Abstract
Hyperuricemia, a common clinical characteristic of preeclamptic pregnancies, has historically been considered a marker of reduced renal function in preeclamptic women. More recently it has been suggested that uric acid may directly contribute to pathological cell signaling events involved in disease progression as well as maternal and fetal pregnancy outcomes including fetal growth restriction. We hypothesize that the increased frequency of restricted fetal growth seen in relation to increasing uric acid concentrations in preeclamptic women is in part the result of uric acid-induced reductions in amino acid transport across the placenta. The objective of the current study was to examine the effects of uric acid on human placental System A amino acid transport using a primary placental villous explant model. Further, we examined the necessity of uric acid uptake and the role of redox signaling as a potential mechanism through which uric acid may attenuate System A activity. Placental uptake of a radiolabeled amino acid analogue, specific to the System A transporter, was reduced in a concentration-dependent fashion with increasing uric acid (0−7 mg/dL), corresponding to uric acid concentrations measured in healthy pregnant and preeclamptic women in the third trimester. Uric acid-induced reduction in System A activity was partially reversed by NADPH oxidase inhibition and completely eliminated by antioxidant treatment. This study demonstrates inhibition of placental System A amino acid transport with uric acid treatment, as a result of uric acid-induced stimulation of intracellular redox signaling cascades. These findings may be relevant to the increased frequency of fetal growth restriction observed in hyperuricemic preeclampsia. Additionally the results of this study, indicating a detrimental effect of hyperuricemia on amino acid transport in the placenta, at concentrations present in women with preeclampsia, also suggest a role for uric acid in the pathophysiology of preeclampsia.
Keywords: Uric acid, Amino acid transport, System A, Antioxidants, Redox signaling, Placenta, Preeclampsia, Fetal growth restriction, Hyperuricemia, NADPH oxidase, Probenecid, Explants
1. Introduction
Appropriate fetal growth depends upon an adequate nutrient supply to the developing fetus throughout gestation. Pathological placental development and/or function can result in compromised fetal growth, or intra-uterine growth restriction (IUGR), indirectly through insufficient placental perfusion or directly through alterations in the transport of nutrients across the placental barrier into the fetal circulation.
Amino acids are essential nutrients serving both as building blocks for fetal/placental protein synthesis as well as an energy source, supplying 20−40% of fetal energy demands [1–3]. Placental transport of amino acids is an active process involving several membrane transporters, with differing amino acid substrate specificities. The System A amino acid transporter carries neutral, short side-chain amino acids in a sodium-dependent fashion from the maternal intervillous blood supply into the fetal circulation [4]. Reduced activity of the System A amino acid transport system is tightly linked to intra-uterine growth restriction. System A activity is reduced up to 60% in microvillus membrane vesicles from placentae of small for gestational age infants compared to appropriately grown infants [5,6]. Animal models of fetal growth restriction, either induced via maternal undernutrition [7,8] or reduced placental perfusion [9,10], also have lower placental System A transport and activity and this reduction is present before reduced fetal growth supporting a causal role.
In addition to nutritional status, maternal smoking, alcohol use, socioeconomic status, parity and race all increase the frequency of IUGR [11], as does the hypertensive disorder of pregnancy, preeclampsia [12]. Nearly one third of all preeclamptic pregnancies are complicated by IUGR [13]. While the pathogenesis of preeclampsia is not completely understood, it is thought that inadequate remodeling of the uterine spiral arterioles by invading placental trophoblast cells with subsequent reductions of placental perfusion set the stage for reduced nutrient delivery and placental oxidative stress, which may in turn result in compromised fetal growth and the maternal syndrome of preeclampsia [14,15]. While new onset hypertension after 20 weeks gestation and proteinuria are the specific clinical characteristics used to diagnose preeclampsia, a very common component of the disorder is hyperuricemia, recognized for over a century to be present in preeclamptic women [16,17]. Higher circulating uric acid concentration, present in 75% of preeclamptic women [17], has long been attributed to altered renal function and considered a marker of disease severity. However, more recent studies of hyperuricemia in non-pregnant populations indicate uric acid as an independent risk factor for hypertension, cardiovascular and renal disease [18]. Furthermore, uric acid has intracellular signaling roles capable of initiating inflammatory and redox cascades [18–21]. These findings led our group, along with others; to re-evaluate the potential role(s) uric acid may play in the pathophysiology of preeclampsia [22,23].
