Abstract
The primary cilium is a microtubule-based organelle implicated as an essential component of a number of signaling pathways. It is present on cells throughout the mammalian body; however, its functions in most tissues remain largely unknown. Herein we demonstrate that primary cilia are present on cells in murine skin and hair follicles throughout morphogenesis and during hair follicle cycling in postnatal life. Using the Cre-lox system, we disrupted cilia assembly in the ventral dermis and evaluated the effects on hair follicle development. Mice with disrupted dermal cilia have severe hypotrichosis (lack of hair) in affected areas. Histological analyses reveal that most follicles in the mutants arrest at stage 2 of hair development and have small or absent dermal condensates. This phenotype is reminiscent of that seen in the skin of mice lacking Shh or Gli2. In situ hybridization and quantitative RT-PCR analysis indicates that the hedgehog pathway is downregulated in the dermis of the cilia mutant hair follicles. Thus, these data establish cilia as a critical signaling component required for normal hair morphogenesis and suggest that this organelle is needed on cells in the dermis for reception of signals such as sonic hedgehog.
Introduction
The development and patterning of many tissues in the mammalian body involves conserved inductive signaling events between the epithelium and underlying mesenchyme. The hair follicle is a prototypic example of an organ formed through such reciprocal inductive interactions. While in most tissues these signaling events occur only during embryogenesis, the hair follicle is exceptional in that it continually regenerates itself throughout life utilizing many of the same signaling pathways that are essential for hair follicle morphogenesis. These properties, along with the abundance and accessibility of hair follicles, and the existence of many murine mutations affecting follicle morphogenesis, have made it an attractive system to analyze reciprocal signaling events between the epithelium and the mesenchyme.
In the mouse, primary hair follicle morphogenesis begins at approximately embryonic day 14.5 (Paus et al., 1999). The signal initiating hair follicle formation (stage 0) is thought to be an unidentified Wnt that is sent from the dermal mesenchyme to the epithelium. This ‘first dermal signal’ results in a clustering of cells in the epidermis to form an epithelial placode (stage 1). In the germ hair stage (stage 2), the epithelial placode sends the ‘first epithelial signal’ back to the mesenchyme promoting clustering of mesenchymal cells into the dermal condensate/dermal papilla (Hardy, 1992). The dermal condensate serves as an organizing center for the developing hair follicle. In response to signals from the dermal condensate (“second dermal signal”), the epidermal cells undergo a period of intense proliferation and down growth to establish the hair peg (stage 3–5); (Paus et al., 1999; Schmidt-Ullrich and Paus, 2005). Sonic hedgehog (Shh) signaling is vital for this epidermal proliferation. It is thought that Shh produced by the epidermal placode helps maintain the dermal condensate and that Shh responsiveness is necessary for epidermal follicular down growth (Nanba et al., 2003; St-Jacques et al., 1998). In keeping with this hypothesis, mice lacking Shh or Gli2, the primary Shh pathway transcriptional activator in the skin, have stage 2 arrested follicles (Botchkarev and Paus, 2003; Mill et al., 2003; St-Jacques et al., 1998). Transgenic expression of wild type Gli2 specifically in epidermal cells was sufficient to rescue hair follicle arrest in Gli2−/− animals, indicating that the Shh pathway in the epidermis is essential for follicular morphogenesis and down growth. A constitutively active form of Gli2 (but not wild type Gli2) expressed in the epidermis of shh−/− mutants was able to promote epidermal proliferation and induce Shh targets; however, this only partially rescued the follicular phenotype. These data suggest there is a Shh-dependent function required to activate Gli2 in the epidermis and a Shh-dependent signal from the dermis back to the epidermis that is needed to complete the down growth necessary for follicle development (Mill et al., 2003).
In the stage 6–8 follicle, the invading follicular epithelium continues to envelop the dermal condensate forming the dermal papilla (DP), which plays an important role in further hair follicle development and cycling in postnatal periods. The number of cells recruited to form the dermal papilla is directly related to the size of the follicle that will form and the thickness of the resulting hair (Paus and Foitzik, 2004). Surrounding the DP at the base of the follicle are the epithelial matrix cells that differentiate into multiple concentric epithelial cell layers that constitute the mature hair follicle including the hair shaft and the inner root sheath surrounding the hair shaft (Blanpain and Fuchs, 2005). Mutations in several genes have been generated that affect the number of cells in the DP, including Shh and platelet-derived growth factor A (PDGF-A, both of which result in significantly fewer DP cells and severely impaired hair follicle formation (Karlsson et al., 1999; Mill et al., 2003).
Intriguingly, primary cilia, which are small microtubule-based appendages extending from the surface of most cells in the body are required for normal signaling activity in several pathways, including Shh and PDGFRαα, as well as for regulating the balance between canonical and noncanonical Wnt signaling (Christensen et al., 2007; Gerdes et al., 2007; Schneider et al., 2005). In the case of PDGF mediated signaling, data indicate that PDGFRαα homodimers localize to and are activated in the ciliary membrane in response to PDGF-A (Schneider et al., 2005). In the absence of cilia, PDGF-AA is unable to activate PDGFR-αα. Similarly, multiple components of the hedgehog signaling pathway, including the Shh receptor Ptch1, the pathway transducer Smoothened (Smo), the negative regulator suppressor of fused (SuFu), and all three of the Gli transcription factors are present in cilia (Corbit et al., 2005; Haycraft et al., 2005; May et al., 2005; Rohatgi et al., 2007a). Cells with mutations in genes required to build cilia are unable to respond to Shh and have defects in both Gli2 activator and Gli3 repressor functions (Haycraft et al., 2005; Huangfu and Anderson, 2005; Rohatgi et al., 2007b). In addition, cilia have a role in regulating Wnt signaling as mutations affecting cilia can prevent noncanonical Wnts (such as Wnt5a) from suppressing the canonical Wnt (Wnt3a) pathway (Simons et al., 2005). Furthermore, using fibroblasts with a mutation in the ciliogenic gene Kif3a, Corbit et al. recently demonstrated that loss of cilia leads to an increased signaling response to canonical Wnts such as Wnt3a (Corbit et al., 2008). Together these data raise the possibility that primary cilia have essential functions in skin and hair follicle morphogenesis through a role in coordinating signaling activities of Shh, PDGF-AA, and the activity ratio of canonical versus noncanonical Wnt signaling (Christensen et al., 2007).
The construction and maintenance of the primary cilium is dependent on intraflagellar transport (IFT). IFT mediates the bidirectional movement of proteins between the tip and base of the cilia axoneme using numerous proteins (e.g. IFT88) involved in the formation of the IFT particle and the molecular motors kinesin II (Kif3a, Kif3b, and Kap3) and cytosolic dynein motor proteins respectively. Disruption of IFT in mice prevents cilia formation and causes early to mid-gestational lethality (Houde et al., 2006; Huangfu and Anderson, 2005; Liu et al., 2005; Murcia et al., 2000) due to defects in left-right axis specification and in neural tube closure and patterning. Ciliary dysfunction is also associated with the formation of cystic kidneys, hepatic and pancreatic abnormalities, skeletal malformations, and obesity (Davenport and Yoder, 2005; Haycraft et al., 2007; Huangfu and Anderson, 2005; Liu et al., 2005; Singla and Reiter, 2006). Thus, primary cilia have essential functions in development and homeostasis in multiple tissues and many of the phenotypes observed in cilia mutant mice have been linked with impaired regulation of Hedgehog and Wnt signaling (Cano et al., 2004; Chizhikov et al., 2007; Haycraft et al., 2007; Zhang et al., 2005).
Despite the prevalence of primary cilia in the mammalian body, their functional importance in most tissues, such as the skin, remains unknown. In part this is due to the early embryonic lethality caused by loss of cilia function. Thus, we utilized conditional alleles of two ciliogenic genes Kif3a (Kif3atm2Gsn) and Ift88 (Ift88tm1Bky) to disrupt cilia assembly specifically in the dermis of the skin to explore ciliary function during skin and hair follicle morphogenesis. These dermal cilia mutants have defects in hair development similar to that seen in Gli2−/− or Shh−/− mutant mice (Mill et al., 2003; St-Jacques et al., 1998). These data demonstrate an unappreciated role for dermal cilia during hair follicle morphogenesis where they appear to be involved in reception of hedgehog signals.
