Abstract
Objective
To determine the effect of meal composition on postprandial testosterone levels in women with polycystic ovary syndrome (PCOS).
Design
Randomized, crossover design.
Setting
Academic research center.
Patients
Fifteen women with PCOS.
Intervention
We evaluated changes in testosterone, sex hormone binding globulin (SHBG), DHEA-S, cortisol, glucose, and insulin for six hours after a high-fat, Western meal (HIFAT) (62% fat, 24% carbohydrate, 1g fiber) and an isocaloric low-fat, high-fiber meal (HIFIB) (6% fat, 81% carbohydrate, 27g fiber).
Main outcome measure
Change in testosterone.
Results
Testosterone decreased 27% within two hours after both meals (P<0.001). However, testosterone remained below premeal values for four hours after the HIFIB meal (P<0.004) and six hours after the HIFAT meal (P<0.004). Insulin was two fold higher for two hours after the HIFIB meal compared with the HIFAT meal (P<0.03). Glucose was higher for one hour after the HIFIB meal compared with the HIFAT meal (P<0.003). DHEA-S decreased 8−10% within 2−3 hours after both meals, then increased over the remainder of the study period (P<0.001). Cortisol decreased over the 6-hour period after both meals (P<0.001).
Conclusions
Diet plays a role in the regulation of testosterone levels in women with PCOS. Further studies are needed to determine the role of diet composition in the treatment of PCOS. (ClinicalTrials.gov Identifier: NCT0455338).
Keywords: insulin resistance, hyperandrogenism, postprandial, testosterone, fiber
INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common causes of infertility and anovulation in women. Hyperandrogenemia is a consistent biochemical feature of PCOS, most commonly documented by elevated total or bioavailable testosterone levels (1). The hyperandrogenemia associated with PCOS can lead to peripheral hyperandrogenism, which can present as hirsutism, acne, and alopecia, and is implicated in the pathological development of PCOS (2, 3). A reduction in circulating testosterone levels is associated with increased menstrual cyclicity and fertility in women with PCOS (1, 4-6). Consequently, reducing circulating testosterone levels is a treatment goal for women with PCOS and is often a surrogate outcome in clinical trials (7).
In women with PCOS, diet is an effective means of reducing testosterone levels and improving reproductive outcomes when accompanied by weight loss of at least five percent of body weight (6, 8-10). Consequently, there is current interest in whether a particular diet composition is more effective in treating PCOS (9-12). Knowing the acute effects of meals with varying nutrient contents on testosterone levels may lend insight into which diet composition is most beneficial for women with PCOS in the long-term. Studies in men and also women with PCOS have demonstrated that testosterone levels are reduced as soon as two hours after a meal (13-16). In addition, a recent study by Kasim-Karakas et al. reported a greater reduction in DHEA-S and cortisol at four and five hours after a 75 g protein drink compared with a 75 g glucose drink (11). However, the short-term effect of varying meal composition on testosterone levels has not been studied in women. Although hyperinsulinemia is implicated in the pathogenesis of PCOS, previous clinical studies have not shown a relationship between the change in testosterone and insulin levels after a glucose load (17, 18) or following a meal (13, 16).
The aim of the present study was to determine if a high-fat, Western meal or a low-fat, high-fiber meal produces a greater postprandial reduction in testosterone levels in women with PCOS. These meals were chosen because they represent two typical American breakfasts with very different macronutrient profiles. Since people are generally in a postprandial state for the majority of the day, there may be long-term improvements if a diet that lowers postprandial testosterone levels is maintained over a longer period of time. We also measured the change in DHEA-S and cortisol to determine if there is a similar response of hormones primarily of adrenal origin, compared to that of testosterone, which may involve an ovarian, adrenal, and peripheral component. Finally, we measured the change in glucose and insulin to determine if there is a relationship between the change in these circulating measures with the change in testosterone levels and to establish the overall effects of the two diets.
MATERIALS AND METHODS
Subjects
We studied 15 women with PCOS between the ages of 19−40. Women were considered to have PCOS if they [1] had a history of chronic anovulation, determined by intermenstrual periods of ≥ 45 or ≤ 8 menstrual cycles/y, and [2] had hyperandrogenism, determined by total T > 50 ng/dL or a free androgen index (FAI) > 1.5 (5, 19-21). Hyperprolactinemia was excluded by a prolactin level ≤ 25 ng/mL, nonclassical adrenal 21-hydroxylase deficiency was excluded by a 17-hydroxyprogesterone level < 2 ng/mL, and thyroid disorders were excluded by a TSH level ≥ 0.2 mIU/mL or ≤ 5.5 mIU/mL. All subjects were in good health, were non-smokers, and were not pregnant or lactating. For at least 3 months before the study, all subjects were not taking any medication known to affect sex hormones or carbohydrate or lipid metabolism. Subjects were excluded if they were anemic (Hct < 36%), had type 1 or type 2 diabetes (fasting glucose > 126 mg/dL or receiving anti-diabetic agents), or drank more than two alcoholic drinks/d. The study was carried out in the General Clinical Research Centers at the Pennsylvania State University campuses located at the Milton S. Hershey Medical Center in Hershey, PA and at the University Park campus in University Park, PA.
