Abstract
Ideomotor apraxia is a disorder mainly of praxis planning, and the deficit is typically more evident in pantomiming transitive (tool related) than intransitive (communicative) gestures. The goal of the present study was to assess differential hemispheric lateralization of praxis production using event-related functional magnetic resonance imaging. Voxel-based analysis demonstrated significant activations in posterior parietal cortex (PPC) and premotor cortex (PMC) association areas, which were predominantly left hemispheric, regardless of whether planning occurred for right or left hand transitive or intransitive pantomimes. Furthermore, region of interest–based calculation of mean laterality index (LI) revealed a significantly stronger left lateralization in PPC/PMC clusters for planning intransitive (LI = −0.49 + 0.10, mean + standard deviation [SD]) than transitive gestures (−0.37 + 0.08, P = 0.02, paired t-tests) irrespective of the hand involved. This differential left lateralization for planning remained significant in PMC (LI = −0.47 + 0.14 and −0.36 + 0.13, mean + SD, P = 0.04), but not in PPC (−0.56 + 0.11 and −0.45 + 0.12, P = 0.11), when both regions were analyzed separately. In conclusion, the findings point to a left-hemispheric specialization for praxis planning, being more pronounced for intransitive gestures in PMC, possibly due to their communicative nature.
Keywords: intransitive, left lateralization, praxis planning, transitive
Introduction
Left-hemispheric lateralization of praxis is well known in clinical neurology, and knowledge about it is based on lesion studies in apraxic patients with stroke and neurodegenerative disorders. It was already postulated in the early 20th century by Hugo Liepmann based on his studies of stroke patients who had apraxia mainly with left-hemispheric lesions (reviewed in Goldenberg 2003). The left lateralization of praxis was suggested by several clinical studies since then (Geschwind 1965; Alexander et al. 1992; Schnider et al. 1997; Haaland et al. 2000). Further indication for left-hemispheric dominance of limb praxis derives from callosotomy patients who demonstrate apraxic errors with only their left hand (Watson and Heilman 1983; Lausberg et al. 2003) because the right hemisphere is disconnected from left-hemispheric movement representation. Ideomotor apraxia is a higher order motor disorder characterized by a predominant deficit of gesture planning as patients show profound difficulties in learning skilled movements (Sunderland and Sluman 2000). The particular planning deficit is further indicated by the fact that pantomime of gestures is typically more affected than is their imitation. In addition, performance of gestures usually significantly improves with feedback-driven actual tool use (Goldenberg et al. 2004). Moreover, clinical studies in stroke patients suggest that particular planning of gestures may be lateralized to the left hemisphere because impaired programming motor sequences were more common in left-hemispheric stroke patients, notably if they were apraxic (Harrington and Haaland 1992).
There is evidence that left lateralization of praxis planning might depend on gesture subtype involved, that is, whether gestures are tool related (transitive) or communicative (intransitive) in nature. However, the findings are not entirely consistent. For instance, based on the analysis of praxis errors in left and right hemisphere lesioned patients, left dominance has been suggested for transitive but not intransitive gestures (Haaland and Flaherty 1984; Heath et al. 2001). By contrast, in another study, similar left lateralization for transitive and intransitive gestures has been found, that is, both gesture types were more affected in left than right hemisphere stroke (Hanna-Pladdy et al. 2001), with the performance of transitive gestures being generally more impaired.
The development of event-related functional magnetic resonance imaging (fMRI) allows addressing cognitive aspects of limb praxis (Buccino et al. 2004; Johnson-Frey et al. 2005; Fridman et al. 2006; Hermsdorfer et al. 2007). These studies identified parietal and premotor networks in planning and executing praxis movements, mostly focusing on tool-use pantomime. Electroencephalography (EEG) coherence studies demonstrated that activation of these networks underlying EEG synchronization began as early as 3 s before onset (Wheaton, Shibasaki, and Hallett 2005; Wheaton, Yakota, and Hallett 2005) and, in fact, specifically for praxis, not in preparation of simple movements (e.g., thumb adduction) (Wheaton, Nolte, et al. 2005).
Although progress has been made in determining the neural basis of praxis within the hemispheres, the relationship of the 2 hemispheres remains less clear. Therefore, we extended the scope by assessing, in healthy controls, hemispheric lateralization of praxis movements for both hands separately with special emphasis on planning transitive and intransitive gestures using event-related fMRI. Differential hemispheric dominance was assessed by calculating the laterality index (LI), an approach that has been widely used in motor and language studies (Cramer et al. 1997; Carey et al. 2004; Seghier et al. 2004) and has the advantage of controlling for individual differences in absolute extent of activated brain volumes. We hypothesized that fMRI activity during planning of praxis movements is left lateralized in posteroparietal premotor networks, particularly for transitive gestures.
