Abstract
Coastal waters of the United States (U.S.) are subject to many of the major harmful algal bloom (HAB) poisoning syndromes and impacts. These include paralytic shellfish poisoning (PSP), neurotoxic shellfish poisoning (NSP), amnesic shellfish poisoning (ASP), ciguatera fish poisoning (CFP) and various other HAB phenomena such as fish kills, loss of submerged vegetation, shellfish mortalities, and widespread marine mammal mortalities. Here, the occurrences of selected HABs in a selected set of regions are described in terms of their relationship to eutrophication, illustrating a range of responses. Evidence suggestive of changes in the frequency, extent or magnitude of HABs in these areas is explored in the context of the nutrient sources underlying those blooms, both natural and anthropogenic. In some regions of the U.S., the linkages between HABs and eutrophication are clear and well documented, whereas in others, information is limited, thereby highlighting important areas for further research.
Keywords: harmful algal blooms, HABs, red tides, eutrophication, nutrients, nitrogen, phosphorus
1. Introduction
Virtually every coastal nation is affected by harmful algal blooms (HABs; Hallegraeff, 1993). It is now widely accepted that there are more toxic algal species, algal toxins, areas affected, fisheries resources impacted, and higher economic losses compared to several decades ago. Reasons for such expansion include natural dispersal of species by currents and storms; dispersal of species through human activities such as ballast water discharge and shellfish seeding; improved detection of HABs and their toxins due to better chemical instrumentation and improved communication among scientists; increased aquaculture operations in coastal waters; and stimulation of HABs as a result of cultural eutrophication (Anderson, 1989; Hallegraeff, 1993; Burkholder, 1998; Glibert et al., 2005a; Glibert and Burkholder, 2006).
In this latter context, there is now consensus on some aspects of the relationship between eutrophication and HABs (Heisler et al., 2008), recognizing that significant questions and challenges remain (Glibert, 2006). Various authors have investigated potential linkages between HABs and eutrophication at the global level (e.g., Smayda, 1989; Burkholder, 1998; Anderson et al., 2002; Glibert and Burkholder, 2006; Glibert et al., 2008). In some instances, the linkage is clear and unequivocal. For example, mandated reductions in pollution inputs to the Inland Sea of Japan in the mid-1970s led to a proportional decrease in the number of red tides and HAB events (Okaichi, 1997). Likewise, increased nutrient loadings to the northwestern Black Sea in the 1970s and 1980s led to an increase and compositional shift in algal blooms (Bodeanu and Ruta, 1998). Subsequently, HABs declined in the 1990s coinciding with a decrease in nutrient loading due to reduced fertilizer usage following the breakup of the former Soviet Union and termination of its agricultural subsidies.
The pathways and mechanisms through which nutrients supplied by human activities can stimulate HABs are often complex and subtle. For example, it is now evident that in addition to the quantity of nutrients supplied through point and non-point sources of pollution, the relative abundance of the major nutrients (e.g., nutrient supply ratios) and the chemical form of those nutrients (e.g., inorganic versus organic) are all important (Smayda, 1989; Anderson et al., 2002; Glibert et al., 2001, 2005a). Furthermore, it is now recognized that nutrient effects cannot be inferred from concentration data alone; in fact, there can be a negative relationship between ambient concentration and biomass due to incorporation of the nutrient into biomass. Nutrient loading or flux rates are the more appropriate measure, but much more difficult to quantify. In addition, the role of mixotrophy, or use of both phototrophic and heterotrophic nutrition, by some harmful algae increasingly has been recognized, and these organisms sometimes have been shown to be indirectly stimulated by nutrient enrichment through consumption of algal prey that are directly stimulated by eutrophication (Burkholder and Glasgow, 1997; Burkholder, 1998; Stoecker, 1999; Lewitus et al., 1999a; Glibert et al., 2005a; Burkholder et al., 2008).
Given that some HABs are stimulated by anthropogenic nutrient inputs, projections of increased nitrogen (N) and phosphorus (P) loadings to coastal waters are worrisome (Seitzinger et al., 2005; Harrison et al., 2005; Burkholder et al., 2006; Howarth, 2008; Bricker et al., 2008). Here we focus on some common HAB events in the U.S., particularly those that cause human illness, but this is not intended to be a comprehensive survey of all blooms, species or regions. For example, some blooms in the Northeast (e.g., Narragansett Bay; Li and Smayda, 2000), eastern Florida or Florida Bay (e.g., Phlips et al., 1999; Glibert et al., 2004b), the Texas coast (e.g., Laguna Madre; Buskey et al., 2001), and San Francisco Bay (Lehman et al., 2005) are not reviewed herein.
2. Regional HAB phenomena
2.1 PSP in the Gulf of Maine
The most significant HAB problem in the northeastern U.S. is PSP caused by Alexandrium fundyense1. The affected resources are predominantly shellfish, but higher levels of the food web are also impacted, including lobsters, fish, and marine mammals. Blooms of A. fundyense fall into two categories: regional outbreaks occurring in open coastal waters, and “self-seeding” localized blooms in isolated embayments and sounds with little or no input or export of cells to the adjacent coastal waters (Anderson, 1997; Anderson et al., 2005c). A key feature of A. fundyense bloom dynamics is a dormant cyst stage that allows this species to overwinter. In the nearshore waters of the Gulf of Maine (GOM), blooms are initiated from two cyst seedbeds, one in the Bay of Fundy, and the other offshore of mid-coast Maine, with delivery of established populations to shore by episodic wind forcings as well as large-scale movements of water masses in the central Gulf (Anderson et al., 2005b). Along this extended transport pathway, cells within the eastern and western segments of the Maine coastal current can enter embayments such as Casco and Massachusetts Bays with downwelling-favorable winds. It is important to recognize, however, that the source populations and associated water masses are delivered from upstream and offshore.
PSP toxicity in the western GOM was sporadic and poorly documented prior to a major bloom in 1972 (Hurst, 1975). PSP toxicity continued at high levels until the early 1990s, decreased for a decade thereafter, and recently has shown signs of increasing again (Anderson et al., 2005a).
2.1.1 Eutrophication linkages
An evaluation of potential linkages between northeastern U.S. PSP outbreaks and eutrophication needs to account for the two types of blooms in the region. Riley (1967) challenged the prevailing dogma that coastal nutrients in the GOM were derived primarily from riverine sources. Instead, he suggested that cross-shelf fluxes from the open ocean were important, an inference later validated by Fournier et al. (1977). Nutrients that enter the GOM at depth through the Northeast Channel overwhelm fluxes from the atmosphere or local rivers (Townsend, 1998; Townsend et al., 2006). There has been and continues to be significant inter-annual variability in the magnitude of that nutrient flux into the Gulf, reflecting the relative importance of the source waters and large-scale forcings such as the North Atlantic Oscillation (Townsend et al., 2006).
Coastal currents of the GOM carry populations of A. fundyense into embayments such as Casco and Massachusetts Bays where anthropogenic influences may be more significant. Local cyst germination might also provide an inoculum. Poulton et al. (2005), Martorano (1997) and Love et al. (2005) all provided evidence for increasing dissolved inorganic nitrogen (DIN) levels (ranging from 1–10 µM during Alexandrium bloom season) along a transect from offshore to inshore within Casco Bay. These data reflect land-based nutrient sources in the Bay (i.e., the city of Portland) and riverine inputs.
