Abstract
Studies in cocaine-dependent human subjects have shown differences in white matter on diffusion tensor imaging (DTI) compared to non-drug using controls. It is not known whether the FA differences seen on DTI in white matter regions of cocaine-dependent humans result from a pre-existing predilection for drug use or purely from cocaine abuse. To study the effect of cocaine on brain white matter, DTI was performed on 24 rats after continuous infusion of cocaine or saline for 4 weeks, followed by brain histology. Voxel-based morphometry analysis showed 18% decrease in fractional anisotropy (FA) in the splenium of corpus callosum (CC) in cocaine-administered animals relative to saline controls (P = 0.0001). On histology, significant increase in neurofilament expression (125%, P=0.0044) and decrease in myelin basic protein (40%, P = 0.031) was observed in the same region in cocaine-administered animals. This study supports the hypothesis that chronic cocaine use alters white matter integrity in human CC. Unlike humans, where the FA in the genu differed between cocaine users and non-users, the splenium was affected in rats. These differences between rodent and human findings could be due to a several factors that include differences in the brain structure and function between species and/or the dose, timing, and duration of cocaine administration.
Keywords: Cocaine, Diffusion Tensor Imaging, Rat, Corpus callosum, Neurofilament, Myelin Basic Protein
1. Introduction
Cocaine dependence has been associated with a number of changes on brain imaging. Structural magnetic resonance imaging (MRI) studies have shown evidence of reduced gray matter volume, particularly in the frontal lobe (Bartzokis et al., 2000; Franklin et al., 2002). White matter volume has also been found to be reduced in cocaine users, with a lack of normal age-related changes in white matter volume (Bartzokis et al., 2002). Recently diffusion tensor imaging (DTI) studies have demonstrated that cocaine-dependent subjects show differences in white matter (WM) in brain compared to healthy controls (Lim et al., 2002, 2008; Moeller et al., 2005, 2007). Studies by Moeller et al (Moeller et al., 2005) have demonstrated significantly reduced fractional anisotropy (FA) in the genu and rostral body of the anterior corpus callosum (CC) in cocaine-dependent subjects relative to controls. Fractional anisotropy (FA) is affected by a number of pathologic processes, including axonal integrity and state of myelin (Beaulieu, 2002; Gulani et al., 2001). In order to improve the pathologic specificity of the observed reduction in FA in the genu and rostral body of CC, Moeller et al (Moeller et al., 2007) have measured both the longitudinal (λl) and radial (λr) diffusivities. They showed that the decreased FA is associated with increased radial diffusivity (λr), with minimal change in the longitudinal diffusivity (λl) (Moeller et al., 2005). Based on the published correlation between the individual diffusivities and the histology (Kinoshita et al., 1999; Ono et al., 1995; Schwartz et al., 2005; Song et al., 2002, 2004, 2005), the observed diffusivity changes in the genu of CC in cocaine dependent subjects have been interpreted as indicative of altered myelin with minimal changes in the axonal morphometry.
Understanding the pathological differences between users and non-users of cocaine is not simple because of difficulties in creating a completely controlled environment. In addition, histological confirmation of the proposed pathologic mechanism is difficult to realize in humans. Thus, there is a need for animal studies under controlled conditions to understand the underlying pathology related to the differences seen on brain imaging between cocaine-dependent individuals and controls. Therefore, the main purpose of these studies was to 1) determine if the DTI observations in humans with cocaine dependence can be replicated by administering cocaine to rodents, and 2) correlate DTI findings with histology.
2. Materials and Methods
2.1. Animals
All the animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. These studies were approved by the Institutional Animal Welfare Committee.
A total of 24 male Sprague-Dawley rats in the weight range of 250-300 g were included in these studies. The animals were divided into two groups of 12 each: one group received cocaine and the other group received saline through osmotic pumps for 28 days. The saline group served as the control. Cocaine or saline was continuously infused through Alzet osmotic minipump (model 2ML4, Durect Corp, CA) that was surgically implanted (described below).
2.1.1. Infusion Pump Preparation
The Alzet osmotic minipumps were filled with 2 ml of 0.9% saline or 200 mg/ml of cocaine. The pump is 5.1 cm long and has a diameter of 1.4 cm. A 6 mm long microdialysis fiber (Spectrum Laboratories, Inc., Ranch Dominguez, CA) was attached to the output portal of the pump to prevent the development of necrotic skin lesions (Joyner et al., 1993). The pumps were primed prior to implantation by warming in a tube of saline in a 37 °C water bath overnight.
2.1.2. Pump Implantation
All the surgical procedures for implanting the osmotic pumps were performed under aseptic conditions. Animals were anesthetized with isoflurane, administered through a Harvard rodent ventilator (Inspira asv, Harvard Apparatus, Holliston, MA). The animal's back was shaved and betadine was applied as a disinfectant prior to making an incision. A 2 cm incision was made along the dorsal midline and a subcutaneous pocket was formed by blunt dissection. Prior to incision, bupivicaine (<1 ml/kg of 0.25% bupivicaine) was administered intradermally in the area of the incision as a local anesthetic agent. The pump was inserted with the outlet pointed towards the head. The incision was closed with a Vicryl 4-0 suture. The animal was kept warm during recovery. Based on an infusion rate of 2.5 to 2.6 μl/hr, the rats received approximately 40mg/kg/day cocaine for 28 days (see results). This corresponds to 2.8 g/day in a 70 kg person, which a previous publication equated to a “light user” (Myers et al., 1995).
