Abstract
Background/aims
Although it is known that drug delivery across the blood-brain barrier (BBB) may be hampered by efflux transport activity of the multidrug resistance (mdr) gene product P-glycoprotein, it is not clear how inflammation regulates efflux transporters. In rat brain endothelial (RBE4) cells of BBB origin, the proinflammatory cytokine TNF mainly induces transcriptional upregulation of mdr1b, and to a lesser extent mdr1a, resulting in greater efflux of the substrates (Yu C et al., Cell Physiol Biochem, 2007). This study further determined the mechanisms by which TNF activates mdr1b promoter activity.
Methods/Results
Luciferase reporter assays and DNA binding studies show that (a) maximal basal promoter activity was conferred by a 476 bp sequence upstream to the mdr1b transcriptional initiation site; (2) TNF induced upregulation of promoter activity by NFkB nuclear translocation; and (3) the NFκB binding site of the mdr1b promoter was solely responsible for basal and TNF-activated gene transcription, whereas the p53 binding site was not involved. Binding of the p65 subunit of NFκB to nuclear DNA from RBE4 cells was shown by electrophoretic mobility shift assay and chromatin immunoprecipitation assays.
Conclusion
Thus, NFκB mediated TNF-induced upregulation of mdr1b promoter activity, illustrating how inflammation activates BBB efflux transport.
Keywords: TNF, mdr1b, p-glycoprotein, NFκB, endothelia, multidrug resistance, efflux transport, blood-brain barrier
Introduction
The goal of this study was to determine how the proinflammatory cytokine tumor necrosis factor α (TNF) induces transcriptional upregulation of multi-drug resistance (mdr)-1b, which, in turn, activates the efflux transporter P-glycoprotein (Pgp). Pgp is a 12-transmembrane protein member of the family of ATP-binding cassette efflux transporters. In blood-brain barrier (BBB) endothelial cells, Pgp is located at the apical surface facing the lumen of blood vessels. This efflux transporter has a variety of substrates, such as anti-cancer reagents, anti-epileptics, serum protease inhibitors used to treat neuroAIDS, and antibiotics [1-6]. Several drugs used to treat Parkinson’s and Alzheimer’s diseases, including selegiline and memantine, are also substrates for Pgp [7]. Activation of Pgp by cytokines indicates that inflammation can further reduce the availability of these therapeutic agents into the central nervous system (CNS).
Many CNS disorders involve elevation of TNF. These include inflammation, infection, tumor, ischemia, and neurodegeneration. TNF not only crosses the BBB by a receptor-mediated transport system itself, but it also induces other proinflammatory cytokines, affects their interactions with the BBB, and modulates endothelial signaling [8-15]. We have shown in RBE4 cerebral microvessel endothelial cells of rat origin that TNF induces time- and dose-dependent activation of mdr1b mRNA and the Pgp transporter activity [16]. In microarray analysis of RBE4 cells at different times after TNF treatment, mdr1b is among the genes upregulated more than 10-fold between 6 and 24 h after TNF stimulation, whereas the increase of mdr1a is less robust. This was further confirmed in-vivo, showing that freshly isolated cerebral microvessels from mice had a significant induction of mdr1b mRNA by LPS stimulation (unpublished observations). The increase of mdr1b correlates with higher efflux transport of the anti-mitotic drug vinblastine [16]. These findings provide a basis to further determine the mechanisms of the regulatory changes of mdr1b.
Although much attention has been given to mdr1a [17, 18], fewer studies have specifically addressed mdr1b gene regulation [19]. The regulation of Pgp activity at the BBB level appears to be present both at the post-transcriptional and gene regulation level. Miller and colleagues showed that TNF and endothelin-1 can initially rapidly decrease Pgp transport activity without affecting the level of Pgp protein expression. After a nadir of several hours, there is a sharp increase of both Pgp protein and transport activity. This study pointed to the involvement of NFκB in the regulatory changes of Pgp [5]. In our study, we further determined the transcriptional regulation of mdr1b in RBE4 cells of cerebral microvascular origin.
Promoter activity constitutes the major means of transcriptional activation of a gene. Although the human, mouse, and rat promoter regions of mdr1 have been characterized in non-BBB cells [20-24], mdr1 promoter activity has not been studied in cerebral microvessel endothelia of the BBB. Rat mdr1b contains binding sites for p53 and NFκB upstream to the TATA box located -38 bp upstream of the transcriptional initiation site. Stress signals, such as UV irradiation, heat shock, chemotherapeutics, and agents inducing cell differentiation can modulate the promoter activity [25]. In rat liver cells and human colon carcinoma cells, TNF has been shown to upregulate mdr1b promoter activity [26, 27]. The BBB comprises specialized endothelia that are joined by tight junctions and convey minimal permeability to non-lipophilic large molecules. Because of structural characteristics, the transporter functions of the BBB may also be specialized and subject to different regulation from that in peripheral tissues. If so, this would allow us to identify unique strategies to enhance CNS drug delivery. Thus, it is important to determine the regulation of the mdr1b promoter at the BBB level.
Materials and Methods
Cells and Reagents
The RBE4 rat cerebral microvessel endothelial cell line was obtained from Dr. Pierre-Olivier Couraud (Institute of Cochin, Paris, France). The pGL3.0-basic luciferase (LUC) reporter vector was purchased from Promega (Madison, WI). PCR primers for cloning were purchased from Integrated DNA Technologies (Coralville, IA). [α-32p]dCTP and poly (dI-dC)·poly (dI-dC) were from GE Healthcare Life Science (Piscataway, NJ). Klenow fragment (3′→5′ exo-) was from New England Biolabs (Ipswich, MA). The monoclonal antibody against the nuclear location signal (NLS) of the p65 subunit of the NFκB heterodimer was purchased from Chemicon (Temecula, CA). Unless specified, other regents were purchased from Sigma (St. Louis, MO).
Plasmid construction containing rat mdr1b promoter sequences
The design of gene constructs was based on the known domains of the rat mdr1b promoter and putative binding sites shown in Table 1. Genomic DNA of RBE4 cells was used as the template for PCR amplification of DNA sequences that are upstream to or flanking the transcriptional initiation site (shown by the number 1) of mdr1b gene. The primers for PCR cloning are listed in Table 2. The PCR products were inserted into the luciferase reporter vector pGL3.0-basic between the NheI and XhoI restriction sites upstream of the reporter gene. The resultant rat mdr1b-LUC recombinants were designated -963/+45, -476/+45, -476/+607, -12/+607, -250/+45, -218/+45, -185/+45, and -159/+45. The numbers correspond to the relative position of the transcription initiation site which is +1.