Materials and Methods
Subjects
The study protocol was approved by the Institutional Review Board of National Institute of Neurological Disorders and Stroke, and informed written consent was obtained from all healthy volunteers. Fifteen subjects (9 men, age range 34–72 years) participated in the study; all were right handed, as assessed by the Edinburgh inventory (Oldfield 1971).
Experimental Procedures
Pantomime Task
We employed a modified instructed delay paradigm for the pantomime task as described previously (Fridman et al. 2006). Accordingly, each run started with the rest instruction followed by a transitive (e.g., show me scissors) or intransitive movement (wave good-bye) command for 2.5 s. After a 6-s planning phase, a “do it” command followed for 1.5 s, leading to an execution phase of 3 s, which, if repetitive, allowed the subjects to perform the movements 2 or 3 times. For nonrepetitive movements (e.g., the “victory sign”), the gesture was held for 3 s until the “rest” command (1.5 s) initiated a 15.5-s rest period. Between commands, a fixation cross was presented. Transitive and intransitive gestures were balanced for repetitive and nonrepetitive as well as proximal and distal movements.
The subjects were asked by visual presentation of written commands through a fiber optic goggle system (Avotec, Stuart, FL) to pantomime randomly 20 transitive and 20 intransitive gestures with their right and left hand in 4 runs, separately. Both gesture subtypes were intermixed within a run. Each command was presented twice. Within a run, only either left or right hand pantomimes were tested. Most pantomimes were distal in nature. If involving more proximal movement (e.g., “paint a wall” or “wave good-bye”), the subjects were asked to perform them mainly with the forelimb to avoid head motion. Performance of gestures and possible mirror movements were monitored by video.
Functional magnetic resonance imaging
fMRI data were collected using a 3-T magnetic resonance imaging (MRI) scanner (Signa, General Electric, Milwaukee, WI) and a standard head coil. Subjects lay supine in the MR scanner and their arms rested beside their trunk. Head motion was reduced by foam pads around the participants’ head. A T2*-weighted gradient echo single-shot echoplanar imaging (EPI) sequence (time echo [TE] = 50 ms, time repetition [TR] = 500 ms, flip angle = 90, field of view = 22 × 22 cm, matrix = 64 × 64) was used to obtain functional images sensitive to blood oxygen level–dependent (BOLD) signal. Each image volume consisted of 22 interleaved 5-mm thick slices. A time course series of 240 volumes was acquired for each trial. The first 4 volumes of each session were discarded to allow for T1 equilibration effects. High-resolution T1-weighted structural images were also acquired (128 slices, TR = 33 ms, TE = 4 ms, flip angle = 25 degrees, matrix = 25 × 192).
Data Analysis
Planning- and execution-related increases in BOLD signal were analyzed for each subject on a Linux workstation (Red Hat 8.0) using statistical parametric map (SPM)2b software (http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 6.51SP1 (MathWorks, Natick, MA). DICOM images were converted to analyze format using the software DCMTK–DICOM Toolkit (http://dicom.offis.de/dcmtk). After slice time correction, functional images were spatially realigned to the first image of each session to correct for head motion. Functional images were spatially normalized to the default EPI template provided in SPM2b and resampled into voxels that were 2 × 2 × 2 mm in size. Images were then smoothed with Gaussian filter of 8-mm full width at half maximum to minimize noise and residual differences in gyral anatomy.
Voxel-Based Analysis
Both first- and second-level analyses were performed. In the first level, data were analyzed for each subject separately on a voxel-by-voxel basis using the principles of the general linear model (Friston et al. 1995). Each individual design matrix included pooled data from all 4 experimental sessions. Column vectors representing planning and execution onset were convolved with a canonical hemodynamic response function and its temporal derivatives to create regressors, which were fitted to the individual fMRI time series. Variance from head motion was considered in the statistical design by adding 6 regressors as covariates of no interest, containing rotation and translation parameters from spatial realignment. A high-pass filter of 128 s was used to remove low-frequency noise.
Eight sets of contrast images were created from analyses testing for increased brain activity for planning and execution of intransitive and transitive gestures, both for left and right hand separately. These contrast images were used in 8 separate second-level random effects analyses. Statistical threshold was set to P < 0.001, uncorrected across the whole brain volume.