The study opened to accrual in March of 2005 and closed to enrollment in October of 2006. The Institutional Review Board at the Pennsylvania State University College of Medicine approved the study, and each subject provided written informed consent. Fifteen women were enrolled in the study and all women completed both study visits. Fifty two women were screened in order to enroll these 15 subjects. The most common reasons for consented subjects not enrolling in the study were that they did not meet the inclusion criteria for testosterone or FAI (n = 14) or they decided not to participate (n = 12).
Study design
A random number table was used to randomize subjects to one of two meal sequences using a 2×2 crossover design: [1] the low-fat, high-fiber meal at visit 1 and the high-fat, Western meal at visit 2, or [2] the high-fat, Western meal at visit 1 and the low-fat, high-fiber meal at visit 2. At least seven days separated each visit to diminish the likelihood of carryover effects. The energy and nutrient composition of the test meals is listed in Table 1 and were calculated using Nutrition Data System (NDS) for Research Software (Version 2005, Nutrition Coordinating Center, University of Minnesota, Minneapolis). The high-fat, Western meal contained a Jimmy Dean sausage, egg, and cheese croissant with whole milk. The low-fat, high-fiber meal was isocaloric and contained Fiber One cereal with 1% milk, Dannon fat-free fruit-flavored yogurt + fiber, and a fruit salad containing apple and banana.
Table 1.
Energy and nutrient composition of the two test meals.
| High-Fat, Western Meal | Low-Fat, High-Fiber Meal | |
|---|---|---|
| Calories | 600 | 600 |
| Carbohydrate, g | 38 (25%) | 133 (81%) |
| Fructose, g | 0.2 | 19.0 |
| Glucose, g | 0.7 | 15.4 |
| Sucrose, g | 0.1 | 8.1 |
| Lactose, g | 14.6 | 18.3 |
| Starch, g | 21.0 | 33.7 |
| Total fiber, g | 1 | 26.8 |
| Protein, g | 20 (13%) | 20 (13%) |
| Total fat, g | 41 (62%) | 4 (6%) |
| Saturated fat, g | 14 (22%) | 2 (2%) |
| Cholesterol, mg | 158 | 12.2 |
For three days prior to both study visits, subjects followed a standard 2,000 calorie meal plan that was approximately 30% fat, 55% carbohydrate, and 15% protein, and recorded all food and drinks consumed in a diet log. Subjects were given a sample menu and asked to follow it as closely as possible during this three-day period. Subjects were instructed to fast from 1900 h the night before each study visit and to avoid strenuous exercise and alcoholic beverages for at least 24 hours prior to each study visit.
On the morning of the two study visits, subjects arrived at the General Clinical Research Center at 0700 h. A venicatheter was inserted into an antecubital or hand vein for collection of blood samples and the catheter was kept open with saline. A baseline blood sample was taken after the IV was inserted for measurement of testosterone, sex hormone binding globulin (SHBG), DHEA-S, cortisol, glucose, and insulin.
Subjects were then served the test meal and asked to consume it within 15 minutes. After each meal, a blood sample was taken at 30 minutes and then every hour for six hours for measurement of testosterone, SHBG, DHEA-S, cortisol, glucose, and insulin. During this time, subjects remained comfortably seated or reclined and were allowed to read, listen to music, and watch TV. Subjects were allowed to consume water ad libitum and were allowed to go to the bathroom as needed. After the last blood draw, the catheter was removed and subjects were given a complementary meal.
Clinical and biochemical measurements
Subjects had a dual energy x-ray absorptiometry (DXA) scan to obtain body composition data including percent body fat and lean body measurements. One subject did not have a DXA scan because her weight was above the limit for the equipment. The DXA scans were performed using a Hologic QDR-4500W in fan-beam mode (Hologic Corp, Waltham MA). Proper operation of the x-ray subsystem was verified daily using a spine phantom, and tissue composition calibration was performed at least once/wk on a tissue equivalent phantom.
Serum and plasma were separated by centrifugation for 15 minutes at 1,465 × g (3,200 rpm) and 4° C, then aliquoted into cryovials and stored at −80° C until analysis. Plasma glucose levels were determined by the glucose oxidase technique using the Yellow Springs Instruments Model 2300 Stat Plus Glucose Analyzer. Insulin levels were measured using RIA kits from Linco (St. Charles, MO) (22). Serum levels of testosterone and progesterone were determined by RIA using commercial reagents (Diagnostic Products Corporation, Los Angeles, CA). SHBG was measured by Immunoradiometric Assay (IRMA) (Diagnostic Systems Laboratories, Webster, TX). Serum levels of DHEA-S and cortisol were measured by solid phase RIA (Siemens Medical Solutions Diagnostics, Tarrytown, NY). Intra-assay CVs were: testosterone 5−7%; progesterone 3−7%; SHBG 4−6%; DHEA-S 4−5%; cortisol 4−5%. Inter-assay CVs were: testosterone 7−11%; progesterone 4−8%; SHBG 6−9%; DHEA-S 4−10%; cortisol 3−8% . Analytical sensitivity for the steroid assays were: testosterone 15 ng/dl and progesterone 0.5 ng/ml.
