Abstract
Sex-related influences on pain and analgesia have become a topic of tremendous scientific and clinical interest, especially in the last 10 to 15 years. Members of our research group published reviews of this literature more than a decade ago, and the intervening time period has witnessed robust growth in research regarding sex, gender, and pain. Therefore, it seems timely to revisit this literature. Abundant evidence from recent epidemiologic studies clearly demonstrates that women are at substantially greater risk for many clinical pain conditions, and there is some suggestion that postoperative and procedural pain may be more severe among women than men. Consistent with our previous reviews, current human findings regarding sex differences in experimental pain indicate greater pain sensitivity among females compared with males for most pain modalities, including more recently implemented clinically relevant pain models such as temporal summation of pain and intramuscular injection of algesic substances. The evidence regarding sex differences in laboratory measures of endogenous pain modulation is mixed, as are findings from studies using functional brain imaging to ascertain sex differences in pain-related cerebral activation. Also inconsistent are findings regarding sex differences in responses to pharmacologic and non-pharmacologic pain treatments. The article concludes with a discussion of potential biopsychosocial mechanisms that may underlie sex differences in pain, and considerations for future research are discussed.
Perspective
This article reviews the recent literature regarding sex, gender, and pain. The growing body of evidence that has accumulated in the past 10 to 15 years continues to indicate substantial sex differences in clinical and experimental pain responses, and some evidence suggests that pain treatment responses may differ for women versus men.
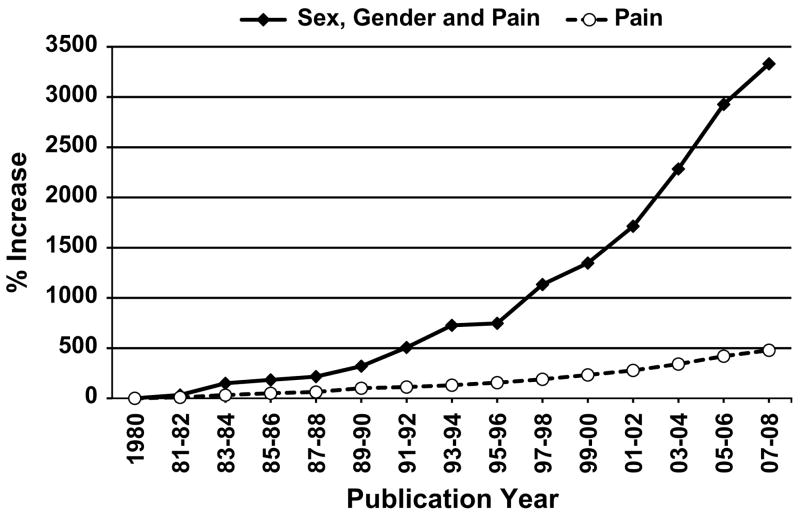
Research regarding sex and gender, differences in pain has increased substantially in recent years. As Fig 1 depicts, publications regarding sex, gender, and pain have increased at a much greater rate over the past 25 to 30 years relative to the pain field in general. In particular, a dramatic increase in publications began in the mid-1990s, which may be attributable to several influential review articles along with other events occurring in the 1990s that drew considerable attention to the topic. In 1992, an important publication by Karen Berkley32 highlighted the importance of sex-related issues in neuroscience research. This brief paper included a survey of 100 articles in reputable neuroscience journals, which found that 45% of the articles failed to report the sex of their subjects, and the author stated “… the differences between females and males, which we all know to be important, can and should be exploited in scientific research.” Shortly thereafter, an editorial appeared in The Journal of Pain, which encouraged studying the differences between women and men, a topic that had been out of favor given the 1980s’ emphasis on equality of the sexes.348 These two publications both reflected and created increased interest in studying sex differences in pain. Subsequently, a review article appeared in Pain Forum, the predecessor of this journal, which discussed the literature regarding sex differences in responses to experimentally induced pain and offered a heuristic model outlining multiple mechanisms underlying these sex differences.137 Subsequently, Karen Berkley’s33 review article appeared in Behavioral and Brain Sciences, accompanied by extensive commentary from several prominent pain scientists, and Unruh418 published a comprehensive review of sex differences in clinical pain in the journal Pain. Thus, the early to mid-1990s was a period of increased scholarly activity regarding sex differences in pain.
Figure 1.
Average annual percentage increase in publications over each 2-year period after 1980, which served as the reference year. These percentages were computed by conducting literature searches using PubMed for every year since 1980. For 2008, the first 6 months was collected and doubled to obtain an annualized estimate. The PubMed search for Sex, Gender, and Pain was completed using the following Boolean combination (Sex differences OR Gender differences) AND Pain.
The burgeoning interest in sex, gender, and pain embodied in this series of prominent publications culminated in two NIH initiatives, which ensured the continued growth of research on the topic. In 1997, NIH issued a request for applications entitled “Sex and Gender-Related Differences in Pain and Analgesic Responses,” which was sponsored by multiple institutes as well as the Office for Research on Women’s Health. This generated substantial interest from the scientific community and launched multiple new research programs related to sex differences in pain. Then, in April 1998, the NIH Pain Research Consortium hosted a scientific conference entitled “Gender and Pain: A Focus on How Pain Impacts Women Differently than Men,” which featured presentations by many prominent basic and clinical pain scientists and garnered considerable attention in the popular media. An additional development began in August 1996 at the World Congress on Pain in Vancouver, where an initial meeting of researchers interested in sex, gender and pain lead to the establishment of an IASP Special Interest Group (SIG) on Sex, Gender, and Pain, which held its first formal meeting in Vienna in 1999. Thus, a combination of events has prompted the recent and dramatic increase in research on issues regarding sex, gender, and pain.
In addition to those alluded to above, several subsequent reviews of this rapidly expanding literature have been provided, often focusing on particular segments of research regarding sex, gender, and pain. More than 10 years ago, a quantitative review of the literature regarding sex differences in experimental pain responses concluded that females show greater sensitivity than males to several modalities of experimental pain.327 Other reviews have been published since this time,35,81,117,441 including reviews of sex differences in responses to analgesic interventions,74,122,221,277 and a recent consensus statement providing recommendations for conducting research on sex, gender, and pain.168 In an attempt to increase the value of this review article, we will focus predominantly (though not exclusively) on findings from human studies that have emerged since the first two reviews by members of our research group.127,327 In addition, rather than emphasizing a specific segment of the literature, we will provide a representative summary of multiple areas of investigation, including sex differences in the prevalence and severity of clinical pain, sex differences in responses to experimental pain, sex differences in treatment responses, as well as discussion of viable biological and psychosocial mechanisms that contribute to sex differences in pain. We will conclude with a synopsis of the current state of the literature followed by a discussion of important issues to be addressed in future research.
Sex Differences in Clinical Pain
Reviews of the pain epidemiology literature have addressed the question “whether there is consistent support for sex differences in the prevalence of pain, or whether sex differences exist only for selected pain conditions.”243,418 These reviews have concluded that the relationship between sex and pain is not simple; nevertheless, most population-based studies have found higher prevalence in women than in men, but there are studies that have found no differences. The goal of this section is to examine whether more recent studies corroborate these findings.
Methodological Considerations
The organization of studies for this review has been challenging as publications have focused on differing dimensions or characteristics of clinical pain. Pain studies can be organized by chronicity (chronic, acute), site (low-back, abdominal), number of sites (regional, widespread), tissue type (musculoskeletal, neuropathic), or etiology (iatrogenic, trauma, insidious). A complete review of sex differences in pain prevalence across all possible pain conditions, sites or etiologies is not feasible given the constraints of this broad review of sex differences in pain. Consequently, we will consider recent findings regarding the following pain conditions: cancer pain, neuropathic pain, musculoskeletal pain, oral pain, headache, abdominal pain, headache, pain in children and adolescents, and postprocedural pain.
Sampling
The general goal of all sampling methods is to obtain a sample that is representative of the target population. The most accurate inferences about sex differences in pain would derive from studies based on a randomly selected representative national or regional sample. However, sex differences in pain have been investigated in samples collected in a variety of ways. Studies that report on clinical samples, often from pain treatment centers, can suffer from the bias associated with health care seeking. Caution must be exercised when interpreting these data because women utilize health care services to a greater extent than men,48,428 consequently a clinical sample does not reflect the general population. Where possible, we will rely on studies drawn from general population-based samples.
As epidemiological studies of pain typically rely on self-report via surveys or telephone interviews, one potential problem can be nonparticipation bias114,224; that is, differences in the outcome of interest between persons willing to participate and those that decline to do so.432 The higher the participation rate, the less bias will be introduced. Some studies report participation rates, and fewer test for differences between participants and nonparticipants as often little information is available from nonparticipants. In complex sampling designs, weighting adjustments can account for some bias, but whether this has occurred is seldom described in the papers we have reviewed.
Another issue concerns geographic or cultural characteristics of the reference population. It cannot be assumed that sex differences are consistent across the world. Because of strong interests in public health, most epidemiological data on pain conditions come from Europe and particularly the Scandinavian countries. However, we have attempted to select studies from a range of geographic regions and cultures.
Measures
Epidemiologic studies of pain typically report point prevalence (currently in pain), period prevalence (ie, experiencing pain during the past month or year), or lifetime prevalence. Some of the studies reviewed have measured pain intensity or severity ratings and depression, a common impact of chronic pain, and when sex differences were tested, we will report the findings. One issue worth mentioning is over interpretation of positive findings for sex differences in pain due to publication biases. It seems plausible that in some cases sex differences were tested, found to be nonsignificant, and then not reported in a manuscript.93 This may be particularly true for population-based studies of prevalence in which pain intensity or severity is of secondary interest.
Pain in Multiple Anatomic Regions
Several studies drawn from multiple geographic locations report prevalence of pain by sex across a number of anatomic sites. Gerdle et al157 found the 7-day prevalence for females was higher than males for all 10 anatomic regions assessed, but no sex difference was found for pain intensity ratings. Several papers from a Dutch population-based study of musculoskeletal complaints have reported higher pain prevalence among females at nearly all body sites.315,442,443 Also, women reported greater functional limitation than men but no differences were found for pain intensity ratings. A study in the Spanish population noted higher prevalence of pain at one or more locations for women (86%) compared with men (72%) and as well as for all individual musculoskeletal sites.24 Another Spanish study reported greater prevalence of pain at any site during the previous day for women (37%) versus men (21%), whereas sex differences in pain prevalence across specific body sites were mixed.60 Significant sex differences in the 1-year prevalence of pain at any body site (F = 40%, M = 35%) were reported in working adults living in Taiwan.170 This sex difference was relatively consistent across the age categories with the largest difference in the 45 to 54 and 55 to 64 age ranges. An estimate of pain prevalence is also available for rural India. Chopra and colleagues69 found higher 7-day point prevalence across all 24 body sites for females compared with males. Small sex differences in pain prevalence emerged for most sites in a representative sample of the US noninstitutionalized population.179
Cancer Pain
Chronic cancer pain is experienced by approximately 30% to 85% of patients with cancer, depending on type of cancer and the stage.423 Because studies of cancer pain prevalence in representative population samples are rare, we have used data from clinical samples to examine sex differences in pain intensity/severity and depression but acknowledge potential for bias. A retrospective study of cancer patients referred for pain treatment found no sex differences in pain intensity or disability.416 Miaskowski276 published a review on sex differences in chronic cancer pain, concluding sex differences were inconsistent. Two studies, one of patients 2 to 3 weeks after their last hospitalization350 and another of oncology outpatients with bone metastasis,100 did not find sex was related to cancer pain. Another study that followed patients with inoperable lung cancer reported that women were more depressed at baseline than men but no differences were found in pain ratings.256 One month after diagnosis, chest pain was reported as more intense by men, whereas women reported more intense pain in areas outside of the chest and arm/shoulder. Schmidt et al366 found that women reported greater pain in the abdomen before rectal cancer surgery, at discharge, and at 3 months after surgery; however, there were no sex differences in pain at later time periods. Valeberg et al421 reported that among outpatients at a large cancer hospital in Norway, females were more likely to have comorbid cancer pain and noncancer pain than males, and these authors also found that women were at increased risk for more severe pain.420
We have identified two studies that used population-level sampling. Reyes-Gibby et al323 reported that among adults ages 50 and older with cancer from the United States, females were more likely to have the symptom cluster of pain, depression, and fatigue than males by a factor of 1.2. A study from the Netherlands found that sex was not associated with prevalence or severity of cancer pain.423 These findings provide little evidence for sex differences in cancer pain; however, greater depression among women with chronic cancer pain has been reported.
Neuropathic Pain
Neuropathic pain is a complex pain state in which the nerve fibers may be damaged, dysfunctional, or injured.275 Until recently, there was little epidemiological data on chronic neuropathic pain at the population level because of the lack of an appropriate assessment instrument to identify the characteristics of neuropathic pain in community samples.44 However, studies have examined sex differences in the epidemiology of specific neurological conditions that are painful.409 These studies report greater disease frequency among females.85,176,358
Recently, questionnaires have been developed based on the analysis of the characteristics of pain (ie, pain descriptors) that discriminate pain due to a definite neurological lesion. Torrance et al409 estimated the prevalence of pain of predominantly neuropathic origin using a random sample of 6000 adults from family practices in three United Kingdom cities using a 5-item neuropathic pain scale developed by Bennett.28 Females (6%) showed greater prevalence of neuropathic pain (lasting longer than 3 months) compared with males (3%). Using a large representative sample of the French population, Bouhassira et al44 assessed neuropathic pain using their symptom-based screen for pain with neuropathic qualities43 and found higher 3-month prevalence in females (8%) compared with males (6%). Neither study reported sex differences in the effects of age, pain intensity, or depression. Consequently, it appears that women are at greater risk for neuropathic pain than men.
Musculoskeletal Pain
Many studies have investigated the prevalence of musculoskeletal pain in men and women, with some assessing chronic musculoskeletal pain irrespective of the site, whereas others have been site specific. In a previous review, Rollman and Lautenbacher341 concluded that women have greater frequency of musculoskeletal pain than men. A number of recent studies have tested for sex differences in chronic musculoskeletal pain at any site. In a study spanning 17 countries across 6 continents with a total sample size of 85,052 adults, the prevalence of any chronic pain condition was higher among females (45%) than males (31%), and females had a higher prevalence of depression comorbid with chronic pain than males.415 Other studies from Australia,41 Europe,47 France,44 the Netherlands,442 Norway,351 Sweden,30,158 and the United Kingdom377 also indicate chronic musculoskeletal pain is more common in females than males (Table 1). In one study, women reported significantly higher ratings of worst and current pain intensity but there were no differences on the rating for least pain.351 We review evidence regarding several specific types of musculoskeletal pain, including back pain, widespread pain/fibromyalgia, and osteoarthritis.
Table 1.
Prevalence of Chronic Pain in Representative Samples
| Study | Country | Prevalence | Female | Male |
|---|---|---|---|---|
| Bergman30 | Sweden | 12-month | 38% | 31% |
| Blythe41,* | Australia | 6-month | 20% | 17% |
| Bouhassira44 | France | Current | 35% | 28% |
| Breivik47 | Europe | 6-month | 11% | 10% |
| Gerdle158 | Sweden | 3-month | 59% | 48% |
| Rustoen351 | Norway | Current | 28% | 23% |
| Smith377 | United Kingdom | Current | 52% | 49% |
| Tsang415 | 17 countries | 12-month | 45% | 31% |
| Von Korff427 | United States | 12-month | 20% | 18% |
| Wijnhoven442 | Netherlands | 12-month | 49% | 41% |
NOTE. Bolded numbers reflect significant sex differences in prevalence.
Blyth et al did not indicate the significance of the difference.
Back Pain
Several investigators have examined sex differences in back pain prevalence and severity, including a number of studies in European samples. A higher point-prevalence of back pain was reported in Swedish females (24%) than males (21%), and women reported greater pain severity than men on the SF-36 bodily pain scale.39 Ihlebaek et al192 tested for sex differences in lifetime, 1 year, and point prevalence of low back pain among working persons in two neighboring regions in Norway and Sweden. Females had a higher prevalence of low back pain than males for both areas across all time periods with the exception that males living in the Norwegian region had a higher lifetime prevalence of low back pain. A German study reported the 7-day prevalence was 40% for women versus 32% for men,368 and another found that sex differences in back pain diminished as the time period lengthened (current, F = 39%, M = 35%; 1-year prevalence, F = 77%, M = 75%; lifetime prevalence, F = 86%, M = 85%), and more men reported low ratings of back pain than women.367 A study using a national representative sample from Spain estimated the current prevalence of low back pain as 18% for females and 11% for males.59 Webb et al434 estimated the prevalence of back pain in over 5000 patients from three general practices in the city of Manchester, England. Twenty-five percent of women and 21% of men reported back pain for at least 1 week in the last month. Interestingly, female sex was no longer a significant predictor following adjustment in multivariate models that included age, body mass index, and several socioeconomic variables. No sex differences were found for pain intensity.
Data are available from other regions as well. In a sample of nearly 14,000 adults from a rural region of China, the 1-year prevalence of low back pain was higher among females than in males across all age groups below 60 years of age.23 In a representative random sample from Turkey, the 2-month prevalence of back pain was consistently higher in women than in men in all age groups with the overall values of 17% for females and 14% for males.301 Two recent studies have reported on sex differences in the 12-month prevalence of back pain from Nigeria. Omokhodion304 reported a higher prevalence for males than females (45% and 36%, respectively), whereas a second study found no sex differences in chronic back or neck problems (F = 17% and M = 16%).172 A population-based postal survey study in Australia429 found few sex differences in current, 1-month, and 12- month prevalence of back pain in females (26%, 55%, 70%) versus males (25%, 50%, 68%). Significant sex differences were not found on pain intensity.
Two studies addressed sex differences in the chronicity of back pain. Thomas and colleagues404 followed 180 patients for 12 months after consultation with acute back pain. After 1 year, 41% of females and 24% males were classified as having both low back pain and disability. Other factors associated with persistent back pain included employment dissatisfaction and history of widespread pain. The predictors of poor outcome were the same for men and women. In a population-based cohort of over 2100 participants in a back pain survey, women with chronic back pain at baseline were more likely than men to still have chronic back pain 4 years later.377 However, the association lost significance in a multivariate model that included age, heath history variables, and social factors. Women without back pain at baseline were no more likely to have developed chronic back pain than pain-free men. Thus, on balance, the recent evidence suggests higher prevalence of back pain in women, but there is limited evidence that females are at greater risk for chronicity.
Widespread Pain and Fibromyalgia
Sex differences in the prevalence of widespread musculoskeletal pain have also been documented. These studies typically include a pain drawing to identity the painful sites. The most common definition is pain present in both the left and right side of the body as well as above and below the waist. Multiple studies from various geographic regions indicate higher prevalence rates across all age groups in women compared to men (see Table 2). In contrast, Gupta and colleagues171 reported no sex differences in 15-month incidence of chronic widespread pain (females = 11% and males = 10%). In a 3-year follow-up of a previous study,30 women without chronic pain or women with regional chronic pain did not develop persistence of chronic widespread pain more often than men.31 Another study also failed to show a sex difference in persistence of chronic widespread pain.311
Table 2.
Prevalence of Widespread Pain in Representative Samples
| Study | Country | Prevalence | Female | Male |
|---|---|---|---|---|
| Bergman30 | Sweden | Chronic | 15% | 8% |
| Buskila53 | Israel | Chronic | 14% | 3% |
| Gerdle157 | Sweden | 1-week | 34% | 22% |
| Hardt179 | United States | 1-month | 4% | 3% |
| Thomas403 | United Kingdom | 1-month | 5% | 3% |
| Winjhoven442 | Netherlands | current | 12% | 6% |
| Winjhoven442 | Netherlands | 1-year | 20% | 11% |
| Winjhoven442 | Netherlands | Chronic | 4% | 1% |
NOTE. Bolded numbers reflect significant sex differences in prevalence.
Other studies have specifically screened for fibromyalgia syndrome (FMS). FMS is a common, chronically painful, soft tissue pain condition. Affected individuals exhibit persistent, widespread pain and tenderness to palpation at anatomically defined tender points located in soft tissue musculoskeletal structures.40 Several studies have used self-report of FMS diagnosed by a health care professional and found similar findings in community samples from North America (F = 2%, M < 0.5%),273 the Netherlands (F = 2%, M < 0.5%),315 and Spain (F = 4%, M < 0.5%).59 Another study that was part of the London Fibromyalgia study has used direct evidence from clinical examinations as the case definition and found a point-prevalence among Canadian adults of 4.9% for women and 1.6% for men.440
Osteoarthritis
A recent meta-analysis on sex differences in osteoarthritis using clinical markers as the case definition (not pain) indicated that females are at significantly increased risk for osteoarthritis (OA) in the knee and hand compared with males.382 Several studies have documented sex differences in pain prevalence, ratings, and depression in OA, and we will review selected studies below.
Two papers have reported sex differences in the prevalence of OA related pain (pain on most days for the past 6 weeks) based on representative samples of adults 60 years and older from the United States. The prevalence of persistent knee pain was estimated as 24% for females and 18% for males11 and 16% for females and 12% for hip pain.70 In contrast, data from a community-based sample in the United Kingdom aged 50 years and older found that females in the 65+ group had a lower 12-month prevalence of knee pain than males (F = 22%, M = 33%), but there were no differences in the 50- to 64-year group.234 Another study of individuals registered with three general practices in the United Kingdom found a 12-month prevalence of pain in and around the knee of 49% for females and 44% for males.200
Jinks200 assessed pain severity using the Western Ontario and McMaster University Osteoarthritis Index (WOMAC)27 and found higher ratings of pain for females than for males in the 50- to 64-year and 65- to 74-year age groups whereas pain scores were higher for males in the 75+ age group. Jinks et al201 followed a prospective cohort of 2982 persons without knee symptoms at baseline for 3 years. At follow-up, sex was not a risk factor to develop mild or severe knee pain. However, in a second analysis, females were more likely than males to have developed severe knee pain at 3-year follow-up. Data from an Italian community based cohort also using the WOMAC found females had significantly greater hip and knee pain than males.355
Several studies have examined sex differences in depression among persons with OA. Data from a large sample of German primary care patients indicated that sex was not a predictor of the depression among patients with OA of the hip or knee as diagnosed by a general practitioner.343 This is in opposition to findings that older Chinese women with knee OA tended to have greater depressive symptoms than men, and the association between sex and pain intensity was moderated by depression.414
Taken together, these findings from studies of musculoskeletal pain indicate that regardless of site or time frame, females consistently are more likely to report musculoskeletal pain than males, though these differences may be less consistent for low back pain. There is limited evidence for increased pain intensity among women with the possible exception of OA, where greater pain severity among women is more common. There is limited evidence that women with musculoskeletal pain are more likely to be depressed than men.
Oral Pain
LeResche243 reviewed several studies of temporomandibular joint pain that demonstrated a higher prevalence in women than men across the lifespan. Studies from Finland,322,352 Germany,159 Sweden,267 Turkey,309 the United States,326 Nigeria,302 and Brazil25 have drawn similar conclusions for tooth pain, jaw joint pain, and other orofacial pain conditions. Riley and colleagues326 found that for most orofacial pain symptoms, significant sex differences in pain ratings were found within the middle aged (45- to 64-year-old) cohort but not the older (65+) group.
Abdominal Pain
Unruh418 reviewed several epidemiological studies and concluded that most studies report a higher prevalence of abdominal pain for women. Several recent population-based studies of abdominal pain of unknown etiology generally support increased prevalence among females. For example, data from the Netherlands indicated point-prevalence was higher for women than men,315 whereas no sex difference emerged in a Spanish study.60 Gerdle157 and Bassols24 reported higher female prevalence across longer time periods, and United States estimates indicate higher prevalence of chronic abdominal pain among females.179
Irritable bowel syndrome (IBS) is currently defined as a chronic syndrome characterized by recurring symptoms of abdominal discomfort or pain and alterations in bowel habits in the absence of detectable organic disease.261 Population-based studies have reported a female-to-male ratio of approximately 3:1 in the diagnosis of IBS in populations from the United States.99,357 In Asian countries, the sex differences have been smaller than in Western countries.173,258 Some evidence suggests similar prevalence rates for pain-related symptoms in IBS, but a greater female predominance in non–pain-associated symptoms of constipation, bloating, and extra intestinal symptoms.376,402,406 Lee et al239 also found no sex difference in the prevalence of painful visceral symptoms or severity of patients’ intensity ratings of abdominal discomfort and pain. However, a study of IBS among Japanese university students found that women reported greater abdominal pain than men.374
Headache
Headache is one of the most common pain conditions.308 Unruh418 reviewed findings from over 60 studies and concluded that the prevalence of headaches and migraines is higher for women than men. Migraine is a severe recurring vascular headache and can occur with and without aura. Estimates of the 1-year prevalence of migraine range from 3% to 33% for women and 1% to 16% for men.252 The American Migraine Study II, a study of over 29,000 adults, has estimated the 1-year prevalence of migraine in the United States as 18% in women and 7% in men.253 A meta-analysis suggests that migraine is most common in North and South America, followed by Europe, and lowest in Africa and Asia.365 Although these regions may vary in overall 1-year prevalence79,174,185,210,253,385,399,431 they show similar female-male differences, with the exception of one study from Saudi Arabia90 (see Table 3).
Table 3.
One-Year Prevalence in National Representative Samples of Migraine Headaches
| Study | Country | Female | Male |
|---|---|---|---|
| Dahlof79 | Sweden | 17% | 10% |
| Deleu90 | Saudi Arabia | 6% | 5% |
| Hagen174 | Norway | 16% | 8% |
| Henry185 | France | 11% | 4% |
| Kececi210 | Turkey | 17% | 8% |
| Lipton253 | United States | 18% | 7% |
| Lipton251 | United States | 17% | 6% |
| Steiner385 | England | 18% | 8% |
| Takeshima399 | Japan | 9% | 2% |
| Wang431,* | China | 5% | 1% |
NOTE. Bolded numbers reflect significant sex differences in prevalence.