Region of Interest–Based Analysis and LI
Lateralization of spatially distributed fMRI activity was assessed using a region of interest (ROI)–based approach. The boundaries of premotor and posterior parietal boxes of interest were defined based on a public library of volume of interest (VOI) masks (Nielsen and Hansen 2004). Accordingly, on the left hemisphere for the posterior parietal cortex (PPC), the Montreal Neurological Institute (MNI) dimensions were x = −52 to 0 mm, y = −85 to −34 mm, and z = 52 to 17 mm and for the premotor cortex (PMC) box of interest x = −50 to −10 mm, y = −28 to 18 mm, and z = 60–0 mm, and on the right hemisphere, x = 52–0 mm, y = −85 to −34 mm, and z = 52–17 mm and x = 50–10 mm, y = −28 to 18 mm, and z = 60–0 mm, respectively. The boxes of interest are delineated in Figure 1. Using the marsbar ROI tool box (http://marsbar.sourceforge.net), we created a binary mask image corresponding to the predefined premotor and posterior parietal VOIs. This binary mask image was used to count all voxels above a specified threshold set at P < 0.001. From each individual analysis, we counted the number of activated voxels at this threshold in the premotor and posterior parietal VOIs on a subject-by-subject basis. We calculated the LI by counting suprathreshold voxels in right (R) and left (L) posterior parietal (PPC) and premotor clusters (PMC) separately according to the formula LI = (R × L)/(R + L). The values range from 1.0 (complete right lateralization) to −1.0 (complete left lateralization).
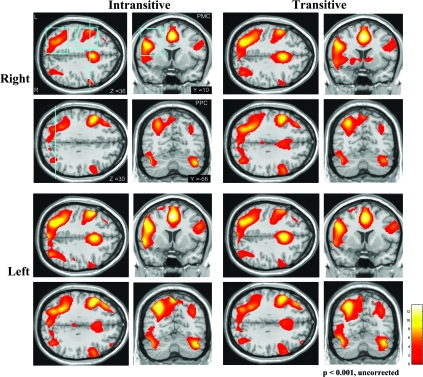
Figure 1.
SPMs superimposed on axial and coronal views (sections indicated by blue lines on axial views) are shown for planning right and left hand intransitive as well as transitive gestures separately (P < 0.001, uncorrected). fMRI activations are clearly left lateralized in parietopremotor areas in all conditions. Boxes of interest for PPC and PMC, on which the calculation of LI scores were done, are delineated in blue. For illustration purposes, only the left-hemispheric boxes are shown.
For statistical analysis, the data of left and right hands were pooled. Mean LI scores across all subjects for combined posterior parietal and premotor (PPC/PMC) as well as the regions separately (PPC and PMC) were calculated. Group differences for the mean LI scores of planning intransitive and transitive gestures were tested using 2-sided paired t-tests.
Results
Behavioral Performance
Performance of gestures during scanning was assessed by video monitoring. The pantomimes were flawless with regard to semantics and content, which is an indirect behavioral control for correctly planning the gestures. The videos were analyzed by blinded raters according to a pantomime recognition scale (PRS) (see Appendix, maximum score 160), which yielded an average score of 156.2 ± 3.8 (Mean ± standard deviation). Minor temporal and spatial errors occurred in some of the gestures, but none of the individual pantomimes scored below 3 in the PRS.
Planning of Intransitive and Transitive Gestures Is Left Lateralized Regardless of the Hand Involved
The results of the voxel-based second-level group analysis for planning and executing transitive and intransitive gestures are summarized in Tables 1 and 2, given in MNI coordinates (maxima within clusters >50 contiguous voxels). The statistical threshold was set to P < 0.001, uncorrected. The strength of activations was derived from the Z score.
Table 1.
