Abstract
Many neuroimaging studies of the mirror neuron system (MNS) examine if certain voxels in the brain are shared between action observation and execution (shared voxels, sVx). Unfortunately, finding sVx in standard group analyses is not a guarantee that sVx exist in individual subjects. Using unsmoothed, single-subject analyses we show sVx can be reliably found in all 16 investigated participants. Beside the ventral premotor (BA6/44) and inferior parietal cortex (area PF) where mirror neurons (MNs) have been found in monkeys, sVx were reliably observed in dorsal premotor, supplementary motor, middle cingulate, somatosensory (BA3, BA2, and OP1), superior parietal, middle temporal cortex and cerebellum. For the premotor, somatosensory and parietal areas, sVx were more numerous in the left hemisphere. The hand representation of the primary motor cortex showed a reduced BOLD during hand action observation, possibly preventing undesired overt imitation. This study provides a more detailed description of the location and reliability of sVx and proposes a model that extends the original idea of the MNS to include forward and inverse internal models and motor and sensory simulation, distinguishing the MNS from a more general concept of sVx.
Keywords: action, execution, fMRI, mirror neurons, observation
Introduction
What other people do is one of the most important stimuli in our environment. Accordingly, our brain devotes significant neural resources to processing these stimuli. The discovery of mirror neurons (MNs; all abbreviations are described in Table 1) in the ventral premotor (PM) cortex (area F5) of the monkey, that respond during the execution of the monkey's own actions and while the monkey observes (di Pellegrino et al. 1992; Gallese et al. 1996; Umilta et al. 2001; Kohler et al. 2002; Keysers et al. 2003) or hears (Kohler et al. 2002; Keysers et al. 2003) other individuals perform similar actions, has suggested that we process the actions of others at least in part by associating them with ours (Gallese et al. 2004; Keysers and Perrett 2004; Keysers and Gazzola 2006). Recent single cell recordings in the rostral inferior parietal lobule have shown that the parietal lobe also contains MNs (Fogassi et al. 2005; Fujii et al. forthcoming). Substantial efforts have thereafter been placed in examining if humans have a similar system and whether additional brain areas may be common to motor execution and observation (Fadiga et al. 1995, 2002; Grafton et al. 1996; Decety et al. 1997; Hari et al. 1998; Iacoboni et al. 1999, 2001; Buccino et al. 2001; Gangitano et al. 2001; Avikainen et al. 2002; Aziz-Zadeh et al. 2002, 2004, 2006; Grezes et al. 2003; Heiser et al. 2003; Buccino, Lui, et al. 2004; Buccino, Vogt, et al. 2004; Leslie et al. 2004; Bangert et al. 2005; Borroni et al. 2005; Calvo-Merino et al. 2005; Fogassi et al. 2005; Iacoboni et al. 2005; Molnar-Szakacs et al. 2005, 2006; Montagna et al. 2005; Mottonen et al. 2005; Nelissen et al. 2005; Calvo-Merino et al. 2006; Cross et al. 2006; Dapretto et al. 2006; Gazzola et al. 2006; Hamilton and Grafton 2006; Jackson et al. 2006; Gazzola, Rizzolatti, et al. 2007; Gazzola, van der Worp, et al. 2007). Functional magnetic resonance imaging (fMRI) has played a prominent role in these efforts: by testing human or primate subjects while executing actions and observing similar actions, fMRI can determine if the blood oxygen–level dependent (BOLD) signal within a certain voxel is augmented both during action observation and execution. If this is the case, the voxel can be said to be “shared” by 2 processes: execution and observation. We will refer to such voxels as “shared voxels” (sVx) instead of mirror voxels because sVx could, but do not necessarily have to, contain MNs: they could contain 1) 2 distinct populations of neurons, one responding only during motor execution and one only during action observation, 2) true MNs, or 3) a combination of both (Morrison and Downing 2007). In addition, the term MNs is so tied to the motor system, that if a voxel outside the motor system is recruited during action execution and observation, a more neutral term, sVx, might be more appropriate.
Table 1.
List of abbreviations used in the paper
| Area | Cyto. reference | Description |
| ACC | Anterior cingulate cortex | |
| BA | Brodmann area | |
| BA1 | (Geyer et al. 1999, 2000) | Part of SI |
| BA2 | (Grefkes et al. 2001) | Part of SI |
| BA3a | (Geyer et al. 1999, 2000) | Part of SI |
| BA3b | (Geyer et al. 1999, 2000) | Part of SI |
| BA44 | (Amunts et al. 1999) | Inferior frontal gyrus pars opercularis |
| BA45 | (Amunts et al. 1999) | Inferior frontal gyrus pars triangularis |
| BA4a | (Geyer et al. 1996) | Anterior part of the primary motor cortex |
| BA4p | (Geyer et al. 1996) | Posterior part of the primary motor cortex |
| BA6 | (Geyer 2003) | Premotor cortex (laterally) and SMA (mesialy) |
| FEF | Frontal eye field | |
| fMRI | Functional magnetic resonance imaging | |
| Hca | Hand complex action—observation condition | |
| Hexe | Hand execution—execution | |
| hlP1 | (Choi et al. 2006) | Human intraparietal area 1 |
| hlP2 | (Choi et al. 2006) | Human intraparietal area 2 |
| Hm | Hand movement—observation condition | |
| Hst | Hand static—observation condition | |
| IPL | Inferior parietal lobule | |
| MCC | Middle cingulate cortex | |
| MFG | Middle frontal gyrus | |
| MNS | Mirror neuron system | |
| MTG | Middle temporal gyrus | |
| OP 1–4 | (Eickhoff, Amunts, et al. 2006; Eickhoff, Grefkes, et al. 2007) | SII in the parietal operculum |
| PF | (Caspers et al. 2006) | Rostral inferior parietal lobule, BA40 |
| PFcm | (Caspers et al. 2006) | Rostral inferior parietal lobule, BA40 |
| PFm | (Caspers et al. 2006) | Rostral inferior parietal lobule, BA40 |
| PFop | (Caspers et al. 2006) | Rostral inferior parietal lobule, BA40 |
| PFt | (Caspers et al. 2006) | Rostral inferior parietal lobule, BA40 |
| PGa | (Caspers et al. 2006) | Caudal inferior parietal lobule, BA39 |
| PGp | (Caspers et al. 2006) | Caudal inferior parietal lobule, BA39 |
| PLSD | Probability according to a LSD post hoc test | |
| Pnk | Probability according to a Newman–Keuls post hoc test | |
| PPC | Posterior parietal cortex | |
| SFG | Superior frontal gyrus | |
| SI | Primary somatosensory cortex, areas 1, 2, 3a, 3b | |
| SII | Secondary somatosensory cortex (OP 1–4) | |
| SMA | Supplementary motor cortex | |
| SPL | Superior parietal lobule | |
| STG | Superior temporal gyrus | |
| sVx | Shared voxels (active during action observation and execution) |
To our knowledge, all fMRI studies investigating the mirror neuron system (MNS) so far have however used conventional group analyses in which the data of each subject is first smoothed, and then only tested at the group level. This specific way of analyzing fMRI data adds 2 additional problems. First, although smoothing can be beneficial (to improve signal to noise ratio and uniform the spatial correlation between adjacent voxels (Worsley and Friston 1995) if the aim is to demonstrate the presence of sVx, it introduces an undesirable side effect: 2 neighboring but not overlapping clusters of voxels (one responding only to action observation and one only to action execution) would seemingly overlap at their common border after a Gaussian kernel has blurred their fringes (Morrison and Downing 2007). Second, although many investigators assume that random effect analyses identify effects that are present in all subjects, a voxel that in half the subjects is only involved in action observation and in the other half only in action execution, could seem to be involved in both task at the group level (see Fig. S1 and Morrison and Downing 2007).
Although fMRI therefore cannot disentangle alternatives (1–3) mentioned above (i.e., whether an sVx really contains MNs), the additional problems associated with smoothing and group analyses can and should be overcome by using single-subject analyses and unsmoothed data. Morrison and Downing (2007) have recently demonstrated the importance of this approach for the study of pain: using smoothed group data, they found that observing the pain of others and experiencing pain causes brain activations that overlap in the anterior cingulate cortex (ACC), confirming previous findings (Singer et al. 2004; Botvinick et al. 2005; Jackson et al. 2005) but using unsmoothed single-subject data, half their subjects entirely failed to show sVx in the ACC, and the other half had only marginal overlaps between pain observation and execution. They concluded that a similar lack of sVx in single subjects may apply to the motor MNS.
Here we examine this alarming possibility that would undermine the credibility of most fMRI studies on the MNS. Our 2 core questions were therefore whether and where sVx can be found at the level of unsmoothed single-subject data during action observation and execution. For this aim, we presented movies of hand actions to 16 healthy participants while recording their brain activity using fMRI and on a following day, we asked them to perform similar actions in the scanner. To overcome the problems of group analyses on smoothed data we then examined this data on a subject-by-subject basis, using unsmoothed data. This led to 3 findings. First, sVx can be reliably identified in single subjects using motor execution and observation and unsmoothed data, showing that sVx in the motor domain are not only the result of smoothing and group analyses. Second, “classical” regions of the MNS (i.e., the ventral PM cortex or anterior inferior parietal lobule in which MNs have been found in monkeys) contain more sVx than expected by chance in all our participants. Third, a number of regions outside of the areas shown to contain MNs in monkeys also contain sVx. This latter finding has 2 related implications discussed at the end of this paper: these novel regions should be investigated further using a variety of techniques including single cell recordings to examine how they contribute to action observation and execution, and we probably need to expand the concept of shared circuits beyond the 2 brain areas in which serendipity has made us first discover MNs.
With the recent advent of cytoarchitectonic probabilistic maps for many of the brain areas involved in action observation and execution (Geyer et al. 1996, 1999, 2000; Amunts et al. 1999; Grefkes et al. 2001; Eickhoff et al. 2005; Caspers et al. 2006; Choi et al. 2006; Eickhoff, Amunts, et al. 2006) the current study will also aim to provide cytoarchitectonic labels to the locations containing most sVx. The use of these labels can facilitate the comparison between species and pave the way to a more systematic comparison of brain location between studies.
Materials and Methods
Subjects and General Procedures
The present report is a combination of 1) innovative analyses of data partially described elsewhere (Gazzola, Rizzolatti, et al. 2007), 2) novel motor execution conditions obtained on the same participants to determine the hand selectivity of regions of interest. Sixteen healthy volunteers participated in the experiment (14 right and 2 left handed; 9 females and 7 males; mean age 31 years ranging 25–45 years; normal or corrected to normal vision; no history of neurological disorders) and were tested with 3 different categories of stimuli in 3 separate days (Fig. S2). To avoid biasing the processing of the stimuli of any given day based on the other conditions, the experiments labeled days 1–3 were always acquired in this particular chronological order to ensure that the motor task (day 3) does not bias the brain responses of the visual tasks (days 1 and 2). All subjects were informed about the content of the study and signed an informed consent on a day-by-day basis, and subjects were therefore unaware of the fact they will need to execute actions in the scanner while watching the actions of others.