In a recent study we demonstrated a striking concentration-dependent increase in the odds for small gestational age infants in preeclamptic women with increasing serum uric acid concentration [17]. This finding, led us to hypothesize that elevated circulating uric acid in preeclamptic women contributes to the fetal growth restriction through inhibition of placental amino acid transport. The objectives of our current study were two fold. First, to examine the effects of increasing concentrations of uric acid on System A amino acid transport across the human placenta using term villous explants. Secondly, to begin to determine the mechanisms by which uric acid may alter amino acid transport in the placenta specifically addressing the potential role of oxidative stress.
2. Methods
2.1. Chemicals and solutions
All placental villous explant dissections and incubations were carried out in a buffer consisting of 1 volume of Dulbecco's modified Eagle's medium (DMEM,) with 3 volumes of Tyrode's buffer [DMEM-Tyrode's (1:3 vol/vol)]. Tyrode's buffer consisted of 135 mM NaCl (or 135 mM choline chloride for sodium-free Tyrode's buffer), 5 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 10 mM HEPES, and 5.6 mM glucose, pH 7.4 (adjusted with NaOH for sodium-containing buffer or KOH for sodium-free buffer). Uric acid (Sigma–Aldrich Co.) was dissolved in DMEM-Tyrode's buffer and filtered through a sterile 0.22 μm vacuum filter to obtain a stock concentration of 7 mg/dL. Probenecid (organic anion inhibitor), N-acetyl cysteine (intracellular antioxidant), dihydroascorbic acid (intracellular antioxidant), ascorbic acid (extra and intracellular antioxidant), catalase (extracellular antioxidant,), superoxide dismutase (extracellular antioxidant) and apocynin (NADPH oxidase inhibitor) were purchased from Sigma–Aldrich. [C14]Methyl amino isobutyric acid ([C14] MeAIB, NEN Life Sciences, Boston, MA), a radiolabeled non-metabolizable amino acid analog with System A specificity, was added to Tyrode's buffer (with and without sodium) in the presence or absence of all the above listed experimental treatments to achieve a final concentration of 0.05 μCi/ml (1.7 nmol/ml). Laemmli protein extraction buffer consisted of 10% glycerol (Sigma–Aldrich Co.), 10 mM Tris pH6.8 (Sigma–Aldrich Co.), 1% SDS (Fisher Bioreagents), 5 mM dithiolthreitol (DTT, Fluka), 0.5 mM phenylmethyl sulfonyl fluoride (PMSF, Sigma), protease inhibitor complex III mixture (Calbiochem), 0.5 mM vanadate (Sigma–Aldrich Co).
2.2. Tissue collection and villous explant dissection
We obtained placental tissue within 10 min of delivery from healthy pregnant women who delivered healthy, full-term infants by caesarean section in the absence of labor (repeat caesarean sections or breech presentation). The University of Pittsburgh Institutional Review Board approved the study. Four-to-five one cubic inch biopsies were dissected from the maternal side of the placenta from cotyledons located midway between the umbilical cord insertion site and the peripheral edge of the placenta. The basal and chorionic plates were removed from each biopsy and the tissue was washed three times in warm phosphate-buffered saline and transported to the laboratory in warm DMEM-Tyrode's (1:3) buffer. Villous fragments (1−2 mg each) were dissected in DMEM-Tyrode's (1:3) buffer and washed several times to remove all blood. We pooled villous fragments into collections of 25 mg tissue that we placed onto Costar netwell supports (15-mm diameter, 74 μm mesh, Cole–Palmer) and then into a 12 well plate containing 2 ml media/well. We performed all dissections and experimental incubations in a sealed glove box at 37 °C maintained at 8% O2/5% CO2/with the balance N2, an oxygen concentration that closely mimics that measured in the intervillous space [24]. All control and experimental treatments were performed in duplicate for each experiment.
2.3. Sodium-dependent System A amino acid uptake assay
The sodium-dependent System A amino acid transport assay was adapted from methodology previously reported [25,26]. All experiments commenced within one hour of delivery. Placental villous explants were initially incubated for two hours in the various sodium-containing DMEM-Tyrode's experimental solutions outlined below and subsequently washed three times in sodium containing (for sodium-dependent uptake) or sodium depleted (for sodium-independent uptake) Tyrode's buffer. Placental explant uptake of the [C14]MeAIB (1.7 nmol/ml) was conducted for 20 min in Tyrode's buffer in the presence or absence of the different experimental effector agents. [C14]MeAIB uptake was stopped by placing the explants in ice-cold Tyrode's buffer and residual adherent [C14]MeAIB was removed by washing the explants several times in ice-cold buffer. The netwells containing the villous explants were placed into a 12 well plate containing 2 ml distilled water at 4 °C for 18 h to release the accumulated [C14]MeAIB. The distilled water was collected and added to 2 ml Scinti-safe Plus 50% scintillation fluid and counts of radioactivity were measured using a liquid scintillation counter (Wallac 1409, Pharmacia). We collected the placental villous explants into 400 μl laemmli protein extraction buffer on ice, sonicated for 30 s and spun for 10 min at 14,000 rpm at 4 °C. A Bradford protein assay performed on the supernatant allowed for normalization of the radioactive counts per minute to protein content in each well. System A amino acid transport is a sodium-dependent process, therefore negative controls for each experimental condition were obtained by performing the above described assay in the absence of sodium (sodium-free Tyrode's buffer).