Table 1. Sequence of rat mdr1b (ABCB1) promoter.
 |
The transcriptional initiation site is designated by the number 1. The first two exon sequences are shown in upper case, bold letters. The TATA box (DNA polym erase binding site) is underlined and located at -38. NFκB and p53 binding sites are located at -182∼-171 and -195∼-214.
Table 2. Cloning primers for the wildtype and mutant rat mdr 1 promoter sequences.
| Name | Sequence |
|---|---|
| -963/+45 forward | 5′-agcgcgctagccccgtggaccatgagttaag |
| -476/+45 and -476/+607 forward | 5′-gcgaagctagcgccagaaaaccgaatggata |
| -250/+45 forward | 5′-gcatggctagcaaccgtgcactatccaggta |
| -218/+45 forward | 5′-gacctgctagcccatatggagagttacctgaaca |
| -185/+45 forward | 5′-gccatgctagctctgtgttaatgtctggggaat |
| -159/+45 forward | 5′-ctcaggctagcgctcccttctcaaaaactcaga |
| -12/+607 forward | 5′-gcgaagctagcgccagaaaaccgaatggata |
| -476/+607 and +12/+607 reverse | 5′-gcaagctcgagaggccctcttcaaactccat |
| 963/+45, -476/+45, -250/+45, -218/+45, -185/+45 and -159/+45 reverse | 5′-atcagctcgagggcctcagcctcttacagc |
| -476/+45 NFκB mutant forward | 5′-catgtctgtgttaatgtctgctcaattccagctc |
| -476/+45 NFκB mutant reverse | 5′-cagacattaacacagacatgtctctacatg |
| -476/+45 p53 mutant forward | 5′-catatggagagttacctgaatcggtagagacatg |
| -476/+45 p53 mutant reverse | 5′-ttcaggtaactctccatatggaggtgtata |
Restriction enzyme sites were included in the 5′ ends of the primers for directional cloning of PCR products. NheI sites are single underlined and XhoI sites are double underlined. In the primers designed for site-directed mutation, the mutated sites are in bold and underlined with dotted lines.
The site-directed mutant constructs were generated by use of the GeneTailor™ Site-Directed Mutagenesis System from Invitrogen (Carlsbad, CA) following the manufacturer’s instructions. Plasmid -476/+45 was the template. The forward and reverse primers for a mutant NFκB or p53 binding site are listed in Table 2. All constructs were verified by DNA-sequencing analysis.
Cell culture, transient transfection, and luciferase assay
RBE4 cells were maintained in F10 and αMEM medium supplemented with 10 % fetal calf serum (FCS), 1 ng/ml basic fibroblast growth factor, 0.3 mg/ml of geneticin, and penicillin /streptomycin. Twenty-four h before transfection, cells were seeded on 24-well cell culture plates at a density of 4 × 105 cell/ml. Before transfection, the medium was changed to F10 and αMEM medium without serum and antibiotics. RBE4 cells were transfected in quadruplicate with 0.8 μg of pGL3.0 firefly luciferase constructs by use of the lipofectamine™ 2000 reagent (Invitrogen). To normalize for transfection efficiency, 0.04 μg of phRL-TK (Renilla luciferase) plasmid was co-transfected into the cells. Twenty-four h after transfection, cells were treated with either 5 ng/ml TNF or phosphate-buffered saline (PBS) for 6 h. In one study with the plasmid -476/+45, an additional group of cells were pretreated with the NFκB inhibitor 6-amino-4-(4-phenoxyphenylethylamino) quinazoline (quinazoline, 1 μM) for 1 h, followed by co-treatment of the inhibitor with 5 ng/ml TNF for 6 h. In all studies, quadruplicate wells were used (i.e., n = 4/group).
At the end of treatment, cellular proteins were extracted by cell lysis buffer. The activities of firefly luciferase and Renilla luciferase were measured on a luminometer (20/20n) according to the manufacturer’s instructions (Turner Biosystems, Sunnyvale, CA). The substrate for firefly luciferase indicative of promoter activity was luciferin, and the substrate for the control Renilla luciferase activity was colenterazine. The luminescent intensity of firefly luciferase was normalized as a ratio to that of Renilla luciferase.
Nuclear extract preparation and western blotting
RBE4 cells were grown on 10 cm cell culture dishes in complete medium until 95 % confluency. After being starved in serum free medium for 24 h, cells were treated with 5 ng/ml of TNF for 10 or 30 min. Nuclear extracts were prepared with the CelLytic Nuclear™ Extraction kit (Sigma) following the manufacturer’s instructions. Protein concentrations were measured by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Fifty μg of nuclear extracts was separated on 12 % sodium dodecyl sulfate (SDS) - polyacrylamide gels, and proteins were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were probed with mouse monoclonal antibody specific to the nuclear location signal (activated form) of the NFκB p65 subunit (Chemicon) and horseradish peroxide-conjugated secondary antibody (Amersham Bioscience, Piscataway, NJ) in PBS containing 0.1 % Tween 20 and 0.5 % milk. The ubiquitous transcription factor Sp3 was also probed as a loading control. The signals were developed with enhanced chemiluminescence (ECL)-plus western blotting detection reagents (Amersham Biosciences).
Electrophoretic mobility shift assay (EMSA)
The NFκB probes were designed as follows: wild type sense: 5′-ggc ctt aat gtc tgg_gga att cca gct ccc ttc t-3′, antisense: probe 5′-gcc gag aag gga gct gga att ccc cag aca tta a-3′; mutant sense: 5′-ggc ctt aat gtc tgC TCca att cca gct ccc ttc t-3′, antisense: 5′-gcc gag aag gga gct gga att GAG cag aca tta a-3′ (the highlighted capital letters stand for the mutation). The probes were synthesized by Integrated DNA Technologies (IDT, Coraville, IA). They were annealed overnight by boiling at 95°C for 5 min and then slowly cooled to room temperature. The annealed double strand probes were subsequently labeled with [α-32P]dCTP by use of Klenow polymerase and purified on Chroma Spin™ Columns (Clontech, Mountain View, CA). Nuclear extracts (5 μg) from groups of serum-starved RBE4 cells with or without TNF treatment were obtained. The nuclear extracts were pre-incubated with 2x binding buffer (24 mM HEPES, pH 7.9, 8 mM Tris-HCl, pH 8.0, 2 mM EDTA, pH 8.0, 1 mM DTT, 12 % glycerol), BSA, and dI-dC polymers, for 20 min at room temperature. The mixture was further incubated with the 32P-labeled wild type and mutant NFκB probes under the same reaction conditions. For competition assay, a 200-fold molar excess of unlabeled wild type or mutant probe was included. In the supershift experiments, an antibody against the p65 subunit of the NFκB complex was used (Chemicon). The DNA-protein complexes were separated on a 7 % native polyacrylamide gel in 0.5x Tris-borate-EDTA buffer at 200 V for 2 h. The gels were dried and exposed to x-ray films at -80 °C for 96 h.