All experiments were approved by the Medical Ethical Commission of the University Medical Center Groningen (NL).
Day 1: Viewing Static Images
Subjects viewed static pictures of a human hand or objects on a table (see Gazzola, Rizzolatti, et al. 2007; for further details) and importantly for the present report, as a control condition, they viewed a scrambled version (scr) of the same pictures to determine activations that can be accounted for by viewing low-level visual patterns.
Day 2: Viewing Movies of Actions
Subjects viewed movies with the same human hand interacting (i.e., grasping, moving, etc) with objects (the same objects showed in the pictures of day 1, e.g., a cup, a glass, etc) placed on a table (hand complex action: Hca). Control conditions included: a hand simply moving to rest on the table (hand movement: Hm) or the same hand simply resting on the table (hand static: Hst) behind the objects used in Hca. All conditions were presented in a block design, with 4 exemplars of each condition picked out pseudorandomly to form 13.5-s blocks containing 4 different actions or 4 different static images, separated by 200-ms intervals of blank screen. The order of blocks was pseudorandom and consecutive blocks were separated by a 10-s pause of blank screen with a fixation cross. The experiment was split in 4 runs with a total of 12 repetitions per condition. Half of the blocks depicted only hands entering from the right of the screen, and half only hands entering from the left of the screen. Subjects were instructed to watch the movies carefully, paying particular attention to the relationship between the hands and the objects (again see Gazzola, Rizzolatti, et al. 2007, for further details). Days 1 and 2 also contained stimuli involving a robotic agent, but these will not be analyzed here. Importantly, days 1 and 2 involved passive viewing only (with the verbal instructions: “We might ask you questions about the stimuli after scanning” to maintain attention) without any motor requirements in order to ensure that hand motor responses would not be primed.
Day 3: Motor Tasks
Subjects performed 4 different motor tasks in separate runs (1–4; order of acquisition counterbalanced across participants).
-
1) HandExecution (Hexe): Before scanning the subject was shown the T-shaped table that would be placed on his/her lap during scanning. The table contained 4 objects. The 2 lateral branches of the T contained a high-stemmed plastic glass. The intersection of the T contained a plastic bowl with a plastic spoon. The bottom of the T contained a plastic cup with a handle. Subjects were then trained on their task. The task sequence was as follows: at the commencement of each trial subjects viewed a diagram of the table on the screen, with a pink rectangle at the left or right to indicate what hand to use, and a red cross in one of the 4 object locations indicated which objects they had to act upon. When the red cross turned to green after 1 s, subjects had to perform the action compatible with the object. For the glass, they had to reach for the glass, grasp it, bring it toward their mouth, but stop before reaching the mouth, and then replace it in its original location. For the cup of coffee, they had to do the same action, but grasping the cup by the handle. For the bowl, they had to perform the same action as above, but with the spoon, as if drinking soup with a spoon. Subjects were then placed in the scanner, with their heads and lower arms firmly strapped onto the scanner bed to avoid that the actions would lead to significant head motion (in all subjects within session head motion remained lower than 1 mm of translation and 3° of rotation). We ensured that subjects were unable to see their own actions and trained them to perform the actions under these conditions. The timing of the actions was rehearsed to last approximately 5 s, but an experimenter within the scanner room documented the beginning and end of each action using a button box to determine the actual duration of the action, that was then used to define the design matrix for data analysis.
Within a single scanning session of 500 s, subjects performed 18, ∼5 s actions with their right hand (HexeR) and 18 with their left (HexeL). Their arms never crossed the table (i.e., right hand only grasped the right glass, and left hand only the left glass), and the 18 actions were composed of 6 actions involving each of the 3 objects. Conditions were fully randomized with 13 ± 2 s lapsing between the onset of 2 conditions.
This motor task is matched closely to the actions shown in the movies (e.g., a glass is grasped in both, etc.) and serves to define voxels shared between observation and execution. Given that grasping a glass in the scanner is not feasible using ones mouth, a separate set of 3 motor conditions was used to assess motor somatotopy, including an additional finger execution condition. These 3 runs were matched in the duration of the actions and the number of repetitions.
2) MouthExecution: subjects had to manipulate a small object hanging from a wooden rod by only moving their lips. The appearance of a central green cross indicated the beginning of the action; its disappearance, the end. The experimenter lowered the rod based on acoustic instructions matched in time with the appearance of the green cross. Each single manipulation lasted for 4 s and was repeated 16 times.
3) FeetExecution: subjects had to manipulate an object using their first and second toe. Again the appearance of a green cross indicated the beginning of the action and its disappearance, the end. The position of the cross relative to the side of the screen (left or right) indicated the foot to be used. The experimenter received acoustic instructions indicating whether the object was to be placed between the toes of the right or left foot. Each manipulation lasted 4 s and was repeated 16 times for each foot.
4) FingerExecution: subjects had to manipulate an object between their fingers. Again the appearance of a green cross indicated the beginning of the action, its location, the hand to be used and its disappearance, the end. The experimenter received acoustic instructions indicating whether the object was to be placed in the right or left hand. Each manipulation lasted 4 s and was repeated 16 times for each hand.
Functional Magnetic Resonance Imaging
Scanning was performed using a Philips Intera 3T Quaser, a synergy SENSE head coil, 30 mT/m gradients and a standard single shot EPI with time echo = 30 ms, TA = time repetition = 2 s, 39 axial slices of 3 mm thickness, with no slice gap and a 3 × 3 mm in plane resolution acquired to cover the entire brain and cerebellum.
General Data Processing
Data were preprocessed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/software/spm2). EPI images from all sessions were slice time corrected and realigned to the first volume of the second day of scanning. High quality T1 images were coregistered to the mean EPI image and segmented. The coregistered gray matter segment was normalized onto the MNI gray matter template and the resulting normalization parameters applied to all EPI images. For each individual, data were then analyzed voxel-by-voxel by applying a general linear model on the unsmoothed normalized data (unless specified otherwise). All conditions were modeled using a box-car function convolved with the hemodynamic response function (HRF). Additional predictors of no interest were modeled to account for translation and rotation along the 3 possible dimensions as determined during the realignment procedure. In particular, we used the following predictors of interest (all of which were convolved with the HRF before estimating the GLM):
Day 1: Boxcar functions with the duration of the pictures.
Day 2: Boxcar functions with the duration of the block containing 3 movies or static controls. Separate predictors were used for the Hca, Hm, and Hst conditions.
Day 3: For Hexe runs, 4 box-car predictors were defined. Two preparatory predictors (one for the right, HprepR, and one for the left, HprepL, hand preparatory phase) started with the beginning of the instruction screen and ended with the go-signal (green cross) one second later. They were defined to capture neural processes time locked to the instruction, therefore reflecting the visual processing involved in decoding the instruction and the visuomotor planning phase of the motor act, and are considered conditions of no interest (see Fig. S7C). The remaining motor execution predictors started when the cross turned to green (go-signal) and lasted for the entire duration of the action. They captured the processes involved in executing the actions. These latter 2 are used in this paper to determine areas used to execute complex actions (right-hand actions are abbreviated HexeR and left-hand actions HexeL). For the MouthExe run, a single box-car predictor was defined, reflecting the duration of the green cross and therefore the movement period. For FingerExe and FootExe runs, separate predictors for right and left movements were used, again reflecting the duration of the green cross and therefore the movement.
Subject-by-Subject sVx
For each subject, at the first level of analysis, using unsmoothed data (except for Fig. 2, where we compare the same analysis with smoothed and group data), we defined a voxel as sVx if the following 3 conditions were satisfied at the same time (Fig. S2). 1) The t-value of the contrast Hca-Hst was above 2.33 & the t-value of the contrast Hca-Hm was above 2.33 (t = 2.33 corresponds to P < 0.01, and the logical “&” means that the overall global null likelihood of a false positive is 0.012 = 0.0001); 2) the maximum t-value during action executions with the right or left hand exceeded 3.13 (t = 3.13 corresponds to P < 0.001); 3) the t-value of the contrast ScramblePicture-Rest was below 3.10 (corresponding to P > 0.001). In other words: (Hca-Hst > 2.33) & (Hca-Hst > 2.33) & (max(HexeR,HexeL)-Rest > 3.13) & (scr-Rest < 3.10). Although the initial visual instructions and motor planning phase of the motor task were modeled separately from the motor execution phase (and not included in the Hexe parameter estimates), to further exclude low-level visual confounds from our estimates of motor areas, we exclusively masked the maps of Hexe with those obtained from viewing scrambled pictures in day 1 (see Fig. S7 for an illustration of the impact of these controls). We took the maximum of the t-value of right or left hand execution to combine structures involved in using the right or the left hand. The results of these logical “&”s is a single Boolean map per subject containing the value 1 when all conditions are satisfied and 0 when they are not. The selection of thresholds was motivated as follows: P < 0.001 is a established threshold in neuroimaging, and was used here for the motor execution and the scrambled images. For observation, the use of a conjunction between Hca-Hst and Hca-Hm reduces the likelihood of false positives, and we therefore relaxed the individual thresholds to P < 0.01, the threshold used in the only other study using single-subject analysis of sVx (Morrison and Downing 2007). This helped preserve statistical power, a critical issue in neuroimaging (Thirion et al. 2007). At the single-subject level, we did not correct statistical thresholds for the number of voxels in which the mass univariate analysis was repeated. This introduces the risk that some of the sVx in Figures 1 and S3 are false positives (within the 44294 voxels of the brain, one has a 0.05 chance of finding up to 56 voxels by chance using P∼0.001 as a voxelwise threshold). However, for the spatial consistency maps (see below) we do control the family-wise error risk by using a Bonferroni correction of 44294 (the number of voxels in the brain).
Figure 2.
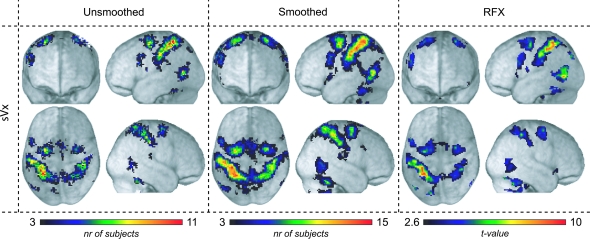
Consistency of sVx rendered on the average T1 image of all 16 subjects. Left and middle columns show the number of subjects showing sVx properties in each voxel using unsmoothed and smoothed data respectively. Only voxels where at least 3 subjects showed sVx are shown (P < 0.025, Bonferroni corrected, see “probabilistic considerations” in Materials and Methods). Note that the color bars of the left and middle panels differ in upper bound to maximize the chromatic range within each panel. The right column shows the t-values of a traditional random effect analysis using smoothed data as in (Gazzola, Rizzolatti, et al. 2007).
Figure 1.
