Abstract
Understanding the benefits of cooperative breeding for group members of different social and demographic classes requires knowledge of their reproductive partitioning and genetic relatedness. From 2004-2007, we examined parentage as a function of relatedness and social interactions among members of 21 American crow (Corvus brachyrhynchos) family groups. Paired female breeders monopolized maternity of all offspring in their broods, whereas paired male breeders sired 82.7% of offspring, within-group auxiliary males sired 6.9% of offspring, and extragroup males sired 10.4% of offspring. Although adult females had fewer opportunities for direct reproduction as auxiliaries than males, they appeared to have earlier opportunities for independent breeding. These different opportunities for direct reproduction probably contributed to the male biased adult auxiliary sex ratio. Patterns of reproductive partitioning and conflict among males were most consistent with a synthetic reproductive skew model, in which auxiliaries struggled with breeders for a limited reproductive share, beyond which breeders could evict them. Counter to a frequent assumption of reproductive skew models, female breeders appeared to influence paternity, although their interests might have agreed with the interests of their paired males. Unusual among cooperative breeders, close inbreeding and incest occurred in this population. Incest avoidance between potential breeders did not significantly affect reproductive skew.
Keywords: American crow, birds, cooperative breeding, copulations, Corvus brachyrhynchos, inbreeding, incest, mate guarding, mating system, reproductive skew
Cooperative breeding in birds occurs when more than two individuals contribute to the care of young in a single brood. Although cooperative systems are highly variable (Brown 1987), cooperative groups in many species are characterized by a single breeding pair, assisted by (presumed) nonbreeding ‘auxiliaries,’ usually adult or subadult offspring from previous broods. Potential benefits derived by auxiliaries (reviewed in Koenig & Dickinson 2004) include enhanced fitness of nondescendent kin (Hamilton 1964), prospecting for extragroup parentage (Young et al. 2007), and territory inheritance (Wiley & Rabenold 1984) or budding (Woolfenden & Fitzpatrick 1984). In some systems, molecular diagnoses of parentage have revealed that apparently nonbreeding auxiliaries also share in direct parentage (e.g. Rabenold et al. 1990). To understand the benefits of cooperative breeding for auxiliaries of different demographic classes, as well as their decisions to remain in their natal group or seek opportunities elsewhere, it is necessary to quantify reproductive opportunities within and outside of cooperative groups, as well as opportunities for independent breeding.
Reproductive skew theory provides a framework for predicting how parentage will be partitioned among same sex group members as a function of parameters such as relatedness, environmental constraints, group productivity and relative competitive ability of group members (reviewed in Clutton-Brock 1998; Johnstone 2000; Magrath & Heinsohn 2000; Reeve & Keller 2001; Magrath et al. 2004). In high skew groups, reproduction is concentrated in a subset of group members, whereas in low skew groups, reproduction is shared more equitably among group members. Different models of skew are built on different assumptions about interactions among group members (often referred to as dominants and subordinates), although the nature of these interactions is likely to vary among taxa. Models variously assume that (1) dominants have complete control over reproductive partitioning (e.g. Vehrencamp 1979; Vehrencamp 1983), (2) dominants have control only over group membership, whereas subordinates regulate their own reproductive share (Johnstone & Cant 1999), or (3) no individual has complete control over reproductive partitioning (Reeve et al. 1998). Also, most models assume that same sex group members decide their own reproductive partitioning (though see Cant & Reeve 2002), even though control by opposite sex group members has been demonstrated in some cooperatively breeding birds (e.g. Williams 2004). Furthermore, current reproductive skew models assume an absence of incest avoidance, an assumption that might be violated in the nuclear family groups typical of many cooperatively breeding birds (Koenig & Haydock 2004). Incest avoidance could drive a pattern in which reproductive share decreases with relatedness of potential breeders, similar to the pattern predicted by concession models of reproductive skew (Emlen 1996; Magrath & Heinsohn 2000; Magrath et al. 2004). Numerous authors have called for tests of these assumptions as well as the predictions of reproductive skew models in the field as a useful approach to distinguishing among models (Clutton-Brock 1998; Johnstone 2000; Cant & Reeve 2002; Magrath et al. 2004).
The American crow (Corvus brachyrhynchos) is a broadly distributed North American corvid, with cooperatively breeding populations in which groups of up to 12 birds raise offspring (McGowan 2001; Verbeek & Caffrey 2002). These groups generally include a socially bonded pair of adults (henceforth ‘breeders’) and auxiliary birds that are usually offspring from previous broods (Caffrey 1992). Although this human commensal, readily observed species has been the subject of numerous behavioural studies (Kilham 1984; Chamberlain-Auger et al. 1990; Marzluff et al. 2001; Yorzinski et al. 2006), nothing has been reported about its genetic mating system, and relatedness among group members has never been quantified. The pair bonded breeders within cooperative crow groups appear socially monogamous (Verbeek & Caffrey 2002), but observations of extrapair copulation attempts suggest that crows might not be genetically monogamous (Kilham 1984; pers. obs.). Also, although long term monitoring in our population of banded birds suggests that most group members are first or second order kin, auxiliary individuals do sometimes immigrate into non-natal groups (Clark et al. 2006; pers. obs.).
Here, we described the genetic mating system of American crows in the context of their social and genetic group structure. Specifically, we (1) quantified behavioural differences between male breeders and auxiliaries, so that reproductive partitioning could be rigorously examined in terms of social role; and (2) described reproductive partitioning among birds within a family group as a function of their social role, age, sex and relatedness. In particular, we tested the hypothesis that reproductive skew differed when auxiliary birds were first order kin to the opposite sex breeder vs. when they were not. We predicted that skew would be greater when auxiliaries were first order kin to the breeder of the opposite sex because of incest avoidance. Because the concession reproductive skew model predicts a similar pattern without a role for incest avoidance, we then examined how the American crow system met the other assumptions of the basic reproductive skew models.
Methods
Study Area and Breeder Classification
From 2004-2007, we examined mating strategies and genetic group structure in a population of American crows in Ithaca, New York, which has been monitored continuously since 1989 (McGowan 1995, 2001; Clark et al. 2006). Auxiliaries in this population are both male and female, and individuals of both sexes help with antipredator vigilance, territory and nest defence (Serrell 2003; Wilson 2008), as well as provisioning the incubating females, nestlings and fledglings. The degree to which provisioning varies with auxiliary age, sex and relatedness to other group members is currently unknown. We collected behavioural and genetic information from 21 focal family groups occupying an area approximately 4 km2. The study site included the Cornell University campus and adjacent natural areas, golf courses, shopping plazas and residential neighborhoods (described as ‘suburban’ in McGowan 2001). We defined a crow ‘family group’ as a cohesive group of birds that maintained the same year-round all purpose territory among years, and that contained some of the same members from year to year. We monitored groups intensively from February-July (2-7 days per week) to document group membership, displacements, mate guarding, copulation, nest building, onset of incubation, hatching, provisioning and fledging. From August-January, we observed each group at least once per month to record membership and interactions among members.
We defined ‘auxiliaries’ as birds that shared the same territory with breeders throughout the breeding period; most auxiliaries helped provision offspring. We defined the ‘female breeder’ as the female that carried out almost all of the incubation and brooding (Kilham 1984; Chamberlain-Auger et al. 1990; Caffrey 2000). In our sample, classification of female breeders was unambiguous, because other females very rarely attempted to brood or incubate. We identified the ‘male breeder’ as the male that appeared dominant in interactions with all other group members (Kilham 1984) and that kept consistently closest to the female breeder from the start of the nest building period until the onset of incubation (Caffrey 1992) during our ad libitum observations (Martin & Bateson 1993).