Areas of statistically significant activations during planning–preparation of right and left hand gestures
| Intransitive |
Transitive |
||||||||
| Hand | Region of activation (BA) | MNI coordinates |
Z value | MNI coordinates |
Z value | ||||
| x | y | z | x | y | z | ||||
| Right | |||||||||
| R inferior frontal gyrus (44/45) | 38 | 22 | 2 | 4.77 | 34 | 24 | −2 | 5.60 | |
| L inferior frontal gyrus (44/45) | −40 | 0 | 36 | 6.03 | −38 | 26 | 6 | 5.99 | |
| R cingulate gyrus (24) | 14 | 2 | 54 | 5.27 | 4 | 12 | 44 | 5.61 | |
| L middle frontal gyrus (46) | −36 | −8 | −54 | 5.62 | |||||
| R superior parietal lobe (5, 7) | 28 | −74 | 38 | 4.40 | 32 | −78 | 36 | 3.76 | |
| L superior parietal lobe (5, 7) | −30 | −66 | 38 | 4.75 | |||||
| L inferior parietal lobe (39, 40) | −40 | −64 | 30 | 4.68 | −32 | −70 | 38 | 4.62 | |
| L superior temporal gyrus (39) | −46 | −52 | 10 | 3.71 | |||||
| R middle temporal gyrus (37) | 60 | −50 | −10 | 3.72 | |||||
| L inferior temporal gyrus (37) | −56 | −54 | −22 | 4.51 | |||||
| R fusiform gyrus (37) | 46 | −54 | −24 | 3.51 | 40 | −52 | −36 | 3.49 | |
| L fusiform gyrus (37) | −48 | −52 | −28 | 5.01 | −48 | −52 | −28 | 4.39 | |
| Left | |||||||||
| R inferior frontal gyrus (44/45) | 44 | 12 | 22 | 4.50 | 46 | 0 | 36 | 4.62 | |
| L inferior frontal gyrus (44/45) | −42 | 2 | 32 | 6.01 | −48 | 2 | 32 | 5.75 | |
| R cingulate gyrus (24) | 10 | 24 | 24 | 4.00 | |||||
| L cingulate gyrus (24) | −6 | 12 | 32 | 4.13 | |||||
| L superior frontal gyrus (6) | −12 | −4 | 72 | 3.93 | |||||
| R superior parietal lobe (5, 7) | 32 | −80 | 36 | 4.40 | |||||
| L superior parietal lobe (5, 7) | −10 | −74 | 44 | 4.91 | −30 | −74 | 44 | 4.56 | |
| R inferior parietal lobe (39, 40) | 32 | −54 | 38 | 4.33 | 32 | −68 | 40 | 4.07 | |
| L inferior parietal lobe (39, 40) | −32 | −64 | 36 | 5.05 | −38 | −52 | 36 | 4.42 | |
| R inferior temporal gyrus (37) | 50 | −44 | −22 | 4.28 | |||||
| L inferior temporal gyrus (37) | −56 | −50 | −24 | 5.23 | −56 | −68 | −12 | 4.45 | |
| R fusiform gyrus (37) | 44 | −50 | −32 | 4.30 | 40 | −46 | 32 | 3.93 | |
| L superior temporal gyrus (39) | −56 | −60 | 2 | 4.34 | |||||
Note: BA, Brodmann area.
Table 2.
Areas of statistically significant activations during execution of right and left hand gestures
| Intransitive |
Transitive |
||||||||
| Hand | Region of activation (BA) | MNI coordinates |
Z value | MNI coordinates |
Z value | ||||
| x | y | z | x | y | z | ||||
| Right | |||||||||
| L precentral gyrus (4) | −34 | −26 | 66 | 5.85 | −42 | −16 | 58 | 6.03 | |
| R medial frontal gyrus (6) | 24 | −4 | 62 | 5.50 | |||||
| L medial frontal gyrus (6) | −8 | −12 | 52 | 5.59 | |||||
| R inferior frontal gyrus (47) | 50 | 16 | −6 | 5.91 | |||||
| L cingulate gyrus (24) | −8 | −12 | 52 | 5.87 | |||||
| R cingulate gyrus (24) | 4 | 4 | 50 | 5.54 | |||||
| R superior temporal gyrus (13) | 60 | −40 | 22 | 5.99 | 68 | −36 | 18 | 5.02 | |
| L superior parietal lobe (7) | −28 | −52 | 54 | 5.48 | −32 | −50 | 64 | 5.59 | |
| L inferior parietal lobe (40) | −52 | −32 | 36 | 5.62 | |||||
| R inferior parietal lobe (40) | 60 | −34 | 28 | 5.41 | 54 | −38 | 34 | 4.22 | |
| Left | |||||||||
| R precentral gyrus (4) | 32 | −24 | 60 | 5.57 | 56 | −20 | 46 | 5.67 | |
| R postcentral gyrus (3) | 38 | −30 | 66 | 5.74 | 48 | −20 | 60 | 5.40 | |
| R cingulate gyrus (24) | 4 | 8 | 52 | 5.29 | |||||
| L cingulate gyrus (24) | −2 | 14 | 40 | 5.84 | |||||
| R medial frontal gyrus (6) | 10 | −2 | 60 | 5.78 | 10 | −2 | 62 | 6.07 | |
| L medial frontal gyrus (6) | −10 | −6 | 60 | 5.27 | |||||
| R superior temporal gyrus (13) | 52 | −32 | 20 | 5.45 | 52 | 14 | −6 | 5.13 | |
| L superior temporal gyrus (13) | −58 | −28 | 12 | 5.22 | −64 | −42 | 24 | 5.78 | |
| L superior parietal lobe (7) | −30 | −60 | 56 | 5.13 | |||||
| R inferior parietal lobe (40) | 38 | −36 | 52 | 5.33 | |||||
| L inferior parietal lobe (40) | −60 | −28 | 26 | 5.69 | |||||
Note: BA, Brodmann area.