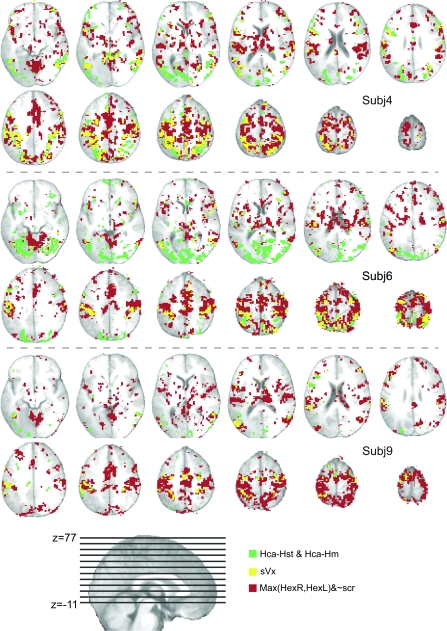
Brain activity for 3 randomly selected single subjects. Activations are shown on 12 axial slices taken at 8-mm steps to range from z = −11 to z = 77, as shown on the sagittal section at the bottom of the figure. Sections are taken from the average T1 image of all 16 participants. Green voxels represent voxels where the contrast Hca-Hst&Hca-Hm was significant. Red voxels, those where the execution of hand actions using the right or the left hand was significant, but where the sight of scrambled images did not produce significant activations. Yellow voxels are those where both conditions are met.
Spatial Consistency Maps
To quantify how consistently a certain voxel was shared between subjects, we summed the 16 Boolean sVx maps defined above (one for each subject). This results in a map containing values ranging from 0 to 16 that quantify the number of subjects for which that particular voxel is sVx (i.e., 0 = in none of the subjects that voxel was sVx; 16 = for all subjects that voxel was sVx).
Likelihood of False-Positive sVx
sVx are defined using a number of logical &s, making the calculation of a false-positive likelihood a nontrivial exercise. Under a global null hypothesis (the null hypothesis is really true for all elements of the conjunction, i.e., Hca = Hm = Hst and Hexe = baseline and Scr > 0) the probability to define a single voxel for a single subject as sVx incorrectly can be calculated based on the multiplication of the probabilities (0.01 × 0.01 × 0.001 considering Hca-Hst, Hca-Hm, and Hexe respectively) resulting in P < 0.0000001. At the other extreme, as argued by Nichols (Nichols et al. 2005), one could play the devil's advocate and focus on the event in which Hca-Hm and Hexe are truly different from zero but Hca-Hst is not. In this particular case, the likelihood to falsely state that the voxel is falsely classified as sVx is P = 0.01. This pessimistic scenario however would not apply for many voxels in the brain (because not that many voxels are both motor and visual as suggested by the thought experiment). Similarly, also the global null scenario will only be true for the relatively rare voxels that are neither visual nor motor according to our situation. Accordingly, the likelihood to make a false-positive decision will be rarely 0.01, and rarely 0.0000001, but usually between these values (0.01 > P > 0.0000001). For all remaining calculations we therefore use a relatively conservative estimate of P = 0.001.
Likelihood of x/16 Subjects Showing sVx in a Certain Voxel
How likely is it to find that x out of 16 subjects show sVx in a given voxel of the brain? This likelihood can be estimated using the cumulative binomial distribution with 16 repetitions and a “success” (i.e., false positive) probability of 0.001. This probability was Bonferroni corrected with a factor of 44294 (the number of voxels in the search volume of the brain). With these assumptions, finding 3 or more subjects showing sVx in a voxel is significant after Bonferroni correction at P < 0.025. All consistency maps in this report will thus be thresholded using a threshold of 3 or more subjects needing to show sVx in a given voxel. Given that the signal of adjacent voxels is correlated, this Bonferroni correction will be overconservative.
Likelihood of x/n Voxels in an Area Showing sVx Properties
Let be xi the number of voxels showing sVx properties in subject i within a certain brain area A containing n voxels. Given the fact that the likelihood of a single voxel to be sVx by chance is ∼0.001 (see section Likelihood of false-positive sVx), we can estimate the likelihood of finding xi or more sVx within the given brain area (under the null hypothesis that the area does not contain sVx at all) using the cumulative density function of the binomial distribution (B) with probability P = 0.001 and number of events n (B(n,P)). Given that the sum of 2 binomial distribution with parameters n and P is a binomial distribution with parameters 2n and P, the sum of the number of voxels found to be sVx in all subjects follows a binomial distribution with parameters 16n and P = 0.001 (∑i=116 xi ∼B(16n,P = 0.001)), and the average (over all 16 subjects) number of voxels (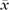 ) found to be sVx in a brain area with size n can be estimated using the cumulative density function of the binomial distribution with parameters 16n and p at the value of 16
) found to be sVx in a brain area with size n can be estimated using the cumulative density function of the binomial distribution with parameters 16n and p at the value of 16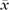
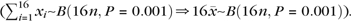 ). It should be noted, that this estimation is overly conservative because it assumes that neighboring voxels represent fully independent measurements. For unsmoothed data, this assumption is more reasonable than for smoothed data, but this assumption is still violated due to intrinsic spatial correlation in the fMRI signal, resulting in a Bonferroni correction being too conservative (i.e., systematic overestimating the likelihood of finding x by chance).
). It should be noted, that this estimation is overly conservative because it assumes that neighboring voxels represent fully independent measurements. For unsmoothed data, this assumption is more reasonable than for smoothed data, but this assumption is still violated due to intrinsic spatial correlation in the fMRI signal, resulting in a Bonferroni correction being too conservative (i.e., systematic overestimating the likelihood of finding x by chance).
Anatomical Descriptions and Regions of Interest
Anatomical description were, in the majority of the cases, performed based on the probabilistic cytoarchitectonic maps of the brain mapping group in Juelich, Germany (Geyer et al. 1996, 1999, 2000; Amunts et al. 1999; Grefkes et al. 2001; Geyer 2003; Caspers et al. 2006; Choi et al. 2006; Eickhoff et al. 2005; Eickhoff, Amunts, et al. 2006), as implemented in the SPM anatomy toolbox (http://www.fz-juelich.de/ime/spm_anatomy_toolbox; Eickhoff et al. 2005). In that approach, a maximum probability map is created of all cytoarchitectonically identified brain areas (BA6 [Brodmann area 6], 44, 45, 1, 2, 3, 4; parietal operculum, inferior parietal lobule; primary visual areas; hippocampus and amygdala). Brain areas BA1–3 will be referred to as SI and OP1–4 as SII. Within the inferior parietal lobe, our analysis includes 7 areas. The 5 areas prefixed with PF correspond to BA40, the 2 with PG to BA39 (Caspers et al. 2006). Outside of these areas, 4 other regions were found to contain a significant number of sVx: MTG, SPL, cerebellum and SFG/MFG. For the MTG and SPL a rough definition of the borders was possible through the map of BAs (BA37 for the MTG and BA 5 and 7 for SPL) provided with MRIcro (xbrodmann.hdr; http://www.sph.sc.edu/comd/rorden/mricro.html). No maps of the cerebellum were available and we therefore drew the maps using the mean anatomical image obtained by averaging the normalized 16 T1 images. For the sVx falling outside of the cytoarchitectonically defined BA6 (Geyer 2003), in location anatomically described as SFG/MFG, defining additional regions of interests (ROIs) is difficult as they still fall within regions that according to the atlas of Talairach and Tournoux (1988) would be described as BA6. We therefore simply refer to these locations as SFG/MFG.
The remaining locations are described macro anatomically (e.g., precentral gyrus). This means that a reference to precentral gyrus indicates that the activation was in a sector of the precentral gyrus that did not fall within any of the cytoarchitectonically identified maximum probability areas. Given that cytoarchitectonic maps are more reliable, a voxel that is attributable to a probabilistic cytoarchitectonic map with a probability of at least 40%, is always attributed to that map and not to the less reliable definition of the other areas. All but the cytoarchitectonically areas should be considered “putative.”
Localization and Quantification of the Overlaps
The spatial consistency map only indicates for how many subjects a particular voxel is sVx, but it does not indicate how many subjects have sVx in a particular area (e.g., a peak value in BA6 of 7 means that for 7 subjects that voxel is sVx, but not that the others do not have any sVx in BA6). We therefore also counted, separately for the right and left hemisphere, how many sVx each subject had in the ROIs specified in the above section “Anatomical descriptions and regions of interests.” We used the anatomy toolbox to obtain the number of sVx that fell in the cytoarchitectonic areas. We then exclusively masked the sVx of each subject with a map containing all the regions included in the toolbox to obtain a map of the voxels that do not belong to these cytoarchitectonically defined areas. Using the Nifti toolbox (http://www.mathworks.com/matlabcentral/fileexchange/loadFile.do?objectId = 8797&objectType = File; http://nifti.nimh.nih.gov/) we then calculated how many of the remaining voxels were within the BA37, SPL, cerebellum and SFG/MFG. The remaining voxels were then regrouped under the label “other.”
We then calculated the average number of sVx for all the regions of interest to illustrate the contribution of each area to the shared circuits. For BA6, BA44, BA45, M1, SI, SII, and IPL, we also calculated the proportion of the total area showing sVx properties (e.g., number of sVx in Area X divided by total number of voxels in Area X) to correct for size difference between the ROIs.
Finally, for each of these cytoarchitectonic defined areas (BA6, BA44, BA45, M1, SI, SII, and IPL) separately, we examined statistically whether the average number of sVx observed is likely to have occurred by chance as described above in the section “Likelihood of x/n voxels in an area showing sVx properties.” We also compared the proportion and absolute number of sVx in the various areas using an Area × Hemisphere ANOVA followed by Newman Keuls post hoc testing (the more conservative NK procedure was chosen here, because no specific comparisons had been planned ahead of time).
Examining the Somatotopical Property of Clusters of sVx
To examine the functional properties of regions with consistent sVx, we extracted the parameter estimates of the MouthExe, FingerExe, and FootExe (separately for right and left side for Finger and FootExe) within the clusters where at least 4 subjects had sVx (We used Marsbar and a traditional GLM with unsmoothed data). Although a threshold of 3 ensures that voxels are highly unlikely to be due to chance (see above), it still leads to relatively large ROIs in certain regions. To focus the analysis toward the central region of each ROI where more subjects show sVx, a threshold of 4 instead of 3 was used for this ROI analysis. We considered execution on the dominant side for this analysis (i.e., left foot and finger execution for the right hemisphere of the cerebrum and the left hemisphere of the cerebellum and vice versa for the right foot and finger). We then examined the parameter estimates using an ANOVA with 13 ROIs and 3 effectors and, given that we found a significant interaction of effector and ROI (P < 10−12), we run 2 planned comparisons per ROI, testing whether FingerExe exceeds FootExe and whether FingerExe exceeds MouthExe (2 one-tailed matched pair t-tests with α = 0.05, uncorrected for multiple comparisons because only 26 out of the possible 861 were planned and tested after finding a significant main effect). Clusters where these t-tests were significant are marked with stars and hats in Figure 7 and are interpreted as hand selective. The analysis was performed at the second level because the 3 effectors were tested in separate sessions, rendering a comparison at the single-subject level potentially confounded by sequence effects, but given that the order of these sessions was counterbalanced across subjects, a comparison at the second level is legitimate.