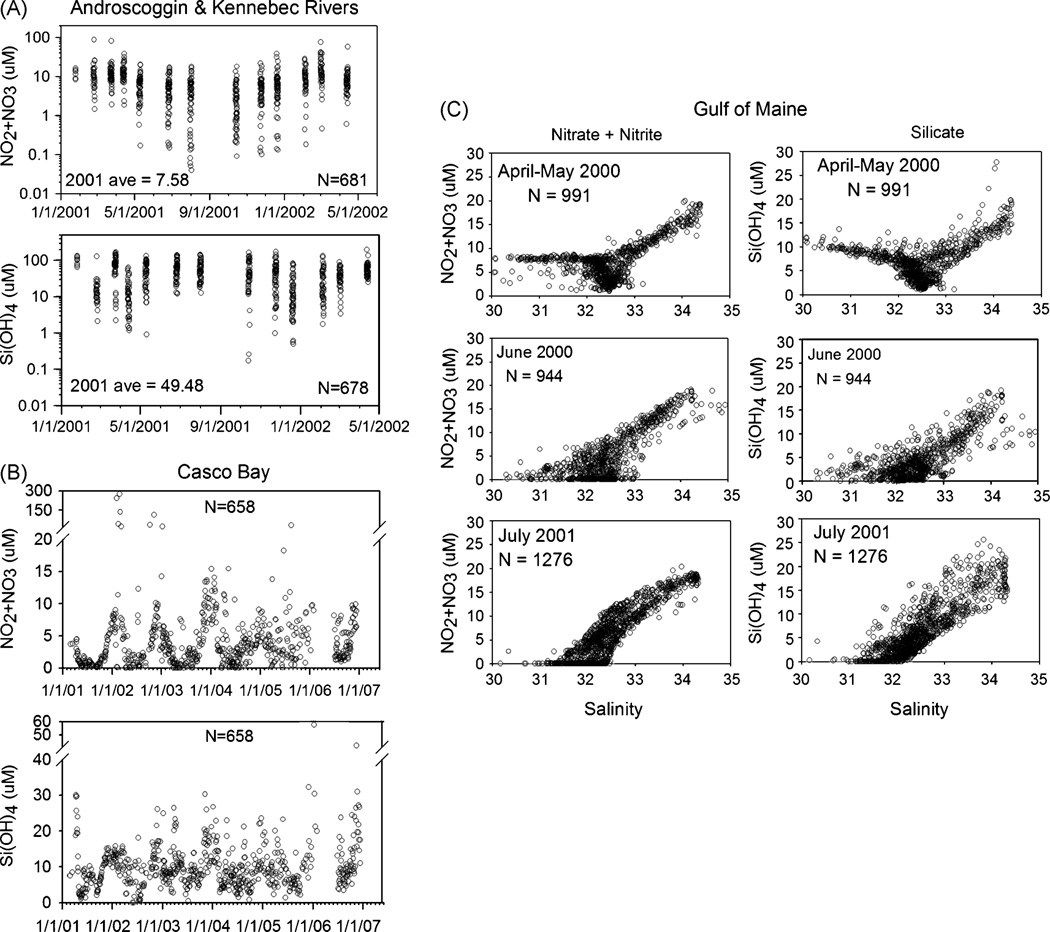
Nutrient measurements in the region’s rivers and coastal waters are relatively scarce, and suggest that nutrient loads in the Androscoggin and Kennebec (two of the four largest rivers emptying into the GOM) are highly variable (Fig. 1A). Nitrate+nitrite (NO3− + NO2−) levels average about 7.6 µM over an annual cycle, with highest levels (12.7 µM) in the high-runoff months (winter-spring) and the lowest (4.6 µM) during low runoff months (Jun-Oct). These are comparable to or lower than values in other urban regions of the U.S, and once those freshwaters mix with coastal waters, the resulting nutrient concentrations are diluted (e.g., to ~2.7 µM NO3−). Episodic high values of NO3− + NO2− in excess of 10 µM can occur during bloom season (Fig. 1B), which could be important to A. fundyense populations in Casco Bay at that time. The average annual cycle shows no clear changes over the last 7 years, although a longer duration may be needed to detect NO3−-related changes (e.g., Rothenberger, 2007). Many HABs also use ammonium (NH4+) or dissolved organic nitrogen (DON) as their primary N substrate. In Casco Bay, NH4+ concentrations are very high, occasionally exceeding 20 µM, and tend to average > 2 µM (Bricker et al., 2007).
Figure 1.
Nutrients in Gulf of Maine nearshore waters. Panel A: Concentrations of nitrate plus nitrite (NO3− + NO2−) and silicate (Si(OH)4) in the Kennebec and Androscoggin Rivers in Maine over the period January 1, 2001 to May 1, 2002 (data from Vorosmarty et al., http://www.gm-wics.sr.unh.edu/). Panel B: Concentrations of NO3− + NO2− and Si(OH)4 collected in Casco Bay at the Southern Maine Community College dock, South Portland, Maine (from Townsend et al., http://grampus.umeoce.maine.edu/). Panel C: Mixing curves for NO3− + NO2− and Si(OH)4 versus salinity for Gulf of Maine samples collected in a high- (April–May 2000), medium- (June 2000) and low-runoff period (July 2001) (Townsend et al., 2005). Assuming a northern Gulf of Maine coast-wide freshwater source concentration of nutrients similar to the Kennebec-Androscoggin (which was 32 µM Si(OH)4 and 12 µM NO3− + NO2− in April 2000; n=49; http://www.gm-wics.sr.unh.edu/), and coastal seawater concentrations of ca. 8 µM of Si(OH)4 and ca. 8 µM NO3− + NO2− and salinity of 32.5, Si(OH)4 concentrations of 9.5 µM and NO3− + NO2− concentrations of 8 µM at salinity of 30 in April–May are expected (in agreement with those values observed in the mixing curve in Panel C).
Analysis of samples collected from inshore and offshore waters throughout the northern GOM in high-, medium-, and low-runoff periods again suggest that terrestrial sources of NO3− + NO2− are not significant, except for localized areas of inner Casco Bay as mentioned, based upon the available data. For example, mixing curves of NO3− concentrations versus salinity show that there was virtually no low-salinity (terrestrial, freshwater) NO3− contribution in July 2001 or June 2000 (Fig. 1C). There was evidence of a terrestrial, freshwater source in these coastal waters in April-May 2000, during and after the high-runoff period but, even then, the NO3− sources were primarily from offshore. Data are not available for other N forms offshore.
Considering localized blooms that occur along the New England coast, a separate analysis in each area would be needed to assess whether eutrophication is affecting A. fundyense blooms. There is a good likelihood that this is occurring, as these embayments, sounds, and estuaries are often surrounded by housing and other development, often with outdated septic systems. One example, the Nauset Marsh System on Cape Cod, lacks major river input, and NO3−-contaminated groundwater from septic systems is the largest source of N (Giblin and Gaines, 1990; Howes et al., 2003). Groundwater NO3− fluxes are ~5-fold greater than inputs from land runoff, and these two sources combined are comparable to inputs in river-dominated urban areas of the U.S. (Portnoy et al., 1998). In the more highly developed areas of the Nauset system, groundwater NO3− concentrations are more than 30-fold higher than in less developed areas. PSP outbreaks within the Nauset Marsh System have increased dramatically over the last several decades. Toxicity occurred in Salt Pond (Eastham) in 8 of 17 years (48%) from 1975 to 1991, and then in 15 of the last 16 years (94%). PSP is also occurring earlier and lasting longer (H. Lind, pers. comm). The growing nutrient load from NO3− contaminated groundwater is a likely reason for the increased frequency of PSP outbreaks within this system.