Older population (65 years and older).
Tension-type headache is the most common form of headache.392 A recent comprehensive review of headaches summarized prevalence by sex for current and lifetime tension-type headache.392 For both sexes, the prevalence peaks between the ages of 30 and 39 years. Unlike for migraine, women (current = 44%, lifetime = 49%) are only slightly more affected than men (37%, 42%). Several studies have disaggregated tension-type headache into episodic and chronic and report a similar female-male ratio for prevalence, with women being at significantly greater risk.188,349,370 Thus, the headache literature consistently shows increased prevalence of headaches and migraine among women.
Pain in Children and Adolescents
The epidemiology of chronic pain in children has been reviewed by McGrath,272 but differences between boys and girls were only briefly discussed. We will review several large studies that compared the prevalence of headaches, musculoskeletal pain, and abdominal pain in children and adolescents.
There is considerable literature on headaches in children. Migraine begins earlier in males than in females, with peak onset between ages of 5 and 10 years and 12 and 17 years, respectively, but new cases of migraine were uncommon in men once they reach their twenties.390 Before puberty, the prevalence of migraine is higher in boys than in girls; however, as adolescence approaches, incidence and prevalence increase more rapidly in girls than in boys.390 Data from the American Migraine Study II estimated that among adolescents 12 to 17 years of age, 7% of girls and 5% of boys reported at least one severe migraine headache in the previous 12 months.253 A meta-review by Stovner and colleagues392 placed the mean point-prevalence for migraine among children/adolescents at 9% for females and 7% for males.
Kroner-Herwig and others231 assessed the distribution and characteristics of headache in German children aged 7 to 14 years using International Classification of Headache Disorders-II criteria to classify headaches into migraine or tension-type. They found that, similar to migraines, boys have an earlier onset to nonmigraine headache than girls. The prevalence of nonmigraine headache was similar for girls and boys of elementary school age years with increasing prevalence for girls during adolescence. There were no significant differences between girls and boys regarding type of headache; however, they did find that girls experience recurrent headaches more than boys. Other studies, however, find the prevalence of tension-type headaches to be higher in girls. For example, in a sample of children from Sweden, Laurell et al236 reported the 1-year prevalence of tension-type headache among girls as 12% and 8% for boys. The prevalence increased with age for both sexes with greater increases for girls. By 13 to 15 years of age, the prevalence was 21% for girls and 9% for boys.
Several authors have reported pain prevalence for multiple sites within the same sample and allow a less biased opportunity to compare the magnitude of sex differences across pain sites. A nationwide study of Swedish students in grades 3, 6, and 9 compared the 7-day prevalence of headache, abdominal, and musculoskeletal pain.49 Girls were more than twice as likely as boys to suffer from headaches (17%, 8%). Abdominal pain was experienced weekly by 10% of the girls and 5% of the boys with sex differences significant only in grades 6 and 9. There were no sex differences for musculoskeletal pain, but prevalence increased with age for girls.
A study of third- and fifth-grade children in Finland found that 32% reported a weekly musculoskeletal pain with significantly more girls reporting pain in chest (7%, 4%) and in the upper back (8%, 5%) compared with boys.278 Sex-related differences were not found for the lower back and neck pain. The pain-free children were reassessed 1 year later, and new-onset nontraumatic musculoskeletal pain was reported in 23% of the girls compared with 16% of the boys.110 There was no sex difference in traumatic-related musculoskeletal pain. Also, females developed pain at multiple sites more often than boys.
A study of more than 700 German school children aged 10 to 18 years old also found that sex differences were increased among adolescents.347 The 3-month prevalence of any pain was significantly higher for girls than for boys at both the 13- to 15-year (F = 98%, M = 92%) and 16- to 18-year (F = 93%, M = 76%) age groups, whereas there was no sex difference among 10- to 12-year-olds (F = 78%, M = 76%). Headache and back pain followed the same pattern, but significant differences between girls and boys only occurred in the oldest group for abdominal pain. There was no sex difference in the duration of any pain symptom at any age group.
Other studies have examined sex differences in chronic pain among children and adolescents. In a study examining the prevalence of chronic pain in a sample of Dutch children (up through 18 years of age), the overall prevalence was 30% in girls compared with 20% in boys.313 Chronic pain increased with age, and sex differences began to appear between 12 and 14 years of age. Girls also rated their chronic pain as more intense on a VAS than boys, but the ratings were not different for non\-chronic pain. Among schoolchildren ages 8 to 16 years living in Catalonia, Spain, the overall prevalence of chronic pain was higher for girls than for boys (47%, 29%).191 Chronic pain at multiple sites was more common among girls than boys (50%, 22%), but lower limb chronic pain was more common among boys than girls (57%, 20% respectively). No sex differences were found for any of the other locations. Petersen et al314 examined pain in schoolchildren ages 6 to 13 living in Sweden. Sex differences in recurrent pain, defined as pain occurring more than once a week for 6 months, were not found for headache, stomach-ache, or backache. However, girls had a higher prevalence of multiple weekly pain symptoms than boys. The most consistent finding across the studies of pain in children and adolescents reviewed above is that sex differences emerge or become larger around puberty.245
Post Procedural Pain
Surgery and other invasive procedures are accompanied by acute pain, and some surgical procedures confer substantial risk for the development of chronic pain.214 Several studies have reported on sex differences in acute pain following a variety of surgical procedures (see Table 4). Unfortunately, there is little standardization in the pain measures used or the time frame for assessing postoperative pain. There are no population level studies of postoperative pain; consequently, we review selected studies in clinical populations.
Table 4.
Sex Differences in Postoperative Pain and Procedural Pain
| Study Author | Surgical Procedure | Sample Size (F,M) | Time/Pain Measure, (Sex Difference) |
|---|---|---|---|
| Chia67 | Outpatient surgery | 1444, 854 | VAS day 1 (F = M); D2 (F < M); D3 (F = M) |
| De Cosmo82 | Cholecystectomy | 49, 31 | 24 h post: VAS rest, F > M, VAS, cough F > M |
| Lau235 | Endoscopic hernia repair | 18, 491 | VAS rest, days 1, 3–5 (F > M), D2 (F = M); VAS cough D1–5 (F > M) |
| Lee241 | Colonoscopy | 431, 569 | 24 h post. Rated as mild, moderate, severe (F > M) |
| Liem250 | Hernia repair | 48, 944 | chronic pain 1-y post (F = M) |
| Mattila269 | Outpatient surgery | 1394, 750 | Week post surgery. VRS pain (F > M); headache (F > M); backache (F > M) |
| Mattila269 | Outpatient surgery | 238, 320 a | Week post surgery. VRS headache (F > M) |
| Nikolajsen297 | Total hip arthroplasty | 294 (total) b | 12–18 mo post. Chronic pain (F = M); NRS (F = M); Constant pain (F > M); Disabling pain (F = M) |
| Ritter329 | Knee arthroplasty | 4379, 2947 | Pre-post. 0–100 VAS (F > M) |
| Rosseland346 | Knee arthroscopic repair | 86, 133 | 2 h post. 5-point VRS (F > M), 0–100 VAS (F = M) |
| Rosseland345 | Knee arthroscopic repair | 46, 54 | 1 year post. Prevalence of pain (F = M) |
| Smith378 | ICD implant | 60, 180 | 1 day post. Pain severity (F > M) |
| Taenzer398 | Knee arthroscopic repair | 186, 230 | VAS day 1 (F > M), days 2, 3 (F = M) |
| Uchiyama417 | Cholecystectomy | 54, 46 | VAS 24 h post (F > M) VAS 48 h post (F > M) |
| Vetrhus425 | Gallbladder removal | 214, 49 | 60 mo post. Prevalence (F = M) |
Abbreviations: VAS, visual analogue scale; NRS, numerical rating scale; VRS, verbal rating scale.
Key: = children,
= Male/female composition not reported.
Two large studies of postoperative pain following out-patient general surgery found conflicting results. Chia and colleagues67 investigated the influence of patient characteristics on postoperative pain at rest and pain on movement in a large sample of Chinese patients. Male sex was associated with increased postoperative pain and morphine requirements. However, a study of 2732 outpatients at a hospital in Finland that included children and adults found that females were more likely to have pain immediately after surgery than males.269 Likewise, greater pain among women has been reported in heterogeneous surgical populations.16,62 These studies included multiple surgical procedures, which often differ across sex (eg, gynecologic surgery vs prostate surgery), which could contribute to sex differences in postoperative pain.
Mixed findings are found in studies of orthopedic surgery. A study that assessed pain in patients who underwent arthroscopic anterior cruciate ligament (ACL) reconstruction at an outpatient facility in the United States did not find sex differences in pain at the immediate postoperative evaluation.398 However, on the first day after surgery, females had higher mean VAS pain scores than males. Rosseland345,346 assessed pain immediately after and 1-year after an arthroscopic ACL procedure. Two hours after the procedure, 84% of the females reported at least moderate pain compared with 57% for men. There were no sex differences on the VAS among those with pain. One year later, there also was no sex difference in pain ratings. However, more females (33%) reported reduced activities of daily living due to pain compared with males (15%). Ritter et al329 followed a large sample of patients that received a total knee arthroplasty for 5 years. Men reported less pain than females before and at all time points after surgery, but the pre-post change in pain did not differ between men and women for any time period. Nikolajsen and others297 found no difference between men and women in the prevalence or intensity of chronic hip pain in 1231 patients who had undergone total hip arthroplasty 12 to 18 months previously.
Mixed findings are also reported for gastrointestinal procedures. Vetrhus425 found that 27% of patients that underwent gallbladder surgery had pain 60 months later. There was no difference in the percentage of males or females reporting pain; although females were more likely than males to report the pain as diffuse. A study of acute pain following endoscopic hernioplasty found that pain scores at rest were significantly higher in females than males.235 Two studies of postoperative pain after cholecystectomy both indicated that female patients had higher VAS pain scores than males.82,417 Females undergoing colonoscopy reported greater abdominal than men.241 Liem et al250 found that sex was not associated with chronic pain 1 year after a laparoscopic hernia repair. Thus, acute postprocedural pain shows a tendency toward greater intensity among females.
This review of recent clinical and epidemiologic findings generally indicates that women are at increased risk for many chronic pain conditions, and women tend to report higher levels of acute procedural pain. These sex differences appear smaller (or nonexistent) in children and appear to emerge or increase in magnitude during adolescence. Inevitably, these sex differences in clinical pain are driven by multiple biopsychosocial factors, which will be discussed below. We have previously suggested that sex differences in nociceptive processing, which would be manifested in responses to experimentally induced pain, represent one potentially important contributing factor. Next, we will review recent findings regarding sex differences in experimental pain sensitivity.
Sex Differences in Responses to Experimental Pain
Multiple studies have examined sex differences in experimentally induced pain, and previous qualitative and quantitative reviews by members of our research group concluded that women display greater sensitivity to multiple pain modalities compared with men.127,327 The current review will extend the findings from these reviews by examining a representative sample of studies published since that time. Sex differences in experimental pain have been evaluated using a wide range of stimulus modalities including pressure, electrical, ischemic, thermal, and other models of experimental pain (eg, chemical). Dynamic models of experimental pain have been used to engage systems underlying summation and inhibition of pain. Pain sensitivity has been assessed by a number of different outcome measures including behavioral indices of threshold (defined by time or intensity to the first sensation of pain) and tolerance, and self-report measures of pain intensity and unpleasantness. Previous reviews have concluded that females are more sensitive to pain compared with males.127,327 The following review will determine whether more recent studies continue to support this conclusion.
Pressure Pain Stimuli
The results from 9 studies that examined sex differences in experimental pressure pain are presented in the upper portion of Table 5. In the meta-analysis by Riley et al,327 pressure pain was determined to produce the largest sex difference. The studies published since that time support the conclusions of the meta-analysis. Females showed lower pain threshold and tolerance compared with men with the exception of one study295 in females had lower pain thresholds than males, but this difference was not significant, likely due to the sample size (12 F, 12 M). One study found that females provided higher ratings of suprathreshold pressure pain than males, with the sex difference increasing in magnitude with greater stimulus intensity.111 In summary, the recent literature continues to provide strong support for the hypothesis that females are more sensitive to pressure pain.
Table 5.
Studies Examining Sex Differences in Pressure, Electrical, and Ischemic Experimental Pain Models
| Authors | Sample Size (M/F) | Stimulation Site | Method | Threshold* | Tolerance* | Ratings† |
|---|---|---|---|---|---|---|
| Pressure pain | ||||||
| Ayesh et al19 | 24/19 | F | PA | F = M | M > F | — |
| Chesterton et al66 | 120/120 | H | PA | M > F | — | — |
| Ellermeir and Westphal 111 | 18/18 | FNG | PA | — | — | F > M |
| Fillingim et al123 | 39/49 | T, M, U | PA | M > F | — | — |
| Fillingim et al132 | 39/61 | T, M, U | PA | M > F | — | — |
| Garcia et al141 | 12/18 | TP | PA | M > F | — | — |
| Komiyama and De Laat 227 | 16/16 | M, H | PA | M > F | M > F | F = M |
| Komiyama et al228 | 44/44 | M, H, L | PA | M > F | M > F | F > M |
| Nie et al295 | 12/12 | H | CCPS | F = M | — | F = M |
| Electrical pain | ||||||
| al’ Absi et al1 | 59/40 | FA | ES | M > F | M > F | — |
| Ashina et al14 | 9/12 | T, TM | ES | M > F | M > F | — |
| Ayesh et al19 | 24/19 | F | ES | F = M | — | — |
| Nyklicek et al298 | 26/23 | FA | ES | M > F | M > F | — |
| Ischemic pain | ||||||
| Bragdon et al45 | 22/20 | A | SETT | F = M | F = M | — |
| Edwards et al107 | 83/115 | A | SETT | F = M | F = M | — |
| Fillingim and Maixner 128 | 25/23 | A | SETT | F = M | M > F | F = M |
| Fillingim et al123 | 39/49 | A | SETT | F = M | F = M | — |
| Fillingim et al132 | 39/61 | A | SETT | F = M | F = M | — |
| Girdler et al161 | 40/37 | A | SETT | M > F | M > F | F = M |
Abbreviations for stimulation sites: A, arm; F, face; FA, forearm; FNG, finger; H, hand; L, Leg; M, masseter muscles; T, trapezius muscles; TM, temporal muscle; U, Ulna.
Abbreviations for methods: CCPS, computer controlled pressure stimulator; ES, electrical stimulation; PA, pressure algometry; SETT, submaximal effort tourniquet test.
Lower levels of threshold and tolerance in females indicate greater pain sensitivity.
Higher subjective ratings in females indicate greater pain sensitivity.
Electrical Pain Stimuli
The results from 3 studies that examined sex differences in perceptual responses to electrical pain are presented in the middle portion of Table 5. Pain threshold and tolerance for electrical stimuli were significantly lower in healthy women compared with men. Even though electrical pain was reported for only 3 studies, it strongly favors the hypothesis that women are more sensitive to this pain modality in comparison to men. These recent findings present a more consistent picture than the 5 studies reviewed by Riley et al,327 who found that electrical stimuli produced inconsistent findings and a moderate effect size for the sex difference.
Ischemic Pain Stimuli
The results from 7 studies that examined sex differences in experimental ischemic pain are presented in the lower portion of Table 5. Studies used several variations of the submaximal effort tourniquet test to induce ischemic pain. Overall, a majority of the studies reported no sex differences in threshold (6 studies), tolerance (5 studies), or pain ratings (2 studies) to ischemic pain. Two studies reported that males displayed higher pain threshold and tolerance.128,161 Despite large sample sizes for several studies,107,124,132,133,161 sex differences in ischemic pain have not been statistically significance due to their small effect sizes.
Heat Pain Stimuli
The results from 22 studies that examined sex differences in experimental heat pain are presented in the upper portion of Table 6. All of the studies used some form of contact heat with the exception of one study that used hot water immersion.360 Also, the forearm was the most common site for stimulus application. The vast majority of studies reported that females were more sensitive to heat pain than males. For the studies examining behavioral measures of heat pain sensitivity, 81% (12/17), and 94% (15/16) of the studies reported lower thresholds and tolerances, respectively, in females. Females were also found to rate heat pain as more intense and unpleasant in the majority of studies that included a suprathreshold protocol (7/9, 78%), and one study found that females required lower temperatures to evoke moderate pain.411 Overall, the hypothesis that heat pain sensitivity differs as a function of sex has been supported.
Table 6.
Studies Examining Sex Differences in Thermal Experimental Pain Models
| Authors | Sample Size (M/F) | Stimulation Site | Method | Threshold* | Tolerance* | Ratings† |
|---|---|---|---|---|---|---|
| Thermal pain – heat | ||||||
| al’ Absi et al5 | 15/11 | FA | CH | M > F | M > F | F > M |
| Bragdon et al45 | 22/20 | A | CH | M > F | M > F | — |
| Edwards et al105 | 28/51 | FA | CH | M > F | M > F | — |
| Edwards et al107 | 83/115 | FA | CH | M > F | M > F | — |
| Fillingim and Maixner128 | 25/23 | FA | CH | M = F | M = F | F > M |
| Fillingim et al130 | 22/27 | FA, F | CH | M = F | M > F | M = F |
| Fillingim et al121 | 92/117 | FA | CH | M > F | M > F | — |
| Fillingim et al126 | 20/20 | FA | CH | M > F | M > F | — |
| Fillingim et al123 | 39/49 | FA | CH | M > F | M > F | — |
| Fillingim et al132 | 39/61 | FA | CH | M > F | M > F | — |
| George et al156 | 16/17 | A | CH | M = F | M > F | — |
| Girdler et al161 | 40/37 | A | CH | M > F | M > F | — |
| Jensen and Petersen198 | 44/41 | FA | CH | M = F | — | F > M |
| Jones et al205 | 69/75 | FA | CH | M = F | M > F | — |
| Kim et al223 | 194/306 | FA | CH | — | — | F > M |
| Kim et al222 | 248/369 | FA | CH | — | — | F > M |
| Nielsen et al296 | 78/110 | FA | CH | — | — | M = F |
| Paulson et al312 | 10/10 | FA | CH | — | — | F > M |
| Sarlani et al360 | 20/20 | HWI | — | — | F > M | |
| Thompson et al405 | 37/88 | H | CH | M > F | M > F | — |
| Tousignant et al412 | 42/41 | H | CH | — | — | F > M3 |
| Wise et al444 | 61/87 | FA | CH | M > F | M > F | — |
| Thermal pain – cold | ||||||
| al’ Absi et al4 | 31/34 | H | CPT | — | — | F > M |
| al’ Absi et al3 | 72/80 | H | CPT | — | — | F > M |
| al’ Absi et al5 | 15/11 | H | CPT | — | — | F > M |
| Baad-Hansen et al20 | 20/34 | H | CPT | — | — | M = F |
| Dixon et al95 | 91/112 | H | CPT | — | M > F | M = F |
| Edwards et al107 | 83/115 | H | CPT | M > F | M > F | — |
| George et al154 | 32/34 | H | CPT | M > F | M > F | — |
| Jackson et al195 | 34/57 | H | CPT | — | M > F | F > M |
| Jones et al205 | 69/75 | H | CPT | M = F | M > F | F > M |
| Keogh et al219 | 50/50 | H | CPT | M = F | M > F | — |
| Keogh et al216 | 31/31 | H | CPT | M > F | — | — |
| Kim et al223 | 194/306 | H | CPT | — | M > F | F > M |
| Kim et al222 | 248/369 | H | CPT | — | M > F | F > M |
| Lowery et al257 | 42/39 | H | CPT | M > F | M > F | F > M |
| Mitchell et al282 | 20/24 | H | CPT | — | M > F | M = F |
| Myers et al290 | 54/50 | H | CPT | M > F | M > F | — |
| Nielsen et al296 | 78/110 | H | CPT | F > M | ||
| Pud et al320 | 15/19 | H | CPT | M = F | M = F | F > M |
| Sarlani et al360 | 20/20 | H | CPT | — | — | F > M |
| Thompson et al405 | 37/88 | H | CPT | M > F | M > F | — |
| Tousignant et al412 | 42/41 | H | CPT | F > M | ||
| Weisenberg et al436 | 40/40 | A | CPT | — | M > F | F > M |
| Zimmer et al455 | 39/37 | H | CPT | — | M > F | M = F |
Abbreviations for stimulation sites: A, arm; F, Face; FA, forearm; H, hand.
Abbreviations for methods: CH, contact heat; CPT, cold water test; HWI, hot water immersion.
Lower levels of threshold and tolerance in females indicate greater pain sensitivity.
Higher subjective ratings in females indicate greater pain sensitivity.
Females required a lower temperature to produce moderate pain
Sex differences were not universal across all heat pain measures within a given study. For example, in a secondary review of unpublished and published studies, Jensen and Petersen198 noted that heat pain thresholds were comparable between males and females, but females reported higher peak pain produced by a prolonged 45.0°C (1 minute) stimulus. The authors also mentioned that the total pain as indicated by area-under-the-curve was larger in females but failed to reach statistical significance. Two studies156,205 reported sex differences for tolerance but not thresholds, whereas others reported equivalent ratings of heat pain in males and females, and differences were observed with lower thresholds and tolerance in females.128,405
Cold Pain Stimuli
The results from 22 studies that examined sex differences in experimental cold pain are presented in the lower portion of Table 6. Most studies have used some form of the cold pressor test in which subjects immerse their arm or hand in circulating cold water for a defined period of time, and their results support the hypothesis that cold pain sensitivity is more pronounced in females. Sex differences in cold pain were observed in 67% (6/9) of studies reporting cold pain threshold, 93% (14/15) of studies reporting cold pain tolerance, and 81% (13/16) of studies reporting continuous or retrospective subjective pain ratings to cold water immersion. Overall, each study reported sex differences in at least one pain outcome, but, similar to heat pain, discrepancies among pain outcomes occur between indices of cold pain within studies. For example, Jones et al205 observed sex-related differences for cold pain tolerance but not threshold. Based on the present set of studies, it appears that sex differences in cold pain are consistent, particularly for suprathreshold measures such as tolerance and pain ratings.
Sex-Related Differences in Dynamic Models of Experimental Pain
A number of investigators have used more dynamic models of pain to evaluate sex differences. One could argue that such pain assays, including temporal summation of pain and tonic pain induced via intramuscular administration of chemical stimuli, may provide more clinically relevant information. These studies generally support the conclusion that sex differences will be more robust with a painful stimulus that produces a deep, tonic sensation of pain.127 Given the recency of these studies, they were not included in the previous meta-analysis327 but will be reviewed below.
Temporal Summation of Pain
The results from 4 studies that examined sex differences in temporal summation of heat pain are presented in the upper portion of Table 7. Temporal summation of heat pain is a commonly used to evaluate differences in the central processing of nociceptive signals (eg, temporal integration of pain). In this model, brief painful heat pulses are repetitively delivered to the skin at intervals at or less than 3 seconds. The temporal response to repetitive thermal stimuli is characterized as a gradual increase in subjective pain ratings associated with C-fiber input (eg, second pain) but not Aδ fiber input (eg, first pain).130,318,338 Females exhibit a more pronounce temporal summation of heat pain,130,338,363 though one study revealed no sex differences.384 Although the authors did not speculate about this observation, differences in testing methodology (eg, preheated thermode with intermittent contact versus Peltier-based thermode) and small sample size may have contributed to the lack of sex-related effects. In addition, sex differences in temporal summation could be influenced by a number of psychological factors. For example, Robinson et al338 reported that sex differences in temporal summation of heat pain were mediated by gender roles and anxiety. Temporal summation of mechanical pain has also been more robust among females than males in most studies,361–363 with one exception.295 Again, differences in methodology (eg, temporal summation of cutaneous vs deep tissues) and site of stimulation (eg, temporal summation of hand vs leg) may be responsible for the discrepant results.
Table 7.
Studies Examining Sex Differences in Experimental Pain Models of Temporal Summation
| Authors | Sample Size (M/F) | Stimulation Site | Method | Summation |
|---|---|---|---|---|
| Temporal summation – heat | ||||
| George et al156 | 16/17 | A | CH | F > M |
| Fillingim et al130 | 22/27 | FA, F | CH | F > M |
| Robinson et al338 | 30/37 | H | CH | F > M |
| Staud et al384 | 11/22 | H | CH | F = M |
| Temporal summation – mechanical | ||||
| Nie et al295 | 12/12 | L, H | CCPS | F = M |
| Sarlani and Greenspan363 | 10/10 | Fng | CCPS | F > M |
| Sarlani et al362 | 25/25 | Fng | CCPS | F > M |
| Sarlani et al361 | 36/27 | Fng | CCPS | F > M |
| Spatial summation | ||||
| Lautenbacher et al238 | 20/20 | FA | CH | F = M |
| Martikainen et al268 | 6/14 | H | CPT | F = M |
| Defrin et al89 | 12/12 | L | CH | F = M |
Abbreviations for stimulation sites: A, arm; F, face; FA, forearm; Fng, finger; H, hand; L, leg.
Abbreviations for methods: CH, contact heat; CPT, cold water test; CCPS, computer controlled pressure stimulator; HWI, hot water immersion.
Finally, sex differences in temporal summation have also been demonstrated in clinical samples. Two studies reported that sex differences in temporal summation to heat156 and mechanical361 stimulation persisted in clinical pain populations with low back pain and temporomandibular disorders, respectively. Thus, on balance, the evidence supports the conclusion that temporal summation is greater among females than males.