2.4. Effects of uric acid on System A activity
To determine the effects of exogenous uric acid on System A activity the assay described above was performed in the presence of increasing concentrations (0.3 mg/dL, 5 mg/dL, 7 mg/dL) of uric acid (n = 6 placentae). These concentrations are similar to those measured in healthy pregnant women (3−5 mg/dL, [27,28]), and women with uric acid values 1 and 2 standard deviations above normal (5.5 mg/dl and 6.5 mg/dl respectively, after 36 weeks gestation [27,28]), the values of uric acid associated with adverse fetal outcomes in preeclampsia [17]. All subsequent assays described below were conducted using the highest concentration of uric acid only. Uric acid was dissolved into both the 2-h incubation media and the radiolabeled amino acid uptake media.
2.5. Effects of uric acid uptake into villous explants on System A Activity
To determine the role of placental uric acid uptake on System A amino acid transport activity the effects of uric acid on System A activity were measured in the presence or absence of probenecid (100 μM, n = 10 placentae) during both the initial 2-h incubation and the 20 min amino acid uptake.
2.6. Effects of uric acid-induced oxidative stress on System A activity
The role of intracellular free radical generation was tested by performing uptake experiments in the presence of either intracellular antioxidants N-acetyl cysteine (NAC, 75 μM, n = 10 placentae), dehydroascorbic acid (DHA, 75 μM, n = 6 placentae), or a combination of extracellular antioxidants (n = 7 placentae), catalase (17 mU/ml) and superoxide dismutase (33 mU/ml). Ascorbic acid (AA, 100 μM, n = 6 placentae), which acts both extracellularly and intracellularly after metabolism to dehydroascorbic acid, was additionally tested.
NADPH oxidase signaling was examined by co-treatment of placental explants with uric acid and apocynin (100 μM, Sigma–Aldrich), an NADPH oxidase inhibitor (n = 4 placentae). In all experiments described the effector agents were present in both the 2-h incubation media and the radiolabeled amino acid uptake media.
2.7. Tissue viability
We assayed for lactate dehydrogenase (In vitro toxicology LDH assay kit, Sigma–Aldrich) in media after the 2 h incubation. LDH values were quantified as a proportion of the 100% LDH control. The control was obtained by sonicating fresh placental villous explants (equivalent to the number of explants used per well) from the same placentae in the same culture media used for experimentation.
2.8. Statistical analysis
System A activity was determined as uptake in the presence minus activity in the absence of sodium. Because absolute System A activity was quite variable in different placentae, System A activity in each experiment was normalized to the activity of untreated controls from the same placenta. Data are presented as median with interquartile ranges.
Since data were not normally distributed we used non-parametric analysis, Kruskal–Wallis one-way analysis of variance test with post-hoc Dunn's analysis. Cuzick's test of trend [29] was used to analyze uric acid concentration response. A Wilcoxon signed rank test was performed to test effects of agents (probenecid, antioxidants, apocynin) on baseline System A activity. Subsequently Mann–Whitney two sample tests compared the individual effectors with and without uric acid. Statistical significance was set at p < 0.05.
3. Results
3.1. Effects of uric acid on system A activity
Uric acid treatment decreased placental System A amino acid uptake in a concentration-dependent fashion (p < 0.05; Fig. 1). The highest concentration of uric acid tested (7 mg/dL) significantly inhibited transporter activity under both 8% O2 and 21% O2 tensions, with reductions of 38% and 35% respectively compared to matched untreated controls. While overall trends in transporter activity were similar between the two oxygen tensions, there was larger inter-assay variability noted at 21% O2 (data not shown). Due to this variation and the physiological relevance of performing experiments at 8% O2 versus 21% O2 all further experiments were conducted at the lower oxygen tension. Non-specific MeAIB accumulation in the tissue, as measured by MeAIB uptake in sodium depleted medium, accounted for 10.2% (±6.8%) of total MeAIB uptake in control wells and 10.5% (±7.3%) in uric acid treated wells (7 mg/dL). Intra-assay variability between duplicate treatment wells was found to be minimal (0.18 ± 0.09 pmol MeAIB/mg protein/20 min).