Chromatin Immunoprecipitation Assay (ChIP)
Groups of RBE4 cells grown confluent on 10 cm culture dish were serum-starved for 24 h and treated with TNF (5 ng/ml) for 0, 10, 30, or 60 min. The cells were then incubated with 1 % formaldehyde for 10 min to crosslink the DNA binding proteins to DNA. The reaction was stopped by 0.125 M of glycine. The cells were collected in PBS containing protease inhibitor cocktail, pelleted, and lysed in the presence of SDS and protease inhibitor cocktail. The lysate was sonicated to shear genomic DNA. The supernatant from each group was brought to a volume of 150 μl by addition of SDS lysis buffer, and further diluted to a total volume of 1.5 ml by use of ChIP dilution buffer containing protease inhibitor cocktail.
The samples (1.4 ml of the above) were pre-cleared with protein A agarose /salmon sperm DNA by 4°C incubation for 30 min, and the supernatant was collected. Immunoprecipitation was achieved by addition of the p65 antibody (3 μg/ml) or isotope-matched IgG, with 16 h incubation at 4°C, further incubation after addition of protein A agarose /salmon sperm DNA at 4 °C for 1 h, and centrifugation. The pellet was washed sequentially with low salt immune complex wash buffer, high salt immune complex wash buffer, LiCl immune complex wash buffer, and TE buffer. After elution, cross-linking was reversed by reaction with 5 M NaCl at 65 °C for 4 h.
DNA was extracted and purified by use of the DNeasy extraction kit (Qiagen, Valencia, CA). Two μl each of the immnoprecipitated samples and the remaining 100 μl of lysate (termed input material) was used for PCR amplification. The PCR primers for the NFκB-binding site of mdr1b were: sense 5′-GCCTGGAAACCATCCCTATT-3′, antisense 5′- GGCCTCAGCCTCTTACAGC-3′. The primers for the house-keeping gene β-actin were: sense 5′-GATCTGGCACCACACCTTCT-3′, antisense 5′-GGGGTGTTGAAGGTCTCAAA-3′. The PCR reaction included denaturing for 5 min at 95 °C, followed by 35 cycles of denaturation (95 °C for 30 sec), annealing (60°C for 30 sec), extension (72 °C for 15 sec), and the final extension at 72°C for 7 min. The PCR products were electrophoresed on 1.5 % agarose gels, and the intensity of the products was quantified with Quantity One. The ratio of the mdr1b promoter fragment in immunoprecipitants to that in the input material was then plotted.
Statistical analysis
All findings were replicated, and representative findings of the consistent results were shown. Group means are presented with their standard errors (SEM). Statistical evaluation of differences among treatments was performed by one-way analysis of variance (ANOVA) with the Statistical Package for Social Sciences (SPSS) 12.0 (SPSS Inc., Chicago, IL). To identify significant differences between treatments, Tukey’s post-hoc test was used for multiple comparisons.
Results
1. Promoter activity of the rat mdr1b gene and its activation by TNF
The structure of the inserts in the luciferase constructs are illustrated in figure 1A. The plasmids are: pGL3.0 vector, -963/+45 plasmid, -476/+45 plasmid, -476/+607 plasmid, and -12/+607 plasmid. The plasmids were induced into RBE4 cells along with the Renilla control plasmid, by use of the Lipofectamine 2000 transfection reagent. At 24 h after transfection, the cells were treated with either PBS vehicle or TNF. The luciferease assay was performed 30 h after transient expression and 6 h after treatment with PBS vehicle or TNF. The group with the promoterless pGL3.0-basic vector (negative control; group 1) showed minimal background firefly luciferase activity. In cells transfected with -963/+45 (group 2), which contains 963 bp promoter sequences upstream to the rat mdr1 transcription initiation site as well as part of the first exon, there was a 30-fold increase of firefly luciferase activity (p<0.001 compared with the control). This indicates that the promoter activated transcription of the luciferase reporter gene. The third group consisted of a transfected construct containing a shorter fragment of the promoter sequence (476 bp). This resulted in even greater activation of luciferase activity (p<0.05 vs group 2). In the fourth group, the shorter construct also included the second exon as well as the first exon and their flanked first intron. This caused a further increase of luciferase activity (p<0.001 vs group 3). However, the absence of the mdr1b promoter despite the inclusion of the first exon, first intron, and second exon in the last group greatly diminished luciferase activity (non-significant vs group 1) (empty bars). In all the constructs containing mdr1 promoter sequences, TNF treatment further increased luciferase activity by 1.6 to 2-fold (p < 0.001) compared with their respective controls (solid bars). These results indicate that the rat mdr1b promoter responds to TNF treatment, and that 476 bp of the upstream sequence is essential for both basal and TNF-induced luciferase activity (Fig.1B).
Fig.1.
Rat mdr1b promoter activity shown by luciferase reporter assays.
A. Promoter constructs used in the study. The sequence of the first exon is shown in the orange box, the second exon in light blue, and the flanking first intron in the shaded box. The first nucleotide in the 5′ of the first exon was designated as +1. The sequences, with the last nucleotides upstream to +1 marked as - and downstream marked as +, were cloned into a pGL3.0 basic firefly luciferase vector and verified by automatic sequencing.
B. Luciferase activity of the promoter constructs was measured 30 h after transfection into RBE4 cells (n = 4 /group). RBE4 cells were treated with PBS vehicle or 5 ng/ml of TNF for 6 h, started 24 h after transfection. All constructs had significantly higher basal luciferase activity than the promotorless pGL3.0 empty vector (***: p < 0.005). The highest promoter activity was seen in the group of RBE4 cells expressing plasmid -476/+607. TNF treatment caused a further increase from their respectively controls (+++, p < 0.005).