Results
Primary cilia in hair follicle development
Primary cilia are solitary, small organelles (normally 2–5 μm long) that are often overlooked or are difficult to detect using standard epifluorescence microscopy on a single section through tissue. Thus, our initial objective was to thoroughly evaluate whether cilia are present on cells in the skin and hair follicle during morphogenesis and during follicular cycling in postnatal stages. Expression of the ciliogenic gene ift88 (previously known as Tg737) was assessed using the β-galactosidase reporter gene incorporated into the Ift88tm1Rpw(Ift88Δ2–3-βGal) targeting construct used to generate the null mutant mouse (Murcia et al., 2000). Ift88tm1Rpw heterozygous skin samples had β-galactosidase activity in both dermal and epidermal cell populations in the developing and cycling follicle. Dermal cells include the dermal condensate, the dermal papilla and interfollicular fibroblasts. Epidermal cells include the matrix, inner and outer root sheaths, as well as cells in the bulge region (Figure 1A, E).
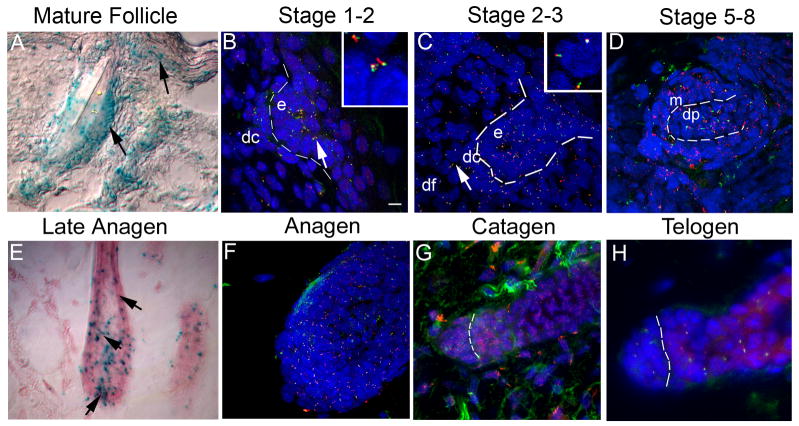
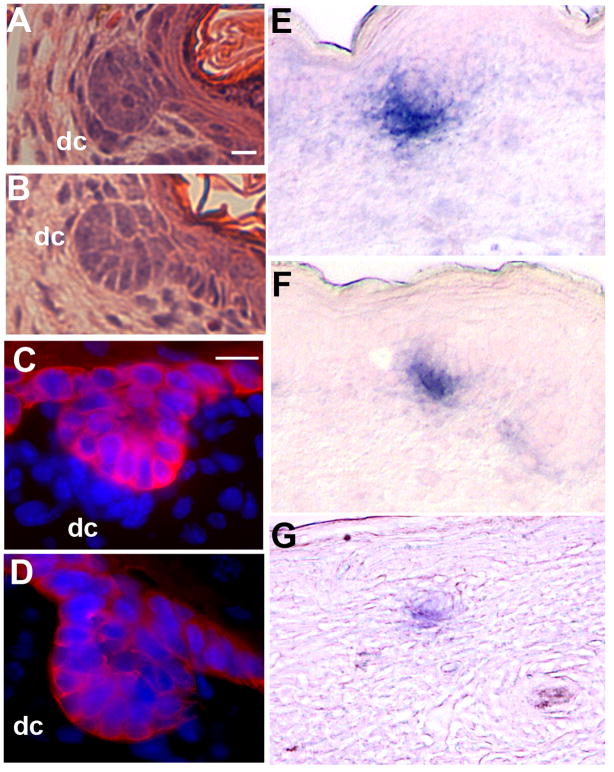
Figure 1.
Temporal and spatial analysis of an IFT88/Tg737 reporter gene (A, E) and protein (B–D, and F–H) reveals the presence of a primary cilium on epithelial and mesenchymal cells of the skin and hair follicle in embryos and adults. (A, E) Ift88 expression was analyzed in heterozygous Ift88tm1Rpw (Ift88Δ2–3-β-Gal) mice using a β-galactosidase reporter gene by X-Gal staining of skin sections. In the mature follicle (A) and late anagen follicle (E) and in the developing skin and hair, Ift88 expression (blue) is detected in most cell types. Expression is prominent in the (arrow) cortex and (indented arrow) ORS (epithelium), (arrowhead) dermal papilla (mesenchyme) (E), and in cells near the (arrow) bulge region (stem cells) of the hair follicle as well as the (arrowhead) interfollicular epidermis (A). (B–D,F–H) IFT88 (red) localizes to primary cilia as shown by immunofluorescence co-localization obtained using antibodies against the ciliary axoneme marker, acetylated α-tubulin (green). The nuclei (blue) were labeled with Hoechst. (B) Analysis in a stage 1–2 developing hair follicle shows primary cilia on cells of both the forming dermal condensate (dc) and epidermal placode (e). The dashed line indicates the region of the basement membrane and the intersection between the epidermis and dermis. The insert shows a magnified image of the region indicated by the arrow. (C) In a stage 2–3 developing hair follicle, cilia were present on the epidermal and dermal portion of the hair germ. The epidermal (e), dermal condensate (dc) and dermal fibroblast (df) cells are indicated. The insert shows a magnification of the region corresponding to the arrow in the dermal fibroblasts. (D) Analysis in a later stage (5–8) follicle shows primary cilia present on cells of both the matrix (m) and dermal papilla (dp). (F–H) Immunofluorescence co-localization of acetylated α-tubulin (green) and IFT88 (red) during hair follicle cycling shows that a single primary cilium is present on most cells at (F) anagen, (G) most dermal cells in catagen with reduced epidermal cilia, and (H) most cells in telogen. Scalebar is 10 microns. 3-D confocal images of cilia in the developing (1) or mature anagen (2) follicle can be seen in supplemental movies 1 and 2.
To determine if cilia are present on skin cells, we immunoprobed 40–50 μm skin cryo-sections with antisera against IFT88 (previously called polaris) and the cilia marker acetylated α-tubulin. Studies in multiple tissues have shown that both proteins localize to the cilia axoneme and that IFT88 is also present in the basal body at the base of cilia (Piperno and Fuller, 1985; Piperno et al., 1987; Taulman et al., 2001). Confocal microscopy was used to reconstruct the entire 3-dimensional architecture of the developing and mature hair follicle (see supplemental movies 1–2). Using this approach, primary cilia were evident on most if not all dermal and epidermal cell populations of the hair follicle and skin (Figure 1). Cilia were present on cells through the development of the follicle, including the placode (stage 1), to advanced hair bulbs (stage 5–8). Cilia were also found in the dermal fibroblasts, cells of the dermal condensate and dermal papilla, and in the epidermis in keratinocytes, as well as the epidermal matrix cells, and cells of the inner root sheath near the follicle bulb and throughout the outer root sheath (ORS). Fewer cilia, with the exception of the ORS, were found in the keratinizing cells of the upper anagen follicle near the hair shaft. Fewer epidermal cilia were also observed in catagen follicles, possibly due to intense epidermal apoptosis during this period. Primary cilia in the epidermis were most prominent on basal keratinocytes in the interfollicular regions. Progressively fewer cilia were observed on differentiated cells in the spinous layer and cilia were lost in the upper layers of the epidermis much as reported by Elofsson et al. in human skin (Elofsson et al., 1984). Most epidermal cilia had an apical orientation that was conserved in outer root sheath cells as the follicle matured, but was lost in the matrix cells. Cilia on dermal cells did not have such an obvious orientation.