Fig. 1.
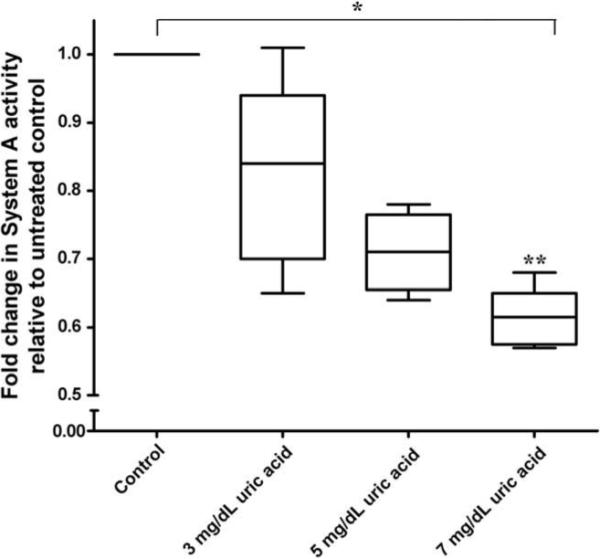
Concentration-dependent decrease in placental System A activity in 8% oxygen, relative to untreated controls, with increasing uric acid concentrations (3, 5, 7 mg/dL). n = 6, *p < 0.05, Kruskal–Wallis one-way analysis of variance on raw data set and Cuzick's test of trend; **p < 0.05 compared to untreated control using Dunn's post-hoc analysis.
3.2. Effects of uric acid uptake into villous explants on System A activity
The organic anion transporter inhibitor probenecid did not change basal System A activity but eliminated the effect of uric acid to reduce System A uptake (Fig. 2).
Fig. 2.
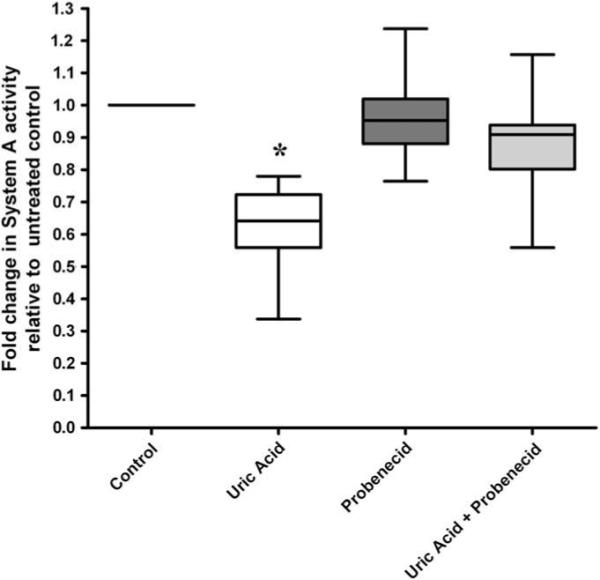
The inhibitory effects of uric acid (7 mg/dL) on System A activity are reversed when placental uric acid uptake is inhibited with probenecid. n = 10 placentae *p < 0.05 compared to untreated control, Wilcoxon sign-ranked test.
3.3. Effects of uric acid-induced oxidative stress on System A activity
Basal System A activity was unchanged by the antioxidants dehydroascorbic acid, N-acetyl cysteine and catalase/SOD alone. However, treatment with ascorbic acid slightly, but significantly, decreased basal System A amino acid uptake. (Fig. 3A). Treatment with these antioxidants completely prevented effects of uric acid to reduce System A activity (Fig. 3B).
Fig. 3.
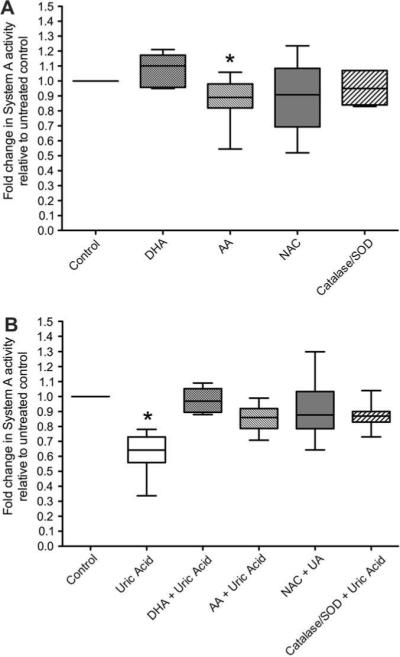
The inhibitory effects of uric acid (7 mg/dL) on System A activity are reversed when uric acid treatment is combined with different antioxidant treatments: dehydroascorbic acid (DHA, n = 6 placentae), ascorbic acid (AA, n = 6 placentae), N-acetyl cysteine (NAC, n = 10 placentae) or a combination of catalase and superoxide dismutase (SOD, n = 7 placentae). Matched placental System A activities were measured in the presence of antioxidants alone (panel A, expressed as a fold change in System A activity relative to untreated control) or with antioxidant and uric acid in combination (panel B; expressed as a fold change in System A activity relative to matched antioxidant treatment alone). *p < 0.05 compared to untreated control, Wilcoxon sign-ranked test. The values with the antioxidants and uric acid in combination was not different than the untreated control nor was the antioxidant and uric acid in combination different than the antioxidant alone (p > 0.173).