2.1.3. Animal Preparation for MRI
Prior to MRI, under isoflurane anesthesia, the jugular vein was catheterized with PE-10 tubing (Intramedic®, Clay Adams, MD, USA). For MRI, animals were intubated and the anesthesia level was maintained by ventilating with a mixture of 2% isoflurane, 30% oxygen and air through an endotracheal tube. Animals were placed on a Plexiglas bed secured with tooth and ear bars. The respiration and rectal temperature were monitored throughout the experiment with a MR compatible physiologic monitoring unit (Model 1025, SA Instruments, Inc., Stonybrook, NY). The oxygen level and heart rate were monitored with a MRI compatible pulse oximeter (NONIN, Plymouth, MN). Throughout the MRI scan, the body temperature was kept at 37 ± 1°C with a feedback controlled warm air system (SA Instruments, Stony Brook, NY).
2.2. MRI Protocol
All MRI studies were performed on a USR 70/30 horizontal bore 7 T MR Scanner (Bruker Biospin, Karlsruhe, Germany). A 71 mm diameter birdcage resonator was used for radio frequency (RF) transmission, and an actively decoupled quadrature surface coil (2.5 cm id) was used for signal reception. High-resolution anatomical RARE images were acquired using the following parameters: RARE factor of 4, TR/TE of 5000 ms/68.4 ms, image matrix of 256 × 256, field of view 3.84 cm, and 20 contiguous, 1mm thick slices. Diffusion weighted images were acquired with identical geometry as the anatomical images using a multi-slice, multi-shot spin echo EPI sequence with the following parameters: repetition time (TR) / echo time (TE) = 3000 ms/38.3 ms, bandwidth = 200 kHz, number of shots = 4, number of gradient encoding directions = 42 with alternating polarity (Madi et al., 2005), diffusion gradient strength = 361 mT/m, δ (gradient duration) = 2.5 ms, Δ (gradient separation) =18 ms, and b= 800 s mm-2. The other parameters were: acquisition matrix = 128 × 128, square field-of-view = 3.84 cm × 3.84 cm, slice thickness =1 mm, and number of slices = 20 (contiguous). Following the acquisition of diffusion weighted images, T1-wighted spin echo images (TR/TE = 500 ms/10 ms) were acquired before and following the administration of GdDTPA (Magnevist; Berlex, Montville, NJ) at a dose of 0.2 ml/kg through a catheter that was surgically inserted into the jugular vein for determining the integrity of the blood-brain barrier. All MRI studies were performed on day 28 following the pump implantation.
2.3. Plasma Cocaine Levels
Blood was drawn twice (baseline and day 28) for measuring the plasma concentrations of cocaine. For the baseline, blood was collected via 24-gauge iv catheter inserted into the tail vein at the time of implantation surgery. On day 28, blood was drawn through the catheter attached to the jugular vein. Approximately 0.5 ml of blood was collected in BD Microtainer ® tubes containing glycolytic inhibitor and immediately centrifuged for 3 minutes at 3000 rpm. Plasma was transferred to separate tubes and frozen at -80 °C.
Liquid chromatography-tandem mass spectrometry (LC-MSMS) was performed at the Center for Human Toxicology at the University of Utah, which is a Good Laboratory Practices (GLP) compliant laboratory. Either 0.1ml or 0.05 ml aliquots were used to determine the plasma concentration of cocaine. Blood samples were analyzed using a modification of the method for cocaine as previously described (Lin et al., 2001). Deuterated cocaine was added to plasma as the internal standards. The pH of the plasma was made acidic (≈ 4.0) by the addition of acetate buffer and the mixture was extracted using mixed-mode (octyl and benzoyl sulphonate) solid phase extraction (SPE). The eluant was evaporated and reconstituted with methanol/0.1% formic acid in water mixture (10:90) and analyzed by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry (LC- APCI-MS/MS). The mass spectrometer was operated in the selected reaction-monitoring (SRM) mode. Quadrupole Q1 was set to pass only the MH+ ions which are caused to undergo collision induced dissociation in quadrupole Q2 to abundant product ions as follow: cocaine and cocaine-d3 at m/z 304 to 182, and 307 to 185, respectively. The product ions were then monitored selectively by quadrupole Q3. The concentration of the analytes was determined from the peak area ratios of the analyte and the analyte's internal standard. The peak area ratio was then converted to concentration using the equation generated from a plot of concentration versus peak area ratio for rat plasma fortified with known concentrations of analyte and internal standard.
2.4. Histology
Following the terminal MRI scans, animals were transcardially perfused with saline followed by 4% formaldehyde. The brain was then removed, post-fixed overnight in 4% formaldehyde, and then immersed in 30% sucrose-phosphate buffered saline (0.1M PBS) for 2-3 days at 4 °C. The brain was sectioned coronally at 40 μm thickness with a cryostat (Leica CM1800, Bannockburn, IL) and stored at -20 °C in tissue storing media.