Disruption of primary cilia in the dermis
Dermal cells, notably the dermal condensate/papilla, have a major influence on hair follicle development and cycling as both an inductive and responding mesenchymal tissue. Thus, to begin assessing the importance of the primary cilium in the skin and hair follicle, we utilized two floxed alleles (Kif3atm2Gsn and Ift88tm1Bky hereafter referred to as Kif3afl and Ift88fl respectively) of ciliogenic genes (Ift88 or Kif3a) to disrupt cilia assembly specifically in cells of the dermis using the Prx1-cre (Tg(Prrx1-cre)1Cjt) line. This transgene drives Cre recombinase activity in the dermal mesenchyme of the skin on the ventrum, limbs, and laterally between the limbs starting at E9.5 (Logan et al., 2002). In contrast to Ift88 null mutant mice (Ift88tm1Rpw), Prx1-cre;Ift88fl/n (n refers to the deleted allele Ift88tm1.1Bky arising from Ift88fl) conditional mutants are viable.
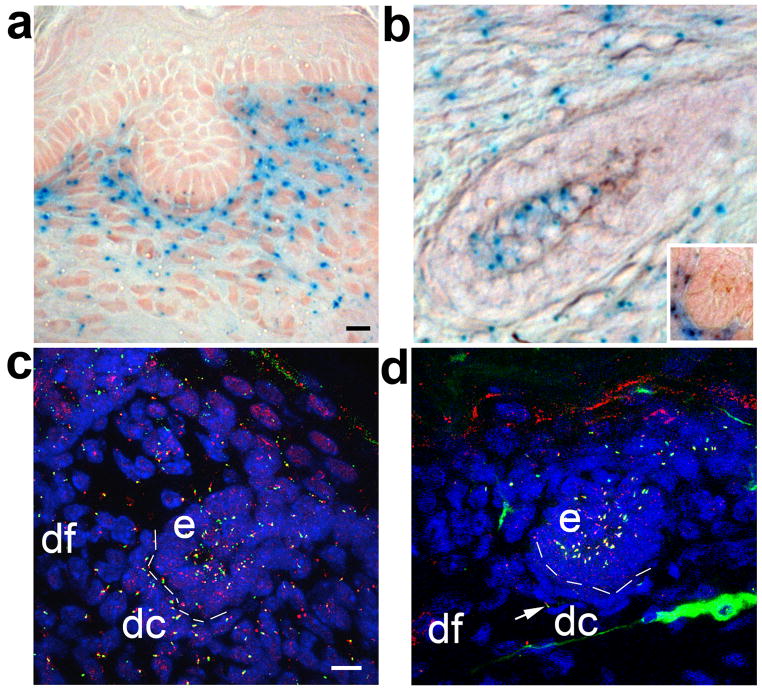
To determine the temporal and spatial locations of Cre activity in the skin and hair follicles, we crossed Prx1-cre positive mice with mice carrying the Cre reporter, R26R (Zambrowicz et al., 1997). Analysis of β-gal staining indicated that Cre activity was tightly restricted to cells in the dermis including dermal condensates/papilla and was not evident in epidermal cells (Fig 2A,B,inset). Conditional cilia mutant mice (Prx1-cre;Ift88fl/n,) were then analyzed using thick sections of ventral skin for loss of cilia by immunofluorescence microscopy (Figure 2). In agreement with the spatial distribution of Cre activity, very few cilia were present on dermal mesenchyme cells of Ift88 conditional mutants. The few cilia remaining on dermal cells likely reflect incomplete activity of the Cre recombinase. In contrast, epidermal cells near the hair follicles in wild-type mice and Prx1-cre conditional mutant mice possessed a primary cilium.
Figure 2.
Cre activity and disruption of cilia in Prx1-cre mice is restricted to the dermal compartment of the skin and hair follicles covering the limbs, flanks, and ventrum. (A) Cre activity in the skin and stage-2 hair follicle from the ventrum of a Prx1-cre;R26R mouse is present only in dermal cells, as revealed by the lacZ reporter gene (blue). (B) Cre activity is also seen in dermal components of advanced follicles including the dermal papilla of an advanced follicle and a stage 2 follicle (inset) from a P5 Prx1-cre;R26R mouse. (C) In wild-type control mice, primary cilia are present in the epidermal (e) and dermal (dc, dermal condensate; df, dermal fibroblast) compartments of the skin and stage 2 hair follicle, as shown by co-localization of antibodies raised against acetylated α-tubulin (green) and IFT88 (red). (D) In Prx1-cre;Ift88fl/n conditional mutant mice, primary cilia are ablated from most dermal condensate (dc) and dermal fibroblast (df) cells, but cilia are unaffected on epidermal (e) cells. Rare dermal cilia are unaffected, likely due to incomplete cre activity (arrow). Scalebar is 10 microns.
Loss of cilia in the dermis of the skin results in hypotrichosis
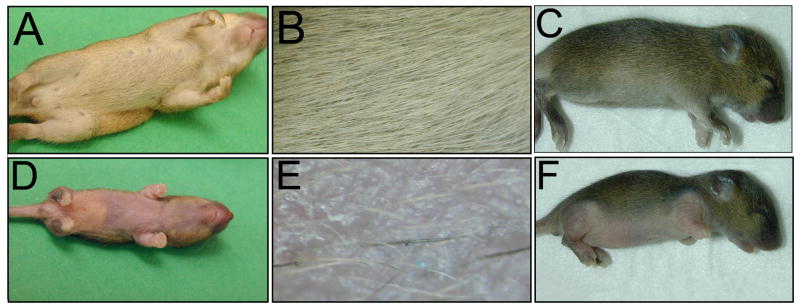
Loss of cilia on dermal cells in the conditional mutants resulted in persistent and severe hypotrichosis (Figure 3). The sparse hair phenotype in both the Prx1-cre;Ift88 and Prx1-cre;Kif3a conditional mutants was identical and was observed specifically in the regions where Prx1-cre is expressed, including the upper ventrum, limbs, and a small domain on top of the head(Logan et al., 2002). Hair follicle number and morphology appeared normal on regions of the body where Cre is not expressed. There were some hair follicles that formed normally on the ventrum in the Prx1-cre conditional cilia mutants (Figure 3E). The mechanism by which these follicles escape developmental arrest is unknown and a similar observation was made in Gli2−/− mutant skin (Mill et al., 2003).
Figure 3.
Conditional disruption of primary cilia in the dermis of the ventral skin in Prx1-cre;Ift88fl/n and Prx1-cre; Kif3afl/n mice results in hypotrichosis. The pelages of (A,B,C) wild-type littermate control mice, (D,E) a Prx1-cre;Ift88fl/n, and (F) Prx1-cre; Kif3afl/n conditional mutant mouse are shown at P14. (B,E) Higher magnification images of the ventrum of the mice in A and D show that the mutant skin has an extremely sparse coat and a few follicles that appear normal. The conditional cilia mutant mice also have deformed limbs that were described previously (Haycraft et al., 2007).