Inhibition of placental NADPH oxidase activity with apocynin did not affect basal System A amino acid uptake. The inhibitory effects of uric acid on placental System A activity were partly reversed by apocynin (p < 0.05, Fig. 4).
Fig. 4.
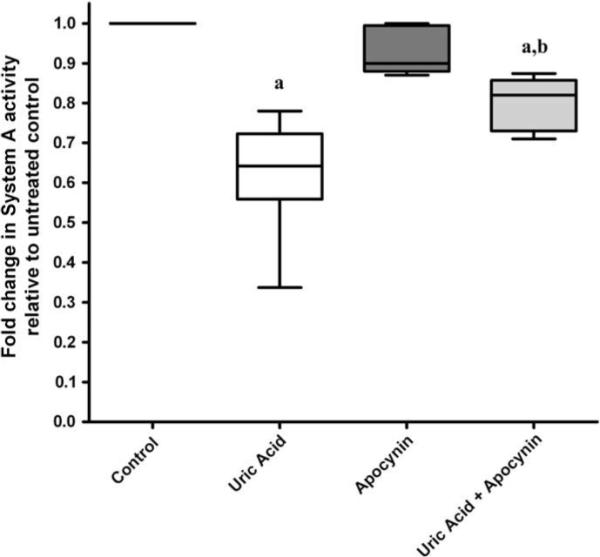
The inhibitory effects of uric acid (7 mg/dL) on System A activity are partly reversed when placental NADPH oxidase activity is inhibited with apocynin. n = 4, (a) p < 0.05 compared to untreated control, Wilcoxon sign rank test. (b) p < 0.05 compared to uric acid treatment alone, Mann–Whitney t-test.
3.4. Tissue viability
Treatment of placental villous explants with uric acid, probenecid, dehydroascorbic acid, ascorbic acid, N-acetyl cysteine, catalase/SOD or apocynin alone or in combination did not decrease villous explant viability as measured by release of LDH (p > 0.05 compared to untreated controls). The different experimental treatments also had no effect on the pH of the culture media (pH 7.4 ± 0.6).
4. Discussion
The primary finding of this study was a concentration-dependent inhibition of placental System A amino acid uptake with increasing uric acid concentrations. This finding supports our hypothesis that compromised fetal growth in preeclamptic pregnancies may be explained in part by inhibition of placental amino acid uptake by hyperuricemia. We examined the placental System A amino acid transporter because of its critical role in fetal growth. Reductions in System A transporter concentration and/or activity are tightly correlated to reduced fetal growth in humans and animals [5–10,30]. Further the reduction in transport in animal studies precedes reduced fetal growth supporting causality [7–10]. The potential clinical relevance of the effect of uric acid to inhibit placental System A activity is supported by the fact that the inhibition (∼40%) of System A activity occurred with uric acid concentrations normally measured in preeclamptic women in the third trimester of pregnancy (5.5−6.9 mg/dL, [27]).
We sought to determine the mechanisms by which uric acid might reduce System A amino acid transport. Probencid prevented the effect of uric acid to inhibit amino acid uptake indicating that uric acid must participate in or modify intracellular signaling to inhibit System A activity. Probenecid is an organic anion transporter inhibitor, which can specifically inhibit the URAT1/OAT 4 uric acid transporters [31,32], present in human placenta [33,34].
The importance of redox signaling in uric acid-induced inhibition of System A activity was specifically highlighted by a series of experiments in which the inhibitory effects of uric acid were lost when uric acid treatment was coupled with antioxidant treatment or NADPH oxidase inhibition. Antioxidants, both those with predominant activity intracellularly (dehydroascorbate, N-acetyl cysteine), or extracellularly (ascorbic acid and catalase/superoxide dismutase), were equally effective at maintaining System A activity. Of the antioxidants tested only ascorbate reduced System A activity on its own. It is likely that this was due to the formation of the ascorbate radical, known to occur in a setting of increased transition metals as would be present with dissected tissues and the absence of chelators [35], as was the case with our experiments.