Brain sections (n=6/animal) were processed as free floating and were incubated in the following primary antibodies: myelin basic protein (1:1000, MBP; Millipore, Billerica, MA) and neurofilament-heavy protein (1:1000, NF-H; Millipore, Billerica, MA). Primary antibodies were diluted with blocking solution (0.1M PBS containing 5% goat serum and 0.3% Triton X-100).
Appropriate secondary antibodies were used at a dilution of 1:500 in 0.1M PBS. The following Alexafluor dye conjugated secondary antibodies were used: goat anti-mouse IgG Alexa Fluor® 488 (Invitrogen, Carlsbad, CA) and goat anti-rabbit IgG (H+L) Alex Fluor® 568 (Invitrogen, Carlsbad, CA). Tissue sections were viewed and captured using a Spot Flex digital camera (Diagnostic Instruments, Inc. Sterling Heights, MI) attached to a Leica RX1500 upright microscope and the images were collected using Spot software (Diagnostic Imaging, Sterling Heights, MI). Operator acquiring the images was blinded to the groups.
2.5. DTI Analysis
The DTI analysis was performed as described by Ramu et al (Ramu et al., 2006). Briefly, all the diffusion weighted images were registered to the rat brain in stereotaxic coordinates (Paxinos and Watson, 2005) using the Automated Image Registration (Woods et al., 1998). These images were then imported into DTI Studio software (Jiang et al., 2006) to calculate the DTI parameters such as FA, mean diffusivity (MD), longitudinal diffusivity (λl) and radial diffusivity (λr) maps. A two-tailed statistical t-test (p < 0.05) was performed using SPM99 for unbiased determination of the significant differences in the FA values between the cocaine and saline administered animals. Maps of these significant differences were generated (t-maps). ROI analysis of FA maps was also performed using DTI studio (Jiang et al., 2006) to confirm that the t-maps correctly identified the regions with significant FA differences.
The t-maps indicated significant differences in the splenium region (see the results) and was later confirmed by the ROI analysis. The same set of ROI's was used to determine the FA values for both the saline and the cocaine-treated groups.
2.6. Histology Analysis
Brain sections were processed at the same time for myelin basic protein and neurofilament heavy protein using the same solutions and antibody dilutions mention above. Images were captured and exposure times were determined for each antibody in pilot experiments. Immunofluorescence analysis was performed using ImagePro Plus software (Media Cybernetics, Inc.; Silver Spring, MD) as described previously (Thornton et al., 2005). Briefly, all images were captured under 20× magnification with no interpolation. Threshold levels were determined to match those of positive staining and to exclude background fluorescence. Image-Pro Plus® Software was then used to measure the percent areas or fluorescent intensity of myelin and neurofilament expression were measured in the region of interest (ROI) containing the splenium. To ensure consistent analysis between sections, the ROI size (sphere with a 700 μm diameter) was maintained throughout the experiment with each section and the intensity levels were determined using consistent threshold levels.
2.8. Statistical Analysis
The results include analysis on only 11 animals in the cocaine group because of severe image artifacts seen in one animal. A two-tailed student's t-test was used to determine differences in the values of FA, MD, λl, λr, percent of expression of myelin and neurofilament in the ROI's. Statistical analysis was performed using GraphPad Prism4 software (GraphPad Software, San Diego, CA). Significance levels were set at 0.05 for all comparisons.
3. Results
The mean SNR values in the b = 0 image (without diffusion weighting) was 104 ± 6.9 (range 97 to 115) in saline controls and 104 ± 5.6 (range 94 to 109) in cocaine animals. The SNR values are not different between the two cohorts. This relatively high SNR assures that the FA values are not artificially increased due to noisy data (Bastin et al., 1998; Hasan et al., 2004).
Visually, we did not detect the presence of lesions either on the high resolution MRI or on any of the diffusion metrics. Also no enhancement was observed on the T1-weighted post-contrast images, suggesting lack of gross abnormalities in the blood-brain barrier.
The average FA maps of a cocaine treated animals in the coronal plane, with and without color coding (modulated by the principal eigenvalue), are shown in Fig. 1. The green, red, and blue colors in this figure represent the principal eigenvalue orientations along left - right, ventral – dorsal, and rostral – caudal, respectively, and the intensity indicates the FA value. These maps were generated by averaging all the FA maps of the cocaine treated animals.
Figure 1.