The concept of extrapair paternity hinges on the presence of clear social pair bonds (defined in Westneat et al. 1990). Although male breeders appeared behaviourally distinct in our sample, and American crows generally have obvious social pair bonds (e.g. Kilham 1984; Caffrey 1992; Clark et al. 2006), we tested the repeatability of our social role assignments by determining whether or not the behaviours of male breeders were quantifiably distinct from the behaviours of male auxiliaries. In 2007, we conducted focal observations on 19 family groups during the nest building, egg laying and early incubation periods (ending observations by the second day of incubation). Using the ‘breeder’ and ‘auxiliary’ classifications that we had generated from our ad libitum observations, we compared the level of pair behaviours between female breeders and male breeders vs. between female breeders and male auxiliaries. During 1-4 focal observations per family group (25-150 minutes per focal observation, depending on how long a family group could be followed on a given day), we recorded all displacements (i.e. one individual supplanting another), allopreens and copulation attempts. At 10 minute intervals within these focal observations, we estimated the distance of each bird in the group from the female breeder. When a bird flew out of sight, we conservatively estimated its distance from the female breeder as the furthest distance we could see in that habitat. In some intervals we were uncertain of the location of some adult males relative to the female breeder; we did not include these intervals in our analyses.
Genetic Sampling and Analyses
On days 24-30 after hatching, we climbed to each nest to mark nestlings with unique combinations of metal bands, colour bands and patagial tags. We collected blood (∼150 ul) from the brachial vein of live nestlings, and collected tissue samples from dead nestlings in and under these nests. By the 2007 field season, 98 of 125 adult birds (78.4%) in our 21 focal groups were banded or identifiable by unique scars or other conspicuous deformities. During most breeding attempts, there was not more than one unbanded or unscarred individual in each group (mean number of unmarked birds per breeding attempt + SE = 1.19 + 0.09; range 0-2). We extracted DNA from blood or feathers from 124 of the 125 marked and unmarked adult birds in these groups. Many had blood samples drawn when they were banded as nestlings. From the remaining adults, we collected passively moulted feathers while they were provisioning nestlings or fledglings on their territories (June-August). Unmarked birds present in multiple years were regenotyped using new feathers collected each year to reconfirm their identity. One auxiliary, which was present in a single year, disappeared before it could be sampled. Yearlings that had not been banded as nestlings could be distinguished from adults (defined here to include birds two years and older) by plumage: yearling crows have browner feathers and more pointed rectrices than adult birds until their definitive prebasic moult at 15 months (Emlen 1936).
DNAs were extracted from blood samples using Perfect gDNA Blood Mini kits (Eppendorf, Westbury, NY, U.S.A.) and from feather tips using DNeasy tissue kits (Qiagen, Valencia, CA, U.S.A.) following the manufacturers' protocols. We sexed all individuals at diagnostic sex linked alleles, using the 2550/2718 primer set (Fridolfsson & Ellegren 1999). We genotyped offspring and family members at 12 polymorphic microsatellite loci, selected from a panel that we previously developed for American crows (Schoenle et al. 2007) and from another panel isolated from the Mariana Crow (Corvus kubaryi; Tarr & Fleischer 1998). The forward primer of each pair was labeled using the fluorescent dyes 6-FAM, NED, PET, or VIC (Applied Biosystems, Foster City, CA, U.S.A.), and polymerase chain reaction (PCR) was carried out with optimized conditions and reagents (Table 1). Genotyping was performed on a 3100 Genetic Analyzer (Applied Biosystems). All alleles were scored automatically and confirmed visually using GENEMAPPER™ version 3.7 software (Applied Biosystems). To validate the reliability of moulted feathers as a DNA source, we compared genotypes of 30 colour marked individuals from which we had both feathers and blood samples. In all comparisons, genotypes derived from these two DNA sources were identical.
Table 1.
Optimized conditions for microsatellite loci
| Locus | Annealing temp (°C) | MgCl2 concentration (mM) | # Alleles | Expected heterozygosity (%) | Source |
|---|---|---|---|---|---|
| CoBr22 | 57 | 3.75 | 15 | 0.88 | Schoenle et al. 2006 |
| CoBr02 | 57 | 3.75 | 9 | 0.76 | Schoenle et al. 2006 |
| CoBr19 | 57 | 3.75 | 24 | 0.92 | Schoenle et al. 2006 |
| CoBr36 | 57 | 3.75 | 36 | 0.93 | Schoenle et al. 2006 |
| CoBr12 | 57 | 3.75 | 13 | 0.82 | Schoenle et al. 2006 |
| CoBr06 | 55 | 1.5 | 6 | 0.71 | Schoenle et al. 2006 |
| CoBr25 | 55 | 1.5 | 4 | 0.57 | Schoenle et al. 2006 |
| CoBr08 | 55 | 1.5 | 5 | 0.59 | Schoenle et al. 2006 |
| CoBr24* | 57 | 3.75 | 48 | 0.95 | Schoenle et al. 2006 |
| CoBr03* | 55 | 1.5 | 7 | 0.72 | Schoenle et al. 2006 |
| Ck1B6G | 55 | 1.5 | 11 | 0.86 | Tarr & Fleischer 1998 |
| Ck5A5F | 55 | 1.5 | 14 | 0.87 | Tarr & Fleischer 1998 |
Loci not used in final analyses because of high inferred null allele frequency.
We used the maximum likelihood approach used for parentage analyses in the program CERVUS 3.0 (Kalinowski et al. 2007). We specified a potential typing error of 1%, and specified the proportion of sampled candidate parents at 90% to account for unsampled adults in areas adjacent to our focal territories. We specified relatedness among 5% of candidate parents at 0.5 to account for kinship of potential breeders within family groups. Two loci (CoBr24 and CoBr03) had high frequencies of inferred null alleles and were not included in the final analyses. The ten remaining loci provided a powerful marker set for parentage discrimination, with a mean allele frequency of 13.7 alleles/locus, combined exclusion probabilities of 0.99915 when neither parent was known, and 0.99998 when one parent was known. Allele frequencies at these ten loci did not deviate significantly from Hardy-Weinburg expectations.
Shared maternity might occur (albeit rarely) in this American crow population, as suggested by a 2001 observation of an exceptionally large clutch incubated by multiple females (K. J. McGowan, unpublished data). Although no clutches were incubated by multiple females in our sample, we tested the assumption that female breeders were the mothers of all nestlings in their nests before assessing paternity. We first used CERVUS to identify mothers (with no ‘known parent’ specified), including all sampled females (yearlings and adults) present in each year as potential mothers. For 192 of 202 offspring, the female breeders of their respective broods were scored as the most likely candidate parents at or above the 95% confidence level. In the remaining ten cases, older siblings of these offspring were selected as the most likely candidate mother, with the female breeders selected as the second most likely candidate mother. This result was not unexpected, because this comparison does not incorporate information on the mate's genotype and therefore has weak power to exclude closely related individuals. In all such cases, however, there were no mismatches between either the sibling-offspring or breeder-offspring dyads. In eight of these ten cases, the selected sister-auxiliary was a yearling and therefore likely to be sexually immature (Black 1941), implicating the breeder as the actual mother. To examine further whether the female breeder or the sister-auxiliary was the most likely candidate, we used a protocol newly implemented in CERVUS 3.0 to run a ‘parental pair’ analysis (sexes known) for these ten offspring, now including all sampled males and females (both yearlings and adults) present in each year as candidate parents. A parental pair analysis seeks the most likely parental pair when neither parent is known. In all ten cases, the expected male and female breeders were selected as the most likely candidate parents; the auxiliary females selected in the simple dyad analysis were not selected. Considered together, these analyses strongly support the assumption that female breeders were the mothers of all nestlings in their nests. When there were allelic mismatches between female breeders and their putative offspring, we regenotyped both members of the dyad to check for typing error. In our final sample, there were only two allele mismatches in all mother-offspring comparisons (out of N = 202 dyads and N = 4040 pairwise comparisons of alleles), which might be attributable to mutation (Slate et al. 2000).