2. Essential involvement of the NFκB binding site in both basal and TNF-induced promoter activity shown by deletion mutation
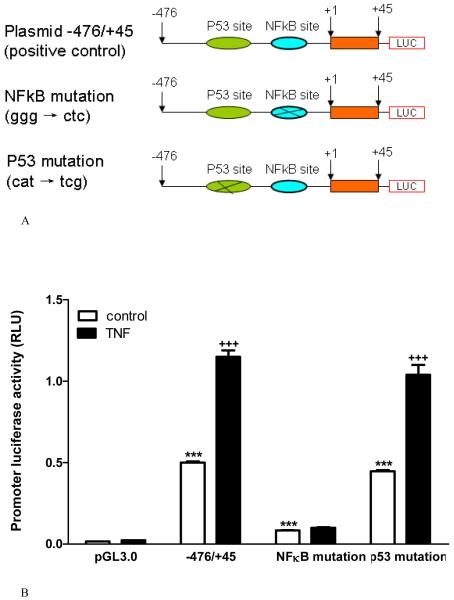
The rat mdr1b promoter contains binding sites for NFκB (-167 bp to -158 bp) and p53 (-200 bp to -180 bp) that are susceptible to regulation by various agents, such as anticancer drugs and insulin [24, 28]. Both p53 and NFκB can be activated by TNF treatment [29, 30]. Therefore, we tested their roles in mdr1 promoter activity by constructing mutant gene fragments with deletion of these binding sites. The plasmid constructs are shown in figure 2A. In groups of RBE4 cells 30 h after transfection of the plasmids, the basal activity is shown in figure 2B with empty bars. The -476/+45 construct conferred promoter activity that drives the transactivation of firefly luciferase in the pGL3.0 plasmid. There was a 31-fold increase compared with the pGL3.0 only (negative control) group (p < 0.001). Removal of the sequences upstream to the p53 binding site (plasmid -218/+45) significantly enhanced the promoter activity (p<0.001 vs -476/+45 plasmid). Further removal of the p53 binding site but with retention of an intact NFκB binding site (plasmid -185/+45) induced the maximal promoter activity (p<0.001 vs -218/+45 plasmid). However, the level of promoter activity did not inversely correlate with the length of the construct. Further deletion of the NFκB binding site in the plasmid -159/+45 greatly reduced basal promoter activity (p<0.001 vs -185/+45 plasmid). The value was comparable to that in the -476/+45 construct. In RBE4 cells expressing the promoter constructs with the NFκB binding sequence, TNF treatment for 6 h (shown in solid bars) caused a further increase of luciferase activity compared with the vehicle-treated control (p < 0.001). TNF failed to cause a significant increase of promoter activity in the absence of the NFκB binding site (Fig.2B).
Fig.2.
Effects of the p53 and NFκB binding sites on mdr1b promoter activity shown by deletion constructs.
A. Nucleotide sequences included in the promoter constructs, with +1 representing the first nucleotide of exon 1.
B. All constructs showed basal luciferase activity. The highest was seen in RBE4 cells expressing plasmid -185/+45 containing the NFκB binding site without the upstream p53 binding site. TNF treatment further increased luciferase activity except in the empty vector and the construct with deletion of NFκB binding site. ***: p < 0.005 compared with the pGL3.0 negative control. +++: p < 0.005 compared with the non-TNF treated control.
3. Effects of point mutation of p53 and NFκB binding sites on promoter activity
The results from deletion constructs might be confounded by the different length of the promoter sequences. Thus, we further performed point mutations of the p53 and NFκB binding sites of the rat mdr1 promoter region (Fig. 3A). The promoter-driven luciferase activity is shown in figure 3B. Construct -476/+45, which has intact binding sites for both p53 and NFκB, conferred more than a 30-fold increase of basal promoter activity compared with the promoterless pGL3.0 luciferase reporter (p < 0.001). TNF treatment of the transfected RBE4 cells caused a further increase of the promoter activity (2-fold, p < 0.001 vs non-TNF treated group). Mutation of the p53 binding site caused no change in promoter activity either before or after TNF treatment. Mutation of the NFκB binding site, by contrast, resulted in a greatly decreased luciferase activity (6-fold, p < 0.001 compared with the wildtype plasmid). There TNF no longer caused further activation of the promoter. Thus, the results are consistent with those shown in figure 2B by deletion constructs. This indicates that the NFκB binding site played a predominant role in mdr1b promoter activation whereas the effect of the p53 binding site was negligible. Exogenous TNF treatment potentiated the promoter activity but was not essential for basal activation.
Fig.3.
Effects of the p53 and NFκB binding sites on mdr1b promoter activity shown by mutation constructs.
A. Point mutation of mdr1b sequence -476/+45 was made at either the NFκB binding site or the p53 binding site. These sequences were cloned into the pGL3.0 luciferase reporter vector.
B. Luciferase assays were performed 30 h after RBE4 cells were transfected with the mutant or wildtype plamids, with (solid bar) or without (empty bar) TNF treatment for 6 h immediately before cell lysis. Mutation of the NFκB binding site nearly completely abolished the promoter activity, and the cells no longer responded to TNF by an increase of luciferase intensity. Mutation of the p53 binding site had no effect.
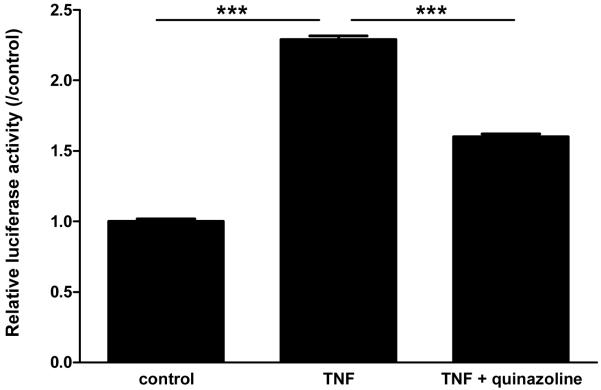
4. Effects of NFκB transcription inhibitor on TNF-induced mdr1b promoter activity
We have shown that inhibitors for NFκB activation can reduce the TNF-induced increase of mdr1b mRNA expression [16]. The activation of NFκB is a complex process involving phosphorylation of the active subunits, dissociation and proteasome degradation of the IκB inhibitory subunit, nuclear translocation of the subunit dimer (p50/p65 heterodimer being a common form), and activation of NFκB-responsive gene elements. To show the direct effect of transcriptional activation, we applied the transcriptional inhibitor quinazoline in TNF-treated cells. Figure 4 shows the findings from luciferase reporter gene assays on RBE4 cells that were transiently transfected with plasmid -476/+45 30 h earlier (n = 3 /group). In the control group, overexpression of the -476/+45 construct caused a significant increase of luciferase activity. TNF treatment caused a 2.3-fold increase of luciferase activity compared with the untreated group, and the difference between the two groups was significant (p < 0.001). This increase was partially blocked (53 % reduction, p < 0.001) by the addition of quinazoline. Thus, NFκB activation participated in the induction of mdr1b gene by TNF.