In summary, PSP outbreaks in the northeastern U.S. take two forms – large regional open-water blooms and localized nearshore outbreaks. For the open-water blooms, the supply of inorganic nutrients from oceanic sources dwarfs inputs from land, so that if there is a eutrophication effect, it is subtle and unquantified. These blooms do impact certain bays and sounds when offshore populations of A. fundyense are delivered by winds, and thus there is a potential for local sources of anthropogenic nutrients to enhance bloom magnitude and duration. The strongest linkage to eutrophication is with localized, nearshore PSP outbreaks that recur annually in small salt ponds, embayments, and sounds where flushing rates are low and development pressures can lead to high nutrient inputs from sewage treatment plants, terrestrial runoff, and groundwater sources.
2.2 Brown tides in the Northeast and Mid-Atlantic
Aureococcus anophagefferens is the picoplanktonic pelagophyte that has caused destructive ‘brown tide’ blooms in northeast and mid-Atlantic U.S. estuaries for two decades (Gobler et al., 2005). The first blooms occurred simultaneously in 1985 in Narragansett Bay, Rhode Island, and Great South Bay and the Peconic Estuary on Long Island, New York (Cosper et al., 1989). Blooms recurred annually in Long Island bays from 1986–88 and sporadically since then, but have not returned to Narragansett Bay. Even during years without blooms, A. anophagefferens populations of up to 104 mL−1 persist (Nuzzi and Waters, 2004). During the 1990s, brown tides expanded south along the U.S. East Coast into bays in New Jersey (Gastrich et al., 2004), Delaware (Popels et al., 2003), Maryland, and Virginia (Trice et al., 2004; Glibert et al., 2007). Low abundance of A. anophagefferens has been observed along the entire eastern seaboard of the U.S. from Maine to Florida (Anderson et al., 1993; Popels et al., 2003).
2.2.1 Eutrophication linkages
Brown tides are not directly caused by eutrophication from inorganic nutrients, but they may be linked to organic nutrients (below). Other phytoplankton such as diatoms often out-compete A. anophagefferens when inorganic nutrient loads are high (Berg et al., 1997; MacIntyre et al., 2004). An examination of spatial and temporal patterns of concentrations of A. anophagefferens cells and inorganic nutrients indicates that blooms occurred when inorganic nutrient levels were low (Cosper et al., 1989; LaRoche et al., 1997; Gobler et al., 2002, 2004). Moreover, NO3− additions during mesocosm and bottle experiments consistently have yielded reduced A. anophagefferens cell densities relative to those of competing algae (e.g., Keller and Rice, 1989; Gobler and Sañudo-Wilhelmy, 2001a). The N-uptake characteristics of A. anophagefferens (low Ks and Vmax for NH4+) suggest that this species is well adapted to low nutrient environments (Lomas et al., 1996). The off-shore rerouting of sewage previously discharged directly into western Great South Bay during the early 1980s led to lower levels of dissolved inorganic nitrogen (DIN) there, thus creating a nutrient regime which reduced total annual phytoplankton biomass, but favored dominance by A. anophagefferens as blooms began to first develop in the late 1980s (Fig. 2A).
Figure 2.
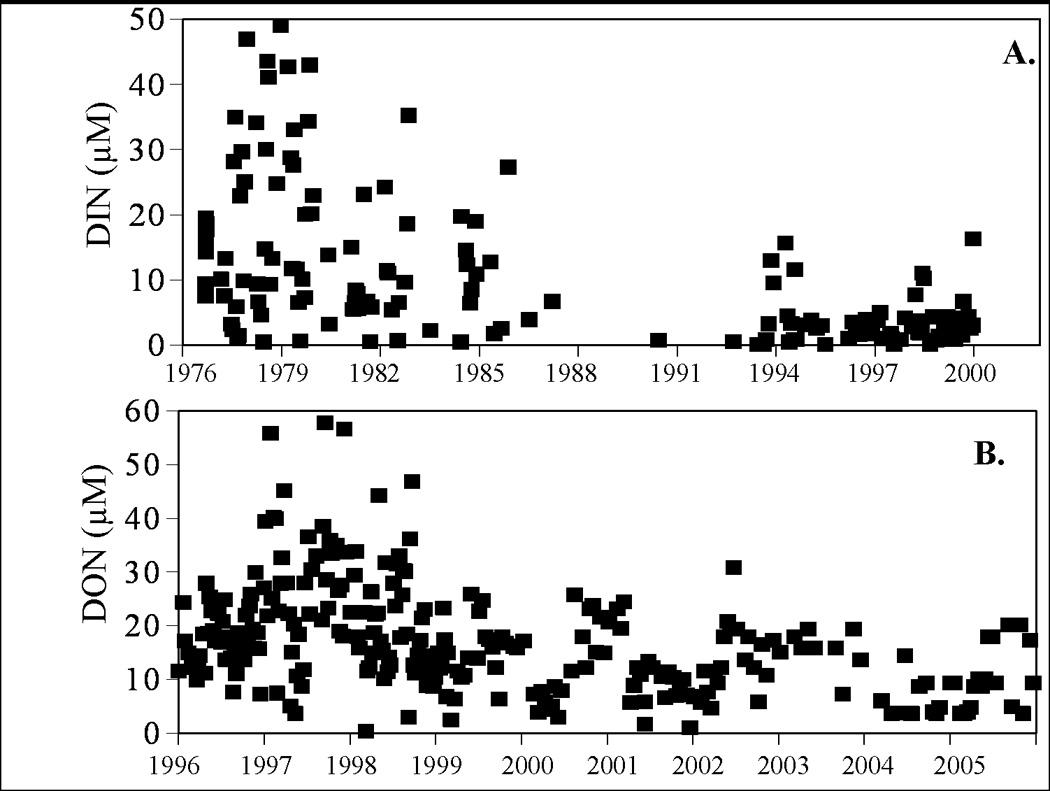
A. Concentrations of DIN in western Great South Bay, NY (Suffolk County Department of Health Services station 250) from 1976 – 2000, before and after the onset of brown tides. B. Concentrations of DON in Great Peconic Bay, NY (Suffolk County Department of Health Services station 130) from 1996 – 2005, since the last occurrence of brown tide in this system.
Blooms of Aureococcus often occur after ‘pre-blooms’ of other algae which draw down inorganic nutrients to low levels. Nutrient remineralization processes during and following these pre-blooms can result in enhanced levels of dissolved organic matter (DOM) which can serve as a source of dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) (LaRoche et al., 1997; Gobler and Sañudo-Wilhelmy, 2001b). Cultures (both axenic and non-axenic) and field populations of A. anophagefferens have been shown to obtain N from a variety of organic compounds, including urea, amino acids, proteins, chitobiose, and acetamide (Berg et al., 1997, 2002, 2003; Mulholland et al., 2002; Kana et al., 2004). Experimental additions of DOM (glucose, amino acids, DOM from macroalgae) have enhanced the growth and relative abundance of A. anophagefferens during field experiments (Gobler and Sañudo-Wilhelmy, 2001a; Kana et al., 2004). Concentrations of DON and DOC often are elevated during bloom initiation, and their drawdown has been associated with bloom development (LaRoche et al., 1997; Gobler et al., 2004).
Brown tides commonly occur in shallow bays where diffusive fluxes from the benthos are important sources of organic nutrients. For example, in the shallow (~1.5 m) lagoonal estuaries of NY, brown tides are persistent events and DON levels are high, due in part to strong benthic-pelagic coupling (Lomas et al., 2004; Gobler et al., 2005). In Maryland’s lagoonal bays, both the DON concentrations and the strength of brown tide blooms have increased steadily over the past decade (Trice et al., 2004; Glibert et al., 2007). Since the mid-1990s, mean concentrations of DON in these bays have more than doubled, and the fraction of total chlorophyll composed of brown tide in the summer has increased to an even greater extent (Glibert et al., 2007).
