Abstract
This study characterized structural abnormalities associated with onset of seizures in children using MRI and a standardized classification system in a large prospective cohort. A total of 281 children aged 6 to 14 years completed an MRI within six months of their first recognized seizure. Most MRI examinations were performed with a standardized, dedicated seizure protocol, and all were scored using a standard scoring system. At least one MRI abnormality was identified in 87 of the 281 (31%) of children with first recognized seizure. Two or more abnormalities were identified in 34 (12%). The most common abnormalities were ventricular enlargement (51%), leukomalacia/gliosis (23%), gray matter lesions such as heterotopias and cortical dysplasia (12%), volume loss (12%), various other white matter lesions (9%), and encephalomalacia (6%). Abnormalities defined as significant, or potentially related to seizures, occurred in 40 (14%). Temporal lobe and hippocampal abnormalities were detected at a higher frequency than in previous studies (13/87). Use of MRI and a standardized reliable and valid scoring system demonstrated a higher rate of abnormal findings than previous investigations, including findings that might have been considered incidental in the past. Practice parameters may need to be revised to expand the definition of significant abnormalities and to support wider use of MRI in children with newly diagnosed seizures.
Keywords: Epilepsy, MRI, Seizures, Child, Brain
Introduction
Few studies have systematically examined structural brain abnormalities in children at seizure onset. Shinnar and colleagues [1] recruited 218 children in New York City, ages 1–19 years, who presented with their first unprovoked afebrile seizure from 1983 to 1992; imaging studies (73% computed tomography, or CT) were obtained over the course of the disorder (personal communication to P. S. Fastenau, February 15, 2006). Of these, 45 (21%) exhibited abnormalities on at least one scan; 39 (17.9%) were presumed to be etiologically related (i.e., “significant” abnormalities). However, CT likely underestimates the extent of structural brain abnormalities. Also, many of the imaging studies were completed many years after onset, which can be a confounding factor because brain structure may change during chronic epilepsy [2,3]. Finally, the sample might not have been representative because the investigators excluded children with typical absence seizures; myoclonic seizures; infantile spasms; generalized tonic-clonic seizures if they had had prior absence or partial seizures; and prior unrecognized seizures, which were reported in approximately one third of children referred to their study [4].
In a parallel study, Berg and colleagues [5] reported on 488 children in Connecticut, of whom 79.9% had completed an imaging study (only 63.3% of all patients had magnetic resonance imaging, or MRI) following a second unprovoked seizure, that is, newly diagnosed epilepsy. In that study, 62 (12.7% of the children who had imaging studies) exhibited significant abnormalities on at least one scan.
Other studies of new-onset seizures focused on children presenting to emergency departments, but these studies were limited with regard to full assessment of the first unprovoked seizure. They relied on CT, included substantial proportions of febrile seizures (30–71%), had small sample sizes [6–10], and/or included relatively few children [11].
This study characterized structural abnormalities associated with onset of seizures in a large prospective cohort of children using MRI and a standardized classification system. The present study extends earlier work by other investigators in several ways. First, imaging was limited to MRI, the current anatomical “gold standard” [12]. Second, imaging was performed nearer to the time of seizure onset, the first recognized seizure. Third, we applied a standardized classification system to the MRI findings and present a more comprehensive and systematic description of imaging findings than these previous investigations. Fourth, data were coded directly from the MR images by a neuroradiologist who read the images firsthand. Finally, MRI findings were examined in relationship to various epileptic syndromes to provide a basis for more informed clinical decision-making in new-onset seizures.
Methods
Subjects
This study was part of a larger NIH-funded study examining cognitive and behavioral measures in children with a first recognized seizure. Children were recruited from clinics at two major medical centers (Indiana University Medical Center and Cincinnati Children's Hospital Medical Center), as well as from physicians in private practice and school nurses in the metropolitan and outlying rural areas. Compared to two other large community-based prospective studies of new-onset seizures in children [4,13,14], our sample was nearly identical with regard to seizure type, proportion of children who had had prior unrecognized seizures at time of first presentation, and proportion of children who experienced a recurrent seizure within their first three years after onset [15].
Children were 6 to 14 years old and had had a first-recognized unprovoked seizure within the 3 months prior to enrollment. This age range was chosen in order to perform adequate neuropsychological testing. Children were not excluded if they had had previous unrecognized/undiagnosed seizures. Children were excluded if their seizure resulted from an acute situational etiology such as toxin, infection, or trauma; if they had a significant neurological deficit; if they had moderate or severe mental retardation; if they had a mass lesion; or if they had a chronic illness that would limit their activities of daily living, such as cerebral palsy or pervasive developmental disorders. Such children had to be excluded by the requirements of the larger study, because these conditions might have affected behavior and cognition, which was the focus of the larger study.
Of the 349 children enrolled, 281 (81%) had an MRI examination within 6 months after their first recognized seizure. Of the 69 children not included in this study, 19 had MRIs that were outside the 6-month window of the date of first recognized seizure, 19 had CT imaging only, and 31 had neither MRI nor CT imaging available. Although MRI was offered to all children, it was not a requirement of the larger study and some parents or children declined. Reasons for declining included need to travel a considerable distance on a separate visit, parent not wanting to miss work, child not wanting to have the MRI, and parent fearing that the child would not be able to tolerate the procedure. Delays in imaging were usually related to family schedule, ability to travel, and scheduling. Compared to the 281 children in this study (using Wilcoxon rank-tests for age and IQ and chi-square tests otherwise), there were no significant differences in IQ, sex, handedness, race, seizure type, or neurological exam results. Children not in the study had median ages at first recognized seizure marginally lower than those in the study (8.5 years vs. 9.3 years, respectively, p = .05). Children who were not in this study were more likely to have an "undetermined" epileptic syndrome compared to those in the study (19% vs. 5% respectively, p = 0.0001) because of the role of imaging results in determining syndrome. Excluding the “undetermined” classification, there were no other epileptic syndrome differences between the groups.
Before participation, parents gave written consent and children gave written assent. This study was approved by the institutional review boards at both participating institutions, and both institutions are compliant with HIPAA regulations.
MR Imaging
MRI examinations at both primary sites were performed on 1.5 Tesla GE Signa Horizon LX scanners (GE Medical Systems, Milwaukee, Wisconsin), running 9.x system software. A standardized pediatric seizure protocol was modified from those in clinical use at both medical centers. The protocol consisted of the following scanning sequences (total scan time approximately 26 minutes):
Sagittal T1-weighted spin echo (SE), with repetition time (TR) = 450ms, echo time (TE) = minimum, slice thickness/interslice gap = 4mm/0mm interleaved, matrix = 256 × 192, field of view (FOV) = 22cm, number of excitations (NEX) = 1.
Axial T2-weighted fast spin echo (FSE), with TR/TE = 3000/102 ms, echo train length (ETL) = 12, 5mm/0mm interleaved, matrix = 256 × 192, FOV = 22 × 16cm, NEX = 1.
Coronal oblique fast fluid attenuated inversion recovery (FLAIR), with TR/TE/TI (inversion time) = 10,000/120/2200, 4mm/0mm interleaved, matrix = 256 × 256, FOV = 18cm, NEX = 1.