Spatial Summation
Table 7 also presents information about 3 studies that examined sex differences in spatial summation. Unlike temporal summation, no differences have been observed between males and females with spatial summation of heat pain88,238 or cold pain.268 Lack of sex differences might be related to the fact that spatial summation was either measured or reflected only for pain threshold in these studies, and sex differences in suprathreshold measures are often more robust than for threshold. Moreover, sample sizes were relatively small in all of these studies, which may have reduced the ability of these studies to detect sex differences. Nonetheless, the currently available data suggest no sex differences in spatial summation of pain, though additional studies with larger sample sizes would increase confidence in this conclusion.
Capsaicin
Responses to the TRPV1 agonist capsaicin have been compared across sexes, and three of these five studies reveal significant sex-related differences in subjective pain ratings, suggesting higher sensitivity in females (Table 8). These sex differences may be due to increased activation of C-fibers among women, as vasomotor responses (eg, axon flare) produced by capsaicin were more pronounced in females.143 Also, sex differences in capsaicin pain appear to be dependent on the menstrual cycle. Females reported less pain during the luteal versus the follicular phase.143 Jensen and Petersen198 used a model with both heat and capsaicin found that females exhibited greater dynamic tactile allodynia but responded similarly to males in subjective ratings of capsaicin pain and secondary hyperalgesia. In response to intraoral capsaicin, women using oral contraceptives provided lower pain ratings than men, whereas normally cycling women and men did not differ.20 Thus, capsaicin-evoked pain does not differ consistently across sex.
Table 8.
Studies Examining Sex Differences in Experimental Models of Chemical and Muscle Pain
| Authors | Sample Size (M/F) | Stimulation Site | Chemical | Pain Ratings |
|---|---|---|---|---|
| Capsaicin pain | ||||
| Baad-Handen20 | 20/34 | IO | CAP | F < M* |
| Gazerani et al143 | 14/14 | F | CAP | F > M |
| Gazerani et al144 | 14/14 | FH, FA | CAP | F > M |
| Frot et al138 | 10/10 | CK, ANK | CAP | F > M |
| Jensen and Petersen198 | 44/41 | FA | CAP | F = M |
| Muscle pain (glutamate and hypertonic saline) | ||||
| Cairns et al54 | 18/20 | M | GLU | F > M |
| Cairns et al55 | 11/13 | M | GLU | F > M |
| Falla et al113 | 9/9 | T | HS | F > M |
| Gazerani et al145 | 15/15 | FH | GLU | F > M |
| Ge et al147 | 11/10 | T, PN | HS | F > M |
| Ge et al146 | 10/9 | T | GLU | F > M |
| Ge et al148 | 14/14 | T | GLU | F > M |
| Ge et al149 | 15/15 | T, PN | HS | F > M |
| Svensson et al396 | 18/17 | M | GLU | F > M |
Abbreviations for stimulation sites: ANK, ankle; CK, cheek; F, face; FA, forearm; FH, forehead; M, masseter muscles; IO, intraoral; PN, posterolateral neck muscles; T, trapezius muscles.
Abbreviations for methods: Cap, Capsaicin; GLU, glutamate; HS, hypertonic saline.
For women using oral contraceptives only.
Hypertonic Saline and Glutamate Muscle Injections
Table 8 also presents data from studies evaluating the effect of intramuscular injections of glutamate and hypertonic saline. Since muscle pain is more prevalent in females, these experimental models may provide clinically relevant information regarding sex differences in pain. Fillingim and Maixner127 suggested that differences in pain sensitivity between men and women would occur most consistently with nociceptive stimuli associated with deep, tonic pain since these stimuli imitate sensations of pain an individual experiences naturally. All nine of the identified studies involving intramuscular injections of glutamate and hypertonic saline found that females reported more pain than males, providing strong support for the hypothesis that females are more sensitive to muscle pain.
Sex Differences in Physiological Measures of Experimental Pain
The studies reviewed above have examined sex differences in self-reported pain, which may be influenced by reporting biases or differences in interpretation or application of pain scales. However, several studies have also examined sex differences in physiological responses to pain, such as pupil dilation,111 muscle reflexes, and cerebral activation.
RIII Reflex
The RIII reflex is a spinal nociceptive reflex detected in the biceps femoris muscle following electrical stimulation of the sural nerve. Three studies observed lower thresholds to elicit the RIII response in females1,137,371 in addition to two studies reporting greater pain ratings after stimulation of the reflex in females.1,137 Two studies reported no sex differences in the RIII reflex,2,136 but one of these studies was conducted in patients with osteoarthritis pain, which may have influenced the results.136
Brain Imaging
Differences in cerebral activation between males and females have been evaluated in a number of studies. Studies have examined brain activity through a number of methods including positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) in response to different pain modalities. Given the reported sex differences in experimental pain perception, the use of neuroimaging is a promising tool that may reveal sex differences in central representations of pain. Table 9 lists several studies that have compared the central processing of pain between males and females. These studies have revealed a number of common cortical and subcortical areas activated by pain, but they have also observed sex-specific areas of activation. This suggests sex differences in central processing of nociceptive information.
Table 9.
Studies Examining Sex Differences in Brain Activation to Experimental Pain
| Authors | Sample Size (M/F) | Imaging Method | Pain Stimulus | Findings |
|---|---|---|---|---|
| Berman et al34 | 7/6 | fMRI | Visceral pressure | M > F: Insula F > M: deactivation in amygdala, mid-cingulate |
| Derbyshire et al91 | 11/10 | PET | Heat (laser) | M > F: Contra PFC, S2, S1, insula F > M: Ipsi perigenual & ventral cingulate |
| Henderson et al184 | 11/11 | fMRI | Hypertonic Saline | M > F: Cerebellar cortex F > M: Mid-cingulate, DLPFC |
| Hobson, et al189 | 8/8 | MEG/EP | Esophageal Electrical | No sex differences |
| Moulton et al285 | 11/17 | fMRI | Contact Heat | F > M: Deactivation in S1, lt anterior insula, DLPFC |
| Naliboff et al292 | 19/23* | fMRI | Visceral pressure | M > F: Rt. DLPFC, Insula, PAG F > M: Lt. VMPFC, rt. ACC, lt. amygdala |
| Paulson et al312 | 10/10 | PET | Heat (contact) | F> M: Contra PFC, Insula, thalamus |
| Straube et al395 | 12/12 | fMRI | Electrical | F > M, MPFC M > F, IC |
Abbreviations for imaging methods sites: fMRI, functional magnetic resonance imaging; MEG/EP, magnetoencephalography/evoked potentials; PET, positron emission tomography.
Abbreviations for findings (F > M, female > male): ACC, anterior cingulated cortex; DLPFC, dorsolateral prefrontal cortex; PFC, prefrontal cortex; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; VMPFC, ventromedial prefrontal cortex.
Two PET studies reported that males and females process thermal pain differently, though both the methodology and the pattern of results differed substantially between studies. Paulson et al312 found that females reported more pain than males in response to a 50 °C contact heat stimulus, with an accompanying higher level of activation in the contralateral thalamus and anterior insula. Derbyshire et al91 tailored a laser heat stimulus to produce comparable pain levels in men and women and found that males showed greater activation in some brain regions (eg, parietal cortex, SII, PFC, insula), whereas females showed greater activation in others (eg, perigenual cortex, cingulate cortex). A more recent study of using fMRI to determine cerebral activation to contact heat reported sex differences in several brain regions, including the somatosensory cortex, insular cortex and dorsolateral prefrontal cortex (DLPFC).285 Interestingly, rather than sex differences in activation, these authors found that the sex differences were due to greater deactivation in these brain regions among females. Others have reported sex differences in cerebral responses to aversive visceral pressure among patients with IBS292 and healthy controls,34 whereas a study using magnetoencephalography and cortical-evoked potential found no sex differences in brain responses to electrical stimulation of the esophagus.189 Overall, these studies suggest that there may be sex differences in cerebral responses to painful stimulation; however, the pattern of results varies across studies, likely due to differences in the stimulation method and in the approaches to brain imaging. Clearly, additional studies are needed to further characterize differences in pain-related brain activity between males and females.
Endogenous Pain Modulation
The experience of pain is endogenously modulated, and several experimental approaches to engaging pain modulatory systems are reviewed in this article. One experimental model of endogenous inhibition is diffuse noxious inhibitory controls (DNIC). Other forms of pain inhibition have included stress-induced analgesia (SIA), in which a laboratory stressor is used to reduce experimental pain, and exercise-induced analgesia. Finally, limited research has addressed sex differences in placebo analgesia. Assuming that basal pain sensitivity may reflect the activity of endogenous pain modulatory systems, it would be expected that females and males may also differ in endogenous pain modulation. The available evidence addressing this possibility is reviewed below.
Diffuse Noxious Inhibitory Controls
Diffuse noxious inhibitory controls (DNIC) refers to a form of endogenous pain modulation in which the perception of one painful stimulus (the test stimulus) is attenuated by a heterotopically applied conditioning stimulus at a remote site. Some investigators have speculated that DNIC may be of substantial clinical relevance, because dysfunction in endogenous pain inhibitory systems is believed to contribute to certain chronic pain conditions.101,384,453 Sex differences in DNIC have been evaluated in a number of studies (Table 11). Approximately half (6/13, 46%) of the studies suggest that DNIC is more pronounced in males than females, based on psychophysical147,165,321,384,438 and neurophysiological371 responses. In contrast, five studies have found that males and females inhibit pain equally during exposure to a conditioning stimulus.20,106,137,268,319,411 Inconsistent findings across studies may be a consequence of methodological differences among the studies; however, no clear pattern emerges when comparing methodological characteristics of studies that have shown a sex differences to those that have not. Most studies used the cold pressor as the conditioning stimulus, whereas a variety of test stimuli have been used. The timing of the conditioning stimulus may also affect the ability to detect inhibition, with the conditioning stimulus producing greater inhibition when administered before the test stimulus compared with weaker inhibition with concurrent administration.268 Additional factors, including sex-specific psychological mediators, may also contribute to the presence or absence of sex differences in DNIC. For example, Weissman-Fogel et al439 found that sex differences in DNIC were no longer significant after controlling for catastrophizing, which suggests a potential mediating role for psychological factors in sex differences in DNIC.
Table 11.
Clinical Studies Regarding Sex Differences in Analgesic Responses
| Authors | Sample Size (F, M) | Type of Pain | Medication | Findings |
|---|---|---|---|---|
| μ-opioids | ||||
| Aubrun et al16 | 1933, 2344 | Multiple surgeries | iv morphine | F > M morphine dose for analgesia |
| Bijur et al38 | 211, 144 | Acute pain in ER | iv morphine (0.1 mg/kg) | F = M |
| Cepeda and Carr62 | 423, 277 | Multiple surgeries | iv morphine | F > M morphine dose for analgesia |
| Chia et al67 | 1444, 854 | Multiple surgeries | iv morphine (PCA) | F < M morphine consumption |
| Gagliese et al140 | 120, 126 | Multiple surgeries | iv morphine (PCA) | F = M |
| Glasson et al162 | 106, 44 | Cholecystectomy | iv morphine or meperidine | F = M (when weight adjusted) |
| Hirasawa et al187 | 15, 15 | Spine surgery | iv morphine (PCA) | F < M morphine consumption |
| Joels et al202 | 246, 235 | Colectomy | iv morphine or meperidine | F < M opioid consumption |
| Kaiko et al206 | 422, 293 | Cancer Pain | im morphine (8, 16 mg) | F = M |
| Miller and Ernst280 | 22, 24 | Acute pain in ER | iv morphine (2.5–5 mg) | F = M (trend for ↑ analgesia in M) |
| Mixed-action opioids | ||||
| Gear et al153 | 69, 62 | Oral surgery | iv nalbuphine (5, 10, or 20 mg) | F > M analgesia at 5, 10 mg doses |
| Gordon et al164 | 22, 12 | Oral surgery | iv pentazocine (30 mg) | F > M analgesia |
| Miller and Ernst280 | 23, 25 | Acute pain in ER | iv butorphanol (0.5–1 mg) | F = M |
| Ryan et al353 | 16, 12 | Dental surgery | 50 mg pentazocine, 0.5 mg naloxone (oral) | F > M analgesia |
| Other analgesics | ||||
| Averbuch and Katzper17 | 195, 119 | Oral surgery | oral ibuprofen | F = M |
| De Cosmo et al82 | 49, 31 | Cholecystectomy | iv tramadol (PCA) | F > M tramadol consumption |
| Ryan et al353 | 15, 14 | Dental surgery | 600 mg ibuprofen (oral) | F = M |
Conclusions related to sex-related differences in DNIC remain tentative pending additional studies. Future research investigating sex differences in DNIC should attend to characteristics of pain induction (eg, stimulus intensity, duration), stimulation site, and possible role of biological (eg, hormonal, genetics, autonomic) and psychological (eg, anxiety, catastrophizing, coping) mediators.
Other Forms of Endogenous Pain Modulation
Several studies have addressed whether physical or psychological interventions differentially influence pain responses in men versus women, and these studies are summarized in the lower portion of Table 10. The majority of these studies (5/6, 81%) indicate that females exhibit more efficient pain inhibitory responses compared with males. For example, using an isometric handgrip exercise, pressure pain threshold was elevated in females but not males, suggesting that exercise-induced analgesia was greater in females.226 However, sex-related differences in pain modulation may be dependent on the type of stressor and pain modality. For example, in response to a laboratory public-speaking stressor, males exhibited a greater stress-related reduction in heat pain, whereas females showed a greater reduction with ischemic pain.45 Sternberg et al387 reported that cold pain was reduced in males during video game competition, whereas pain was more substantially reduced by physical exercise in females.
Table 10.
Studies Examining Sex Differences in Experimental Models of Pain Inhibition
| Authors | Sample Size (M/F) | Primary Testing Stimulus (Site) | Conditioning Stimulus | Pain Inhibition |
|---|---|---|---|---|
| DNIC | ||||
| Baad-Hansen et al20 | 20/34 | Cap (IO) | CPT (H) | F = M |
| Edwards et al108 | 29/48 | TS-CH (A) | CPT (H) | F = M |
| France and Suchowiecki137 | 39/44 | TS-NFR (L) | ISC (A) | F = M |
| Ge et al147 | 11/10 | HS (T) | HS (Trap) | F < M |
| Granot et al165 | 21/10 | CH (A) | CPT (H) | F < M |
| Martikainen et al268 | 6/14 | CPT (H) | CPT (H) | F = M |
| Pud et al319 | 23/17 | MS (H) | CPT (H) | F = M |
| Quiton and Greenspan321 | 32/30 | CH (L) | E (A) | F < M |
| Rosen et al344 | 15/15 | P, E (Fng, F) | CPT (H) | F = M |
| Serrao et al371 | 16/20 | TS-NFR (L) | CPT (H) | F < M |
| Staud et al384 | 11/22 | TS-CH (H) | HWI (H) | F < M |
| Tousignant et al411 | 42/41 | CH (H) | CPT | F = M |
| Weissman-Fogel et al439 | 19/29 | CH (H) | MP (H) | F < M |
| Miscellaneous | ||||
| al’ Absi et al3 | 72/80 | CPT (H) | PS | F = M |
| Bragdon et al45 | 22/20 | ISC (A), CH (A) | IS | F = M |
| Girdler et al161 | 40/37 | CPT | TSST | F > M |
| Koltyn et al226 | 15/15 | P (Fng) | Exercise | F > M |
| Rhudy et al324 | 20/20 | RH (Fng) | Noise | F > M |
| Sternberg et al387 | 19/22 | CPT (A) | VG, Exercise | F < M VG; F > M Exercise |
Abbreviations for stimulation sites: A, arm; F, face; Fng, finger; H, hand; L, leg; IO, Intraoral; T, Trapezius muscle.
Primary test stimulus: Cap, Capsaicin; CH, contact heat; CPT, cold pressor test; E, electrical stimulation; HS, Hypertonic saline; ISC, ischemic; MS, mechanical stimuli; P, pressure; RH, Radiant heat; TS-CH, temporal summation of contact heat pain; TS-NFR, temporal summation of nociceptive flexion reflex (electrical stimulus). Conditioning stimulus: CPT, cold pressor test; E, electrical stimulation; IS, Interpersonal stressor; ISC, Ischemic; HS, Hypertonic saline; HWI, hot water immersion; MP, muscle pain by physical effort; P, pressure pain; PS, Public speaking stressor; TSST, Trier Social Stress Test; VG, Video game.
NOTE. F > M indicates greater pain inhibition among females than males.
Another form of endogenous modulation that may be sensitive to sex differences is placebo analgesia. This section was not included in Table 10 since only a limited number of studies have addressed this issue. Sex differences in placebo response are often not discussed or reported, although these differences may contribute to the large variability in the magnitude of placebo responses.145,225 Clinical studies have generally reported no sex differences in placebo responses.18,153 Regarding laboratory findings, one study reported that men exhibited a greater increase in cold pain tolerance with placebo compared with females.72 Another study reported greater placebo and morphine responses in females as indicated by increase cold pain thresholds and lower pain ratings compared with men.320 The ability of placebo to reduced pain outcomes in males and females was not different in psychophysical studies comparing alfentanil303 and topical lidocaine334 to placebo. However, all of these studies were conducted in typical “clinical trial” fashion, which can reduce the magnitude of placebo responses.424 One study that included a placebo manipulation demonstrated that males who were informed that a “powerful pain reliever” had been administered showed significant increases in ischemic pain tolerance, but there was no effect in females.135 On balance, these studies of endogenous pain modulation suggest inconsistent sex differences, which may not be surprising given the variety of methods used to engage pain modulatory systems and to assess their effects.
Since our two previous reviews127,327 a large number of studies using widely varying methodologies have investigated sex differences in experimental pain sensitivity. Based on the overall findings, it can be concluded that females are more sensitive to painful stimulation as assessed in the laboratory. From the pattern of results, it is difficult to pinpoint any specific mechanism(s), because the sex differences appear relatively consistently across multiple stimulus modalities. However, the recently developed literature on pain in response to intramuscular injections of algesic substances reveals robust and unanimous differences, suggesting that deep, tonic stimuli that mimic clinical musculoskeletal pain may be particularly sensitive to sex differences. Moreover, the inconsistency of findings from brain imaging studies can be attributed not only to the vast methodological differences across studies but also to their small sample sizes. Sex differences in endogenous pain modulation have received more limited attention, but the available evidence suggests that males and females may differ in this regard as well, though the direction and magnitude of the effects are quite variable. The mechanisms and practical importance of these sex differences merit further investigation in future studies.
Sex Differences in Responses to Pain Treatment
Gender Bias in Pain Treatment
Another important issue to consider is the possibility of sex and gender differences in the context of pain treatment. One topic that has received attention is the possibility of gender bias in the provision of pain treatment. Although the use of both prescription and nonprescription analgesics is significantly higher among women than men,109,115,193 there is concern that women are at greater risk for undertreatment of pain.190 It has been observed that women presenting with chest pain are less likely than men to receive both invasive and noninvasive diagnostic and interventional cardiac procedures,80,339,369 though sex differences in symptom presentation and diagnostic test results may contribute to these disparities in management of chest pain.163,447 A frequently cited study reported that after cardiac surgery, women were more likely than men to be prescribed sedatives, whereas men were more likely to receive analgesics.57 More recently, in the emergency department, women were less likely than men to receive analgesics for abdominal pain.65 In contrast, in the hospital setting, more women than men received analgesics, although differences in the reasons for hospitalization could have contributed.422 It has also been reported that women with temporomandibular disorders were treated more frequently by surgical intervention than men, which may have been due to self-selection or an increased tendency for clinicians to recommend surgery for women.264 Vignette studies have also explored gender biases in pain treatment. Using this approach, Hamberg and colleagues177 found that when the neck pain case was a woman, female and male medical students were more likely to provide nonspecific somatic diagnoses, address psychosocial variables in the history, and to prescribe analgesic and psychoactive medications. Another vignette study showed an interaction between physician and patient sex, in that female physicians prescribed higher doses of opioid pain medication for women than men with low back pain, whereas the reverse pattern emerged for male physicians.437 Subsequently, male but not female physicians were more likely to recommend activity restrictions for female than male medical patients.354 However, nurse anesthetists showed no gender bias in pain treatment in a vignette regarding patients who had undergone orthopedic surgery.78 Thus, while not unanimous, evidence suggests potential gender biases in pain treatment; however, the clinical characteristics of the patient and the sex of the provider may influence the magnitude and direction of the effect.
Sex Differences in Analgesia: Clinical Studies
In addition to gender differences in the provision of pain treatment, some investigators have examined whether females and males respond differently to pain treatment. For example, sex differences in responses to analgesic medications have been explored, and these findings have been reviewed previously by several authors.74,122,221,277 While not a direct measure of analgesic response, studies of self-administration of opioids using patient-controlled analgesia (PCA) have revealed lower postoperative opioid consumption among women than men in several studies, as previously reviewed by Miaskowski and Levine.277 Of course, this lower opioid consumption among women could be driven by factors other than pain relief, such as increased adverse effects, which have been well documented among females.38,63,132,457 Additional findings emerging since that review provide a mixed picture of sex differences in opioid analgesia (see Table 11). Lower postoperative opioid consumption among women versus men has been reported in several studies.67,187,202 One study indicated lower opioid consumption among women for lower abdominal surgery, but opioid use was similar across sexes for all other surgical subtypes.64 Other PCA studies have reported no sex differences in opioid consumption.202,140 Others have indicated higher opioid requirements to achieve pain relief when medication was administered by providers rather than via PCA.16,62 More recently, women were found to consume significantly more tramadol, a weak μ-opioid, than men after cholecystectomy, and women also reported greater postoperative pain.86 In multivariate analysis, the sex difference in pain remained significant, whereas the sex difference in tramadol consumption did not. Other studies of clinical pain have reported no sex differences in morphine analgesia for cancer pain,206 acute pain in the emergency department,38,280 or pain after oral surgery.164,206
In addition to the above findings addressing sex differences in responses to μ-opioid agonists, others have investigated analgesic responses to mixed-action opioid agonist-antagonists among women relative to men. In several studies of pain after oral surgery, women have shown more robust and longer lasting analgesic responses than men in response to pentazocine, nalbuphine, and butorphanol.150,152,153,164 Interestingly, these investigators have shown that low dose nalbuphine actually increases pain in men, an effect that can be reversed with a subanalgesic dose of morphine.151 After endodontic surgery, women showed significantly greater pain relief with a pentazocine/naloxone combination compared with men.353 In contrast, no sex differences in butorphanol analgesia were observed among patients treated in the emergency room for trauma-related pain.280 Clinical data regarding sex differences in response to nonopioid analgesics is limited; however, analgesic responses to ibuprofen after dental surgery were similar in women and men.17,353 Taken together, these clinical findings suggest more robust analgesic responses to mixed-action opioids among women, particularly with dental pain models; however, sex differences in μ-opioid analgesia have been inconsistent. Combining the data from Table 11 with the studies reviewed by Miaskowski and Levine,277 there is some suggestion that when using PCA women consume lower doses of morphine. Interestingly, the two studies showing lower morphine requirements for men involved provider administered morphine.16,62 It is tempting to speculate that men may be less willing to report pain or request analgesics from a provider, which would explain their lower opioid consumption in the provider-administered settings. Alternatively, one could argue that women benefit more from the increased sense of control that accompanies self-administration of opioids. Additional research will be required to confirm or refute these possibilities.
Sex Differences in Analgesia: Experimental Studies
In recent years, several investigators have examined sex differences in analgesic responses using experimental pain models, and these studies are summarized in Table 12. As with clinical pain, most of these studies have examined μ-opioid analgesia, and overall the findings suggest minimal sex differences. The vast majority of studies have reported no sex differences analgesia with a variety of opioids. One exception was a study using electrical pain, which reported longer lasting and higher peak morphine analgesia among women than men; however, this study did not include a placebo condition.364 This may be important, because Pud and colleagues320 recently found that women showed greater increases than men in cold pain threshold and tolerance after oral morphine; however, women also showed greater analgesia in response to the placebo. Therefore, when placebo responses were controlled for, no sex difference in morphine response emerged. The effects of some nonopioid pain medications have been compared for women versus men. Analgesic responses to ibuprofen produced greater analgesia for electrical pain among men than women.430 In a study of another NSAID, no significant sex differences the effects of ketorolac on cold pressor pain tolerance were found.72 Inspection of the means indicates that males showed substantially increased tolerance in response to both placebo and ketorolac, whereas females showed no placebo response and a very modest increase in response to ketorolac. Also, using pressure pain, Robinson et al334 reported that lidocaine produced greater cutaneous anesthesia in men than women. Thus, experimental pain models provide no consistent evidence of sex differences in analgesic responses to opioid or nonopioid medications; however, two of the studies indicate the importance of a placebo condition in the experimental design.72,320
Table 12.