In deeper estuaries where benthic-pelagic coupling is weaker, DON concentrations typically are lower than in shallow lagoons and brown tides occur less frequently. This is the case, for example, in the Peconic Estuary (mean depth > 5 m; Gobler et al., 2005). Both brown tide and DON levels there have decreased since 1996 (Fig. 2B), presumably due to decreased N loading, but DIN levels have remained unchanged. Hence, it is possible that lower DON levels, in conjunction with higher grazing rates on A. anophagefferens by the zooplankton community (Deonarine et al., 2006), may be responsible for the absence of brown tides in the Peconic Estuary since the 1990s.
In summary, A. anophagefferens thrives in environments where nutrients are dominated by organic rather than inorganic forms. In regions where inorganic nutrient loading is elevated, other phytoplankton with faster growth rates tend to dominate. Thus, brown tides are not directly related to inorganic nutrient loading, but may be indirectly related to eutrophication processes as inorganic nutrients are assimilated and regenerated as organic forms. In some regions, such as the Maryland Coastal Bays, DON is increasing from agricultural sources, animal operations and organic fertilizers (e.g. Glibert et al., 2007) and direct sources of DON should be considered as contributing to eutrophication in these regions, and therefore to brown tides.
2.3 HABs in the Mid-Atlantic region
In the mid-Atlantic, the frequency of occurrence and, in many cases, the intensity of HABs increasing relative to previous decades. The region has a wide range of high-biomass and/or toxic HABs involving species such as Prorocentrum minimum, A. anophagefferens, Microcystis aeruginosa, Pfiesteria piscicida, P. shumwayae, Karlodinium veneficum (formerly Karlodinium micrum, Gyrodinium galatheanum), Heterosigma akashiwo, Chattonella subsalsa and Chattonella cf. vericulosa, and Fibrocapsa japonica (Marshall et al., 2005a; Burkholder et al., 2005; Moeller et al., 2007; Lewitus et al., 2008). Numerous other potentially toxic or harmful species are also present; for example, six species of Pseudo-nitzschia have been found, and of these, 46% contained low levels of domoic acid (Thessen and Stoecker, 2008). Marshall et al. (2005b) identified 34 harmful species for Chesapeake Bay, while Tango et al. (2004) identified 13 in Maryland Coastal Bays. The trend in brown tides in Maryland’s Coastal Bays was discussed above, and only a few species and trends are highlighted here.
2.3.1 Eutrophication linkages
A recent comprehensive assessment of eutrophication in the nation’s waters revealed that most of the estuaries affected by HABs are in the mid-Atlantic, and that this region is the most affected by eutrophication of all coastal regions of the U.S. (Bricker et al., 2007, 2008). With the exception of the Mississippi plume, N loads were found to be highest in the mid-Atlantic. The mid-Atlantic has the nation’s two largest estuaries in areal extent (Chesapeake Bay and the Albemarle-Pamlico Estuarine System), which drain watershed areas that are rapidly growing in human and animal populations and changing in land use and N loads. Since 1960, for example, the watershed for Chesapeake Bay has sustained more than a doubling in human population and in use of N- based fertilizers (Kemp et al., 2005; Fig. 3).
Figure 3.
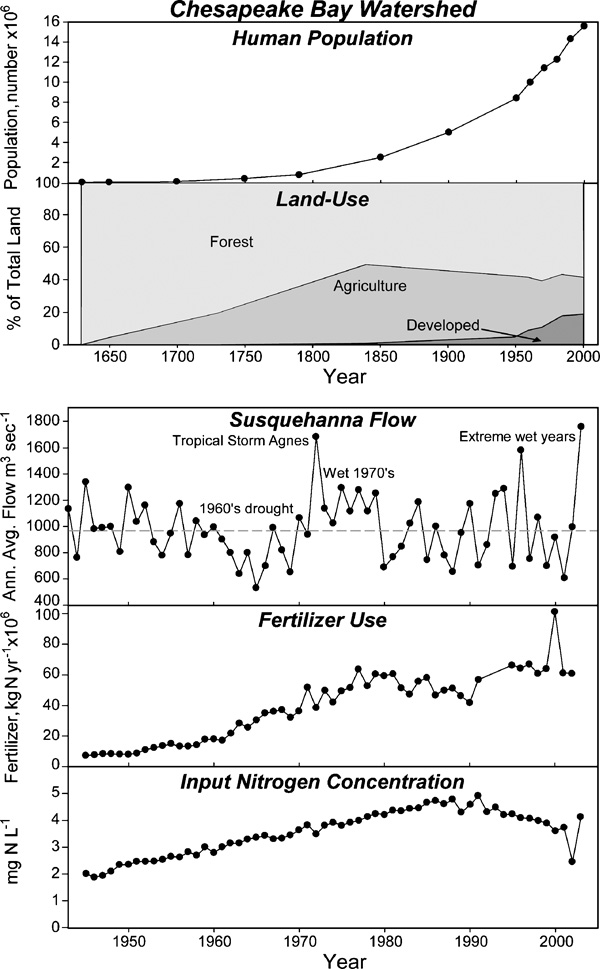
Temporal changes in land uses, river flow, fertilizer use, and river N concentration in the Chesapeake Bay watershed. From Kemp et al. 2005 with permission of the publisher.
Land use in the region also has changed with a large expansion of confined animal feed operations (CAFOs), primarily swine and poultry (Mallin and Cahoon, 2003; Rothenberger et al., submitted). These have contributed to nutrient loads through surface runoff, leachate into groundwater, and enhanced atmospheric emissions (Burkholder et al., 1997; Mallin, 2000); approximately 80% of emissions of NH3 from swine operations in the U.S. originate in North Carolina (EPA, 1998; Aneja et al., 2003). Thus, the total N load to the Albemarle-Pamlico water from atmospheric deposition is among the highest for U.S. watersheds (EPA, 2000). CAFOs also have altered the composition of nutrients reaching the estuarine and coastal waters, in particular increasing NH4+ and in some cases, urea (Glibert et al., 2005b; Burkholder et al., 2006). Retention ponds also have been shown to serve as incubation sites for many species (Lewitus et al., 2003; 2008). These major estuaries are also sustaining major loss of wetlands, depletion of shellfish stocks, and global warming, all of which can act synergistically to increase nutrient loads and alter trophic relationships.
An example of frequent high-biomass bloom formers is P. minimum. Well-established to be stimulated by eutrophication (Heil et al., 2005; Rothenberger, 2007; Glibert et al., 2008), this species frequently blooms following precipitation events that add significant quantities of nutrients. Blooms are most common in late winter-spring (Albemarle-Pamlico) through early summer (Chesapeake Bay) following major agricultural fertilizer applications (Glibert et al., 2001; Springer et al., 2005; Tango et al., 2005). These blooms now appear to be 10- to 100-fold higher in maximum density in Chesapeake Bay than blooms of the same organism recorded a few decades ago (Tyler and Seliger, 1978).
Blooms of K. veneficum are also increasing in frequency in Chesapeake Bay, and have been linked to some fish kills (Deeds et al., 2002; Goshorn et al., 2004). These blooms tend to occur when NO3− concentrations are low, and when NH4+ or urea concentrations and ambient PO43− are elevated. Mixotrophy through consumption of algal prey that are directly stimulated by eutrophication also contributes to K. veneficum nutrition and supports faster growth rates (Adolf et al., 2006, 2008).