We then examined paternity, specifying female breeders as ‘known parents,’ and including all sampled adult males present in a given year as potential fathers. Confidence levels of 80% or greater might be sufficient to identify true genetic parents when combined with behavioural data (Slate et al. 2000). We therefore accepted males suggested by CERVUS 3.0 as true sires when (1) they were selected as the most likely candidate at the 95% confidence level or above (N = 117); (2) they were selected at the 80% confidence level and had no allelic pair mismatches (N = 62); or (3) the male breeder was selected at the 80% confidence level, with a single allelic pair mismatch (N = 7). When the male breeder was not the suggested sire and the confidence level for the suggested candidate fell below 95%, we denoted those offspring as having extrapair sires of unknown identity (N = 17). None of the proposed candidates in these latter 17 cases were auxiliary males within the family group of the respective offspring, and in each case, all adult males in the group had been sampled; the extrapair sires were therefore further described as ‘extragroup.’ In four cases, candidate fathers selected at the 95% confidence level were sons of the male and female breeder (i.e. mother-son incestuous matings). In each case, there were no pair or trio mismatches between the incestuous candidate sire and putative offspring, whereas the male breeder shared 2-3 mismatches with that offspring.
We used our microsatellite genotypes to generate pairwise genetic relatedness coefficients between all pairs of family members using the program RELATEDNESS v.5.0.8 (Queller & Goodnight 1989). Negative coefficients suggest that two individuals are less related than expected by chance if the two genotypes were randomly selected, whereas positive coefficients suggest that the individuals are related (e.g. mean coefficients of 0.5, 0.25 and 0.125 are expected between first, second and third order kin, respectively). In accordance with these expectations, preliminary results showed a mean + SE coefficient of relatedness between female breeders and their putative offspring of 0.52 + 0.009 (N = 156).
Statistical Analyses
Statistical analyses were conducted in JMP version 7.0, using nonparametric tests when variables deviated from normal distributions. To compare the level of mate guarding between males that we had qualitatively classified as ‘breeders’ or ‘auxiliaries,’ we compared the mean distance of the male breeder vs. the mean distance of the closest adult male auxiliary from the female breeder in a given family group (Wilcoxon signed-ranks test, with pairing done by family group), averaged over all 10 minute intervals of our focal observations. In certain analyses of group structure (specified in the Results), we tested information from only a single, arbitrarily selected year (2007) from each family group to avoid analysing the same individuals repeatedly. All statistical tests were two-tailed.
To estimate reproductive skew, we calculated the binomial skew index (B) in Skew Calculator 2003 v 1.2.1. (Nonacs 2000, 2003), which corrects observed with expected variance in reproductive success of each group member, with the null expectation that each member has an equal probability of reproduction. Positive B values indicate that observed skew is greater than expected by random chance, whereas zero or negative values indicate that parentage is randomly or more equally shared than expected by chance, respectively, from a simulated random distribution. Only adult birds were included as potential breeders. Offspring sired by extragroup males were not included in the analysis, as skew indices apply only to reproductive partitioning within groups.
Ethical Note
All capture, handling, marking, observation and blood sampling of American crows was carried out under permits from the U.S. Geological Survey Bird Banding Lab (#22263) and New York State (#33), and under protocols approved by the Binghamton University (# 537-03 and 607-07) and Cornell University (# 1988-0210) Institutional Animal Care and Use Committees.
Results
Classification of Individuals by Dominance and Mating Behaviour
To test whether our 2004-2007 qualitative classifications of male social role based on ad libitum behavioural observations were supported by quantitative focal observations of pair behaviours, we observed 19 family groups for 80.9 hours between 17 March and 7 April 2007 (mean + SE = 3.32 + 0.24 focal observation periods per group, N = 63 focal observations). There was more than one adult male in 13 of these 19 family groups. In comparisons of within-group male breeders to male auxiliaries, we limit our sample to these 13 groups.
Males that had been previously classified as breeders were behaviourally distinct from auxiliary males. In the 13 groups with more than one adult male, male breeders were likely to be closer to the female breeders than adult male auxiliaries (Wilcoxon signed-ranks test: T = 45.5, N = 13, P = 0.0001): they were the closest adult male to the female breeders in 299 of 359 (83.3%) intervals, whereas adult male auxiliaries were closer in only 20 of 359 (5.5%) intervals; breeders and adult male auxiliaries were equidistant from the female breeder in 40 of 359 (11.1%) intervals. Seven of the 19 male breeders were allopreened by female breeders, whereas adult male auxiliaries were not observed being allopreened. Male breeders displaced other birds a mean + SE of 0.83 + 0.22 times per hour and were not displaced by other birds of any sex or age.
From 2004-2007, we observed six copulations between male and female breeders from five different family groups. In each case, the male breeder approached the female from the front and she performed precopulatory displays (described in Kilham 1984; Verbeek & Caffrey 2002), lowering herself to the ground and quivering her wings. Females appeared passive and receptive during these copulations. During one within pair copulation, the female uttered loud, monotonous calls, but in all other cases, the females were silent. These within-pair copulations were observed approximately seven days prior to the onset of incubation until the first day of incubation. We observed seven extrapair copulation attempts by within-group auxiliary males from five family groups; six attempted copulations by extragroup males in four family groups; and one additional copulation attempt by an unidentified extrapair male. All 14 attempted extrapair copulations were interrupted by the male breeder. Females did not perform precopulatory displays prior to these copulation attempts and appeared to actively resist them, flapping their wings throughout and vocalizing loudly. Extrapair males were observed to (1) approach a female breeder from behind and attempt to mount her while she foraged (N = 1); (2) drop on her while she was collecting nest material (N = 2); (3) chase her below her nest tree and pin her to a branch (N = 1); and (4) land on her while she was incubating (N = 10). In four of these ten extrapair copulation attempts with incubating females, multiple (4-6) birds landed on the incubating female simultaneously. Extrapair copulation attempts were observed approximately 12 days prior to the onset of incubation until the fourth day of incubation. Three within-group auxiliary males that were observed in these apparently unsuccessful copulation attempts did ultimately attain paternity in that brood with their respective female breeders (Table 1).