The hypotrichosis in the cilia mutants is caused by an arrest in follicle morphogenesis
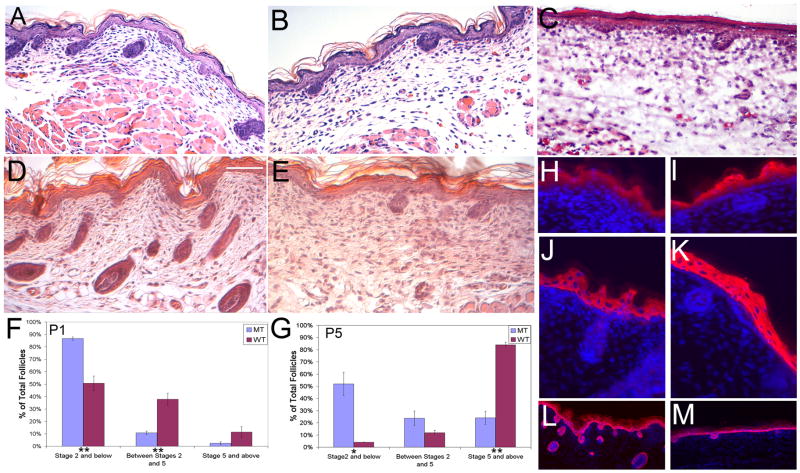
To further assess the follicular phenotype, we conducted histological analysis of the ventral skin from Prx1-cre;Ift88 conditional mutant mice at P1 and P5. The data indicate there is a significant delay in follicle development in the conditional mutants (Figure 4B, C, E), with most arresting at stage 2(Figure 4F, G). This is in contrast to the wildtype controls (Figure 4A, D) where most follicles advanced to stages 5–8 by P5 (Figure 4F, G). Interfollicular skin appeared normal as determined by staining for different cell populations using antibodies for K5 (basal layer), K10 (suprabasal layers), and loricrin (granular layer) (Figure 4H-M). Mild fibrosis was seen in the Prx1-cre; Ift88 mutant animals via trichrome staining (supplemental Fig. 1).
Figure 4.
Hair follicle morphogenesis is arrested at stage 2 (hair germ) in mice with primary cilia ablated from dermal cells of the skin and hair follicles. Histological sections of skin from (A) P1 wildtype, (B) mutant P1 Prx1-cre;Ift88fl/n,and (C) P1 Prx1-cre; Kif3afl/n mice show fewer and less advanced follicles in the mutant mice. This phenotype worsens at P5 in (E) mutant animals when compared to age matched (D) wildtype controls. Histomorphometric analyses of the hair follicle phenotypes in skins harvested from mutant and control mice at (F) P1 and (G) P5 indicate that most follicles arrest at stage 2 of morphogenesis in Prx1-cre;Ift88fl/n mice. The analyses were performed using a minimum of 40 longitudinal follicles in each group from (F) P1 (n=5 pairs) and (G) P5 (n=3 pairs) mice. Error bars represent SEM. Statistical comparisons were conducted using the two-tailed independent Student’s t test. * p < 0.05; ** p < 0.01. (H–M) Defects in differentiation were analyzed by immunofluorescence using antibodies against (H,I) loricrin (granular layer), (J,K) K1 (stratum spinosum), and (L,M) K5 (basal layer) of the epidermis reveal no overt differences in staining between (H,J,L) WT and (I,K,M) MT interfollicular epidermis. Scale bar is 50 microns.
Comparison between the conditional cilia mutant and wild type skin revealed that there were also defects in the dermal condensate in cilia mutants. Using endogenous alkaline phosphatase as a marker for dermal condensate cells as well as by histological and immunofluorescent (K5 stain) approaches, it is evident that in most follicles of the mutant mice, the dermal condensate was not detected or had fewer cells than in wild-type controls (Figure 5, Table 1, and supplemental Figure 2). This dermal condensate hypoplasia was less pronounced at P1 than at P5. Additionally, wildtype stage 2 dermal condensates at P5 appeared similar to those at P1, though there were too few present for statistical analysis (Table 1). At P21, the arrested hair germs in Ift88 and Kif3a mutants are no longer visible (data not shown) indicating that they likely degenerate.
Figure 5.
Disruption of cilia in the dermis of Prx1-cre;Ift88fl/n conditional mutant mice results in a marked reduction or absence of cells in the dermal condensate of stage 2 hair follicles. Histological analysis of (A and B) Hematoxylin and Eosin stained sections and (C and D) thick section immunofluorescence analysis using epidermal marker Keratin 5 (red) antibodies shows a marked reduction in the number of cells in the dermal condensate (dc) of (B and D) Prx1-cre;Ift88fl/n conditional mutants compared to (A and C) controls. The reduced number of dermal condensate cells was also evident in (F and G) Prx1-cre;Ift88fl/n conditional mutants when compared to (E) controls as determined by staining for endogenous alkaline phosphatase activity. (E, F) are stage 2 follicles from P1 mice and (G) is a stage 2 follicle at P5. Scalebar is 10 microns.
Table 1.
Statistical representation of dermal condensate cell number in wild-type and dermal cilia disrupted mutants at P1 and P5
| Genotype | Timepoint | No. of DC cells | Standard error | n |
|---|---|---|---|---|
| Wild-type 1 | P1 | 7.18 | 0.48 | 11 |
| Wild-type 2 | P1 | 6 | 0.65 | 10 |
| Wild-type 3 | P1 | 5.75 | 0.31 | 12 |
| Avg. wild-type | P1 | 6.31 | 0.44 | 3 animals |
| Mutant 1 | P1 | 4 | 0.42 | 10 |
| Mutant 2 | P1 | 4.43 | 0.59 | 14 |
| Mutant 3 | P1 | 5.1 | 0.66 | 10 |
| Avg. mutant | P1 | 4.51 | 0.32 | 3 animals |
| Avg. wild type | P5 | Only 5 stage 2 follicles | 3 animals | |
| Mutant 1 | P5 | 2.8 | 0.55 | 10 |
| Mutant 2 | P5 | 2.18 | 0.44 | 10 |
| Mutant 3 | P5 | 1.8 | 0.42 | 10 |
| Avg. mutant | P5 | 2.26* | 0.29 | 3 animals |
Abbreviations: Avg., average; DC, dermal condensate.
Bold connotes significant f-test comparing wild-type condensates at P1 to mutant condensates at PI (P<0.05) or P5 (*P<0.01).
Loss of dermal cilia impairs hedgehog signaling activity
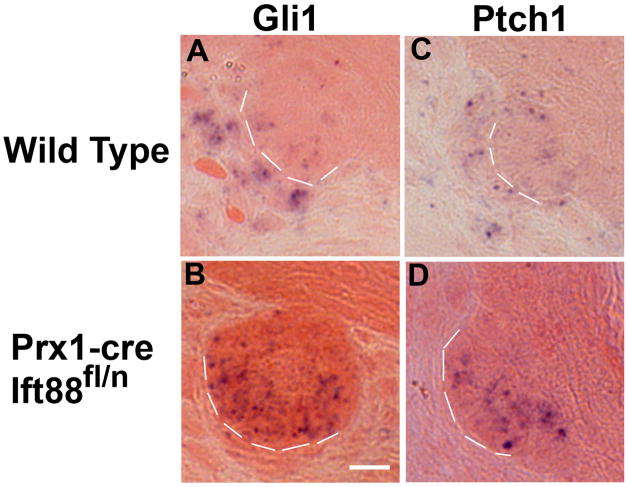
Together, the phenotypes observed in the Prx1-cre;Ift88 and Prx1-cre;Kif3a conditional mutant mice reveal that ciliary function in the dermal mesenchyme is essential for normal hair follicle morphogenesis. Interestingly, the skin and hair follicle phenotype seen in these mutant mice recapitulates that seen in Gli2 −/− and Shh −/− mutants. Thus, we analyzed the activity of the Shh pathway in the dermal cilia mutants by in situ hybridization and real-time quantitative RT-PCR using RNA isolated from laser microdissected stage 2 dermal condensates (Supplemental figure 3). At P1, hedgehog pathway activity was impaired as shown by qRT-PCR where Gli1 expression was reduced by 4.7 (p<0.01 (p=0.0073)) fold. Similarly, in situ hybridization analysis indicated that expression of the hedgehog responsive genes Ptch1 and Gli1 were reduced in the dermal region of the follicle at P5. However, this was difficult to assess due to the reduced size of the dermal condensates in most of the mutant follicles (Figure 6). Unexpectedly, levels of expression of Ptch1 and Gli1 in epidermal cells, which retain their cilia, appear to be elevated compared to the controls.
Figure 6.
