Abstract
Objective
To investigate the role of very late antigen 1 (VLA-1) (also known as integrin receptor α1β1) in corneal transplantation inflammation and allograft survival.
Methods
Cell infiltration and vasculogenesis (both angiogenesis and lymphangiogenesis) associated with allodisparate corneal transplantation were assessed in VLA-1—deficient conditions and controls by immunofluorescent microscopic studies. Corneal allograft survival was also assessed after anti—VLA-1 antibody treatment and in VLA-1 knockout recipient mice.
Results
Anti—VLA-1 antibody treatment leads to a profound reduction in the granulocytic, monocytic, and T-cell infiltration after corneal transplantation. In addition, corneal angiogenesis and lymphangiogenesis were both significantly suppressed in VLA-1 knockout mice. Remarkably, universal graft survival was observed in both anti—VLA-1 antibody treatment and knockout mice.
Conclusions
Very late antigen 1 blockade markedly reduces inflammation and inflammation-induced tissue responses, including vasculogenic responses, associated with corneal transplantation and promotes allograft survival.
Clinical Relevance
These studies offer insights into important integrin-mediated mechanisms of corneal transplant—related inflammation and provide possible new integrin-based immunotherapies for transplant rejection.
INTRODUCTION
Integrins are a diverse family of heterodimeric cell surface transmembrane glycoproteins that mediate cell × cell and cell × matrix interactions. Very late antigen 1 (VLA-1), integrin α1β1, is primarily a receptor for collagens and laminins.1-4 It has been shown that deletion of integrin α1 in mouse permits normal development but gives rise to a specific deficit in cell adhesion5 and attenuated delayed-type hypersensitivity.6 Furthermore, VLA-1 blockade ameliorates certain immunoinflammatory diseases such as arthritis.6 However, the roles of VLA-1 in organ transplant survival have never been investigated before, which is the major goal of this study. Though corneal transplantation is by far the most common form of solid tissue transplantation in humans, its pharmacotherapy has changed little over the past several decades, though it is well known that the mainstay regimen with corticosteroids is only variably effective and associated potentially with serious adverse effects such as glaucoma, cataracts, and opportunistic infections.7 It is therefore important to explore more effective strategies to improve corneal transplant survival. Lymphatic and blood vessels play important roles in transplant immunity: lymphatics, allowing for antigen-presenting cell migration to lymph nodes and blood vessels, facilitating immune cell targeting of the graft.7-8 Indeed, previous data from our laboratory have shown that surgical excision of the local draining lymph nodes leads to indefinite and universal graft acceptance without any form of immunosuppression.9 However, surgical lymphadenectomy to promote graft survival is not practical. It is hence critical to investigate the molecular mechanisms underlying this pathway. Unlike angiogenesis, which has been extensively studied, the molecular regulation of lymphangiogenesis has historically been neglected for decades until recently when several lymphatic-specific markers were discovered.10-11 We have recently shown that blockade of vascular endothelial growth factor receptor 3 (VEGFR-3), a lymphatic molecule, greatly suppresses corneal transplant rejection.12 Though it has been shown previously that angiogenesis is suppressed in 1-deficient mice,13 the issue of lymphangiogenesis has never been addressed directly in those studies.