Other harmful algae that have been linked both directly and indirectly to eutrophication are the potentially toxic, heterotrophic Pfiesteria spp. (Pfiesteria piscicida, P. shumwayae), which mostly have affected the Albemarle-Pamlico and Chesapeake Bay (Burkholder and Glasgow, 1997; Burkholder et al., 2005; Glibert et al., 2001, 2006a; Marshall et al., 2006; Moeller et al., 2007; Lewitus et al., 2008). Field studies with supporting laboratory experiments have shown that Pfiesteria spp. thrive in nutrient over-enriched estuaries where they consume cryptophytes and other microbial prey when preferred fish resources are not available (Burkholder and Glasgow, 1997; Lewitus et al., 1999b; Glasgow et al., 2001; Parrow et al., 2002). They can also act as mixotrophs by retaining kleptochloroplasts from cryptophytes for short periods, apparently used to augment their nutrition (Lewitus et al., 1999b). Pfiesteria spp. depend mostly on grazing for their nutrient supplies, but can also take up dissolved nutrients directly (Burkholder and Glasgow, 1997; Lewitus et al., 1999a,b; Burkholder et al., 2001; Glibert et al. 2006a). Pfiesteria outbreaks in Chesapeake Bay tributaries also have been associated with high DON and DOC levels (Glibert et al., 2001). For all samples collected from 2000–2001 in Chesapeake Bay (n = 1,614 for water and 156 for sediment), the average urea concentration was an excellent predictor of the percent positive detection of Pfiesteria in sediments and in the water column (Glibert et al., 2004a). Laboratory studies have shown that Pfiesteria can be stimulated by P (Burkholder and Glasgow, 1997), and that the preference for N form follows a trend of amino acids>urea>NH4+> NO3− (Glibert et al., 2006a). Urea is now the dominant agricultural N form in the region, and is also a dominant N form in poultry manure (Glibert et al., 2005b). The major effect of eutrophication on Pfiesteria spp., nonetheless, appears to be indirectly mediated through abundance of prey that is directly stimulated (Burkholder and Glasgow, 1997; Glibert et al., 2006a).
Large blooms of the toxic cyanobacterium M. aeruginosa are common in the summer months in the low-salinity reaches of the estuaries and tributaries of the region (e.g., Jaworski, 1990). In recent years, these blooms have increased as nutrient concentrations have been escalating (Marshall et al., 2005b).
Raphidophytes have caused fish kills and recurring impacts to some mid-Atlantic ecosystems, especially H. akashiwo. Based on an analysis of historical phytoplankton monitoring data, these raphidophytes, as well as Fibrocapsa japonica and Chattonella subsalsa, have increased significantly in abundance over the past 20 years in the Coastal Bays of Maryland (Tango et al., 2004). While inorganic nutrient concentrations decreased from the 1980s to the mid 1990s in the bays where these species are most common (Wazniak et al., 2007), significant increases have since been documented in both P and N, especially as NH4+ and DON (Wazniak et al., 2007; Glibert et al., 2007). In laboratory experiments, Zhang et al. (2006) found that cell yield for C. subsalsa and Heterosigma akashiwo was higher on NH4+ and NO3−, respectively, and that C. subsalsa did not use urea while H. akashiwo readily used this N form. The levels of N used in these experiments were comparable to natural conditions in the highly eutrophic Delaware inland bays.
In summary, the mid-Atlantic region has many HAB species, most of which have increased in frequency, abundance, regional extent and impact over the past few decades as population, agriculture and animal operations, and fertilizer usage have increased. In addition to increased nutrient loads, the estuaries, rivers and embayments of the region are also highly modified by declines in shellfish stocks and wetlands (e.g. Steel, 1991; Rothschild et al. 1994), leading to multiple stressors which collectively and synergistically lead to habitat change and alterations in trophic structure, including HAB proliferations.
2.4 Karenia brevis blooms in the Gulf of Mexico
The most significant HAB species in the Gulf of Mexico is the ichthyotoxic dinoflagellate Karenia brevis (formerly Gymnodinium breve Ptychodiscus brevis), although other HAB species (e.g., Pseudo-nitzschia spp., Prorocentrum minimum) are present (Parsons et al., 2002). Published accounts of widespread west Florida shelf K. brevis blooms predate extensive development on that coast (Lund, 1936; Tester and Steidinger, 1997; Magaña et al., 2003). Although K. brevis is the primary species associated with toxic blooms in the Gulf of Mexico, there are now at least five described Karenia species known from Gulf waters (Haywood et al., 2004). Recent blooms are now known to exhibit a sequence of Karenia species (Heil et al., in press) but the toxicity of species other than K. brevis is presently under investigation.
2.4.1 Eutrophication linkages
There are two views on the role of nutrients and eutrophication in Karenia brevis blooms. Walsh et al. (2006) examined potential linkages between K. brevis outbreaks on the west Florida shelf and the Texas shelf with riverine flux and eutrophication. Increases in nutrient loads to the Caloosahatchee River during the 1970s and 1980s were followed by decreases in the 1990s (Fig. 4). Overall, Walsh et al. (2006) did not discern overall differences in the magnitude or frequency of west Florida shelf red tides, or correlations with nutrient data, although the data records were sporadic and the change in total flux relative to earlier periods is unknown. Using a similar approach, Vargo et al. (2004, 2008) evaluated nutrient sources for support of blooms after initiation and transport into coastal waters and estimated that estuarine fluxes of N and P from Tampa Bay, Charlotte Harbor, and the Caloosahatchee River were sufficient to support a major portion of the nutrients needed to sustain moderate K. brevis blooms (3 × 105 cells L−1) in localized nearshore areas, but would contribute a low proportion of the nutrients needed to support larger blooms over broader areas.
Figure 4.
Available data for total and inorganic N and P concentrations for a 2 square km area at the Caloosahatchee River mouth and the extent of Everglades Agricultural Area (EAA) which is devoted to sugar cane fields for the time frame of 1949 through 2005. Nutrient data has been retrieved from the state of Florida STORET database; available data for the river from a variety of sources are listed on the figure. The change in the areal extent of the EAA has been modified from Brand (2002).
On the other hand, Brand and Compton (2007) suggested that there has been a significant increase in nutrient inputs to the nearshore waters of west Florida over the past 50 years. Based on their analysis of the State of Florida long-term HAB database, which heavily emphasized bloom periods, they reported that higher nearshore abundance of K. brevis during blooms over the 1994–2002 timeframe, compared to the 1954–1963 timeframe, appears to be related to elevated nutrient flux into nearshore coastal waters from urban and agricultural development in the Caloosahatchee River watershed, and eutrophication of Lake Okeechobee, the headwaters for the Caloosahatchee. Turner et al. (2006) used paleo-indicators to assess long-term water quality changes in Charlotte Harbor and found evidence of a 3-fold increase in N loadings between the 1800s and the present, with significant increases in water column NO3− concentrations between 1960 and 1980 which have remained essentially constant since the 1980s (see their Fig. 3).
Walsh and Steidinger (2001) and Walsh et al. (2006) hypothesized that N2 fixation by blooms of the pelagic cyanobacterium Trichodesmium spp. and subsequent release of DON, coupled with use of remineralized nutrients from dead fish can lead to K. brevis bloom initiation, growth and maintenance on the west Florida and Texas shelves. They estimated that N2 fixation could supply up to 0.16 µM L−1 day−1 based on Trichodesmium biomass and N excretion rates measured over a 3-yr period (Mulholland et al., 2006). Subsequent decay of dead fish might then be sufficient to supply all of the required N and P for blooms on the order of 106 cells L−1 or greater. Mulholland et al. (2006) also concluded that observed N2 fixation and subsequent release of DON by Trichodesmium could provide substantial support for a moderate K. brevis bloom. A source of P would be required for the Saharan dust-Trichodesmium-dead fish hypothesis since P concentrations are extremely low in shelf waters (Vargo and Shanley, 1985; Vargo et al., 2008) and Trichodesmium spp. competes with K. brevis for available P. Further, based on stable N-isotope measurements of particulate organic matter (POM) collected during 4 years of west Florida shelf red tides, Havens et al. (2004) reported values of δ15NPOM from −2 to 6 ‰, which excludes sewage and upwelled N as a source, but does not exclude commercial fertilizers derived from atmospheric N, which have an isotopic composition close to 0 (e.g., del Amor 2008). However, the low δ15NPOM values indicative of fixed N recorded by Havens et al. (2004) were found only in samples collected in close proximity to a Trichodesmium bloom whereas the majority of bloom values (71 in 5 separate blooms) were in the range of 3 to 5 ‰. Thus N from a variety of sources appears to fuel west Florida shelf K. brevis blooms.
