Abstract
This study explored sex effects on the process of risk-taking. We observed that the female participants (n = 10) showed stronger activation in the right insula and bilateral orbitofrontal cortex (OFC) than did the male participants (n = 12) while they were performing in the Risky-Gains task. The female participants also showed stronger activations in the precentral, postcentral, and paracentral regions after receiving punishment feedback. In addition, the strength of neural activity in the insula correlated with the rate of risky behaviors for the female participants but not for the male participants. Similarly, the percent signal changes in the right OFC correlated negatively with the rate of selecting risky choices for the female group. These findings strongly suggest a sex-related influence modulating brain activity during risk-taking tasks. When taking the same level of risk, relative to men, women tend to engage in more neural processing involving the insula and the OFC to update and valuate possible uncertainty associated with risk-taking decision making. These results are consistent with the value-based decision-making model and offer insights into the possible neural mechanisms underlying the different risk-taking attitudes of men and women.
Keywords: insula, neuroimaging, orbitofrontal cortex, risk taking, sex differences
Introduction
Risk taking involves a set of cognitive and affective processes that aim to balance the potential losses and benefits of an action (Arce et al. 2006). Previous behavioral studies have found that men show greater impulsivity, tend to be more sensation seeking, and engage in risk-taking behaviors more frequently than women (Rosenblitt et al. 2001; Whiteside and Lynam 2003). Such a differential pattern of risk-taking behavior between men and women suggests that there could be sex-related differences in the neural activity associated with risk-taking-related cognition. Nonetheless, studies have rarely addressed this question. In a recent study examining activation of the orbitofrontal cortex (OFC) and the dorsolateral prefrontal cortex during performance of the Iowa Gambling task (Bolla et al. 2004), differential patterns of neural activation between men and women were observed. The male participants showed better task performance and greater right lateralized prefrontal activity than the female participants. Although the findings contribute to the understanding of the effects of sex on risk-taking behavior, it has been argued that the dissimilar performances of the male and female groups confound the findings on neural activation of the proposed sex-related effect (Li et al. 2006). Indeed, when Li et al. (2006) studied sex-related differences in neural activity associated with response inhibition, they first matched the performance of the male and female groups in the response inhibition task (Li et al. 2006). Taking note of Li et al.’s experimental design, the present study controlled differences in the behavioral performance of the male and female groups in the belief that this would enable any neural activity observed to be different to results from the sex rather than the task performance effects.
Studies on the risk-taking behavior of healthy adults have reported activation of the OFC (Krain et al. 2006), the inferior prefrontal cortex (Paulus et al. 2001), the ventrolateral and ventromedial frontal cortices (Elliott et al. 1999, 2000; Rogers et al. 1999), the insula (Critchley et al. 2001), and the anterior cingulate cortex (Elliott et al. 2000). In this connection, Paulus et al. (2003) demonstrated that right insula activation was significantly stronger for participants when selecting risky versus safe responses on the Risky-Gains task. They also revealed that the degree of insula activation was related to the probability of selecting a safe response following a punished response (Paulus et al. 2003). Literature has suggested that the insula plays a significant role in estimating risks in uncertain situations, and in guiding behavior based upon the anticipation of aversive emotional consequences (Sanfey et al. 2003). According to the somatic marker hypothesis, external stimuli could initiate a state that is associated with pleasurable or aversive somatic markers, which then guide the individual's behavior toward a nonaversive state (Naqvi et al. 2006). Uncertainty about the outcome of a risky behavior may bias an individual's response toward the choice, whether risky or safe. The operation of these biases and aversive emotions associated with risk-taking decisions and behaviors, usually acting at an implicit level, was found to be mediated by the activity of the insula (Hastie and Dawes 2001).
In this study, we used the Risky-Gains task (Paulus et al. 2003) to examine sex effects on the neural activity associated with risk-taking behaviors. We adopted Li et al.’s (2006) approach and matched the male and female groups according to 1) impulsive tendencies as measured by the Barratt Impulsiveness Scale (BIS; Patton et al. 1995), and 2) behavioral outcomes in terms of the rates and response times for making a safe or risky response. Following Paulus et al.’s (2003) study, we performed one contrast comparing the brain activity associated with risky responses with that associated with safe responses (risk taking), and another contrast comparing risky responses that were punished with those that were not (punishment). We also explored whether the neural activity associated with receiving punishment feedback would have an immediate effect on subsequent risk-taking behaviors. We performed region of interest (ROI) analysis of the bilateral insula and the bilateral OFC, the 2 areas most involved in the risk-taking decision-making process (Paulus et al. 2003; Krain et al. 2006). We hypothesized that the male and female participants would show differential patterns of neural activation associated with risk taking. We further hypothesized that when the female and the male participants showed comparable rates of risky selection, the neural activations in the insula and the OFC would be stronger for the female participants than for the male participants. The rationale for this is that previous studies (e.g., Rosenblitt et al. 2001; Whiteside and Lynam 2003) have found that women are more conservative than men when it comes to taking risks and tend to elicit stronger neural responses from a risky decision. To further support our hypothesis, we correlated the neural activities of the right insula and the OFC with the rate of risky selection and punishment for both the male and female groups. We hypothesized that stronger correlations would be obtained for the female participants than for the male participants.