Experimental Studies Regarding Sex Differences in Analgesic Responses
| Authors | Sample Size (F, M) | Medication | Type of Pain | Findings |
|---|---|---|---|---|
| μ-opioids | ||||
| Fillingim et al132 | 61, 39 | iv morphine (0.08 mg/kg) | Heat, pressure, ischemic pain | F = M |
| Olofsen et al303 | 8, 8 (E) 5, 5 (H) |
iv alfentanil (30 min infusion to 150 ng/mL) | Electrical Heat Pain |
F = M |
| Pud, et al320 | 15, 19 | Oral morphine (0.5 mg/kg) | Cold pressor | F = M (placebo controlled) |
| Romberg, et al342 | 10, 10 | iv M6 G (0.3 mg/kg) | Electrical | F = M |
| Sarton, et al364 | 10, 10 | iv morphine (0.13 mg/kg) | Electrical | F > M analgesia peak/duration |
| Mixed-action opioids | ||||
| Fillingim et al133 | 41, 38 | iv pentazocine (0.5 mg/kg) | Heat, pressure, ischemic pain | F = M |
| Zacny and Beckman454 | 8, 8 | iv butorphanol (0.5, 1, 2 mg/70 kg) | Cold pressor | F = M (M > F trend) |
| Nonopioid analgesics | ||||
| Walker and Carmody430 | 10, 10 | Oral ibuprofen 800 mg | Electrical | M > F |
| Compton et al72 | 25, 25 | oral ketorolac 10 mg | Cold pressor | F = M |
| Robinson et al334 | 23, 21 | lidocaine iontophoresis | Pressure pain | M > F |
Sex Differences in Responses to Nonpharmacologic Interventions
In contrast to research on sex differences in responses to pharmacologic treatments, whether nonpharmacologic interventions for pain produce differential effects in women and men has received relatively little attention. Some research has addressed this issue in the context of experimentally induced pain. For example, a cognitive intervention in which subjects were instructed to focus on the sensations of pain they experienced was effective for reducing pain intensity in men but not women.219 In another experimental study, women but not men reported lower ratings of cold pressor pain after exercising on a treadmill, whereas men but not women showed reduced pain ratings after playing video games.387 In another study, pleasant odors significantly reduced the intensity and unpleasantness of heat pain in females but not in males.265 It has also been reported that ingestion of sucrose produced a longer-lasting suppression of the R3 reflex in males than in females.37
Limited clinical research has addressed sex differences in outcomes from physical medicine or interdisciplinary treatments. Several studies have examined potential sex differences in outcomes of treatments for back pain. For example, conventional physical therapy was more effective for men, whereas intensive dynamic back exercises produced better pain reduction among women.178 Similarly, women but not men with back pain undergoing cognitive behavioral treatment with or without physical therapy exhibited improved health-related quality of life.197 Moreover, women in active treatment showed reduced likelihood of permanent disability than women in the standard care control group, but no such effect emerged for men. In contrast to these results, other findings indicate similar treatment gains for women and men after active rehabilitation for chronic low back pain.155,208,263 In a study of conservative multidisciplinary treatment for orofacial pain, women showed significant reductions in pain 2 years after treatment, whereas men showed no pain improvements.230 Keogh and colleagues220 recently reported that men and women showed similar reductions in pain intensity and pain-related distress after interdisciplinary pain management, but men maintained their treatment gains 3 months later, whereas women did not.
Importantly, even when men and women show treatment responses of similar magnitude, the determinants of outcome may be sex-related. For example, anger expression was associated with functional and mood improvements among men but not women undergoing interdisciplinary pain treatment.51 Another study of responses to interdisciplinary pain treatment found that pretreatment pain tolerance more strongly predicted reductions in pain severity and pain-related interference among women than men.104 Also, in a study evaluating responses to three physical therapy interventions for acute back pain, baseline levels of pain and disability as well as duration of symptoms predicted outcomes in women, whereas type of treatment and fear avoidance beliefs were significant predictors among men.155 This somewhat limited literature suggests the possibility of sex differences in responses to nonpharmacological pain treatment and the predictors of treatment response, but additional research is needed to further explore these issues.
Mechanisms Underlying Sex Differences in Pain and Responses to Treatment
The preceding review clearly demonstrates the presence of sex differences in pain responses, and some evidence suggests that endogenous and exogenous modulation of pain may vary in women versus men. However, the mechanisms underlying these sex differences have yet to be fully uncovered. Importantly, as in other fields of pain research, mechanisms underlying sex-related variability in pain responses are often portrayed as either psychosocial or biological. This dualistic conceptualization should be recognized as artificial and based primarily on the level of analysis rather than the actual mechanism of action. For example, at a psychosocial level, gender differences in expression of pain are often attributed to the effects of stereotypic sex roles. However, from a more biological perspective, hormonal and neurobiological factors are inevitably associated with and influenced by masculine versus feminine sex roles, and these underlying neurobiological processes can directly affect nociceptive responses. Thus, when considering the putative mechanisms underlying sex differences in pain, the terms “psychosocial” versus “biological” are used for convenience, but it is recognized that these terms may actually refer to the same underlying processes described at different levels of analysis. Previous reviews have addressed potential “biological” contributions to sex differences in pain, including gonadal hormones,7,75,77,131 endogenous pain modulation,73,127 as well as “psychosocial” influences including gender roles35,289,335 and other psychosocial factors.203,289,335 We will briefly discuss each of these issues: “biological” mechanisms and gonadal hormones and pain.
In addition to their reproductive role, gonadal hormones produce far-reaching effects throughout the peripheral and central nervous systems, and these hormones likely contribute importantly to sex differences in pain. The concentrations and temporal characteristics of estrogens, progesterone and testosterone differ substantially between sexes. For women, hormone levels change during and after pregnancy, after menopause, and monthly throughout most of the female’s reproductive lifetime (menstrual cycle), whereas men are exposed to less impressive fluctuations in hormone levels across the lifespan, with the most significant change being the reduction of testosterone with aging. After a brief overview of findings regarding hormonal effects on clinical and experimental pain in humans, we will highlight several pathways whereby gonadal hormones can influence pain.75
Hormonal Influences on Clinical Pain
Abundant evidence suggests hormonal contributions to many clinical pain conditions. For example, as noted previously, prepubertal girls and boys have an approximately equal prevalence of migraine; however, the lifetime prevalence of migraine increases to 18% for women and 6% for men after puberty, suggesting a hormonal link between female sex and migraine.253,391 Similar prevalence patterns have been observed for temporomandibular disorders, with no difference between boys and girls in childhood and higher prevalence in women after puberty.242 In addition, the prevalence of one or more common pain complaints was similar among girls and boys before puberty but increased more dramatically in girls as puberty progressed.245 The severity of symptoms appears to vary across the menstrual cycle for several pain conditions, including irritable bowel syndrome,180,181 TMD,244 headache,13,213 and fibromyalgia.9,10,310 However, data suggesting no menstrual cycle effect are also available.240,300 Additional support for hormonal modulation of pain comes from findings that during pregnancy migraine frequency declines and TMD pain is reduced.46,75,247 Interestingly, as the estradiol level sharply declines postpartum, frequency of migraine attacks increases.356
Exogenous hormone use has also been associated with clinical pain. Postmenopausal women using hormone replacement have shown increased risk for back pain50,288,397 and TMD,246 and oral contraceptive use has been related to increased risk for TMD246 and carpal tunnel syndrome.116 Moreover, Wise and colleagues445 found that postmenopausal women on hormone replacement seeking treatment for orofacial pain reported significantly more severe pain compared to facial pain patients not using hormones. However, other research suggests no association of exogenous hormone use with clinical pain.260 Moreover, discontinuation of hormone replacement therapy in postmenopausal women was associated with higher levels of reported pain or stiffness,299 and after sustained estradiol administration, estradiol withdrawal has been shown to precipitate migraine headaches.249,381 Finally, a study of transsexuals undergoing hormonal treatment to acquire somatic characteristics of the opposite sex revealed a change in response to pain.6 Approximately one-third of the male-to-female subjects undergoing estradiol/antiandrogen treatment developed chronic pain, whereas about half of the female-to-male subjects treated with testosterone reported a significant improvement of the chronic pain (headache) already present before the start of treatment. Taken together, these data provide evidence for hormonal contributions to clinical pain, in that both administration and withdrawal of estrogens have been shown to increase risk for pain.
Hormonal Influences on Experimental Pain
Studies of laboratory pain provide additional evidence of hormonal influences on pain responses. In a meta-analytic review of 16 publications related to pain perception across the menstrual cycle, Riley and colleagues concluded that pain thresholds for mechanical, thermal, and ischemic muscle pain were higher during the follicular phase of the menstrual cycle (low to moderate levels of estradiol and progesterone) than during perimenstrual phases of the cycle (decreasing levels of estradiol and progesterone), and the effect sizes were generally small to moderate.328 Since this systematic review, additional studies have yielded conflicting results. Electrical pain thresholds were lower in the luteal versus the follicular phase in one study,401 but two other studies reported no menstrual cycle effects on electrical pain thresholds.305,408 Three studies reported no menstrual cycle effects on heat pain perception83,166,380 and another reported lower heat pain thresholds only on the abdomen during the ovulatory phase.21 In several studies, pressure pain thresholds generally did not vary across the menstrual cycle in healthy women or women with TMD,71,373,426 one study reported lower thresholds during the perimenstrual versus luteal and follicular phases,194 others reported that PPT tested on the back was lower during the ovulatory phase,21 and temporalis PPTs were higher in the menstrual than the follicular phase.98 Cimino and colleagues71 found that masseter and temporalis PPTs were lowest during the periovulatory phase. Two studies reported no menstrual cycle effects on ischemic pain.373,393 Cold pressor pain threshold showed menstrual cycle effects in two studies, with lower thresholds in perimenstrual183 and luteal386 phases; however, these authors reported no menstrual cycle effects on pain ratings or pain tolerance, and others have reported no menstrual cycle effect on cold pressor pain.229 Gazerani et al143 reported greater capsaicin-induced pain, allodynia, and mechanical hyperalgesia during the menstrual versus the luteal phase. In addition to subjective pain responses, pain-related cerebral activation,68,84 laser evoked potentials,166 and nociceptive muscle reflexes have varied across the menstrual cycle.401 As previously noted328,372 these inconsistent menstrual cycle effects are likely related to the tremendous variability in how investigators have defined cycle phases, along with other methodological inconsistencies, including varying pain modalities and testing sites.
Additional evidence of hormonal contributions to pain sensitivity has been reported. For example, in pre-menopausal women, higher estradiol levels were associated with increased pain in response to thermal stimuli.129 In contrast, higher progesterone levels were associated with increased cold pressor pain sensitivity, and this association was attenuated in the presence of higher estradiol levels.386 Further, we reported that postmenopausal women taking hormone replacement therapy (HRT) displayed lower thermal pain thresholds and tolerances than postmenopausal women not taking HRT, whose pain responses did not differ from men.120 Thus, menstrual cycle and hormonal influences on pain sensitivity have been reported, but the direction and magnitude of these associations is highly variable.
The exact mechanisms whereby hormones influence pain remain complex and poorly understood, because hormonal effects vary in both magnitude and direction based on numerous factors, including (1) the dose and timing of hormonal exposure; (2) the type of pain under consideration; (3) the entire hormonal complement (ie the presence of multiple hormonal factors); (4) the target tissues (eg peripheral vs spinal vs supraspinal). Moreover, gonadal steroids exert both organizational and activational effects, which refer to long-term developmental influences versus transient effects in adulthood, respectively. The impact of sex hormones on pain responses can be broadly dichotomized into peripheral versus central nervous system effects, which are discussed below.
Peripheral Effects of Sex Hormones
Sex hormones can affect disease pathophysiology, which can affect disease-related pain. For example, the effects of estrogens on bone deposition and cartilage homeostasis could influence the development of articular pathology and pain.400 Of more direct relevance to pain are the hormonal contributions to inflammation. In general, women show a heightened inflammatory response compared with men.394 Although beneficial for wound healing and response to infection, this more robust inflammatory response places women at significantly greater risk for a variety of painful inflammatory autoimmune conditions, including rheumatoid arthritis, osteoarthritis, and systemic lupus erythematosus. The inflammatory response to various triggering events involves plasma extravasation, chemotactic attraction of leukocytes, and, in turn, stimulated release of inflammatory cytokines and growth factors. Additional peptides are released from C fibers, and spinal N-methyl-D-aspartate (NMDA) receptor activation and nitric oxide production occur.450 This cascade of events can alter the transduction properties of nociceptors, lowering their activation thresholds, and this peripheral sensitization could ultimately lead to central sensitization. For example, stress-induced activation of the sympathoadrenal system attenuated the inflammatory response (ie plasma extravasation) to bradykinin in male but enhanced plasma extravasation in female rats.167 The effects of estrogens on inflammatory responses are highly complex and depend on the level of estrogens, the cell type being examined, the specific inflammatory factor, the type of tissue that is inflamed, the time course of the inflammatory response (eg acute vs chronic), and the time point at which estrogen exposure occurs.394 For example, very high estrogen concentrations tend to inhibit inflammation, whereas lower levels of estrogens produce either no effect or a proinflammatory effect.56,394 Regarding inflammatory pain, systemically administered estradiol reduced formalin-induced nociceptive behaviors in gonadectomized male and female rats,142,233,262 whereas centrally administered estradiol heightened formalin-induced nociceptive responses in male rats.8,61 Thus, peripheral and central effects of estrogens may be divergent.75 A complete review of the literature regarding hormonal effects on inflammation is beyond the scope of this manuscript, and interested readers are referred to other recent reviews for more detail.56,75,281,394 Suffice it to say that hormonal effects on inflammation represent one important albeit complex pathway whereby gonadal hormones can influence pain responses.
Another peripheral mechanism whereby gonadal hormones can affect pain responses is through their effects on peripheral afferents. Indeed, estrogen receptors are found on primary afferents,29 and estradiol has been shown to increase trigeminal afferent discharges evoked by injection of NMDA.96 Also, estrogen increased C-fiber activity evoked by uterine cervical distension, and this enhanced afferent activity was reversed by administration of a TRPV1 receptor antagonist.452 However, another group recently demonstrated that estrogen reduced capsaicin-induced TRPV1 activation of lumbosacral afferents.451 Thus, estrogenic influences on peripheral afferent unction have been reported, though the direction of the effects can be variable.
Gonadal steroids exert wide-ranging effects in the central nervous system, including direct and indirect effects on pain processing. Sex hormones may influence multiple central nervous system pathways, including effects on the functioning of endogenous opioid systems, dopaminergic function, serotonergic activity, and other endogenous components involved in the nociceptive processing, as discussed below.
Endogenous Opioid Systems
The most studied of the endogenous pain modulatory systems is the endogenous opioid system, and sex differences in the functioning of this system could arise based on several different mechanisms. First, sex differences could result from differences in the distribution, expression or sensitivity of opioid receptors in regions of the central nervous system involved in nociceptive processing. At rest, women have shown higher μ-opioid receptor binding in various cortical and subcortical brain regions than men,456 whereas men exhibited greater μ-opioid receptor binding in several brain regions than women in response to experimentally induced muscle pain. These sex differences in both resting and pain-related μ-opioid receptor binding may contribute not only to sex differences in basal pain perception but also to differences in sensitivity to opioid medications.
Sex differences in opioid function could be partially mediated by the well-known interaction between gonadal hormones and the opioid system.42 In rodents, estradiol site-specifically modulates peripheral and supraspinal but not spinal μ-opioid receptor activity.199 In the brain, intact and estradiol-treated ovariectomized female rats had significantly fewer opioid-binding sites than their untreated ovariectomizedcounterparts.435 Women in low estradiol states show decreased μ-opioid receptor availability. Smith et al379 showed that women in a high estradiol, low progesterone state, reported less pain and displayed increased pain-related brain μ-opioid receptor binding than women in a low estradiol state.
Additional evidence of hormonal modulation of opioid function comes from studies investigating responses to exogenous opioids under different hormonal conditions. For example, hormonal manipulations affect opioid antinociception in rodents, though the magnitude and direction of these effects can depend on multiple factors.77,131 We have previously concluded131 that the preclinical evidence suggests that conditions characterized by relatively high estradiol levels are associated with reduced sensitivity to opioid agonists. However, the influence of gonadal steroids on responses to opioids in humans has not been determined.
Dopamine
Another neurotransmitter system that could contribute to sex differences in pain responses is the dopaminergic system. Recent insights indicate a central role for dopaminergic neurotransmission in modulating pain perception,448 and evidence suggests that there are sex-specific differences in the dopaminergic function, and estrogens play an important role in maintaining the integrity and modulating the functional activity of the dopamine system in the CNS.270,271 Estrogens and progestins have complex effects on dopamine turnover, which vary across brain regions and depend on the dose and time course of administration.255,271 Also, some data indicate sex differences in dopamine transporter (DAT) function. This transporter plays a critical role in regulating dopaminergic function.139,448 The density of DATs are greater in female versus male rats,330,448 and clinical reports have shown greater densities of DATs within healthy adult women versus men.286,383,448 Moreover, assays of DAT function indicate a more active DAT system within females versus males.36,448 Such sex differences may be related to estrogens.
Although these studies do not confirm that dopamine influences sex differences in pain perception, it seems plausible that hormonal influences on dopaminergic function could contribute to sex differences in pain. Some have suggested that dysfunction of dopaminergic neurotransmission may explain the primary clinical symptoms of fibromyalgia (ie, chronic widespread pain and generalized hyperalgesia); therefore, dopamine represents an important and physiologically relevant target for the treatment of fibromyalgia.449 Additional research is needed to determine the role of the dopamine system in sex-related influences on pain.
Serotonin
Serotonin represents another potential contributor to sex differences in pain. Serotonin (5-hydroxtryptamine [5-HT]) influences pain processing in complex fashion, depending on the site of action and receptor subtype. In the CNS, 5-HT generally has been associated descending pain inhibition, whereas peripheral 5-HT is an inflammatory mediator and is generally pronociceptive. Although peripheral effects of serotonin are thought to contribute to sex-related pain conditions, such as migraine and IBS, serotonin’s contribution to sex differences in pain processing is primarily associated with its CNS actions. For example, compared with male rats, female rats have demonstrated higher serotonin levels and/or synthesis in multiple brain regions.94,175,209,433 A similar sex difference in rat brain serotonin turnover, an indication of serotonergic activity, has also been reported.58 Furthermore, brain serotonergic function is modulated by ovarian hormones.270,271 Clinical research also suggests that the greater brain 5-HT synthesis in female IBS patients versus controls may be related to the visceral hypersensitivity that characterizes IBS patients, the female predominance of the disorder, and the sex difference of the efficacy of the 5-HT3 antagonist in treatment for this syndrome.291 Thus, it seems plausible that central serotonin function may contribute to sex differences in pain.
NMDA Receptor Function
Sex differences in pain modulation may also be influenced by NMDA receptor function. NMDA receptors are expressed in the dorsal horn and their sustained activation by the release of glutamate from tonically active primary afferents enhances the excitability of the second-order neurons on which they are expressed, producing enhanced nociceptive responses. McRoberts and colleagues274 recently reported that the application of agonists (NMDA and glycine) to cultured dorsal root ganglion (DRG) neurons from female animals produced significantly larger currents compared with DRG neurons from males, and the addition of estradiol increased the NMDA receptor currents more in females than males. Moreover, NMDA antagonism can enhance opioid anti-nociception, and this effect has shown sex dependence, with generally greater enhancement in male versus female animals,169,254,294 although these effects depend on the particular NMDA antagonist used, its site of action, as well as its dose.76 It seems plausible that estrogenic enhancement of NMDA receptor excitability could contribute to more robust central sensitization among women than men.186 These sex-related influences on NMDA receptor function could help explain sex differences in temporal summation (or “windup”) of pain.130,338,362,363
“Psychosocial” Mechanisms
Gender Roles and Pain
Within gender studies, sex has generally been seen as a biological marker, used to categorize human beings into males and females based on physical characteristics such as chromosomes, hormones, external genitalia, and secondary characteristics.35,87 The assignment of a sex category involves social processes whereby a human being is classified as man or woman based on socially agreed-on biological criteria (eg, genitalia at birth, chromosomal typing); however, biological characteristics are often inferred based on social characteristics, such as how people dress or present themselves.35 Some investigators argue that the use of the dichotomous variable sex as a proxy for presumed biologic aspects of being female or male may obscure the contribution to sex-correlated differences that could be ascribed to the ways in which women and men are socialized.289 Thus, the terms “sex” and “gender,” while related, are not interchangeable. Sex refers to biological distinctions characterizing male and female, whereas gender reflects sex-related social roles with which an individual identifies that presumably reflect learned femininity and masculinity.289, 446
The differences that exist between males and females in the perception, expression, and tolerance of pain are likely influenced by a variety of social and psychological processes.279 Gender roles have been associated with pain response, with the masculine gender norm dictating increased tolerance of pain among males, whereas feminine gender norms accept pain as a normal part of life and are more permissive of pain expression.289,418 Using standardized measures of gender roles, several studies have investigated the association of masculinity and femininity to experimental pain responses. In one study, higher masculinity relative to femininity was associated with higher mechanical pain thresholds among men but not women, whereas greater masculinity relative to femininity predicted higher mechanical pain tolerance in both sexes.307 Similar findings were reported for cold pain tolerance in a more recent study.290 Sanford and colleagues359 reported that higher levels of femininity predicted lower cold pain tolerance, whereas masculinity was not associated with pain response. Subsequently, these investigators reported that higher masculinity relative to femininity was associated with higher cold pain tolerance and lower cold pain ratings.407 In two of these studies, sex differences in pain responses remained significant after controlling for gender roles290,307 whereas gender roles partially mediated the sex difference in pain tolerance in the other two studies.359,407
In addition to these studies using general measures of gender roles, Robinson and colleagues have developed a pain-specific gender role measure, the Gender Role Expectations of Pain (GREP).336 Their findings indicate that both women and men consider women more sensitive to pain, less enduring of pain, and more willing to report pain compared with men.336,338,444 Willingness to report pain was significantly associated with heat pain threshold and heat pain tolerance, and sex differences in pain threshold were not significant after controlling for willingness to report pain, whereas sex differences in pain tolerance remained significant.444 These authors also found that sex differences in temporal summation of heat pain were partially mediated by willingness to report pain.338 Using a different measure of pain-related gender norms, Nayak and colleagues293 found that females viewed overt pain expression as more acceptable than did males, and these beliefs predicted cold pain tolerance, which was lower in females than males. Pool et al317 found that both men and women agreed that the ideal man should tolerate more pain than the ideal woman, suggesting that gender norms are indeed associated with pain tolerance. They then assessed the degree to which participants identified with these gender norms and demonstrated that strong identification with the male gender norm was associated with higher electrical pain tolerance in men, whereas gender norm identification was not associated with pain tolerance among women.
Experimental manipulations have also been used to examine the influence of gender roles on pain perception. Levine and DeSimone248 reported that men reported less cold pressor pain in the presence of a female versus a male experimenter, whereas pain ratings for females were not influenced by experimenter sex. Similar findings were reported by Gijsbers and colleagues,160 who found that males showed a higher pressure pain threshold when tested by a female versus a male experimenter, whereas females’ pain threshold was not influenced by the sex of the experimenter. Interestingly, experimenter gender effects for both of these studies may have been enhanced, as one study stated that “experimenters were dressed in a manner that emphasized their gender roles,”160 and the other reported that “to evoke gender-related motives, experimenters were selected for their attractiveness.”248 Aslaksen et al15 also reported an interaction between participant and experimenter gender, such that males tested by a female experimenter provided lower heat pain ratings and lower ratings of arousal compared with those tested by a male experimenter, whereas experimenter gender did not influence the pain or arousal ratings of female participants. Another study demonstrated that cold pain tolerance was higher for both males and females when tested by an experimenter of the opposite sex.207 On balance, these studies indicate that males report less pain in the presence of a female experimenter; however, other investigators have failed to show any effect of experimenter gender on pain responses.52,112,290,307 Based on these findings, Greenspan and colleagues168 recommend documenting and reporting experimenter sex in the experimental pain setting because such factors may influence pain report in the laboratory and clinic setting.
Additional research has attempted to manipulate other gender-related variables. Fillingim et al119 provided instructions designed to manipulate females’ and males’ perceived ability to tolerate ischemic pain, hypothesizing that enhancing perceived ability would produce greater effects in females, because males report higher perceived ability to tolerate pain at baseline. Surprisingly, the group with the highest pain tolerance was males who had been informed that females tolerate the procedure better. In contrast, Robinson and colleagues333 found that sex differences in cold pain tolerance were nonsignificant when participants were given gender-specific expectations for pain tolerance. The findings appear to indicate that females exhibited an increase in their pain tolerance when given the expectation that women would tolerate the pain for a longer time, whereas males showed similar pain tolerance regardless of expected tolerance time. Another study examined the effects of high versus low monetary incentives on pain tolerance among females and males, anticipating that high external incentives would produce stronger effects among females, because males possess higher endogenous motivation to tolerate pain.257 However, the incentive manipulation had no effect on pain tolerance for females or males.
More limited research has addressed the contribution of gender roles to clinical pain, and the results have been mixed. For example, higher scores on one aspect of masculinity were associated with lower pain-related symptoms among patients with rheumatoid arthritis.413 Also, higher femininity scores in college aged males predicted an increased number of pain complaints 30 years later, whereas the masculinity-femininity scale did not predict future pain complaints among females.12 In contrast, Helgeson182 reported that higher masculinity predicted greater chest pain after myocardial infarction among men and women, and others have reported no association between gender role measures and clinical pain.121,232
The available research indicates a potentially important contribution of gender roles to sex differences in responses to experimentally induced pain, with masculinity and femininity predicting higher and lower pain sensitivity, respectively. Findings regarding clinical pain are more limited and less consistent. The exact mechanisms mediating the association of gender roles and pain responses have yet to be elucidated. An important question is whether these findings simply reflect gender-related response biases (ie, men under-report and/or women over-report pain) or might they reflect gender-based differences in endogenous pain modulation? More research is needed to further characterize the contribution of gender roles to the relationship between sex, gender, and pain.
Cognitive/Affective Variables
Cognitive/affective factors are important determinants of pain responses and likely contribute to sex differences in pain.289,335 The cognitive and affective mechanisms that have been investigated most widely in the context of sex and gender differences include coping processes, catastrophizing, and affective factors (eg, anxiety, depression). The following sections discuss the evidence regarding sex differences in the associations among psychological factors and pain report.