Coronal oblique fast multiplanar inversion recovery (FMPIR), with TR/TE/TI = 5000/100/120ms, ETL = 16, slice thickness/gap = 3mm/0mm, matrix = 512 × 256, FOV = 16cm, NEX = 2.
Axial diffusion weighted single-shot spin-echo echoplanar, b = 1000, with TR/TE = 7000ms/min, slice thickness/gap = 5/1 mm, matrix = 128 × 128, FOV = 22cm, NEX = 1.
Axial 3D inversion recovery prepped fast SPGR (spoiled gradient recalled), with TR/TE = 30ms/min; flip angle = 20, slice thickness = 1–1.3mm, matrix = 256 × 256, FOV = 22cm, NEX = 1.
Some examinations (64 of 281, 23%) were performed on MRI units at affiliated or outside facilities. These were performed on magnet systems varying from 0.5–1.5 Tesla, using routine clinical sequences. These consisted of sagittal, axial and, in most cases, coronal images. When compared to the primary academic medical center examinations, all of which were performed at 1.5 Tesla, the examinations performed at affiliated or outside facilities showed no significant differences in the proportion of significant abnormalities identified. Contrast administration was not part of the standard protocol for study sites and was not considered in the analysis.
MRI examinations were subjectively rated as “Good,” “Adequate,” or “Limited.” Limited exams typically demonstrated significant motion artifact on multiple sequences. Adequate exams typically demonstrated motion artifact on one or more sequences, but were considered diagnostic.
MRI Scoring System
The scoring system for MRI examinations utilized in this study (see Appendix) was adapted from a system previously developed at one of our study sites [16,17]. The scoring system classifies 12 abnormal features by location. Abnormal features include volume loss, leukomalacia/gliosis, other white matter lesion, encephalomalacia, heterotopia, cortical dysplasia, other gray matter lesion, mass lesion, increased or decreased (restricted) diffusion, hemorrhage, and vascular lesion. Locations are classified by lobe, hemisphere, and periventricular region, as well as cerebellar, brainstem, or generalized. The scoring system also classifies ventricular enlargement (maximal width of the midbody of the lateral ventricle on the axial images), prominence of the extra-axial spaces (width over the frontal and cerebellar convexities), presence or absence of hippocampal atrophy or signal abnormality, and presence or absence of other structural abnormalities (developmental anomalies or notable variants not otherwise classified, such as Chiari I malformation and arachnoid cyst). Abnormal features and locations are listed in Table 2.
Table 2.
MRI abnormalities by location (n = 281, 87 with at least 1 abnormality)
| Abnormality |  |
F | T | Par | O | H | Peri | C | BS | G | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume loss | 10 | 3.6 | 11.5 | 3 | 2 | 4 | 3 | 2 | 1 | ||||
| Leukomalacia/gliosis | 20 | 7.1 | 23.0 | 8 | 3 | 6 | 2 | 1 | 8 | 2 | |||
| Other WML | 8 | 2.8 | 9.2 | 5 | 1 | 4 | 1 | 1 | |||||
| Encephalomalacia | 5 | 1.8 | 5.7 | 3 | 2 | 3 | 2 | ||||||
| Gray matter lesion - heterotopias | 4 | 1.4 | 4.6 | 3 | 1 | 2 | |||||||
| Gray matter lesion-Cortical dysplasias | 3 | 1.1 | 3.4 | 3 | |||||||||
| Other gray matter lesion | 4 | 1.4 | 4.6 | 1 | 1 | 2 | |||||||
| Increased diffusion† | 2 | 0.7 | 2.3 | 2 | 2 | 1 | 1 | ||||||
| Decreased diffusion† | 0 | ||||||||||||
| Hemorrhage | 0 | ||||||||||||
| Vascular lesion | 3 | 1.1 | 3.4 | 2 | 2 | ||||||||
| Ventricular Enlargement | |||||||||||||
| 1.0–1.5 cm | 39 | 13.9 | 44.8 | 39 | |||||||||
| 1.6–2.5 cm | 5 | 1.8 | 5.7 | 5 | |||||||||
| >2.5 | 0 | ||||||||||||
| Extra-axial Spaces – width over frontal | |||||||||||||
| 0.5–1.0 cm | 3 | 1.1 | 3.4 | 3 | |||||||||
| 1.1–1.5 cm | 0 | ||||||||||||
| >1.5 cm | 0 | ||||||||||||
| Hippocampal Abnormality – Atrophy‡ | 4 | 1.4 | 4.6 | 4 | |||||||||
| Hippocampal Signal Abnormality‡ | 3 | 1.1 | 3.4 | 3 | |||||||||
| Other Structural Abnormality | |||||||||||||
| Not Significant | 8 | 2.8 | 9.2 | 8 | |||||||||
| Significant | 1 | 0.4 | 1.1 | 1§ | |||||||||
| No. of Children by Location# | 44 | 23 | 13 | 17 | 3 | 1 | 11 | 11 | 1 | 2 | |||
| % of 87 | 50.6 | 26.4 | 14.9 | 19.5 | 3.4 | 1.1 | 12.6 | 12.6 | 1.1 | 2.3 | |||
| % of Total | 15.7 | 8.2 | 4.6 | 6.0 | 1.1 | 0.4 | 3.9 | 3.9 | 0.4 | 0.7 | |||
NLS = not location specific, F = frontal, T = temporal, Par = parietal, O = occipital, H = hemisphere, Peri = periventricular, C = cerebellar, BS = brainstem, G = generalized.
There were no extra axial spaces – cerebellar convex abnormalities.
For a given abnormality, a child can have more than one location, so the total number of abnormalities across locations for any given abnormality may be more than the number of children with that abnormality.
Diffusion was associated with other abnormalities in all three children and was not counted as a separate abnormality when reporting the number of children with multiple abnormalities.
A few scans were judged to be inadequate to assess hippocampal atrophy (8 scans) and hippocampal signal abnormality (7 scans).
The significant structural abnormality was agenesis of corpus callosum.
For a given location, a child can have more than one abnormality, so the total number of abnormalities across abnormalities for any given location may be more than the number of children with any abnormality at that location.
Note. Empty cells = 0. Shaded rows represent “significant abnormalities” (i.e., those that were judged to be etiologically related to the seizure condition).
It was recognized that there is some potential overlap in scoring encephalomalacia and leukomalacia/gliosis. Encephalomalacia was generally considered to signify “cystic encephalomalacia” and to involve cortical regions. If there was some signal abnormality consistent with gliosis adjacent to an area of encephalomalacia, this was scored as encephalomalacia. If in addition there were other discrete areas of abnormal signal consistent with gliosis, then these were scored as (additional) areas of leukomalacia/gliosis. Signal changes consistent with perivascular spaces were recorded if prominent or unusual, but were not considered abnormalities.
Significant MRI Abnormalities
A significant abnormality was defined as an MRI finding reasonably likely to be related to seizure disorder, consistent with other classifications of epileptogenic lesions [12]. These were defined a priori as at least one of the following: leukomalacia/gliosis, encephalomalacia, any gray matter lesion, mass lesion, hemorrhage, vascular lesion, hippocampal abnormality, ventricular enlargement > 1.5 cm, or prominence of extra-axial fluid spaces > 1.0 cm. Structural abnormalities were examined on a case-by-case basis.