Ablation of dermal cilia in the skin of Prx1-cre;Ift88fl/n conditional mutant mice results in downregulation of the hedgehog responsive genes, Gli1 and Patched1, during hair follicle morphogenesis. Analysis of the hedgehog signaling pathway was performed in sections of P5 skin by in situ hybridization to detect expression of the hedgehog responsive genes (A, B) Gli1 and (C, D) Patched1 (Ptch1) in (A, C) control and (B, D) Prx1-cre;Ift88fln conditional mutant mice. The mutants show reduced or absent expression for both genes in the dermal condensates and an increase in the epidermal cells. Dashed lines separate the epidermal components of the hair follicle from the underlying dermal condensate. Scalebar is 10 microns.
Disruption of Dermal cilia and the canonical Wnt pathway
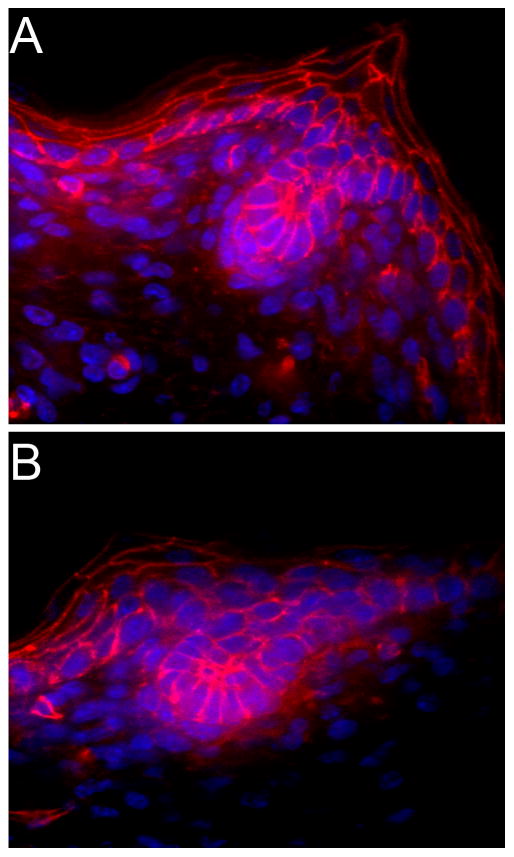
In several tissues, ciliary dysfunction has been shown to cause an increase in β-catenin levels and over activation of the canonical wnt pathway. To determine whether this is also the case in the skin and hair follicle, we analyzed β-catenin expression and localization by immunofluorescent confocal microscopy in the dermal cilia mutants (Figure 7). There were no overt changes evident in the mutants when compared to similarly staged control follicles at P1.
Figure 7.
There are no overt changes in Canonical Wnt signaling in dermal cilia conditional mutants as determined by thick section immunofluorescent confocal analysis of β-catenin (red) localization between (A) wild type and (B) Prx1-cre;Ift88fl/n mutant mice at P1. Nuclei are stained with Hoescht (blue).
Discussion
There have been sporadic reports of primary cilia in mammalian skin, often these were in association with disease states such as basal cell carcinoma (Daroczy and Feldmann, 1974; Wilson and Mc, 1963). In addition, in vivo cilia have been found in basal keratinoctyes and a minority of primary keratinocytes in culture also express a cilium under certain growth conditions; however, the functional importance of this organelle in the skin is unexplored(Elofsson et al., 1984; Strugnell et al., 1996). Discrepancies in the literature regarding the extent of ciliation in the skin may reflect the technical difficulties related to fixation (such as Karnovsky’s solution) or that serial section electron microscopic analysis was required to observe this small organelle (Elofsson et al., 1984; Elofsson, 1981; Roberto F, 1999; Wandel et al., 1984). Hair follicle cilia, by contrast, have remained unexamined, with the exception of the mature anagen wool follicle where cilia were found on a few epidermal cells of the bulb and the dermal papilla (only 41 epidermal cilia and 6 dermal cilia in a total of 9 follicles were found) (Orwin and Woods, 1982).
Using immunofluorescent confocal microscopy, we were able to analyze cells in the complex three-dimensional structure of the epidermis and dermis of the skin and hair follicle and demonstrate conclusively that primary cilia are found on most if not all cells throughout development of the skin. The presence of cilia on epidermal and dermal cells in the skin and particularly in the dermal condensate/papilla, a cell population that functions to regulate cycling and development of the follicle, supports the idea that primary cilia are important for normal skin and hair follicle morphogenesis.
This study was initiated to directly address the question of whether cilia are functionally important in skin. Due to the mid-gestation lethality associated with the loss of cilia in mice, we utilized conditional alleles of two genes required for ciliogenesis, Kif3a and Ift88, to disrupt cilia in the dermal mesenchyme by crossing them with the Prx1-cre mice. Analysis of the phenotype in either line (Prx1-cre;Ift88 and Prx1-cre;Kif3a) revealed that the disruption of dermal cilia results in stage 2 follicular arrest and severe hypotrichosis. Therefore, ciliary function in the dermis is essential for hair follicle development. Histological analysis indicates that cilia on cells of the dermal condensate/dermal papilla are particularly important, since the dermal condensate is often absent or severely hypocellular compared to controls. Mice with mutations in one of several components of the Shh and PDGF signaling pathways exhibit defects in dermal papilla maintenance or formation (Karlsson et al., 1999; Mill et al., 2003; St-Jacques et al., 1998). The fact that loss of dermal cilia, with intact epidermal cilia, leads to follicular arrest suggests that cilia are required for reception of a signal, possibly originating from the epidermis, needed for their maintenance, proliferation, or recruitment of cells into the dermal condensate.
The Prx1-cre;Ift88 and Prx1-cre;Kif3a conditional cilia mutants are the first dermal specific knockouts with a follicular arrest phenotype at stage 2 of development. Previous work has implicated signaling in the epidermis as critical for progression past this stage of follicle morphogenesis (Mill et al., 2003; Mill et al., 2005). Our data with the conditional cilia mutant mice indicate that reception of signals in the dermal cells is also critically important for follicular morphogenesis. Since identical phenotypes are obtained with both the Kif3a and the Ift88 conditional mutant mice, the data suggest that their phenotypic sequelae stem from a loss of ciliary function as opposed to other unknown cellular activities of these proteins. Although we have not fully evaluated the pathway(s) affected in the Kif3a and Ift88 conditional cilia mice, as indicated below, defects in hedgehog signaling likely contribute to this phenotype.
Recently, cilia dysfunction has been associated with impaired hedgehog signaling activity leading to abnormalities in the patterning of the limb bud and neural tube, and to defects in the expansion of cerebellar progenitor cells (Chizhikov et al., 2007; Haycraft et al., 2005; Huangfu and Anderson, 2005). Similarly, both our qRT-PCR analysis using laser capture enriched dermal condensates and in situ hybridization data show that there is reduced expression of Shh target genes in the dermal condensate region in ciliary conditional mutant mice; although this could also be associated with the hypocellularity of the dermal condensates in older mutants. Moreover, the observed arrest of hair follicle morphogenesis at stage 2 and the defects in dermal condensate/papilla maintenance are similar to the phenotypes observed in mice lacking Shh, Gli2, or other likely mediators of Shh, such as the Lama5−/− mice (Li et al., 2003; Mill et al., 2003; St-Jacques et al., 1998). These results are also supported by the previously characterized limb patterning defects observed in these mutants which we have shown involve defects in hh signaling(Haycraft et al., 2007). Together these data support a model in which the loss of dermal cilia results in arrested follicle development and hypotrichosis due to dysregulated Shh signaling activity.
Our expression data also suggest there is a feedback effect resulting in an increase in gli1 and ptch1 expression in the epidermal placode of the mutant stage 2 follicles. A possible explanation for this phenomenon is that the reduction in ptch1 expression in the dermis leads to a local increase in the availability of Shh in the neighboring epidermal placodes upregulating ptch1 and gli1. A similar observation was made when smoothened was disrupted in the epidermis. In these mice, dermal Shh signaling activity is elevated due to an increase in Shh availability caused by the reduction in Ptch1 expression in the epidermis (Gritli-Linde et al., 2007).