Coping and Catastrophizing
Coping refers to cognitive and behavioral efforts to manage demands judged to tax or exceed one’s resources, and this might be one factor contributing to gender differences in responses to pain.195 It seems plausible that biological and psychosocial influences may predispose males and females to utilize different coping strategies, and several studies have demonstrated sex differences in pain coping. In a sample of patients with musculoskeletal pain, Jensen et al196 found that women reported higher levels of catastrophizing and increasing behavioral activities compared with men, and higher catastrophizing was associated with poorer perceived health status among women. In a telephone survey, Un-ruh419 found that women reported having more intense pain and used more coping strategies than men, including positive self-statements and the use of more social and emotional support than men, but men and women did not differ in catastrophizing. Among patients with osteoarthritis, Keefe and colleagues212 found that women reported higher levels of pain, disability, and pain behavior. Also, women reported higher levels of catastrophizing, which mediated the sex differences in pain-related outcomes. In a daily diary study, these investigators also found that women with osteoarthritis reported greater use of problem-focused coping than men.211 Also, catastrophizing was more strongly related to negative mood among men than women. In children and adolescents with chronic pain, girls reported greater use of social support–seeking as a pain coping method, whereas boys made greater use of distraction.259 Another study in adolescents revealed that girls used more social support, positive statements, and internalizing/catastrophizing, whereas males reported more behavioral distraction, and the authors reported that internalizing/catastrophizing mediated sex differences in clinical pain.217 Sex differences in coping have also been reported in healthy populations. Several investigators have reported higher levels of catastrophizing among healthy women compared with their male counterparts.107,134,306 In one of these studies, catastrophizing mediated sex differences in reports of recent daily pain but did not affect the sex differences in heat pain sensitivity.107 Thus, sex differences in pain coping have been widely reported and have mediated sex differences in pain in some studies.
Affective Distress
Anxiety represents a negative emotional response to an anticipated threat, and higher levels of anxiety have been associated with increased clinical pain and heightened experimental pain sensitivity.325,331,337 Sex differences in anxiety have been reported, such that women tend to report higher levels of anxiety and are at increased risk for many anxiety disorders,26,410 and anxiety has been suggested as a potential mediator of sex differences in pain sensitivity.340 However, increasing evidence suggests that anxiety may be more strongly associated with pain responses among males than females.203 Several studies of laboratory pain have indicated that anxiety is positively associated with pain sensitivity among males but not females.125,204,205 In patients with chronic low back pain, Robinson and colleagues332 demonstrated that anxiety was more strongly related to both ongoing clinical pain and pain induced via low back exercise among men than women. Edwards and colleagues102 also reported that anxiety was more strongly related to clinical pain severity among male versus female patients with chronic pain, and they subsequently found that higher pretreatment anxiety predicted greater pain reductions after interventional therapy for men but not women.103 Thus, anxiety is more strongly related to experimental and clinical pain and to treatment-related pain reductions among men.
Another anxiety-related construct is anxiety sensitivity, which refers to the fear of anxiety-related bodily sensations.388,405 Sex, differences in anxiety sensitivity have been reported, such that females report higher levels of anxiety sensitivity, especially for the physical concerns component of the construct (ie, fear of the physical sensations associated with anxiety, such as rapid heartbeat, shortness of breath).389 Measures of anxiety sensitivity have been associated with both clinical97,218,388 and experimental pain responses.215,388,405 Moreover, anxiety sensitivity was more strongly related to pain among women than men with chest pain, and the authors found that the association of anxiety sensitivity to pain severity in women was mediated by the tendency to negatively interpret bodily sensations.218 Sex differences in the associations of anxiety sensitivity to experimental pain have also been reported. Specifically, in a study of responses to cold pressor pain higher anxiety sensitivity predicted lower pain threshold and tolerance only among men, whereas higher anxiety sensitivity was associated with greater sensory and affective pain ratings among women.215 Taken together, these findings suggest that anxiety sensitivity may contribute differently to pain responses among women and men.
Depression
Another component of affective distress that is relevant to pain is depression. Abundant evidence demonstrates that depression and pain are highly comor-bid,92,287 and depression is more prevalent among women than men,287 especially somatic depression.375 Moreover, among individuals with depression, women are more likely to report pain complaints than men,266 and some of the evidence reviewed above suggests that women with some forms of chronic pain (eg, cancer, OA) may be more likely to experience depression compared to men. Although depression has been associated with experimental pain sensitivity,22,237,316 whether depression influences pain perception differently among women versus men is not yet known.
Conclusions and Recommendations
We have attempted to thoroughly, if not comprehensively, review the recent literature regarding sex differences in clinical pain, experimental pain sensitivity, and response to pain treatment, and several conclusions can be confidently drawn from the available evidence. First, the prevalence of most common forms of pain is higher among women than men, and women report greater pain after invasive procedures than men, though these findings are less consistent. Second, compared with men, women display enhanced sensitivity to most forms of experimentally induced pain (with the exception of is-chemic pain). Although this has been noted in previous reviews,127,327 a substantial increase in the number of studies has occurred, some of which have used more clinically relevant experimental pain models. For example, findings indicate that women show more robust temporal summation of pain and experience higher levels of pain after intramuscular injection of algesic substances, such as glutamate and hypertonic saline. Also, only recently has evidence emerged indicating that men may exhibit greater DNIC than women, and recent findings suggest that DNIC may be particularly predictive of clinical pain.101,108,453 Additional data regarding sex differences in responses to analgesic medications have been quite mixed and general conclusions are difficult to draw. We have also discussed multiple “biological” and “psychosocial” mechanisms that may contribute to sex differences in pain and analgesic responses, including gonadal hormones, endogenous pain modulatory systems, gender roles, and cognitive/affective factors.
Although research regarding sex, gender, and pain has continued to expand and generate novel findings, to date there has been limited clinical impact of this new knowledge. We would like to highlight several issues, consideration of which could promote more rapid progress in the field. First, without compelling scientific justification limited research to one sex or the other, both preclinical and human studies should routinely include subjects of both sexes. The NIH requires this for human studies; however, nonhuman pain research continues to eschew females.32,283 Given that the clinical pain conditions to which preclinical research is intended to apply are female-predominant, one could argue that preclinical research that excludes females is incomplete at best and invalid at worst. Moreover, clinical studies, which typically include participants of both sexes, should consistently analyze for sex differences and report the findings, whether positive or negative. This would help overcome publication biases, which could overestimate sex differences based on the reduced likelihood of reporting negative findings. Another important conceptual and analytical concern is the distinction between qualitative and quantitative sex differences. Most of the studies reviewed above address quantitative differences, which refers to whether females and males display different amounts of pain or analgesia. In contrast, qualitative sex differences are present when a given variable influences pain or analgesia differently in women versus men. Because qualitative differences may indicate sex-specific pain mechanisms they represent the most compelling rationale for the development of sex-specific pain treatments. Thus, even in the absence of quantitative sex differences, researchers should conduct analyses to uncover potential qualitative sex differences, which simply involves including sex as a moderator in the statistical model.
In this era of translational science, an important goal for future research in this area is to generate information that will enhance pain treatment for both sexes. Despite the challenges of translational research, several opportunities that could be exploited to enhance translation have previously been suggested.118 For example, human laboratory pain models and genetic research could both serve as translational bridges between laboratory findings from nonhuman animals and clinical populations. For example, Mogil and colleagues284 successfully translated a novel sex-related genetic association (ie, the melanocortin-1-receptor gene, MC1R) with analgesic responses across species using experimental pain models in both mice and humans. To complete the translational continuum, it is important to determine whether sex-related genetic associations such as these discovered in the laboratory setting will extend to clinical populations. Human brain imaging represents another methodology that holds promise for facilitating mechanistic and translational advancements, and increased application of imaging to enhance understanding of sex differences in pain and analgesia is strongly recommended. We would like to echo the important issues demanding future investigation as delineated in the recent consensus report from the IASP Special Interest Group on Sex, Gender, and Pain, including identifying hormonal versus chromosomal contributions to sex differences in pain/analgesia; understanding the contribution of local (versus gonadal release) hormonal effects; elucidating the role of psychological factors; understanding whether pain chronicity contributes to sex differences; distinguishing the roles of sexual dimorphism in ascending versus descending modulatory pathways; determining the cellular and molecular bases of sex differences in pain/analgesia; understanding sex differences across the lifespan; and considering whether diagnostic criteria for some pain disorders should be sex-specific.168 Empirical attention to these issues will further advance knowledge regarding sex, gender, and pain and could lead to sex-specific enhancements in clinical pain management in the not too distant future.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida. This work was also supported by NIH grant NS41670 (R.B.F.).
References
- 1.al’Absi M, France C, Harju A, France J, Wittmers L. Adrenocortical and nociceptive responses to opioid blockade in hypertension-prone men and women. Psychosom Med. 2006;68:292–298. doi: 10.1097/01.psy.0000203240.64965.bd. [DOI] [PubMed] [Google Scholar]
- 2.al’Absi M, France CR, Ring C, France J, Harju A, McIntyre D, Wittmers LE. Nociception and baroreceptor stimulation in hypertension-prone men and women. Psychophysiology. 2005;42:83–91. doi: 10.1111/j.1469-8986.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 3.al’Absi M, Petersen KL. Blood pressure but not cortisol mediates stress effects on subsequent pain perception in healthy men and women. Pain. 2003;106:285–295. doi: 10.1016/S0304-3959(03)00300-2. [DOI] [PubMed] [Google Scholar]
- 4.al’Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- 5.al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- 6.Aloisi AM, Bachiocco V, Costantino A, Stefani R, Ceccarelli I, Bertaccini A, Meriggiola MC. Cross-sex hormone administration changes pain in transsexual women and men. Pain. 2007;132(Suppl 1):S60–S67. doi: 10.1016/j.pain.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Horm Behav. 2006;50:1–7. doi: 10.1016/j.yhbeh.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Aloisi AM, Ceccarelli I. Role of gonadal hormones in forma-lininducedpainresponsesofmalerats:Modulationbyestradiol and naloxone administration. Neuroscience. 2000;95:559–566. doi: 10.1016/s0306-4522(99)00445-5. [DOI] [PubMed] [Google Scholar]
- 9.Alonso C, Loevinger BL, Muller D, Coe CL. Menstrual cycle influences on pain and emotion in women with fibromyalgia. J Psychosom Res. 2004;57:451–458. doi: 10.1016/j.jpsychores.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Anderberg UM, Marteinsdottir I, Hallman J, Backstrom T. Variability in cyclicity affects pain and other symptoms in female fibromyalgia syndrome patients. J Musculoskel Pain. 1998;6:5–22. [Google Scholar]
- 11.Andersen RE, Crespo CJ, Ling SM, Bathon JM, Bartlett SJ. Prevalence of significant knee pain among older Americans: results from the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 1999;47:1435–1438. doi: 10.1111/j.1532-5415.1999.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 12.Applegate KL, Keefe FJ, Siegler IC, Bradley LA, McKee DC, Cooper KS, Riordan P. Does personality at college entry predict number of reported pain conditions at mid-life? A longitudinal study. J Pain. 2005;6:92–97. doi: 10.1016/j.jpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Arjona A, Rubi-Callejon J, Guardado-Santervas P, Serrano-Castro P, Olivares J. Menstrual tension-type headache: Evidence for its existence. Headache. 2007;47:100–103. doi: 10.1111/j.1526-4610.2007.00656.x. [DOI] [PubMed] [Google Scholar]
- 14.Ashina S, Bendtsen L, Ashina M, Magerl W, Jensen R. Generalized hyperalgesia in patients with chronic tension-type headache. Cephalalgia. 2006;26:940–948. doi: 10.1111/j.1468-2982.2006.01150.x. [DOI] [PubMed] [Google Scholar]
- 15.Aslaksen PM, Myrbakk IN, Hoifodt RS, Flaten MA. The effect of experimenter gender on autonomic and subjective responses to pain stimuli. Pain. 2007;129:260–268. doi: 10.1016/j.pain.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Averbuch M, Katzper M. A search for sex differences in response to analgesia. Arch Intern Med. 2000;160:3424–3428. doi: 10.1001/archinte.160.22.3424. [DOI] [PubMed] [Google Scholar]
- 18.Averbuch M, Katzper M. Gender and the placebo analgesic effect in acute pain. Clin Pharmacol Ther. 2001;70:287–291. doi: 10.1067/mcp.2001.118366. [DOI] [PubMed] [Google Scholar]
- 19.Ayesh EE, Jensen TS, Svensson P. Somatosensory function following painful repetitive electrical stimulation of the human temporomandibular joint and skin. Exp Brain Res. 2007;179:415–425. doi: 10.1007/s00221-006-0801-3. [DOI] [PubMed] [Google Scholar]
- 20.Baad-Hansen L, Poulsen HF, Jensen HM, Svensson P. Lack of sex differences in modulation of experimental intraoral pain by diffuse noxious inhibitory controls (DNIC) Pain. 2005;116:359–365. doi: 10.1016/j.pain.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clin J Pain. 2002;18:180–190. doi: 10.1097/00002508-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Bar KJ, Brehm S, Boettger MK, Boettger S, Wagner G, Sauer H. Pain perception in major depression depends on pain modality. Pain. 2005;117:97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Barrero LH, Hsu YH, Terwedow H, Perry MJ, Dennerlein JT, Brain JD, Xu X. Prevalence and physical determinants of low back pain in a rural Chinese population. Spine. 2006;31:2728–2734. doi: 10.1097/01.brs.0000244583.35982.ea. [DOI] [PubMed] [Google Scholar]
- 24.Bassols A, Bosch F, Campillo M, Canellas M, Banos JE. An epidemiological comparison of pain complaints in the general population of Catalonia (Spain) Pain. 1999;83:9–16. doi: 10.1016/s0304-3959(99)00069-x. [DOI] [PubMed] [Google Scholar]
- 25.Bastos JL, Gigante DP, Peres KG. Toothache prevalence and associated factors: A population based study in southern Brazil. Oral Dis. 2008;14:320–326. doi: 10.1111/j.1601-0825.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 26.Bekker MH, van Mens-Verhulst J. Anxiety disorders: Sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend Med. 2007;4(Suppl B):S178–S193. doi: 10.1016/s1550-8579(07)80057-x. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 28.Bennett M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 29.Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol. 2005;50:971–979. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Bergman S, Herrstrom P, Hogstrom K, Petersson IF, Svensson B, Jacobsson LT. Chronic musculoskeletal pain, prevalence rates, and sociodemographic associations in a Swedish population study. J Rheumatol. 2001;28:1369–1377. [PubMed] [Google Scholar]
- 31.Bergman S, Herrstrom P, Jacobsson LT, Petersson IF. Chronic widespread pain: A three year followup of pain distribution and risk factors. J Rheumatol. 2002;29:818–825. [PubMed] [Google Scholar]
- 32.Berkley KJ. Vive la difference. TINS. 1992;15:331–332. doi: 10.1016/0166-2236(92)90048-d. [DOI] [PubMed] [Google Scholar]
- 33.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 34.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R268–R276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- 35.Bernardes SF, Keogh E, Lima ML. Bridging the gap between pain and gender research: A selective literature review. Eur J Pain. 2008;12:427–440. doi: 10.1016/j.ejpain.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Bhatt SD, Dluzen DE. Dopamine transporter function differences between male and female CD-1 mice. Brain Res. 2005;28:188–195. doi: 10.1016/j.brainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharjee M, Bhatia R, Mathur R. Gender specificity of sucrose induced analgesia in human adults. Ind J Physiol Pharmacol. 2007;51:410–414. [PubMed] [Google Scholar]
- 38.Bijur PE, Esses D, Birnbaum A, Chang AK, Schechter C, Gallagher EJ. Response to morphine in male and female patients: analgesia and adverse events. Clin J Pain. 2008;24:192–198. doi: 10.1097/AJP.0b013e31815d3619. [DOI] [PubMed] [Google Scholar]
- 39.Bingefors K, Isacson D. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain: A gender perspective. Eur J Pain. 2004;8:435–450. doi: 10.1016/j.ejpain.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Bliddal H, Nneskiold-Samsoe B. Chronic widespread pain in the spectrum of rheumatological diseases. Best Pract Res Clin Rheumatol. 2007;21:391–402. doi: 10.1016/j.berh.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: A prevalence study. Pain. 2001;89:127–134. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 42.Bodnar RJ, Commons K, Pfaff DW. Central Neural States Relating Sex and Pain. Baltimore: Johns Hopkins University Press; 2002. [Google Scholar]
- 43.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lanteri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Bragdon EE, Light KC, Costello NL, Sigurdsson A, Bunting S, Bhalang K, Maixner W. Group differences in pain modulation: Pain-free women compared to pain-free men and to women with TMD. Pain. 2002;96:227–237. doi: 10.1016/S0304-3959(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 46.Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006;295:1824–1830. doi: 10.1001/jama.295.15.1824. [DOI] [PubMed] [Google Scholar]
- 47.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Briscoe ME. Why do people go to the doctor? Sex differences in the correlates of GP consultation. Soc Sci Med. 1987;25:507–513. doi: 10.1016/0277-9536(87)90174-2. [DOI] [PubMed] [Google Scholar]
- 49.Brun Sundblad GM, Saartok T, Engstrom LM. Prevalence and co-occurrence of self-rated pain and perceived health in school-children: Age and gender differences. Eur J Pain. 2007;11:171–180. doi: 10.1016/j.ejpain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Brynhildsen JO, Bjors E, Skarsgard C, Hammar ML. Is hormone replacement therapy a risk factor for low back pain among postmenopausal women? Spine. 1998;23:809–813. doi: 10.1097/00007632-199804010-00014. [DOI] [PubMed] [Google Scholar]
- 51.Burns JW, Johnson BJ, Devine J, Mahoney N, Pawl R. Anger management style and the prediction of treatment outcome among male and female chronic pain patients. Behav Res Ther. 1998;36:1051–1062. doi: 10.1016/s0005-7967(98)00080-1. [DOI] [PubMed] [Google Scholar]
- 52.Bush FM, Harkins SW, Harrington WG, Price DD. Analysis of gender effects on pain perception and symptom presentation in temporomandibular joint pain. Pain. 1993;53:73–80. doi: 10.1016/0304-3959(93)90058-W. [DOI] [PubMed] [Google Scholar]
- 53.Buskila D, Abramov G, Biton A, Neumann L. The prevalence of pain complaints in a general population in Israel and its implications for utilization of health services. J Rheumatol. 2000;27:1521–1525. [PubMed] [Google Scholar]
- 54.Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2001;86:782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- 55.Cairns BE, Wang K, Hu JW, Sessle BJ, Arendt-Nielsen L, Svensson P. The effect of glutamate-evoked masseter muscle pain on the human jaw-stretch reflex differs in men and women. J Orofac Pain. 2003;17:317–325. [PubMed] [Google Scholar]
- 56.Calabrese EJ. Estrogen and related compounds: biphasic dose responses. Crit Rev Toxicol. 2001;31:503–515. doi: 10.1080/20014091111785. [DOI] [PubMed] [Google Scholar]
- 57.Calderone KL. The influence of gender on the frequency of pain and sedative medication administered to postoperative patients. Sex Roles. 1990;23:713–725. [Google Scholar]
- 58.Carlsson M, Svensson K, Eriksson E, Carlsson A. Rat brain serotonin: biochemical and functional evidence for a sex difference. J Neural Transm. 1985;63:297–313. doi: 10.1007/BF01252033. [DOI] [PubMed] [Google Scholar]
- 59.Carmona L, Ballina J, Gabriel R, Laffon A. The burden of musculoskeletal diseases in the general population of Spain: Results from a national survey. Ann Rheum Dis. 2001;60:1040–1045. doi: 10.1136/ard.60.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Catala E, Reig E, Artes M, Aliaga L, Lopez JS, Segu JL. Prevalence of pain in the Spanish population: Telephone survey in 5000 homes. Eur J Pain. 2002;6:133–140. doi: 10.1053/eujp.2001.0310. [DOI] [PubMed] [Google Scholar]
- 61.Ceccarelli I, Fiorenzani P, Grasso G, Lariviere WR, Massafra C, Massai L, Muscettola M, Aloisi AM. Estrogen and mu-opioid receptor antagonists counteract the 17 beta-estradiol-induced licking increase and interferon-gamma reduction occurring during the formalin test in male rats. Pain. 2004;111:181–190. doi: 10.1016/j.pain.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 62.Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97:1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- 63.Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: Effect of age, gender, and race. Clin Pharmacol Ther. 2003;74:102–112. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 64.Chang KY, Tsou MY, Chan KH, Sung CS, Chang WK. Factors affecting patient-controlled analgesia requirements. J Formos Med Assoc. 2006;105:918–925. doi: 10.1016/S0929-6646(09)60177-7. [DOI] [PubMed] [Google Scholar]
- 65.Chen EH, Shofer FS, Dean AJ, Hollander JE, Baxt WG, Robey JL, Sease KL, Mills AM. Gender disparity in analgesic treatment of emergency department patients with acute abdominal pain. Acad Emerg Med. 2008;15:414–418. doi: 10.1111/j.1553-2712.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 66.Chesterton LS, Barlas P, Foster NE, Baxter GD, Wright CC. Gender differences in pressure pain threshold in healthy humans. Pain. 2003;101:259–266. doi: 10.1016/S0304-3959(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 67.Chia YY, Chow LH, Hung CC, Liu K, Ger LP, Wang PN. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled iv analgesia: A prospective survey of 2,298 Chinese patients. Can J Anaesth. 2002;49:249–255. doi: 10.1007/BF03020523. [DOI] [PubMed] [Google Scholar]
- 68.Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS, Kim JW, Kim SY, Lee SG, Lee MS. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology. 2006;105:120–127. doi: 10.1097/00000542-200607000-00021. [DOI] [PubMed] [Google Scholar]
- 69.Chopra A, Saluja M, Patil J, Tandale HS. Pain and disability, perceptions and beliefs of a rural Indian population: A WHO-ILAR COPCORD study: WHO-International League of Associations for Rheumatology: Community Oriented Program for Control of Rheumatic Diseases. J Rheumatol. 2002;29:614–621. [PubMed] [Google Scholar]
- 70.Christmas C, Crespo CJ, Franckowiak SC, Bathon JM, Bartlett SJ, Andersen RE. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345–348. [PubMed] [Google Scholar]
- 71.Cimino R, Farella M, Michelotti A, Pugliese R, Martina R. Does the ovarian cycle influence the pressure-pain threshold of the masticatory muscles in symptom-free women? J Orofac Pain. 2000;14:105–111. [PubMed] [Google Scholar]
- 72.Compton P, Charuvastra V, Ling W. Effect of oral ketorolac and gender on human cold pressor pain tolerance. Clin Exp Pharmacol Physiol. 2003;30:759–763. doi: 10.1046/j.1440-1681.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 73.Craft RM. Sex differences in drug- and non-drug-induced analgesia. Life Sci. 2003;72:2675–2688. doi: 10.1016/s0024-3205(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 74.Craft RM. Sex differences in opioid analgesia: From mouse to man. Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Craft RM. Modulation of pain by estrogens. Pain. 2007;132(Suppl 1):S3–S12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 76.Craft RM, Lee DA. NMDA antagonist modulation of morphine antinociception in female vs male rats. Pharmacol Biochem Behav. 2005;80:639–649. doi: 10.1016/j.pbb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: The role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Criste A. Do nurse anesthetists demonstrate gender bias in treating pain? A national survey using a standardized pain model. AANA J. 2003;71:206–209. [PubMed] [Google Scholar]