Rating Methodology
A total of three raters participated in this study; all were senior attending neuroradiologists with 14–26 years of experience. Raters were blind to the other raters’ scoring and to other clinical data (e.g., EEG) except knowing that the child had presented with a seizure. This served two functions: to minimize the bias of the raters and to simulate more closely the radiological practice scenario in many institutions. Because of the prospective nature of the study and the sizeable number of exams at each site, each MRI exam was scored by one rater. Exams were generally reviewed on standard PACS workstations, although the outside, non-affiliated exams were reviewed on hardcopy.
Validity
As preliminary evidence of content validity, the scoring system was reviewed and endorsed by five experts (three board- and CAQ-certified neuroradiologists and two board-certified pediatric neurologists) prior to use in the study. Criterion-related validity was demonstrated in another paper showing that significant abnormalities but not non-significant abnormalities were associated with neuropsychological deficiencies at onset [18].
Inter-Rater Reliability
To measure the inter-rater reliability of the scoring system, three neuroradiologists coded MRI exams on a selected group of 30 children. Pairwise comparisons of the reviewers yielded a mean percent agreement of 99%. The minimum percent agreement for any individual child’s scan was 96%. Upon completion of the blinded ratings, discrepancies among the raters were discussed, differences in scoring were resolved, and modifications were made to the criteria to improve consistency and reduce ambiguity. Other scans were also reviewed and rescored as necessary using the revised criteria. The initial rating discrepancies included classification of borderline low cerebellar tonsils and coding of perivascular spaces.
Results
Of the 349 children enrolled in the study, 281 (81%) had an MRI examination within 6 months after their first recognized seizure. Demographic and clinical characteristics of this sample are presented in Table 1. Age of onset was distributed across the entire age range, with roughly equal numbers of boys and girls. Estimated IQ covered a broad range with the mean and median falling in the middle of the average range for healthy children, consistent with other studies of new- or recent-onset seizures [19]. Seizure type and epileptic syndrome were classified based on the history and clinical data including results of the EEG. EEG results were available for all 281 children. The majority of children (91%) had only one seizure type, and a variety of seizure types and epileptic syndromes were represented. Children with partial seizures slightly outnumbered those with generalized seizures, which is characteristic of this population [20]. The etiology was equally distributed between idiopathic/familial and symptomatic, which is somewhat different from population estimates that favor idiopathic/familial by approximately 2:1 [21]. Among children with symptomatic epileptic syndromes, there were roughly equal proportions of common causes (malformation, neonatal, trauma, infection), which is consistent with population estimates [21].
Table 1.
Demographic and Clinical Characteristics of the Sample (n = 281)
| Mean (SD) or % | |
|---|---|
| Age | 9.7 (2.5) |
| IQ (Estimated from K-BIT)* | 101.8 (15.1) |
| Gender (% Female) | 51% |
| Handedness (% Left-Handed)† | 11% |
| Race | |
| White/Non-Hispanic | 81% |
| African-American | 13% |
| Multiracial | 2% |
| Hispanic | 1% |
| Asian | 1% |
| Other/Unknown | 2% |
| Primary Seizure Type§ | |
| Generalized Tonic-Clonic | 29% |
| Atonic, Akinetic, Myoclonic | 1% |
| Complex Partial Seizures | 22% |
| Simple Partial | 6% |
| Partial with | 28% |
| Absence | 12% |
| Unknown/Unclassified | 2% |
| Epileptic Syndrome§ | |
| Generalized Idiopathic Generalized Tonic Clonic | 17% |
| Generalized Idiopathic Absence | 12% |
| Generalized Symptomatic/Cryptogenic | 3% |
| Idiopathic Partial | 17% |
| Partial Symptomatic/Cryptogenic | 46% |
| Undetermined | 5% |
| Neurological Exam | |
| Normal | 94% |
| Abnormal | 5% |
| Not Done | 1% |
n = 249
n = 248
Seizure type and epileptic syndrome were classified based on data available at the time of the imaging scan, consistent with a realistic clinical scenario.
Of the 281 children, 141 (50%) completed an MRI examination within 1 month following the first recognized seizure, 76 (27%) from 1–2 months following the first recognized seizure, and 64 (23%) from 2–6 months following the first recognized seizure. The MRI examination was obtained within an average of 5.8 weeks (SD = 5.4) of the first recognized seizure.
Overall, 76% of the MRI studies were rated as good, 20% adequate, and only 4% were considered limited. Of the studies completed at outside facilities, 60 (94%) were rated as adequate or good in scan quality. All studies were considered interpretable and were included in the analysis. Any differences between study (inside) sites versus outside sites in imaging technique or quality appear inconsequential inasmuch as there were no differences between sites on the major outcome variable, proportion of significant abnormalities (16% inside vs. 9% outside, χ2 = 1.60, p = 0.21).
Of the 281 children in this study, 87 (31%) had at least one MRI abnormality. Two or more abnormalities were identified in 34 (12%) children. Forty children (14%) had MRI abnormalities that were classified as significant in that they were considered to be potentially etiologically related to the seizure condition.
Table 2 presents the number of children with MRI abnormalities by type of abnormality and by location. Because some abnormalities span multiple locations for a given child, the sum of location values for a given type of abnormality may exceed the total number of children with that abnormality type. Similarly, because different abnormalities can affect the same locations for a given child, the sum of abnormalities for a given location may exceed the total number of children at a particular location.
Of the 87 children with at least one MRI abnormality, the most common abnormalities were ventricular enlargement greater than 1 cm (44/87, 51%), leukomalacia/gliosis (20/87, 23%), gray matter lesions (10/87, 12%), volume loss (10/87, 12%), various other white matter lesions (8/87, 9%), and encephalomalacia (5/87, 6%). Mild ventricular enlargement, in the range of 1.0–1.5 cm, was found in 39 children, with 7 of these showing other significant abnormalities. All five children with moderate ventricular enlargement (1.6–2.5 cm) had other significant abnormalities. By location, the most common were right frontal (18/87, 21%), left parietal (14/87, 16%), left frontal (11/87, 13%), periventricular (11/87, 13%), cerebellar (11/87, 13%), and right parietal (9/87, 10%). Temporal and hippocampal abnormalities were observed in 13 children (15%; 6 left, 6 right, 1 bilateral). Figure 1–Figure 3 show examples of leukomalacia/gliosis and encephalomalacia. Figure 4 shows an example of ventricular enlargement at the level of measurement.