Statistical analysis
This 2×2 crossover study was designed to detect a 20 ng/dL difference in the change in testosterone from baseline between the two meals. We assumed the inter-subject variances for each meal, as well as their covariance, were equivalent and that the intra-subject standard deviations for the meals were equivalent and equal to 20 ng/dL. On the basis of these assumptions, we needed to enroll 16 participants for the study to have a power of 80% for a two-sided test with a type I error rate of 0.05 to detect a 20 ng/dL difference in the change in testosterone between the two meals. However, the study period ended before the final subject could be enrolled and we proceeded with the analysis as we had experienced no dropouts between study visits.
The FAI was calculated as the ratio of total testosterone divided by SHBG, multiplied by 100 (23). The area under the curve (AUC) was computed for each subject using the trapezoidal rule. For all outcome variables, mixed effects models were fit to assess within-meal and between-meal differences (24). The mixed effects model is an extension of the traditional analysis of variance model that accounts for the within- and between-subject correlation inherent in crossover trials with repeated measurements over time (e.g., the blood draws) within each period of the crossover. If necessary to meet modeling assumptions such as normality, the outcome variable was logarithmically transformed. When significant effects of time were found, the effect of treatment on change scores was examined (postprandial level – fasted level). Data are presented as mean ± SE, all hypothesis tests were two-sided, and P < 0.05 was considered significant. All analyses were performed using SAS software (version 9.1; SAS Institute Inc., Cary, NC, USA).
RESULTS
The fifteen subjects were age 19 to 39 years and had a BMI ranging from 19.9 to 53.5 kg/m2. Seven subjects were normal weight (BMI 18.5−24.9), three were overweight (BMI 25−29.9), and five were obese (BMI ≥ 30). Descriptive statistics of the study subjects are listed in Table 2. Fasting glucose and SHBG were significantly different between the two randomization groups at baseline (P=0.002 and P=0.03, respectively). However, there was no effect of group on the outcomes measured. Of the 15 subjects, 12 were Caucasian, 1 was Hispanic, 1 was Asian, and 1 was African American. Fourteen out of fifteen participants had a T level ≥ 50 ng/dL at screening. The last woman had a T of 47 and a FAI of 2.4 at screening. No participants had any medical conditions or were taking any medications known to affect nutrient absorption. All subjects were compliant with the diet and exercise recommendations during the three days prior to their study visits.
Table 2.
Baseline characteristics of the study subjects (n = 15) a
| Characteristic | Value | Range |
|---|---|---|
| Age (years) | 26.9 ± 6.5 | 19−39 |
| Weight (kg) | 80.9 ± 32.5 | 50.7−160.1 |
| BMI (kg/m2) | 29.6 ± 10.7 | 19.9−53.5 |
| Systolic blood pressure (mmHg) | 108.2 ± 13.6 | 82−132 |
| Diastolic blood pressure (mmHg) | 65.7 ± 7.8 | 54−87 |
| Waist circumference (cm) | 93.5 ± 25.0 | 71−152 |
| Body fat percentage b | 33.7 ± 7.9 | 18.1−45.8 |
| Lean mass (g) b | 44,993 ± 10,065 | 33,182−67,455 |
| Fat mass (g) b | 26,128 ± 10,065 | 9,867−59,264 |
| Prolactin (ng/mL) c | 9.9 ± 6.3 | 3−24 |
| 17-hydroxyprogesterone (ng/mL) c | 0.9 ± 0.6 | 0.4−1.1 |
| TSH (mIU/mL) c | 1.7 ± 1.3 | 0.6−5.0 |
| Testosterone (ng/dL) | 59.1 ± 19.2 | 31−89 |
| SHBG (nmol/L) | 45.9 ± 33.4 | 10−131 |
| Free Androgen Index | 6.5 ± 4.2 | 1.7−19.8 |
| Fasting glucose (mg/dL) | 86.2 ± 9.2 | 75−113 |
| Fasting insulin (μU/ml) | 12.9 ± 10.8 | 4−57 |
| Cholesterol (mg/dL) c | 161.2 ± 21.6 | 110−193 |
| Triglycerides (mg/dL ) c | 108.5 ± 72.3 | 27−283 |
Data represent mean ± SD. BMI, body mass index; SHBG, sex hormone binding globulin; TSH, thyroid-stimulating hormone.