Methods
Participants
Ethics approval was obtained from the institutional review board of the University of Texas. Participants were recruited using convenience sampling by posting open advertisements on campus inviting members of the university to participate. Twelve male volunteers (mean age = 29.9 ± 6.2 years, mean education = 17.2 ± 1.8 years) and 10 female volunteers (mean age = 30.2 ± 5.6 years, mean education = 16.4 ± 0.9 years) participated in this study. All were strongly right handed (Snyder and Harris 1993), and they were not on any medications at the time of the study. Impulsivity scores did not differ between the 2 groups (BIS total score for males = 61.8 ± 7.0 and for females = 64.6 ± 6.9, t20 = 0.96, P = 0.350; BIS attentional impulsiveness subscore for males = 16.8 ± 1.9 and for females = 16.6 ± 2.7, t20 = 0.15, P = 0.881; BIS motor impulsiveness subscore for males = 20.8 ± 3.2 and for females = 22.9 ± 2.8, t20 = 1.56, P = 0.126; BIS nonplanning impulsiveness subscore for males = 24.2 ± 4.6 and for females = 25.1 ± 2.9, t20 = 0.56, P = 0.584). None of the participants had a history of neurological or psychiatric illnesses. The study was explained to the participants prior to obtaining their informed consent.
Experimental Task
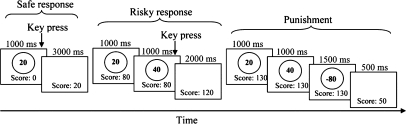
We adopted Paulus et al.’s (2003) Risky-Gains task in this study. In brief, participants are required to decide whether to select a safe or a risky response. Their goal is to gain as many points as possible through carrying out the task. For each trial, the participants are offered 20, 40, and 80 points in a fixed sequential order. They then decide whether to claim 20, 40, or 80 points by pressing a button when the points appear. A selection of 20 points is always a safe response as the participants will always get +20 points. However, selections of 40 and 80 points are risky responses because it is possible that the points could represent a gain (+40/+80) or a loss (−40/−80). Specifically, the +40 and +80 are rewarding risky responses, and the −40 or −80 is a punished response.
The experiment consists of 96 trials, with each trial lasting 4 s irrespective of the participant's choice. The 96 trials, presented in a random sequence, are made up of 54 nonpunished trials (i.e., + 20, + 40, and + 80), 24 punished trials involving −40 points, and 18 punished trials involving −80 points (Fig. 1). The points accumulated from trial to trial are shown at the bottom of the computer screen. A final score is shown after the last trial when the experiment is completed. One feature of the Risky-Gains task is that there is no advantage in selecting the risky over the safe response on the final score because the probabilities of presenting −40 or −80 are designed in such a way that a participant's final score would be the same had he/she consistently select 20, 40, or 80 (Paulus et al. 2003). To familiarize them with the task, the participants carried out a practice exercise before the scanning.
Figure 1.
Schematic diagram of the Risky-Gains task.
Behavioral Measures
A participant's performance on the risk-taking task is presented as the mean and standard deviation for the selected options. The rate of selecting the safe (+20) and risky choices (+40 and +80) and the rate of being punished (−40 and −80) were calculated for both the male and female participants. The analysis also included differences between the 2 sex groups on the rates of making a safe or a risky response immediately after being punished. We performed all analyses of the distribution of the participants’ responses and reaction times between the 2 sex groups using the Mann–Whitney U test with the significance level set at P < 0.050.
Data Acquisition
The present study used event-related functional magnetic resonance imaging (fMRI). The 96 trials were presented in four 24-trial blocks, each separated by 12 s of fixation. A back-projection was used to display the stimuli during the risk-taking task, and the participants were required to make a response during each trial. The imaging was conducted on a 3 T Siemens MRI scanner (Siemens, Erlangen, Germany) at the Research Imaging Center, University of Texas Health Science Center, San Antonio, TX. The participants lay supine on the scanning table and were fitted with plastic ear-canal molds. Twenty-four contiguous gradient-echo planar images, sensitive to blood oxygen level–dependent (BOLD) contrast, were acquired parallel to the anterior commissure–posterior commissure plane with parameters set as follows: time repetition (TR) = 2 s, time echo (TE) = 30 ms, field of view (FOV) = 256 mm × 256 mm, matrix size = 128 × 128, flip angle = 90°, and slice thickness = 6 mm. For each slice, 222 images were acquired, with a total scan time of 7 min 24 s. The anatomical MRI was acquired using a T1-weighted, three-dimensional (3D) gradient-echo pulse sequence (TR = 20 ms, TE = 5.15 ms, FOV = 256 mm × 256 mm, slice thickness = 6 mm).
Imaging Data Processing and Analysis
We analyzed the functional images using the Statistical Parametric Map (SPM2) software package (Wellcome Department of Cognitive Neurology, Institute of Neurology, Queen Square, London, UK), running under Matlab 6.5 (MathWorks, Natick, MA). During the normalization process, the individual data were resliced into 4-mm isotropic voxels. The resulting images were then spatially smoothed by convolution with a 3D Gaussian kernel (full width half maximum = 8 mm). There were a total of 5 regressors classified as follows: one for making a safe response (i.e., +20 point trials); 2 for making a risky response (i.e., +40 and +80 point trials); and 2 for receiving punishment feedback (i.e., −40 and −80 point trials). The trial duration for each event in the model was set in accordance with the response time of the participant. That is, the first 3 regressors on the neural activity were captured from the beginning of the trial to the time the participant made a response, whereas the last 2 regressors were captured from the time the participant received the punishment feedback to the end of the trial. The resulting time series data were high-pass filtered with a default threshold of 128 s to remove low frequency drift. We performed subject-level statistical analyses by setting up contrasts between the risky and safe responses and between the punishment feedback and risky responses at a threshold of P < 0.001. Cortical findings were reported. We conducted a one-sample t-test to examine the neural activations of the male and female participants on each of the 2 contrasts. We then compared the activations of the male and female participants on these 2 contrasts using a 2-sample t-test with a voxel-wise intensity threshold of P < 0.001 corrected at a cluster level of 10 voxels. For the ROI analysis, we first used the Automated Anatomical Labeling template available in the WFU PickAtlas (Maldjian et al. 2003), a toolbox for SPM, to define the anatomical regions of each participant. The regions for the left and right insula were equivalent to Brodmann's area (BA) 13, whereas those for the OFC were equivalent to BA 10, 11, and 47. We then calculated the percent signal change in each of these ROIs using the MarsBaR ROI toolbox for SPM available on the Web at http://marsbar.sourceforge.net (Brett et al. 2002). We then used the Mann–Whitney U test to compare the differences in the percent signal change between the male and female participants in each of the ROIs. We further conducted exploratory whole-brain analysis between the 2 sex groups. The risky versus safe-response contrast and punishment feedback versus risky-response contrast were conducted. Group threshold with cluster volume was used for correcting the Type I errors resulting from the multiple comparisons conducted in the analysis. Significant activation was defined as having a voxel-wise intensity threshold of P < 0.001 corrected at a cluster level of 10 voxels.