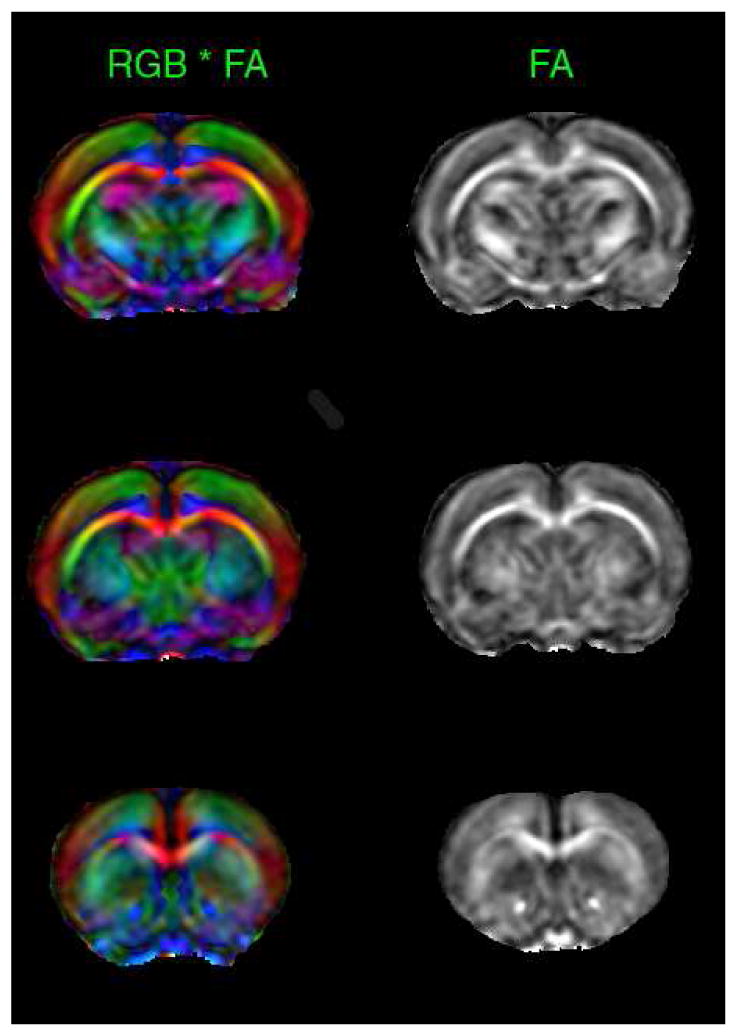
FA maps in the coronal plane with (left) and without color coding at three different levels in cocaine treated animals. These maps were averaged over all the cocaine treated animals. This figure demonstrates both the quality of DTI data and the quality of image registration necessary for group analysis. Red, green and blue represent left-right, anterior-posterior, and rostral-cuadal directions, respectively.
The t-maps (shown in green) representing the significant group differences in the FA values between the saline and cocaine treated groups, superimposed on the FA maps, are shown Fig. 2. As can be observed from this figure, significant differences were observed only in the splenium of CC.
Figure 2.
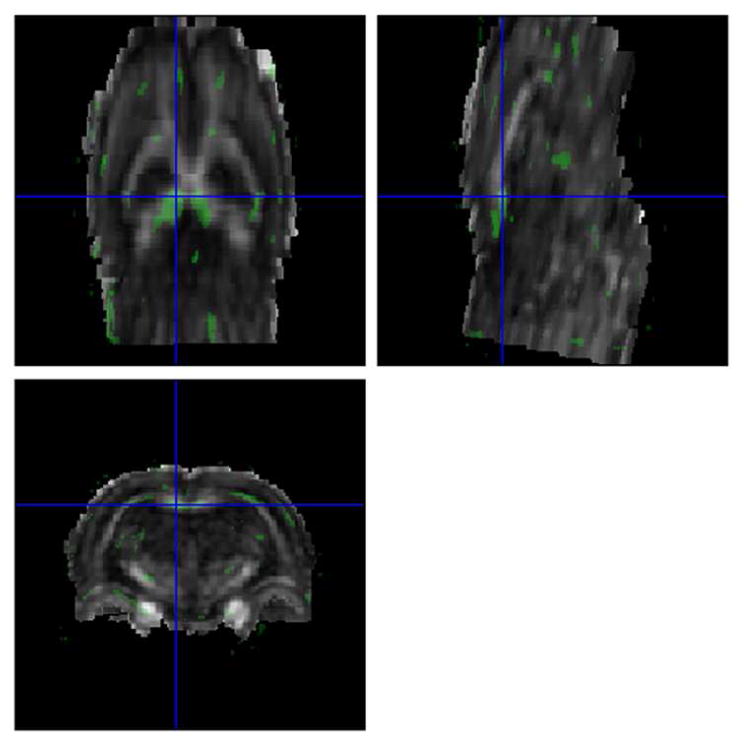
Fractional anisotropy (FA) differences (t-maps; shown in green) between the brains of saline and cocaine treated rats in axial, sagittal, and coronal planes. The cross hairs (in blue) correspond to the splenium where the differences were observed
Even though cocaine was administered to all the animals under identical conditions, the plasma concentration varied considerably from animal-to-animal. The variations of diffusion metrics with plasma concentration of cocaine, along with a linear regression analysis, are shown in Fig. 3. As can be seen from this figure, FA, λl, and, MD showed a negative correlation with the cocaine concentration. However, there was no statistically significant correlation between λr and plasma cocaine concentration. But at all the blood plasma cocaine concentrations, the value of λr was observed to be larger in saline than the cocaine administered animals. Over all, this data suggests an association between the changes in the DTI metrics and cocaine levels. Figure 4 shows the quantitative ROI analysis of the DTI measures in the splenium for the saline and cocaine groups. As seen from this figure the values of FA and λl are significantly smaller while λr is significantly higher in the cocaine treated group relative to the saline treated group. MD showed a decrease in the cocaine treated animals relative to saline controls; this difference, however, did not reach statistical significance.