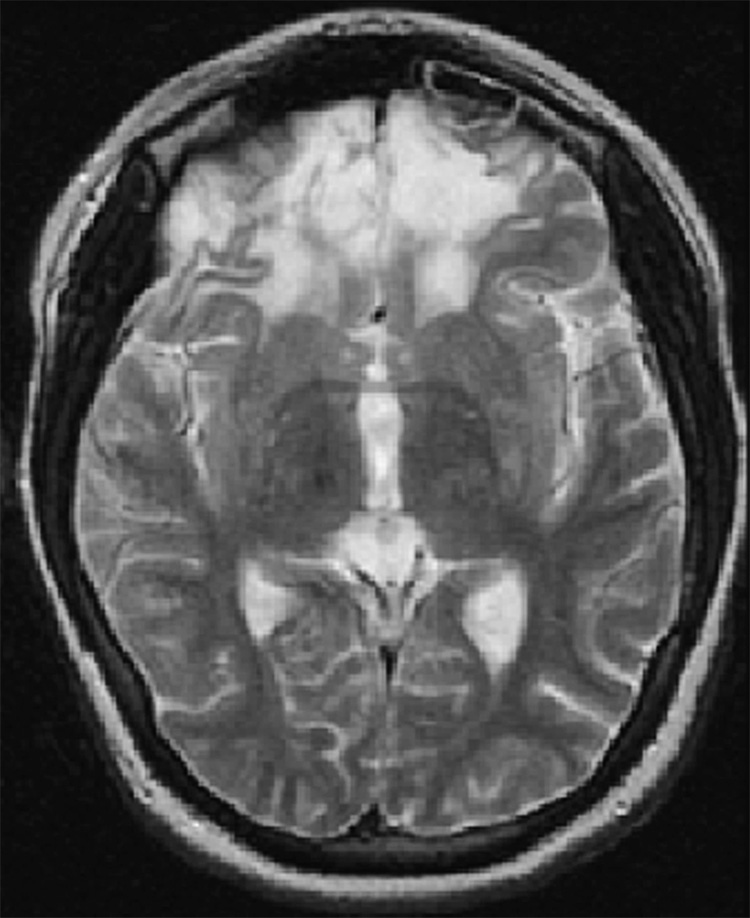
Figure 1.
Subject #212: Bilateral frontal encephalomalacia with gliosis and volume loss. Transverse T2-weighted fast spin echo MR image (TR/TE = 3000/102 ms, ETL = 12, slice thickness 5mm with 0mm gap interleaved, matrix = 256 × 192, FOV = 22 × 16cm).
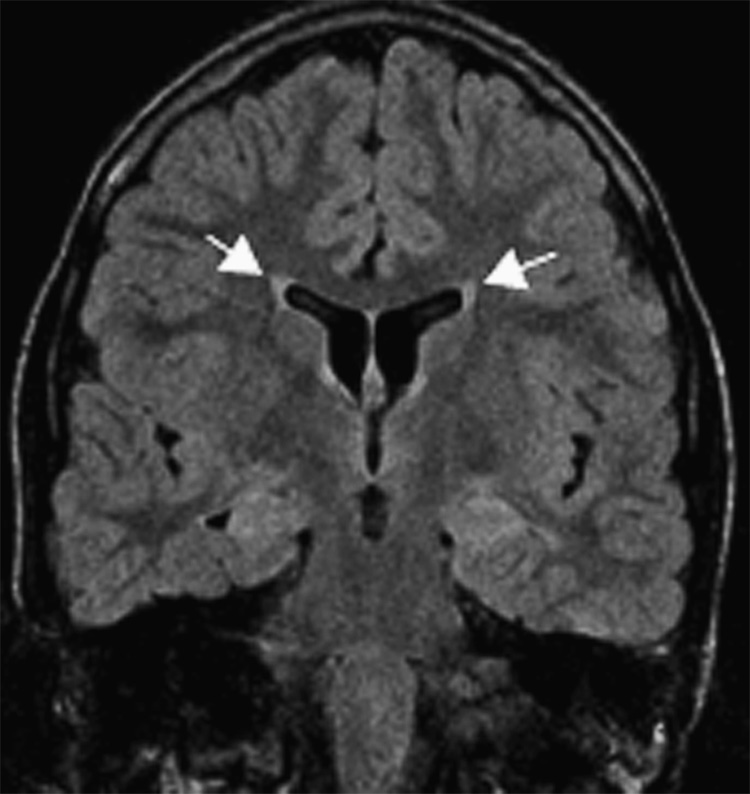
Figure 3.
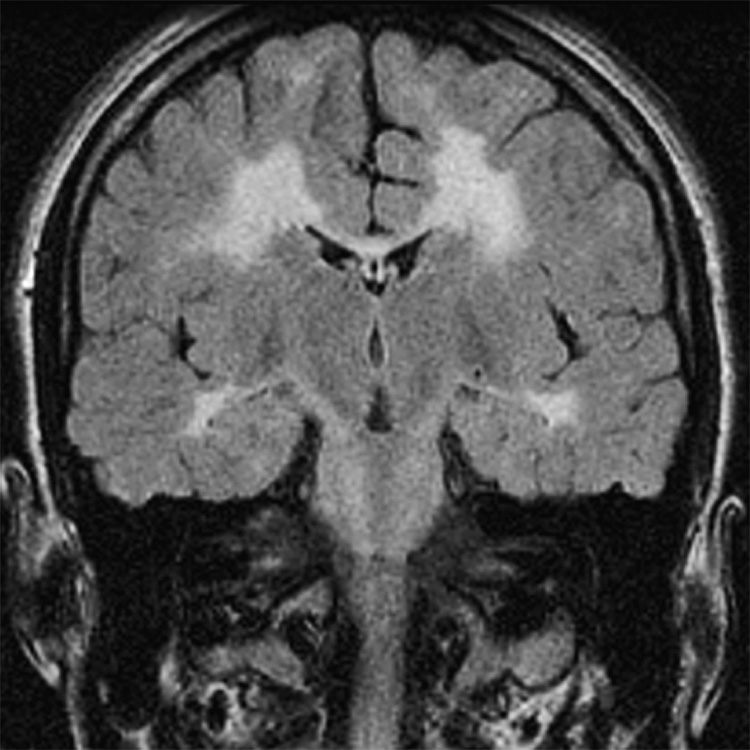
Subject #223: Leukomalacia/gliosis with ventricular enlargement (arrows). Coronal oblique fast fluid attenuated inversion recovery MR image (TR/TE/TI = 10000/120/2200, slice thickness 4mm with 0mm gap interleaved, matrix = 256 × 256, FOV = 18cm).
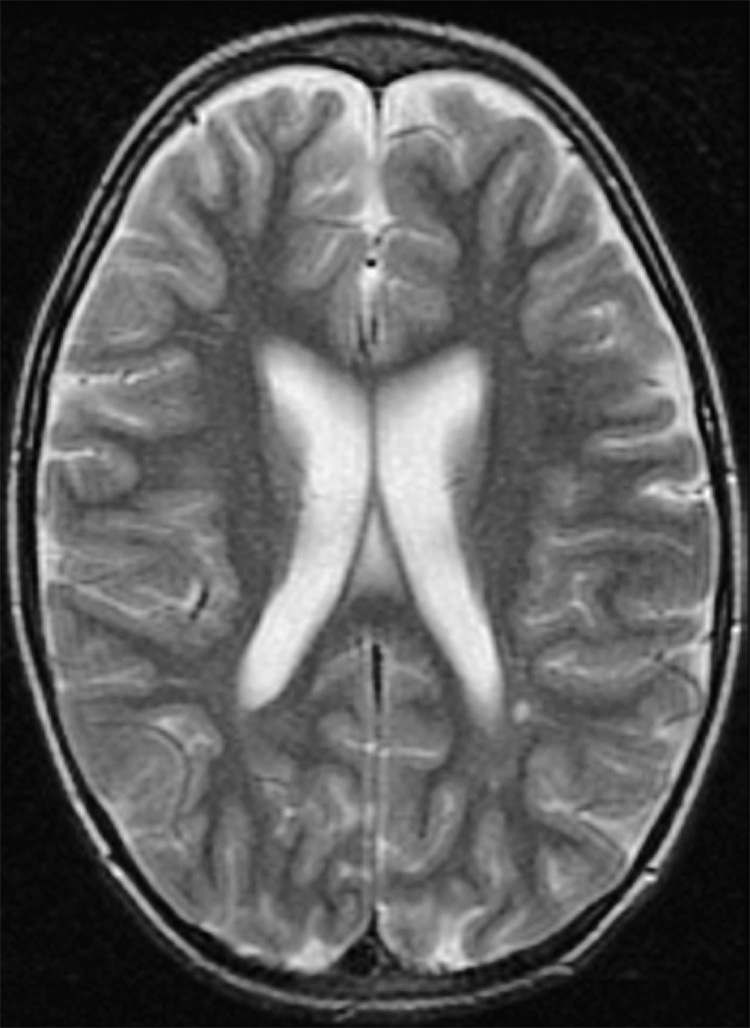
Figure 4.