METHODS
MICE AND ANTIBODIES
Very late antigen 1 knockout BLAB/c mice were generated as described previously5 and kindly provided by Biogen Idec (Cambridge, Mass). Seven- to 10-week-old, male, wild-type BALB/c or C57BL6 mice (Taconic Farms, Germantown, NY, or from our own breeding facility) were used in all other experiments. All protocols were approved by the Schepens Eye Research Institute Animal Care and Use Committee, and all animals were treated according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were anesthetized using a mixture of ketamine hydrochloride and xylazine (120 and 20 mg per kilogram of body weight, respectively) for each surgical procedure. The following antibodies were used for this study: mouse Gr1—fluorescein isothiocyanate conjugated (FITC), mouse Mac1-FITC, mouse CD31-FITC (Santa Cruz Biotechnology, Santa Cruz, Calif), purified antimouse LYVE-1 (lymphatic vessel endothelial hyaluronan receptor 1) (a kind gift of David Jackson, PhD, Weatherall Institute of Molecular Medicine, United Kingdom14), purified antimouse CD3, rat antimouse CD16/32, Rhodamine-conjugated donkey antirabbit IgG (Santa Cruz Biotechnology), Cy3-conjugated antihamster IgG (Jackson ImmunoResearch Laboratories, Inc, West Grove, Pa). Isotype controls included rat IgG2b-FITC, hamster IgG1, and rabbit serum. Purified VLA-1—blocking antibody (Ha 31/8) and the isotype control antibody (Ha 4/8) were kindly supplied by Biogen Idec. All the other antibodies (except where noted) and isotype-matched controls were purchased from BD PharMingen, San Diego, Calif. For each antibody staining study on whole-mount tissues, 3 to 5 samples were examined. For cross-section studies, multiple sections derived from at least 3 mice were examined. All studies were repeated at least 3 times to confirm the results.
CORNEAL TRANSPLANTATION AND ASSESSMENT OF GRAFT SURVIVAL
Two sets of orthotopic corneal transplantation were performed according to our standard protocol9: (1) naive C57BL6 (donors) to BALB/c (recipients) mice for anti—VLA-1 antibody treatment experiment and (2) naive C57BL6 (donors) to VLA-1 knockout (recipients) BALB/c mice for VLA-1 knockout experiment (n = 10 for each experimental group). Briefly, the central (1.5-mm diameter) cornea of the recipient was excised with Vannas scissors (Storz Instruments Co, San Dimas, Calif) and replaced with a donor button (2.0-mm diameter) with 8 interrupted 11-0 nylon sutures (Sharppoint; Vanguard, Houston, Tex). All grafted eyes were first examined after 3 days and corneal sutures were removed 1 week later. Corneal grafts were observed biweekly by slitlamp biomicroscopy for 8 weeks. A standard grading scheme was adapted for evaluation, and scoring of the grafts and the survival were assessed by Kaplan-Meier analysis.9 Briefly, the degree of opacification was graded between 0 and 5+ (0 = clear and compact graft, 1+ = minimal superficial opacity, 2+ = mild deep [stromal] opacity with pupil margin visible, 4+ = intense stromal opacity with the anterior chamber visible, 5+ = maximal corneal opacity with total obscuration of the anterior chamber). Grafts with an opacity score of 2+ or higher after 3 weeks or an opacity score of 3+ or higher at 2 weeks were regarded as rejected.
PHARMACEUTICAL INTERVENTIONS
To study the effect of anti-α1 on corneal cell infiltration, the recipient naive BALB/c mice were randomly selected to receive either 200 μg of anti-α1 monoclonal antibody or isotype control antibody via intraperitoneal injection 1 hour before surgeries and biweekly thereafter until 28 days when the treatment was discontinued. Eyes were excised 24 hours, 7 days, 14 days, and 28 days after transplantation for epifluorescent microscopic studies as stated in the following subsection. To study the effect of anti-α1 on corneal graft survival, the treatment was given similarly up to 8 weeks.