NADPH oxidase is a membrane bound enzyme complex, which generates superoxide through the transfer of electrons from NADPH to molecular oxygen. NADPH oxidase is activated by uric acid in adipocytes [36]. We tested whether this source of oxidative stress might be relevant to the effect of uric acid on System A uptake. Inhibition of placental NADPH oxidase with apocynin partially rescued the inhibitory effects of uric acid suggesting that uric acid stimulates placental NADPH oxidase resulting in localized increases of superoxide. Since NADPH oxidase inhibition did not completely reverse the effect of uric acid other pro-oxidant effects are likely, perhaps directly by uric acid. Although uric acid is usually considered an antioxidant, in settings of low antioxidants and in particular low ascorbic acid concentration, as is present in preeclampsia [37,38], uric acid functions as a free radical [39].
While we could find no literature describing direct inhibitory effects of free radicals on the System A transporter there is evidence supporting an inhibitory effect of free radicals on the sodium–potassium-ATPase (Na+–K+-ATPase) pump, whose activity is essential in establishing the intracellular sodium gradient to drive the System A transporter [4,25]. In rat cerebellar granule cells, induction of redox signaling resulted in reduction of Na+–K+-ATPase activity, with equal inhibitory effects when the conjugating agents were restricted to either the extracellular or intracellular compartments alone [40]. Additionally, uncoupling of nitric oxide synthase and subsequent generation of superoxide mediates inhibition of the Na+–K+-ATPase pump in cardiac myocytes [41]. Interestingly, uric acid treatment, at concentrations similar to those used in this study, reduced Na+–K+-ATPase pump activity by 50% in rat striatum [42]. The results of this study, along with the evidence of altered Na+–K+-ATPase activity in the presence of increased free radicals, suggest that the inhibitory effects of uric acid-induced redox signaling on System A transport activity may be the result of inadequate establishment of intracellular sodium gradients. Further studies specifically examining the effects of uric acid on placental Na+–K+-ATPase activity are warranted.
Hyperuricemia is a common finding in preeclampsia primarily considered a marker of disease severity attributed to altered kidney function. Evidence for uric acid-induced pathological cell signaling has been mounting in the non-pregnant population with links to heightened pro-inflammatory cascades, oxidative stress, hypertension and cardiovascular disease [18]. More recently our group, along with others, have begun to question whether elevations in circulating uric acid throughout pregnancy may contribute to pathological cell signaling events in the mother, fetus and placenta [22,23]. Specifically relevant to growth restriction, there is a striking correlation between uric acid concentration and the odds ratio for preeclamptic women to have small for gestational age infants [17]. The effects of uric acid to reduce amino acid supports a pathogenic role for uric acid is relevant to this finding.
Nonetheless there remains some puzzling discordances. Nearly 75% of all preeclamptic women are hyperuricemic [17] yet only one third of preeclamptic women have growth restricted infants [13]. It is possible that this could be explained by our experimental design. The placental explants in these experiments were obtained from term uncomplicated pregnancies. These placental tissues would have been exposed to relatively low concentrations of uric acid throughout gestation prior to the acute exposure to pathological concentrations of uric acid during experimentation. In other settings the acute effects of uric acid are different than those after chronic exposure. In animal studies acute exposure to increased uric acid recruits endothelial progenitor cells and this response is lost with chronic hyperuricemia [43]. It is also possible that some placentae have the ability to up-regulate System A, Na+–K+-ATPase and/or down-regulate the expression of the uric acid transporters (URAT1/OAT 4), under conditions of chronic uric acid exposure. Additionally our experiments were done with uric acid in isolation and the effect of uric acid in a particular pregnancy may depend on the actions of hormones or other cofactors. All pregnant women have measurable basal levels of circulating uric acid (3.8−5.1 mg/dL in 3rd trimester uncomplicated pregnancies, [27]), and the concentration-dependent response relationship for uric acid to reduce System A activity appears present at normal pregnancy uric acid concentrations. Therefore the placenta must have protective mechanisms to ensure adequate fetal growth under normal conditions. Fetal and/or maternal pre-disposing factors (i.e. genetics) may dictate what concentrations of uric acid can be adequately tolerated and only in those instances in which the uric acid levels overcome these threshold values does the placenta become overwhelmed and amino acid transport is affected.
The results of our study provide some insight into the molecular mechanisms relating uric acid to fetal growth restriction, specifically uric acid-induced inhibition of amino acid transport across placental membranes through redox signaling. Whether therapeutic reductions in maternal uric acid concentrations would be beneficial to fetal growth is still unknown. However, in view of the effects of antioxidants to prevent the effects of uric acid on system A transport the NICHD multi-center trial in which 5000 women have received antioxidant therapy to attempt to prevent preeclampsia which has just been completed (but not yet published) should help shed some light on the fetal effects of reducing placental redox signaling.