Fig.4.
In RBE4 cells transfected with plasmid -476/+45, TNF caused a further increase of luciferase activity while co-treatment with quinazoline dampened this increase.
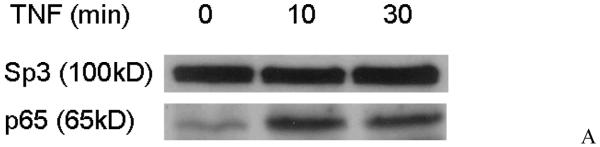
5. Direct binding of NFκB with nuclear DNA after TNF stimulation in RBE4 cells
RBE4 cells were treated with TNF (5 ng/ml) or PBS vehicle, and nuclear proteins were extracted for western blotting of phosphorylated NFκB. As shown in figure 5A, only very small amounts of activated NFκB existed in the nucleus in the absence of TNF treatment. After the cells were stimulated with TNF for 10 min, the amount of activated NFκB was increased substantially and persisted for 30 min of stimulation. Since the activation of NFκB was more robust at 10 min than 30 min, we chose 10 min to detect direct binding of the activated NFκB complex to DNA by EMSA. Consistent with the low level of activated NFκB in the nucleus of non-stimulated RBE4 cells shown in figure 5A, the nuclear extract from non-stimulated RBE4 cells did not show detectable specific binding to the 32P-NFκB probe (Fig.5B, lane 1). By contrast, there was a strong signal when the binding reaction was performed with the nuclear extract from cells treated with TNF (5 ng/ml for 10 min) (lane 2, indicated by arrow). This suggests that TNF induced nuclear translocation of the NFκB signaling complex where it binds to the target gene. The signal representing the binding of the 32P-labeled nucleotide sequence with the NFκB signaling complex was abolished by inclusion of 200-fold molar excess of the unlabeled probe in the binding reaction (lane 3). In the supershift assay, pre-incubation of the reaction mixture with a p65 antibody resulted in a slower migrating band (lane 4). Thus, the NFκB complex contains the p65 subunit that binds specifically to the antibody, forming a DNA-p65-anbitody complex of higher molecular weight. When 200-fold excess of the mutant probe (in which the NFκB binding site has been disrupted) was used, there was no competition of the signal standing for DNA binding of the 32P-NFκB probe (lane 5). Further, by contrast to the specific signals shown by the wildtype 32P-NFκB DNA binding site probe, the 32P-labeled mutant probe failed to show specific binding (lane 6). Therefore, mutation of the NFκB binding site abolished the binding of nuclear-translocated NFκB to its DNA target.
Fig.5.

TNF induced nuclear translocation and DNA binding of the p65 subunit of NFκB.
A. Western blotting of activated p65 (subunit of NFκB) in the nuclear fraction of RBE4 cells which had been treated with TNF for 10 - 30 min after 24 h of serum starvation. The nuclear protein Sp3 was probed as an internal control.
B: EMSA of 5 μg of nuclear extract from RBE4 cells, with or without 10 min of TNF treatment before cell lysis. Lane 1: negative control without TNF treatment or probing antibody. Lane 2: TNF treatment resulting in binding of the 32P-NFκB probe to DNA. Arrow indicates molecular size at which the signal occurs. Lane 3: Inclusion of a 200-fold molar excess of unlabeled probe abolished the signal seen in lane 2. Lane 4: Incubation of the nuclear extract with the p65 antibody slowed the migration of the 32P-NFκB probe from the native gel. The size of a higher molecular weight signal is indicated by an arrowhead. Lane 5: A mutant unlabeled probe in which NFκB binding was abolished did not displace the 32P-NFκB probe from its DNA binding shown at the position of the arrow. Lane 6: A 32P-labeled mutant NFκB probe did not show DNA binding. Most radioactivity was resolved as lower molecular weight bands, probably oligomers and dimers. The dye-front, containing free probes (monomers), was included in all images. wt: wildtype; mt: mutant.
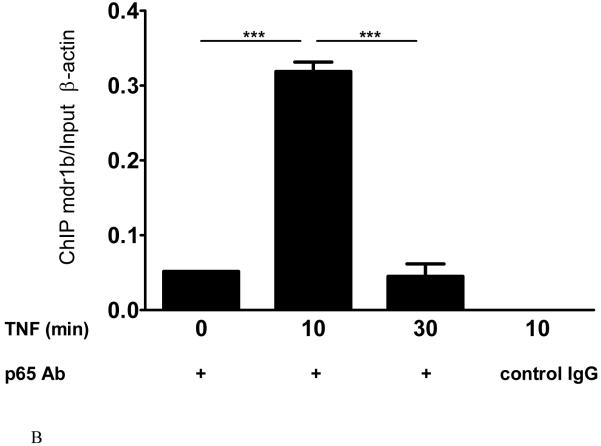
6. Specific binding of NFκB with the NFκB binding site of the mdr1 promoter after TNF stimulation in RBE4 cells
After immunoprecipitation with the p65 NFκB antibody, the mdr1b DNA was amplified in the cell lysate of RBE4 cells 10 min after TNF treatment (lane 2, Fig.6A). At the same treatment time, the control group with isotype-matched IgG did not pull down mdr1b DNA (lane 4). In the absence of TNF (lane 1), or after prolonged TNF treatment (30 min, lane 3), the binding of mdr1b DNA to p65 protein was significantly lower than in the 10 min group (Fig.6B). In the non-immunoprecipitated input samples, mdr1b and β-actin DNA showed similar amplification among the groups.
Fig.6.

TNF induced p65-mdr1b complex in RBE4 cells.
A. ChIP assay on lysates of RBE4 cells treated with TNF for 0, 10, or 30 min. In the lysate obtained after 10 min of TNF treatment, the control IgG failed to pull down mdr1b (lane 4, negative control), despite the presence of mdr1b and β-actin DNA in the input. In the absence of TNF treatment, there was minimal pull-down of mdr1b by the p65 antibody (lane 1). Abundant mdr1b signal was seen in the group 10 min after TNF treatment (lane 2), whereas a longer treatment interval of 30 min did not retain the p65-mdr1b binding (lane 3).
B. Densitometry measurement of the PCR product, expressed as immunoprecipitated mdr1b /total β-actin. The mdr1b signal in the group of cells treated with TNF for 10 min was significantly higher than that of untreated cells or the 30 min treatment group (n = 2 /group). ***: p < 0.005.