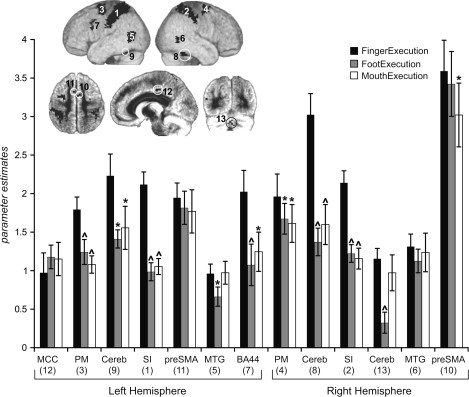
Figure 7.
Parameter estimates for FingerExe, FootExe, and MouthExe (lower panel) relative to a passive baseline for the 13 ROIs illustrated in the upper left panel. “*” over a Foot or MouthExe bar indicates the parameter estimate is lower than FingerExe at P < 0.05, “∧” the same at P < 0.001.
Results
Descriptive Single-Subject Analysis
Figure 1 illustrates for 3 subjects (s4, s6, and s9) the areas that showed significant activation during the execution of hand actions in red (after exclusion of low-level visual responses and the preparation phase, see Methods, Hexe), those involved in observation of actions compared with the observation of a static controls and human movements in green (Hca-Hst&Hca-Hm). Voxels involved in both execution and vision are shown in yellow (sVx). Supplementary Figure S3 shows the same results for the remaining 13 subjects. Although an extensive description of the areas involved in motor execution or observation alone would go beyond the scope of this paper, motor execution consistently activated premotor (BA6, SMA, BA44), cingulate (ACC, MCC), prefrontal (SFG, MFG), motor (M1), primary and secondary somatosensory, posterior parietal (SPL, precuneus, IPL), temporal (STG, MTG) cortices, insula, basal ganglia, thalamus, cerebellum, and additional smaller clusters. During action observation, activations included visual and visual association areas (V17/18, MOG, MTG, STG/S, ITG-fusiform), SI, SII, IPL, SPL, MCC, BA6, SFG, MFG, IFG, BA44, insula, thalamus, cerebellum, and other smaller clusters.
Most interestingly, sVx were consistently observed in all our 16 subjects. Quantifying the proportion of red and green voxels around sVx (see Supplementary Materials 4: sVx Island Analysis and Fig. S5) suggests that sVx were not generally located at the border between distinct regions of red and green. Instead, in most brain areas, they appeared as yellow islands on a red background. sVx were most prominent in: BA6, SFG, MFG, BA44, MCC, SI, SPL, IPL, and MTG/MOG (see Table S1)
Spatial Consistency Maps
To examine how similar the distribution of sVx was between subjects, we determined for each voxel the number of subjects that showed sVx properties in that location. In such an analysis a value of 0 signifies that none of the 16 subjects showed an sVx in that location and a value of 16 that all subjects had sVx in that location (see Methods). Figure 2 left column shows the results of this analysis. Particularly consistent sVx locations were observed in premotor, postcentral, parietal, temporal, and cerebellar locations (see Table S1). The most consistent voxel (11/16 subjects) fell at the border between the SPL and SI (BA2). The next most consistent locations were in SI (BA2; 10/16), followed by the dorsal PM cortex (border between BA6 and SFG; 9/16), PFt, MTG/MOG, and Cerebellum (8/16), Pfop, and precuneus (7/16), BA44, MCC, and SMA (6/16). To examine the impact of smoothing data, Figure 2 middle column illustrates the results of the same analysis but using smoothed (6 × 6 × 6 mm full width at half maximum Gaussian kernel) data. The overall pattern is very similar but consistency is increased both in terms of peak (peak 15/16 in SI, BA2) and extension of the highly consistent zones. Comparing the results of these consistency maps with traditional random effect analyses using smoothed data (Fig. 2, right) reveals that similar voxels are considered to be significant using the 3 approaches in this particular data set. To quantify this similarity, we calculated the pair-wise correlation between these 3 maps (obtained by flattening each volume into a one-dimensional vector, and calculating the correlation between 2 such volume-vectors). The unsmoothed single-subject map correlated significantly with both the smoothed single subject (r = 0.72, P < 10−14) and random effect t-map (r = 0.49, P < 10−14).
In addition, we created consistency maps for the Hexe condition (with and without exclusive masking with scrambled images, Fig. S7B,D) and the preparation phase preceding the go-signal of the Hexe condition (Fig. S7C). This preparation phase consistently activated primary and association areas of the visual system, most of which are absent from the Hexe map. Excluding voxels responding to scrambled images from the Hexe map further removes activity in low-level visual areas. Modeling preparation separately and masking exclusively with the vision of scrambled images therefore indeed seems to be an effective way of minimizing task-specific visual activity from our estimate of motor execution. The preparation map, however, also includes activity in motor structures (vPM, dPM, SMA, and PPC [posterior parietal cortex]) involved in motor preparation, leading to a conservative estimate of brain areas involved in motor execution in the remaining Hexe.
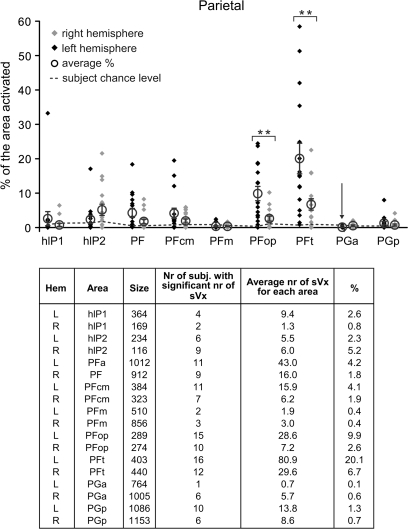
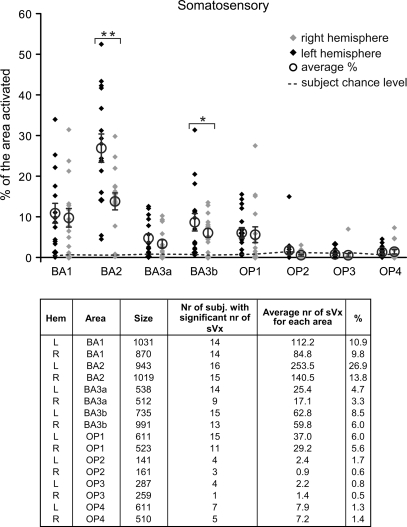
The peak overlap in the above analysis using unsmoothed data in BA6 for instance was 9/16. This does NOT mean that the remaining 7/16 do not show sVx in BA6 but only that within this very specific voxel, they do not. To directly examine how many individuals show sVx in BA6, we counted the number of sVx in each subject within the boundaries of the cytoarchitectonically defined BA6 (Geyer, 2003), and expressed the resulting count either as an absolute number of voxels or as a proportion of the total number of voxels in BA6 (i.e. % of BA6 with sVx properties). The same analysis was performed for all other cytoarchitectonically defined areas (BA 1, 2, 3a, 3b; OP 1–4; BA 4a, 4p; BA 44, 45; hIP [human intraparietal] 1, 2; PF-proper, PFcm, PFop, PFt; PGa, PGp) for which at least one subject had an sVx. Figures 3–5 report the results of this analysis. Using the percentage of voxels within an area showing sVx properties is particularly useful for comparing the number of sVx between hemispheres (see Supplementary Material 5: Lateralization of sVx).
Figure 3.
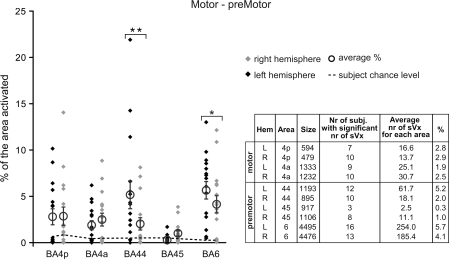
Percentage of premotor and motor areas showing sVx properties. Each black diamond represents the value of a single subject in the left hemisphere, each gray one that in the right hemisphere. Open circles represent the average percentage and error bars, the standard error of the mean (SEM) over the 16 subjects. Stars over square brackets represent significant differences in the percentage of the areas showing sVx if the right and left hemisphere are compared using a Fisher's Least Significant Difference planned comparisons tests (*P < 0.05, **P < 0.001). For all areas, the average number of sVx exceeds the number expected by chance (P < 0.001 uncorrected for the number of ROIs). The dotted line indicates for each area how much sVx would be expected for single subjects by chance, and subjects above this line therefore show more sVx than expected by chance at P < 0.001 uncorrected for number of ROIs and Subjects). The table below the graph indicates for each area: hemisphere, name, size, number of subjects with significant number of sVx (P < 0.001), average number of sVx, and percentage of voxels of this area with sVx properties averaged over the 16 subjects.
Figure 5.
Proportion of voxels in the inferior parietal lobule showing sVx properties. Only for left PGa (arrow) was the average number of sVx below that expected by chance. Conventions as in Figure 3.
Frontal Lobe
Figure 3 indicates the proportion of sVx in the various motor and premotor areas. All areas showed on average more sVx than expected by chance (P < 0.001, uncorrected for the number of ROIs. Given that we tested 44 ROIs in total—22 in each hemisphere—this corresponds to P < 0.044 after Bonferroni correction for the number of ROIs). The table included in Figure 3 details how many individual subjects had more sVx than expected by chance for each of the areas. Left area BA44 and BA6 had a significantly larger proportion of voxels showing sVx properties than all other areas (Pnk < 0.007 except for right BA6), whereas BA45 was the area with the proportionally smallest number of sVx. A similar pattern is true if the absolute number of voxels is considered. The primary motor cortex (BA 4a, 4p) did show evidence of sVx but only at its borders with SI and the MCC, whereas the regions of 4a and 4p most involved in hand execution were deactivated (see Supplementary Material 7: BOLD reduction in M1). Comparing the right and left hemisphere revealed that both BA6 (PLSD < 0.04) and BA44 (PLSD < 10−4) contain proportionally more sVx in the left hemisphere (see Supplementary Material 5: Lateralization of sVx).
Somatosensory Areas of the Parietal Lobe
Figure 4 shows the results of the same analysis for the somatosensory areas. All areas showed on average more sVx than expected by chance (P < 0.001 uncorrected or P < 0.044 corrected for the number of ROIs). BA2 (left and right) had proportionally more sVx than all other areas (all Pnk < 0.0002 for the left and all Pnk < 0.02 for the right BA2), and within SII, OP1 had proportionally more sVx (all Pnk < 0.008). The same pattern was true if absolute numbers of sVx were considered instead.