To further understand an individual's risk-taking behavior, we also studied the possible effects of punishment feedback on subsequent risky choices. The fMRI data were re-run by adding 3 new regressors to the analyses. The 3 regressors used in the previous analysis remained unchanged; that is, one for the safe response (i.e., +20 point trials) and 2 for receiving the punishment feedback (i.e., −40 and −80 point trials). The 3 new regressors were as follows: 1) a safe response followed by a risky response (safe-then-risky response); 2) a risky response followed by a risky response (risky-then-risky response); and 3) receiving punishment feedback followed by a risky response (punished-then-risky response). All the male participants were entered into this re-run but only 9 of the 10 female participants were because one female participant did not make any risky responses after receiving the punishment feedback. We conducted subject-level statistical analyses by carrying out 5 contrasts, punished-then-risky response versus the safe response, punished-then-risky response versus the safe-then-risky response, punished-then-risky response versus the risky-then-risky response, risky-then-risky response versus the safe response, and risky-then-risky response versus the safe-then-risky response. For each of these contrasts, we performed a whole-brain analysis comparing the neural activations between the sex groups using a 2-sample t-test with a voxel-wise intensity threshold of P < 0.001 corrected at a cluster level of 10 voxels. To further explore the relationship between the behavioral responses and the neural activations of the insula and the OFC during a risky response following receipt of punishment feedback, we extracted the percent signal change in the 2 regions and correlated it with the performance on the task. Specifically, we obtained correlations separately for the male and female groups on 1) the rate of risky responses after receiving punishment feedback, and 2) the rate of risky responses after receiving reward feedback. The extraction of the percent signal change and the definition of the ROI (i.e., the insula and the OFC) were the same as mentioned above. All correlations were performed using Spearman rank correlation, rs, and the significance level was set at P < 0.050. We similarly conducted correlations between the behavioral response and the percent signal change at the insula and the OFC. The extraction procedure and definitions of the ROIs were the same as previously described.
Results
Behavioral Data
Table 1 shows the rates and response times of making a safe or risky response or of being punished in the male and female groups. The Mann–Whitney U test revealed no significant differences between the 2 groups for both the rate (safe: Z = 0.033, P = 0.974; risky: Z = 0.429, P = 0.674; punished: Z = 0.166, P = 0.872) and the response time (safe: Z = 1.517, P = 0.140; risky: Z = 0.132, P = 0.923). Additional analysis revealed that the female participants made significantly more safe responses (choosing +20 points) immediately after receiving punishment feedback than did the male participants (Z = 1.946, P = 0.050).
Table 1.
Mean (SD), rate (%), and response time (ms) of safe and risky responses by male participants (n = 12) and female participants (n = 10)
| Rate (%) |
Response time (ms) |
||||
| Safe |
Risky |
Punish |
Safe |
Risky |
|
| +20 | +40 and +80 | −40 and −80 | +20 | +40 and +80 | |
| Male | 29.60 (11.28) | 46.53 (9.67) | 23.87 (2.86) | 474.85 (62.62) | 386.18 (40.92) |
| Female | 30.94 (14.76) | 44.58 (10.05) | 24.38 (6.54) | 524.27 (101.58) | 388.25 (57.80) |
ROI Analysis
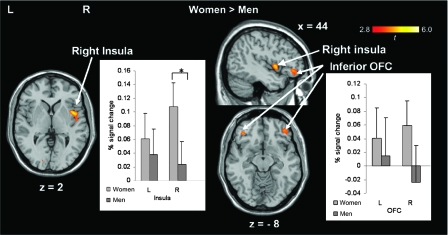
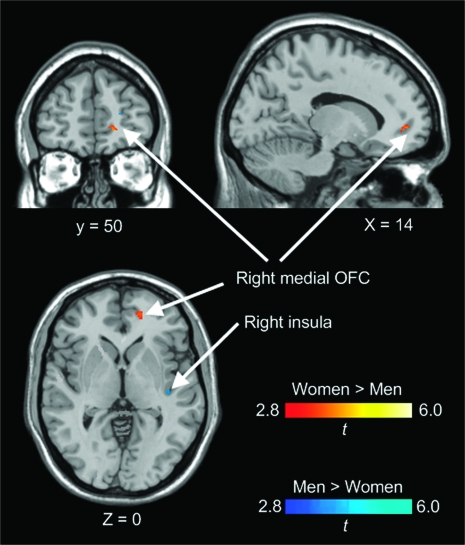
In the risky-response versus safe-response contrast, the female participants showed stronger activation than the male participants in the right insula (BA 13) and the bilateral OFC (BA 47) (Fig. 2). However, there was no stronger activation in these regions in the male than the female participants. The Mann–Whitney U test revealed that the female participants showed a significantly greater percent signal change than the male participants in the right insula for the same contrast (Z = 2.176, P = 0.030, Fig. 2). In the punishment feedback versus risky-response contrast, the female participants showed stronger activation than the male participants in the right medial OFC (BA 10), whereas the male participants showed stronger activation than the female participants in the right insula (BA 13) (Fig. 3).
Figure 2.