n = 14
Values measured at screening
Testosterone, SHBG, and FAI
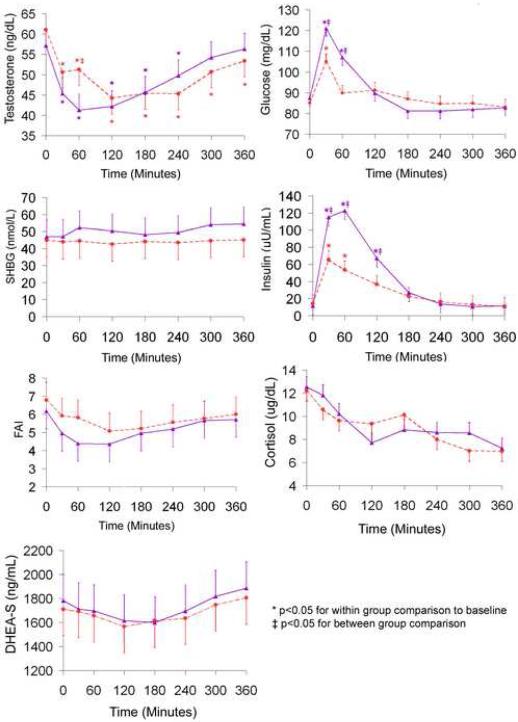
Testosterone, SHBG, and the FAI at baseline and after the low-fat, high-fiber meal and the high-fat, Western meal are shown in Figure 1. There was a significant meal * time interaction for the change in T after the test meals (P = 0.004). Testosterone decreased within two hours after both meals by 27% (P < 0.001). However, T remained below premeal values for four hours after the low-fat, high-fiber meal (P < 0.004) and for six hours after the high-fat, Western meal (P < 0.004). Testosterone began to return to baseline 60 minutes after the low-fat, high-fiber meal, whereas T continued to drop 60 minutes after the high-fat, Western meal and did not begin to rise until 120 minutes after the meal.
Figure.
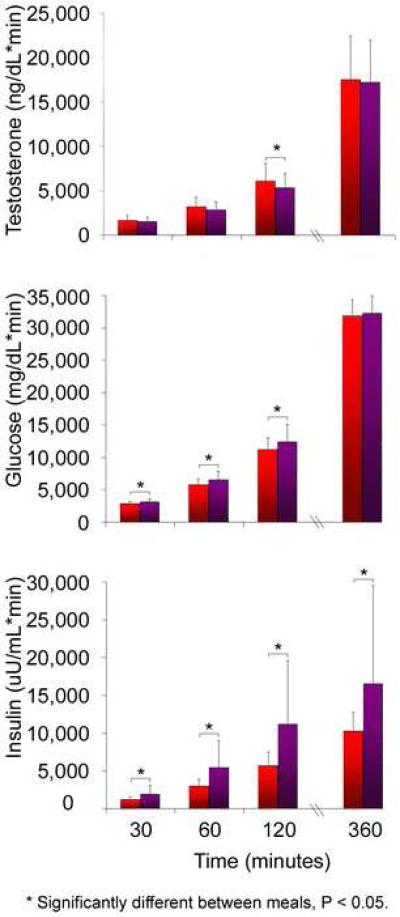
Pairwise comparisons of the adjusted means showed that T was significantly lower at 60 minutes after the low-fat, high-fiber meal compared with the high-fat, Western meal (P = 0.008). In addition, the area under the curve for testosterone was significantly lower after the low-fat, high-fiber meal at 120 minutes compared with the high-fat, Western meal (P = 0.04) (Figure 2). There was no difference in the area under the curve for T at any other time point.
Figure.
The effects of meal and time on SHBG were not significant. There was a significant effect of time for the FAI (P < 0.001), however, the meal * time interaction was not significant. There were no significant differences in the area under the curve for SHBG or the FAI at any time point after the two test meals (data not shown).
DHEA-S and cortisol
DHEA-S and cortisol levels at baseline and after the low-fat, high-fiber meal and the high-fat, Western meal are shown in Figure 1. DHEA-S decreased 8−10% within 2−3 hours after both test meals and then increased over the remainder of the study period. The effect of time for DHEA-S was significant (P < 0.001), but there was not a significant meal * time interaction. Cortisol decreased over the 6 hour period after both test meals. The effect of time for cortisol was significant (P < 0.001), but the meal * time interaction was not significant. There were no significant differences in the area under the curve for DHEA-S or cortisol at any time point after the two test meals (data not shown).