Figure 4.
Proportion of voxels in somatosensory areas showing sVx properties. Conventions as in Figure 3.
The Inferior Parietal Lobule
Results for the IPL are reported in Figure 5. All areas except for PGa had on average more sVx than expected by chance (P < 0.001 uncorrected or P < 0.044 corrected for the number of ROIs). The IPL has recently been divided into cytoarchitectonically distinct subfields according to observer-independent cytoarchitectonic criteria, 5 of them are in the rostral sector corresponding to BA40 (PF, PFcm, PFm, PFop, PFt) and 2 in the caudal sector corresponding to BA39 (PGa and PGp, see Caspers et al. 2006 for details). In addition 2 fields in the ventral bank of the anterior intraparietal sulcus have been cytoarchitectonically specified (hIP 1 and 2; Choi et al. 2006). Of these areas left PFt had proportionally more sVx than all other areas (all Pnk < 0.0002), followed by left PFop (all Pnk < 0.05). If the absolute number of sVx is considered instead, left PFt still has most sVx, but PF now becomes the second most sVx-containing area.
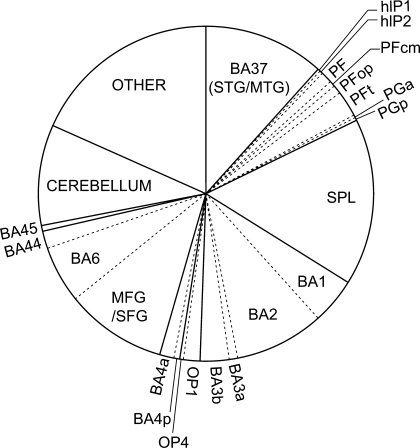
Proportion of sVx Contributed by Each Brain Area
Figure 6 indicates the relative contribution of the different brain regions to the total shared circuit. In addition to the cytoarchitectonically defined regions used above, we counted the number of sVx in the superior parietal lobule, the cerebellum, the middle temporal area (putative BA37) and SFG and MFG based on macro anatomical landmarks (see Methods). These areas were included because of their prominence in the analyses of Figures 1 and 2. All other areas were pooled under the name “other” because of the lack of reliable criteria for defining their borders.
Figure 6.
Relative contribution to the total number of sVx in the brain. Areas OP2–3 and PFm are omitted because they contained less than 5 sVx. The pie represents the total average number of sVx in the brain and each slice the proportion of total sVx contributed by a particular area (left and right hemisphere combined).
Next to the premotor and parietal regions generally associated with the MNS, the somatosensory areas, the mid-temporal gyrus (BA37), the cerebellum, the middle and superior frontal gyri contribute considerably to the total number of sVx. The slice “Other” includes mainly MCC, followed by thalamus, basal ganglia (caudate and putamen) and insular regions. Examination of the consistency maps indicates that of the other areas the MCC is characterized by relatively consistent loci of activation with a peak of 6 subjects at x = −10, y = −20, z = 42, followed by the thalamus (5/16, x = 12, y = −14, z = 10) and middle insula (5/16, x = −38, y = −2, z = 12). The low spatial consistency of the remaining “other” sVx (peaks ≤ 3 subjects) suggests they may reflect idiosyncrasies or false positives.
Examining the Somatotopical Property of Clusters of sVx
To examine the specificity of activations in sVx, we asked the same participants to perform 3 additional motor tasks involving the manipulation of an object using their fingers, their toes and their lips. We then examined brain activity in the main 13 clusters of the spatial consistency map (see renders in Fig. 7) during these tasks. Given that all the actions in the movies were performed with the hand, we examined in particular which sVx clusters were more active while participants themselves manipulated objects with their fingers (as the actors) compared with their toes or lips (see methods and Fig. 7). Importantly, the finger execution data used for this comparison (FingerExe) is not the same used in the definition of sVx (Hexe), which would have unduly biased the analysis.
Performing an ANOVA with 13 ROI and 3 effectors (FingerExe vs. FootExe vs. MouthExe), we found a main effect of effector (F2,30 = 14.2, P < 0.00005), with the FingerExe determining stronger BOLD signal than the other 2 effectors (both P < 0.0002) suggesting an overall preference for the Hand in our sVx. There was also a significant interaction of ROI and effector (F24,360 = 5.36, P < 10−12) suggesting differences in selectivity across ROIs. To examine this interaction, we tested, for each ROI separately, whether FingerExe exceeded FootExe and MouthExe using planned one-tailed t-tests (cutoff for both of the 2 comparison P < 0.05). According to this criteria, about half the ROIs, including premotor, somatosensory and lateral cerebellum, were hand selective (left BA44, left and right dorsal PM, left and right SI, left and right cerebellar hemispheres). In addition, in 2 areas FingerExe exceeded FootExe but not MouthExe (left MTG and sagittal cerebellum) and in the right pre-SMA FingerExe exceeds mouth but not FootExe. Finally for 3 ROIs (left MCC, left pre-SMA and right MTG) both comparisons yielded no significant differences. Interestingly MouthExe or FootExe was never significantly larger than FingerExe, confirming that none of the ROIs had a preference for another effector than the one seen in the movies. An additional analysis (see Supplementary Materials 6: Somatotopy) revealed that the prevalence of FingerExe was significantly more pronounced in the sVx ROIs than in the whole brain.
Discussion
With this study, we aimed to provide an unbiased description of the areas that are involved both in observation and execution of actions. Fully aware that not all these areas might contain MNs we use the term “shared voxels” to reflect the duality of the activation without implying the necessary existence of MNs in these voxels. We used unsmoothed data and single-subject analyses to examine whether the overlaps between action observation and execution found in previous studies were not simply an effect of smoothing data and pooling multiple subjects as suggested by Morrison and Downing (2007). In addition, we examined how consistent these sVx are between subjects. Finally, we took advantage of cytoarchitectonic maps (Geyer et al. 1996, 1999, 2000; Amunts et al. 1999; Grefkes et al. 2001; Geyer 2003; Eickhoff et al. 2005; Caspers et al. 2006; Choi et al. 2006; Eickhoff, Amunts, et al. 2006) to introduce a more detailed and comparable description of the area contributing to sVx.
Our results indicate that all subjects have more sVx than expected by chance in at least some brain areas (left BA6, BA2, PFt) even using unsmoothed data. In general the sVx were not observed at the border between larger, distinct areas responding exclusively to action observation or execution, but mainly as islands within areas responding to execution and more rarely, on areas responding to observation. The similarity between the unsmoothed consistency map and the classical group analysis of the same data (see Fig. 2) may give the wrong impression that performing single-subject analyses is superfluous. This however is not the case: the seminal examination of overlaps for the case of pain (Morrison and Downing 2007) has shown that finding sVx in a group analysis does not guarantee the existence of such sVx in single subjects. This finding begged the frightening question of whether the same lack of sVx in single subjects may apply to the case of actions. The present paper, by developing a set of methods for analyzing and visualizing sVx in single subjects, shows that for actions, sVx in the group do correspond, to a large extent, to those at the single-subject level—at least in our data set. This is an important novel finding that strengthens the current literature on action. Given the potential risks of group analyses for the case of sVx, we would however encourage future investigations in sVx, to include a single-subject consistency map similar to the ones shown here (e.g., Fig. S1C of Gazzola et al. 2006). For the case of actions we can therefore state that the overlaps found in the group reflect sVx in the single subjects, confirming that the actions of other individuals reliably recruit part of the voxels involved in executing similar actions even at the level of individual participants.
Using methods to minimize the risk of overlaps between observation and execution due to smoothing and group analysis our results therefore do advance our understanding of the putative human mirror system by confirming that the ventral PM cortex and the inferior parietal lobe, known to contain MNs in monkeys, show sVx in humans. In addition, we quantified the number of sVx in a variety of cytoarchitectonically defined brain regions separately for the right and left hemisphere. Why conduct such a numerical analysis? Is it meaningful to state that BA6 contains more sVx than BA44? Such a numerical comparison of sVx between different brain areas is not intended to indicate which area is functionally more important: each is likely to contribute to the overall neural computation, and asking which is most important is as uninteresting as asking whether the motor, the transmission or the wheels of a car are more important for moving it. Instead, the numerical comparison within brain areas across hemispheres is useful as it provides to our knowledge the first quantitative evidence that BA44, BA6, BA2, BA3b, PFop, and PFt are more extensively recruited in the left compared with the right hemisphere during action observation even after correcting for size differences between these areas in the 2 hemispheres. This observation could be due to the actors in the movies performing all actions with the right hand and/or because the majority of our subjects were right handed with their left hemisphere therefore being dominant for motor control. We explore these issues in more detain in the Supplementary Materials (see Supplementary Material 5 Lateralization of sVx). In addition the quantification indicate that areas in which MNs have been found in monkeys (F5 corresponding to BA44 and PF corresponding to PF + PFop + PFt + PFcm + PFm) only contribute 7% of the total number of sVx in our study. This proportion increases to 17% if one includes all of BA6. Although the precise number is inconsequential (e.g., neurons might be more “important” or tightly packed in some areas), this low number does beg the intriguing question of what the remaining sVx—in particular in the dorsal premotor, SMA, superior parietal, temporal, primary and secondary somatosensory, dorsal middle cingulate cortices and cerebellum—code for, and how they contribute to the overall computations in social perception. The fact that only 7% of our sVx fall within the regions that are known to contain MNs in monkeys should not be misinterpreted to mean that 93% fall in regions that have been shown not to contain MNs. Instead 93% fall within regions in which MNs have not yet been extensively looked for in monkeys using single cell recordings. Indeed Mukamel et al. (2008), recording from single cells in humans, have found neurons responding during action execution and observation in the SMA and temporal lobe, giving further support to the idea that fMRI can find sVx outside the ventral PM cortex and IPL and be accurate. Deoxyglucose autoradiography studies (Raos et al. 2004, 2007; Evangeliou et al. 2008) performed in monkeys also support the idea that the mirror system may encompass regions not yet explored using single cell recordings. In the supplemental materials, we discuss findings relevant to the main sVx-containing regions reported in this paper in some detail. Here we will attempt instead to propose a speculative functional model derived from motor control theory to attempt an integration of sVx found in areas containing MNs in monkeys with sVx found outside of these areas. We will also discuss the main caveats of the present study and ways in which they could be addressed in future experiments
Hebbian Learning and Forward and Inverse Internal Models
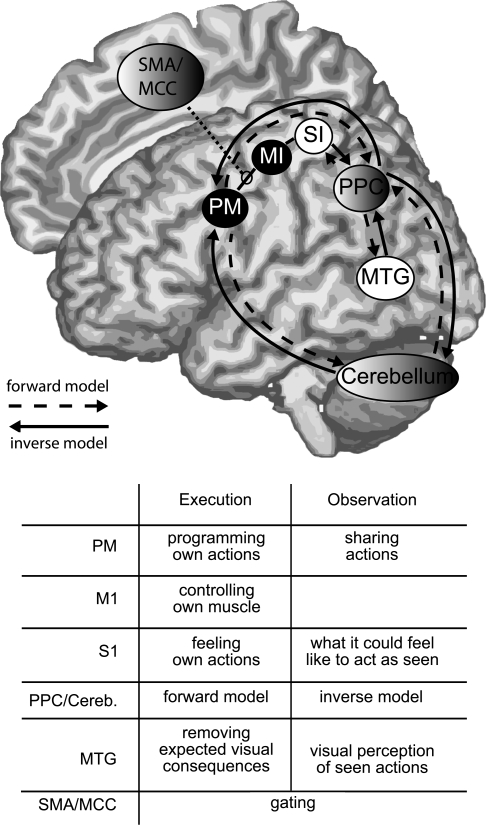
Current theories of motor control assume that an efficient planning and execution of actions requires 2 types of internal models (Wolpert and Ghahramani 2000). Forward internal models predict the sensory consequences of a motor command (i.e., what the intended action should feel, look and sound like); inverse internal models transform a desired sensory end-state (e.g., having the glass at my lips) into a suitable motor command. Two main routes may implement such internal models in the brain (see Fig. 8 and Miall 2003). The PM cortex sends cortico-cortical projections to the PPC, which in turn is connected with somatosensory, visual, and auditory brain regions, including SI and the MTG (Seltzer and Pandya 1978, 1994; Matelli et al. 1986; Selemon and Goldman-Rakic 1988; Andersen et al. 1997; Luppino et al. 1999; Tanne-Gariepy et al. 2002; Rizzolatti and Matelli 2003; Keysers and Perrett 2004). In addition to the cortico-cortical route linking PM and PPC, the PM also sends projections to the cerebellum, which in turn sends connections back to the PPC (Schmahmann and Pandya 1989; Stein and Glickstein 1992; Dum and Strick 2003). In particular, the lateral cerebellar hemispheres are reciprocally connected with PM, MI, and PPC through the dentate nucleus and the thalamus (Thach et al. 1992) and are particularly involved in the planning of actions. The intermediate cerebellar cortex also receives visual and motor input, but appears to be involved in a more automatic control of ongoing movement (Thach et al. 1992). Both the cortico-cortical and cortico-cerebellar–cortical routes linking the premotor areas to sensory areas could implement a forward model (Wolpert et al. 1998; Miall 2003).