Results of the ROI analyses. The activation map shows the activation in the predefined regions, the insula and the OFC, on a standard template, and the bar charts show the plot of percent signal change for the risky versus safe-response contrast in female and male participants. No significantly stronger activation was revealed for the male participants than for the female participants. Right (R) is right. L = left hemisphere; R = right hemisphere; x, y, z in Montreal Neurological Institute coordinates. Error bars show standard error of means.
Figure 3.
Results of the ROI analyses comparing activations in the insula and the OFC between female and male participants. x, y, z in Montreal Neurological Institute coordinates.
Risky versus Safe Response and Punishment Feedback versus Risky Response
Whole-Brain Analysis
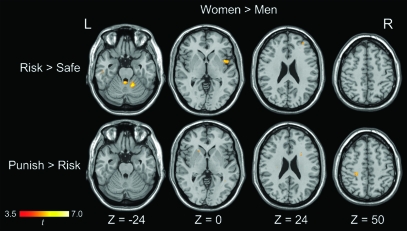
Exploratory whole-brain analysis generally revealed stronger activations in the right insula (BA 13), the middle frontal gyrus (BA 46), and the middle temporal gyrus (BA 20) for the female participants than for the male participants during the risky versus safe-response contrast. In the punishment feedback versus risky-response contrast, stronger activations in the precentral gyrus (BA 6), the paracentral lobule (BA 4), and the postcentral gyrus (BA 3) were observed among the female participants relative to the male participants. Table 2 and Figure 4 show details of the neural activations. No stronger activations among the male participants, relative to the female participants, were observed.
Table 2.
Differences in brain activations between female and male participants in risky versus safe response and punishment feedback versus risky-response contrasts in whole-brain analysis
| BA | Side | Coordinate |
Cluster | T | ||||
| x | y | z | ||||||
| Females > males | ||||||||
| Risky versus safe response | Middle frontal gyrus | 46 | R | 28 | 46 | 26 | 16 | 4.01 |
| (Risk taking) | Insula | 13 | R | 42 | 6 | 2 | 59 | 4.50 |
| Middle temporal gyrus | 20 | L | −58 | −20 | −20 | 22 | 4.33 | |
| Punishment feedback versus risky response | Inferior frontal gyrus | 48 | L | −30 | 30 | 14 | 41 | 4.78 |
| (Punishment) | Precentral gyrus | 6 | L | −20 | −22 | 62 | 25 | 4.46 |
| Paracentral lobule | 4 | L | −10 | −22 | 74 | 18 | 4.19 | |
| Postcentral gyrus | 3 | L | −28 | −28 | 50 | 28 | 4.18 | |
Note: No significantly stronger activation in male participants, relative to female participants, was observed. Extended cluster = 10 voxels. L = left hemisphere; R = right hemisphere; x, y, z in Montreal Neurological Institute coordinates.
Figure 4.
Whole-brain analysis comparing sex effects on risky versus safe-response contrast and punishment feedback versus risky-response contrast at a voxel-wise intensity threshold of P < 0.001 corrected at a cluster level of 10 voxels. No significantly stronger activation was revealed for the male participants than for the female participants. L = left hemisphere; R = right hemisphere; x, y, z in Montreal Neurological Institute coordinates.
Correlational Analysis
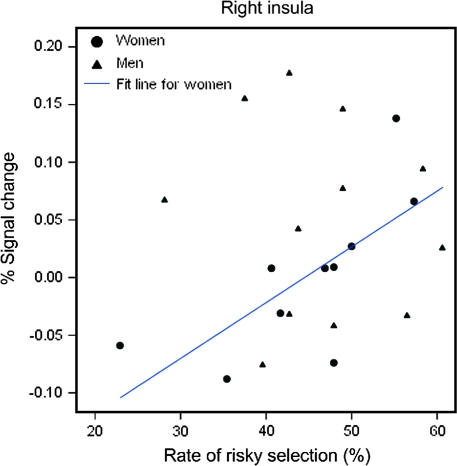
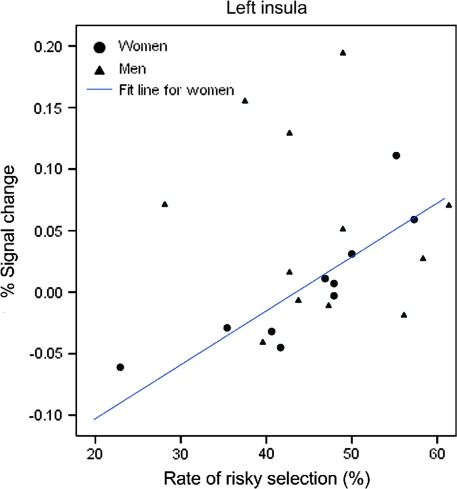
The female participants showed a significant and positive correlation between the rate of risky-response making (i.e., +40 and +80 points) and the percent signal changes in the right and left insula (right: rs = 0.793, P = 0.006, and left: rs = 0.900, P < 0.001) (Figs 5 and 6). No significant correlations were found for the male participants.
Figure 5.
Scatter plot between the rate of risky responses and percent signal change in the right insula.
Figure 6.
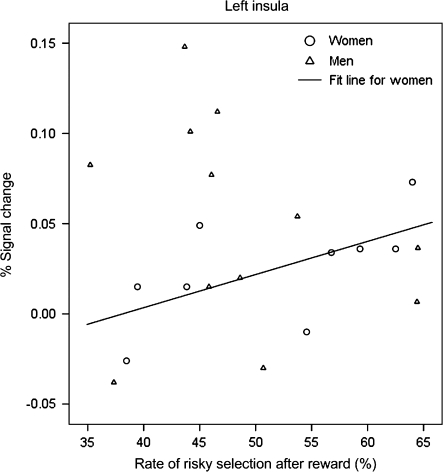
Scatter plot between the rate of risky responses and percent signal change in the left insula.