IMMUNOHISTOCHEMICAL STUDY AND EPIFLUORESCENCE MICROSCOPY
Briefly, eyeballs or whole-mount corneas were excised from mice. For cell infiltrate studies, 8μm frozen sections were fixed in acetone for immunofluorescent staining as described previously.15 To block nonspecific staining, sections were blocked with 2% bovine serum albumin and anti-FcR monoclonal antibody (CD16/CD32) for 30 minutes before they were stained with primary or control antibodies for 2 hours. Thereafter, the sections were incubated with secondary antibodies for 1 hour. For vasculogenesis studies, the whole-mount flat corneas of VLA-1 knockout BALB/c mice were sampled 7 days posttransplantation and stained overnight with FITC anti-CD31 (PECAM-1) antibody and then anti—LYVE-1 (to specifically detect lymphatics) antibody for 1 hour. Finally, sections were covered with mounting medium (Vector, Burlingame, Calif) and examined by an epifluorescence microscope (Eclipse E800; Nikon, Japan). Digital pictures were taken using the Spot Image Analysis system, and vascular structures stained as CD31+LYVE-1- were identified as blood vessels while those stained as CD31+LYVE-1+ were defined as lymph vessels.16 Angiogenesis and lymphangiogenesis were graded according to our standard protocol as described previously, with some modifications.16-17 Briefly, the quantification was based on 2 primary parameters: (1) the circumferential extent (12 areas around the clock) of the vasculogenesis. A score of 1 was given to each area if the vasculogenesis was present in the sector. (2) the centripetal growth of the longest vascular frond in each area. A grade between 0 (no growth) and 2 (vasculogenesis to the donor-graft border) was given to each area. Scores for each area were then summed to derive the vasculogenic index (range, 0-24). The mean difference between vasculogenesis scores was analyzed by the Mann-Whitney U test. For cross-section studies, the Gr-1+, Mac-1+, or CD3+ cells were counted for each section, covering the entire thickness and span of the corneal tissues, and correlation analysis between the number of Gr-1+, Mac-1+, and CD3+ cells per section was performed with a t test. P<.05 was considered significant.
RESULTS
VLA-1 IS EXPRESSED ON GRAFTED CORNEAS
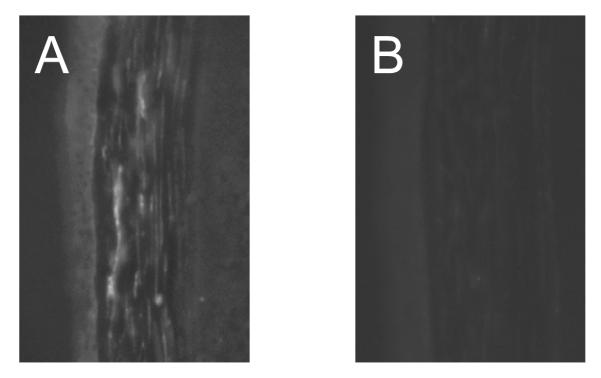
We first set to confirm the expression of VLA-1 on grafted corneas. As shown in Figure 1, VLA-1 staining was detected in the grafted corneal stroma (Figure 1A) while the isotype control sample staining was negative (Figure 1B).
Figure 1.
Representative cross-section micrographs showing very late antigen 1 expression on the mouse cornea 14 days after transplantation (A) and its negative control (B) (original magnification × 200).
VLA-1 BLOCKADE DOWN-REGULATES CELL INFILTRATION AFTER CORNEAL TRANSPLANTATION
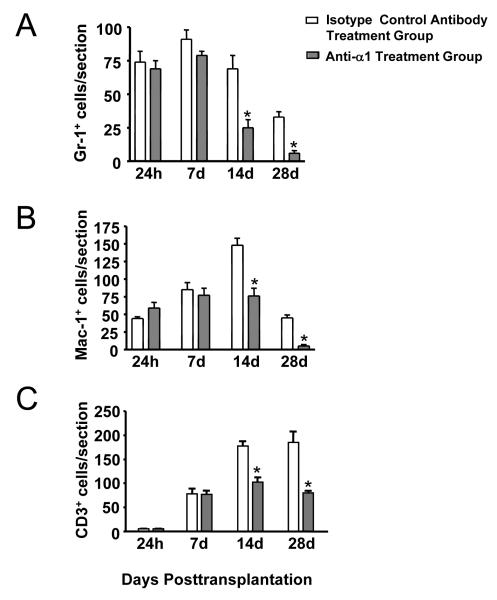
We next investigated whether VLA-1 plays a role in the cell infiltration associated with corneal transplantation by studying the effect of VLA-1 blockade on both inflammatory-cell (Gr-1+ neutrophils and Mac-1+ monocytes/macrophages) and T-cell (CD3+) infiltration into corneal grafts at various points. As seen in Figure 2, compared with the isotype control treatment groups, significant suppression of cell infiltration was observed with all the cell types studied in VLA-1 blockade groups at day 14 and day 28 (P<.05), before the onset of murine corneal allograft rejection (which typically occurs at 4 weeks after transplantation).