Mixotrophy may also provide nutrients for K. brevis blooms (e.g., Bronk et al., 2004; Jeong et al. 2005a,b; Glibert et al., submitted), as described above for K. veneficum and Pfiesteria spp. Dissolved organic N (DON) concentrations in west Florida estuaries and coastal waters are about 10-fold higher than inorganic N concentrations, and while inorganic N:P ratios are low, DON:DOP (dissolved organic P) ratios are consistently higher than the Redfield ratio (Heil et al., 2007). Notably, Hu et al. (2006) found much higher DON flux compared to DIN and DIP. Collectively these data suggest that species capable of using DON, such as K. brevis, would be favored over those relying on inorganic N sources (Heil et al., 2007). Concentrations of DON, presumably both terrestrial and marine derived, are sufficient to support blooms >105 cells L−1 but the extent to which this complex N is used by K. brevis and other Karenia spp. is unknown (Hu et al., 2006; Vargo et al., 2008). Thus, N from various sources appears to fuel west Florida shelf and nearshore K. brevis blooms and different nutrient sources may play a role in initiation versus maintenance stages of these blooms.
Hu et al. (2006) hypothesized that “submarine ground water discharge provides the “missing” nutrients that can trigger and support Karenia red-tides off west-central Florida”. They suggest that increased development and population growth has increased groundwater nutrient levels and this, in turn, contributes to longer and higher biomass west Florida shelf blooms. While Vargo et al. (2008) report elevated near-bottom nutrient concentrations, they generally detected only at the 50 m isobath during years when shelf-break upwelling due to Loop Current meanders occurred.
Elsewhere in the Gulf of Mexico, a link between eutrophication and the frequency and magnitude of K. brevis blooms along the Texas coast has been suggested (Buskey et al., 1996; Denton and Contreras, 2004; Biegalski and Villareal, 2005; Walsh et al., 2006). This linkage has been based upon data for nutrient inputs from the Mississippi River (Turner and Rabalais, 1994; Villareal et al., 2001), combined with fish kill and bloom information (Trebatoski, 1988). Walsh et al. (2006), for example, postulated that doubling of the NO3− loads in the Mississippi River every decade since the 1950’s has led to massive increases in phytoplankton biomass in the river plume. Considering measured water column and near bottom N:P ratios off both Texas and southwest Florida coastal areas where red tides are prevalent, together with modeling results (Walsh et al., 2006), remineralization of this elevated biomass leads to low N:P ratios that favor development of K. brevis blooms. Notably, the first documented bloom off Texas occurred in 1955 followed by another in 1976 and 1986, but blooms have occurred almost annually after 1996. Similarly, the increased NO3− loading from the Mississippi River coupled with reduced N:Si ratios have been presented as causative factors leading to increased abundance of potentially toxic Pseudo-nitzschia spp. in the northern Gulf of Mexico (Parsons et al., 2002).
In summary, K. brevis populations may derive nutrients as DON from the cyanobacterial blooms and other aquatic food web sources such as decomposition of fish killed by K. brevis, as well as land-based sources. Initiation and development of K. brevis in open waters along the west Florida shelf are apparently supported by sources within the aquatic food web, whereas in localized nearshore areas, land-based nutrients may also supply a proportion of the nutrients that sustain the blooms. While it is well known that eutrophication in coastal regions is increasing globally as a result of development and fertilizer use (e.g., Cloern, 2001; Glibert et al., 2005a, 2006b; Howarth, 2008; Bricker et al., 2007), clear evidence to support hypotheses about increased bloom frequency and biomass on the west Florida shelf is still not yet available. Blooms of K. brevis along the Texas coast, which are influenced by major nutrient loads from the Mississippi River, have been more clearly linked to stimulation from land-based sources, and additional experimental data will help to clarify the strength of that linkage.
2.5 ASP and PSP in California
California coastal HAB problems are dominated by two organisms: Alexandrium catenella which produces saxitoxin (STX), the causative agent of PSP, and several Pseudo-nitzschia species whose toxic strains produce domoic acid (DA), the causative agent for Amnesic Shellfish Poisoning (ASP; alternately called Domoic Acid Poisoning). While other HAB species are present, some of which are linked to nutrient loading (e.g., the dinoflagellate Lingulodinium polyedrum and the raphidophyte H. akashiwo; Kudela and Cochlan, 2000; Herndon et al., 2003), here we emphasize ASP and PSP syndromes that are regularly monitored by state agencies in California.
Unlike many other ecosystems impacted by HABs, the physical, chemical, and ecological characteristics of the coastal waters of California are largely dominated by upwelling. The boundary along the coast between the upwelled water and the warmer adjacent surface water is usually a front with an associated equatorward jet (Smith, 1992). Consequently, upwelling circulation overrides both the nutrient limitation of stratified waters and the light limitation of well-mixed waters (Hood et al., 1992), and generally nourishes these waters with macronutrients in excess of anthropogenic sources.
2.5.1 Eutrophication Linkages
ASP
Potentially toxic Pseudo-nitzschia spp. are ubiquitous in California coastal waters, and major toxin events often occur over large spatial and temporal scales (Trainer et al., 2000). Prior to 2000, toxic blooms were considered rare and unusual in southern California (Lange et al., 1994); however, in recent years ASP has become increasingly important in southern regions (e.g. Trainer et al., 2000; Busse et al., 2006; Schnetzer et al., 2007). The apparent synchrony of ASP events in California suggests that there must be large-scale forcings, such as upwelling relaxation, responsible for the otherwise coincidental timing of major DA outbreaks.
Multiple factors have been shown to trigger the production of DA by Pseudo-nitzschia (Bates, 1998), but the most thoroughly characterized are macronutrient limitation by either PO43− or silicate (Si(OH)4) in cultures (Pan et al., 1996a,b). Pseudo-nitzschia previously has been associated with both eutrophication and a reduction in the ratio of N:Si (c.f. review by Bates et al., 1998; Parsons et al., 2002), and in California there is circumstantial evidence that a massive DA event in Monterey Bay in 1998 was triggered by post-El Niño runoff (Scholin et al., 2000). Recently, Anderson et al. (2006) reported a correspondence between limiting Si concentrations, the ratios of NO3−:Si(OH)4 and PO43−:Si(OH)4 and the concentrations of Pseudo-nitzschia and particulate DA, among other factors.
DA production by Pseudo-nitzschia spp. has also been linked to iron (Fe) and copper (Cu) stress. These elements may be indirectly linked to anthropogenic changes, as excess availability of Cu is associated with runoff (Ladizinsky, 2003), and decreasing Fe is associated with modifications to stream and river flow ( Johnson et al., 2001). Limitation by Fe directly modulates Si:N ratios in diatoms, as DA may serve as an Fe-acquisition mechanism (Rue and Bruland, 2001; Wells et al., 2005). Recent laboratory and field data also demonstrate that Pseudo-nitzschia spp. may increase toxicity when using urea as an N source (Howard et al., 2006), which would come predominantly from anthropogenic inputs (Cochlan et al., 2008). Thus, cultural eutrophication may have the unanticipated consequence of both selecting for Pseudo-nitzschia spp. and promoting toxin production (Kudela et al., 2008).