Effects of Punishment Feedback on Subsequent Responses
Whole-Brain Analysis
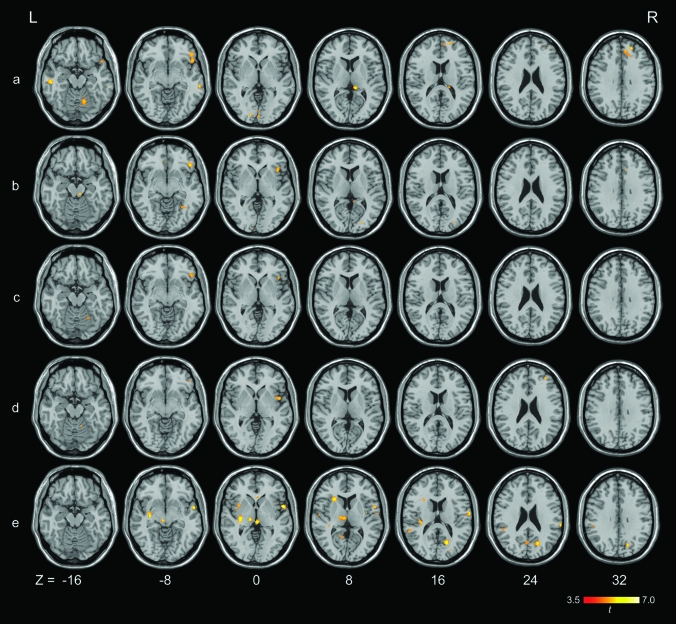
Stronger brain activation was observed for the female participants than for the male participants in all the 5 contrasts related to the effect of punishment feedback. Table 3 and Figure 7 show details of the neural activation.
Table 3.
Differences in brain activation between female and male participants in whole-brain analyses
| BA | Side | Coordinate |
Cluster | T | |||
| x | y | z | |||||
| Females > males | |||||||
| Punished-then-risky versus safe responses | |||||||
| Insula | 13 | R | 46 | 20 | −10 | 108 | 4.53 |
| Superior medial frontal gyrus | 32 | R | 4 | 52 | 18 | 11 | 3.78 |
| Superior frontal gyrus | 9 | R | 16 | 30 | 36 | 228 | 5.36 |
| 9 | R | 18 | 46 | 30 | 43 | 4.89 | |
| 10 | R | 18 | 56 | 18 | 15 | 4.31 | |
| Precentral gyrus | 6 | L | −36 | −26 | 66 | 15 | 4.22 |
| Superior temporal gyrus | 38 | R | 56 | 14 | −4 | 13 | 3.95 |
| Middle temporal gyrus | 20 | R | 58 | −32 | −14 | 55 | 4.54 |
| Inferior temporal gyrus | 20 | L | −54 | −22 | −20 | 102 | 5.49 |
| Calcarine region | 17 | L | −4 | −90 | 4 | 34 | 4.26 |
| Punished-then-risky versus safe-then-risky responses | |||||||
| Inferior orbitofrontal gyrus | 47 | R | 42 | 30 | −4 | 106 | 5.27 |
| Middle cingulate gyrus | 32 | R | 10 | 20 | 40 | 18 | 4.56 |
| Superior occipital gyrus | 19 | R | 22 | −80 | 22 | 10 | 4.10 |
| Fusiform gyrus | 37 | R | 30 | −54 | −6 | 16 | 3.94 |
| Punished-then-risky versus risky-then-risky responses | |||||||
| Insula | 13 | R | 40 | 22 | 0 | 4.02 | 49 |
| Inferior temporal gyrus | 20 | L | −52 | −22 | −22 | 5.31 | 22 |
| Fusiform | 19 | R | 26 | −62 | −14 | 4.19 | 11 |
| Risky-then-risky versus safe responses | |||||||
| Middle frontal gyrus | 46 | R | 30 | 46 | 26 | 4.12 | 21 |
| Insula | 13 | R | 42 | 6 | 2 | 4.63 | 37 |
| Risky-then-risky versus safe-then-risky responses | |||||||
| Postcentral gyrus | 2 | L | −28 | −42 | 58 | 55 | 4.21 |
| Rolandic operculum | 48 | R | 52 | 6 | 0 | 155 | 5.88 |
| 48 | R | 60 | −6 | 14 | 64 | 4.71 | |
| Insula | 13 | L | −26 | 22 | 8 | 99 | 5.01 |
| 13 | L | −36 | 6 | 2 | 14 | 3.99 | |
| Superior temporal lobe | 42 | R | 64 | −30 | 20 | 31 | 5.63 |
| 22 | L | −60 | −10 | 6 | 17 | 4.06 | |
| 41 | L | −50 | −40 | 22 | 22 | 4.03 | |
| 44/22 | L | −58 | −44 | 16 | 15 | 3.88 | |
| Supramarginal gyrus | 48 | L | −54 | −32 | 30 | 11 | 3.89 |
| Superior parietal lobe | 7 | R | 28 | −64 | 54 | 11 | 4.13 |
| Calcarine | 17 | L | −12 | −58 | 10 | 28 | 4.15 |
| Cuneus | 23/17 | L | −8 | −68 | 26 | 15 | 4.12 |
| 18 | R | 16 | −70 | 28 | 176 | 5.73 | |
| Thalamus | L | −14 | −20 | 0 | 216 | 5.39 | |
| Males > females | |||||||
| Risky-then-risky versus safe responses | — | — | — | — | — | — | — |
| Punished-then-risky versus safe-then-risky responses | — | — | — | — | — | — | — |
| Punished-then-risky versus risky-then-risky responses | — | — | — | — | — | — | — |
| Risky-then-risky versus safe responses | |||||||
| Parahippocampal gyrus | 30 | R | 26 | −26 | −26 | 19 | 5.27 |
| Risky-then-risky versus safe-then-risky responses | — | — | — | — | — | — | — |
Note: There were 5 contrasts set in accordance with the responses before or after the feedback. Extended cluster = 10 voxels. L = left hemisphere; R = right hemisphere; x, y, z in Montreal Neurological Institute coordinates.