Subject #204: Ventricular enlargement with volume loss and enlarged extra-axial spaces. Slice at level of ventricular measurement. Transverse T2-weighted fast spin echo MR image (TR/TE = 3000/102 ms, ETL = 12, slice thickness 5mm with 0mm gap interleaved, matrix = 256 × 192, FOV = 22 × 16cm).
MRI abnormalities are reported by epileptic syndromes [22] (Table 3). Children with symptomatic/cryptogenic seizures, both generalized and localization-related, were most likely to have at least one MRI abnormality (42.9% and 39.2%, respectively). Also, 23% – 30% of children with generalized idiopathic epilepsies had at least one MRI abnormality.
Table 3.
MRI Abnormalities by Epileptic Syndrome
| Generalized Idiopathic GTC |
Generalized Idiopathic Absence |
Generalized Symptomatic/ Cryptogenic |
Localization- Related Idiopathic |
Localization- Related Symptomatic/ Cryptogenic |
Undetermined | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Children | 48 | 33 | 7 | 49 | 130 | 14 | ||||||||||||
| Number of Children with at least one Abnormality | 11 (22.9%) | 10 (30.3%) | 3 (42.9%) | 9 (18.4%) | 51 (39.2%) | 3 (21.4%) | ||||||||||||
| Abnormality | ||||||||||||||||||
| n | % of 48 | % of 11 | n | % of 33 | % of 10 | n | % of 7 | % of 3 | n | % of 49 | % of 9 | n | % of 130 | % of 51 | n | % of 14 | % of 3 | |
| Volume loss | 2 | 4.2 | 18.2 | 1 | 3.0 | 10.0 | 1 | 14.3 | 33.3 | 1 | 2.0 | 11.1 | 4 | 3.1 | 7.8 | 1 | 7.1 | 33.3 |
| Leukomalacia/gliosis | 2 | 4.2 | 18.2 | 2 | 28.6 | 66.7 | 2 | 4.1 | 22.2 | 14 | 10.8 | 27.5 | ||||||
| Other WML | 3 | 6.3 | 27.3 | 1 | 3.0 | 10.0 | 4 | 3.1 | 7.8 | |||||||||
| Encephalomalacia | 1 | 2.1 | 9.1 | 1 | 14.3 | 33.3 | 3 | 2.3 | 5.9 | |||||||||
| Gray matter lesion - heterotopias | 1 | 2.1 | 9.1 | 3 | 2.3 | 5.9 | ||||||||||||
| Gray matter lesion - Cortical dysplasias | 1 | 3.0 | 10.0 | 2 | 1.5 | 3.9 | ||||||||||||
| Other gray matter lesion | 2 | 4.2 | 18.2 | 2 | 1.5 | 3.9 | ||||||||||||
| Increased diffusion* | 1 | 14.3 | 33.3 | 1 | 0.8 | 2.0 | ||||||||||||
| Decreased diffusion* | 1 | 0.8 | 2.0 | |||||||||||||||
| Hemorrhage | ||||||||||||||||||
| Vascular lesion | 2 | 6.1 | 20.0 | 1 | 0.8 | 2.0 | ||||||||||||
| Ventricular Enlargement | ||||||||||||||||||
| 1.0 – 1.5 cm | 3 | 6.3 | 27.3 | 6 | 18.2 | 60.0 | 1 | 14.3 | 33.3 | 7 | 14.3 | 77.8 | 19 | 14.6 | 37.3 | 3 | 21.4 | 100 |
| 1.6 –2.5 cm | 1 | 14.3 | 33.3 | 4 | 3.1 | 7.8 | ||||||||||||
| >2.5 cm | ||||||||||||||||||
| Extra-axial fluid spaces - Width over frontal | ||||||||||||||||||
| 0.5 – 1.0 cm | 1 | 3.0 | 10.0 | 2 | 1.5 | 3.9 | ||||||||||||
| 1.1–1.5 cm | ||||||||||||||||||
| >1.5 cm | ||||||||||||||||||
| Hippocampal Abnormality - Atrophy | 1 | 2.1 | 9.1 | 1 | 2.0 | 11.1 | 2 | 1.5 | 3.9 | |||||||||
| Hippocampal Signal Abnormality | 3 | 2.3 | 5.9 | |||||||||||||||
| Other Structural Abnormality | ||||||||||||||||||
| Not Significant | 2 | 6.1 | 2.0 | 6 | 4.6 | 11.8 | ||||||||||||
| Significant | 1 | 0.8 | 2.0 | |||||||||||||||
Note. Empty cells = 0. Shaded rows represent “significant abnormalities” (i.e., those that were judged to be etiologically related to the seizure condition).
There were no extra axial spaces – cerebellar convex abnormalities.
Diffusion was associated with other abnormalities in all three children and was not counted as a separate abnormality when reporting the number of children with multiple abnormalities.
Discussion
The major finding was that using a standardized scoring system with good quality MR imaging for new-onset seizures demonstrated a higher rate of total abnormal findings (31%) compared to previous studies. A number of general observations can be made from our data. Only a minority of children at their first recognized seizure displayed classic epileptogenic lesions involving the cortex or gray matter. Rather, white matter lesions were more commonly associated with the first recognized seizure. In addition, volume loss, considered a priori as a non-significant abnormality, was also more frequent than expected in this population, consistent with Shinnar and colleagues’ findings [1]. Mild ventricular enlargement (1.0–1.5 cm) was also fairly frequent (14% of total sample), and approximately one fifth of those were accompanied by other significant abnormalities. It is surprising that such a large number of cases of white matter abnormalities and ventricular enlargement were identified. This might be because we used a standardized scoring system, and because we measured ventricular size in a consistent manner rather than by informal judgment. We also used contemporary MR imaging and in most cases a standardized seizure protocol.