Figure 2.
Significant down-regulation of Gr-1+—cell (A), Mac-1+—cell (B), and CD3+ T-cell (C) infiltration into the corneal grafts in anti—very late antigen 1 antibody treatment groups of 14 and 28 days posttransplantation. *P<.05.
VASCULOGENESIS IS SUPPRESSED IN THE CORNEAL GRAFTS OF VLA-1—DEFICIENT MICE
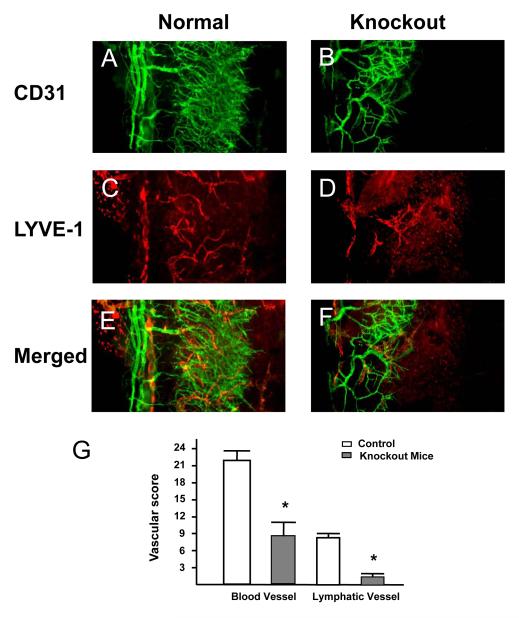
Because growth of blood and lymphatic vessels into the normally avascular corneal bed is a critical facet of the local inflammatory response to grafts, and a major risk factor for subsequent graft rejection,7-8 we subsequently examined the effect of VLA-1 deficiency in corneal transplantation—associated angiogenesis and lymphangiogenesis. As demonstrated in Figure 3, 7 days after transplantation, both blood (CD31+LYVE-1-) and lymph vessels (CD31+LYVE-1+) were significantly decreased in the VLA-1 knockout recipients (Figure 3B, D, and F) compared with wild-type controls (Figure 3A, C, and E) (results are summarized in Figure 3G) (P<.01).
Figure 3.
Representative whole-mount micrographs showing that both angiogenesis and lymphangiogenesis are suppressed in the corneal grafts in very late antigen 1—deficient mice. A, CD31 in controls. B, CD31 in knockout mice. C, LYVE-1 (lymphatic vessel endothelial hyaluronan receptor 1) in controls. D, LYVE-1 in knockout mice. E, Merged CD31 and LYVE-1 in controls. F, Merged CD31 and LYVE-1 in knockout mice. G, Summary of results. Error bars represent SEM. *P<.01 (original magnification × 200).
CORNEAL GRAFT SURVIVAL IS MARKEDLY ENHANCED WITH VLA-1 BLOCKADE OR IN VLA-1—DEFICIENT MICE
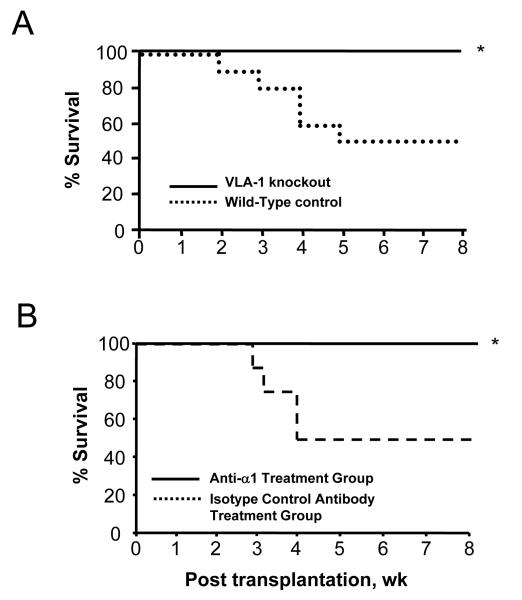
The earlier data implicate VLA-1 in corneal transplantation—associated cell infiltration, as well as angiogenesis and lymphangiogenesis, which are all critical factors in corneal graft rejection. We then tested the central hypothesis that VLA-1 blockade improves corneal allograft survival by assessing the transplant survival in both anti—VLA-1 antibody treatment and in VLA-1 knockout recipient mice. Results from these studies are presented in Figure 4, summarized by Kaplan-Meier survival curves. Remarkably, universal graft survival was observed in both groups compared with their corresponding wild-type controls (Figure 4) (P<.05). The disparities between the VLA-1 blockade or VLA-1—deficient groups, and their controls, were seen as early as 2 to 3 weeks posttransplantation.