Relatedness and Group Structure
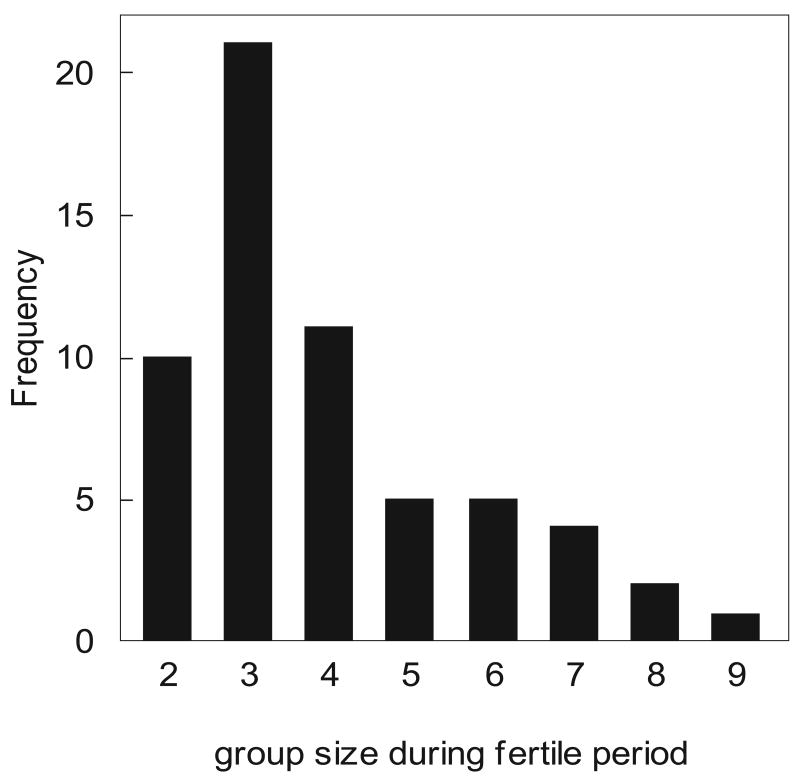
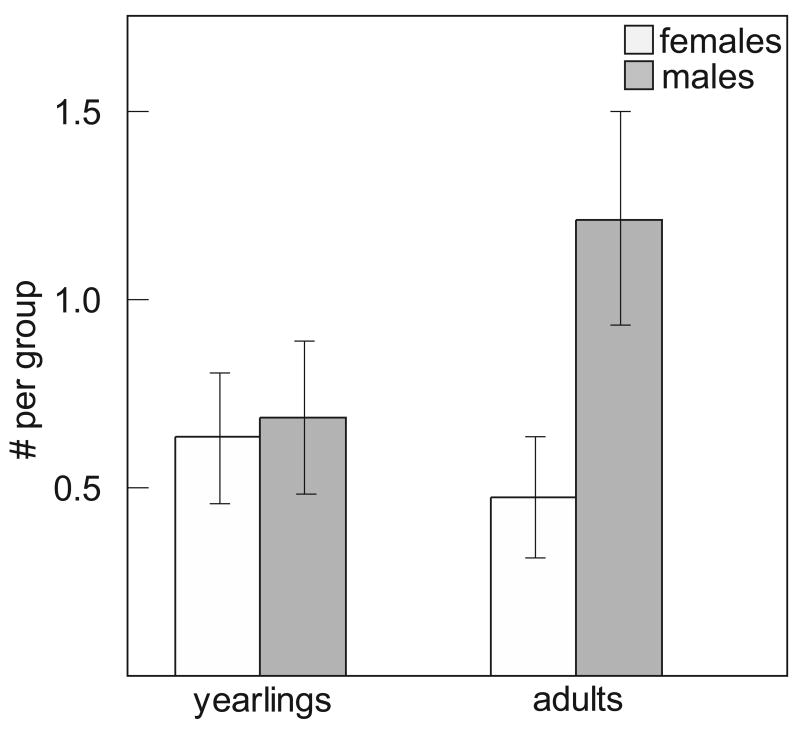
The mean + SE size of the focal groups at the beginning of the breeding period was 4 + 0.22 adults, N = 60 breeding attempts (range = 2-9 birds; Fig. 1; note that these summary statistics include multiple years of data from most groups). This distribution of group sizes was very similar to the distribution described in this population from 1989-1999 (McGowan 2001). There were 48 unique male and 26 unique female auxiliaries in these groups from 2004-2007. Considering only a single year (2007) from each family group to avoid analyzing the same individuals over time, there was no difference in the number of yearling male and female auxiliaries (Wilcoxon signed-ranks test: T = -4.0, N = 21, P two tailed = 0.78; mean + SE = 0.76 + 0.22 males and 0.71 + 0.18 females per family group); there were, however, significantly more adult male than female auxiliaries (Wilcoxon signed-ranks test: T = -37.0, N = 21, P two tailed = 0.017; mean + SE = 1.19 + 0.25 males and 0.43 + 0.15 females per family group; Fig. 2). Overall, male auxiliaries were significantly older than female auxiliaries (Mann-Whitney U test: U = -2.24, N1 = 22, N2 = 39, P = 0.025; mean + SE = 2.28 + 0.25 years for males and 1.45 + 0.13 years for females). This latter result is congruent with a previous report showing that males are older than females in the same social class (Clark et al. 2006). From 2004-2007, five female breeders and only one male breeder died or disappeared from the 21 focal groups.
Figure 1.
Frequency distribution of group sizes at the beginning of each breeding attempt (N = 60 breeding attempts).
Figure 2.
Mean number of auxiliaries per group + SE by age class and sex. Only one year of data (2007) is shown to avoid analysing birds repeatedly across years.
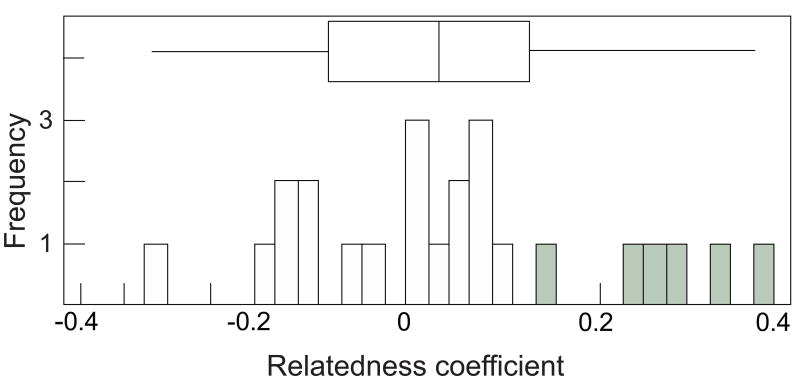
We genotyped 24 breeding pairs from our 21 family groups, because we collected DNA from three of the pairs that changed over the course of this study. The mean relatedness coefficient between male breeders and their paired female breeders was close to zero (mean + SE = 0.03 + 0.04, N = 24 dyads, range -0.31-0.38), although six of these 24 dyads (25.0%) appeared to be related at the level of second or third order kin (i.e. with coefficients of relatedness > 0.125; Fig. 3). Dyads of female breeders and adult male auxiliaries within a family group shared a lower mean coefficient of relatedness than dyads of male breeders and adult male auxiliaries in that group (Paired t test: t16 = -2.75, P = 0.014). Sixteen adult male auxiliaries unrelated to the female breeder vs. three adult male auxiliaries unrelated to the male breeder were distributed among 11 and 2 different family groups, respectively. Adult male auxiliaries unrelated to the breeders occurred for three reasons, which were not mutually exclusive: (1) the disappearance and replacement of female breeders in three groups, (2) the presence of nondescendent kin of the male breeder in seven groups, and (3) the immigration of three males, unrelated to either breeder, into two groups.
Figure 3.
Frequency distribution of pairwise related coefficients between male and female breeders. Gray bars indicate relatedness coefficients at or above the level predicted for third-order kin (r > 0.125). The median coefficient of relatedness, shown by the box-and-whiskers plot, is 0.015.
Genetic Parentage and Reproductive Skew
We genotyped 202 offspring from 60 broods (mean + SE = 3.36 + 0.19 offspring per brood, range = 1-6 offspring), belonging to 21 family groups (2.86 + 0.19 broods per family group, range = 1-4 broods). Of these offspring, 35 (17.3%) were sired by males other than the social breeder. Extrapair offspring were distributed in 17 (28.81%) different broods, among 13 of the 21 family groups (61.9%). Extragroup males sired 21 offspring (10.4%), distributed in 13 broods among 11 family groups. Five of these 21 offspring were sired by male breeders from adjacent territories (N = 3 adjacent male breeders), whereas 16 were sired by extragroup males of unknown identity. Auxiliaries in this sample did not obtain parentage outside of their family group. Within-group auxiliary males sired 14 offspring (6.9%), distributed in seven broods among six family groups. Auxiliary sons incestuously sired four offspring (2.0%), stepsons sired six offspring (3.0%), and nondescendent kin of the male breeders sired four (2.0%) of the 202 offspring (Table 2). All auxiliaries that gained paternity were related to the male breeder; the three unrelated auxiliary males did not gain paternity. Yearling birds did not achieve parentage, supporting our behavioural observations that suggest they are sexually immature until they are two years old.
Table 2.