Figure 3.
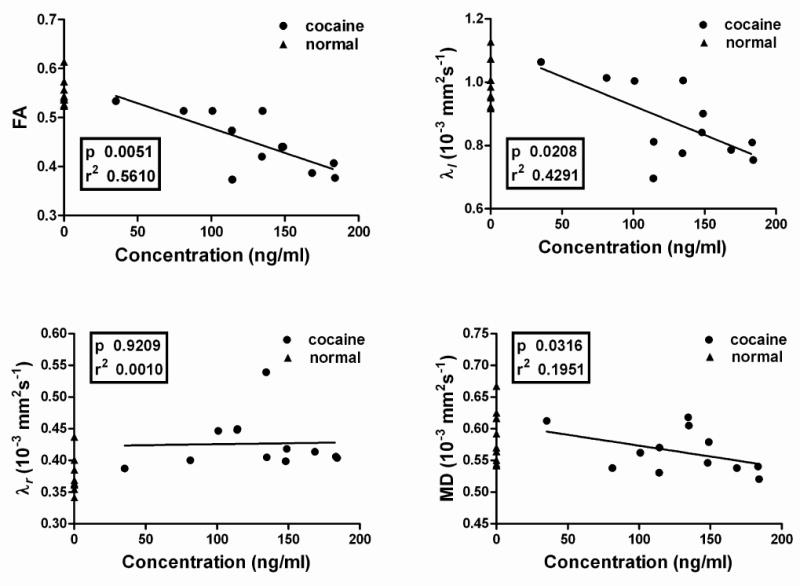
Variations of diffusion parameters FA, λl, λt and MD with plasma cocaine concentration. The triangles on the y-axis (corresponding to zero concentration) represent the values in the saline-treated animals. The p and r2 values are also shown for the linear fit. p and r2 are based on Student's two tailed t-test.
Figure 4.
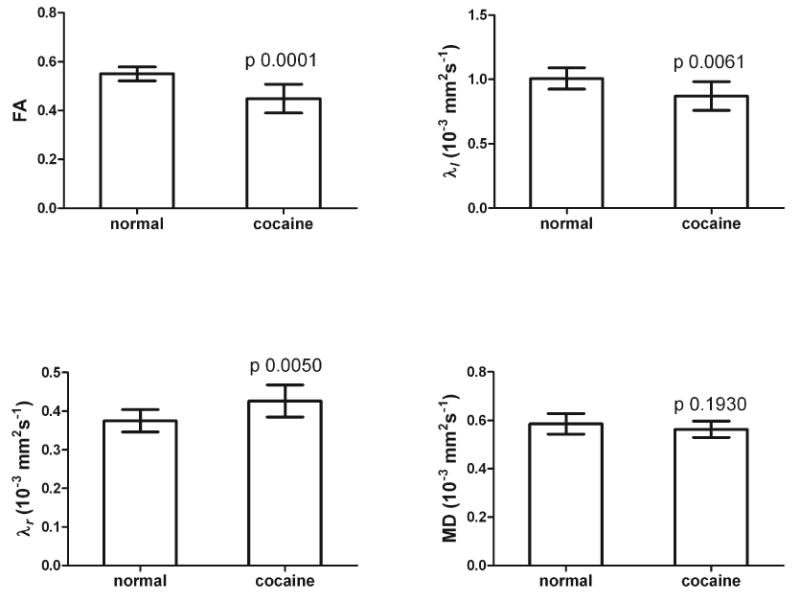
Quantitative ROI analysis in the splenium of the CC of all the diffusion parameters in saline- and cocaine-treated groups. The p-values are shown. P values are based on Student's two-tailed t-test.
As an example, the immunofuorescent images of saline and cocaine treated brains labeled for neurofilament in the splenium and hippocampal regions are shown in Fig. 5. This representative figure shows higher neurofilament expression in the splenium of a cocaine treated subject compared to saline control. Quantitative results of the percentage expressions of MBP and NF-H, based on immunohistochemistry in splenium are shown in Fig. 6. There was significant difference in the both the MBP and neurofilament levels in cocaine-treated animals relative to saline controls.
Figure 5.