Glucose and insulin
Glucose and insulin levels at baseline and after the low-fat, high-fiber meal and the high-fat, Western meal are shown in Figure 1. There was a significant meal * time interaction for the change in glucose (P = 0.007) and for insulin (P < 0.001) after the two test meals. Pairwise comparisons of the adjusted means showed that glucose levels were significantly higher 30 and 60 minutes after the low-fat, high-fiber meal compared with the high-fat, Western meal (P < 0.003). Insulin levels were approximately two fold higher 30, 60, and 120 minutes after the low-fat, high-fiber meal compared with the high fat, Western meal (P < 0.03). The area under the curve was significantly higher after the low-fat, high-fiber meal compared with the high-fat, Western meal at 30, 60, and 120 minutes for glucose, and at all time points after the meal for insulin (Figure 2). We noted minimal absolute change and also did not find a significant association between changes in glucose or insulin levels with testosterone levels. We found that for every 10 mg/dL increase in glucose, testosterone decreased 0.56 ng/dL (P = 0.08). In addition, for every 10 μU/mL increase in insulin, testosterone decreased 0.10 ng/dL P = 0.41)
DISCUSSION
To our knowledge, this is the first study to evaluate the short-term effects of meal composition on testosterone levels in women with PCOS. We observed a 27% reduction in testosterone levels within two hours after eating a high-fat, Western meal and a low-fat, high-fiber meal in women with PCOS. However, testosterone levels were reduced for two hours longer after the high-fat, Western meal compared with the low-fat, high-fiber meal, indicating that there is an effect of meal composition on the postprandial testosterone response, independent of caloric load. There was no difference in the area under the curve for testosterone at six hours after the two test meals, indicating that there were similar circulating levels over the six-hour study period. As expected, glucose and insulin levels were higher after the low-fat, high-fiber meal compared with the high-fat, Western meal.
The postprandial reduction in testosterone levels that we observed is consistent with previous findings in men and in women with PCOS. In two studies in men, testosterone levels decreased 20−25% within two hours after ingestion of a meal (13, 16). Likewise, Panidis et al. reported that serum testosterone levels significantly decreased 19−42% three hours after oral administration of 75g dextrose in insulin resistant and insulin sensitive women with PCOS (BMI 27.7−39.0 and 19.1−21.5 kg/m2, respectively), as well as in overweight and lean women without PCOS (BMI 26.5−38.3 and 19.0−23.5 kg/m2, respectively) (25). In another study, Parra et al. measured changes in serum androgens after a 725-calorie breakfast (55% CHO, 31% fat) in six overweight and obese women with PCOS (BMI 26.2−41.4 kg/m2) (14). In five of the six women, free testosterone levels decreased an average of 62% at 90 minutes. The greater reduction in testosterone levels in this study could possibly be due to a higher calorie content of the meal, or perhaps to the mixed composition of the meal.
A few studies in men have investigated the role of diet composition on testosterone levels after a meal. Although these studies have demonstrated that meal composition affects postprandial testosterone levels, the specific effects of individual nutrients are uncertain. Meikle et al. reported that total and free testosterone levels were approximately 30% lower than baseline for four hours after consuming an 800-calorie high-fat milkshake (57% fat, 34% carbohydrate). However, they observed no significant change in testosterone levels following a mixed carbohydrate and protein milkshake (26% protein, 73% carbohydrate) (15). Habito and Ball compared meals with different sources of protein (soy vs. meat), different amounts of saturated fat (lean meat vs. meat with animal fat) and different sources of fat (animal fat vs. vegetable oil) in 15 healthy men (16). Mean testosterone levels decreased significantly two hours after all of the meals except the high animal fat meal, suggesting that the type of fat influenced postprandial testosterone levels. Although Habito and Ball did not observe a change in testosterone levels after a high saturated fat meal, Volek et al. observed a 22% and 23% decrease in total and free testosterone levels following a fat-rich meal (86% fat) containing 52g saturated fat, 59g monounsaturated fat and 12g polyunsaturated fat in 11 healthy men (13). Taken together, there is not a consistent effect of diet composition on circulating testosterone levels in men in previous studies.
The results of the present study suggest that in women with PCOS there is a delayed reduction in testosterone levels after a high-fat, Western meal compared with a low-fat, high-fiber meal. One mechanism for the postprandial reduction in testosterone levels could be from an increase in hepatic blood flow and increased hepatic extraction of steroids after a meal. The delayed reduction in testosterone levels after the high-fat meal could be due to slower postprandial blood flow to the mesenteric artery and portal vein, resulting in a longer period of nutrient absorption and metabolism. Previous studies have reported that there is a faster peak in mean velocity and volume flow in the superior mesenteric artery following a high-carbohydrate meal compared with a high-fat meal (26, 27). In addition, Høst et al. observed that Doppler estimated portal blood flow increased 107% one hour after a fat-rich meal (80−83% fat), compared with a 62% increase after an isocaloric, isovolumetric carbohydrate rich meal (81% carbohydrate) in healthy non-obese males, which could be due to a delayed increase in portal blood flow after the fat-rich meal (28). The differing responses in mesenteric and portal blood flow after high-carbohydrate and high-fat meals could be due to differences in gastric emptying, release of gastrointestinal hormones, and sympathetic nervous system activation (26, 29). However, the specific mechanisms for the postprandial reduction in testosterone levels are unknown (28).
Although hyperinsulinemia is implicated in the pathogenesis of PCOS, it is unlikely that insulin is directly responsible for the postprandial changes in testosterone levels that we observed. Several investigators have reported no relationship between the change in insulin and testosterone levels after a meal (13, 16) or after an oral glucose load (17, 18). This is in agreement with the results of the present study. In addition, no relationship was observed between androgen and insulin levels following IV infusion of glucose or insulin (17, 30-32). In support of this, Parra et al. conducted an elegant study in which women with PCOS were fed a 725 calorie breakfast with simultaneous infusion of epinephrine and propranolol to cause an acute blockade on pancreatic insulin secretion. After 90 minutes, the infusion was stopped and blood samples were collected for the remaining 90 minutes. The investigators observed that testosterone levels decreased after the breakfast despite the suppression of pancreatic insulin secretion. There was also no acute increase in androgens after cessation of the epinephrine and propranol infusion. These data suggest that insulin itself is not responsible for the postprandial decline in testosterone levels.