Discussion
The BBB serves as a regulatory interface in CNS targeted delivery of therapeutic agents as well permeation of CNS toxins. As inflammation is frequently present in CNS disorders, it is important to understand how inflammatory mediators regulate the efflux transporters. To do this, we used the proinflammatory cytokine TNF as a prototypic stimulus, and determined the molecular targets of its action in upregulating mdr1b. To our knowledge, this is the first mdr1b promoter assay reported for the specialized endothelial cells of the BBB.
The rationale to perform promoter analysis is supported by reports that TNF activates Pgp activity in BBB endothelial cells, with increased mRNA and protein expression of the transporter [5, 16, 31]. Yet it is not clear how TNF induces transcriptional activation of mdr1. There are two isoforms of the mdr1 gene in rodents: mdr1a and mdr1b. Both forms are present in primary cultured rodent brain microvessel endothelial cells and immortalized brain endothelial cells [32, 33]. Our recent studies by microarray and real-time PCR have shown that mdr1b is highly expressed and shows stronger upregulation by TNF in both mouse cerebral microvessels and cultured RBE4 cells. Six h after TNF treatment of RBE4 cells, mdr1b gene expression was increased 50-fold, remaining at least 10-fold enhanced for 24 h [16], while mdr1a expression was induced only 20-fold at 6 h, falling to 7-fold at 12 h and 4-fold at 24 h (unpublished data). Thus, we mainly focused on mdr1b in this study.
The regulatory site in the promoter region of the mdr1 gene differs among species [25] and shows both increases and decreases of promoter activity in studies of parenchymal cells [34-36]. Nonetheless, multiple regulators have been identified, indicating that disease processes can affect the status of drug resistance and response to toxic substances. Using a series of constructs containing different lengths of the rat mdr1b gene, we showed by luciferase reporter assays that the sequence 476 bp upstream of the transcription initiation site was mainly responsible for basal promoter activity. This does not eliminate the possibility that distal enhancers and repressors, as well as posttranscriptional processing, might also participate in the regulation of mRNA expression.
Luciferase reporter assays further indicated that TNF treatment caused a significant increase of the promoter activity from the basal state. This is consistent with studies of several cell lines of non-CNS origin. As inflammation and hypoxia are among the strongest inducers of multi-drug resistance, TNF as well as hypoxia-inducible factor (HIF)-1 have been the most studied regulators of mdr1. In Caco-2 intestinal epithelial cells and bovine aortic endothelial cells, the promoter activity of mdr1 can be induced by hypoxia mediated by transcription activation through HIF-1. This may be implicated in the resistance of hypoxic cells to chemotherapeutic agents [37]. TNF also upregulates mdr1 and renders the cells more resistant to TNF as well as the resulting apopotosis caused by caspase activation [38]. However, reduction of multidrug resistance by TNF and other proinflammatory cytokines in hepatoma cells can also reduce the resistance to chemotherapy [39]. These findings suggest that regulatory changes of the mdr1 gene by TNF appear to be tissue-and cell-specific.
The rat mdr1b promoter contains two major regulatory sites: NFκB and p53. The ubiquitous transcription factor NFκB has been shown to induce drug resistance by upregulation of Pgp expression and function in kidney proximal tubule cells [40] and in a variety of cancer cells [41-44]. When NFκB interacts with c-Fos, downregulation of mdr1 can occur [35]. Regulation by p53 may proceed in either directions [45, 46]. To determine the relative contributions of NFκB and p53 in TNF-induced mdr1b activation in RBE4 cells, we used additional promoter constructs where the p53 binding site, NFκB binding site, or both, had been deleted. Plasmid -476/+45, which contains both p53 and NFκB binding sites and part of the first exon sequence, was used as a positive control. As seen in figure 3B, deletion of the sequence upstream to the p53 binding site (-476 to -217) further increased promoter activity, both in the basal and TNF-stimulated cells. Deletion of the p53 binding site caused increases in both basal and TNF-stimulated states. Further deletion of the NFκB binding site, however, brought the luciferase activity down to a level similar to that of the positive control (plasmid -476/+45), though the increase was still significantly higher than the negative control (pGL3.0 empty vector). Moreover, TNF stimulation did not further increase the luciferase activity in the absence of the NFκB binding site. Taken together, these results indicate that (a) the DNA sequence 159 nucleotide upstream to the first exon confers basal mdr1b promoter activity; (b) the NFκB binding site provides greater mdr1b promoter activity in the resting state, and is responsible for TNF-induced upregulation of mdr1b; and (c) the p53 binding site and sequence further upstream may negatively regulate mdr1b promoter activity.
Since the length of the plasmid may affect its efficiency of expression in RBE4 cells, we further conducted point mutation studies to test the basal and TNF-induced mdr1b promoter activity. Mutation of the NFκB binding site by substitution of ctc for ggg caused a striking suppression of the basal promoter activity, and there was no response of the cells to TNF treatment. Mutation of the p53 binding site (tcg for cat substitution), however, did not cause a significant change of either basal or TNF-stimulated promoter activity in comparison with the positive control (plasmid -476/+45). Thus, the NFκB binding site plays a major role in mdr1 promoter activity in unstimulated cells and in the TNF-treated condition.
Quinazoline is a nitroquinazoline derivative that inhibits NFκB activation [47, 48]. Its inhibitory effect on the promoter activity of plasmid -476/+45 indicates that NFκB is stimulatory, though not essential, in mdr1b promoter activity. To further illustrate that nuclear translocation and binding of activated NFκB are involved in TNF-induced mdr1 promoter activation, EMSA assays were performed. There was specific binding of the radioactive probe to the nuclear extract from TNF-stimulated RBE4 cells, shown by the absence of signals in groups of naïve cells and in the presence of excess unlabeled probe. Moreover, incubation of the nuclear extract with an antibody against the nuclear localization sequence of p65 caused a supershift of the radioactive probe to a higher molecular weight complex. These results show that TNF induces nuclear translocation and DNA binding of the NFκB subunit p65.
Selective binding of p65 to mdr1b was confirmed by chromatin immunoprecipitation and PCR amplification of mdr1b DNA. In the absence of TNF stimulation, there was minimal binding of p65 to mdr1b. TNF treatment for 10 min induced maximal binding, whereas longer treatment duration (30 min) did not show the same effect, consistent with the dynamic nature of p65-DNA association. The time course coincided with the signal intensity of p65 protein in the nuclear fraction by western blot analysis in figure 5A. The specificity was further supported by the absence of mdr1b when the isotype IgG control substituted p65 antibody for ChIP, and by equal amplification of mdr1b and β-actin among the groups in non-ChIP samples (the input). Therefore, TNF treatment induced NFκB activation, p65 nuclear translocation, binding and upregulation of the mdr1b promoter activity in the RBE4 cells.