Overall, planning intransitive and transitive gestures significantly activated premotor and posterior parietal association areas, which were predominantly left hemispheric regardless as to whether the planning occurred for the right or left hand (Fig. 1). Accordingly, clear left lateralization of planning is also evidenced by the negative mean LI scores in all conditions (see Table 3). As depicted in Figure 1 on axial and coronal views, activated premotor areas included mainly the inferior and middle frontal gyri. Furthermore, in PPC, significant activations were found in the inferior and superior parietal lobes, including the precuneus. In addition, strong BOLD signals were observed in the anterior cingulate cortex involving supplementary motor area (SMA) bilaterally as well as cerebellum. Finally, significant activations were also found in posterior temporal regions.
Table 3.
LI scores of ROIs (mean ± SD), for right (R) and left (L) hand, and pooled for both hands (R + L)
| Gesture subtype | PPC | PMC | PPCR+L | PMCR+L | PPC/PMCR+L | |
| Planning | ||||||
| Intransitive | R | −0.59 ± 0.08 | −0.52 ± 0.11 | −0.56 ± 0.11 | −0.47 ± 0.14 | −0.49 ± 0.10 |
| L | −0.53 ± 0.15 | −0.42 ± 0.17 | ||||
| * | ** | *** | ||||
| Transitive | R | −0.49 ± 0.12 | −0.41 ± 0.10 | −0.45 ± 0.12 | −0.36 ± 0.13 | −0.37 ± 0.08 |
| L | −0.41 ± 0.12 | −0.32 ± 0.17 | ||||
| Execution | ||||||
| Intransitive | R | −0.31 ± 0.05 | −0.12 ± 0.04 | −0.19 ± 0.09 | −0.05 ± 0.06 | −0.10 ± 0.06 |
| L | −0.08 ± 0.10 | 0.02 ± 0.07 | ||||
| Transitive | R | −0.31 ± 0.08 | −0.14 ± 0.07 | −0.18 ± 0.08 | −0.04 ± 0.05 | −0.09 ± 0.05 |
| L | −0.06 ± 0.04 | 0.06 ± 0.01 | ||||
Note: *P = 0.11, mean difference ± standard deviation (SD) = −0.11 ± 0.35; **P = 0.04, mean difference ± SD = −0.11 ± 0.28; ***P = 0.02, mean difference ± SD = −0.12 ± 0.27, 2-tailed paired t-tests.
As demonstrated in Figure 2, in posterior parietal and premotor clusters, fMRI activity during execution of gestures (indicated in green) was generally more bilateral (particularly for left hand gestures) compared with planning (indicated in red). This global activation pattern during execution is reflected by only modest left lateralization for right hand and absent right lateralization for left hand gestures, as indicated by mean LI scores (see Table 3). Furthermore, fMRI activities in the PPC were located more anteriorly and superiorly during gesture execution, whereas in the premotor areas, including SMA, fMRI activation of planning and execution largely overlapped.
Figure 2.
Superior and lateral overviews of significant fMRI activations (P < 0.001, uncorrected) rendered on template hemispheres for intransitive and transitive gestures, right and left hand separately. fMRI activation of planning (red) is separated from execution (green) along an inferior–superior as well as posterior–anterior gradient in PPC, whereas in PMC, including SMA, activities largely overlap (yellow).
Planning Intransitive Gestures Is More Left Lateralized than Transitive Gestures
Differential left lateralization between planning intransitive and transitive gestures was quantified by calculating mean LI scores based on voxel counts in PPC and PMC. The results are summarized in Table 3. The comparison of mean LI scores derived from combined PPC–PMCR+L clusters revealed a significantly stronger left lateralization for planning intransitive than transitive gestures. When analyzed separately, left lateralization for planning intransitive gestures remained significant in the premotor cluster PMCR+L, whereas the difference in posterior parietal cluster PPCR+L was not statistically significant. There were no significant differences in LI scores between intransitive and transitive gestures during execution.
Discussion
The findings of the present study demonstrate strong left lateralization of fMRI activities while planning normal praxis movements for both hands. This concurs with clinical observations in ideomotor apraxia. As a higher order motor disorder, it usually affects both hands similarly and is caused mainly by left-hemispheric lesions. The left-lateralized control of praxis for both hands is clinically relevant because disability by apraxic deficits of the ipsilesional left hand may be underestimated or incorrectly ascribed to handedness. Furthermore, the bilateral nature allows assessment of apraxia in the ipsilesional hand, in which the disorder is not masked by elementary sensorimotor impairment often associated with the contralesional hand.