In the present study, we did not observe an effect of meal composition on postprandial DHEA-S, cortisol, and SHBG levels. The similar postprandial changes in DHEA-S and cortisol after the two test meals differs from a recent study by Kasim-Karakas et al. that reported a significant effect of meal composition on the change in DHEA and cortisol levels after a high-protein drink (75 g 98% pure, intact whey protein isolate) compared with a 75g OGTT in women with PCOS (11). The different response of DHEA-S and cortisol in the two studies suggests that individual nutrients affect of the response of adrenal hormones, but these differences become less apparent following mixed meals.
Although we did not observe a change in postprandial SHBG levels following the two test meals, there is evidence that dietary factors including fructose, glucose, sucrose, and insulin affect SHBG expression and secretion (33, 34). For example, a recent study by Selva et al. demonstrated that SHBG levels were reduced by 40−80% in mice with human SHBG transgenes expressed as soon as three days after consuming a diet high in sucrose, glucose or fructose (33). In humans, a increase in SHBG has been reported following a long-term (2−7 months) hypocaloric diet (6, 10, 35), however SHBG levels were unchanged following 3−12 week eucaloric diets enriched with monounsaturated fatty acids (17% energy) or polyunsaturated fatty acids (14% energy), or low in carbohydrates (43% energy) (12, 36). The lack of change in SHBG following a eucaloric diet suggests that manipulating diet composition alone (in contrast to weight loss) may not be enough to dramatically alter SHBG levels. Alternatively, our period of observation during this study could have been too short to expect changes in a hepatically synthesized and cleared protein.
A limitation of the present study is that since we did not control for a single nutrient, it is not possible to determine what specific dietary component(s) causes the prolonged reduction in testosterone levels after the high-fat, Western meal. In designing this study, our aim was to test two meals that had very different nutrient compositions to increase the likelihood of detecting any differences in postprandial testosterone levels. Another limitation is our small sample size based on studies done in men (13, 15, 16). A larger sample size would have increased the strength of our conclusions. The unfavorable relative effects of the low-fat, high-fiber meal on postprandial glucose and insulin levels due to its high carbohydrate content may be argument enough to dissuade the use of a high-fiber, high-carbohydrate diet as a therapy for hyperandrogenemia in PCOS. A final limitation is that including lean, overweight, and obese women in the study may have increased the variability in biochemical outcomes measured. However, excluding subjects based on BMI was not warranted since previous studies have not demonstrated an effect of BMI on changes in postprandial testosterone levels.
Since testosterone levels are reduced for as long as six hours after a meal, eating small, frequent meals throughout the day could be a means of reducing testosterone levels in women with PCOS. However, this is speculation and further studies would be needed to test this hypothesis. Spreading out food intake over 6 or more small meals compared with three or fewer large meals could also have additional benefits in women with PCOS. In clinical trials, eating small, frequent meals reduced day-long insulin levels and reduced cholesterol and triglyceride levels compared with an isocaloric diet with three or fewer meals (37-39). Some observational studies have also reported a lower body weight and body fat percentage in people who consume frequent meals compared with fewer meals (40-42). However these findings have not been confirmed in clinical studies. Further studies are needed to test the effect of meal frequency on testosterone levels in women with PCOS.
Despite limitations, our results indicate that meal composition affects postprandial testosterone levels in women with PCOS. In particular, we find that altering meal composition improves testosterone levels in women with PCOS without weight loss, which is an interesting concept that warrants further investigation. This is significant because whereas treatment of PCOS with weight loss is applicable only to overweight and obese women, altering diet composition is a potential treatment for all women with PCOS. Other areas for follow up experiments likely to enhance our mechanistic understanding of the effect of diet composition in PCOS include reducing the variability in diet composition to a single nutrient, and prolonging the study to understand cumulative diet effects on women with PCOS.
ACKNOWLEDGEMENTS
Many thanks to the Pennsylvania State University General Clinical Research Center and Core Endocrine Laboratory for their help with the clinical tests and laboratory assays.
Support provided by the National Institutes of Health (K24 HD01476) and by the General Clinical Research Center of The Pennsylvania State University (NIH #M01RR10732 and construction grant C06 RR016499).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was done at The Pennsylvania State University General Clinical Research Centers in Hershey, PA and University Park, PA.
Presented at the 89th Annual Meeting of the Endocrine Society, Toronto, Ont, CA, 2007.