Our data cannot be compared easily to other studies because the populations are somewhat different. Our study attempted to acquire MRI scans on all children, whereas previous studies excluded some syndromes such as absence epilepsy. Also, specific abnormalities may occur in different proportions in our study as compared to the Berg [5] and Shinnar [1] studies because they included children who had seizure onset in infancy and early childhood. Many of those early epileptic syndromes have unique structural abnormalities that would generally not be associated with a new presentation of seizures at age 6 and beyond. For example, a substantial proportion of their children had conditions such as cerebral dysgenesis, genetic abnormalities, and neurocutaneous syndromes, as well as much higher rates of encephalomalacia compared to our sample. Thus, had our study extended down to infancy (like prior studies), use of MRI would likely have revealed an even higher proportion of abnormalities.
The standardized scoring system for rating MRI examinations in this population was found to have excellent content validity and inter-rater agreement. Moreover, comparisons to previous studies revealed some important benefits of using MRI with a standardized scoring system. First, our study identified a much higher proportion of total abnormalities (31%) compared to the study that relied mostly on CT scans (16%) [1]. Second, the relatively low rate of total abnormalities (21%) in a new-onset sample that used MRI for 80% of the sample [5] raises the possibility that use of a standardized scoring system may further improve detection of abnormalities.
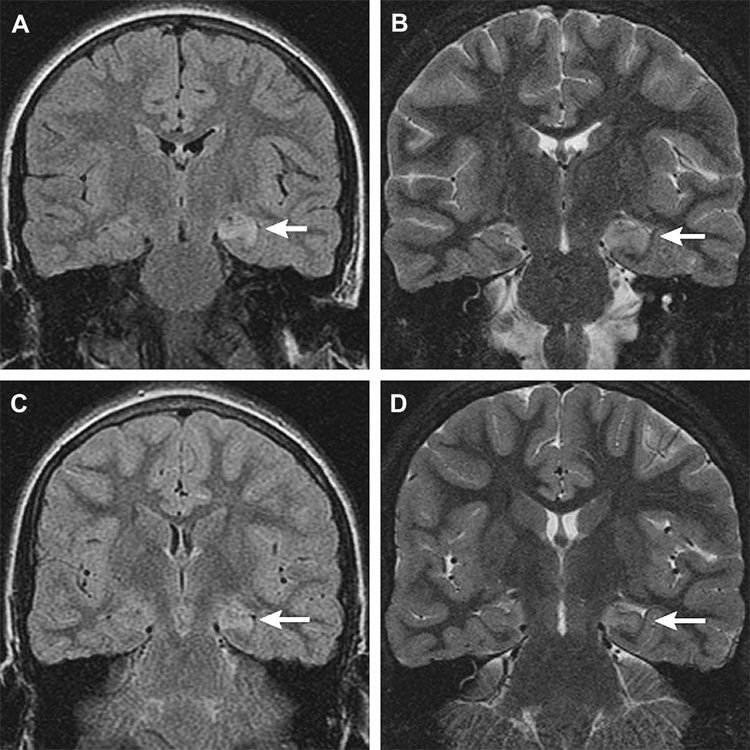
It is especially noteworthy in this new-onset sample that mesial temporal lobe and hippocampal abnormalities constituted 15% of all abnormalities observed (e.g., Figure 5). This is much higher than the 5% reported by Berg and her colleagues [5], even though most of their exams used MRI. This is also considerably higher than the 7% reported by Shinnar and his colleagues [1], even though many of their imaging studies were obtained later in the course of the disorder when mesial temporal sclerosis could be more likely to appear [2]. As stated earlier, use of updated, high-resolution MRI together with a standardized scoring system might have increased sensitivity to subtle findings in the temporal lobes and elsewhere. This is particularly important given the relationship of pathological changes in this region with concurrent cognitive deficits [2], with future cognitive deficits [23], with future intractability [24], and with prognosis following surgery [25], especially when using MRI volumetrics to quantify the extent of the structural abnormalities.
Figure 5.
Subject #209: Left hippocampal signal abnormality (arrows) in a ten-year-old. Coronal MR images, initial scan (a, b), and follow up 36 days later (c,d). (a) Increased signal left hippocampus, coronal fast fluid attenuated inversion recovery (FLAIR) MR image (TR/TE/TI = 10000/127/2250, slice thickness 4mm with 0mm gap interleaved, matrix = 256 × 192, FOV = 18cm). (b) Swelling and increased signal left hippocampus, coronal oblique fast multiplanar inversion recovery (FMPIR) MR image (TR/TE/TI = 5000/104/120ms, ETL =16, slice thickness/gap = 3mm/0mm, matrix = 512 × 256, FOV = 16cm). (c) Persistent increased signal left hippocampus, coronal FLAIR MR image (TR/TE/TI = 10000/142/2200, slice thickness 4mm with 0mm gap interleaved, matrix = 256 × 192, FOV = 18cm). (d) Subtle volume loss with persistent T2 prolongation left hippocampus, coronal FMPIR MR image (TR/TE/TI = 5000/96/120ms, ETL =16, slice thickness/gap = 3mm/0mm, matrix = 512 × 256, FOV = 16cm).
The present findings are consistent with two smaller studies. In a study of adults with temporal lobe epilepsy, Hermann and colleagues [23] observed significantly reduced brain volume (but only in white matter) in 37 participants with childhood onset (< age 14 yrs; M = 7.8 yrs) compared to 16 with later onset (> age 14 yrs; M = 23.3 yrs), even after controlling for duration of epilepsy. Taken together with our findings, white matter changes might be more characteristic of childhood onset. Hermann [23] reported that these white matter abnormalities were associated with neuropsychological impairment, which underscores the potential importance of early detection of MRI abnormalities in providing prognostic data to treating clinicians.
In a very small study of 12 children imaged within two years of diagnosis with partial seizures, Kolk and colleagues [26] observed structural abnormalities in 6 (50%), which is not surprising for partial seizures. Hippocampal lesions accounted for 4 of the 6 abnormalities (67%), and the other two children had ventricular enlargement or bilateral cortical atrophy. However, we observed structural abnormalities in fewer (n = 60, or 34%) of the 179 children with partial seizures in our sample, who were imaged closer to diagnosis; hippocampal abnormalities accounted for only 6 of the 60 (10%).
In addition, in light of increasing evidence of cerebellar atrophy in adults with epilepsy [27], the absence of cerebellar atrophy in this sample of new onset children is noteworthy. This is consistent with the findings in adults by Hagemann and colleagues [28] who found no cerebellar atrophy in a new-onset group but rather only in the chronic group; furthermore, the extent of atrophy was correlated with duration of disorder and with number of lifetime seizures, suggesting that it is a progressive consequence of seizures themselves.
Two recent studies indicate that the presence of a lesion on MRI is associated with continuing seizures at follow-up. In a temporal lobe epilepsy report, a lesion on MRI was the only independent predictor of seizure outcome [24]. Similarly, in a report on partial seizures, the initial MRI results predicted outcome [29]. These studies and our findings indicate the possibility that certain (more or less subtle) imaging abnormalities that are associated with poor long-term outcome in childhood onset epilepsy are present at the onset of the epilepsy. This is an important reason to extend outcome studies in childhood-onset seizures towards adulthood.