In the dermal cilia mutants we also observed that there were a number of follicles that escaped developmental arrest. In part this could reflect an inefficiency of Cre activity in the Prx1-cre line. An alternate explanation is that loss of cilia in the dermis also disrupts proteolytic processing of Gli3 to its repressor form. In the absence of a Shh signal (i.e. Shh mutants or in regions where shh signal is low), Gli3 is normally cleaved into a repressor; however, in mesenchymal cells lacking cilia this processing is impaired despite the inability of these cells to respond to Shh signals. Thus, some of the follicles may escape the arrest expected for cells unable to respond to Shh due to the additional loss of Gli3 repressor activity. This was shown previously in Shh−/− mutants where subsequent loss of Gli3 (Shh−/−;Gli3−/− double mutants) were able to rescue many aspects of the Shh−/− mutant phenotype, including a partial rescue of the dermal condensate in hair follicle morphogenesis(Mill et al., 2005).
While our analyses indicate that cilia on cells in the dermis of the skin are essential for normal hair follicle morphogenesis and that they likely function in Shh reception, our findings are perplexing in light of data from Mill et al. (Mill et al., 2003). These authors demonstrated that the arrested hair follicle phenotype in Gli2−/− mutants can be rescued by expression of Gli2 specifically in the epidermis. In contrast to our results, their data suggest that Shh reception in the epidermis, rather than dermis, is critical for morphogenesis of the hair follicle past stage 2. However, it is interesting that Mill et al. were unable to completely rescue the follicular phenotypes in Shh−/− mutants by exogenous expression of an activated form of Gli2 in the epidermis. Thus, the understanding of how cilia mediate hedgehog signaling during hair follicle morphogenesis is incomplete, and it is likely that loss of cilia from the DC/DP affects both the activator and repressor functions of the Gli transcription factors. To fully address this issue will require further analyses using cre lines that that disrupt ciliary function specifically in the epidermis or in more defined cell populations in the follicle, such as the dermal condensate.
The hair follicle phenotypes displayed by the dermal cilia mutants phenocopy those seen in mice lacking Shh or Gli2. However, in addition to its role in hedgehog signaling, the primary cilium has been implicated in several other pathways that are known to be important for hair follicle and skin development including Wnt and PDGFR-αα signaling. Cilia influence the Wnt pathway by regulating noncanonical Wnt (i.e. Wnt5a) repression of the canonical Wnt signals (i.e. Wnt3a) and by restricting the strength of a cell’s response to canonical Wnt signals. In the absence of cilia, the canonical pathway is no longer repressed by noncanonical Wnts leading to an increase in β-catenin and canonical Wnt signaling (Benzing and Walz, 2006; Corbit et al., 2008; Simons et al., 2005). However, in our dermal cilia knockouts the epidermal cilia are intact and β-catenin appears to be properly regulated. This may be due to the fact that in later hair follicle development the canonical wnts are primarily expressed in the epidermis (Reddy et al., 2001), though early loss of dermal canonical function leads to defects in ventral dermal specification(Ohtola et al., 2008). The lack of an observed change in β-catenin localization may indicate that canonical wnt activity is more important in the epidermis, or that disruption of cilia that theoretically leads to a mild to moderate increase in activation of the canonical pathway would, unlike loss of canonical wnt signaling, not impact dermal development significantly. ] Thus, future studies using epidermal specific cilia knockout lines may be more informative concerning the effects of ciliary disruption on canonical versus noncanonical signaling in the skin and hair follicle, particularly considering the recent data indicating that β-catenin signaling in the epidermis is important for specification of hair follicle fates (Zhang et al., 2008). Unfortunately, the importance of the noncanonical wnt pathway is less well characterized in the hair follicle and the role of this pathway in hair follicle morphogenesis as opposed to polarity is not understood.
Cilia have also been implicated as important regulators of PDGF signaling. The receptor (PDGF receptor-alpha, PDGFR-α) localizes to the ciliary membrane and cells with defects in ciliogenesis are unable to respond to the PDGF-AA ligand (Schneider et al., 2005). In the skin, PDGF-A is expressed in the developing epithelial portions of the epidermis and hair follicle, whereas the dermal condensates cells express PDGFR-α. Furthermore, Pdgf-a−/− mutant mice develop skin and hair phenotypes characterized by a constellation of dermal phenotypes, including a hypoplastic dermis, small dermal papillae, abnormal dermal sheaths, and thin hair with misshapen follicles. The expression patterns and mutant phenotypes suggest that epidermal PDGF-A stimulates proliferation of dermal mesenchymal cells that contribute to the dermal papillae, mesenchymal sheaths, and dermal fibroblasts (Karlsson et al., 1999). Thus, disruption of PDGFR-αα signaling in the mesenchyme of the dermal cilia mutants may be a contributing factor, in addition to loss of Shh responsiveness, to the reduced size of the dermal condensate. However, these conditional mutants do not display the general dermal atrophy phenotype normally associated with loss of PDGF-A signaling in the skin, in fact, trichrome staining suggests mild fibrosis or thickening of the dermis (supplemental figure 1). This argues against a predominant role for PDGFR-αα signaling in these mutant phenotypes. This may reflect compensation by PDGF-AB and PDGF-BB signaling, which function normally in cells lacking cilia(Schneider et al., 2005). Indeed, both PDGF-BB and PDGF-AA have shown anagen inducing effects upon injection into skin, and a study in lung fibroblast suggests that PDGF-AB is stronger at driving fibroblast proliferation than PDGF-AA (Bonner et al., 1991; Tomita et al., 2006).
The crosstalk between the Wnt, PDGF, and Shh pathways in hair follicle development remains incompletely understood, but may contribute to the phenotypes observed. Previous analysis of shh knockout mice has ruled out a direct epistatic interaction, but shh knockout mice were missing positive PDGFRα mesenchymal cells neighboring Shh−/− follicles suggesting a role for Shh in the clustering of these dermal condensate cells (Karlsson et al., 1999). It has also been suggested that the Shh pathway initiates stage specific activation of Wnt signaling components β-catenin and Lef1, but this was evaluated in the context of epidermal shh pathway activation (Mill et al., 2005) and changes in β-catenin expression or localization were not evident in our dermal conditional mutants. Expression of noncanonical wnt5a in the dermal condensate does require the sonic hedgehog pathway, suggesting wnt5a may be a downstream Shh target in the dermis (Reddy et al., 2001). Although data indicate that cilia are essential for hair follicle development, the role of this organelle in regulating the crosstalk between the Shh, PDGF-AA, and canonical versus noncanonical signaling pathways remains to be fully explored.
The primary cilium is an organelle with a previously unappreciated and wide distribution in the developing hair follicle and skin. Evolving research on cilia in the last decade has changed the perspective on this organelle from a functionless remnant to a signal transduction center in multiple tissues. In this study, we have localized primary cilia to both epidermal and dermal populations of the skin and developing follicle. We further show that dermal cilia are essential for normal hair follicle morphogenesis with disruption of this organelle leading to hair follicle arrest that is associated with abnormal Shh signaling activity. Furthermore, the involvement of cilia in multiple signaling pathways such as canonical and noncanonical Wnt, PDGFR-αα and Hedgehog has exciting implications for understanding human skin biology and disease processes where these signaling pathways become dysregulated and contribute to hair loss or carcinogenesis.
Methods
Mice
Ift88tm1Rpw (previously referred to as Tg737Δ2–3-β-gal) mice on the FVB/N genetic background that carry a knockout allele of the Ift88 (previously called Tg737) gene were generated at the Oak Ridge National Laboratory and described previously (Murcia et al., 2000). The Ift88 conditional allele, Ift88fl (Ift88tm1Bky), was generated at the University of Alabama at Birmingham and was described previously (Haycraft et al., 2007). The conditional Kif3a allele, Kif3afl (Kif3atm2Gsn), was obtained from Dr. Goldstein (UCSD) (Marszalek et al., 2000). The Prx1-cre mice (Tg(Prx1-cre)1Cjt) were generated by Logan et al. (Logan et al., 2002) and obtained from Jackson Laboratory. Conditional cilia mutant mice were analyzed on a mixed C57BL/6 X 129 genetic background. The experimental mice were generated using male Prx1-cre mice due to the germline activity of Prx1-cre in females. PCR analysis of DNA obtained from tail biopsies was used to genotype mice as described previously (Murcia et al., 2000; Yoder et al., 1997). All animals in this study were maintained in AALAC certified mouse facilities at UAB and in accordance with IACUC regulations and protocols at the University of Alabama at Birmingham.