- 79.Dahlof C, Linde M. One-year prevalence of migraine in Sweden: A population-based study in adults. Cephalalgia. 2001;21:664–671. doi: 10.1046/j.1468-2982.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- 80.Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–498. doi: 10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]
- 81.Dao TT, LeResche L. Gender differences in pain. J Orofac Pain. 2000;14:169–184. [PubMed] [Google Scholar]
- 82.De Cosmo G, Congedo E, Lai C, Primieri P, Dottarelli A, Aceto P. Preoperative psychologic and demographic predictors of pain perception and tramadol consumption using intravenous patient-controlled analgesia. Clin J Pain. 2008;24:399–405. doi: 10.1097/AJP.0b013e3181671a08. [DOI] [PubMed] [Google Scholar]
- 83.De Leeuw R, Albuquerque RJ, Andersen AH, Carlson CR. Influence of estrogen on brain activation during stimulation with painful heat. J Oral Maxillofac Surg. 2006;64:158–166. doi: 10.1016/j.joms.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 84.de Leeuw R, Albuquerque RJ, Andersen AH, Carlson CR. Influence of estrogen on brain activation during stimulation with painful heat. J Oral Maxillofac Surg. 2006;64:158–166. doi: 10.1016/j.joms.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 85.de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 86.De CG, Congedo E, Lai C, Primieri P, Dottarelli A, Aceto P. Preoperative psychologic and demographic predictors of pain perception and tramadol consumption using intravenous patient-controlled analgesia. Clin J Pain. 2008;24:399–405. doi: 10.1097/AJP.0b013e3181671a08. [DOI] [PubMed] [Google Scholar]
- 87.Deaux K, Major B. Putting gender into context: An interactive model of gender-related behavior. Psychol Rev. 1987;94:369–389. [Google Scholar]
- 88.Defrin R, Benstein-Sheraizin A, Bezalel A, Mantzur O, Arendt-Nielsen L. The spatial characteristics of the painful thermal grill illusion. Pain. 2008;26 doi: 10.1016/j.pain.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 89.Defrin R, Pope G, Davis KD. Interactions between spatial summation, 2-point discrimination and habituation of heat pain. Eur J Pain. 2008;13 doi: 10.1016/j.ejpain.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 90.Deleu D, Khan MA, Al Shehab TA. Prevalence and clinical characteristics of headache in a rural community in Oman. Headache. 2002;42:963–973. doi: 10.1046/j.1526-4610.2002.02225.x. [DOI] [PubMed] [Google Scholar]
- 91.Derbyshire SW, Nichols T, Firestone L, Townsend D, Jones A. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain. 2002;3:401–411. doi: 10.1054/jpai.2002.126788. [DOI] [PubMed] [Google Scholar]
- 92.Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: Research findings and theoretical considerations. Psychosom Med. 2002;64:773–786. doi: 10.1097/01.psy.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- 93.Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA. 1990;263:1385–1389. [PubMed] [Google Scholar]
- 94.Dickinson SL, Curzon G. 5-Hydroxytryptamine-mediated behaviour in male and female rats. Neuropharmacology. 1986;25:771–776. doi: 10.1016/0028-3908(86)90094-8. [DOI] [PubMed] [Google Scholar]
- 95.Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: A path analytic description. Pain. 2004;112:188–196. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 96.Dong XD, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, Sessle BJ, Cairns BE. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience. 2007;146:822–832. doi: 10.1016/j.neuroscience.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Drahovzal DN, Stewart SH, Sullivan MJ. Tendency to catastrophize somatic sensations: Pain catastrophizing and anxiety sensitivity in predicting headache. Cogn Behav Ther. 2006;35:226–235. doi: 10.1080/16506070600898397. [DOI] [PubMed] [Google Scholar]
- 98.Drobek W, Schoenaers J, De Laat A. Hormone-dependent fluctuations of pressure pain threshold and tactile threshold of the temporalis and masseter muscle. J Oral Rehabil. 2002;29:1042–1051. doi: 10.1046/j.1365-2842.2002.00988.x. [DOI] [PubMed] [Google Scholar]
- 99.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch Jensen P, Corazziari E, et al. US householder survey of functional gastrointestinal disorders: Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 100.Edrington JM, Paul S, Dodd M, West C, Facione N, Tripathy D, Koo P, Schumacher K, Miaskowski C. No evidence for sex differences in the severity and treatment of cancer pain. J Pain Symptom Manage. 2004;28:225–232. doi: 10.1016/j.jpainsymman.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 101.Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology. 2005;9(65):437–443. doi: 10.1212/01.wnl.0000171862.17301.84. [DOI] [PubMed] [Google Scholar]
- 102.Edwards RR, Augustson E, Fillingim RB. Sex-specific effects of pain-related anxiety on adjustment to chronic pain. Clin J Pain. 2000;16:46–53. doi: 10.1097/00002508-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 103.Edwards RR, Augustson E, Fillingim RB. Differential relationships between anxiety and treatment-associated pain reduction among male and female chronic pain patients. Clin J Pain. 2003;19:208–216. doi: 10.1097/00002508-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 104.Edwards RR, Doleys DM, Lowery D, Fillingim RB. Pain tolerance as a predictor of outcome following multidisciplinary treatment for chronic pain: Differential effects as a function of sex. Pain. 2003;106:419–426. doi: 10.1016/j.pain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61:346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 106.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: A comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 107.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: Differential effects for daily pain versus laboratory-induced pain. Pain. 2004;111:335–341. doi: 10.1016/j.pain.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 108.Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): Association with clinical variables. Pain. 2003;106:427–437. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 109.Eggen AE. The Tromso Study: Frequency and predicting factors of analgesic drug use in a free-living population (12–56 years) J Clin Epidemiol. 1993;46:1297–1304. doi: 10.1016/0895-4356(93)90098-l. [DOI] [PubMed] [Google Scholar]
- 110.El-Metwally A, Salminen JJ, Auvinen A, Macfarlane G, Mikkelsson M. Risk factors for development of non-specific musculoskeletal pain in preteens and early adolescents: A prospective 1-year follow-up study. BMC Musculoskel Disord. 2007;8:46. doi: 10.1186/1471-2474-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ellermeier W, Westphal W. Gender differences in pain ratings and pupil reactions to painful pressure stimuli. Pain. 1995;61:435–439. doi: 10.1016/0304-3959(94)00203-Q. [DOI] [PubMed] [Google Scholar]
- 112.Essick G, Guest S, Martinez E, Chen C, McGlone F. Site-dependent and subject-related variations in perioral thermal sensitivity. Somatosens Mot Res. 2004;21:159–175. doi: 10.1080/08990220400012414. [DOI] [PubMed] [Google Scholar]
- 113.Falla D, Arendt-Nielsen L, Farina D. Gender-specific adaptations of upper trapezius muscle activity to acute nociceptive stimulation. Pain. 2008;15(138):217–225. doi: 10.1016/j.pain.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 114.Fejer R, Hartvigsen J, Kyvik KO, Jordan A, Christensen HW, Hoilund-Carlsen PF. The Funen Neck and Chest Pain study: Analysing non-response bias by using national vital statistic data. Eur J Epidemiol. 2006;21:171–180. doi: 10.1007/s10654-006-0006-x. [DOI] [PubMed] [Google Scholar]
- 115.Fernandez-Liz E, Modamio P, Catalan A, Lastra CF, Rodriguez T, Marino EL. Identifying how age and gender influence prescription drug use in a primary health care environment in Catalonia, Spain. Br J Clin Pharmacol. 2008;65:407–417. doi: 10.1111/j.1365-2125.2007.03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferry S, Hannaford P, Warskyj M, Lewis M, Croft P. Carpal tunnel syndrome: A nested case-control study of risk factors in women. Am J Epidemiol. 2000;15(151):566–574. doi: 10.1093/oxfordjournals.aje.a010244. [DOI] [PubMed] [Google Scholar]
- 117.Fillingim RB. Sex, gender and pain: Women and men really are different. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- 118.Fillingim RB. Sex differences in pain: Translational challenges and opportunities. In: Mao J, editor. Translational Pain Research. New York: Nova Science Publishers, Inc; 2006. pp. 293–314. [Google Scholar]
- 119.Fillingim RB, Browning AD, Powell T, Wright RA. Sex differences in perceptual and cardiovascular responses to pain: The influence of a perceived ability manipulation. J Pain. 2002;3:439–445. doi: 10.1054/jpai.2002.128067. [DOI] [PubMed] [Google Scholar]
- 120.Fillingim RB, Edwards RR. The association of hormone replacement therapy with experimental pain responses in postmenopausal women. Pain. 2001;92:229–234. doi: 10.1016/s0304-3959(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 121.Fillingim RB, Edwards RR, Powell T. The relationship of sex and clinical pain to experimental pain responses. Pain. 1999;83:419–425. doi: 10.1016/S0304-3959(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 122.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 123.Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related psychological predictors of baseline pain perception and analgesic responses to pen-tazocine. Biol Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 124.Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wallace MR. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 125.Fillingim RB, Keefe FJ, Light KC, Booker DK, Maixner W. The influence of gender and psychological factors on pain perception. J Gender Cult Health. 1996;1:21–36. [Google Scholar]
- 126.Fillingim RB, Maddux V, Shackelford JM. Sex differences in heat pain thresholds as a function of assessment method and rate of rise. Somatosensory Mot Res. 1999;16:57–62. doi: 10.1080/08990229970654. [DOI] [PubMed] [Google Scholar]
- 127.Fillingim RB, Maixner W. Gender differences in the responses to noxious stimuli. Pain Forum. 1995;4:209–221. [Google Scholar]
- 128.Fillingim RB, Maixner W. The influence of resting blood pressure and gender on pain responses. Psychosom Med. 1996;58:326–332. doi: 10.1097/00006842-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 129.Fillingim RB, Maixner W, Girdler SS, Light KC, Harris MB, Sheps DS, Mason GA. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom Med. 1997;59:512–520. doi: 10.1097/00006842-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 130.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 131.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 132.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R. Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 133.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Price DD, Staud R. Experimental pain models reveal no sex differences in pentazocine analgesia in humans. Anesthesiology. 2004;100:1263–1270. doi: 10.1097/00000542-200405000-00031. [DOI] [PubMed] [Google Scholar]
- 134.Fillingim RB, Wilkinson CS, Powell T. Self-reported abuse history and pain complaints among healthy young adults. Clin J Pain. 1999;15:85–91. doi: 10.1097/00002508-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 135.Flaten MA, Simonsen T, Olsen H. Drug-related information generates placebo and nocebo responses that modify the drug response. Psychosom Med. 1999;61:250–255. doi: 10.1097/00006842-199903000-00018. [DOI] [PubMed] [Google Scholar]
- 136.France CR, Keefe FJ, Emery CF, Affleck G, France JL, Waters S, Caldwell DS, Stainbrook D, Hackshaw KV, Edwards C. Laboratory pain perception and clinical pain in post-menopausal women and age-matched men with osteoarthritis: Relationship to pain coping and hormonal status. Pain. 2004;112:274–281. doi: 10.1016/j.pain.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 137.France CR, Suchowiecki S. A comparison of diffuse noxious inhibitory controls in men and women. Pain. 1999;81:77–84. doi: 10.1016/s0304-3959(98)00272-3. [DOI] [PubMed] [Google Scholar]
- 138.Frot M, Feine JS, Bushnell MC. Sex differences in pain perception and anxiety: A psychophysical study with topical capsaicin. Pain. 2004;108:230–236. doi: 10.1016/j.pain.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 139.Fumagalli F, Jones S, Bosse R, Jaber M, Giros B, Missale C, Wightman RM, Caron MG. Inactivation of the dopamine transporter reveals essential roles of dopamine in the control of locomotion, psychostimulant response, and pituitary function. Adv Pharmacol. 1998;42:179–182. doi: 10.1016/s1054-3589(08)60722-x. [DOI] [PubMed] [Google Scholar]
- 140.Gagliese L, Gauthier LR, Macpherson AK, Jovellanos M, Chan VW. Correlates of postoperative pain and intravenous patient-controlled analgesia use in younger and older surgical patients. Pain Med. 2008;9:299–314. doi: 10.1111/j.1526-4637.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- 141.Garcia E, Godoy-Izquierdo D, Godoy JF, Perez M, Lopez-Chicheri I. Gender differences in pressure pain threshold in a repeated measures assessment. Psychol Health Med. 2007;12:567–579. doi: 10.1080/13548500701203433. [DOI] [PubMed] [Google Scholar]
- 142.Gaumond I, Arsenault P, Marchand S. Specificity of female and male sex hormones on excitatory and inhibitory phases of formalin-induced nociceptive responses. Brain Res. 2005;2:105–111. doi: 10.1016/j.brainres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 143.Gazerani P, Andersen OK, Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization: Gender-specific differences. Pain. 2005;118:155–163. doi: 10.1016/j.pain.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 144.Gazerani P, Andersen OK, Arendt-Nielsen L. Site-specific, dose-dependent, and sex-related responses to the experimental pain model induced by intradermal injection of capsaicin to the foreheads and forearms of healthy humans. J Orofac Pain. 2007;21:289–302. [PubMed] [Google Scholar]
- 145.Gazerani P, Wang K, Cairns BE, Svensson P, Arendt-Nielsen L. Effects of subcutaneous administration of glutamate on pain, sensitization and vasomotor responses in healthy men and women. Pain. 2006;124:338–348. doi: 10.1016/j.pain.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 146.Ge HY, Arendt-Nielsen L, Farina D, Madeleine P. Gender-specific differences in electromyographic changes and perceived pain induced by experimental muscle pain during sustained contractions of the upper trapezius muscle. Muscle Nerve. 2005;32:726–733. doi: 10.1002/mus.20410. [DOI] [PubMed] [Google Scholar]
- 147.Ge HY, Madeleine P, Arendt-Nielsen L. Sex differences in temporal characteristics of descending inhibitory control: An evaluation using repeated bilateral experimental induction of muscle pain. Pain. 2004;110:72–78. doi: 10.1016/j.pain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 148.Ge HY, Madeleine P, Arendt-Nielsen L. Gender differences in pain modulation evoked by repeated injections of glutamate into the human trapezius muscle. Pain. 2005;113:134–140. doi: 10.1016/j.pain.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 149.Ge HY, Madeleine P, Cairns BE, Arendt-Nielsen L. Hypoalgesia in the referred pain areas after bilateral injections of hypertonic saline into the trapezius muscles of men and women: A potential experimental model of gender-specific differences. Clin J Pain. 2006;22:37–44. doi: 10.1097/01.ajp.0000149799.01123.38. [DOI] [PubMed] [Google Scholar]
- 150.Gear RW, Gordon NC, Heller PH, Paul S, Miaskowski C, Levine JD. Gender difference in analgesic response to the kappa-opioid pentazocine. Neurosci Lett. 1996;205:207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- 151.Gear RW, Gordon NC, Hossaini-Zadeh M, Lee JS, Miaskowski C, Paul SM, Levine JD. A subanalgesic dose of morphine eliminates nalbuphine anti-analgesia in postoperative pain. J Pain. 2008;9:337–341. doi: 10.1016/j.jpain.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 153.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 154.George SZ, Dannecker EA, Robinson ME. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: An experimental investigation in pain-free individuals. Eur J Pain. 2006;10:457–465. doi: 10.1016/j.ejpain.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 155.George SZ, Fritz JM, Childs JD, Brennan GP. Sex differences in predictors of outcome in selected physical therapy interventions for acute low back pain. J Orthop Sports Phys Ther. 2006;36:354–363. doi: 10.2519/jospt.2006.2270. [DOI] [PubMed] [Google Scholar]
- 156.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J Pain. 2007;8:2–10. doi: 10.1016/j.jpain.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 157.Gerdle B, Bjork J, Coster L, Henriksson K, Henriksson C, Bengtsson A. Prevalence of widespread pain and associations with work status: A population study. BMC Musculoskel Disord. 2008;9:102. doi: 10.1186/1471-2474-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gerdle B, Bjork J, Henriksson C, Bengtsson A. Prevalence of current and chronic pain and their influences upon work and healthcare-seeking: A population study. J Rheumatol. 2004;31:1399–1406. [PubMed] [Google Scholar]
- 159.Gesch D, Bernhardt O, Alte D, Schwahn C, Kocher T, John U, Hensel E. Prevalence of signs and symptoms of temporomandibular disorders in an urban and rural German population: Results of a population-based Study of Health in Pomerania. Quintessence Int. 2004;35:143–150. [PubMed] [Google Scholar]
- 160.Gijsbers K, Nicholson F. Experimental pain thresholds influenced by sex of experimenter. Percept Mot Skills. 2005;101:803–807. doi: 10.2466/pms.101.3.803-807. [DOI] [PubMed] [Google Scholar]
- 161.Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 162.Glasson JC, Sawyer WT, Lindley CM, Ginsberg B. Patient-specific factors affecting patient-controlled analgesia dosing. J Pain Palliat Care Pharmacother. 2002;16:5–21. doi: 10.1080/j354v16n02_02. [DOI] [PubMed] [Google Scholar]
- 163.Goldberg R, Goff D, Cooper L, Luepker R, Zapka J, Bittner V, Osganian S, Lessard D, Cornell C, Meshack A, Mann C, Gilliland J, Feldman H. Age and sex differences in presentation of symptoms among patients with acute coronary disease: The REACT Trial: Rapid Early Action for Coronary Treatment. Coron Artery Dis. 2000;11:399–407. doi: 10.1097/00019501-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 164.Gordon NC, Gear RW, Heller PH, Paul S, Miaskowski C, Levine JD. Enhancement of morphine analgesia by the GABAB agonist baclofen. Neuroscience. 1995;69:345–349. doi: 10.1016/0306-4522(95)00335-g. [DOI] [PubMed] [Google Scholar]
- 165.Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136:142–149. doi: 10.1016/j.pain.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 166.Granot M, Yarnitsky D, Itskovitz-Eldor J, Granovsky Y, Peer E, Zimmer EZ. Pain perception in women with dysmenorrhea. Obstet Gynecol. 2001;98:407–411. doi: 10.1016/s0029-7844(01)01465-x. [DOI] [PubMed] [Google Scholar]
- 167.Green PG, Levine JD. Sexual dimorphism in the effect of nonhabituating stress on neurogenic plasma extravasation. Eur J Neurosci. 2005;21:486–492. doi: 10.1111/j.1460-9568.2005.03872.x. [DOI] [PubMed] [Google Scholar]
- 168.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: A consensus report. Pain. 2007;132(S1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grisel JE, Allen S, Nemmani KV, Fee JR, Carliss R. The influence of dextromethorphan on morphine analgesia in Swiss Webster mice is sex-specific. Pharmacol Biochem Behav. 2005;81:131–138. doi: 10.1016/j.pbb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 170.Guo HR, Chang YC, Yeh WY, Chen CW, Guo YL. Prevalence of musculoskeletal disorder among workers in Taiwan: A nationwide study. J Occup Health. 2004;46:26–36. doi: 10.1539/joh.46.26. [DOI] [PubMed] [Google Scholar]
- 171.Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, Macfarlane GJ, Chiu YH, Nicholl B, McBeth J. The role of psychosocial factors in predicting the onset of chronic widespread pain: Results from a prospective population-based study. Rheumatology (Oxford) 2007;46:666–671. doi: 10.1093/rheumatology/kel363. [DOI] [PubMed] [Google Scholar]
- 172.Gureje O, Akinpelu AO, Uwakwe R, Udofia O, Wakil A. Comorbidity and impact of chronic spinal pain in Nigeria. Spine. 2007;32:E495–E500. doi: 10.1097/BRS.0b013e31810768fc. [DOI] [PubMed] [Google Scholar]
- 173.Gwee KA, Wee S, Wong ML, Png DJ. The prevalence, symptom characteristics, and impact of irritable bowel syndrome in an Asian urban community. Am J Gastroenterol. 2004;99:924–931. doi: 10.1111/j.1572-0241.2004.04161.x. [DOI] [PubMed] [Google Scholar]
- 174.Hagen K, Zwart JA, Vatten L, Stovner LJ, Bovim G. Prevalence of migraine and non-migrainous headache–head-HUNT, a large population-based study. Cephalalgia. 2000;20:900–906. doi: 10.1046/j.1468-2982.2000.00145.x. [DOI] [PubMed] [Google Scholar]
- 175.Haleem DJ, Kennett GA, Curzon G. Hippocampal 5-hydroxytryptamine synthesis is greater in female rats than in males and more decreased by the 5-HT1A agonist 8-OH-DPAT. J Neural Transm Gen Sect. 1990;79:93–101. doi: 10.1007/BF01251004. [DOI] [PubMed] [Google Scholar]
- 176.Hall GC, Carroll D, Parry D, McQuay HJ. Epidemiology and treatment of neuropathic pain: The UK primary care perspective. Pain. 2006;122:156–162. doi: 10.1016/j.pain.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 177.Hamberg K, Risberg G, Johansson EE, Westman G. Gender bias in physicians’ management of neck pain: A study of the answers in a Swedish national examination. J Womens Health Gend Based Med. 2002;11:653–666. doi: 10.1089/152460902760360595. [DOI] [PubMed] [Google Scholar]
- 178.Hansen FR, Bendix T, Skov P, Jensen CV, Kristensen JH, Krohn L, Schioeler H. Intensive, dynamic back-muscle exercises, conventional physiotherapy, or placebo-control treatment of low-back pain: A randomized, observer-blind trial. Spine. 1993;18:98–108. doi: 10.1097/00007632-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 179.Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;11 doi: 10.1111/j.1526-4637.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 180.Heitkemper MM, Cain KC, Jarrett ME, Burr RL, Hertig V, Bond EF. Symptoms across the menstrual cycle in women with irritable bowel syndrome. Am J Gastroenterol. 2003;98:420–430. doi: 10.1111/j.1572-0241.2003.07233.x. [DOI] [PubMed] [Google Scholar]
- 181.Heitkemper MM, Jarrett M. Pattern of gastrointestinal and somatic symptoms across the menstrual cycle. Gastroenterology. 1992;102:505–513. doi: 10.1016/0016-5085(92)90097-i. [DOI] [PubMed] [Google Scholar]
- 182.Helgeson VS. The effects of masculinity and social support on recovery from myocardial infarction. Psychosom Med. 1991;53:621–633. doi: 10.1097/00006842-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 183.Hellstrom B, Lundberg U. Pain perception to the cold pressor test during the menstrual cycle in relation to estrogen levels and a comparison with men. Integr Physiol Behav Sci. 2000;35:132–141. doi: 10.1007/BF02688772. [DOI] [PubMed] [Google Scholar]
- 184.Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: A retrospective study of single-trial fMRI data. Neuroimage. 2008;39:1867–1876. doi: 10.1016/j.neuroimage.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 185.Henry P, Auray JP, Gaudin AF, Dartigues JF, Duru G, Lanteri-Minet M, Lucas C, Pradalier A, Chazot G, El HA. Prevalence and clinical characteristics of migraine in France. Neurology. 2002;59:232–237. doi: 10.1212/wnl.59.2.232. [DOI] [PubMed] [Google Scholar]
- 186.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 187.Hirasawa M, Hasegawa J, Nishiyama J, Suzuki T. Utilization of PCIA (patient-controlled intravenous analgesia) for postoperative analgesia of spine fusion. Tokai J Exp Clin Med. 2003;28:17–20. [PubMed] [Google Scholar]
- 188.Ho KH, Ong BK. A community-based study of headache diagnosis and prevalence in Singapore. Cephalalgia. 2003;23:6–13. doi: 10.1046/j.0333-1024.2002.00272.x. [DOI] [PubMed] [Google Scholar]
- 189.Hobson AR, Furlong PL, Worthen SF, Hillebrand A, Barnes GR, Singh KD, Aziz Q. Real-time imaging of human cortical activity evoked by painful esophageal stimulation. Gastroenterology. 2005;128:610–619. doi: 10.1053/j.gastro.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 190.Hoffmann DE, Tarzian AJ. The girl who cried pain: A bias against women in the treatment of pain. J Law Med Ethics. 2001;29:13–27. doi: 10.1111/j.1748-720x.2001.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 191.Huguet A, Miro J. The severity of chronic pediatric pain: An epidemiological study. J Pain. 2008;9:226–236. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 192.Ihlebaek C, Hansson TH, Laerum E, Brage S, Eriksen HR, Holm SH, Svendsrod R, Indahl A. Prevalence of low back pain and sickness absence: A “borderline” study in Norway and Sweden. Scand J Public Health. 2006;34:555–558. doi: 10.1080/14034940600552051. [DOI] [PubMed] [Google Scholar]
- 193.Isacson D, Bingefors K. Epidemiology of analgesic use: a gender perspective. Eur J Anaesthesiol Suppl. 2002;26:5–15. doi: 10.1097/00003643-200219261-00003. [DOI] [PubMed] [Google Scholar]
- 194.Isselee H, De Laat A, Bogaerts K, Lysens R. Long-term fluctuations of pressure pain thresholds in healthy men, normally menstruating women and oral contraceptive users. Eur J Pain. 2001;5:27–37. doi: 10.1053/eujp.2000.0213. [DOI] [PubMed] [Google Scholar]
- 195.Jackson T, Iezzi T, Chen H, Ebnet S, Eglitis K. Gender, interpersonal transactions, and the perception of pain: An experimental analysis. J Pain. 2005;6:228–236. doi: 10.1016/j.jpain.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 196.Jensen I, Nygren A, Gamberale F, Goldie I, Westerholm P. Coping with long-term musculoskeletal pain and its consequences: Is gender a factor? Pain. 1994;57:167–172. doi: 10.1016/0304-3959(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 197.Jensen IB, Bergstrom G, Ljungquist T, Bodin L, Nygren AL. A randomized controlled component analysis of a behavioral medicine rehabilitation program for chronic spinal pain: Are the effects dependent on gender? Pain. 2001;91:65–78. doi: 10.1016/s0304-3959(00)00420-6. [DOI] [PubMed] [Google Scholar]
- 198.Jensen MT, Petersen KL. Gender differences in pain and secondary hyperalgesia after heat/capsaicin sensitization in healthy volunteers. J Pain. 2006;7:211–217. doi: 10.1016/j.jpain.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 199.Ji Y, Murphy AZ, Traub RJ. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. J Pain. 2007;8:494–502. doi: 10.1016/j.jpain.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 200.Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain. 2002;100:55–64. doi: 10.1016/s0304-3959(02)00239-7. [DOI] [PubMed] [Google Scholar]