Is neuroimaging necessary after a first recognized seizure? The evidence for the utility of neuroimaging in children was reviewed in a practice parameter issued by the American Academy of Neurology, the Child Neurology Society, and the American Epilepsy Society [30]. They concluded that the data were limited and were insufficient for issuing a guideline other than recommending MRI as the preferred modality. They suggested that clinicians had the option of obtaining an emergent MRI if a postictal neurological defect was slow to resolve and a nonurgent MRI if a child had cognitive or motor disability, an abnormal neurological examination, or a focal seizure without EEG evidence of idiopathic focal seizure or generalized seizure. In a new statement based on empirical data from both children and adults, the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology [31] stated that emergent noncontrast CT is "possibly" useful after a seizure if there is a history to suggest a CNS lesion (e.g., head trauma, CSF shunt, malignancy, or neurocutaneous disorder), an abnormal neurological examination, or focal onset of the seizure. That report suggested that future research is needed to define the role of MRI in this setting.
Our current data suggest that clinicians should consider obtaining an MRI after a first recognized seizure in children. Further support for this contention comes from Betting and colleagues [32] who reported MRI abnormalities in 24% of patients 9–50 years of age with idiopathic generalized epilepsy, from Gelisse and colleagues [33] who noted abnormal CT or MRI imaging in 14.8% of children with benign epilepsy with centrotemporal spikes, and from Labate and colleagues [34] who found abnormal MRIs in 38.6% of patients 10–83 years of age with benign temporal lobe epilepsy. Although many of these abnormalities may be nonspecific, they could prove to be important in the treatment or prognosis of epilepsy in this population.
Our study was designed to determine the frequency of MRI abnormalities in any child with a first recognized seizure. We were interested in changes that would not only assist in defining the etiology of seizures and guiding therapy, but might contribute to the understanding of the risk factors for behavioral and cognitive comorbidities. Although the majority of changes found on MRI did not lead to an alteration in therapy, we think our data are potentially helpful in two ways. First, we have shown that MRI abnormalities may be present even in children with apparently benign syndromes such as childhood absence epilepsy and benign childhood epilepsy with centrotemporal spikes. Second, MRI abnormalities might predict comorbidity. In a smaller sample of children from this study, we found an association between MRI abnormality and lower scores on neuropsychological testing. We plan on following this sample to see if MRI abnormalities are associated with subsequent changes in behavior and academic functioning.
Our study has some limitations. Although as a whole 76% of the MRI studies were rated as good and only 4% as limited, motion artifacts on individual sequences were not infrequent. Diagnostic yield might be improved by sedation in younger children, which could not be done routinely in this research study. Because MR imaging in practice, particularly in Emergency Departments, is often performed close to the ictus, the 6-month window for MRI after the first recognized seizure may seem long; however, acute provoked seizures related to a known etiology were specifically excluded from the study. Detailed neonatal and pediatric medical histories were not available on all subjects, which limited our ability to determine the etiology of the changes on MRI. Finally, although this study would have been strengthened by a control group, obtaining MRI on healthy controls was not part of the larger study.
Conclusion
Use of MRI and a reliable, valid standardized scoring system in a large sample of children following their first recognized seizure identified a high rate of abnormalities (87/281, 31%), which may have important implications for practice guidelines with this population. First, some findings that might have been regarded as incidental in the past (e.g., volume loss/ventricular enlargement, white matter abnormalities) appear to be present at the onset of seizures and might therefore be clinically significant. Second, the detection of a high proportion of abnormalities in epileptic syndromes other than localization-related symptomatic/cryptogenic syndromes challenges the current imaging practice parameters and supports an argument for routine MRI at the onset of any seizure condition. Finally, a systematic approach to reading imaging studies in this population might help the radiologist to detect subtle abnormalities that could contribute to predicting clinical morbidities or comorbidities (e.g., medical intractability, cognitive impairment, poor response to interventions). Future studies correlating these abnormalities to seizure outcomes and comorbidities would be especially helpful in further defining significance from the radiologic standpoint. It will be important to balance the potential benefits of MRI in identifying more subtle imaging findings with the need to avoid contributing unnecessarily to rising costs of medical imaging.
Figure 2.
Subject #236: Leukomalacia/gliosis without ventricular enlargement. Coronal oblique fast fluid attenuated inversion recovery MR image (TR/TE/TI = 10000/120/2200, slice thickness 4mm with 0mm gap interleaved, matrix = 256 × 256, FOV = 18cm).
Acknowledgments
The scoring system used in this study was originally developed at Cincinnati Children's Hospital Medical Center by William S. Ball, MD, and was elaborated by the authors for the present study. The authors express their gratitude to the many staff who assisted in all phases of the study; to Phyllis Dexter for editorial assistance and Paul Buelow for help with manuscript preparation; and, most of all, to the children and families who gave so generously of their time, energy, and patience to this research endeavor.
Funding
National Institutes of Health [NS22416 to J.K.A.]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shinnar S, O'Dell C, Mitnick R, Berg AT, Moshe SL. Neuroimaging abnormalities in children with an apparent first unprovoked seizure. Epilepsy Res. 2001;43:261–269. doi: 10.1016/s0920-1211(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 2.Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol. 2003;53:413–416. doi: 10.1002/ana.10509. [DOI] [PubMed] [Google Scholar]
- 3.Salmenpera T, Kononen M, Roberts N, Vanninen R, Pitkanen A, Kalviainen R. Hippocampal damage in newly diagnosed focal epilepsy: a prospective MRI study. Neurology. 2005;64:62–68. doi: 10.1212/01.WNL.0000148643.36513.2A. [DOI] [PubMed] [Google Scholar]
- 4.Shinnar S, Berg AT, Moshe SL, Petix M, Maytal J, Kang H, Goldensohn ES, Hauser WA. Risk of seizure recurrence following a first unprovoked seizure in childhood: a prospective study. Pediatrics. 1990;85:1076–1085. [PubMed] [Google Scholar]