Pairwise relatedness coefficients and apparent relationships between the six within-group subordinate sires and their male breeders and females
| Extrapair sire # | Relatedness between extrapair sire and female breeder | Relatedness between extrapair sire and male breeder | Extrapair sire's apparent relationships |
|---|---|---|---|
| 1 | -0.0656 | 0.2574 | half-brother of male breeder; rose in rank during breeding season* |
| 2 | 0.1557 | 0.4908 | son of male breeder; fertilized stepmother* |
| 3 | 0.0471 | 0.4698 | son of male breeder; fertilized stepmother* |
| 4 | -0.1973 | 0.4375 | brother of male breeder |
| 5 | 0.5811 | 0.6326 | son of male and female breeder; fertilized mother |
| 6 | 0.5918 | 0.5814 | son of male and female breeder; fertilized mother |
Resisted copulation attempts observed between extrapair sire and female breeder; see details in text.
The mean value for the binomial skew index B + SE was 0.18 + 0.06 (N = 10 groups, range = -0.02-0.58) among males that were not first order kin of the female breeder and 0.21 + 0.06 (N = 9 groups; range = 0.03-0.53) when auxiliary males were first order kin of the female breeder. Reproduction was significantly skewed among males of both relatedness classes (p<0.001). Skew was not different between male breeders and auxiliary males that were related and unrelated to the female breeder (t test: t17 = 0.46, P = 0.65).
Discussion
American crow groups in this population were characterized by paired male and female breeders that were behaviourally distinct from auxiliary birds. During the nest building and early incubation period, male breeders were usually the closest adult male to the female, displaced other birds (particularly adult male auxiliaries) but were not themselves displaced, and disrupted copulation attempts by other males but themselves copulated undisturbed. Reproduction was skewed towards the paired breeders in both sexes. Female breeders monopolized all maternity within their broods, whereas male breeders sired 82.7% of the offspring in their broods, within-group auxiliary males sired 6.9% of offspring and extragroup males sired 10.4% of offspring. Focal auxiliaries did not sire offspring in neighboring broods, suggesting that prospecting for extragroup parentage (Young et al. 2007) was not important for any class of auxiliary. It is possible, however, that offspring sired by unknown extragroup males were sired by adult male auxiliaries outside of the focal family groups. Almost all auxiliaries were relatives of the male breeders, either as offspring from previous broods or nondescendent kin (e.g. nephews, brothers). Only three of 48 auxiliary males were unrelated to male breeders, and they did not contribute to direct reproduction. Contrary to our prediction, reproduction was not significantly more skewed towards male breeders when the auxiliaries were first order kin of the female breeders.
A substantial proportion (25.0%) of paired birds shared coefficients of relatedness greater than the level of third order kin, and a small proportion of offspring (2%) were sired incestuously through fertilizations of mothers by their adult sons. Proximately, inbreeding in this population might have been promoted by delayed dispersal and short natal dispersal distances of many individuals of both sexes (mean dispersal distance + SE for females = 5.2 + 1.81 km, range =0.04-59.6, N = 33; for males = 0.85 + 0.29 km, range =0-7.8, N = 27; K. J. McGowan, unpublished data). Although true mean natal dispersal distance was likely underestimated (birds that stayed close to home were more detectable), it was clear that many individuals, both male and female, did not disperse far from their natal territory to breed.
The ubiquity of female choice of their extrapair partners in birds has been questioned (Arnqvist & Kirkpatrick 2005; Eliassen & Kokko 2008), particularly as direct observations of extrapair copulations are rare (Westneat & Stewart 2003). Our data suggest that female American crows might not have complete control over their extrapair reproductive partners. The six within-pair copulations that we observed were all solicited by female breeders, whereas the fourteen extrapair copulation attempts that we observed (both by auxiliary and extragroup males) appeared to be resisted by female breeders; these observations were congruent with copulatory behaviour described in a Florida population of American crows (Kilham 1984), as well as in the congeneric Northwestern crow (Corvus caurinus; Verbeek & Butler 1999). Several (but not all) of the extrapair males that we observed attempting resisted copulations gained paternity with these female breeders. Whether or not forced copulations can lead to fertilizations in birds that lack an intromittant organ is controversial (Gowaty & Buschhaus 1998; Westneat & Stewart 2003), and we do not know if these males successfully gained paternity during a resisted copulation attempt, or if females solicited copulations from them (Double & Cockburn 2000) or submitted to these copulations to reduce the costs of harassment (Arnqvist & Kirkpatrick 2005; Eliassen & Kokko 2008) at other times. Even when females do appear to resist copulations, they might be selecting males that can overcome their resistance (Kokko et al. 2003), or attempting to increase mate guarding by their consort males while encouraging copulation attempts by other males (Westneat & Stewart 2003; Pradhan et al. 2006). In support of the idea of female control over extrapair fertilizations, the five extragroup offspring with identified sires were all acquired by neighboring breeders (not auxiliaries). As an argument against the idea of complete female control, however, four offspring were sired by adult auxiliary sons of the female breeders; such incestuous fertilizations are unlikely to be in the interest of female breeders (Emlen 1996).
Group Composition and Opportunities for Direct Reproduction
The sex ratio of adult auxiliaries was male biased and male auxiliaries were older, on average, than female auxiliaries. The observed adult auxiliary sex ratio and age structure might have been influenced by gender differences in opportunities for direct reproduction within and outside natal groups. Adult male auxiliaries (even those related to female breeders) did occasionally sire offspring in their group as early as their second year, whereas female auxiliaries did not contribute at any age. Females in this population, however, bred independently as early as their second year (possibly facilitated by the relatively high rate of death and disappearance of female breeders in this population; McGowan 2001; this paper), whereas in the 19 years that this population has been monitored, marked males have not bred independently until at least their third year (K. J. McGowan, unpublished data). More opportunities for early independent breeding, combined with fewer opportunities for direct reproduction within their natal groups, could thus create incentives for adult auxiliary females to leave their natal group earlier than auxiliary males.
The auxiliary sex ratio in cooperatively breeding birds is typically biased towards males (Williams & Rabenold 2005). In our population, the adult auxiliary sex ratio was male biased, whereas the sex ratio of yearlings was unbiased. Our behavioural and parentage data suggested that yearlings are sexually immature, which might be characteristic of the genus (reviewed in Caffrey 1992). The absence of variation in reproductive opportunities between the sexes probably contributed to our unbiased yearling sex ratio. However, the sex ratio of yearling auxiliaries in a California population of American crows (Corvus brachyrhynchos) was female biased (N = 29 yearlings), even though these yearlings also appeared sexually immature (Caffrey 1992). The factors driving the unusual female biased sex ratio in this population were unclear (Caffrey 1992), although it is apparent that opportunities for immediate, direct reproduction cannot explain all variation in auxiliary sex ratio biases in the American crow.
Predictions and Assumptions of Reproductive Skew Models
The two basic categories of skew models (reviewed in Magrath et al. 2004) are sometimes referred to as ‘transactional’ (including ‘concession’ and ‘restraint’ models) and ‘tug-of-war’ models. In concession models, a dominant allows a subordinate the minimum share of reproduction that will compensate it for remaining in the group (Vehrencamp 1979, 1983). In restraint models, a subordinate limits its own share of reproduction to a level above which it will be evicted by the dominant (Johnstone & Cant 1999). In contrast, in tug-of-war models, reproductive share is based on the relative competitive ability of dominants and subordinates, without regard for group stability (Reeve et al. 1998). Concession models predict that reproductive skew will increase with relatedness, restraint models predict that reproductive skew will decrease with relatedness, whereas tug-of-war models variously predict that skew will increase with (Cant 1998), decrease with, or else be unaffected by relatedness (Reeve et al. 1998), depending on the how dominance is described (Beekman et al. 2003) or reproductive share gained (reviewed in Johnstone 2000). Within this simplified framework, the pattern of reproductive partitioning and male relatedness that we observed in American crows was most consistent with restraint and certain tug-of-war models: only auxiliaries related to the male breeder shared in reproduction. We can gain further insight about the factors that might (and might not) influence reproductive partitioning in male American crows by considering behavioural information on the nature of their interactions in light of the assumptions of the different reproductive skew models.