Figure 7.
Whole-brain analysis showing stronger brain activation in the female participants than in the male participants on a) punished-then-risky versus safe responses; b) punished-then-risky versus safe-then-risky responses; c) punished-then-risky versus risky-then-risky responses; d) risky-then-risky versus safe responses; and e) risky-then-risky versus safe-then-risky responses at a voxel-wise intensity threshold of P < 0.001 corrected at a cluster level of 10 voxels. L = left hemisphere; R = right hemisphere; x, y, z in Montreal Neurological Institute coordinates.
Correlational Analysis
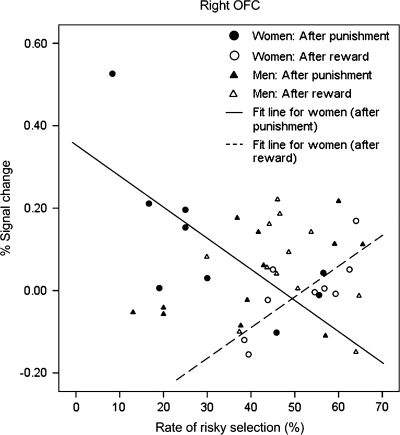
Significant correlations were found between the rate of risky responses and the percent signal change in the OFC region for the female participants (Fig. 8). They were negatively correlated in the punished-then-risky responses (rs = −0.678, P = 0.045) but positively correlated in the risky-then-risky responses (rs = 0.803, P = 0.009). However, no significant correlations were found for the male participants (rs = 0.420, P = 0.174, and rs = −0.189, P = 0.557, respectively). In the left insula, we found a significant and positive correlation only for the risky-then-risky response (rs = 0.740, P = 0.023), but this was not observed for the male participants (rs = −0.315, P = 0.319) (Fig. 9).
Figure 8.
Scatter plot between the rate of risky responses after receiving punishment feedback (i.e., punished-then-risky response) and its percent signal change in the right OFC.
Figure 9.
Scatter plot between the rate of risky responses after making a risky response (i.e., risky-then-risky response) and its percent signal change in the left insula.
Discussion
Consistent with our a priori prediction, sex was associated with differential patterns of neural activity during the risk-taking process. Stronger BOLD responses were observed in the right insula and bilateral OFC for the female participants than for the male participants when making a risky response. Among the female participants, the rate of risky responses was related to the strength of the neural activity in the insula when risky choices were made subsequent to risky responses (i.e., risky-then-risky response). The female participants also showed stronger neural activity than their male counterparts in the precentral, paracentral, and postcentral areas when receiving punishment feedback. Only among the female participants did we observe a correlation of OFC activity with the rate of risky responses: the neural activity of the right OFC correlated negatively with the rate of making risky choices after receiving punishment feedback (i.e., punished-then-risky response) and positively with the rate of making risky responses but after selecting a risky response (i.e., risky-then-risky response). The 2 main findings revealed in this study are, first, that there is a strong sex-related influence modulating the neural activities when performing the risk-taking task; and, second, that the insula and the OFC possibly have differential roles in updating and valuating the options involved in risk-taking actions. Although risk-taking behavior is strongly associated with measures of sensation seeking and impulsivity (Leland and Paulus 2005), it is unlikely that the present findings are confounded by the risk-seeking tendencies of the participants because both the male and female participants were matched in terms of their impulsivity scores and exhibited similar rates of risk-taking behavior during the in-scanner experiment.
Valuation of Actions in the Risky-Gains Task
Valuation of actions is one of the 5 processes of the value-based decision-making model proposed by Rangel et al. (2008). Valuation of actions is the process by which values are assigned to different possible actions in accordance with the rewards and the costs, or “prospects,” associated with an action as perceived by the individual (Kahneman and Tversky 1979; Neumann and Morgenstern 2004). Within the valuation process, a goal-directed system is among the 3 systems involved in assigning a value to an action (the other 2 are the Pavlovian and Habitual systems; see Rangel et al. 2008). Previous research has suggested that activity in the OFC is an important neural substrate of the goal-directed system (Wallis 2007). Specifically, the OFC plays a significant role in assigning the goal and decision values (Hare et al. 2008) and in positive outcome evaluation (Breiter et al. 2001).
In the Risky-Gains task used in this study, making a safe response (+20) could be a habitual response that did not seem to involve much updating and valuating of the value associated with the choice of response. In contrast, making a safe-then-risky response required the participant to value the action of adhering to a habitual response against the prospect of gaining higher scores (+40/+80), whereas making a risky-then-risky response required the updating of reward feedback gained from the +40 response and valuating the prospect of gaining even higher scores (+80). It was expected that the participants would exhibit an emotional reaction on receiving punishment feedback after making a risky response (−40/−80 trials). Hence, making a punished-then-risky response would require updating the emotional reaction associated with the punishment and at the same time valuating the prospect of gaining higher scores.
Making Safe and Risky Responses
The female participants showed stronger neural activations than the male participants in the right insula and the bilateral OFC when making risky responses. Risk-taking decision-making involves resisting the habitual selection of a safe response (+20 trials) in order to achieve a higher score. Our findings regarding the involvement of the right insula in risk-taking behaviors are consistent with other studies on risk-taking processes (e.g., Ernst et al. 2002; Krain et al. 2006) and decisions of higher versus lower risks (e.g., Paulus et al. 2003). The insula has been previously found to play an important role in risk estimation (Sanfey et al. 2003). Thus, activity in the insula observed in this study may be associated with the anticipation of making a risky response. In other words, the insula may mediate the anticipation of punishment or loss rather than the actual emotional consequences of being punished. Indeed, in studies of emotion processing, activity in the insula was observed to be associated with anticipating the presentation of aversive visual images (Simmons et al. 2004; Phan et al. 2006), making judgments about different emotional stimuli (Gorno-Tempini et al. 2001), processing fearful faces (Morris et al. 1998), and being aware of threats and internal states of the body (Critchley et al. 2002; Critchley et al. 2004). Because the male and female groups were matched in terms of their rates of response selection, the stronger right insula activation observed in women than men may suggest that more neural activity is involved in risk estimation in women than men. This process would probably involve the anticipation of potential aversive outcomes associated with selecting a risky choice.