Footnotes
Sources of support: National Institute of Health (grant # P01 HD303967), Magee-Womens Research Institute Postdoctoral Fellowship and State of Pennsylvania Health Research Formula Fund, Pennsylvania Department of Health.
References
- 1.Bauer MK, Harding JE, Bassett NS, Breier BH, Oliver MH, Gallaher BH, et al. Fetal growth and placental function. Mol Cell Endocrinol. 1998;140:115–20. doi: 10.1016/s0303-7207(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 2.Cramer S, Beveridge M, Kilberg M, Novak D. Physiological importance of system A-mediated amino acid transport to rat fetal development. Am J Physiol Cell Physiol. 2002;282:C153–60. doi: 10.1152/ajpcell.2002.282.1.C153. [DOI] [PubMed] [Google Scholar]
- 3.Malina A, Daftary A, Crombleholme W, Markovic N, Roberts JM. Placental system a transporter mRNA is not different in preeclampsia, normal pregnancy, or pregnancies with small-for-gestational-age infants. Hypertens Pregnancy. 2005;24:65–74. doi: 10.1081/PRG-45780. [DOI] [PubMed] [Google Scholar]
- 4.Jansson T. Amino acid transporters in the human placenta. Pediatr Res. 2001;49:141–7. doi: 10.1203/00006450-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Dicke JM, Henderson GI. Placental amino acid uptake in normal and complicated pregnancies. Am J Med Sci. 1988;295:223–7. doi: 10.1097/00000441-198803000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Mahendran D, Donnai P, Glazier JD, D’Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34:661–5. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Rosso P. Maternal malnutrition and placental transfer of alpha-aminoisobutyric acid in the rat. Science. 1975;187:648–50. doi: 10.1126/science.803709. [DOI] [PubMed] [Google Scholar]
- 8.Varma DR, Ramakrishnan R. Effects of protein-calorie malnutrition on transplacental kinetics of aminoisobutyric acid in rats. Placenta. 1991;12:277–84. doi: 10.1016/0143-4004(91)90009-5. [DOI] [PubMed] [Google Scholar]
- 9.Nitzan M, Orloff S, Schulman JD. Placental transfer of analogs of glucose and amino acids in experimental intrauterine growth retardation. Pediatr Res. 1979;13:100–3. doi: 10.1203/00006450-197902000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Jansson T, Persson E. Placental transfer of glucose and amino acids in intrauterine growth retardation: studies with substrate analogs in the awake guinea pig. Pediatr Res. 1990;28:203–8. doi: 10.1203/00006450-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–6. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JM, Pearson GD, Cutler JA, Lindheimer MD. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertens Pregnancy. 2003;22:109–27. doi: 10.1081/PRG-120016792. [DOI] [PubMed] [Google Scholar]
- 13.Eskenazi B, Fenster L, Sidney S, Elkin EP. Fetal growth retardation in infants of multiparous and nulliparous women with preeclampsia. Am J Obstet Gynecol. 1993;169:1112–8. doi: 10.1016/0002-9378(93)90265-k. [DOI] [PubMed] [Google Scholar]
- 14.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 16.Voto LS, Illia R, Darbon-Grosso HA, Imaz FU, Margulies M. Uric acid levels: a useful index of the severity of preeclampsia and perinatal prognosis. J Perinat Med. 1988;16:123–6. doi: 10.1515/jpme.1988.16.2.123. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JM, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46:1263–9. doi: 10.1161/01.HYP.0000188703.27002.14. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–90. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 19.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–93. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 20.Abuja PM. Ascorbate prevents prooxidant effects of urate in oxidation of human low density lipoprotein. FEBS Lett. 1999;446:305–8. doi: 10.1016/s0014-5793(99)00231-8. [DOI] [PubMed] [Google Scholar]
- 21.Maples KR, Mason RP. Free radical metabolite of uric acid. J Biol Chem. 1988;263:1709–12. [PubMed] [Google Scholar]
- 22.Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta. 2008;29(Suppl A):S67–72. doi: 10.1016/j.placenta.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang DH, Finch J, Nakagawa T, Karumanchi SA, Kanellis J, Granger J, et al. Uric acid, endothelial dysfunction and pre-eclampsia: searching for a pathogenetic link. J Hypertens. 2004;22:229–35. doi: 10.1097/00004872-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Soothill PW, Nicolaides KH, Rodeck CH, Campbell S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Ther. 1986;1:168. doi: 10.1159/000262264. [DOI] [PubMed] [Google Scholar]