Assumption 1: Control over reproductive partitioning and group membership
Reproductive skew models vary in their assumptions about who controls same sex reproductive partitioning and group membership. Concession models assume that a dominant individual has perfect control over reproductive partitioning, and that subordinate individuals leave the group voluntarily if they have larger fitness payoffs elsewhere (Vehrencamp 1979, 1983), whereas restraint models assume that subordinates have control over their reproductive share, but can be forcibly ejected or excluded by the dominant to prevent or limit their reproductive share. In contrast, tug-of-war models assume that neither subordinates nor dominants have complete control over reproductive partitioning, and make no explicit assumptions about control over group membership (Reeve et al. 1998).
Our observations suggest that American crow male breeders did not have complete control over reproductive partitioning, but had the ability to forcibly evict auxiliaries. Incomplete control over the fertilizations of their females was suggested by the occurrence of extragroup paternity, which is unlikely to have any benefit for male breeders. Incomplete control by either the breeders or auxiliary males over reproductive shares was further suggested by the observed auxiliary copulation attempts, all of which were interrupted by male breeders, suggesting that there was continual conflict over reproductive share. Some, but not all, of these auxiliaries successfully secured a reproductive share. Control by male breeders over group membership, however, was suggested both by the low incidence of auxiliaries unrelated to male breeders and by an observation of a three year old male in the process of emigrating into a non-natal group. He first spent several days attempting to join one non-natal group, where he was repeatedly attacked by the male breeder. He was then accepted into another non-natal group without apparent conflict. This evidence for incomplete control over reproductive partitioning, combined with breeder control over group membership, was most consistent with the assumptions of synthetic models of tug-of-war and restraint (but not concession), in which male auxiliaries struggled with breeders over a reproductive share in a ‘window of selfishness’ (sensu Reeve 2000) above which the subordinate could be evicted (Johnstone 2000; Reeve 2000; Magrath et al. 2004).
Most models of reproductive skew, including the synthetic models that might best predict reproductive partitioning among American crow males, assume that decisions about reproductive partitioning are limited to same sex group members. In some systems, however, decisions about reproductive partitioning among males are made by females, which appeared to be the case in another cooperative corvid, the brown jay (Cyanocorax morio; Williams 2004). This intersexual control over reproductive skew can confound interpretations of classical skew models (Magrath & Heinsohn 2000; Magrath et al. 2004). Our observations suggested that female American crows might have had some influence over reproductive partitioning among males, but that female breeders reinforced the reproductive share of their pair males: females appeared to resist fertilizations outside of their pair bond. When the interests of female and male breeders are in agreement, the predictions of classical skew models are unchanged (Cant & Reeve 2002).
Assumption 2: No incest avoidance
Current models of reproductive skew do not account for potential incest avoidance among group members, which might have a major effect on reproductive partitioning in nuclear families (Emlen 1996; Magrath & Heinsohn 2000). If matings between relatives are avoided (a pattern that appears frequently, but not universally true in cooperatively breeding birds; Koenig & Haydock 2004), a pattern of reproductive partitioning will emerge in nuclear families in which reproductive share is lower for related birds, consistent with concession models of skew (Magrath & Heinsohn 2000). When there is strong evidence for incest avoidance in a given system (e.g. Koenig et al. 1998), one might reasonably exclude incestuous pairs from the set of potential breeders (Magrath et al. 2004). The assumption of incest avoidance is not appropriate to all systems, however, as demonstrated in this American crow population. These crows did not completely avoid incest and inbreeding, and reproduction was not significantly more skewed towards male breeders when auxiliaries were first order kin of the female breeders. This latter result might have been influenced by the small sample size of groups, as well as the high degree of overall skew. None the less, we can conclude that, in our sample of American crows, incest avoidance did not create a pattern in which skew increased with relatedness. The occurrence of incest in cooperatively breeding birds, however, appears to vary even among the cooperative corvids. Although incest was detected in relatively small samples of American crows (this study) and brown jays (Williams & Rabenold 2005), incest appears absent in the carrion crow (Corvus corone corone; Baglione et al. 2002) and extremely rare in the Florida scrub-jay (Aphelocoma coerulescens; Quinn et al. 1999). The importance of incest avoidance in driving patterns of reproductive partitioning must therefore be assessed separately in each system.
Comparison with Other Cooperative Corvids
Out of the 26 known cooperative jays and crows (Ligon & Burt 2004), the six species with described genetic mating systems exhibit wide variation in both social mating systems and patterns of reproductive partitioning (Table 1). For example, the proportion of mixed paternity broods in two cooperative Aphelocoma jays ranges from among the highest reported in birds (63% of broods in the plural breeding Mexican Jay, Aphelocoma ultramarina; Li & Brown 2000) to the lowest (0% of broods in the monogamous Florida scrub-jay; Quinn et al. 1999). Likewise, mixed maternity within single broods occurs with apparent regularity in the white-throated magpie-jay (Calocitta formosa; Berg 2005), but rarely (Quinn et al. 1999; Baglione et al. 2002) or inconsistently (Lawton & Lawton 1985; Williams 2004) in the other corvids. Even when the proportion of polygamous broods appeared superficially similar across taxa, as exhibited by congeneric American crows and carrion crows (Baglione et al. 2002), the identity of the auxiliary sires and their interactions with the male breeders appeared to differ substantially. In the American crow, four of the six reproducing auxiliaries bred on their natal territories, whereas in the carrion crow, shared reproduction appeared to be limited to non-natal immigrants. Aggressive interactions among potential male breeders were obvious in American crows, but not in carrion crows (Baglione et al. 2002).
Describing a single pattern of reproductive partitioning for an entire species can be misleading, because patterns of reproductive partitioning vary even within populations over time. Evidence of joint nesting in a population of brown jays (Lawton & Lawton 1985), for example, was absent in the same population in subsequent studies (Williams 2004). Such variation in patterns of reproductive partitioning might be partly driven by environmental conditions. For example, reproductive skew in a population of cooperatively breeding white-winged choughs (Corcorax melanorhamphos) decreased after a drought disrupted previously stable nuclear family groups (Heinsohn et al. 2000). American crows have also experienced a recent change in environmental conditions in the form of West Nile virus, which elevated breeder mortality in 2002-2003 in the Ithaca population (Clark et al. 2006). Although we have not yet examined patterns of reproductive skew before and after the epidemic, it might have led to an increase in population level reproductive skew, as predicted by the restraint model (Johnstone & Cant 1999), if it lowered ecological constraints by creating more opportunities for independent breeding.
Recent emphasis has been placed on synthesizing reproductive skew models into a universal model applicable to many group-living species (e.g. Reeve & Shen 2006; Buston et al. 2007). A single model predicting reproductive partitioning among corvids would need to accommodate variation in important processes contributing to these patterns, such as incest avoidance and intersexual control. A thorough comparison of the factors influencing reproductive skew across more corvid populations and species, with careful attention to both the assumptions and predictions of the different skew models, might illuminate the most important and general processes driving patterns of reproductive skew among them. Such comparative approaches have been taken for the social insects (Reeve & Keller 2001) and primates (Kutsukake & Nunn 2006). The application of this approach to taxa as disparate as corvids, primates and insects might provide insights to the factors of the most universal importance in predicting patterns of reproductive skew.
In conclusion, our observations of reproductive partitioning in the American crow are most consistent with a synthetic skew model (Johnstone 2000) of tug-of-war and restraint (but not concession), in which male auxiliaries struggle with breeders over a reproductive share in a ‘window of selfishness’ (Reeve 2000), beyond which they will be evicted. Counter to a frequent assumption of reproductive skew models, females did appear to influence paternity, although they might have reinforced the interests of their mates (Cant & Reeve 2002). Inbreeding and incest did occur in this population, and incest avoidance between potential breeders did not significantly affect skew in our sample. It would be useful, however, to create and test predictions of a model that incorporates potential costs of inbreeding on optimal skew (e.g. in terms of lower group productivity; Johnstone 2000; Magrath et al. 2004) in a larger sample. Also, a complete test of reproductive skew in American crows should ultimately consider many additional factors, such as ecological constraints on independent breeding, relative competitive ability of group members (Beekman et al. 2003), the degree to which one or more auxiliaries influence reproductive productivity (Johnstone et al. 1999) and relatedness asymmetry among potential breeders (Reeve & Keller 1996).