The OFC is known for its role in decision-making, especially when the outcome of a decision is uncertain (e.g., Bechara et al. 2000; O'Doherty et al. 2001). Recent studies have further demonstrated that the OFC is involved in reward-related decision making (Bechara et al. 2003; Elliot et al. 2003; O'Doherty et al. 2003; Paulus et al. 2003; Rolls 2004; Cohen et al. 2005; Eshel et al. 2007). Our findings regarding the stronger activation in the OFC for the female participants than for the male participants suggest that women need to partake in a higher degree of mental consideration before making a risky response.
The intensity of the left and right insula activities was found to correlate positively with the rate of making risky responses among the female participants but not among the male participants. This observation is inconsistent with the findings of Paulus et al.’s (2003) study, which revealed a nonsignificant correlation with sex. We argue this might be due to the methodological discrepancies between the 2 studies. For example, in Paulus et al.’s study, the participants’ personality traits were found to correlate with the percent signal change in the insula, whereas in this study we attempted to control some of the personality traits, for instance, impulsiveness, when studying this sex effect.
Reward/Punishment Feedback and Making Subsequent Risk Responses
When the participants received punishment feedback after making a risky response, stronger activation was observed in the inferior frontal gyrus as well as in the precentral and postcentral regions for the female participants than for the male participants. This stronger activation suggests that the female participants were more reactive to being punished. This responsiveness may arise because the female participants are more mentally alert than the male participants when updating and valuating their subsequent actions during the task. When the participants made a risky response subsequent to being punished, stronger and more extensive neural activities were observed among the female participants than among the male participants in the superior frontal gyrus; the precentral gyrus; the superior, middle, and inferior temporal lobes; and the calcarine regions. This pattern of neural activation appears to be associated with decision making in situations of uncertainty (Paulus et al. 2001). The stronger activation among the female participants in the right insula and the superior temporal may mediate the decision to resist making a habitual response (i.e., a safe response) and instead make a risky response (Paulus et al. 2005). In other words, after receiving the punishment feedback, the female participants may hesitate more before making a subsequent risky response than the male participants.
The contrast between risky-then-risky and safe responses sheds light on the neural processes associated with risk taking after receiving reward feedback. The female participants showed stronger activity than their male counterpart in the right insula and the middle frontal gyrus. Previous reports have suggested that the association between activity in the insula and anticipation of negative emotions as we have previously discussed (e.g., Simmons et al. 2004; Phan et al. 2006). Hence, when deciding to make a risky response (40/80) after a previous risky response (40/80), the female participants might anticipate a negative outcome (the possibility of losing points), and such information would then be incorporated into the valuation of subsequent actions in making the risky response (Critchley et al. 2002, 2004). In contrast, the male participants showed stronger activity in the right parahippocampal gyrus than did the female participants. Harrington et al. (2004) proposed that right parahippocampal activity is associated with encoding activity during the process of decision making. Following this line of thoughts, it is likely that the male participants, relative to their female counterparts, were more active in encoding the positive experience from the reward feedback. The encoded positive experience may render men than women more ready for committing to subsequent risk-taking behaviors.
In this study, we observed some moderate relationships between the rate of making risky responses after receiving punishment or reward feedback, and the activities of the right OFC only for the female participants, suggesting that there may be a sex-related difference in the role played by the OFC in valuation of action options and subsequently in the regulation of the responses to be made based on the updated information. It is noteworthy that in female participants, the OFC appeared to play differential roles when punishment feedback was received versus when reward feedback was received. There was a negative relationship between the OFC activity and the rate of risky responses after receiving reward feedback (i.e., risky-then-risky response), and a positive relationship between the OFC activity and the rate of risky response after receiving punishment feedback (i.e., punished-then-risky response).
Sex-Related Differences in Risk Taking
According to the literature on the evolution of gender differences in risk taking, risk averse psychological mechanisms are better developed in the female sex and risk prone behaviors are better developed in the male sex (Hawkes 1991; Miller 2000; Gray 2004). This accords with the sexual selection theory (Darwin 1871; Andersson 1994). Findings of this study may provide a neuroscientific perspective of understanding the proposed sex-related difference in risk-taking attitude. In the process of risk-taking decision making, when knowledge of the risk parameters is incomplete, such as when the participants in this study had no knowledge of the probability of the outcomes of the trials on the Risky-Gains task, ambiguity is created. The parameter of such ambiguity needs to be encoded in the brain during the process of value assignment, via activity in brain regions such as the OFC and insula, to modulate the goal-directed system. The outcome of this is a modification of values assigned to actions in the ambiguous situation that would lead the individual to show an aversion to choices that are ambiguous (Camerer and Weber 1992). The stronger OFC and insula activities in women than in men may reflect the sensitivity of women to situations of ambiguity. This sensitivity may activate neural resources to understand the parameters of the ambiguity (OFC and insula activities) and the outcome of previous actions (OFC activity) in order to modulate value assignment so as to make a beneficial decision. Also, this sensitivity to ambiguity could make women more risk averse than men.