- 25.Shibata E, Powers RW, Rajakumar A, von Versen-Hoynck F, Gallaher MJ, Lykins DL, et al. Angiotensin II decreases system A amino acid transporter activity in human placental villous fragments through AT1 receptor activation. Am J Physiol Endocrinol Metab. 2006;291:E1009–16. doi: 10.1152/ajpendo.00134.2006. [DOI] [PubMed] [Google Scholar]
- 26.Parrott MS, von Versen-Hoeynck F, Ness RB, Markovic N, Roberts JM. System A amino acid transporter activity in term placenta is substrate specific and inversely related to amino acid concentration. Reprod Sci. 2007;14:687–93. doi: 10.1177/1933719107306895. [DOI] [PubMed] [Google Scholar]
- 27.Powers RW, Bodnar LM, Ness RB, Cooper KM, Gallaher MJ, Frank MP, et al. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am J Obstet Gynecol. 2006;194:160. doi: 10.1016/j.ajog.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 28.Lind T, Godfrey KA, Otun H, Philips PR. Changes in serum uric acid concentrations during normal pregnancy. Br J Obstet Gynaecol. 1984;91:128–32. doi: 10.1111/j.1471-0528.1984.tb05895.x. [DOI] [PubMed] [Google Scholar]
- 29.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 30.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–46. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price KL, Sautin YY, Long DA, Zhang L, Miyazaki H, Mu W, et al. Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol. 2006;17:1791–5. doi: 10.1681/ASN.2006030264. [DOI] [PubMed] [Google Scholar]
- 32.Pea F. Pharmacology of drugs for hyperuricemia. Mechanisms, kinetics and interactions. Contrib Nephrol. 2005;147:35–46. doi: 10.1159/000082540. [DOI] [PubMed] [Google Scholar]
- 33.Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275:4507–12. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- 34.Rizwan AN, Krick W, Burckhardt G. The chloride dependence of the human organic anion transporter 1 (hOAT1) is blunted by mutation of a single amino acid. J Biol Chem. 2007;282:13402–9. doi: 10.1074/jbc.M609849200. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B, Vitamin C. antioxidant or pro-oxidant in vivo? Free Radic Res. 1996;25:439–54. doi: 10.3109/10715769609149066. [DOI] [PubMed] [Google Scholar]
- 36.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–96. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 37.Davidge ST, Hubel CA, Brayden RD, Capeless EC, McLaughlin MK. Sera antioxidant activity in uncomplicated and preeclamptic pregnancies. Obstet Gynecol. 1992;79:897–901. [PubMed] [Google Scholar]
- 38.Hubel CA, Kagan VE, Kisin ER, McLaughlin MK, Roberts JM. Increased ascorbate radical formation and ascorbate depletion in plasma from women with preeclampsia: implications for oxidative stress. Free Radic Biol Med. 1997;23:597–609. doi: 10.1016/s0891-5849(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 39.Benzie I, Strain J. Uric acid: friend or foe? Redox Rep. 1996;2:231–4. doi: 10.1080/13510002.1996.11747055. [DOI] [PubMed] [Google Scholar]
- 40.Petrushanko I, Bogdanov N, Bulygina E, Grenacher B, Leinsoo T, Boldyrev A, et al. Na–K-ATPase in rat cerebellar granule cells is redox sensitive. Am J Physiol Regul Integr Comp Physiol. 2006;290:R916–25. doi: 10.1152/ajpregu.00038.2005. [DOI] [PubMed] [Google Scholar]
- 41.White CN, Hamilton EJ, Garcia A, Wang D, Chia KK, Figtree GA, et al. Opposing effects of coupled and uncoupled NOS activity on the Na+–K+ pump in cardiac myocytes. Am J Physiol Cell Physiol. 2008;294:C572–8. doi: 10.1152/ajpcell.00242.2007. [DOI] [PubMed] [Google Scholar]
- 42.Bavaresco CS, Zugno AI, Tagliari B, Wannmacher CM, Wajner M, Wyse AT. Inhibition of Na+, K+-ATPase activity in rat striatum by the metabolites accumulated in Lesch–Nyhan disease. Int J Dev Neurosci. 2004;22:11–7. doi: 10.1016/j.ijdevneu.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Patschan D, Patschan S, Gobe GG, Chintala S, Goligorsky MS. Uric acid heralds ischemic tissue injury to mobilize endothelial progenitor cells. J Am Soc Nephrol. 2007;18:1516–24. doi: 10.1681/ASN.2006070759. [DOI] [PubMed] [Google Scholar]