Table 3.
Described variation in social and genetic mating systems among cooperative corvids
| Species | Social mating system | % Polyandrous broods | Mixed maternity | Source |
|---|---|---|---|---|
| Florida scrub-jay Aphelocoma coerulescens | Monogamous | 0% | Rare | Quinn et al. 1999 |
| carrion crow Corvus corone corone | Monogamous or polyandrous | 26% | Rare | Baglione et al. 2002; pers. comm. V. Baglione1 |
| American crow Corvus brachyrhynchos | Monogamous | 28.8% | No | This study |
| brown jay Cyanocorax morio | Plural breeding; joint nesting; polygynandrous | 31-43% | Yes1; but see Williams 2004 | Lawton & Lawton 19851; Williams 2004; Williams & Rabenold 20052 |
| white-throated magpie-jay Calocitta formosa | Monogamous1 | 33.3%-61.5% | Yes | Langen 1996; Berg 2005; pers. comm. J. Ellis1 |
| Mexican Jay Aphelocoma ultramarina | Plural breeding; monogamous | 63.0% | No | Li & Brown 2000 |
Acknowledgments
We thank L. Schoenle for assistance in the field and lab; L. Stenzler, C. Makarewich and A. Talaba for help with molecular analyses; R. Heiss, J. Murray, J. Montagna, T. Wilson, Z. Adaila, G. Barr, J. McGowan and A. Tringali for their contributions in the field; and D. Robinson, J. Fitzpatrick and J. Dickinson for helpful advice. Population banding and field work supported in part by NSF-SGER 29444 and NIH (R21)-1064305. AKT's work was supported by the Animal Behaviour Society, Cornell Sigma Xi Grant-in-Aid of Research, the Frank M. Chapman Memorial Fund, the Kieckhefer Adirondacks Fellowship, the Cooper Ornithological Society, the Wilson Ornithological Society, the Andrew Mellon Foundation, an Eloise Gerry Fellowship from Sigma Delta Epsilon/Graduate Women in Science, the American Association of University Women and the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnqvist G, Kirkpatrick M. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. American Naturalist. 2005;165:S26–S37. doi: 10.1086/429350. [DOI] [PubMed] [Google Scholar]
- Baglione V, Marcos JM, Canestrari D, Ekman J. Direct fitness benefits of group living in a complex cooperative society of carrion crows (Corvus corone corone) Animal Behaviour. 2002;64:887–893. [Google Scholar]
- Beekman M, Komdeur J, Ratnieks FLW. Reproductive conflicts in social animals: who has power? Trends in Ecology & Evolution. 2003;18:277–282. [Google Scholar]
- Berg E. Parentage and reproductive success in the white-throated magpie-jay, Calocitta formosa, a cooperative breeder with female helpers. Animal Behaviour. 2005;70:375–385. [Google Scholar]
- Black C. PhD Thesis. University of Illinois; Urbana: 1941. Ecological and economic relations of the crow, with special reference to Illinois. [Google Scholar]
- Brown JL. Helping and Communal Breeding in Birds. Princeton, New Jersey: Princeton University Press; 1987. [Google Scholar]
- Buston PM, Reeve HK, Cant MA, Vehrencamp SL, Emlen ST. Reproductive skew and the evolution of group dissolution tactics: a synthesis of concession and restraint models. Animal Behaviour. 2007;74:1643–1654. doi: 10.1016/j.anbehav.2007.03.003. [DOI] [Google Scholar]
- Caffrey C. Female biased delayed dispersal and helping in American crows. The Auk. 1992;109:609–619. [Google Scholar]
- Caffrey C. Correlates of reproductive success in cooperatively breeding western American crows: if helpers help, it's not by much. The Condor. 2000;102:333–341. [Google Scholar]
- Cant MA, Reeve HK. Female control of the distribution of paternity in cooperative breeders. American Naturalist. 2002;160:602–611. doi: 10.1086/342820. [DOI] [PubMed] [Google Scholar]
- Chamberlain-Auger JA, Auger PJ, Strauss EG. Breeding biology of American crows. Wilson Bulletin. 1990;102:615–622. [Google Scholar]
- Clark AB, Robinson DA, McGowan KJ. Effects of West Nile virus mortality on social structure of an American crow (Corvus brachyrhynchos) population in New York state. Ornithological Monographs. 2006;60:65–78. [Google Scholar]
- Clutton-Brock TH. Reproductive skew, concessions and limited control. Trends in Ecology & Evolution. 1998;13:288–292. doi: 10.1016/s0169-5347(98)01402-5. [DOI] [PubMed] [Google Scholar]
- Double M, Cockburn A. Pre-dawn infidelity: females control extrapair mating in superb fairy-wrens. Proceedings of the Royal Society of London Series B-Biological Sciences. 2000;267:465–470. doi: 10.1098/rspb.2000.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliassen S, Kokko H. Current analyses do not resolve whether extrapair paternity is male or female driven. Behavioral Ecology and Sociobiology. 2008;62:1795–1804. [Google Scholar]
- Emlen JT., Jr Age determination in the American crow. Condor. 1936;38:99–102. [Google Scholar]
- Emlen ST. Reproductive sharing in different types of kin associations. American Naturalist. 1996;148:756–763. [Google Scholar]
- Fridolfsson AK, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. Journal of Avian Biology. 1999;30:116–121. [Google Scholar]
- Gowaty PA, Buschhaus N. Ultimate causation of aggressive and forced copulation in birds: female resistance, the CODE hypothesis and social monogamy. American Zoologist. 1998;38:207–225. [Google Scholar]
- Hamilton WD. The genetical evolution of social behavior. Journal of Theoretical Biology. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Heinsohn R, Dunn P, Legge S, Double M. Coalitions of relatives and reproductive skew in cooperatively breeding white-winged choughs. Proceedings of the Royal Society of London Series B-Biological Sciences. 2000;267:243–249. doi: 10.1098/rspb.2000.0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RA. Models of reproductive skew: a review and synthesis. Ethology. 2000;106:5–26. [Google Scholar]
- Johnstone RA, Cant MA. Reproductive skew and the threat of eviction: a new perspective. Proceedings of the Royal Society of London Series B-Biological Sciences. 1999;266:275–279. [Google Scholar]
- Johnstone RA, Woodroffe R, Cant MA, Wright J. Reproductive skew in multimember groups. American Naturalist. 1999;153:315–331. doi: 10.1086/303169. [DOI] [PubMed] [Google Scholar]
- Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Molecular Ecology. 2007;16:1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x. [DOI] [PubMed] [Google Scholar]
- Kilham L. Intra- and extrapair copulatory behavior in American Crows. Wilson Bulletin. 1984;96 [Google Scholar]
- Koenig WD, Dickinson JL. Ecology and Evolution of Cooperative Breeding in Birds. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- Koenig W, Haydock J. Incest avoidance. In: Koenig WD, Dickinson JL, editors. Ecology and Evolution of Cooperative Breeding in Birds. Cambridge: Cambridge University Press; 2004. pp. 142–156. [Google Scholar]
- Koenig WD, Haydock J, Stanback MT. Reproductive roles in the cooperatively breeding acorn woodpecker: incest avoidance versus reproductive competition. American Naturalist. 1998;151:243–255. doi: 10.1086/286115. [DOI] [PubMed] [Google Scholar]
- Kutsukake N, Nunn CL. Comparative tests of reproductive skew in male primates: the roles of demographic factors and incomplete control. Behavioral Ecology and Sociobiology. 2006;60:695–706. [Google Scholar]