Figure 8.
Forward and inverse models of sVx. Brain areas indicated with circles filled in black are thought to contain primarily motor; areas filled in white, sensory; and areas filled in white-to-black gradient, intermediate representations. The table within the figure details the function of the main nodes during execution and observation. See text for details.
Sensory brain areas, including the MTG and auditory areas however also project to the PPC which projects to PM along a direct cortico-cortical route and through the cerebellum, potentially implementing the inverse model (Seltzer and Pandya 1978, 1994; Matelli et al. 1986; Andersen et al. 1997; Luppino et al. 1999; Dum and Strick 2003; Miall 2003; Rizzolatti and Matelli 2003; Rizzolatti and Craighero 2004; Selemon and Goldman-Rakic 1988; Schmahmann and Pandya 1989; Stein and Glickstein 1992; Thach et al. 1992; Wolpert et al. 1998; Wolpert and Ghahramani 2000; Tanne-Gariepy et al. 2002; Wolpert et al. 2003). The brain regions involved in these forward and inverse models correspond strikingly well with those in which we find sVx.
Recently, we have argued that shared circuits for hand actions could be the result of Hebbian associations trained during motor execution (DelGiudice et al. forthcoming; Keysers and Perrett 2004), (see Heyes 2001; Catmur et al. 2007) for a related account). The rationale behind this proposal is that motor control requires sensation and action to be linked. Given that while we act we can sense the consequences of our own actions (proprioception, somatosensation, vision, and audition), the sensory consequences of our own actions are systematically and synchronously paired with motor commands. This predicts the emergence of Hebbian connections that link motor programs to sensory consequences (forward models) and vice versa, sensory consequences to motor programs (inverse models). Although we witness the actions of other individuals, the visual and auditory similarity between other people's movements and our own trigger the inverse models that have been Hebbianly trained to associate the vision and sound of our own actions to motor and somatotosensory representations. In this process, orientation insensitive neurons in the temporal lobe are essential, as our own actions are usually seen from a different perspective, but such neurons exist in the STS of the monkey (Keysers and Perrett 2004). Once the motor representations have been triggered, the observer can then utilize its own forward models to predict the forthcoming actions of others (Umilta et al. 2001).
Combining Hebbian learning and the idea of internal models of motor control provides a powerful, albeit speculative, framework in which the set of sVx regions found in the current experiment can be functionally organized. During action observation, the MTG provides a high level representation of other people's actions in a relatively sensory (visual) code that is subsequently projected onto the PPC. From there it is:
1) Sent to SI (BA2) and SII, where it triggers representations of what it feels like to move in this way (proprioceptive) and touch objects in that way (tactile simulation). This process is a sensory association process rather than a forward or inverse model
2) Transformed into motor commands adequate for achieving similar goals in the PM through both cortico-cortical and cortico-cerebellar–cortical forward model routes (motor simulation). The study of neurological patients and repetitive transcranial magnetic stimulation studies suggest that this activation of motor programs in premotor regions during action observation seems essential for a normal perception, understanding and imitation of other people's actions (Heiser et al. 2003; Goldenberg and Karnath 2006; Pobric and Hamilton 2006; Avenanti et al. 2007; Goldenberg et al. 2007; Urgesi et al. 2007; Pazzaglia et al. 2008).
The posterior parietal cortex and cerebellum would then implement a necessary computational step that requires mixing sensory, motor, attentional, and intentional signals, and activity in such neurons will fill the entire continuum between relatively sensory, neither sensory nor motor to relatively motor (Fogassi et al. 2005). In these mixed regions in particular, demands of the task compared with the baselines may trigger many processes involved in visuomotor transformation that have often been given functional labels such as attention or intention (Andersen et al. 1997).
During motor execution on the other hand a go-signal will be rapidly transformed into appropriate motor commands which in turn will lead to activity in SI through sensory reafference (i.e., sensing the arm move). In addition, forward models involving the projections to the cerebellum and the PPC will generate expected sensory representations, which will result in activity in the MTG, PPC, cerebellum, and SI. Again, during this process, the MTG and SI will be relatively sensory in nature (and hand specific in SI), whereas representations in the posterior parietal and cerebellum will represent mixed representations along a computational continuum between action and sensations, and will incorporate many attentional and computational factors common to the observation and execution task. If the forward and inverse model are both trained through Hebbian associations during action execution and work accurately, we would expect that many of these representations triggered by the forward models and proprioception share the same neural substrates as those recruited by inverse models and visual perception during the observation of similar actions (Keysers and Perrett 2004).
This speculative model receives support on the one hand from our observation that in all the key nodes of this model (MTG, PPC, cerebellum, SI, and PM) we find reliable sVx even without smoothing or group analyses. It receives further support from the single cell recordings that show that neurons in the PPC (PF; Fogassi et al. 2005) and IPS (Fujii et al. forthcoming) and F5 (Gallese et al. 1996; Umilta et al. 2001; Kohler et al. 2002; Keysers et al. 2003; Fujii et al. forthcoming) respond both during the observation and execution of an action in a way specific for a particular action. In addition, the observation that single cells in the STS are activated during the observation of actions (Keysers and Perrett 2004) and modulated during the execution of actions (Hietanen and Perrett 1993, 1996) provides evidence for embedding the MTG in the forward model.
Embedding SI and SII into this model is unusual, and these nodes have so far been missing from most models of action observation. SI however contained most of the most reliable sVx is our study, and because we have used no spatial smoothing, these sVx are unlikely to represent PPC activity that was blurred into SI. In addition, many studies now show evidence that SI and SII are modulated by the vision of actions (Avikainen et al. 2002; Rossi et al. 2002; Raos et al. 2004, 2007; Cross et al. 2006; Gazzola et al. 2006; Gazzola, Rizzolatti, et al. 2007; Grezes et al. 2003; Hasson et al. 2004; Oouchida et al. 2004; Mottonen et al. 2005; Molnar-Szakacs et al. 2006) and these regions are also activated while participants simply see other individuals being touched (Keysers and Perrett 2004; Blakemore et al. 2005). Two studies have additionally shown that making the perceived action more salient from a tactile or proprioceptive point of view by involving objects that are painful to touch (Morrison, Bach, et al. 2007) or movements that would be painful to perform (Costantini et al. 2005) causes increases of activity in SII and SI respectively. Finally, we find all 4 subdivisions of SI to contain reliable sVx, including the more proprioceptive subdivisions 3a and 2 and the more tactile subdivisions 3b and 1 (Nelson et al. 1980). This suggests that SI and SII may associate somatosensory sensations of both tactile and proprioceptive nature with both the execution and observation of actions. The exact role played by these areas is manifold. During action observation/listening, SI and SII embody sensory associations between the sight/sound of an action and what the action would feel like. During execution, SI and SII could represent a convergence between an initial forward model (i.e., what the planned action should feel like) and reafference (what the action actually feels like while executed) and sense motor errors. Joint actions finally render the role of SI even more manifold: during Argentinean tango for instance, one partner senses the actions of the other through SI, so that SI would becomes an input node for social perception, that would then send an inverse model, through PPC and cerebellum to PM, in order to program a movement suitable to bring about a certain proprioceptively defined change in the position of the partner.
Finally, including the cerebellum in action observation models is also unusual, although a number of studies have reported cerebellar activity (Leslie et al. 2004; Calvo-Merino et al. 2006; Jackson et al. 2006; Gazzola, Rizzolatti, et al. 2007). The cerebellum, however, has generally been considered a key player in forward and inverse models of motor control (Schmahmann and Pandya 1989; Stein and Glickstein 1992; Thach et al. 1992; Wolpert et al. 1998; Desmurget and Grafton 2000; Dimitrova et al. 2006) and considering action observation an instance of inverse model synergistically integrates our knowledge of motor control with out interpretation of cerebellar activity in the context of action observation. The presence of sVx despite the sharpened spatial accuracy of our analysis, together with the observation that the lateral sVx clusters are hand specific, and fall in the vicinity of those found in other studies of finger movements (Dimitrova et al. 2006) suggests that the cerebellar activations do reflect overlapping forward and inverse models instead of unspecific attentional effects.
Overall, our model therefore includes a number of sources of overlap between execution and observation that go beyond the original notion of MNs. First, in the MTG and SI, representations according to this model are not motor, but sensory. Second, in the PPC and cerebellum representations would be half way between motor and sensory codes. The observation of relatively classic MNs in PF (Fogassi et al. 2005) and IPS (Fujii et al. forthcoming) are probably examples on the motor extreme of this continuum, but many cells in the PPC have much more abstract properties, being affected by vision, sound, proprioception, and motor intentions in a way that often only makes sense at the level of populations of neurons (Andersen et al. 1997), and we would therefore expect many of the PPC sVx to contain neurons that are modulated by both the observation and execution task but deviate from the simple response pattern of classic MNs.