Action planning involves the selection and initiation of motor programs. During planning of pantomime, internal images of gestures are generated. It is a complex process integrating visuospatial, kinaesthetic, and cognitive (action goal) information, which is challenging to dissect experimentally from action control. Our design is based on the instructed delay paradigm originally developed in primates (Weinrich and Wise 1982). It allows planning the gestures for several seconds, followed by visually cued execution, which may not satisfactorily reflect the spontaneity of action planning in a naturalistic context. However, although the planning phase is artificially prolonged, the following execution phase ensures that the intentional aspect of planning is preserved beyond pure motor imagination.
Strong left-lateralized fMRI activations in the inferior and middle frontal cortices are consistent with recent evidence from lesion subtraction analysis in a series of stroke patients with impaired and normal pantomime, suggesting that pantomime of gestures is mediated mainly by left inferior frontal areas (Goldenberg et al. 2007). Furthermore, analysis in the same patient cohort revealed that impaired imitation of hand postures was associated mainly with inferior parietal lesions (Goldenberg and Karnath 2006). Therefore, the findings from quantitative structural analysis point to functional dichotomy of inferior frontal cortex supporting primarily pantomime and parietal cortex guiding imitation of gestures. However, the present study, with strong posterior parietal fMRI activation during both planning and executing pantomimes, does not seem to corroborate their limited representation in frontal cortex. It is likely that lesion overlap studies alone may not be able to adequately elucidate networks of distributed and interactive parietofrontal regions underlying cognitive processing of praxis but may have to be combined with functional studies for that purpose. It has been argued that left lateralization of parietal activity, also found in other fMRI studies using pantomime paradigms (Ohgami et al. 2004; Johnson-Frey et al. 2005; Fridman et al. 2006; Hermsdorfer et al. 2007), may be explained by the artificial scanner environment (Goldenberg et al. 2007) because the lack of visual feedback and the movements in the narrow space are particularly demanding with respect to body-centered spatial processing. Furthermore, it may be argued that the left lateralization of fMRI activation in general is related to the scanner condition because it has been suggested that feedback-independent open loop processing is left predominant (Haaland et al. 2004). The experimental setting may even influence the fMRI activity in the planning phase before actual pantomime. However, EEG coherence studies (Wheaton et al. 2005), which allowed studying pantomime of gestures in a naturalistic setting without spatial restrictions, showed synchronized activation of parietal–premotor networks during preparation and onset of gestures in line with findings of fMRI studies. Moreover, EEG coherence analysis confirmed left lateralization for planning and executing ipsilateral left gestures (Wheaton et al. 2008) and others. Left lateralization of fMRI activity was stronger in posterior parietal than premotor areas during pantomime planning, which supports the concept that this region may store motor engrams of gestures (Heilman et al. 1982; Rothi et al. 1985; Buxbaum et al. 2007).
Our findings demonstrated only minimal overlap of fMRI activities between planning and executing gestures in PPC, agreeing with previous reports (Johnson-Frey et al. 2005; Fridman et al. 2006). Furthermore, the present study confirms a posterior–anterior and inferior–superior gradient from planning to executing gestures. However, in PMC, demonstrating largely overlapping activities for planning and executing pantomimes, a similar separation of activations could not be reproduced. The discrepancy may be explained by the different experimental design, which involved a NoGo task in earlier studies allowing better delineation of activation between planning and executing (Fridman et al. 2006).
We explored differential left lateralization of intransitive and transitive gestures using ROI analysis in premotor and posterior parietal clusters because these regions were shown to be most consistently involved in apraxic patients (Haaland et al. 2000). The distinction of transitive and intransitive gestures is important, both clinically and theoretically. Pantomiming transitive and intransitive gestures is sensitive in the assessment of apraxia because it provides the fewest cues and therefore strongly depends on stored motor engrams (Mozaz et al. 2002). In ideomotor apraxia due to left-hemispheric lesions, transitive gestures are typically more affected than intransitive gestures (Haaland and Flaherty 1984; Roy et al. 1991; Foundas et al. 1999). It has been hypothesized that transitive gestures depend more on intact left parietopremotor function because they are more remote from natural context than intransitive gestures (Leiguarda 2003). Therefore, we expected that the transitive gestures would be more left lateralized. However, our findings, with intransitive gestures being significantly more left lateralized, apparently do not corroborate this view. There is no straightforward explanation for the discrepancy in the lateralization pattern between the present fMRI findings and lesion studies. It is conceivable that under physiological conditions, left parietopremotor networks operate more efficiently, thereby requiring less planning activity to prepare for transitive gestures. On the other hand, findings from lesioned patients may not be easily translated into functional organization of intact networks. For instance, lesioned hemispheres have been demonstrated to be influenced by interhemispheric inhibition as demonstrated for motor function, at least in subacute stages after stroke (Murase et al. 2004).