Disclosure information: Dr. Legro reports having served as a consultant to Glaxo Smith Kline and Ferring. He has had paid lecture fees by Serono, meeting support from Abbott, and grant support from Pfizer and Solvay. Dr. Kris-Etherton serves as a consultant to J & J Merck, Unilever, Heinz, and the California Walnut Commission. She has had paid lecture fees by Frito Lay and current grant support from the National Cattleman's Beef Association.
CAPSULE
A randomized, clinical trial in 15 women with polycystic ovary syndrome demonstrated that testosterone decreases within two hours after a meal challenge and returns to baseline, but with varying patterns based on diet composition.
REFERENCES
- 1.Robinson S, Rodin DA, Deacon A, Wheeler MJ, Clayton RN. Which hormone tests for the diagnosis of polycystic ovary syndrome? Br J Obstet Gynaecol. 1992;99:232–8. doi: 10.1111/j.1471-0528.1992.tb14505.x. [DOI] [PubMed] [Google Scholar]
- 2.Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67:155–63. doi: 10.1016/s0015-0282(97)81873-0. [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 4.Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86:1626–32. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- 5.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–66. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 6.Moran LJ, Noakes M, Clifton PM, Wittert GA, Williams G, Norman RJ. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr. 2006;84:77–87. doi: 10.1093/ajcn/84.1.77. [DOI] [PubMed] [Google Scholar]
- 7.Nestler JE. Insulin regulation of human ovarian androgens. Hum Reprod. 1997;12(Suppl 1):53–62. doi: 10.1093/humrep/12.suppl_1.53. [DOI] [PubMed] [Google Scholar]
- 8.Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update. 2004;10:267–80. doi: 10.1093/humupd/dmh018. [DOI] [PubMed] [Google Scholar]
- 9.Stamets K, Taylor DS, Kunselman A, Demers LM, Pelkman CL, Legro RS. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004;81:630–7. doi: 10.1016/j.fertnstert.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Moran LJ, Noakes M, Clifton PM, Tomlinson L, Galletly C, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:812–9. doi: 10.1210/jc.2002-020815. [DOI] [PubMed] [Google Scholar]
- 11.Kasim-Karakas SE, Cunningham WM, Tsodikov A. Relation of nutrients and hormones in polycystic ovary syndrome. Am J Clin Nutr. 2007;85:688–94. doi: 10.1093/ajcn/85.3.688. [DOI] [PubMed] [Google Scholar]
- 12.Douglas CC, Gower BA, Darnell BE, Ovalle F, Oster RA, Azziz R. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril. 2006;85:679–88. doi: 10.1016/j.fertnstert.2005.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volek JS, Gomez AL, Love DM, Avery NG, Sharman MJ, Kraemer WJ. Effects of a high-fat diet on postabsorptive and postprandial testosterone responses to a fat-rich meal. Metabolism. 2001;50:1351–5. doi: 10.1053/meta.2001.25648. [DOI] [PubMed] [Google Scholar]
- 14.Parra A, Barron J, Mota-Gonzalez M, Ibarra V, Espinosa de los Monteros A. Serum androgen changes during meal-induced hyperinsulinemia and after acute sequential blockade and hyperstimulation of insulin release in women with polycystic ovary syndrome. Arch Med Res. 1995;26:S209–17. Spec No. [PubMed] [Google Scholar]
- 15.Meikle AW, Stringham JD, Woodward MG, McMurry MP. Effects of a fat-containing meal on sex hormones in men. Metabolism. 1990;39:943–6. doi: 10.1016/0026-0495(90)90305-v. [DOI] [PubMed] [Google Scholar]
- 16.Habito RC, Ball MJ. Postprandial changes in sex hormones after meals of different composition. Metabolism. 2001;50:505–11. doi: 10.1053/meta.2001.20973. [DOI] [PubMed] [Google Scholar]
- 17.Buyalos RP, Geffner ME, Watanabe RM, Bergman RN, Gornbein JA, Judd HL. The influence of luteinizing hormone and insulin on sex steroids and sex hormone-binding globulin in the polycystic ovarian syndrome. Fertil Steril. 1993;60:626–33. doi: 10.1016/s0015-0282(16)56212-8. [DOI] [PubMed] [Google Scholar]
- 18.Tropeano G, Lucisano A, Liberale I, Barini A, Vuolo IP, Martino G, et al. Insulin, C-peptide, androgens, and beta-endorphin response to oral glucose in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 1994;78:305–9. doi: 10.1210/jcem.78.2.8106616. [DOI] [PubMed] [Google Scholar]
- 19.Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, et al. The Pregnancy in Polycystic Ovary Syndrome study: baseline characteristics of the randomized cohort including racial effects. Fertil Steril. 2006;86:914–33. doi: 10.1016/j.fertnstert.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Legro RS, Zaino RJ, Demers LM, Kunselman AR, Gnatuk CL, Williams NI, et al. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am J Obstet Gynecol. 2007;196:402 e1–10. doi: 10.1016/j.ajog.2006.12.025. discussion e10−1. [DOI] [PubMed] [Google Scholar]