Immunofluorescence Microscopy
Hair and skin from appropriate regions as indicated in the figures was adhered to nitrocellulose filter paper in a PBS bath then longitudinally trimmed. The samples were embedded in Optimal Cutting Temperature (O.C.T.) compound (Sakura, Torrance CA) and flash frozen in a 2-Methyl butane bath cooled in liquid nitrogen. To evaluate the presence of cilia on cells of the skin, 40–50 μm thick skin sections were cut using a Leica CM1900 cryostat and fixed either for 2 hrs in 4% PFA or for 30 minutes in ice-cold methanol. The sections were permeablized by incubation for 30 minutes in either 0.02% SDS in PBS or in ice-cold methanol. Sections were washed in PBS, blocked with 1% BSA for 30 minutes, and incubated with primary antibodies and sequentially secondary antibodies overnight at 4°C in PBS with 1% BSA and 0.01% Triton. Antibodies used in this analysis include anti-polaris/IFT88 (B1700, 1:1,000, (Haycraft et al., 2007)), anti-acetylated alpha-tubulin (Sigma, St. Louis, Missouri, Cat#T 6793, 1:1,000), anti-β-catenin (Sigma, St. Louis, Missouri cat#C 2206, 1:1000), anti-K5 (Covance, Berkeley, CA, Cat#PRB-160P, 1:1000), anti-K1 (Covance, Berkeley, CA, Cat#PRB-165P, 1:500) and anti-loricrin (Covance, Berkeley, CA, Cat#PRB-145P, 1:500). Nuclei were stained with Hoechst 33258 (Sigma) diluted 1:1,000 in PBS. After extensive washing in PBS, the sections were covered in mounting medium consisting of 1mg/ml p-phenylenediamine in 90% glycerol and coverslips were attached with nail polish. Images were captured using either an inverted Nikon TE200 epifluorescence microscope with a CoolSnap HQ/FX (Roper Scientific) CCD camera operated through MetaMorph (Molecular Devices, Downington PA) imaging software, a Leica Confocal Imaging Spectrophotometer TCS SP unit (UAB High Resolution Imaging Core), or a Perkin Elmer Spinning Disc confocal microscope. The resulting images were viewed and analyzed using Adobe Photoshop (Adobe). For confocal microscopy, optical sections were captured at ~0.4–0.5 μm intervals and the three-dimensional structure of skin and hair follicles was rendered from the Z-stacks using Voxx2 imaging software (available from the Indiana Center for Biological Microscopy, Indiana University http://nephrology.iupui.edu/imaging/voxx/index.htm) (Clendenon et al., 2002).
β-galactosidase assays
Analysis of Ift88/Tg737 expression was performed as described by (Taulman et al., 2001) using 8–10 μm sections of skin biopsies obtained from Ift88tm1Rpw (Tg737Δ2–3-β-gal) heterozygous mice. The location of Cre recombinase activity was analyzed in sections of mice doubly transgenic for Prx1-cre and the ROSA26 Cre-reporter, R26R (Zambrowicz et al., 1997), using X-gal as a substrate.
Histomorphometry, Endogenous Alkaline Phosphatase Staining, and Trichrome staining
Histomorphological analysis was performed on Haematoxylin and Eosin stained sections (5 μm) obtained from ventral skin over the sternum and analyzed using the staging guidelines described by (Paus et al., 1999). Longitudinal sections were separated by at least 40 μm to prevent double counting of hair follicles. Statistical analysis was performed using a two-tailed independent student’s t test (p<=0.05) on at least three littermate controls or five age-matched controls with at least 40 staged follicles per mouse. Endogenous Alkaline Phosphatase staining was performed on 8 μm cryosections post fixed for 8 minutes in 4% PFA/PBS, washed with NTMT,(100mM NaCl, Tris-HCl pH 8.0, 50mM MgCl2, 1%Tween-20) and incubated in BM Purple substrate (Roche) for 2 hours at 4 C. Trichrome staining was performed using Lillie’s trichrome on 5um paraffin embedded sections.
In situ hybridization
In situ hybridization for Ptch1 and Gli1 probes was performed as described in (Sheng et al., 2002) using Ptch1 and Gli1 probes previously described in (Haycraft et al., 2007).
Laser Cutting Microdissection and Quantitative Real Time PCR
Laser microdissection was performed using a Zeiss P.A.L.M microdissection instrument. Captures were enriched for dermal condensate using the dissection and capture regimen indicated in supplemental figure 3. Briefly, 8 μm cryosections were cut and H+E stained then laser dissected and catapulted. A minimum of 80 mutant or 40 wild type stage 2 follicles were microdissected per sample. RNA was extracted using Ambion RNaqueous and converted to cDNA. Quantitative RT-PCR analysis was performed using a Roche Lightcycler 480 and resulting fold differences and statistical significance was assessed using the REST (Relative Expression Software Tool) program (Pfaffl et al., 2002) using a pairwise fixed reallocation randomization test with 50,000 iterations. Gene expression was referenced to 18sRNA (to compensate for cell size/loading) and normalized to Ift88 levels using an amplicon in the deleted region (to compensate for variable contamination of samples with neighboring epidermal placodes in the laser microdissection process and cre efficiency). Taqman probes for Gli1 (Mm00494645_m1), 18sRNA(Hs99999901_s1), and IFT88 (Mm0133466_m1) were obtained from Applied Biosystems.
Supplementary Material
Supplemental Figure 1: Mild fibrosis and possible dermal expansion was present in (B,D) Prx1cre;Tg737fl/fl animals compared to (A,C) wildtype littermates at (A,B) P1 and (C,D) P5 as determined by trichrome staining (green).
Supplemental Figure 2: Representative 8um images prior to Laser Microdissection of stage 2 follicles in P1 (A) wildtype and (B) conditional cilia knockout mice showing the reduced number of dermal condensate cells in mutants compared to control samples.
Supplemental Figure 3: Representation of the Laser Capture Microdissection strategy used to analyze expression in the dermal condensates. (A) Stage 2 hair follicles are identified based on previously described criteria (Paus et al., 1999). Dermal condensate capture relies on (B) laser microdissection of dermal condensates (outlined) followed by (C) sample catapulting.
Supplemental Movie 1: Immunofluorescence analysis of a stage 2 hair germ showing that cilia are present on most epidermal and dermal cells including the interfollicular epidermis, epidermal hair germ, dermal fibroblasts, and forming dermal papilla as shown by cilia axoneme markers acetylated α-tubulin (green) IFT88 (red), and Hoescht nuclear staining(blue).
Supplemental Movie 2: Immunofluorescent analysis of a P25 anagen follicle with cilia present on most epidermal and dermal cells including the matrix and dermal papilla (center) as shown by ciliary axoneme markers acetylated α-tubulin (green), IFT88 (red), and Hoescht nuclear staining (blue).
Acknowledgments
We gratefully acknowledge Dr. Trenton Schoeb for guidance in pathology as well as the fellow members of the Yoder lab for critical reading and comments on the manuscript. We acknowledge the UAB Comparative Pathology lab for histology services and the Indiana Center for Biological Microscopy for developing the program Voxx2. We also thank the UAB Laser Microdissection Facility and Natalya Frolova for assistance and acknowledge the UAB Heflin Genomics Core and Dr. Michael Crowley for providing facilities and assistance with qRT-PCR analysis. This work was supported in part by a Pilot and Feasibility award (BKY) from the UAB Skin Diseases Research Center (SDRC, P30 AR050948, to Dr. Craig Elmets), as well an RO1 award from NIAMS (AR052792, BKY), and by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory, managed by UT-Battelle, LLC for the U.S. Department of Energy under contract no. DE-AC05-00OR22725 (EJM).