Irrespective of the clinical data, a possible reason for the stronger left predominance of intransitive gestures is their communicative nature because they are also frequently coupled with verbal communication (indeed all subjects were right handed in the present study). Similarly, a significant difference in left lateralization between planning intransitive and transitive gestures was found only in premotor clusters, areas involved in language processing. Interestingly, a recent fMRI study demonstrated stronger activation of left inferior frontal gyrus during recognition of intransitive than transitive gestures corroborating the findings in the present work (Villarreal et al. 2008). Pantomiming transitive gestures is more complex than intransitive gestures because it involves the imagined interaction with a tool, that is, it requires integrating both peripersonal and extrapersonal (tool) space. Likewise, in transitive acts, the tool has to be aimed at the target of its action, thereby involving more visuospatial processing. Therefore, one may speculate whether planning tool-related pantomimes requires more spatial attention in extrapersonal space, a well-known cognitive function of right hemisphere (Gazzaniga 1995), particularly of premotor region (Committeri et al. 2007). Finally, pantomiming transitive acts is less familiar than intransitive gestures, which might be a reason why searching for access to these movement representations during planning activates premotor areas more bilaterally. Left lateralization was different only during gesture planning, suggesting that differential cognitive requirements are less important during online control of praxis. In other words, gestures differ in their representation not in action execution, pointing to the relevance of internal models guiding skilled action (Buxbaum et al. 2005).
Funding
Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Acknowledgments
We are very grateful to Devera Schoenberg for skillful editing. Conflict of Interest: None declared.
Appendix
Pantomime Recognition Scale
0 Unrecognizable.
1 Movement present, but hard to decipher, prolonged with pauses.
2 Movement is borderline recognizable with moderately severe temporal and spatial errors.
3 Movement is fair, but with any of the following errors: temporal and/or spatial errors, context errors, slightly prolonged movement sequences.
4 Movement is error-free.
References
- Alexander MP, Baker E, Naeser MA, Kaplan E, Palumbo C. Neuropsychological and neuroanatomical dimensions of ideomotor apraxia. Brain. 1992;115(Pt 1):87–107. doi: 10.1093/brain/115.1.87. [DOI] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Deficient internal models for planning hand-object interactions in apraxia. Neuropsychologia. 2005;43:917–929. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle K, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: evidence from stroke and corticobasal degeneration. Cortex. 2007;43:411–423. doi: 10.1016/s0010-9452(08)70466-0. [DOI] [PubMed] [Google Scholar]
- Carey JR, Anderson KM, Kimberley TJ, Lewis SM, Auerbach EJ, Ugurbil K. fMRI analysis of ankle movement tracking training in subject with stroke. Exp Brain Res. 2004;154:281–290. doi: 10.1007/s00221-003-1662-7. [DOI] [PubMed] [Google Scholar]
- Committeri G, Pitzalis S, Galati G, Patria F, Pelle G, Sabatini U, Castriota-Scanderbeg A, Piccardi L, Guariglia C, Pizzamiglio L. Neural bases of personal and extrapersonal neglect in humans. Brain. 2007;130:431–441. doi: 10.1093/brain/awl265. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Macauley BL, Raymer AM, Maher LM, Rothi LJ, Heilman KM. Ideomotor apraxia in Alzheimer disease and left hemisphere stroke: limb transitive and intransitive movements. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:161–166. [PubMed] [Google Scholar]
- Fridman EA, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, Wheaton L, Wu T, Hallett M. The role of the dorsal stream for gesture production. Neuroimage. 2006;29:417–428. doi: 10.1016/j.neuroimage.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Principles of human brain organization derived from split-brain studies. Neuron. 1995;14:217–228. doi: 10.1016/0896-6273(95)90280-5. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia and beyond: life and work of Hugo Liepmann. Cortex. 2003;39:509–524. doi: 10.1016/s0010-9452(08)70261-2. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hentze S, Hermsdorfer J. The effect of tactile feedback on pantomime of tool use in apraxia. Neurology. 2004;63:1863–1867. doi: 10.1212/01.wnl.0000144283.38174.07. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hermsdorfer J, Glindemann R, Rorden C, Karnath HO. Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb Cortex. 2007;17:2769–2776. doi: 10.1093/cercor/bhm004. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Karnath HO. The neural basis of imitation is body part specific. J Neurosci. 