- 21.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236–42. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 24.Vonesh EF, Chinchilli VM. Linear and Nonlinear Models for the Analysis of Repeated Measurements. Marcel Dekker; New York, NY: 1997. [Google Scholar]
- 25.Panidis D, Rousso D, Skiadopoulos S, Vavilis D, Karayannis B, Petropoulos P. Does postprandial hypersinsulinemia contribute to hyperandrogenism in patients with polycystic ovary syndrome? Clin Exp Obstet Gynecol. 1997;24:88–91. [PubMed] [Google Scholar]
- 26.Moneta GL, Taylor DC, Helton WS, Mulholland MW, Strandness DE., Jr. Duplex ultrasound measurement of postprandial intestinal blood flow: effect of meal composition. Gastroenterology. 1988;95:1294–301. doi: 10.1016/0016-5085(88)90364-2. [DOI] [PubMed] [Google Scholar]
- 27.Qamar MI, Read AE. Effects of ingestion of carbohydrate, fat, protein, and water on the mesenteric blood flow in man. Scand J Gastroenterol. 1988;23:26–30. doi: 10.3109/00365528809093842. [DOI] [PubMed] [Google Scholar]
- 28.Hoost U, Kelbaek H, Rasmusen H, Court-Payen M, Christensen NJ, Pedersen-Bjergaard U, et al. Haemodynamic effects of eating: the role of meal composition. Clin Sci (Lond) 1996;90:269–76. doi: 10.1042/cs0900269. [DOI] [PubMed] [Google Scholar]
- 29.de Aguilar-Nascimento JE. The role of macronutrients in gastrointestinal blood flow. Curr Opin Clin Nutr Metab Care. 2005;8:552–6. doi: 10.1097/01.mco.0000170755.32996.1d. [DOI] [PubMed] [Google Scholar]
- 30.Herbert CM, 3rd, Hill GA, Diamond MP. The use of the intravenous glucose tolerance test to evaluate nonobese hyperandrogenemic women. Fertil Steril. 1990;53:647–53. doi: 10.1016/s0015-0282(16)53458-x. [DOI] [PubMed] [Google Scholar]
- 31.Fox JH, Licholai T, Green G, Dunaif A. Differential effects of oral glucose-mediated versus intravenous hyperinsulinemia on circulating androgen levels in women. Fertil Steril. 1993;60:994–1000. [PubMed] [Google Scholar]
- 32.Mehta RV, Patel KS, Coffler MS, Dahan MH, Yoo RY, Archer JS, et al. Luteinizing hormone secretion is not influenced by insulin infusion in women with polycystic ovary syndrome despite improved insulin sensitivity during pioglitazone treatment. J Clin Endocrinol Metab. 2005;90:2136–41. doi: 10.1210/jc.2004-1040. [DOI] [PubMed] [Google Scholar]
- 33.Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. 2007;117:3979–87. doi: 10.1172/JCI32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67:460–4. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 35.Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;36:105–11. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 36.Kasim-Karakas SE, Almario RU, Gregory L, Wong R, Todd H, Lasley BL. Metabolic and endocrine effects of a polyunsaturated fatty acid-rich diet in polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:615–20. doi: 10.1210/jc.2003-030666. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins DJ, Ocana A, Jenkins AL, Wolever TM, Vuksan V, Katzman L, et al. Metabolic advantages of spreading the nutrient load: effects of increased meal frequency in non-insulin-dependent diabetes. Am J Clin Nutr. 1992;55:461–7. doi: 10.1093/ajcn/55.2.461. [DOI] [PubMed] [Google Scholar]
- 38.Bertelsen J, Christiansen C, Thomsen C, Poulsen PL, Vestergaard S, Steinov A, et al. Effect of meal frequency on blood glucose, insulin, and free fatty acids in NIDDM subjects. Diabetes Care. 1993;16:4–7. doi: 10.2337/diacare.16.1.4. [DOI] [PubMed] [Google Scholar]
- 39.Rashidi MR, Mahboob S, Sattarivand R. Effects of nibbling and gorging on lipid profiles, blood glucose and insulin levels in healthy subjects. Saudi Med J. 2003;24:945–8. [PubMed] [Google Scholar]
- 40.Fabry P, Hejl Z, Fodor J, Braun T, Zvolankova K. The Frequency Of Meals. Its Relation To Overweight, Hypercholesterolaemia, And Decreased Glucose-Tolerance. Lancet. 1964;18:614–5. doi: 10.1016/s0140-6736(64)90510-0. [DOI] [PubMed] [Google Scholar]
- 41.Metzner HL, Lamphiear DE, Wheeler NC, Larkin FA. The relationship between frequency of eating and adiposity in adult men and women in the Tecumseh Community Health Study. Am J Clin Nutr. 1977;30:712–5. doi: 10.1093/ajcn/30.5.712. [DOI] [PubMed] [Google Scholar]
- 42.Louis-Sylvestre J, Lluch A, Neant F, Blundell JE. Highlighting the positive impact of increasing feeding frequency on metabolism and weight management. Forum Nutr. 2003;56:126–8. [PubMed] [Google Scholar]