- Langen TA. The mating system of the white-throated magpie-jay, Calocitta formosa, and Greenwood's hypothesis for sex biased dispersal. Ibis. 1996;138:506–513. [Google Scholar]
- Lawton MF, Lawton RO. The breeding biology of the brown jay in Monteverde, Costa Rica. Condor. 1985;87:192–204. [Google Scholar]
- Li SH, Brown JL. High frequency of extrapair fertilization in a plural breeding bird, the Mexican jay, revealed by DNA microsatellites. Animal Behaviour. 2000;60:867–877. doi: 10.1006/anbe.2000.1554. [DOI] [PubMed] [Google Scholar]
- Ligon JD, Burt DB. Evolutionary origins. In: Koenig WD, Dickinson JL, editors. Ecology and Evolution of Cooperatively Breeding in Birds. Cambridge: Cambridge University Press; 2004. pp. 5–34. [Google Scholar]
- Magrath RD, Heinsohn RG. Reproductive skew in birds: models, problems and prospects. Journal of Avian Biology. 2000;31:247–258. [Google Scholar]
- Magrath RD, Johnstone RA, Heinsohn R. Reproductive skew. In: Koenig WD, Dickinson JL, editors. Ecology and Evolution of Cooperatively Breeding in Birds. Cambridge: Cambridge University Press; 2004. pp. 157–176. [Google Scholar]
- Martin P, Bateson P. Measuring Behaviour. 2nd. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Marzluff JM, McGowan KJ, Donnelly R, Knight RL. Causes and consequences of expanding American Crow populations. In: Marzluff JM, Bowman R, Donnelly R, editors. Avian Ecology and Conservation in an Urbanizing World. Norwell, MA: Kluwer Academic Press; 2001. pp. 365–381. [Google Scholar]
- McGowan KJ. A test of whether economy or nutrition determines fecal sac ingestion in nesting corvids. Condor. 1995;97:50–56. [Google Scholar]
- McGowan KJ. Demographic and behavioral comparisons of suburban and rural American Crows. In: Marzluff JM, Bowman R, Donnelly R, editors. Avian Ecology and Conservation in an Urbanizing World. Norwell MA: Kluwer Academic Press; 2001. pp. 365–381. [Google Scholar]
- Nonacs P. Measuring and using skew in the study of social behavior and evolution. American Naturalist. 2000;156:577–589. doi: 10.1086/316995. [DOI] [PubMed] [Google Scholar]
- Nonacs P. Measuring the reliability of skew indices: is there one best index? Animal Behaviour. 2003;65:615–627. [Google Scholar]
- Pradhan GR, Engelhardt A, van Schaik CP, Maestripieri D. The evolution of female copulation calls in primates: a review and a new model. Behavioral Ecology and Sociobiology. 2006;59:333–343. doi: 10.1007/s00265-005-0075-y. [DOI] [Google Scholar]
- Queller DC, Goodnight KF. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. [DOI] [PubMed] [Google Scholar]
- Quinn JS, Woolfenden GE, Fitzpatrick JW, White BN. Multi-locus DNA fingerprinting supports genetic monogamy in Florida Scrub-jays. Behavioral Ecology and Sociobiology. 1999;45:1–10. [Google Scholar]
- Rabenold PP, Rabenold KN, Piper WH, Haydock J, Zack SW. Shared paternity revealed by genetic analysis in cooperatively breeding tropical wrens. Nature. 1990;348:538–540. doi: 10.1038/348538a0. [DOI] [Google Scholar]
- Reeve HK. A transactional theory of within-group conflict. American Naturalist. 2000;155:365–382. doi: 10.1086/303322. [DOI] [PubMed] [Google Scholar]
- Reeve HK, Keller L. Asymmetry and reproductive sharing in animal societies. American Naturalist. 1996;148:764–769. [Google Scholar]
- Reeve HK, Keller L. Tests of reproductive-skew models in social insects. Annual Review of Entomology. 2001;46:347–385. doi: 10.1146/annurev.ento.46.1.347. [DOI] [PubMed] [Google Scholar]
- Reeve HK, Shen SF. A missing model in reproductive skew theory: the bordered tug-of-war. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8430–8434. doi: 10.1073/pnas.0603005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve HK, Emlen ST, Keller L. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behavioral Ecology. 1998;9:267–278. [Google Scholar]
- Schoenle LA, Townsend AK, Lovette IJ. Isolation and characterization of microsatellite loci in a cooperatively breeding corvid, the American crow (Corvus brachyrhynchos) Molecular Ecology Notes. 2007;7:46–48. [Google Scholar]
- Serrell R. Master's thesis. Binghamton University; 2003. Sentinel behavior in the American crow Corvus brachyrhynchos. [Google Scholar]
- Slate J, Marshall T, Pemberton J. A retrospective assessment of the accuracy of the paternity inference program CERVUS. Molecular Ecology. 2000;9:801–808. doi: 10.1046/j.1365-294x.2000.00930.x. [DOI] [PubMed] [Google Scholar]
- Tarr CL, Fleischer RC. Primers for polymorphic GT microsatellites isolated from the Mariana crow, Corvus kubaryi. Molecular Ecology. 1998;7:253–255. [PubMed] [Google Scholar]
- Vehrencamp SL. The roles of individual, kin and group selection in the evolution of sociality. In: Marler P, Vandenbergh JG, editors. Handbook of Behavioral Neurobiology: Social Behavior and Communication. New York: Plenum Press; 1979. pp. 351–394. [Google Scholar]
- Vehrencamp SL. A model for the evolution of despotic vs. egalitarian societies. Animal Behaviour. 1983;31:667–682. [Google Scholar]
- Verbeek NAM, Butler RW. Northwestern crow (Corvus caurinus) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 1999. [Google Scholar]
- Verbeek NAM, Caffrey C. American crow (Corvus brachyrhynchos) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 2002. [Google Scholar]
- Westneat DF, Stewart IRK. Extrapair paternity in birds: causes, correlates and conflict. Annual Review of Ecology Evolution and Systematics. 2003;34:365–396. [Google Scholar]
- Westneat DF, Sherman PW, Morton ML. The ecology and evolution of extrapair copulations in birds. In: Power DM, editor. Current Ornithology. New York & London: Plenum Press; 1990. [Google Scholar]
- Wiley RH, Rabenold KN. The evolution of cooperative breeding by delayed reciprocity and queuing for favorable social positions. Evolution. 1984;38:609–621. doi: 10.1111/j.1558-5646.1984.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Williams DA. Female control of reproductive skew in cooperatively breeding brown jays (Cyanocorax morio) Behavioral Ecology and Sociobiology. 2004;55:370–380. [Google Scholar]
- Williams DA, Rabenold KN. Male biased dispersal, female philopatry and routes to fitness in a social corvid. Journal of Animal Ecology. 2005;74:150–159. [Google Scholar]
- Wilson TM. Master's Thesis. Binghamton University; 2008. Patterns of sentinel behavior at the nest in the cooperatively breeding American crow (Corvus brachyrhynchos) [Google Scholar]
- Woolfenden GE, Fitzpatrick JW. The Florida Scrub-jay: Demography of a Cooperative-Breeding Bird. Princeton, NJ: Princeton University Press; 1984. [Google Scholar]
- Yorzinski JL, Vehrencamp SL, Clark AB, McGowan KJ. The inflected alarm caw of the American crow: differences in acoustic structure among individuals and sexes. Condor. 2006;108:518–529. [Google Scholar]
- Young AJ, Spong G, Clutton-Brock T. Subordinate male meerkats prospect for extra-group paternity: alternative reproductive tactics in a cooperative mammal. Proceedings of the Royal Society B-Biological Sciences. 2007;274:1603–1609. doi: 10.1098/rspb.2007.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]