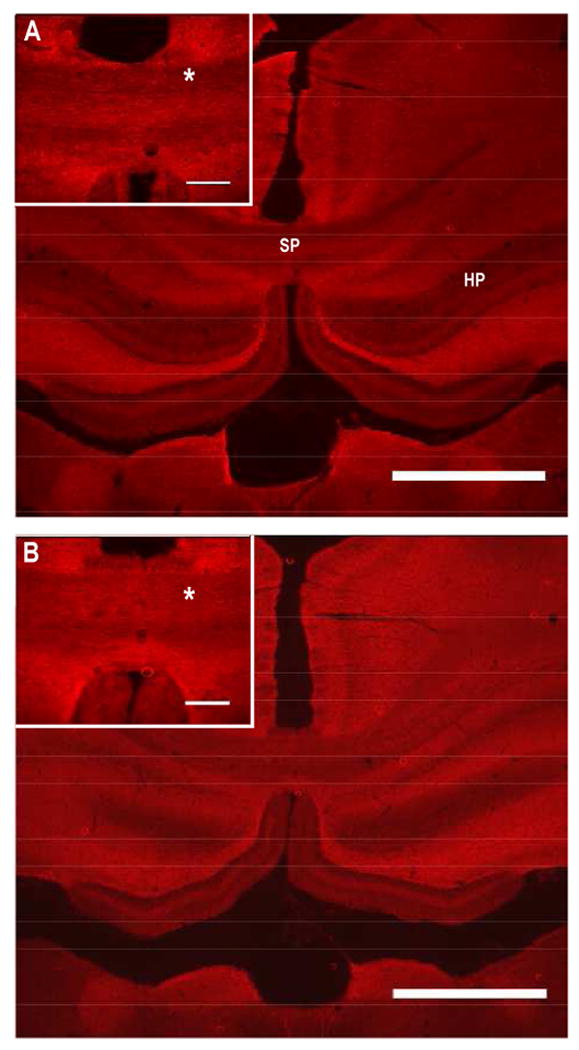
Immunofuorescent of images of saline (A) and cocaine (B) treated brains labeled for neurofilament. Inlet images zoom on the splenium. SP- splenium, HP-Hippocampal region, and “*” indicates area of the splenium where differences in fluorescent intensities were observed. Scale bars: 1 mm and inlet image 300μm.
Figure 6.
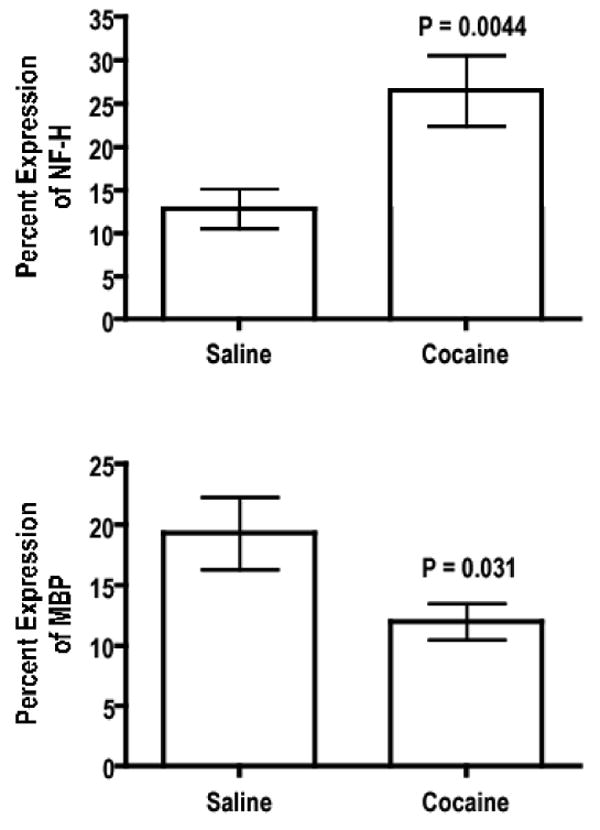
Quantitative analysis of MBP and NF-H saline and cocaine-treated groups. P values are based on Student's two-tailed t-test.
4. Discussion
The main findings of this study are that: 1) based on DTI and confirmed by immunohistochemistry, cocaine affects white matter in rats with significant differences seen in the splenium of CC of cocaine-treated rats compared to saline-treated rats; 2) both radial and longitudinal diffusivities are affected in the cocaine-treated animals; and 3) immunohistochemistry shows a significant decrease in myelin and increased neurofilament expression in cocaine-treated animals relative to controls. The approximately linear relationship between plasma cocaine levels and the diffusion anisotropy indices (FA, λl, and λr) metrics strongly suggests an association between the observed changes in the anisotropy indices and cocaine administration.
The overall findings of the current study of reduced FA in the CC in cocaine-administered animals are consistent with previous work in cocaine-dependent subjects (Moeller et al., 2005). In addition, the finding of an increased radial diffusivity is consistent with other human work (Moeller et al., 2007). However, unlike in human studies where the DTI changes were observed in the genu and rostral body, we observed changes in the splenium of the CC in rats. Specifically, we observed decreased FA in the cocaine-administered rats to be associated with increased radial diffusivity and decreased longitudinal diffusivity. Based on immunohistochemistry, the increased radial diffusivity in rats appears to be associated with decreased MBP expression. The correlation between radial diffusivity and MBP is consistent with recent suggestions that radial diffusivity mainly reflects the state of myelination. (Schwartz et al., 2005; Song et al., 2004, 2005).
An interesting observation made in the current studies is the increased neurofilament staining in cocaine-treated animals relative to controls (Fig. 5). This increased neurofilament expression was observed to be associated with reduced longitudinal diffusivity. This is somewhat surprising since increased neurofilament implies increased axonal density that should result in increased longitudinal diffusivity. However, lack of correlation between the longitudinal diffusivity and state of neurofilament expression is consistent with more recent reports (Budde et al., 2007; DeBoy et al., 2007; Herrera et al., 2007). These studies strongly suggest that the original concept of longitudinal diffusivity being solely related to axonal injury might be overly simplistic.