What role finally would the motor structures containing sVx in the mesial wall play in this model? The SMA, pre-SMA, and MCC have been shown to contain neurons in monkeys that modulate their firing based on whether the execution of a particular action is adequate in a particular context (Rizzolatti et al. 1990, 1996), and fMRI studies in humans suggest a similar role (Nakata et al. 2008). Also during the observation of pain, mesial motor structures could inhibit overt motor output (Morrison, Peelen, et al. 2007). The regions have projections to PM and M1 (Vogt and Vogt 2003) suggesting that they may serve as role of gatekeeper, deciding when to allow premotor activity to gain access to M1 and produce overt behavior (as in our action execution task), and when to inhibit M1 in order for premotor shared activity not to spill out into overt motor behavior (as evidenced by the reduction of BOLD signal we report here in M1 during Hca). Interestingly, recent single cell recordings in these regions support this vision (Mukamel et al. 2008): some neurons responded with increased firing rates during action observation and reduced firing during execution and some showed the opposite pattern. Both these patterns would be expected from neurons participating in promoting overt motor activity during execution and suppressing motor activity during observation. Averaged over the volume of a voxel, such neurons would cause augmentations of BOLD in the SMA, pre-SMA, and MCC during both observation and execution, a finding not only confirmed by our own study but also by an elegant 2DG studies in macaque monkeys (Raos et al. 2007).
Caveats and Questions
As noted in the introduction, sVx do not need to contain individual neurons involved in both tasks (Morrison and Downing 2007). An interesting question for future research will therefore be to use single cell recordings in the regions where we found sVx but where single cell recordings in the context of action observation and execution have so far not been conducted, including in particular the dorsal PM cortex, SI, SII, the cerebellum and the superior parietal lobule. An alternative approach may be to use repetition suppression across modality, where participants would perform one of 2 types of actions and then see someone else perform either the same type of actions or a different type. If the sVx contains neurons involved in observation and execution, they might show a more pronounced reduction of BOLD if the observed and executed action match (Dinstein et al. 2007). A problem with this approach, however, is that not all neurons may show suppression, and negative findings are therefore hard to interpret (Dinstein et al. 2007).
Second, our motor execution task contrasted reach-and-manipulate actions against a passive baseline. This weak control condition differs from the experimental condition in many ways. Action execution requires the transformation of a visual go-signal into motor plans, motor execution, sensing ones own body move but also creating spatial maps of where the object is and concentrating ones attention toward the task, that are all absent from the passive baseline condition. This means that there might be multiple ways in which this task might recruit processes that overlap with action observation. On the more meaningful end, both somatosensory processes involved in feeling ones own limb more, and motor processes involved in making it move may therefore overlap with action observation, an aspect we have discussed above. However, less “desirable” sources of overlap are also difficult to exclude. For instance, the visual go-signal needs to be transformed into a motor command that has to be based on a visuospatial memory of where the target objects are in space and what to do with them. This visuomotor and visuospatial transformation and memory processes could overlap with the inverse models we suspect to be triggered by action observation. We have tried to minimize such confounds by removing activity time locked to the onset of the instructions, by excluding voxels that respond to the sight of geometric patterns (our scrambled images; see Fig. S7) and by examining if sVx clusters respond more to finger compared with toe or lip actions. For the PM, SI and lateral cerebellar clusters the latter additional experiment renders such generic explanations unlikely but negative findings in the remaining areas are difficult to interpret: we have not included this hand selectivity criterion into our basic sVx definition because many MNs in PF and F5 respond to an action independently of the effector used (Gallese et al. 1996; Fogassi et al. 2005), and sVx without effector selectivity are therefore not necessarily artifactual. In future experiments, the execution of an action resembling those viewed in the movie should however be contrasted against actions such as eye movements with similar visuospatial, attentional, and memory requirements but with very different somatosensory and premotor representations.
A third and related caveat regards the functional interpretation of the various sVx. How for instance do the dorsal and ventral PM sVx differ? Do some hand selective sVx represent tactile and others proprioceptive aspects of hand actions? How do sVx in the various locations of the PPC differ? Experiments in which certain aspects of the stimulus are systematically varied while others are kept constant will be needed to answer these questions. Seeing an actor grasp a ball vs. grasping a cactus for instance (Morrison, Bach, et al. 2007) indicates that SII might be particularly important for coding aversive tactile properties, and a similar approach could be used to dissociate the coding of grasping vs. reaching, or tactile and kinesthetic coding etc. Repetition suppression might again be a powerful tool to identify which dimensions are most salient in the various sVx (Hamilton and Grafton 2006).
A related issue is that perceiving other people's actions is a multilayered problem. If we see someone shut the door on someone else, we can ask at least 3 questions: 1) How is he doing it? Answer: with his hand. 2) What is he doing? Answer: shutting the door. 3) Why is he doing it? Answer: because he is upset. Disentangling how various nodes of shared circuits contribute to these levels and how they interact with structures classically associated with mentalizing will remain an import question for future investigation (Keysers and Gazzola 2007; Thioux et al. 2008). The few studies that have examined this question so far have lead to rather conflicting results and beg the need for further experiments. Three studies for instance have looked at where the brain might process why individuals perform certain actions, but have identified 3 different brain regions: the IFG (Iacoboni et al. 2005), the temporoparietal junction (Brass et al. 2007) and midline structures (de Lange et al. 2008). The same applies to studies localizing the what of action perception: repetition suppression experiments have shown either that the PPC is more important than the PM (Hamilton and Grafton 2006) or that the PM is more important than the PPC (Lestou et al. 2008), whereas studies examining the observation of actions for which the observer lacks a corresponding effector suggest that the PM might be just as sensitive as the PPC to the goal of an action (Gazzola, Rizzolatti, et al. 2007; Gazzola, van der Worp, et al. 2007).
Fourth, the idea that certain sVx-containing areas (e.g., cerebellum and PPC) can implement a forward model during motor execution and an inverse model during action observation should be tested empirically. During action execution, the information should arrive from premotor areas, whilst during observation, from visual/auditory areas. Comparing the temporal dynamics of information flow during action observation/listening and action execution using Granger Causality (on BOLD; Jabbi and Keysers forthcoming) or magnetoencephalographic data in humans or local field potentials or single cell recordings in nonhuman primates) could test these predictions.
Finally, our observation of stimulus dependent reduction of BOLD in MI (see Supplementary Materials 7. BOLD reduction in M1) begs the question of how this finding can be integrated with other techniques suggesting a facilitation of M1 activity: 1) motor evoked potential facilitations during action observation (Fadiga et al. 1995; Stefan et al. 2005), 2) MI activity measured by EEG/MEG (Hari et al. 1998; Nishitani and Hari 2000), 3) increased MI metabolism in monkeys (Raos et al. 2004, 2007). Next to replications of our finding of reduced BOLD activity in MI during observation, the combination of fMRI with TMS, EEG or single cell recordings might be particular important here, as they have been for understanding discrepancies between techniques in the study of binocular rivalry.
General Conclusion
By using unsmoothed data of 16 subjects analyzed separately during the observation and execution of hand actions, we show that although the classic MNS areas (ventral PM cortex and rostral inferior parietal lobule) indeed do contain voxels shared between execution and observation, many additional brain areas contain such consistently sVx, including in particular the dorsal PM cortex, the supplementary and cingulate motor areas, the superior parietal lobe, the somatosensory cortices and the cerebellum. In all these areas, using methods that minimize the risk of finding overlaps between execution and observation by chance, we found reliable evidence for the fact that within the volume of a single functional voxel (27 mm3) the BOLD signal was augmented both while observing hand actions (be it compared against a static baseline or a control movement) and while executing similar actions. In all of these regions, voxels showing these properties were found in the same location in more of our single subjects than would be expected by chance. Additional experiments will however be needed to determine if sVx indeed contain shared neurons: voxels contain millions of neurons, and increases of BOLD signal during action observation and execution could be the result of 1) separate populations of neurons responding exclusively during action observation or execution or 2) neurons responding similarly during action observation and execution or 3) a combination or both (Morrison and Downing 2007). Nevertheless, the present finding contributes to strengthening the evidence in favor of the existence of motor and somatosensory simulation during action observation and provide a tentative model of how this vast array of brain regions may cooperate in forward and inverse models to associate other people's actions with our own actions and sensations, and our own actions with their somatosensory, visual and auditory consequences. By localizing sVx using cytoarchitectonic maps and quantifying the consistency of the finding in individual subjects, we hope that the present study will stimulate and facilitate fMRI and single cell recording studies of action observation and execution aiming at elucidating the neural basis and informational content of shared activity and hope that this study will pave the way to a more general use of single-subject data analysis within the investigation of simulation theories of social cognition.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
N.W.O., VIDI, and a Marie Curie Excellence Grant to C.K. Funding to pay the Open Access publication charges for this article were provided by the Marie Curie Excellence Grant by the European Commission.
Acknowledgments
We thank S. Caspers, S. Eickhoff, and S. Geyer for sharing their cytoarchitectonic maps of the IPL with us, enabling a more quantitative analysis of subregions of the IPL; Anita Kuiper for help with scanning; L. Cerliani for useful comments on the manuscript and our subjects for being willing to be scanned multiple times. Conflict of Interest: None declared.