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr Opin Nephrol Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal Stem Cells of the Skin. Annu Rev Cell Dev Biol. 2005 doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JC, Osornio-Vargas AR, Badgett A, Brody AR. Differential proliferation of rat lung fibroblasts induced by the platelet-derived growth factor-AA, -AB, and -BB isoforms secreted by rat alveolar macrophages. Am J Respir Cell Mol Biol. 1991;5:539–547. doi: 10.1165/ajrcmb/5.6.539. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Paus R. Molecular biology of hair morphogenesis: development and cycling. J Exp Zoolog B Mol Dev Evol. 2003;298:164–180. doi: 10.1002/jez.b.33. [DOI] [PubMed] [Google Scholar]
- Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory Cilia and Integration of Signal Transduction in Human Health and Disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- Clendenon JL, Phillips CL, Sandoval RM, Fang S, Dunn KW. Voxx: a PC-based, near real-time volume rendering system for biological microscopy. Am J Physiol Cell Physiol. 2002;282:C213–218. doi: 10.1152/ajpcell.2002.282.1.C213. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Daroczy J, Feldmann J. [Significance of cilia in cells of the epidermis and the dermis (author’s transl)] Zentralbl Allg Pathol. 1974;118:314–320. [PubMed] [Google Scholar]
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289:F1159–1169. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- Elofsson R, Andersson A, Falck B, Sjoborg S. The ciliated human keratinocyte. J Ultrastruct Res. 1984;87:212–220. doi: 10.1016/s0022-5320(84)80061-1. [DOI] [PubMed] [Google Scholar]
- Elofsson R, Andersson A, Falck B, Sjoborg S. The human epidermal melanocyte-aciliated cell. Acta Dermatovener. 1981;99:49–52. [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007 doi: 10.1038/ng.2007.12. advanced online publication. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Hallberg K, Harfe BD, Reyahi A, Kannius-Janson M, Nilsson J, et al. Abnormal hair development and apparent follicular transformation to mammary gland in the absence of hedgehog signaling. Dev Cell. 2007;12:99–112. doi: 10.1016/j.devcel.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends in Genetics. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 Localize to Cilia and Require the Intraflagellar Transport Protein Polaris for Processing and Function. PLoS Genetics. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, et al. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Houde C, Dickinson RJ, Houtzager VM, Cullum R, Montpetit R, Metzler M, et al. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev Biol. 2006;300:523–533. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126:2611–2621. doi: 10.1242/dev.126.12.2611. [DOI] [PubMed] [Google Scholar]
- Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, et al. Laminin-10 is crucial for hair morphogenesis. Embo J. 2003;22:2400–2410. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, et al. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Developmental Biology. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003:282–294. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill P, Mo R, Hu MC, Dagnino L, Rosenblum ND, Hui CC. Shh controls epithelial proliferation via independent pathways that converge on N-Myc. Dev Cell. 2005;9:293–303. doi: 10.1016/j.devcel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Nanba D, Nakanishi Y, Hieda Y. Role of Sonic hedgehog signaling in epithelial and mesenchymal development of hair follicles in an organ culture of embryonic mouse skin. Dev Growth Differ. 2003;45:231–239. doi: 10.1046/j.1524-4725.2003.691.x. [DOI] [PubMed] [Google Scholar]
- Ohtola J, Myers J, Akhtar-Zaidi B, Zuzindlak D, Sandesara P, Yeh K, et al. {beta}-Catenin has sequential roles in the survival and specification of ventral dermis. Development. 2008;135:2321–2329. doi: 10.1242/dev.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwin DF, Woods JL. Number changes and development potential of wool follicle cells in the early stages of fiber differentiation. J Ultrastruct Res. 1982;80:312–322. doi: 10.1016/s0022-5320(82)80044-0. [DOI] [PubMed] [Google Scholar]
- Paus R, Foitzik K. In search of the “hair cycle clock”: a guided tour. Differentiation. 2004;72:489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, van der Veen C, Maurer M, Eichmuller S, Ling G, et al. A Comprehensive Guide for the Recognition and Classification of Distinct Stages of Hair Follicle Morphogenesis. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mechanisms of Development. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Roberto FMC, Varricchio E, Di Guardo G, Bruno F. Primary cilium expression in cells from normal and pathological caprine skin. J Submicrosc Cytol Pathol. 1999;31:169–171. [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007a;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 Regulates Hedgehog Signaling at the Primary Cilium. Science. 2007b;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, et al. PDGFR[alpha][alpha] Signaling Is Regulated through the Primary Cilium in Fibroblasts. Current Biology. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Sheng H, Goich S, Wang A, Grachtchouk M, Lowe L, Mo R, et al. Dissecting the oncogenic potential of Gli2: deletion of an NH(2)-terminal fragment alters skin tumor phenotype. Cancer Res. 2002;62:5308–5316. [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Strugnell GE, Wang AM, Wheatley DN. Primary cilium expression in cells from normal and aberrant human skin. J Submicrosc Cytol Pathol. 1996;28:215–225. [PubMed] [Google Scholar]
- Taulman PD, Haycraft CJ, Balkovetz DF, Yoder BK. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell. 2001;12:589–599. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Akiyama M, Shimizu H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J Dermatol Sci. 2006;43:105–115. doi: 10.1016/j.jdermsci.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Wandel A, Steigleder GK, Bodeux E. [Primary cilia in cells of the epidermis and dermis] Z Hautkr. 1984;59:382, 389–392. [PubMed] [Google Scholar]
- Wilson RB, Mc WC. Isolated flagella in human skin. Election microscopic observations. Lab Invest. 1963;12:242–249. [PubMed] [Google Scholar]
- Yoder BK, Richards WG, Sommardahl C, Sweeney WE, Michaud EJ, Wilkinson JE, et al. Differential rescue of the renal and hepatic disease in an autosomal recessive polycystic kidney disease mouse mutant. A new model to study the liver lesion. Am J Pathol. 1997;150:2231–2241. [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Davenport JR, Croyle MJ, Haycraft CJ, Yoder BK. Disruption of IFT results in both exocrine and endocrine abnormalities in the pancreas of Tg737(orpk) mutant mice. Lab Invest. 2005;85:45–64. doi: 10.1038/labinvest.3700207. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, et al. Activation of {beta}-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 2008;135:2161–2172. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Mild fibrosis and possible dermal expansion was present in (B,D) Prx1cre;Tg737fl/fl animals compared to (A,C) wildtype littermates at (A,B) P1 and (C,D) P5 as determined by trichrome staining (green).
Supplemental Figure 2: Representative 8um images prior to Laser Microdissection of stage 2 follicles in P1 (A) wildtype and (B) conditional cilia knockout mice showing the reduced number of dermal condensate cells in mutants compared to control samples.
Supplemental Figure 3: Representation of the Laser Capture Microdissection strategy used to analyze expression in the dermal condensates. (A) Stage 2 hair follicles are identified based on previously described criteria (Paus et al., 1999). Dermal condensate capture relies on (B) laser microdissection of dermal condensates (outlined) followed by (C) sample catapulting.
Supplemental Movie 1: Immunofluorescence analysis of a stage 2 hair germ showing that cilia are present on most epidermal and dermal cells including the interfollicular epidermis, epidermal hair germ, dermal fibroblasts, and forming dermal papilla as shown by cilia axoneme markers acetylated α-tubulin (green) IFT88 (red), and Hoescht nuclear staining(blue).
Supplemental Movie 2: Immunofluorescent analysis of a P25 anagen follicle with cilia present on most epidermal and dermal cells including the matrix and dermal papilla (center) as shown by ciliary axoneme markers acetylated α-tubulin (green), IFT88 (red), and Hoescht nuclear staining (blue).