In the rat, the splenium is the posterior 20% of the total callosal length and carries axons mainly from the visual cortex (Kim et al., 1996). Recent electron microscopy studies have demonstrated that the splenium in rats contains the largest number of myelinated axons (Sargon et al., 2003). In humans, again based on electron microscopy, the genu of the CC contains the largest number of myelinated axons (Sargon et al., 2007b). It has been reported, based on microarray analysis studies, that cocaine abusers show a striking decrease in myelin related genes (Albertson et al., 2004; Sokolov, 2007). In rats the splenium alone shows pathological changes with age in myelin-containing axons (Sargon et al., 2007a), whereas humans show age related changes in the anterior CC on DTI (Sullivan et al., 2006). These reports suggest that callosal fibers with high degree of myelin may be more susceptible to damage by cocaine. This perhaps explains why cocaine has an effect on splenium in rats rather than genu in humans. The observed association between the MBP expression and cocaine administration is consistent with the hypothesis that highly myelinated fibers are more vulnerable to cocaine abuse.
Our model uses young adult rats with a continuous 4-week infusion of purified cocaine at doses that correspond to light cocaine use in humans. In contrast, human users binge smoke impure crack cocaine repeatedly for several hours 10-15 times per month (Myers et al., 1995). Without the binge use of smoked cocaine, the pharmacokinetics of cocaine could be different. Another confound that could explain the differences between the results observed in our animal studies and cocaine-dependent humans is a pre-existing predilection for drug use in humans. Another potential reason for the difference between rat and human findings includes the fact that in the current studies, rats received continuous infusion of cocaine that may represent some aspects of high-dose cocaine abuse, but unlike the pattern of cocaine self-administration that humans use (King et al., 1992). Studies on animal models with self administration might be closer to human cocaine abuse (see for example (Valles et al., 2006).
One potential limitation of the current study is that the animals were only scanned at one time point (i.e., after treatment with cocaine or saline). Thus, it is unknown whether the differences in DTI and histological findings between treatment groups could have been at least partially related to pretreatment differences. However, this possibility is unlikely because of the genomic and environmental homogeneity of the laboratory animals that were used in this study. It would be desirable for future studies to have repeated measures design by acquiring MRI scans pretreatment as well as at other time points during treatment.
Osmotic minipumps are commonly used for delivering substances over a long period of time (Harbaugh et al., 1988). In our studies, plasma cocaine concentrations were measured to determine whether there was any variability due to partial occlusion of the pumps, which can occur over a period of time (Jones et al., 2001). There was some variation in the plasma cocaine levels in rats that allowed us to establish a correlation between cocaine levels and altered DTI parameters, which lend support for an association between cocaine administration and DTI findings (Fig. 3). It is also worth pointing out that we have measured only the plasma levels of cocaine. These levels might not reflect the actual tissue cocaine concentrations.
The present study is one of the few imaging studies on the effects of cocaine on animals that measured plasma cocaine concentrations. The observed correlation between cocaine levels and altered DTI parameters underscores the need to measure cocaine concentrations for a proper interpretation of the results.
In conclusion, our studies suggest that cocaine affects the corpus callosum. However, we have also observed significant differences in terms of which corpus-callosum subregion was affected between cocaine dependent humans and cocaine-administered animals. This study lends some support to the findings of altered white matter on DTI in humans with cocaine dependence being related to cocaine use, but differences between rat and human findings warrant further investigation.