Figure 4.
Kaplan-Meier survival curves showing the role of very late antigen 1 (VLA-1) in corneal graft survival. Universal graft survival was observed in both VLA-1 knockout (A) and VLA-1—neutralizing antibody treatment groups (B) (n = 10 for each experimental group). *P<.05.
COMMENT
Very late antigen 1 is an important molecular facet of a number of inflammatory responses involving macrophages, T cells, angiogenesis, and fibrosis.3-4,6 However, there have been no studies to date on the effect of VLA-1 blockade on organ transplant survival. Our data, for the first time, to our knowledge, suggest that VLA-1 blockade has a profound effect as an immunomodulatory strategy for improving corneal graft survival. We have previously studied the effect of blockade of a number of molecular pathways in corneal transplantation immunity, including: (1) proinflammatory cytokines IL-1 and tumor necrosis factor α; (2) intercellular adhesion molecule 1; (3) the costimulatory CD40L(CD154)-CD40 pathway; and (4) the vascular endothelial growth factors (VEGFs) VEGF-A and VEGFR-3.12, 18-21 Though the graft survival rate is improved in all these cases, we have only seen results approaching what we see with blockade of VLA-1 with systemic anti-CD40L treatment, in both these cases achieving universal graft survival. The development of the anti-CD40L strategy in the clinic was impeded, however, by serious thrombotic adverse effects in human subjects receiving the anti-CD40L treatment for systemic autoimmune diseases.22
The surprisingly high survival rate in VLA-1—blockade or VLA-1—deficient conditions may be explained by the fact that this molecular pathway is involved in both innate and adaptive aspects of corneal transplantation immunity, since both innate (neutrophil and macrophage) and T-cell infiltrations are suppressed. Our finding that corneal transplantation—related angiogenesis is significantly suppressed in VLA-1 knockout beds is consistent with previous reports on suppression of tumor-associated angiogenesis in α1-null mice13 and in VEGF-induced angiogenesis when α1-blocking antibodies are administered.23-24 However, these studies shed no light on lymphangiogenesis, a critical factor in tumor metastasis and the generation of immune responses in the cornea. In fact, only scarce references have been made for the role of integrins in lymphangiogenesis. A recent in vitro study on integrin α9β1 showed that this molecule interacts with VEGF-C and VEGF-D, the ligands for the lymphangiogenic receptor VEGFR-3.25 However, no in vivo studies have been reported regarding the α9β1 molecule. Moreover, α9 knockout mice die shortly after birth because of severe lymphatic deficiency. Indeed, knockout mice cannot survive most of the specific lymphatic factors (such as VEGFR-3, Prox-1, VEGF-C, and podoplanin).26-29 The luxury of viable VLA-1 knockout mice, combined with the unique feature of the alymphatic status of normal cornea, enables us to present herein the first report, to our knowledge, on the in vivo role of VLA-1 on transplantation-related lymphangiogenesis. Additionally, our data that VLA-1 treatments lead to both the decrease in Mac-1+—cell infiltration and the suppression of corneal lymphangiogenesis further support the recently reported role for macrophages in lymphangiogenesis.16
The lymphatic system penetrates most tissues in the body, and its dysfunctions are involved in a diverse array of diseases including lymphedema (primary to secondary to cancer surgeries or radiation therapy), delayed wound healing, diabetes mellitus, and cancer cell metastasis, which can be disabling, disfiguring, and even life threatening.10-11 To date, there are no effective treatments for them. It is anticipated that this study, beyond its contributions to corneal transplant immunity, will also shed light on the development of new therapeutic strategies for other disorders associated with lymphangiogenesis and inflammation.