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Curr Biol. 2007;17:2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Avikainen S, Forss N, Hari R. Modulated activation of the human SI and SII cortices during observation of hand actions. Neuroimage. 2002;15:640–646. doi: 10.1006/nimg.2001.1029. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Iacoboni M, Zaidel E, Wilson S, Mazziotta J. Left hemisphere motor facilitation in response to manual action sounds. Eur J Neurosci. 2004;19:2609–2612. doi: 10.1111/j.0953-816X.2004.03348.x. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M. Lateralization of the human mirror neuron system. J Neurosci. 2006;26:2964–2970. doi: 10.1523/JNEUROSCI.2921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Maeda F, Zaidel E, Mazziotta J, Iacoboni M. Lateralization in motor facilitation during action observation: a TMS study. Exp Brain Res. 2002;144:127–131. doi: 10.1007/s00221-002-1037-5. [DOI] [PubMed] [Google Scholar]
- Bangert M, Peschel T, Schlaug G, Rotte M, Drescher D, Hinrichs H, Heinze HJ, Altenmuller E. Shared networks for auditory and motor processing in professional pianists: evidence from fMRI conjunction. Neuroimage. 2005;30:917–926. doi: 10.1016/j.neuroimage.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Bristow D, Bird G, Frith C, Ward J. Somatosensory activations during the observation of touch and a case of vision-touch synaesthesia. Brain. 2005;128:1571–1583. doi: 10.1093/brain/awh500. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. Cyclic time course of motor excitability modulation during the observation of a cyclic hand movement. Brain Res. 2005;1065:115–124. doi: 10.1016/j.brainres.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Brass M, Schmitt RM, Spengler S, Gergely G. Investigating action understanding: inferential processes versus action simulation. Curr Biol. 2007;17:2117–2121. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro CA, Rizzolatti G. Neural circuits involved in the recognition of actions performed by nonconspecifics: an FMRI study. J Cogn Neurosci. 2004;16:114–126. doi: 10.1162/089892904322755601. [DOI] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P. Action observation and acquired motor skills: an FMRI study with expert dancers. Cereb Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Grezes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol. 2006;16:1905–1910. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Catmur C, Walsh V, Heyes C. Sensorimotor learning configures the human mirror system. Curr Biol. 2007;17:1527–1531. doi: 10.1016/j.cub.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol. 2006;495:53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini M, Galati G, Ferretti A, Caulo M, Tartaro A, Romani GL, Aglioti SM. Neural systems underlying observation of humanly impossible movements: an FMRI study. Cereb Cortex. 2005;15:1761–1767. doi: 10.1093/cercor/bhi053. [DOI] [PubMed] [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST. Building a motor simulation de novo: observation of dance by dancers. Neuroimage. 2006;31:1257–1267. doi: 10.1016/j.neuroimage.2006.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Curr Biol. 2008;18:454–457. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F. Brain activity during observation of actions. Influence of action content and subject's strategy. Brain. 1997;120(Pt 10):1763. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- DelGiudice M, Manera V, Keysers C Forthcoming. Programmed to learn? The ontogeny of mirror neurons. Dev Sci. doi: 10.1111/j.1467-7687.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dimitrova A, de Greiff A, Schoch B, Gerwig M, Frings M, Gizewski ER, Timmann D. Activation of cerebellar nuclei comparing finger, foot and tongue movements as revealed by fMRI. Brain Res Bull. 2006;71:233–241. doi: 10.1016/j.brainresbull.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. J Neurophysiol. 2007;98:1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex. 2007;17:1800–1811. doi: 10.1093/cercor/bhl090. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Evangeliou MN, Raos V, Galletti C, Savaki HE. Functional imaging of the parietal cortex during action execution and observation. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn116. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Buccino G, Rizzolatti G. Speech listening specifically modulates the excitability of tongue muscles: a TMS study. Eur J Neurosci. 2002;15:399–402. doi: 10.1046/j.0953-816x.2001.01874.x. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hihara S, Iriki A Forthcoming. Social cognition in premotor and parietal cortex. Social Neurosci. doi: 10.1080/17470910701434610. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport. 2001;12:1489–1492. doi: 10.1097/00001756-200105250-00038. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in human. Curr Biol. 2006;16:1824–1829. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage. 2007;35:1674–1684. doi: 10.1016/j.neuroimage.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gazzola V, van der Worp H, Mulder T, Wicker B, Rizzolatti G, Keysers C. Aplasics born without hands mirror the goal of hand actions with their feet. Curr Biol. 2007;17:1235–1240. doi: 10.1016/j.cub.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Wien: Springer; 2003. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hermsdorfer J, Glindemann R, Rorden C, Karnath HO. Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb Cortex. 2007;17:2769–2776. doi: 10.1093/cercor/bhm004. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Karnath HO. The neural basis of imitation is body part specific. J Neurosci. 2006;26:6282–6287. doi: 10.1523/JNEUROSCI.0638-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Grezes J, Armony JL, Rowe J, Passingham RE. Activations related to “mirror” and “canonical” neurones in the human brain: an fMRI study. Neuroimage. 2003;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. J Neurosci. 2006;26:1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci USA. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Heiser M, Iacoboni M, Maeda F, Marcus J, Mazziotta JC. The essential role of Broca's area in imitation. Eur J Neurosci. 2003;17:1123–1128. doi: 10.1046/j.1460-9568.2003.02530.x. [DOI] [PubMed] [Google Scholar]
- Heyes C. Causes and consequences of imitation. Trends Cogn Sci. 2001;5:253–261. doi: 10.1016/s1364-6613(00)01661-2. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Perrett DI. Motion sensitive cells in the macaque superior temporal polysensory area. I. Lack of response to the sight of the animal's own limb movement. Exp Brain Res. 1993;93:117–128. doi: 10.1007/BF00227786. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Perrett DI. Motion sensitive cells in the macaque superior temporal polysensory area: response discrimination between self-generated and externally generated pattern motion. Behav Brain Res. 1996;76:155–167. doi: 10.1016/0166-4328(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, Mazziotta JC, Rizzolatti G. Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci USA. 2001;98:13995–13999. doi: 10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Keysers C Forthcoming. Inferior frontal gyrus activity triggers anterior Insula response to emotional facial expressions. Emotion. doi: 10.1037/a0014194. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. Neural circuits involved in imitation and perspective-taking. Neuroimage. 2006;31:429–439. doi: 10.1016/j.neuroimage.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Towards a unifying neural theory of social cognition. Prog Brain Res. 2006;156:383–406. doi: 10.1016/S0079-6123(06)56021-2. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn Sci. 2007;11:194–196. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kohler E, Umilta MA, Nanetti L, Fogassi L, Gallese V. Audiovisual mirror neurons and action recognition. Exp Brain Res. 2003;153:628–636. doi: 10.1007/s00221-003-1603-5. [DOI] [PubMed] [Google Scholar]
- Keysers C, Perrett DI. Demystifying social cognition: a Hebbian perspective. Trends Cogn Sci. 2004;8:501–507. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: action representation in mirror neurons. Science. 2002;297:846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Lestou V, Pollick FE, Kourtzi Z. Neural substrates for action understanding at different description levels in the human brain. J Cogn Neurosci. 2008;20:324–341. doi: 10.1162/jocn.2008.20021. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;251:281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Miall RC. Connecting mirror neurons and forward models. Neuroreport. 2003;14:2135–2137. doi: 10.1097/00001756-200312020-00001. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cereb Cortex. 2005;15:986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Kaplan J, Greenfield PM, Iacoboni M. Observing complex action sequences: the role of the fronto-parietal mirror neuron system. Neuroimage. 2006;33:923–935. doi: 10.1016/j.neuroimage.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Montagna M, Cerri G, Borroni P, Baldissera F. Excitability changes in human corticospinal projections to muscles moving hand and fingers while viewing a reaching and grasping action. Eur J Neurosci. 2005;22:1513–1520. doi: 10.1111/j.1460-9568.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- Morrison I, Bach P, Tipper S. Cognitive neuroscience society annual meeting. New York: MIT Press: 2007. Selective responses in SII for observed pain and action. [Google Scholar]
- Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage. 2007;37:642–651. doi: 10.1016/j.neuroimage.2007.03.079. [DOI] [PubMed] [Google Scholar]
- Morrison I, Peelen MV, Downing PE. The sight of others’ pain modulates motor processing in human cingulate cortex. Cereb Cortex. 2007;17:2214–2222. doi: 10.1093/cercor/bhl129. [DOI] [PubMed] [Google Scholar]
- Mottonen R, Jarvelainen J, Sams M, Hari R. Viewing speech modulates activity in the left SI mouth cortex. Neuroimage. 2005;24:731–737. doi: 10.1016/j.neuroimage.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Iacoboni M, Fried I. Visuo-motor mirror responses in human medial temporal lobe. J Cogn Neurosci. 2008;20:143. [Google Scholar]
- Nakata H, Sakamoto K, Ferretti A, Gianni Perrucci M, Del Gratta C, Kakigi R, Luca Romani G. Somato-motor inhibitory processing in humans: an event-related functional MRI study. Neuroimage. 2008;39:1858–1866. doi: 10.1016/j.neuroimage.2007.10.041. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Luppino G, Vanduffel W, Rizzolatti G, Orban GA. Observing others: multiple action representation in the frontal lobe. Science. 2005;310:332–336. doi: 10.1126/science.1115593. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Sur M, Felleman DJ, Kaas JH. Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol. 1980;192:611–643. doi: 10.1002/cne.901920402. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R. Temporal dynamics of cortical representation for action. 2000;97:913–918. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oouchida Y, Okada T, Nakashima T, Matsumura M, Sadato N, Naito E. Your hand movements in my somatosensory cortex: a visuo-kinesthetic function in human area 2. Neuroreport. 2004;15:2019–2023. doi: 10.1097/00001756-200409150-00005. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M, Smania N, Corato E, Aglioti SM. Neural underpinnings of gesture discrimination in patients with limb apraxia. J Neurosci. 2008;28:3030–3041. doi: 10.1523/JNEUROSCI.5748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Hamilton AF. Action understanding requires the left inferior frontal cortex. Curr Biol. 2006;16:524–529. doi: 10.1016/j.cub.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Raos V, Evangeliou MN, Savaki HE. Observation of action: grasping with the mind's hand. Neuroimage. 2004;23:193–201. doi: 10.1016/j.neuroimage.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Raos V, Evangeliou MN, Savaki HE. Mental simulation of action in the service of action perception. J Neurosci. 2007;27:12675–12683. doi: 10.1523/JNEUROSCI.2988-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Gentilucci M, Camarda RM, Gallese V, Luppino G, Matelli M, Fogassi L. Neurons related to reaching-grasping arm movements in the rostral part of area 6 (area 6a beta) Exp Brain Res. 1990;82:337–350. doi: 10.1007/BF00231253. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The classic supplementary motor area is formed by two independent areas. Adv Neurol. 1996;70:45–56. [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Rossi S, Tecchio F, Pasqualetti P, Ulivelli M, Pizzella V, Romani GL, Passero S, Battistini N, Rossini PM. Somatosensory processing during movement observation in humans. Clin Neurophysiol. 2002;113:16–24. doi: 10.1016/s1388-2457(01)00725-8. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomical investigation of projections to the basis pontis from posterior parietal association cortices in rhesus monkey. J Comp Neurol. 1989;289:53–73. doi: 10.1002/cne.902890105. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J Comp Neurol. 1994;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci. 2005;25:9339–9346. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JF, Glickstein M. Role of the cerebellum in visual guidance of movement. Physiol Rev. 1992;72:967–1017. doi: 10.1152/physrev.1992.72.4.967. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Thioux M, Gazzola V, Keysers C. Action understanding: how, what and why. Curr Biol. 2008;18:R431–R434. doi: 10.1016/j.cub.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing. A neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Ionta S, Aglioti SM. Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nat Neurosci. 2007;10:30–31. doi: 10.1038/nn1815. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L. Cytology of human dorsal midcingulate and supplementary motor cortices. J Chem Neuroanat. 2003;26:301–309. doi: 10.1016/j.jchemneu.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Doya K, Kawato M. A unifying computational framework for motor control and social interaction. Philos Trans R Soc Lond B Biol Sci. 2003;358:593–602. doi: 10.1098/rstb.2002.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3(Suppl.):1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.