- 201.Jinks C, Jordan KP, Blagojevic M, Croft P. Predictors of onset and progression of knee pain in adults living in the community: A prospective study. Rheumatology (Oxford) 2008;47:368–374. doi: 10.1093/rheumatology/kem374. [DOI] [PubMed] [Google Scholar]
- 202.Joels CS, Mostafa G, Matthews BD, Kercher KW, Sing RF, Norton HJ, Heniford BT. Factors affecting intravenous analgesic requirements after colectomy. J Am Coll Surg. 2003;197:780–785. doi: 10.1016/S1072-7515(03)00671-9. [DOI] [PubMed] [Google Scholar]
- 203.Jones A, Zachariae R. Gender, anxiety, and experimental pain sensitivity: An overview. J Am Med Womens Assoc. 2002;57:91–94. [PubMed] [Google Scholar]
- 204.Jones A, Zachariae R. Investigation of the interactive effects of gender and psychological factors on pain response. Br J Health Psychol. 2004;9:405–418. doi: 10.1348/1359107041557101. [DOI] [PubMed] [Google Scholar]
- 205.Jones A, Zachariae R, Arendt-Nielsen L. Dispositional anxiety and the experience of pain: Gender-specific effects. Eur J Pain. 2003;7:387–395. doi: 10.1016/S1090-3801(02)00139-8. [DOI] [PubMed] [Google Scholar]
- 206.Kaiko RF, Wallenstein SL, Rogers AG, Houde RW. Sources of variation in analgesic responses in cancer patients with chronic pain receiving morphine. Pain. 1983;15:191–200. doi: 10.1016/0304-3959(83)90018-0. [DOI] [PubMed] [Google Scholar]
- 207.Kallai I, Barke A, Voss U. The effects of experimenter characteristics on pain reports in women and men. Pain. 2004;112:142–147. doi: 10.1016/j.pain.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 208.Kankaanpaa M, Taimela S, Airaksinen O, Hanninen O. The efficacy of active rehabilitation in chronic low back pain: Effect on pain intensity, self-experienced disability, and lumbar fatigability. Spine. 1999;24:1034–1042. doi: 10.1097/00007632-199905150-00019. [DOI] [PubMed] [Google Scholar]
- 209.Kawakami M, Yoshioka E, Konda N, Arita J, Visessuvan S. Data on the sites of stimulatory feedback action of gonadal steroids indispensable for luteinizing hormone release in the rat. Endocrinology. 1978;102:791–798. doi: 10.1210/endo-102-3-791. [DOI] [PubMed] [Google Scholar]
- 210.Kececi H, Dener S. Epidemiological and clinical characteristics of migraine in Sivas, Turkey. Headache. 2002;42:275–280. doi: 10.1046/j.1526-4610.2002.02080.x. [DOI] [PubMed] [Google Scholar]
- 211.Keefe FJ, Affleck G, France CR, Emery CF, Waters S, Caldwell DS, Stainbrook D, Hackshaw KV, Fox LC, Wilson K. Gender differences in pain, coping, and mood in individuals having osteoarthritic knee pain: A within-day analysis. Pain. 2004;110:571–577. doi: 10.1016/j.pain.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 212.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: The role of catastrophizing. Pain. 2000;87:325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 213.Keenan PA, Lindamer LA. Non-migraine headache across the menstrual cycle in women with and without premenstrual syndrome. Cephalalgia. 1992;12:356–359. doi: 10.1111/j.1468-2982.1992.00356.x. [DOI] [PubMed] [Google Scholar]
- 214.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 215.Keogh E, Barlow C, Mounce C, Bond FW. Assessing the relationship between cold pressor pain responses and dimensions of the anxiety sensitivity profile in healthy men and women. Cogn Behav Ther. 2006;35:198–206. doi: 10.1080/16506070600898330. [DOI] [PubMed] [Google Scholar]
- 216.Keogh E, Bond FW, Hanmer R, Tilston J. Comparing acceptance- and control-based coping instructions on the cold-pressor pain experiences of healthy men and women. Eur J Pain. 2005;9:591–598. doi: 10.1016/j.ejpain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 217.Keogh E, Eccleston C. Sex differences in adolescent chronic pain and pain-related coping. Pain. 2006;123:275–284. doi: 10.1016/j.pain.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 218.Keogh E, Hamid R, Hamid S, Ellery D. Investigating the effect of anxiety sensitivity, gender and negative interpretative bias on the perception of chest pain. Pain. 2004;111:209–217. doi: 10.1016/j.pain.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 219.Keogh E, Hatton K, Ellery D. Avoidance versus focused attention and the perception of pain: Differential effects for men and women. Pain. 2000;85:225–230. doi: 10.1016/s0304-3959(99)00270-5. [DOI] [PubMed] [Google Scholar]
- 220.Keogh E, McCracken LM, Eccleston C. Do men and women differ in their response to interdisciplinary chronic pain management? Pain. 2005;114:37–46. doi: 10.1016/j.pain.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 221.Kest B, Sarton E, Dahan A. Gender differences in opioid-mediated analgesia: Animal and human studies. Anesthesiology. 2000;93:539–547. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- 222.Kim H, Neubert JK, Rowan JS, Brahim JS, Iadarola MJ, Dionne RA. Comparison of experimental and acute clinical pain responses in humans as pain phenotypes. J Pain. 2004;5:377–384. doi: 10.1016/j.jpain.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 223.Kim H, Neubert JK, San MA, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–496. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 224.Kjoller M, Thoning H. Characteristics of non-response in the Danish Health Interview Surveys, 1987–1994. Eur J Public Health. 2005;15:528–535. doi: 10.1093/eurpub/cki023. [DOI] [PubMed] [Google Scholar]
- 225.Klosterhalfen S, Enck P. Neurophysiology and psychobiology of the placebo response. Curr Opin Psychiatry. 2008;21:189–195. doi: 10.1097/YCO.0b013e3282f50c36. [DOI] [PubMed] [Google Scholar]
- 226.Koltyn KF, Trine MR, Stegner AJ, Tobar DA. Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sports Exerc. 2001;33:282–290. doi: 10.1097/00005768-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 227.Komiyama O, De Laat A. Tactile and pain thresholds in the intra- and extra-oral regions of symptom-free subjects. Pain. 2005;115:308–315. doi: 10.1016/j.pain.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 228.Komiyama O, Kawara M, De Laat A. Ethnic differences regarding tactile and pain thresholds in the trigeminal region. J Pain. 2007;8:363–369. doi: 10.1016/j.jpain.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 229.Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain. 2006;7:151–160. doi: 10.1016/j.jpain.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 230.Krogstad BS, Jokstad A, Dahl BL, Vassend O. The reporting of pain, somatic complaints, and anxiety in a group of patients with TMD before and 2 years after treatment: Sex differences. J Orofacial Pain. 1996;10:263–269. [PubMed] [Google Scholar]
- 231.Kroner-Herwig B, Heinrich M, Morris L. Headache in German children and adolescents: A population-based epidemiological study. Cephalalgia. 2007;27:519–527. doi: 10.1111/j.1468-2982.2007.01319.x. [DOI] [PubMed] [Google Scholar]
- 232.Ku JH, Jeon YS, Kim ME, Lee NK, Park YH. Psychological problems in young men with chronic prostatitis-like symptoms. Scand J Urol Nephrol. 2002;36:296–301. doi: 10.1080/003655902320248272. [DOI] [PubMed] [Google Scholar]
- 233.Kuba T, Wu HB, Nazarian A, Festa ED, Barr GA, Jenab S, Inturrisi CE, Quinones-Jenab V. Estradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female rats. Horm Behav. 2006;49:441–449. doi: 10.1016/j.yhbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 234.Lacey RJ, Thomas E, Duncan RC, Peat G. Gender difference in symptomatic radiographic knee osteoarthritis in the Knee Clinical Assessment–CAS(K): A prospective study in the general population. BMC Musculoskel Disord. 2008;9:82. doi: 10.1186/1471-2474-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lau H, Patil NG. Acute pain after endoscopic totally extraperitoneal (TEP) inguinal hernioplasty: Multivariate analysis of predictive factors. Surg Endosc. 2004;18:92–96. doi: 10.1007/s00464-003-9068-y. [DOI] [PubMed] [Google Scholar]
- 236.Laurell K, Larsson B, Eeg-Olofsson O. Prevalence of headache in Swedish schoolchildren, with a focus on tension-type headache. Cephalalgia. 2004;24:380–388. doi: 10.1111/j.1468-2982.2004.00681.x. [DOI] [PubMed] [Google Scholar]
- 237.Lautenbacher S, Krieg JG. Pain perception in psychiatric disorders: a review of the literature. J Psychiatr Res. 1994;28:109–122. doi: 10.1016/0022-3956(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 238.Lautenbacher S, Nielsen J, Andersen T. Arendt-Nielsen L:. Spatial summation of heat pain in males and females. Somatosens Mot Res. 2001;18:101–105. doi: 10.1080/135578501012006192-1. [DOI] [PubMed] [Google Scholar]
- 239.Lee OY, Mayer EA, Schmulson M, Chang L, Naliboff B. Gender-related differences in IBS symptoms. Am J Gastroenterol. 2001;96:2184–2193. doi: 10.1111/j.1572-0241.2001.03961.x. [DOI] [PubMed] [Google Scholar]
- 240.Lee SY, Kim JH, Sung IK, Park HS, Jin CJ, Choe WH, Kwon SY, Lee CH, Choi KW. Irritable bowel syndrome is more common in women regardless of the menstrual phase: A Rome II-based survey. J Korean Med Sci. 2007;22:851–854. doi: 10.3346/jkms.2007.22.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee YC, Wang HP, Chiu HM, Lin CP, Huang SP, Lai YP, Wu MS, Chen MF, Lin JT. Factors determining post-colonoscopy abdominal pain: Prospective study of screening colonoscopy in 1000 subjects. J Gastroenterol Hepatol. 2006;21:1575–1580. doi: 10.1111/j.1440-1746.2006.04145.x. [DOI] [PubMed] [Google Scholar]
- 242.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 243.LeResche L. Gender considerations in the epidemiology of chronic pain. In: Crombie IK, editor. Epidemiology of Pain. Seattle: IASP Press; 1999. pp. 43–52. [Google Scholar]
- 244.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 245.LeResche L, Mancl LA, Drangsholt MT, Saunders K, Korff MV. Relationship of pain and symptoms to pubertal development in adolescents. Pain. 2005;118:201–209. doi: 10.1016/j.pain.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 246.LeResche L, Saunders K, Von Korff MR, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69:153–160. doi: 10.1016/s0304-3959(96)03230-7. [DOI] [PubMed] [Google Scholar]
- 247.LeResche L, Sherman JJ, Huggins K, Saunders K, Mancl LA, Lentz G, Dworkin SF. Musculoskeletal orofacial pain and other signs and symptoms of temporomandibular disorders during pregnancy: a prospective study. J Orofac Pain. 2005;19:193–201. [PubMed] [Google Scholar]
- 248.Levine FM, De Simone LL. The effects of experimenter gender on pain report in male and female subjects. Pain. 1991;44:69–72. doi: 10.1016/0304-3959(91)90149-R. [DOI] [PubMed] [Google Scholar]
- 249.Lichten EM, Lichten JB, Whitty A, Pieper D. The confirmation of a biochemical marker for women’s hormonal migraine: The depoestradiol challenge test. Headache. 1996;36:367–371. doi: 10.1046/j.1526-4610.1996.3606367.x. [DOI] [PubMed] [Google Scholar]
- 250.Liem MS, van Duyn EB, van der GY, van Vroonhoven TJ. Recurrences after conventional anterior and laparoscopic inguinal hernia repair: A randomized comparison. Ann Surg. 2003;237:136–141. doi: 10.1097/00000658-200301000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 252.Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J. Classification of primary headaches. Neurology. 2004;63:427–435. doi: 10.1212/01.wnl.0000133301.66364.9b. [DOI] [PubMed] [Google Scholar]
- 253.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 254.Lomas LM, Terner JM, Picker MJ. Sex differences in NMDA antagonist enhancement of morphine antihyperalgesia in a capsaicin model of persistent pain: Comparisons to two models of acute pain. Pharmacol Biochem Behav. 2008;89:127–136. doi: 10.1016/j.pbb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 255.Lookingland KJ, Moore KE. Effects of estradiol and prolactin on incertohypothalamic dopaminergic neurons in the male rat. Brain Res. 1984;323:83–91. doi: 10.1016/0006-8993(84)90267-1. [DOI] [PubMed] [Google Scholar]
- 256.Lovgren M, Tishelman C, Sprangers M, Koyi H, Hamberg K. Symptoms and problems with functioning among women and men with inoperable lung cancer: A longitudinal study. Lung Cancer. 2008;60:113–124. doi: 10.1016/j.lungcan.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 257.Lowery D, Fillingim RB, Wright RA. Sex differences and incentive effects on perceptual and cardiovascular responses to cold pressor pain. Psychosom Med. 2003;65:284–291. doi: 10.1097/01.psy.0000033127.11561.78. [DOI] [PubMed] [Google Scholar]
- 258.Lu CL, Chen CY, Lang HC, Luo JC, Wang SS, Chang FY, Lee SD. Current patterns of irritable bowel syndrome in Taiwan: The Rome II questionnaire on a Chinese population. Aliment Pharmacol Ther. 2003;18:1159–1169. doi: 10.1046/j.1365-2036.2003.01711.x. [DOI] [PubMed] [Google Scholar]
- 259.Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Sex and age differences in coping styles among children with chronic pain. J Pain Symptom Manage. 2007;33:208–216. doi: 10.1016/j.jpainsymman.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 260.Macfarlane TV, Blinkhorn A, Worthington HV, Davies RM, Macfarlane GJ. Sex hormonal factors and chronic widespread pain: A population study among women. Rheumatology (Oxford) 2002;41:454–457. doi: 10.1093/rheumatology/41.4.454. [DOI] [PubMed] [Google Scholar]
- 261.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8:334–342. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 263.Mannion AF, Junge A, Taimela S, Muntener M, Lorenzo K, Dvorak J. Active therapy for chronic low back pain, part 3: Factors influencing self-rated disability and its change following therapy. Spine. 2001;26:920–929. doi: 10.1097/00007632-200104150-00015. [DOI] [PubMed] [Google Scholar]
- 264.Marbach JJ, Ballard GT, Frankel MR, Raphael KG. Patterns of TMJ surgery: Evidence of sex differences [see comments] J Am Dental Assoc. 1997;128:609–614. doi: 10.14219/jada.archive.1997.0260. [DOI] [PubMed] [Google Scholar]
- 265.Marchand S, Arsenault P. Odors modulate pain perception: A gender-specific effect. Physiol Behav. 2002;76:251–256. doi: 10.1016/s0031-9384(02)00703-5. [DOI] [PubMed] [Google Scholar]
- 266.Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, Ritz L, Kornstein S, Young EA, Trivedi MH. Sex differences in depression symptoms in treatment-seeking adults: Confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Compr Psychiatry. 2008;49:238–246. doi: 10.1016/j.comppsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Marklund S, Wanman A. Incidence and prevalence of myofascial pain in the jaw-face region: A one-year prospective study on dental students. Acta Odontol Scand. 2008;66:113–121. doi: 10.1080/00016350802010372. [DOI] [PubMed] [Google Scholar]
- 268.Martikainen IK, Narhi MV, Pertovaara A. Spatial integration of cold pressor pain sensation in humans. Neurosci Lett. 2004;361:140–143. doi: 10.1016/j.neulet.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 269.Mattila K, Toivonen J, Janhunen L, Rosenberg PH, Hynynen M. Postdischarge symptoms after ambulatory surgery: First-week incidence, intensity, and risk factors. Anesth Analg. 2005;101:1643–1650. doi: 10.1213/01.ANE.0000184189.79572.28. [DOI] [PubMed] [Google Scholar]
- 270.McEwen BS. Invited review: Estrogens effects on the brain: Multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 271.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 272.McGrath PA. Chronic pain in children. In: Crombie IK, editor. Epidemiology of Pain. Seattle: IASP Press; 1999. pp. 81–101. [Google Scholar]
- 273.McNally JD, Matheson DA, Bakowsky VS. The epidemiology of self-reported fibromyalgia in Canada. Chronic Dis Can. 2006;27:9–16. [PubMed] [Google Scholar]
- 274.McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;21(148):1015–1020. doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Merskey H, Bogduk N. Classification of Chronic Pain. 2. Seattle: IASP Press; 1994. [Google Scholar]
- 276.Miaskowski C. Gender differences in pain, fatigue, and depression in patients with cancer. J Natl Cancer Inst Monogr. 2004:139–143. doi: 10.1093/jncimonographs/lgh024. [DOI] [PubMed] [Google Scholar]
- 277.Miaskowski C, Levine JD. Does opioid analgesia show a gender preference for females? Pain Forum. 1999;8:34–44. [Google Scholar]
- 278.Mikkelsson M, Salminen JJ, Kautiainen H. Non-specific musculoskeletal pain in preadolescents: Prevalence and 1-year persistence. Pain. 1997;73:29–35. doi: 10.1016/s0304-3959(97)00073-0. [DOI] [PubMed] [Google Scholar]
- 279.Miller C, Newton SE. Pain perception and expression: The influence of gender, personal self-efficacy, and lifespan socialization. Pain Manag Nurs. 2006;7:148–152. doi: 10.1016/j.pmn.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 280.Miller PL, Ernst AA. Sex differences in analgesia: A randomized trial of mu versus kappa opioid agonists. South Med J. 2004;97:35–41. doi: 10.1097/01.smj.0000085743.68121.a9. [DOI] [PubMed] [Google Scholar]
- 281.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mitchell LA, MacDonald RA, Brodie EE. A comparison of the effects of preferred music, arithmetic and humour on cold pressor pain. Eur J Pain. 2006;10:343–351. doi: 10.1016/j.ejpain.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 283.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 284.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. The melanocor-tin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100:4867–4862. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- 286.Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 287.Munce SE, Stewart DE. Gender differences in depression and chronic pain conditions in a national epidemiologic survey. Psychosomatics. 2007;48:394–399. doi: 10.1176/appi.psy.48.5.394. [DOI] [PubMed] [Google Scholar]
- 288.Musgrave DS, Vogt MT, Nevitt MC, Cauley JA. Back problems among postmenopausal women taking estrogen replacement therapy. Spine. 2001;26:1606–1612. doi: 10.1097/00007632-200107150-00023. [DOI] [PubMed] [Google Scholar]
- 289.Myers CD, Riley JL, III, Robinson ME. Psychosocial contributions to sex-correlated differences in pain. Clin J Pain. 2003;19:225–232. doi: 10.1097/00002508-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 290.Myers CD, Robinson ME, Riley JL, III, Sheffield D. Sex, gender, and blood pressure: Contributions to experimental pain report. Psychosom Med. 2001;63:545–550. doi: 10.1097/00006842-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 291.Nakai A, Kumakura Y, Boivin M, Rosa P, Diksic M, D’Souza D, Kersey K. Sex differences of brain serotonin synthesis in patients with irritable bowel syndrome using alpha-[11C]methyl-L-tryptophan, positron emission tomography and statistical parametric mapping. Can J Gastroenterol. 2003;17:191–196. doi: 10.1155/2003/572127. [DOI] [PubMed] [Google Scholar]
- 292.Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: Central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 293.Nayak S, Shiflett SC, Eshun S, Levine FM. Culture and gender effects in pain beliefs and the prediction of pain tolerance: Cross-Cultural Research. J Comparative Soc Sci. 2000;34:135–151. [Google Scholar]
- 294.Nemmani KV, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific N-methyl-D-aspartate receptor antagonists: Dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109:274–283. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 295.Nie H, Arendt-Nielsen L, Andersen H, Graven-Nielsen T. Temporal summation of pain evoked by mechanical stimulation in deep and superficial tissue. J Pain. 2005;6:348–355. doi: 10.1016/j.jpain.2005.01.352. [DOI] [PubMed] [Google Scholar]
- 296.Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, Harris JR. Individual differences in pain sensitivity: Genetic and environmental contributions. Pain. 2008;136:21–29. doi: 10.1016/j.pain.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 297.Nikolajsen L, Brandsborg B, Lucht U, Jensen TS, Kehlet H. Chronic pain following total hip arthroplasty: A nationwide questionnaire study. Acta Anaesthesiol Scand. 2006;50:495–500. doi: 10.1111/j.1399-6576.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- 298.Nyklicek I, Vingerhoets AJ, Van Heck GL. Hypertension and pain sensitivity: Effects of gender and cardiovascular reactivity. Biol Psychol. 1999;50:127–142. doi: 10.1016/s0301-0511(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 299.Ockene JK, Barad DH, Cochrane BB, Larson JC, Gass M, Wassertheil-Smoller S, Manson JE, Barnabei VM, Lane DS, Brzyski RG, Rosal MC, Wylie-Rosett J, Hays J. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–193. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- 300.Okifuji A, Turk DC. Sex hormones and pain in regularly menstruating women with fibromyalgia syndrome. J Pain. 2006;7:851–859. doi: 10.1016/j.jpain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 301.Oksuz E. Prevalence, risk factors, and preference-based health states of low back pain in a Turkish population. Spine. 2006;31:E968–E972. doi: 10.1097/01.brs.0000247787.25382.3c. [DOI] [PubMed] [Google Scholar]
- 302.Okunseri C, Hodges JS, Born D. Self-reported toothache experience in an adult population in Benin City, Edo State, Nigeria. Oral Health Prev Dent. 2005;3:119–125. [PubMed] [Google Scholar]
- 303.Olofsen E, Romberg R, Bijl H, Mooren R, Engbers F, Kest B, Dahan A. Alfentanil and placebo analgesia: no sex differences detected in models of experimental pain. Anesthesiology. 2005;103:130–139. doi: 10.1097/00000542-200507000-00020. [DOI] [PubMed] [Google Scholar]
- 304.Omokhodion FO. Low back pain in a rural community in South West Nigeria. West Afr J Med. 2002;21:87–90. [PubMed] [Google Scholar]
- 305.Oshima M, Ogawa R, Londyn D. Current perception threshold increases during pregnancy but does not change across menstrual cycle. J Nippon Med Sch. 2002;69:19–23. doi: 10.1272/jnms.69.19. [DOI] [PubMed] [Google Scholar]
- 306.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: Further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 307.Otto MW, Dougher MJ. Sex differences and personality factors in responsivity to pain. Percept Mot Skills. 1985;61:383–390. doi: 10.2466/pms.1985.61.2.383. [DOI] [PubMed] [Google Scholar]
- 308.Overdad K. Epidemiological study design and measurement of associations. In: Olesen J, editor. Headache Classification and Epidemiology. New York: Raven Press; 1994. pp. 189–198. [Google Scholar]
- 309.Ozan F, Polat S, Kara I, Kucuk D, Polat HB. Prevalence study of signs and symptoms of temporomandibular disorders in a Turkish population. J Contemp Dent Pract. 2007;8:35–42. [PubMed] [Google Scholar]
- 310.Pamuk ON, Cakir N. The variation in chronic widespread pain and other symptoms in fibromyalgia patients: The effects of menses and menopause. Clin Exp Rheumatol. 2005;23:778–782. [PubMed] [Google Scholar]
- 311.Papageorgiou AC, Silman AJ, Macfarlane GJ. Chronic widespread pain in the population: A seven year follow up study. Ann Rheum Dis. 2002;61:1071–1074. doi: 10.1136/ard.61.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Paulson PE, Minoshima S, Morrow TJ, Casey KL. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76:223–229. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, Bohnen AM, van Suijlekom-Smit LW, Passchier J, van der Wouden JC. Pain in children and adolescents: A common experience. Pain. 2000;87:51–58. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 314.Petersen S, Brulin C, Bergstrom E. Recurrent pain symptoms in young schoolchildren are often multiple. Pain. 2006;121:145–150. doi: 10.1016/j.pain.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 315.Picavet HS, Hazes JM. Prevalence of self reported musculoskeletal diseases is high. Ann Rheum Dis. 2003;62:644–650. doi: 10.1136/ard.62.7.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Pinerua-Shuhaibar L, Prieto-Rincon D, Ferrer A, Bonilla E, Maixner W, Suarez-Roca H. Reduced tolerance and cardiovascular response to ischemic pain in minor depression. J Affect Disord. 1999;56:119–126. doi: 10.1016/s0165-0327(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 317.Pool GJ, Schwegler AF, Theodore BR, Fuchs PN. Role of gender norms and group identification on hypothetical and experimental pain tolerance. Pain. 2007;129:122–129. doi: 10.1016/j.pain.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 318.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 319.Pud D, Sprecher E, Yarnitsky D. Homotopic and hetero-topic effects of endogenous analgesia in healthy volunteers. Neurosci Lett. 2005;380:209–213. doi: 10.1016/j.neulet.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 320.Pud D, Yarnitsky D, Sprecher E, Rogowski Z, Adler R, Eisenberg E. Can personality traits and gender predict the response to morphine? An experimental cold pain study. Eur J Pain. 2006;10:103–112. doi: 10.1016/j.ejpain.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 321.Quiton RL, Greenspan JD. Sex differences in endogenous pain modulation by distracting and painful conditioning stimulation. Pain. 2007;132(Suppl 1):S134–S149. doi: 10.1016/j.pain.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Rauhala K, Oikarinen KS, Jarvelin MR, Raustia AM. Facial pain and temporomandibular disorders: An epidemiological study of the Northern Finland 1966 Birth Cohort. Cranio. 2000;18:40–46. doi: 10.1080/08869634.2000.11746112. [DOI] [PubMed] [Google Scholar]
- 323.Reyes-Gibby CC, Aday LA, Anderson KO, Mendoza TR, Cleeland CS. Pain, depression, and fatigue in community-dwelling adults with and without a history of cancer. J Pain Symptom Manage. 2006;32:118–128. doi: 10.1016/j.jpainsymman.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Rhudy JL, Meagher MW. Noise stress and human pain thresholds: Divergent effects in men and women. J Pain. 2001;2:57–64. doi: 10.1054/jpai.2000.19947. [DOI] [PubMed] [Google Scholar]
- 325.Rhudy JL, Williams AE. Gender differences in pain: Do emotions play a role? Gend Med. 2005;2:208–226. doi: 10.1016/s1550-8579(05)80051-8. [DOI] [PubMed] [Google Scholar]
- 326.Riley JL, III, Gilbert GH. Orofacial pain symptoms: An interaction between age and sex. Pain. 2001;90:245–256. doi: 10.1016/S0304-3959(00)00408-5. [DOI] [PubMed] [Google Scholar]
- 327.Riley JL, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: A meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 328.Riley JLI, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 329.Ritter MA, Wing JT, Berend ME, Davis KE, Meding JB. The clinical effect of gender on outcome of total knee arthroplasty. J Arthroplasty. 2008;23:331–336. doi: 10.1016/j.arth.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 330.Rivest R, Falardeau P, Di PT. Brain dopamine transporter: Gender differences and effect of chronic haloperidol. Brain Res. 1995;692:269–272. doi: 10.1016/0006-8993(95)00611-s. [DOI] [PubMed] [Google Scholar]