2006;26:6282–6287. doi: 10.1523/JNEUROSCI.0638-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland KY, Flaherty D. The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain Cogn. 1984;3:370–384. doi: 10.1016/0278-2626(84)90029-0. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123(Pt 11):2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127:1145–1158. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Daniels SK, Fieselman MA, Thompson K, Vasterling JJ, Heilman KM, Foundas AL. Praxis lateralization: errors in right and left hemisphere stroke. Cortex. 2001;37:219–230. doi: 10.1016/s0010-9452(08)70569-0. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY. Motor sequencing with left hemisphere damage. Are some cognitive deficits specific to limb apraxia? Brain. 1992;115(Pt 3):857–874. doi: 10.1093/brain/115.3.857. [DOI] [PubMed] [Google Scholar]
- Heath M, Roy EA, Black SE, Westwood DA. Intransitive limb gestures and apraxia following unilateral stroke. J Clin Exp Neuropsychol. 2001;23:628–642. doi: 10.1076/jcen.23.5.628.1240. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32:342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Terlinden G, Muhlau M, Goldenberg G, Wohlschlager AM. Neural representations of pantomimed and actual tool use: evidence from an event-related fMRI study. Neuroimage. 2007;36(Suppl 2):T109–T118. doi: 10.1016/j.neuroimage.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausberg H, Cruz RF, Kita S, Zaidel E, Ptito A. Pantomime to visual presentation of objects: left hand dyspraxia in patients with complete callosotomy. Brain. 2003;126:343–360. doi: 10.1093/brain/awg042. [DOI] [PubMed] [Google Scholar]
- Leiguarda RC. Apraxias and the lateralization of motor functions in the human parietal lobe. Adv Neurol. 2003;93:235–248. [PubMed] [Google Scholar]
- Mozaz M, Rothi LJ, Anderson JM, Crucian GP, Heilman KM. Postural knowledge of transitive pantomimes and intransitive gestures. J Int Neuropsychol Soc. 2002;8:958–962. doi: 10.1017/s1355617702870114. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nielsen FA, Hansen LK. Finding related functional neuroimaging volumes. Artif Intell Med. 2004;30:141–151. doi: 10.1016/S0933-3657(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Ohgami Y, Matsuo K, Uchida N, Nakai T. An fMRI study of tool-use gestures: body part as object and pantomime. Neuroreport. 2004;15:1903–1906. doi: 10.1097/00001756-200408260-00014. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rothi LJ, Heilman KM, Watson RT. Pantomime comprehension and ideomotor apraxia. J Neurol Neurosurg Psychiatry. 1985;48:207–210. doi: 10.1136/jnnp.48.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy EA, Square-Storer P, Hogg S, Adams S. Analysis of task demands in apraxia. Int J Neurosci. 1991;56:177–186. doi: 10.3109/00207459108985414. [DOI] [PubMed] [Google Scholar]
- Schnider A, Hanlon RE, Alexander DN, Benson DF. Ideomotor apraxia: behavioral dimensions and neuroanatomical basis. Brain Lang. 1997;58:125–136. doi: 10.1006/brln.1997.1770. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Zimine I, Mayer E, Michel CM, Khateb A. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp. 2004;23:140–155. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland A, Sluman SM. Ideomotor apraxia, visuomotor control and the explicit representation of posture. Neuropsychologia. 2000;38:923–934. doi: 10.1016/s0028-3932(00)00021-x. [DOI] [PubMed] [Google Scholar]
- Villarreal M, Fridman EA, Amengual A, Falasco G, Gerscovich ER, Ulloa ER, Leiguarda RC. The neural substrate of gesture recognition. Neuropsychologia. 2008;46:2371–2382. doi: 10.1016/j.neuropsychologia.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Watson RT, Heilman KM. Callosal apraxia. Brain. 1983;106(Pt 2):391–403. doi: 10.1093/brain/106.2.391. [DOI] [PubMed] [Google Scholar]
- Weinrich M, Wise SP. The premotor cortex of the monkey. J Neurosci. 1982;2:1329–1345. doi: 10.1523/JNEUROSCI.02-09-01329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton LA, Bohlhalter S, Nolte G, Shibasaki H, Hattori N, Fridman E, Vorbach S, Grafman J, Hallett M. Cortico-cortical networks in patients with ideomotor apraxia as revealed by EEG coherence analysis. Neurosci Lett. 2008;433:87–92. doi: 10.1016/j.neulet.2007.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton LA, Nolte G, Bohlhalter S, Fridman E, Hallett M. Synchronization of parietal and premotor areas during preparation and execution of praxis hand movements. Clin Neurophysiol. 2005;116:1382–1390. doi: 10.1016/j.clinph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Shibasaki H, Hallett M. Temporal activation pattern of parietal and premotor areas related to praxis movements. Clin Neurophysiol. 2005;116:1201–1212. doi: 10.1016/j.clinph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Yakota S, Hallett M. Posterior parietal negativity preceding self-paced praxis movements. Exp Brain Res. 2005;163:535–539. doi: 10.1007/s00221-005-2314-x. [DOI] [PubMed] [Google Scholar]