Despite correlative evidence for a connection between ASP and coastal runoff and/or eutrophication, studies are, as yet, lacking to test whether there are direct linkages. In general, blooms of Pseudo-nitzschia in California occur during anomalously weak (but not absent) upwelling conditions, typically during a transition from excess to limiting macronutrients (Kudela et al., 2004). However, there is no consistent evidence that Pseudo-nitzschia blooms are correlated with runoff events, nor is there direct evidence for trace-metal limitation or stimulation of DA during most blooms (Kudela et al., 2004). An apparent south-to-north trend in bloom events in coastal California waters is consistent with large-scale physical forcing, suggesting that the spatial pattern is indicative of a change in environmental conditions.
PSP
PSP toxin events associated with Alexandrium catenella occur most years (Price et al., 1991), and large-scale outbreaks appear to exhibit a northward temporal trend (Langlois and Smith, 2001). As with ASP, there are correlative links between PSP occurrences in California and cultural eutrophication, but direct linkages have not yet been established. PSP outbreaks typically initiate on the open coast, and only then move into bays and estuaries (Langlois, 2001). A consistent pattern associated with PSP events is an increase of A. catenella in offshore waters, followed by onshore transport during relaxation-favorable winds, with subsequent intoxication of shellfish (Price et al., 1991; Langlois and Smith, 2001). Thus, as with ASP, PSP events appear to be correlated with large-scale oceanographic events, in particular the upwelling-relaxation cycle and the onshore transport of toxic cells.
In summary, both PSP and ASP dynamics in California appear to be dominated by large-scale oceanographic forcings in nutrient dynamics. This does not, however, preclude the possibility that the growth of these algae, their toxicity, and the frequency or duration of toxic events may be exacerbated by anthropogenic nutrient inputs once these populations reach nearshore waters. For example, there is both direct (Cochlan et al., 2008) and indirect (Collos et al., 2004) evidence that some toxic strains of Alexandrium catenella and Pseudo-nitzschia spp. can use DON for growth, such as urea (presumed to be from anthropogenic sources) that has been measured in appreciable concentrations in California nearshore waters (Cochlan et al., 2008). Although the extent to which such nutrient pulses occur during natural upwelling or runoff is not known for this region, their utilization by HAB species normally found in coastal California suggests that anthropogenic N sources could be potential factors in bloom initiation or maintenance. As with the Gulf of Maine, these localized blooms are significant since they generally occur in populated coastal zones, despite the fact that the total flux from these nutrient sources likely is a minor component in comparison to seasonal upwelling inputs along the open coast (Kudela et al., 2008).
2.6 PSP, ASP, and Heterosigma akashiwo in the Pacific Northwest
The dinoflagellate Alexandrium catenella is responsible for shellfish harvesting closures in the Pacific Northwest due to PSP toxins. The occurrence of PSP toxins in Washington shellfish was once restricted to the open coast, the Strait of Juan de Fuca and northern Puget Sound. A large bloom originating in the Whidbey Island basin in 1978 spread through large areas of central Puget Sound that were previously unaffected (Nishitani and Chew, 1988; Rensel, 1993) and since then PSP incidents have progressed southward into the remaining inlets of southern Puget Sound (Trainer et al., 2002).
More than ten Pseudo-nitzschia spp. are found in the oceanic and inland waterways of Washington. The primary species believed to cause domoic acid DA-related shellfish harvesting closures are P. pseudodelicatissima, P. cuspidata, and P. australis (Trainer et al., 2007). Since 1991, domoic acid has been a recognized problem on the outer Washington coast (e.g., Trainer and Bill, 2004), but over this same period low levels of DA have been measured in Puget Sound shellfish (Trainer et al., 1998). Puget Sound is presumed to be less susceptible to DA closures due to the absence of DA-retaining razor clams in this region.
Fish-killing blooms of the raphidophyte H. akashiwo have occurred in Puget Sound and adjacent inland waters of British Columbia, Canada for an unknown period. This alga was observed in the 1960s before marine fish farms were first installed in either region. All evidence from Puget Sound to date indicates that blooms originate in areas remote from the fish farms such as the U.S.-Canada border waters of North Puget Sound and the southern Georgia Strait or in shallow backwaters of central and northern Puget Sound. The blooms are transported by estuarine circulation (seaward moving brackish river plume) tidal action and winds to net-pen locations in Puget Sound where finfish have been killed (Rensel, 2007).
In Washington inland waterways, there is evidence that both flagellate and non-flagellate HAB species are expanding their scope and magnitude, in some cases possibly due to anthropogenic influences (below). Most of this area is naturally replete with high levels of DIN (10 to 25 µM) due to oceanic upwelling and advection into inland marine waters, but there are seasonally nutrient-sensitive backwaters where HAB development may be related to human-caused perturbations as discussed below.
2.6.1 Eutrophication Linkages
Along the open coast, episodic upwelling is the dominant source of nutrients to surface waters, and thus blooms of A. catenella and potentially toxic Pseudo-nitzschia spp. in those areas are not likely to be sensitive to anthropogenic influence. However, there are two general systems where the linkage between HABs and nutrient pollution in the Pacific Northwest should be examined more closely. Within Puget Sound and the adjacent Strait of Juan de Fuca, inorganic N concentrations are usually high year-round, and are considered saturating for phytoplankton growth except in poorly flushed terminal inlets which make up only ~7% of the area (Mackas and Harrison, 1997). Anthropogenic sources likely have contributed to the elevated NH4+ concentrations commonly found in surface waters of the region (W. Cochlan, unpubl. data; Rensel, 2007). Ambient concentrations of reduced N substrates (such as NH4+ and urea) are generally about 10-fold lower than NO3−, which is primarily of oceanic origin within Puget Sound. In the absence of directed studies to test influences of anthropogenic nutrient enrichment, linking nutrient loading to blooms of H. akashiwo remains an elusive possibility in the Pacific Northwest.
With regard to PSP, some anthropogenic stimulation may be occurring since the magnitude, frequency and geographical distribution of shellfish bed closures from PSP toxins have increased over the past 50 years since monitoring began (Trainer et al., 2003). Comparison of maximum yearly PSP toxin values for Puget Sound (averaged for each of the last 5 decades using all shellfish monitoring data) with population estimates of the counties bordering Puget Sound shows a high correlation. The data suggest that increased nutrient loadings to Puget Sound may be contributing to the spatial and temporal increases in PSP in nutrient-sensitive regions that are vertically stratified in the summer months and poorly flushed.
For the occurrence of DA closures, there also may be a linkage to anthropogenic nutrients within Puget Sound. Several species of Pseudo-nitzschia are present in Puget Sound and have been for decades (Bill et al., 2006; Horner, 2003), but only since 2003 has their toxicity resulted in shellfish harvesting closures (Bill et al., 2006; Trainer et al., 2007). One possibility is that more potentially toxic strains have entered Puget Sound from offshore initiation sites such as the Juan de Fuca Eddy (Trainer et al., 2002) via occasional summertime reverse flow of surface waters along the south shore of the Strait of Juan de Fuca. It is also possible that environmental factors within the inland waters are stimulating Pseudo-nitzschia growth and/or DA production as discussed below.