If sex-related differences in risk-taking attitude are at least partly imprinted during human evolution, one should expect corresponding sex-related genotypical and hence phenotypical differences. Indeed, Gur et al. (2002) have found sex differences in the frontal brain volumes of healthy adults: women were found to have larger OFCs than men, which meant there was a highly significant difference in the ratio of orbital gray to amygdala volume. The observed sex-related difference in neural activity of the OFC during risk taking may relate to the underlying sex differences in the neuroanatomy of the neural substrates in the prefrontal cortex. Furthermore, neural mechanisms may have evolved to allow for the development of a risk prone attitude in men and a risk averse attitude in women when facing risky situations. Thus, stronger activity in the OFC and the insula in women during risk taking, which are required for ambiguity encoding, outcome updating, and value assignment, may have a genetic basis. Whether this is the case remains unverified, but it is worth examining because it would have significant implications for interventions for disorders relating to maladaptive risk-taking behaviors such as pathological gambling.
In summary, the findings of stronger activities in the OFC and insula during risk taking in females, relative to males, clearly indicates that sex could be a significant modulator of the goal-directed valuation system in situations of ambiguity. The differential influence of sex on the other valuation systems in the value-based decision-making model (Rangel et al. 2008), namely the Pavlovian and Habitual valuation systems, remains unclear and awaits future investigation.
Limitations
Although we controlled for the impulsive tendencies of the participants in order to depict a clearer picture of the sex differences in neural activity during risk taking, the study still has some limitations worth noting. First, although we controlled for impulsiveness, we did not do so for other personality traits that may have impacted the observed sex effect. Second, due to resource constraint, physiological information such as the menstrual phase of the female participants, which might have affected the results, was not collected. Third, the male and female groups in this study might have differed in their social and cultural backgrounds. These factors may have influenced their risk-taking behavior as well as their brain activation. Hence, future studies should consider including more cohorts for a better delineation of the relationship between sex and the changes in neural activity associated with risk taking.
Conclusion
This study has extended previous research on risk-taking behaviors by examining sex effects on the neural activity associated with risky decision making. The findings clearly support the speculation that sex-related differences exist in the neural activity associated with risk taking. Men and women do call upon very different neural processes and mental resources even when their behavioral outcomes are comparable.
Funding
This project was supported by the Research Output Prize of The University of Hong Kong, Collaboration Research Award of the national Natural Science Foundation of China (30828012), and the Niche Area Research Grant on “Applied Cognitive Neuroscience and Neuroplasticity” of The Hong Kong Polytechnic University.
Acknowledgments
Conflict of Interest: None declared.
References
- Andersson M. Sexual selection. Princeton: Princeton University Press; 1994. [Google Scholar]
- Arce E, Miller DA, Feinstein JS, Stein MB, Paulus MP. Lorazepam dose-dependently decreases risk-taking related activation in limbic areas. Psychopharmacology. 2006;189:105–116. doi: 10.1007/s00213-006-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003;19:23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cereb Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahnerman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Presented at the 8th international conference on Functional Mapping of the Human Brain, June 2–6, Sendai, Japan. 2002. Region of interest analysis using an SPM toolbox [abstract] Available on CD-ROM in Neuroimage 16(2) [Google Scholar]
- Camerer CF, Weber M. Recent developments in modeling preferences: uncertainty and ambiguity. J Risk Uncertain. 1992;5:325–370. [Google Scholar]
- Cohen MX, Heller AS, Ranganath C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cogn Brain Res. 2005;23:61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Darwin C. The descent of man, and selection in relation to sex. Princeton: Princeton University Press; 1871. [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Elliot R, Newman JL, Longe OA, William Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rees G, Dolan RJ. Ventromedial prefrontal cortex mediates guessing. Neuropsychologia. 1999;37:403–411. doi: 10.1016/s0028-3932(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, Nicoletti R, Umita C, Nichelli P. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Gray PB. Evolutionary and cross-cultural perspectives. J Gambl Stud. 2004;20:347–371. doi: 10.1007/s10899-004-4579-6. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, Rao SM. Neural representation of interval encoding and decision making. Brain Res Cogn Brain Res. 2004;21:193–205. doi: 10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Hastie R, Dawes RM. Rational choice in an uncertain world. Thousand Oaks (CA): Sage Publications; 2001. [Google Scholar]
- Hawkes K. Showing off: tests of a hypothesis about men's foraging goals. Ethol Sociobiol. 1991;12:29–54. [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision making. Neuroimage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Leland DS, Paulus MP. Increased risk-taking decision-making but not altered response to punishment in stimulus-using young adults. Drug Alcohol Depend. 2005;78:83–90. doi: 10.1016/j.drugalcdep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage. 2006;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Miller G. The mating mind. New York: Anchor Books; 2000. [Google Scholar]
- Morris JS, Friston KJ, Beuchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Naqvi N, Shiv B, Bechara A. The role of emotion in decision making: a cognitive neuroscience perspective. Curr Dir Psychol Sci. 2006;15:260–264. [Google Scholar]
- Neumann JV, Morgenstern O. Theory of games and economic behavior. Princeton: Princeton University Press; 2004. pp. 17–20. [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–744. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005;25:607–615. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Zauscher B, McDowell JE, Frank L, Brown GG, Braff DL. Prefrontal, parietal, and temporal cortex networks underlie decision-making in the presence of uncertainty. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Rogers RD, Owen AM, Middleton HC, Williams EJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates interior and orbital prefrontal cortex. J Neurosci. 1999;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rosenblitt JC, Soler H, Johnson SE, Quadagno DM. Sensation seeking and hormones in men and women: exploring the link. Horm Behav. 2001;40:396–402. doi: 10.1006/hbeh.2001.1704. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Harris LJ. Handedness, sex, and familial sinistrality effects on spatial tasks. Cortex. 1993;29:115–134. doi: 10.1016/s0010-9452(13)80216-x. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Exp Clin Psychopharmacol. 2003;11:210–217. doi: 10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]