Acknowledgment
We thank David Jackson, PhD, from the Weatherall Institute of Molecular Medicine, United Kingdom, for providing anti—LYVE-1 antibodies.
Grant information: NIH/NEI Grant EY-12963 (to M.R.D.) and EY-014786 (to L.C.); an Award from Research to Prevent Blindness (to M.R.D.).
REFERENCES
- 1.Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 2.Berman AE, Kozlova NI, Morozevich GE. Integrins: structure and signaling. Biochemistry (Mosc) 2003;68:1284–1299. doi: 10.1023/b:biry.0000011649.03634.74. [DOI] [PubMed] [Google Scholar]
- 3.De Fougerolles AR. Integrins in immune and inflammatory disease. In: Gullberg D, editor. I Domains in Integrins. Landes Biosciences; Georgetown, Tex: 2003. pp. 165–184. [Google Scholar]
- 4.Ben-Horin S, Bank I. The role of very late antigen-1 in immune-mediated inflammation. Clin Immunol. 2004;113:119–129. doi: 10.1016/j.clim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Gardner H, Kreidberg J, Koteliansky V, et al. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 6.de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, et al. Regulation of inflammation by collagen-binding integrins alpha1beta1 and alpha2beta1 in models of hypersensitivity and arthritis. J Clin Invest. 2000;105:721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Cursiefen C, Chen L, Dana MR, et al. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci. 2001;42:1293–1298. [PubMed] [Google Scholar]
- 10.Brown P. Lymphatic system: unlocking the drains. Nature. 2005;436:456–458. doi: 10.1038/436456a. [DOI] [PubMed] [Google Scholar]
- 11.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 13.Pozzi A, Moberg PE, Miles LA, et al. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97:2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson DG, Prevo R, Clasper S, et al. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhu SN, Yamada J, Streilein JW, et al. ICAM-1 deficiency suppresses host allosensitization and rejection of MHC-disparate corneal transplants. Transplantation. 2000;69:1008–1013. doi: 10.1097/00007890-200003150-00061. [DOI] [PubMed] [Google Scholar]
- 16.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dana MR, Zhu SN, Yamada J. Topical modulation of interleukin-1 activity in corneal neovascularization. Cornea. 1998;17:403–409. doi: 10.1097/00003226-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Dana MR, Yamada J, Streilein JW. Topical interleukin 1 receptor antagonist promotes corneal transplant survival. Transplantation. 1997;63:1501–1507. doi: 10.1097/00007890-199705270-00022. [DOI] [PubMed] [Google Scholar]
- 19.Yamada J, Streilein JW, Dana MR. Role of tumor necrosis factor receptors TNFR-I (P55) and TNFR-II (P75) in corneal transplantation. Transplantation. 1999;68:944–949. doi: 10.1097/00007890-199910150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Qian Y, Boisgerault F, Benichou G, et al. Blockade of CD40-CD154 costimulatory pathway promotes survival of allogeneic corneal transplants. Invest Ophthalmol Vis Sci. 2001;42:987–994. [PubMed] [Google Scholar]
- 21.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 22.Sidiropoulos PI, Boumpas DT. Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus. 2004;13:391–397. doi: 10.1191/0961203304lu1032oa. [DOI] [PubMed] [Google Scholar]
- 23.Perruzzi CA, de Fougerolles AR, Koteliansky VE, et al. Functional overlap and cooperativity among alphav and beta1 integrin subfamilies during skin angiogenesis. J Invest Dermatol. 2003;120:1100–1109. doi: 10.1046/j.1523-1747.2003.12236.x. [DOI] [PubMed] [Google Scholar]
- 24.Senger DR, Perruzzi CA, Streit M, et al. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol. 2002;160:195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlahakis NE, Young BA, Atakilit A, et al. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wigle JT, Oliver G. Prox-1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 27.Dumont DJ, Jussila L, Taipale J, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 28.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 29.Schacht V, Ramirez MI, Hong YK, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]