- 5.Berg AT, Testa FM, Levy SR, Shinnar S. Neuroimaging in children with newly diagnosed epilepsy: A community-based study. Pediatrics. 2000;106:527–532. doi: 10.1542/peds.106.3.527. [DOI] [PubMed] [Google Scholar]
- 6.Garvey MA, Gaillard WD, Rusin JA, Ochsenschlager D, Weinstein S, Conry JA, Winkfield DR, Vezina LG. Emergency brain computed tomography in children with seizures: who is most likely to benefit? J Pediatr. 1998;133:664–669. doi: 10.1016/s0022-3476(98)70109-x. [DOI] [PubMed] [Google Scholar]
- 7.Landfish N, Gieron-Korthals M, Weibley RE, Panzarino V. New onset childhood seizures. Emergency department experience. J Fla Med Assoc. 1992;79:697–700. [PubMed] [Google Scholar]
- 8.Maytal J, Krauss JM, Novak G, Nagelberg J, Patel M. The role of brain computed tomography in evaluating children with new onset of seizures in the emergency department. Epilepsia. 2000;41:950–954. doi: 10.1111/j.1528-1157.2000.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Riviello JJ, Harper MB, Baskin MN. The role of emergent neuroimaging in children with new-onset afebrile seizures. Pediatrics. 2003;111:1–5. doi: 10.1542/peds.111.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Warden CR, Brownstein DR, Del Beccaro MA. Predictors of abnormal findings of computed tomography of the head in pediatric patients presenting with seizures. Ann Emerg Med. 1997;29:518–523. doi: 10.1016/s0196-0644(97)70226-9. [DOI] [PubMed] [Google Scholar]
- 11.King MA, Newton MR, Jackson GD, Fitt GJ, Mitchell LA, Silvapulle MJ, Berkovic SF. Epileptology of the first-seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet. 1998;352:1007–1011. doi: 10.1016/S0140-6736(98)03543-0. [DOI] [PubMed] [Google Scholar]
- 12.Chuang NA, Otsubo H, Chuang SH. Magnetic resonance imaging in pediatric epilepsy. Top Magn Reson Imaging. 2002;13:39–60. doi: 10.1097/00002142-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40:445–452. doi: 10.1111/j.1528-1157.1999.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 14.Shinnar S, Berg AT, Moshe SL, O'Dell C, Alemany M, Newstein D, Kang H, Goldensohn ES, Hauser WA. The risk of seizure recurrence after a first unprovoked afebrile seizure in childhood: an extended follow-up. Pediatrics. 1996;98:216–225. [PubMed] [Google Scholar]
- 15.Fastenau PS, Johnson CS, Byars AW, Dunn DW, Austin JK. A 3-Year Prospective Study of Neuropsychological Changes in Children Following the First Recognized Seizure: Relationship to Neurological and Psychosocial Variables. J Int Neuropsychol Soc. 2007;13:126. [Google Scholar]
- 16.Grueneich R, Ris MD, Ball W, Kalinyak KA, Noll R, Vannatta K, Wells R. Relationship of structural magnetic resonance imaging, magnetic resonance perfusion, and other disease factors to neuropsychological outcome in sickle cell disease. J Pediatr Psychol. 2004;29:83–92. doi: 10.1093/jpepsy/jsh012. [DOI] [PubMed] [Google Scholar]
- 17.Klug JM, de Grauw A, Taylor CNR, Egelhoff JC. Magnetic resonance evaluation in children with new onset seizure. Ann Neurol. 1996;40:304. [Google Scholar]
- 18.Byars AW, Degrauw TJ, Johnson CS, Fastenau PS, Perkins SM, Egelhoff JC, Kalnin A, Dunn DW, Austin JK. The association of MRI findings and neuropsychological functioning after the first recognized seizure. Epilepsia. 2007;48:1067–1074. doi: 10.1111/j.1528-1167.2007.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oostrom KJ, van Teeseling H, Smeets-Schouten A, Peters AC, Jennekens-Schinkel A. Three to four years after diagnosis: cognition and behaviour in children with 'epilepsy only'. A prospective, controlled study. Brain. 2005;128:1546–1555. doi: 10.1093/brain/awh494. [DOI] [PubMed] [Google Scholar]
- 20.Hauser W. Epidemiology of epilepsy in children. In: Pellock J, Dodson W, Bourgeois B, editors. Pediatric epilepsy: Diagnosis and therapy. New York: Demos; 2001. pp. 81–96. [Google Scholar]
- 21.Cowan LD, Bodensteiner JB, Leviton A, Doherty L. Prevalence of the epilepsies in children and adolescents. Epilepsia. 1989;30:94–106. doi: 10.1111/j.1528-1157.1989.tb05289.x. [DOI] [PubMed] [Google Scholar]
- 22.CCT-ILAE Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 23.Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, Wendt G, O'Leary D, Magnotta V. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–1071. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- 24.Spooner CG, Berkovic SF, Mitchell LA, Wrennall JA, Harvey AS. New-onset temporal lobe epilepsy in children: lesion on MRI predicts poor seizure outcome. Neurology. 2006;67:2147–2153. doi: 10.1212/01.wnl.0000248189.93630.4f. [DOI] [PubMed] [Google Scholar]
- 25.Jack CR, Jr, Sharbrough FW, Cascino GD, Hirschorn KA, O'Brien PC, Marsh WR. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol. 1992;31:138–146. doi: 10.1002/ana.410310204. [DOI] [PubMed] [Google Scholar]
- 26.Kolk A, Beilmann A, Tomberg T, Napa A, Talvik T. Neurocognitive development of children with congenital unilateral brain lesion and epilepsy. Brain Dev. 2001;23:88–96. doi: 10.1016/s0387-7604(01)00180-2. [DOI] [PubMed] [Google Scholar]
- 27.Hermann BP, Bayless K, Hansen R, Parrish J, Seidenberg M. Cerebellar atrophy in temporal lobe epilepsy. Epilepsy Behav. 2005;7:279–287. doi: 10.1016/j.yebeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Hagemann G, Lemieux L, Free SL, Krakow K, Everitt AD, Kendall BE, Stevens JM, Shorvon SD. Cerebellar volumes in newly diagnosed and chronic epilepsy. J Neurol. 2002;249:1651–1658. doi: 10.1007/s00415-002-0843-9. [DOI] [PubMed] [Google Scholar]
- 29.Gaillard WD, Weinstein S, Conry J, Pearl PL, Fazilat S, Fazilat S, Vezina LG, Reeves-Tyer P, Theodore WH. Prognosis of children with partial epilepsy: MRI and serial 18FDG-PET. Neurology. 2007;68:655–659. doi: 10.1212/01.wnl.0000255942.25101.8d. [DOI] [PubMed] [Google Scholar]
- 30.Hirtz D, Ashwal S, Berg A, Bettis D, Camfield C, Camfield P, Crumrine P, Elterman R, Schneider S, Shinnar S. Practice parameter: evaluating a first nonfebrile seizure in children: report of the quality standards subcommittee of the American Academy of Neurology, The Child Neurology Society, and The American Epilepsy Society. Neurology. 2000;55:616–623. doi: 10.1212/wnl.55.5.616. [DOI] [PubMed] [Google Scholar]
- 31.Harden CL, Huff JS, Schwartz TH, Dubinsky RMMM, Zimmerman RDM, Weinstein SM, Foltin JCMF, Theodore WHM. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007;69:1772–1780. doi: 10.1212/01.wnl.0000285083.25882.0e. [DOI] [PubMed] [Google Scholar]
- 32.Betting LE, Mory SB, Lopes-Cendes I, Li LM, Guerreiro MM, Guerreiro CA, Cendes F. MRI reveals structural abnormalities in patients with idiopathic generalized epilepsy. Neurology. 2006;67:848–852. doi: 10.1212/01.wnl.0000233886.55203.bd. [DOI] [PubMed] [Google Scholar]
- 33.Gelisse P, Corda D, Raybaud C, Dravet C, Bureau M, Genton P. Abnormal neuroimaging in patients with benign epilepsy with centrotemporal spikes. Epilepsia. 2003;44:372–378. doi: 10.1046/j.1528-1157.2003.17902.x. [DOI] [PubMed] [Google Scholar]
- 34.Labate A, Ventura P, Gambardella A, Le Piane E, Colosimo E, Leggio U, Ambrosio R, Condino F, Messina D, Lanza P, Aguglia U, Quattrone A. MRI evidence of mesial temporal sclerosis in sporadic "benign" temporal lobe epilepsy. Neurology. 2006;66:562–565. doi: 10.1212/01.wnl.0000198208.59347.96. [DOI] [PubMed] [Google Scholar]