Acknowledgments
We thank Dr. Shi-Jie Liu for his help with animal surgery and Tessy Chacko, and Alex Li for their help with histology. The 7 T Bruker scanner was purchased with funding from NRCC/NIH under High End Instrumentation grant (#S10 RR17205) to PAN. Dr. Moeller is supported by K02DA00403.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–9. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry. 2002;51:605–11. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000;98:93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Bastin ME, Armitage PA, Marshall I. A theoretical study of the effect of experimental noise on the measurement of anisotropy in diffusion imaging. Magn Reson Imaging. 1998;16:773–85. doi: 10.1016/s0730-725x(98)00098-8. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57:688–95. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- DeBoy CA, Zhang J, Dike S, Shats I, Jones M, Reich DS, Mori S, Nguyen T, Rothstein B, Miller RH, Griffin JT, Kerr DA, Calabresi PA. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007;130:2199–210. doi: 10.1093/brain/awm122. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–42. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45:191–5. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Harbaugh RE, Saunders RL, Reeder RF. Use of implantable pumps for central nervous system drug infusions to treat neurological disease. Neurosurgery. 1988;23:693–8. doi: 10.1227/00006123-198812000-00001. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Alexander AL, Narayana PA. Does fractional anisotropy have better noise immunity characteristics than relative anisotropy in diffusion tensor MRI? An analytical approach. Magn Reson Med. 2004;51:413–7. doi: 10.1002/mrm.10682. [DOI] [PubMed] [Google Scholar]
- Herrera JJ, Chacko T, Narayana PA. Histological correlation of diffusion tensor imaging metrics in experimental spinal cord injury. J Neurosci Res. 2007 doi: 10.1002/jnr.21481. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–16. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH. Chronic intrathecal infusions after spinal cord injury cause scarring and compression. Microsc Res Tech. 2001;54:317–24. doi: 10.1002/jemt.1144. [DOI] [PubMed] [Google Scholar]
- Joyner C, King G, Lee TH, Ellinwood EH., Jr Technique for the continuous infusion of high doses of cocaine by osmotic minipump. Pharmacol Biochem Behav. 1993;44:971–3. doi: 10.1016/0091-3057(93)90033-p. [DOI] [PubMed] [Google Scholar]
- Kim JH, Ellman A, Juraska JM. A re-examination of sex differences in axon density and number in the splenium of the rat corpus callosum. Brain Res. 1996;740:47–56. doi: 10.1016/s0006-8993(96)00637-3. [DOI] [PubMed] [Google Scholar]
- King GR, Joyner C, Lee T, Kuhn C, Ellinwood EH., Jr Intermittent and continuous cocaine administration: residual behavioral states during withdrawal. Pharmacol Biochem Behav. 1992;43:243–8. doi: 10.1016/0091-3057(92)90664-2. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Ohnishi A, Kohshi K, Yokota A. Apparent diffusion coefficient on rat brain and nerves intoxicated with methylmercury. Environ Res. 1999;80:348–54. doi: 10.1006/enrs.1998.3935. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–5. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–72. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SN, Moody DE, Bigelow GE, Foltz RL. A validated liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry method for quantitation of cocaine and benzoylecgonine in human plasma. J Anal Toxicol. 2001;25:497–503. doi: 10.1093/jat/25.7.497. [DOI] [PubMed] [Google Scholar]
- Madi S, Hasan KM, Narayana PA. Diffusion tensor imaging of in vivo and excised rat spinal cord at 7 T with an icosahedral encoding scheme. Magn Reson Med. 2005;53:118–25. doi: 10.1002/mrm.20304. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–7. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, Swann AC, Narayana PA. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154:253–8. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Myers MG, Rohsenow DJ, Monti PM, Dey A. Patterns of cocaine use among individuals in substance abuse treatment. Am J Drug Alcohol Abuse. 1995;21:223–31. doi: 10.3109/00952999509002690. [DOI] [PubMed] [Google Scholar]
- Ono J, Harada K, Takahashi M, Maeda M, Ikenaka K, Sakurai K, Sakai N, Kagawa T, Fritz-Zieroth B, Nagai T, et al. Differentiation between dysmyelination and demyelination using magnetic resonance diffusional anisotropy. Brain Res. 1995;671:141–8. doi: 10.1016/0006-8993(94)01335-f. [DOI] [PubMed] [Google Scholar]
- Sargon MF, Celik HH, Aksit MD, Karaagaoglu E. Quantitative analysis of myelinated axons of corpus callosum in the human brain. Int J Neurosci. 2007a;117:749–55. doi: 10.1080/00207450600910119. [DOI] [PubMed] [Google Scholar]
- Sargon MF, Denk CC, Celik HH, Surucu HS, Aldur MM. Electron microscopic examination of the myelinated axons of corpus callosum in perfused young and old rats. Int J Neurosci. 2007b;117:999–1010. doi: 10.1080/00207450600934382. [DOI] [PubMed] [Google Scholar]
- Sargon MF, Mas N, Senan S, Ozdemir B, Celik HH, Cumhur M. Quantitative analysis of myelinated axons of commissural fibers in the rat brain. Anat Histol Embryol. 2003;32:141–4. doi: 10.1046/j.1439-0264.2003.00446.x. [DOI] [PubMed] [Google Scholar]
- Schwartz ED, Chin CL, Shumsky JS, Jawad AF, Brown BK, Wehrli S, Tessler A, Murray M, Hackney DB. Apparent diffusion coefficients in spinal cord transplants and surrounding white matter correlate with degree of axonal dieback after injury in rats. AJNR Am J Neuroradiol. 2005;26:7–18. [PMC free article] [PubMed] [Google Scholar]
- Sokolov BP. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int J Neuropsychopharmacol. 2007;10:547–55. doi: 10.1017/S1461145706007322. [DOI] [PubMed] [Google Scholar]
- Song SK, Kim JH, Lin SJ, Brendza RP, Holtzman DM. Diffusion tensor imaging detects age-dependent white matter changes in a transgenic mouse model with amyloid deposition. Neurobiol Dis. 2004;15:640–7. doi: 10.1016/j.nbd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–61. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Thornton MR, Mantovani C, Birchall MA, Terenghi G. Quantification of N-CAM and N-cadherin expression in axotomized and crushed rat sciatic nerve. J Anat. 2005;206:69–78. doi: 10.1111/j.0021-8782.2005.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles R, Rocha A, Nation JR. The effects of acquisition training schedule on extinction and reinstatement of cocaine self-administration in male rats. Exp Clin Psychopharmacol. 2006;14:245–53. doi: 10.1037/1064-1297.14.2.245. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–52. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]


