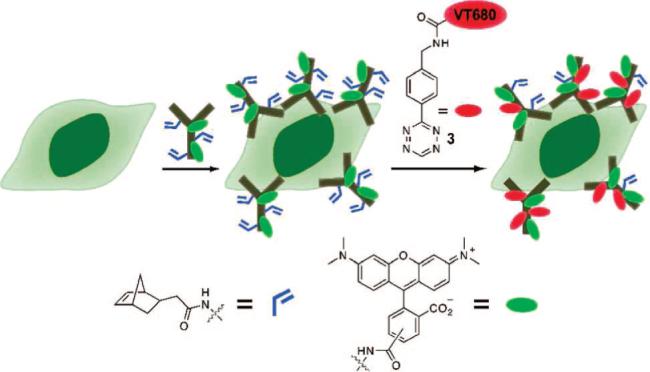
Pretargeting of SKBR3 cells with norbornene and tetramethylrhodamine co-labeled trastuzumab, followed by tagging the live cells with tetrazine-VT680 (3) via an inverse electron demand Diels–Alder coupling.
Figure 2.
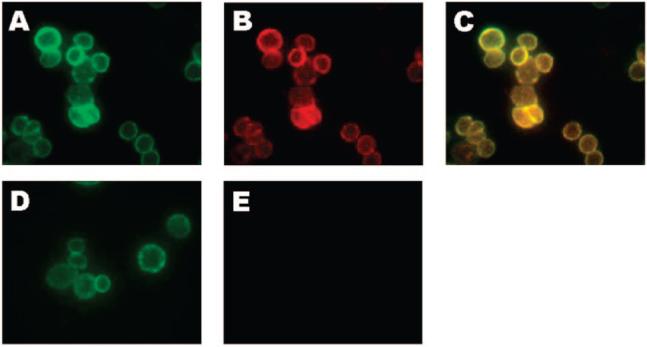
Fluorescent microscope images of SKBR3 human breast cancer cells after pretargeting trastuzumab antibodies modified with rhodamine and norbornene and subsequent labeling with 3. (A) Rhodamine channel. (B) Near-IR channel (tetrazine-VT680). (C) Merged image of A and B. Images were also taken of cell surfaces targeted with a control antibody modified with only rhodamine and exposed subsequently to 3. (D) Rhodamine channel. (E) Near-IR channel.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Drs. Fangwei Shao and Jered Haun for their assistance in the preparation of this manuscript. This research was supported in part by NIH grants U01-HL080731 and T32 – CA 79443.
Footnotes
Supporting Information Available: Detailed synthetic and cell labeling procedures, kinetic data, and full characterization for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Link JA, Mock ML, Tirrell DA. Non-canonical amino acids in protein engineering. Curr. Opin. Biotechnol. 2003;14:603–609. doi: 10.1016/j.copbio.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 3.Diamandis EP, Christopoulos TK. The biotin-(strept)avidin system: principles and applications in biotechnology. Clin. Chem. 1991;37:625–636. [PubMed] [Google Scholar]
- 4.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Link JA, Tirrell DA. Cell surface labeling of Escherichia coli via copper(I)-catalyzed [3 + 2] cycloaddition. J. Am. Chem. Soc. 2003;125:11164–11165. doi: 10.1021/ja036765z. [DOI] [PubMed] [Google Scholar]
- 6.Prescher JA, Dube DH, Bertozzi CR. Chemical remodelling of cell surfaces in living animals. Nature. 2004;430:873–877. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- 7.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper (l)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem., Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. Second-generation difluorinated cyclooctynes for copper-free click chemistry. J. Am. Chem. Soc. 2008;130:11486–11493. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ning XH, Guo J, Wolfert MA, Boons GJ. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast huisgen cycloadditions. Angew. Chem., Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rideout DC, Breslow R. Hydrophobic acceleration of Diels-Alder reactions. J. Am. Chem. Soc. 1980;102:7816–7817. [Google Scholar]
- 12.Graziano G. Rate enhancement of Diels-Alder reactions in aqueous solutions. J. Phys. Org. Chem. 2004;17:100–101. [Google Scholar]
- 13.Kwart H, King K. The reverse Diels-Alder or retrodiene reaction. Chem. Rev. 1968;68:415–447. [Google Scholar]
- 14.Sauer J, Mielert A, Lang D, Peter D. Umsetzungen von 1.2.4.5-tetrazinen mit olefinen. zur struktur von dihydropyridazinen. Chem. Ber. 1965;98:1435–1445. [Google Scholar]
- 15.Balcar J, Chrisam G, Huber FX, Sauer J. Reaktivität von stickstoff-heterocyclen gegenüber cyclooctin als dienophil. Tetrahedron Lett. 1983;24:1481–1484. [Google Scholar]
- 16.Thalhammer F, Wallfahrer U, Sauer J. Reaktivität einfacher offenkettiger und cyclisher dienophile bei Diels-Alder-reaktionen mit inversem elektronenbedarf. Tetrahedron Lett. 1990;47:6851–6854. [Google Scholar]
- 17.Kämpchen T, Massa W, Overheu W, Schmidt R, Seitz G. Zur kenntnis von reaktionen des 1,2,4,5-tetrazin-3,6-dicarbonsäure-dimethylesters mit nucleophilen. Chem. Ber. 1982;115:683–694. [Google Scholar]
- 18.Lin C-H, Lieber E, Horwitz JP. The synthesis of sym-diaminotetrazine. J. Am. Chem. Soc. 1954;76:427–430. [Google Scholar]
- 19.Soloducho J, Doskocz J, Cabaj J, Roszak S. Practical synthesis of bis-substituted tetrazines with two pendant 2-pyrrolyl or 2-thienyl groups, precursors of new conjugated polymers. Tetrahedron. 2003;59:4761–4766. [Google Scholar]
- 20.During preparation of this manuscript the use of a cycloaddition reaction between a tetrazine and a strained trans-cyclooctene was reported for labeling a small protein: Blackman ML, Royzen M, Fox JM. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels–Alder reactivity. J. Am. Chem. Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805.
- 21.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J. Clin. Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 23.Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C, Shepard HM. Differential responses of human tumor-cell lines to anti-P185(Her2) monoclonal-antibodies. Cancer Immunol. Immun. 1993;37:255–263. doi: 10.1007/BF01518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