- 331.Robin O, Vinard H, Vernet Maury E, Saumet JL. Influence of sex and anxiety on pain threshold and tolerance. Funct Neurol. 1987;2:173–179. [PubMed] [Google Scholar]
- 332.Robinson ME, Dannecker EA, George SZ, Otis J, Atchison JW, Fillingim RB. Sex differences in the associations among psychological factors and pain report: A novel psychophysical study of patients with chronic low back pain. J Pain. 2005;6:463–470. doi: 10.1016/j.jpain.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 333.Robinson ME, Gagnon CM, Riley JL, III, Price DD. Altering gender role expectations: Effects on pain tolerance, pain threshold, and pain. ratings. J Pain. 2003;4:284–288. doi: 10.1016/s1526-5900(03)00559-5. [DOI] [PubMed] [Google Scholar]
- 334.Robinson ME, Riley JL, Brown FF, Gremillion H. Sex differences in response to cutaneous anesthesia: A double blind randomized study. Pain. 1998;77:143–149. doi: 10.1016/S0304-3959(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 335.Robinson ME, Riley J, III, Myers CD. Psychosocial contributions to sex-related differences in pain responses. In: Fillingim RB, editor. Sex, Gender, and Pain. Seattle: IASP Press; 2000. pp. 41–68. [Google Scholar]
- 336.Robinson ME, Riley JL, III, Myers CD, Papas RK, Wise EA, Waxenberg LA, Fillingim RB. Gender role expectations of pain: Relationship to sex differences in pain. J Pain. 2001;2:251–257. doi: 10.1054/jpai.2001.24551. [DOI] [PubMed] [Google Scholar]
- 337.Robinson ME, Riley JLI. The role of emotion in pain. In: Gatchel RJ, Turk DC, editors. Psychosocial Factors in Pain. New York: Guilford Press; 1999. pp. 74–88. [Google Scholar]
- 338.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 339.Roger VL, Farkouh ME, Weston SA, Reeder GS, Jacobsen SJ, Zinsmeister AR, Yawn BP, Kopecky SL, Gabriel SE. Sex differences in evaluation and outcome of unstable angina. JAMA. 2000;283:646–652. doi: 10.1001/jama.283.5.646. [DOI] [PubMed] [Google Scholar]
- 340.Rollman GB. Gender differences in pain: Role of anxiety. Pain Forum. 1995;4:231–234. [Google Scholar]
- 341.Rollman GB, Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain. 2001;17:20–24. doi: 10.1097/00002508-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 342.Romberg R, Olofsen E, Sarton E, den HJ, Taschner PE, Dahan A. Pharmacokinetic-pharmacodynamic modeling of morphine-6-glucuronide-induced analgesia in healthy volunteers: Absence of sex differences. Anesthesiology. 2004;100:120–133. doi: 10.1097/00000542-200401000-00021. [DOI] [PubMed] [Google Scholar]
- 343.Rosemann T, Backenstrass M, Joest K, Rosemann A, Szecsenyi J, Laux G. Predictors of depression in a sample of 1,021 primary care patients with osteoarthritis. Arthritis Rheum. 2007;57:415–422. doi: 10.1002/art.22624. [DOI] [PubMed] [Google Scholar]
- 344.Rosen A, Feldreich A, Dabirian N, Ernberg M. Effect of heterotopic noxious conditioning stimulation on electrical and pressure pain thresholds in two different anatomical regions. Acta Odontol Scand. 2008;66:181–188. doi: 10.1080/00016350802169111. [DOI] [PubMed] [Google Scholar]
- 345.Rosseland LA, Solheim N, Stubhaug A. Pain and disability 1 year after knee arthroscopic procedures. Acta Anaesthesiol Scand. 2008;52:332–337. doi: 10.1111/j.1399-6576.2007.01541.x. [DOI] [PubMed] [Google Scholar]
- 346.Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: Women report more pain than men after arthroscopic surgery. Pain. 2004;112:248–253. doi: 10.1016/j.pain.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 347.Roth-Isigkeit A, Thyen U, Raspe HH, Stoven H, Schmucker P. Reports of pain among German children and adolescents: An epidemiological study. Acta Paediatr. 2004;93:258–263. [PubMed] [Google Scholar]
- 348.Ruda MA. Gender and pain [editorial] Pain. 1993;53:1–2. doi: 10.1016/0304-3959(93)90048-T. [DOI] [PubMed] [Google Scholar]
- 349.Russell MB, Levi N, Saltyte-Benth J, Fenger K. Tension-type headache in adolescents and adults: A population based study of 33,764 twins. Eur J Epidemiol. 2006;21:153–160. doi: 10.1007/s10654-005-6031-3. [DOI] [PubMed] [Google Scholar]
- 350.Rustoen T, Fossa SD, Skarstein J, Moum T. The impact of demographic and disease-specific variables on pain in cancer patients. J Pain Symptom Manage. 2003;26:696–704. doi: 10.1016/s0885-3924(03)00239-2. [DOI] [PubMed] [Google Scholar]
- 351.Rustoen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Gender differences in chronic pain: Findings from a population-based study of Norwegian adults. Pain Manag Nurs. 2004;5:105–117. doi: 10.1016/j.pmn.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 352.Rutkiewicz T, Kononen M, Suominen-Taipale L, Nordblad A, Alanen P. Occurrence of clinical signs of tempo-romandibular disorders in adult Finns. J Orofac Pain. 2006;20:208–217. [PubMed] [Google Scholar]
- 353.Ryan JL, Jureidini B, Hodges JS, Baisden M, Swift JQ, Bowles WR. Gender differences in analgesia for endodontic pain. J Endod. 2008;34:552–556. doi: 10.1016/j.joen.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 354.Safran DG, Rogers WH, Tarlov AR, McHorney CA, Ware JE., Jr Gender differences in medical treatment: The case of physician-prescribed activity restrictions. Soc Sci Med. 1997;45:711–722. doi: 10.1016/s0277-9536(96)00405-4. [DOI] [PubMed] [Google Scholar]
- 355.Salaffi F, Leardini G, Canesi B, Mannoni A, Fioravanti A, Caporali R, Lapadula G, Punzi L. Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index in Italian patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2003;11:551–560. doi: 10.1016/s1063-4584(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 356.Sances G, Granella F, Nappi RE, Fignon A, Ghiotto N, Polatti F, Nappi G. Course of migraine during pregnancy and postpartum: A prospective study. Cephalalgia. 2003;23:197–205. doi: 10.1046/j.1468-2982.2003.00480.x. [DOI] [PubMed] [Google Scholar]
- 357.Sandler RS. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology. 1990;99:409–415. doi: 10.1016/0016-5085(90)91023-y. [DOI] [PubMed] [Google Scholar]
- 358.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: Incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 359.Sanford SD, Kersh BC, Thorn BE, Rich MA, Ward LC. Psychosocial mediators of sex differences in pain responsivity. J Pain. 2002;3:58–64. doi: 10.1054/jpai.2002.xb30066. [DOI] [PubMed] [Google Scholar]
- 360.Sarlani E, Farooq N, Greenspan JD. Gender and laterality differences in thermosensation throughout the perceptible range. Pain. 2003;106:9–18. doi: 10.1016/s0304-3959(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 361.Sarlani E, Garrett PH, Grace EG, Greenspan JD. Temporal summation of pain characterizes women but not men with temporomandibular disorders. J Orofac Pain. 2007;21:309–317. [PMC free article] [PubMed] [Google Scholar]
- 362.Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. Pain. 2004;109:115–123. doi: 10.1016/j.pain.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 363.Sarlani E, Greenspan JD. Gender differences in temporal summation of mechanically evoked pain. Pain. 2002;97:163–169. doi: 10.1016/s0304-3959(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 364.Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A. Sex differences in morphine analgesia: An experimental study in healthy volunteers. Anesthesiology. 2000;93:1245–1254. doi: 10.1097/00000542-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 365.Scher AI, Stewart WF, Lipton RB. Migraine and headache: A meta-analytic approach. In: Crombie IK, editor. Epidemiology of Pain. Seattle: IASP Press; 1999. pp. 159–170. [Google Scholar]
- 366.Schmidt CE, Bestmann B, Kuchler T, Longo WE, Rohde V, Kremer B. Gender differences in quality of life of patients with rectal cancer: A five-year prospective study. World J Surg. 2005;29:1630–1641. doi: 10.1007/s00268-005-0067-0. [DOI] [PubMed] [Google Scholar]
- 367.Schmidt CO, Raspe H, Pfingsten M, Hasenbring M, Basler HD, Eich W, Kohlmann T. Back pain in the German adult population: Prevalence, severity, and sociodemo-graphic correlates in a multiregional survey. Spine. 2007;32:2005–2011. doi: 10.1097/BRS.0b013e318133fad8. [DOI] [PubMed] [Google Scholar]
- 368.Schneider S, Randoll D, Buchner M. Why do women have back pain more than men? A representative prevalence study in the federal republic of Germany. Clin J Pain. 2006;22:738–747. doi: 10.1097/01.ajp.0000210920.03289.93. [DOI] [PubMed] [Google Scholar]
- 369.Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, Dube R, Taleghani CK, Burke JE, Williams S, Eisenberg JM, Escarce JJ. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;25(340):618–626. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 370.Schwartz BS, Stewart WF, Simon D, Lipton RB. Epidemiology of tension-type headache. JAMA. 1998;279:381–383. doi: 10.1001/jama.279.5.381. [DOI] [PubMed] [Google Scholar]
- 371.Serrao M, Rossi P, Sandrini G, Parisi L, Amabile GA, Nappi G, Pierelli F. Effects of diffuse noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain. 2004;112:353–360. doi: 10.1016/j.pain.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 372.Sherman JJ, LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. Am J Physiol Regul Integr Comp Physiol. 2006;291:R245–R256. doi: 10.1152/ajpregu.00920.2005. [DOI] [PubMed] [Google Scholar]
- 373.Sherman JJ, LeResche L, Mancl LA, Huggins K, Sage JC, Dworkin SF. Cyclic effects on experimental pain response in women with temporomandibular disorders. J Orofac Pain. 2005;19:133–143. [PubMed] [Google Scholar]
- 374.Shiotani A, Miyanishi T, Takahashi T. Sex differences in irritable bowel syndrome in Japanese university students. J Gastroenterol. 2006;41:562–568. doi: 10.1007/s00535-006-1805-2. [DOI] [PubMed] [Google Scholar]
- 375.Silverstein B. Gender differences in the prevalence of somatic versus pure depression: A replication. Am J Psychiatry. 2002;159:1051–1052. doi: 10.1176/appi.ajp.159.6.1051. [DOI] [PubMed] [Google Scholar]
- 376.Simren M, Abrahamsson H, Svedlund J, Bjornsson ES. Quality of life in patients with irritable bowel syndrome seen in referral centers versus primary care: The impact of gender and predominant bowel pattern. Scand J Gastroenterol. 2001;36:545–552. doi: 10.1080/003655201750153476. [DOI] [PubMed] [Google Scholar]
- 377.Smith BH, Elliott AM, Chambers WA, Smith WC, Hannaford PC, Penny K. The impact of chronic pain in the community. Fam Pract. 2001;18:292–299. doi: 10.1093/fampra/18.3.292. [DOI] [PubMed] [Google Scholar]
- 378.Smith G, Dunbar SB, Valderrama AL, Viswanathan B. Gender differences in implantable cardioverter-defibrillator patients at the time of insertion. Prog Cardiovasc Nurs. 2006;21:76–82. doi: 10.1111/j.0889-7204.2006.04843.x. [DOI] [PubMed] [Google Scholar]
- 379.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotrans-mission in women. J Neurosci. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Soderberg K, Sundstrom PI, Nyberg S, Backstrom T, Nordh E. Psychophysically determined thresholds for thermal perception and pain perception in healthy women across the menstrual cycle. Clin J Pain. 2006;22:610–616. doi: 10.1097/01.ajp.0000210904.75472.63. [DOI] [PubMed] [Google Scholar]
- 381.Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355–365. doi: 10.1212/wnl.22.4.355. [DOI] [PubMed] [Google Scholar]
- 382.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 383.Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- 384.Staud R, Robinson ME, Vierck CJ, Jr, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–174. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 385.Steiner TJ, Scher AI, Stewart WF, Kolodner K, Liberman J, Lipton RB. The prevalence and disability burden of adult migraine in England and their relationships to age, gender and ethnicity. Cephalalgia. 2003;23:519–527. doi: 10.1046/j.1468-2982.2003.00568.x. [DOI] [PubMed] [Google Scholar]
- 386.Stening K, Eriksson O, Wahren L, Berg G, Hammar M, Blomqvist A. Pain sensations to the cold pressor test in normally menstruating women: Comparison with men and relation to menstrual phase and serum sex steroid levels. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1711–R1716. doi: 10.1152/ajpregu.00127.2007. [DOI] [PubMed] [Google Scholar]
- 387.Sternberg WF, Bokat C, Kass L, Alboyadjian A, Gracely RH. Sex-dependent components of the analgesia produced by athletic competition. J Pain. 2001;2:65–74. doi: 10.1054/jpai.2001.18236. [DOI] [PubMed] [Google Scholar]
- 388.Stewart SH, Asmundson GJ. Anxiety sensitivity and its impact on pain experiences and conditions: A state of the art. Cogn Behav Ther. 2006;35:185–188. doi: 10.1080/16506070601090457. [DOI] [PubMed] [Google Scholar]
- 389.Stewart SH, Taylor S, Baker JM. Gender differences in dimensions of anxiety sensitivity. J Anxiety Disord. 1997;11:179–200. doi: 10.1016/s0887-6185(97)00005-4. [DOI] [PubMed] [Google Scholar]
- 390.Stewart WF, Linet MS, Celentano DD, Van NM, Ziegler D. Age- and sex-specific incidence rates of migraine with and without visual aura. Am J Epidemiol. 1991;134:1111–1120. doi: 10.1093/oxfordjournals.aje.a116014. [DOI] [PubMed] [Google Scholar]
- 391.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States: Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–69. [PubMed] [Google Scholar]
- 392.Stovner LJ, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, Steiner T, Zwart JA. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 393.Straneva PA, Maixner W, Light KC, Pedersen CA, Costello NL, Girdler SS. Menstrual cycle, beta-endorphin and pain sensitivity in premenstrual dysphoric disorder. Health Psychol. 2002;21:358–367. [PubMed] [Google Scholar]
- 394.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 395.Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum Brain Mapp. 2008;24 doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101:221–227. doi: 10.1016/S0304-3959(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 397.Symmons DP, van Hemert AM, Vandenbroucke JP, Valkenburg HA. A longitudinal study of back pain and radiological changes in the lumbar spines of middle aged women, I: Clinical findings. Ann Rheum Dis. 1991;50:158–161. doi: 10.1136/ard.50.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Taenzer AH, Clark C, Curry CS. Gender affects report of pain and function after arthroscopic anterior cruciate ligament reconstruction. Anesthesiology. 2000;93:670–675. doi: 10.1097/00000542-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 399.Takeshima T, Ishizaki K, Fukuhara Y, Ijiri T, Kusumi M, Wakutani Y, Mori M, Kawashima M, Kowa H, Adachi Y, Urakami K, Nakashima K. Population-based door-to-door survey of migraine in Japan: The Daisen study. Headache. 2004;44:8–19. doi: 10.1111/j.1526-4610.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 400.Tanko LB, Sondergaard BC, Oestergaard S, Karsdal MA, Christiansen C. An update review of cellular mechanisms conferring the indirect and direct effects of estrogen on articular cartilage. Climacteric. 2008;11:4–16. doi: 10.1080/13697130701857639. [DOI] [PubMed] [Google Scholar]
- 401.Tassorelli C, Sandrini G, Cecchini AP, Nappi RE, Sances G, Martignoni E. Changes in nociceptive flexion reflex threshold across the menstrual cycle in healthy women. Psychosom Med. 2002;64:621–626. doi: 10.1097/01.psy.0000021945.35402.0d. [DOI] [PubMed] [Google Scholar]
- 402.Taub E, Cuevas JL, Cook EW, III, Crowell M, Whitehead WE. Irritable bowel syndrome defined by factor analysis: Gender and race comparisons. Dig Dis Sci. 1995;40:2647–2655. doi: 10.1007/BF02220455. [DOI] [PubMed] [Google Scholar]
- 403.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: Cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110:361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 404.Thomas E, Silman AJ, Croft PR, Papageorgiou AC, Jayson MI, Macfarlane GJ. Predicting who develops chronic low back pain in primary care: A prospective study. BMJ. 1999;318:1662–1667. doi: 10.1136/bmj.318.7199.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Thompson T, Keogh E, French CC, Davis R. Anxiety sensitivity and pain: Generalisability across noxious stimuli. Pain. 2008;134:187–196. doi: 10.1016/j.pain.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 406.Thompson WG. Gender differences in irritable bowel symptoms. Eur J Gastroenterol Hepatol. 1997;9:299–302. doi: 10.1097/00042737-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 407.Thorn BE, Clements KL, Ward LC, Dixon KE, Kersh BC, Boothby JL, Chaplin WF. Personality factors in the explanation of sex differences in pain catastrophizing and response to experimental pain. Clin J Pain. 2004;20:275–282. doi: 10.1097/00002508-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 408.Tofoli GR, Ramacciato JC, Volpato MC, Meechan JG, Ranali J, Groppo FC. Anesthetic efficacy and pain induced by dental anesthesia: The influence of gender and menstrual cycle. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e34–e38. doi: 10.1016/j.tripleo.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 409.Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin: Results from a general population survey. J Pain. 2006;7:281–289. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 410.Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 411.Tousignant-Laflamme Y, Page S, Goffaux P, Marchand S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Res. 2008;9 doi: 10.1016/j.brainres.2008.06.120. [DOI] [PubMed] [Google Scholar]
- 412.Tousignant-Laflamme Y, Rainville P, Marchand S. Establishing a link between heart rate and pain in healthy subjects: A gender effect. J Pain. 2005;6:341–347. doi: 10.1016/j.jpain.2005.01.351. [DOI] [PubMed] [Google Scholar]
- 413.Trudeau KJ, noff-Burg S, Revenson TA, Paget SA. Agency and communion in people with rheumatoid arthritis. Sex Roles. 2003;49:303–311. [Google Scholar]
- 414.Tsai YF. Gender differences in pain and depressive tendency among Chinese elders with knee osteoarthritis. Pain. 2007;130:188–194. doi: 10.1016/j.pain.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 415.Tsang A, Korff MV, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, de GG, De GR, Gureje O, Lepine JP, Haro JM, Levinson D, Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;3 doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 416.Turk DC, Okifuji A. Does sex make a difference in the prescription of treatments and the adaptation to chronic pain by cancer and non-cancer patients? Pain. 1999;82:139–148. doi: 10.1016/S0304-3959(99)00041-X. [DOI] [PubMed] [Google Scholar]
- 417.Uchiyama K, Kawai M, Tani M, Ueno M, Hama T, Yamaue H. Gender differences in postoperative pain after laparoscopic cholecystectomy. Surg Endosc. 2006;20:448–451. doi: 10.1007/s00464-005-0406-0. [DOI] [PubMed] [Google Scholar]
- 418.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 419.Unruh AM, Ritchie J, Merskey H. Does gender affect appraisal of pain and pain coping strategies? Clin J Pain. 1999;15:31–40. doi: 10.1097/00002508-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 420.Valeberg BT, Miaskowski C, Hanestad BR, Bjordal K, Paul S, Rustoen T. Demographic, clinical, and pain characteristics are associated with average pain severity groups in a sample of oncology outpatients. J Pain. 2008;19 doi: 10.1016/j.jpain.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 421.Valeberg BT, Rustoen T, Bjordal K, Hanestad BR, Paul S, Miaskowski C. Self-reported prevalence, etiology, and characteristics of pain in oncology outpatients. Eur J Pain. 2008;12:582–590. doi: 10.1016/j.ejpain.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 422.Vallano A, Malouf J, Payrulet P, Banos JE. Analgesic use and pain in the hospital settings. Eur J Clin Pharmacol. 2007;63:619–626. doi: 10.1007/s00228-007-0303-7. [DOI] [PubMed] [Google Scholar]
- 423.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van KM, Patijn J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain. 2007;132:312–320. doi: 10.1016/j.pain.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 424.Vase L, Riley JL, III, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 425.Vetrhus M, Berhane T, Soreide O, Sondenaa K. Pain persists in many patients five years after removal of the gallbladder: Observations from two randomized controlled trials of symptomatic, noncomplicated gallstone disease and acute cholecystitis. J Gastrointest Surg. 2005;9:826–831. doi: 10.1016/j.gassur.2005.01.291. [DOI] [PubMed] [Google Scholar]
- 426.Vignolo V, Vedolin GM, de Araujo CR, Rodrigues Conti PC. Influence of the menstrual cycle on the pressure pain threshold of masticatory muscles in patients with masticatory myofascial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:308–315. doi: 10.1016/j.tripleo.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 427.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R. Chronic spinal pain and physical-mental comorbidity in the United States: Results from the national comorbidity survey replication. Pain. 2005;113:331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 428.Waldron I. Sex differences in illness incidence, prognosis and mortality: Issues and evidence. Soc Sci Med. 1983;17:1107–1123. doi: 10.1016/0277-9536(83)90004-7. [DOI] [PubMed] [Google Scholar]
- 429.Walker BF, Muller R, Grant WD. Low back pain in Australian adults: prevalence and associated disability. J Manipulative Physiol Ther. 2004;27:238–244. doi: 10.1016/j.jmpt.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 430.Walker JS, Carmody JJ. Experimental pain in healthy human subjects: Gender differences in nociception and in response to ibuprofen. Anesth Analg. 1998;86:1257–1262. doi: 10.1097/00000539-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 431.Wang SJ, Liu HC, Fuh JL, Liu CY, Lin KP, Chen HM, Lin CH, Wang PN, Hsu LC, Wang HC, Lin KN. Prevalence of headaches in a Chinese elderly population in Kinmen: Age and gender effect and cross-cultural comparisons. Neurology. 1997;49:195–200. doi: 10.1212/wnl.49.1.195. [DOI] [PubMed] [Google Scholar]
- 432.Wasan AD, Taubenberger SP, Robinson WM. Reasons for participation in pain research: Can they indicate a lack of informed consent? Pain Med. 2008;24 doi: 10.1111/j.1526-4637.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 433.Watts AG, Stanley HF. Indoleamines in the hypothalamus and area of the midbrain raphe nuclei of male and female rats throughout postnatal development. Neuroen-docrinol. 1984;38:461–466. doi: 10.1159/000123934. [DOI] [PubMed] [Google Scholar]
- 434.Webb R, Brammah T, Lunt M, Urwin M, Allison T, Symmons D. Prevalence and predictors of intense, chronic, and disabling neck and back pain in the UK general population. Spine. 2003;28:1195–1202. doi: 10.1097/01.BRS.0000067430.49169.01. [DOI] [PubMed] [Google Scholar]
- 435.Weiland NG, Wise PM. Estrogen and progesterone regulate opiate receptor densities in multiple brain regions. Endocrinology. 1990;126:804–808. doi: 10.1210/endo-126-2-804. [DOI] [PubMed] [Google Scholar]
- 436.Weisenberg M, Tepper I, Schwarzwald J. Humor as a cognitive technique for increasing pain tolerance. Pain. 1995;63:207–212. doi: 10.1016/0304-3959(95)00046-U. [DOI] [PubMed] [Google Scholar]
- 437.Weisse CS, Sorum PC, Sanders KN, Syat BL. Do gender and race affect decisions about pain management? J Gen Intern Med. 2001;16:211–217. doi: 10.1046/j.1525-1497.2001.016004211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Weissman-Fogel I, Dashkovsky A, Rogowski Z, Yarnitsky D. Vagal damage enhances polyneuropathy pain: Additive effect of two algogenic mechanisms. Pain. 2008;18 doi: 10.1016/j.pain.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 439.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res. 2008;186:79–85. doi: 10.1007/s00221-007-1206-7. [DOI] [PubMed] [Google Scholar]
- 440.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: The prevalence of fibro-myalgia syndrome in London, Ontario. J Rheumatol. 1999;26:1570–1576. [PubMed] [Google Scholar]
- 441.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 442.Wijnhoven HA, de Vet HC, Picavet HS. Prevalence of musculoskeletal disorders is systematically higher in women than in men. Clin J Pain. 2006;22:717–724. doi: 10.1097/01.ajp.0000210912.95664.53. [DOI] [PubMed] [Google Scholar]
- 443.Wijnhoven HA, de Vet HC, Picavet HS. Sex differences in consequences of musculoskeletal pain. Spine. 2007;32:1360–1367. doi: 10.1097/BRS.0b013e31805931fd. [DOI] [PubMed] [Google Scholar]
- 444.Wise EA, Price DD, Myers CD, Heft MW, Robinson ME. Gender role expectations of pain: Relationship to experimental pain perception. Pain. 2002;96:335–342. doi: 10.1016/s0304-3959(01)00473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Wise EA, Riley JLI, Robinson ME. Clinical pain perception and hormone replacement therapy in post-menopausal females experiencing orofacial pain. Clin J Pain. 2000;16:121–126. doi: 10.1097/00002508-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 446.Wizemann TM, Pardue ML. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 447.Wong Y, Rodwell A, Dawkins S, Livesey SA, Simpson IA. Sex differences in investigation results and treatment in subjects referred for investigation of chest pain. Heart. 2001;85:149–152. doi: 10.1136/heart.85.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8:781–797. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- 449.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 450.Woolf CJ. Central sensitization: Uncovering the relation between pain and plasticity. Anesthesiology. 2007;106:864–867. doi: 10.1097/01.anes.0000264769.87038.55. [DOI] [PubMed] [Google Scholar]
- 451.Xu S, Cheng Y, Keast JR, Osborne PB. 17{beta}-Estradiol activates ER{beta} signalling and inhibits TRPV1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology. 2008;10 doi: 10.1210/en.2008-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Yan T, Liu B, Du D, Eisenach JC, Tong C. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesth Analg. 2007;104:1246–1250. doi: 10.1213/01.ane.0000263270.39480.a2. [DOI] [PubMed] [Google Scholar]
- 453.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain. 2007;11 doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 454.Zacny JP, Beckman NJ. The effects of a cold-water stimulus on butorphanol effects in males and females. Pharmacol Biochem Behav. 2004;78:653–659. doi: 10.1016/j.pbb.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 455.Zimmer C, Basler HD, Vedder H, Lautenbacher S. Sex differences in cortisol response to noxious stress. Clin J Pain. 2003;19:233–239. doi: 10.1097/00002508-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 456.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Zun LS, Downey LV, Gossman W, Rosenbaumdagger J, Sussman G. Gender differences in narcotic-induced emesis in the ED. Am J Emerg Med. 2002;20:151–154. doi: 10.1053/ajem.2002.32631. [DOI] [PubMed] [Google Scholar]