In summary, we have provided novel evidence showing that the rat mdr1b promoter activity in RBE4 cells resides mainly in a short sequence between the NFκB binding site and the first exon, upstream to the transcription initiation site. The NFκB binding sequence is responsible for TNF-induced potentiation of the mdr1 activity. We propose that in situations with elevated TNF production, such as inflammation, immune reaction, hypoxia, tumor, and neurodegeneration, NFκB activation stimulates multi-drug resistance by transcriptional activation of the efflux transporter. Thus, the targeting of NFκB activation in cerebral endothelia may reduce drug resistance and enhance the delivery of CNS therapeutics across the BBB.
Acknowledgement
Supported by NIH (DK54880, NS45751, NS46528). We thank Dr. Pierre-Olivier Couraud (Institute of Cochin, Paris, France) for the RBE4 cell line, and Ms. Loula Burton for editorial assistance.
References
- 1.Begley DJ. Strategies for delivery of peptide drugs to the central nervous system: exploiting molecular structure. J Control Release. 1994;29:293–306. [Google Scholar]
- 2.Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J Pharmacol Exp Ther. 2005;312:44–52. doi: 10.1124/jpet.104.073320. [DOI] [PubMed] [Google Scholar]
- 3.Mealey KL. Therapeutic implications of the MDR-1 gene. J Vet Pharmacol Ther. 2004;27:257–264. doi: 10.1111/j.1365-2885.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Ercal N, Price TO. The blood-brain barrier in neuroAIDS. Curr HIV Res. 2006;4:259–266. doi: 10.2174/157016206777709447. [DOI] [PubMed] [Google Scholar]
- 5.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 6.Baltes S, Gastens AM, Fedrowitz M, Potschka H, Kaever V, Loscher W. Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology. 2007;52:333–346. doi: 10.1016/j.neuropharm.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 7.Gendelman HE. Biomarkers, laboratory, and animal models for the design and development of adjunctive therapies for HIV-1 dementia and other neuroinflammatory disorders. J Neuroimmune Pharmacol. 2007;2:8–13. doi: 10.1007/s11481-006-9050-2. [DOI] [PubMed] [Google Scholar]
- 8.Pan W, Zadina JE, Harlan RE, Weber JT, Banks WA, Kastin AJ. Tumor necrosis factor α: a neuromodulator in the CNS. Neurosci Biobehav Rev. 1997;21:603–613. doi: 10.1016/s0149-7634(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 9.Pan W, Kastin AJ. TNFα transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- 10.Pan W, Csernus B, Kastin AJ. Upregulation of p55 and p75 receptors mediating TNF alpha transport across the injured blood-spinal cord barrier. J Mol Neurosci. 2003;21:173–184. doi: 10.1385/JMN:21:2:173. [DOI] [PubMed] [Google Scholar]
- 11.Pan W, Akerstrom V, Zhang J, Pejovic V, Kastin AJ. Modulation of feeding-related peptide/protein signals by the blood-brain barrier. J Neurochem. 2004;90:455–461. doi: 10.1111/j.1471-4159.2004.02502.x. [DOI] [PubMed] [Google Scholar]
- 12.Pejovic V, Soskic V, Pan W, Kastin AJ. Brain proteome of mice lacking the receptors for tumor necrosis factor alpha. Proteomics. 2004;4:1461–1464. doi: 10.1002/pmic.200300687. [DOI] [PubMed] [Google Scholar]
- 13.Yu C, Kastin AJ, Tu H, Pan W. Opposing effects of proteasomes and lysosomes on LIFR: modulation by TNF. J Mol Neurosci. 2007;32:80–89. doi: 10.1007/s12031-007-0017-4. [DOI] [PubMed] [Google Scholar]
- 14.Yu C, Kastin AJ, Pan W. TNF reduces LIF endocytosis despite increasing NFkappaB-mediated gp130 expression. J Cell Physiol. 2007;213:161–166. doi: 10.1002/jcp.21105. [DOI] [PubMed] [Google Scholar]
- 15.Pan W, Hsuchou H, Yu C, Kastin AJ. Permeation of blood-borne IL15 across the blood-brain barrier and the effect of LPS. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05390.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu C, Pan W, Tu H, Waters S, Kastin AJ. TNF activates MDR1 (P-glycoprotein) in cerebral microvascular endothelial cells. Cell Physiol Biochem. 2007;20:853–858. doi: 10.1159/000110445. [DOI] [PubMed] [Google Scholar]
- 17.Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. Influence of breast cancer resistance protein (Abcg2) and p-glycoprotein (Abcb1a) on the transport of imatinib mesylate (Gleevec) across the mouse blood-brain barrier. J Neurochem. 2007;102:1749–1757. doi: 10.1111/j.1471-4159.2007.04808.x. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsuki S, Yamaguchi H, Katsukura Y, Asashima T, Terasaki T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem. 2008;104:147–154. doi: 10.1111/j.1471-4159.2007.05008.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanderson L, Khan A, Thomas S. Distribution of suramin, an antitrypanosomal drug, across the blood-brain and blood-cerebrospinal fluid interfaces in wild-type and P-glycoprotein transporter-deficient mice. Antimicrob Agents Chemother. 2007;51:3136–3146. doi: 10.1128/AAC.00372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda K, Pastan I, Gottesman MM. Isolation and sequence of the promoter region of the human multidrug-resistance (P-glycoprotein) gene. J Biol Chem. 1987;262:17432–17436. [PubMed] [Google Scholar]
- 21.Raymond M, Gros P. Cell-specific activity of cis-acting regulatory elements in the promoter of the mouse multidrug resistance gene mdr1. Mol Cell Biol. 1990;10:6036–6040. doi: 10.1128/mcb.10.11.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu L, Cohen D, Piekarz RL, Horwitz SB. Three distinct nuclear protein binding sites in the promoter of the murine multidrug resistance mdr1b gene. J Biol Chem. 1993;268:7520–7526. [PubMed] [Google Scholar]
- 23.Borst P, Schinkel AH. Genetic dissection of the function of mammalian P-glycoproteins. Trends Genet. 1997;13:217–222. doi: 10.1016/S0168-9525(97)01112-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhou G, Kuo MT. Wild-type p53-mediated induction of rat mdr1b expression by the anticancer drug daunorubicin. J Biol Chem. 1998;273:15387–15394. doi: 10.1074/jbc.273.25.15387. [DOI] [PubMed] [Google Scholar]
