Abstract
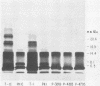
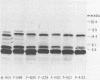
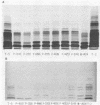
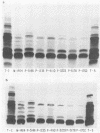
Lipopolysaccharides (LPSs) purified from 16 reference somatic serotypes of Pasteurella multocida were examined and compared by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Resolution of LPS patterns in a gel was optimum when sample wells were cast separately from the stacking gel and the running gel consisted of 15% T (total monomer) polyacrylamide and 4 M deionized urea. Band patterns of P. multocida LPSs in a gel differed from control Salmonella minnesota wild-type and core mutant LPSs. Although the band patterns and mobilities of LPSs from some P. multocida reference serotypes were similar, none were identical. Evidence for O antigens similar to those produced by enterobacteria was not observed. Proteinase K digestion of whole P. multocida cells resulted in LPS band patterns similar to those of purified LPS. The presence or absence of a capsule on a strain had no major influence on band patterns in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Comparisons of LPS patterns of group B and E hemorrhagic septicemia strains with those of serologically related group A strains of P. multocida indicated that they were similar. Typing antisera made with purified serotype 2 or 5 LPS reacted with electroblots of all these strains. However, the reactions did not distinguish strains as being serotype 2 or 5.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Heddleston K. L., Gallagher J. E., Rebers P. A. Fowl cholera: gel diffusion precipitin test for serotyping Pasteruella multocida from avian species. Avian Dis. 1972 Jul-Sep;16(4):925–936. [PubMed] [Google Scholar]
- Hitchcock P. J. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect Immun. 1984 Oct;46(1):202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., van Boxtel R., de Jong M. Atrophic rhinitis in swine: correlation of Pasteurella multocida pathogenicity with membrane protein and lipopolysaccharide patterns. Infect Immun. 1984 Oct;46(1):48–54. doi: 10.1128/iai.46.1.48-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. J., Naasz M. A., DeLong D., Leary S. L. Pasteurellosis in laboratory rabbits: characterization of lipopolysaccharides of Pasteurella multocida by polyacrylamide gel electrophoresis, immunoblot techniques, and enzyme-linked immunosorbent assay. Infect Immun. 1986 Sep;53(3):460–463. doi: 10.1128/iai.53.3.460-463.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen S., Häyrinen J., Finne J. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J Bacteriol. 1988 Jun;170(6):2646–2653. doi: 10.1128/jb.170.6.2646-2653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimler R. B., Angus R. D., Phillips M. Evaluation of the specificity of Pasteurella multocida somatic antigen-typing antisera prepared in chickens, using ribosome-lipopolysaccharide complexes as inocula. Am J Vet Res. 1989 Jan;50(1):29–31. [PubMed] [Google Scholar]
- Rimler R. B., Brogden K. A. Pasteurella multocida isolated from rabbits and swine: serologic types and toxin production. Am J Vet Res. 1986 Apr;47(4):730–737. [PubMed] [Google Scholar]
- Rimler R. B., Phillips M. Fowl cholera: protection against Pasteurella multocida by ribosome-lipopolysaccharide vaccine. Avian Dis. 1986 Apr-Jun;30(2):409–415. [PubMed] [Google Scholar]
- Rimler R. B., Rebers P. A., Phillips M. Lipopolysaccharides of the Heddleston serotypes of Pasteurella multocida. Am J Vet Res. 1984 Apr;45(4):759–763. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]