- 25.Sukhai M, Piquette-Miller M. Regulation of the multidrug resistance genes by stress signals. J Pharm Pharm Sci. 2000;3:268–280. [PubMed] [Google Scholar]
- 26.Stein U, Walther W, Shoemaker RH. Modulation of mdr1 expression by cytokines in human colon carcinoma cells: an approach for reversal of multidrug resistance. Br J Cancer. 1996;74:1384–1391. doi: 10.1038/bjc.1996.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ros JE, Schuetz JD, Geuken M, Streetz K, Moshage H, Kuipers F, Manns MP, Jansen PL, Trautwein C, et al. Induction of Mdr1b expression by tumor necrosis factor-alpha in rat liver cells is independent of p53 but requires NF-kappaB signaling. Hepatology. 2001;33:1425–1431. doi: 10.1053/jhep.2001.24667. [DOI] [PubMed] [Google Scholar]
- 28.Zhou G, Kuo MT. NF-kappaB-mediated induction of mdr1b expression by insulin in rat hepatoma cells. J Biol Chem. 1997;272:15174–15183. doi: 10.1074/jbc.272.24.15174. [DOI] [PubMed] [Google Scholar]
- 29.Trickler WJ, Mayhan WG, Miller DW. Brain microvessel endothelial cell responses to tumor necrosis factor-alpha involve a nuclear factor kappa B (NF-kappaB) signal transduction pathway. Brain Res. 2005;1048:24–31. doi: 10.1016/j.brainres.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Hong Q, Hsu LJ, Schultz L, Pratt N, Mattison J, Chang NS. Zfra affects TNF-mediated cell death by interacting with death domain protein TRADD and negatively regulates the activation of NF-kappaB, JNK1, p53 and WOX1 during stress response. BMC Mol Biol. 2007;8:50. doi: 10.1186/1471-2199-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer B, Hartz AMS, Fricker G, Miller DS. Modulation of p-glycoprotein transport function at the blood-brain barrier. Experimental Biology and Medicine. 2005;230:118–127. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi K, Pu H, Tian J, Andras IE, Lee YW, Hennig B, Toborek M. HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J Neurochem. 2005;93:1231–1241. doi: 10.1111/j.1471-4159.2005.03114.x. [DOI] [PubMed] [Google Scholar]
- 33.Demeuse P, Fragner P, Leroy-Noury C, Mercier C, Payen L, Fardel O, Couraud PO, Roux F. Puromycin selectively increases mdr1a expression in immortalized rat brain endothelial cell lines. J Neurochem. 2004;88:23–31. doi: 10.1046/j.1471-4159.2003.02071.x. [DOI] [PubMed] [Google Scholar]
- 34.Jin S, Scotto KW. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol. 1998;18:4377–4384. doi: 10.1128/mcb.18.7.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogretmen B, Safa AR. Negative regulation of MDR1 promoter activity in MCF-7, but not in multidrug resistant MCF-7/Adr, cells by cross-coupled NF-kappa B/p65 and c-Fos transcription factors and their interaction with the CAAT region. Biochemistry. 1999;38:2189–2199. doi: 10.1021/bi982236+. [DOI] [PubMed] [Google Scholar]
- 36.Yin Y, Allen PD, Jia L, MacEy MG, Kelsey SM, Newland AC. Constitutive levels of cAMP-dependent protein kinase activity determine sensitivity of human multidrug-resistant leukaemic cell lines to growth inhibition and apoptosis by forskolin and tumour necrosis factor alpha. Br J Haematol. 2000;108:565–573. doi: 10.1046/j.1365-2141.2000.01903.x. [DOI] [PubMed] [Google Scholar]
- 37.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- 38.Johnstone RW, Cretney E, Smyth MJ. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood. 1999;93:1075–1085. [PubMed] [Google Scholar]
- 39.Lee G, Piquette-Miller M. Cytokines alter the expression and activity of the multidrug resistance transporters in human hepatoma cell lines; analysis using RT-PCR and cDNA microarrays. J Pharm Sci. 2003;92:2152–2163. doi: 10.1002/jps.10493. [DOI] [PubMed] [Google Scholar]
- 40.Thevenod F, Friedmann JM, Katsen AD, Hauser IA. Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-kappaB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem. 2000;275:1887–1896. doi: 10.1074/jbc.275.3.1887. [DOI] [PubMed] [Google Scholar]
- 41.Yin Y, Allen PD, Jia L, MacEy MG, Kelsey SM, Newland AC. Constitutive levels of cAMP-dependent protein kinase activity determine sensitivity of human multidrug-resistant leukaemic cell lines to growth inhibition and apoptosis by forskolin and tumour necrosis factor alpha. Br J Haematol. 2000;108:565–573. doi: 10.1046/j.1365-2141.2000.01903.x. [DOI] [PubMed] [Google Scholar]
- 42.Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe S, Mills GB, Unate H. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene. 2002;21:1945–1954. doi: 10.1038/sj.onc.1205117. [DOI] [PubMed] [Google Scholar]
- 43.Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Gielen J, Merville MP, Bours V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 44.McCarty MF, Block KI. Preadministration of high-dose salicylates, suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy. Integr Cancer Ther. 2006;5:252–268. doi: 10.1177/1534735406291499. [DOI] [PubMed] [Google Scholar]
- 45.Li ZH, Zhu YJ, Lit XT. Wild-type p53 gene increases MDR1 gene expression but decreases drug resistance in an MDR cell line KBV200. Cancer Lett. 1997;119:177–184. doi: 10.1016/s0304-3835(97)00267-x. [DOI] [PubMed] [Google Scholar]
- 46.Thottassery JV, Sun D, Zambetti GP, Troutman A, Sukhatme VP, Schuetz EG, Schuetz JD. Sp1 and egr-1 have opposing effects on the regulation of the rat Pgp2/mdr1b gene. J Biol Chem. 1999;274:3199–3206. doi: 10.1074/jbc.274.5.3199. [DOI] [PubMed] [Google Scholar]
- 47.Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, Hayashi H. A novel structural class of potent inhibitors of NF-kappa B activation: structure-activity relationships and biological effects of 6-aminoquinazoline derivatives. Bioorg Med Chem. 2003;11:3869–3878. doi: 10.1016/s0968-0896(03)00438-3. [DOI] [PubMed] [Google Scholar]
- 48.Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, Fukazawa T, Hayashi H. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation. Bioorg Med Chem. 2003;11:383–391. doi: 10.1016/s0968-0896(02)00440-6. [DOI] [PubMed] [Google Scholar]