Aquaculture activities (e.g., fish farms) have been suggested to be stimulatory to the raphidophycean flagellate H. akashiwo and eutrophication has been linked to H. akashiwo blooms in various locations worldwide (e.g., Wang et al., 2008). In Puget Sound, however, where fish farms are few in number, the evidence is to the contrary. Salmon excrete mostly NH3 and low levels of urea (Brett and Zala, 1975), but elevated concentrations of reduced N substrates have not been measured downstream of the fish farms in Puget Sound. NH4+ found within the pens is partly oxidized to NO3− and diluted within a few meters downstream so that the DIN increase represents only about 5% of the high background flux levels at farm sites (Rensel, 1991).
All commercial fish farms in Puget Sound are, by state aquatic lease requirement, located in non-nutrient sensitive (N-replete) areas (SAIC, 1986) where sunlight, not macronutrients, limits phytoplankton productivity. Certain subareas of Puget Sound are seasonally nutrient-sensitive either by short-term, river-induced vertical stratification during the spring or by summer and fall solar-heating induced vertical stratification of backwaters that allows nutrient stripping of DIN by algae in the surface mixed layer. As H. akashiwo cells can migrate to sub-surface, nutrient-rich waters, they might potentially have a competitive advantage over non-motile species. Only some blooms (e.g., Rensel, 1995) occur in such conditions, however, whereas others occur, or at least are transported through, moderately N-depleted mixed layers (Rensel, 2007). The geographic scale of many blooms of H. akashiwo (affecting nearly all of Puget Sound in several cases), the brevity of the blooms (a few days to at most a week in duration), and the apparent dependency on warm weather periods and physical transport mechanisms all highlight the importance of naturally occurring physical forcing factors (Rensel, 2007).
Isolates of both H. akashiwo and P. cuspidata from the Pacific Northwest can grow equally well on NH4+ and NO3− when supplied as the sole N source (Auro et al., 2006; Herndon and Cochlan, 2007; Auro, 2007), so there is a potential for anthropogenic stimulation of these blooms. For example, several H. akashiwo blooms in central Puget Sound have co-occurred precisely with summertime municipal sewage spills into nutrient-sensitive backwater areas in Kitsap County, Washington (Rensel, 2007). Likewise,Trainer et al. (2007) suggested that a toxic bloom of P. pseudodelicatissima developed following a pulse of inorganic N (up to 13 µM NO3− and 13 µM NH4+) after weeks of N-limited conditions in Sequim Bay in 2005. They speculated that a failing septic system was the source of the elevated NH4+ concentration in this poorly flushed bay.
In summary, anthropogenic sources of nutrients are considered insignificant for HABs along the outer coast of Washington, but within Puget Sound and the Strait of Juan de Fuca, the potential contribution of pollution-related loading to HAB development remains to be fully understood and quantified. The ability of H. akashiwo and Pseudo-nitzschia spp. to achieve good growth by using various N sources suggests that reduced N may contribute to their recent success. In these and other cases, the spread of a toxic HAB species into or within new areas such as Puget Sound may be linked to anthropogenic nutrient sources, but as yet directed studies to confirm or refute a link are largely lacking.
2.7 Ciguatera
Ciguatera fish poisoning (CFP) is a circumtropical seafood poisoning caused by the ingestion of marine fish (especially reef fish) that have accumulated toxins produced by species of the benthic dinoflagellate genus, Gambierdiscus, especially G. toxicus (Yasumoto et al., 1977) and possibly other co-occurring dinoflagellates (e.g., Ostreopsis llenticularis; Tosteson et al., 1986).
2.7.1 Eutrophication Linkages
The role played by nutrient enrichment in CFP remains unclear because the topic is understudied. Fewer than ten known field-based studies have measured nutrient concentrations concurrently with the abundance of ciguatera dinoflagellates, and only four of these studies were conducted for at least 1 year and reported statistical results (Yasumoto et al., 1980; Carlson and Tindall, 1985; Parsons and Preskitt, 2007). Of these, Carlson and Tindall (1985) reported a significant, positive correlation between the abundance of ciguatera dinoflagellates and nutrient concentrations. Parsons and Preskitt (2007) did not report a positive correlation in their study of six Hawaiian sites, but Gambierdiscus concentrations were highest at the site (Puako) exhibiting the highest amounts of NO3− + NO2−.
Culture-based studies also have been limited. Lechat et al. (1985) reported that growth rates of G. toxicus were greater when using higher media enrichments (ES 4%) versus more diluted enrichments (ES 1% and 2%). Durand-Clement (1986) reported that growth of G. toxicus increased when the urea concentration was increased from 0 to 1 mM, although this concentration is higher than would be expected in natural habitats. Sperr and Doucette (1996) reported that growth rates did not vary over a range of N:P ratios from 5:1 to 50:1.
The role of nutrients in toxin production is unclear as well. Durant-Clement (1986) reported no differences in toxin production across different media recipes or nutrient concentrations. In contrast, Lechat et al. (1985) indicated that cellular ciguatoxin content increased 10-fold when the metallic salt content (PII-mix) was increased 10-fold. Bomber (1987) did not discern any difference in cellular toxicity for G. toxicus grown in regular ES media and low-N ES media. Cellular toxicity also was constant over the range of N:P ratios used by Sperr and Doucette (1996), although toxin levels spiked at an N:P of 30:1 for an unknown reason.
Ciguatera dinoflagellates have two potential sources of nutrients, the water column and benthic substrata. Since they are primarily epiphytic, they can obtain nutrients from macroalgal substatra or co-occurring epiphytes (i.e., the thallisphere;Yasumoto et al., 1980; Withers, 1981). The most li kely role of eutrophication in CFP is through coral reef degradation (Bagnis, 1994). Researchers early on noted that CFP outbreaks often followed disturbances to coral reef environments (e.g., Randall, 1958; Cooper, 1964). The role of nutrient loading in coral reef degradation is complex and some reef sites appear to be more susceptible to nutrient degradation than others (Lapointe, 1999; Parsons et al., 2008). Eutrophication can cause an increase in macroalgae (Lapointe et al., 2004; Smith et al., 2001), providing more habitat for ciguatera dinoflagellates.
In summary, the limited evidence available indicates that any linkages between eutrophication and increased incidents of CFP remain inconclusive. There may be indirect linkages between eutrophication and increased growth of ciguatera dinoflagellates, but further assessment is required to determine the strength of these linkages, and the potential for direct links remains to be examined. Outbreaks of CFP often follow impacts to coral reef ecosystems of which nutrient loading can be a factor, including the expansion of macroalga-based habitat. As ciguatera dinoflagellates are benthic organisms, rigorous assessment of eutrophication linkages will require analysis of not only water-column nutrient loadings, but also of indirect effects mediated through benthic nutrient sources.
3. Conclusions
HAB events in the U.S. are diverse in many ways, spanning a wide range of algal species, poisoning syndromes, and other negative impacts. A common observation is that the initation of large-scale HABs along open coasts appears to be unrelated to anthropogenic nutrients, since nutrients supplied by upwelling or advection from offshore water masses (e.g., New England and the Pacific Northwest), or by N2 fixed from co-occurring blooms (e.g., Gulf of Mexico) can be much larger than terrestrial or atmospheric sources, although data on the diversity of nutrient sources (including organic) are often lacking in these regions. Localized, nearshore pollution effects are possible in embayments, estuaries, and sounds, and thus HAB cells delivered to these locations from open waters can be stimulated and sustained by anthropogenic inputs. In regions where HABs originate within estuaries, embayments or nearshore coasts, the role of anthropogenic nutrients is much larger, and in some cases, a dominant factor in HAB species success. Overall, there is an obvious need for additional research on the relationship between HABs and eutrophication in U.S. estuaries and coastal waters, including many species and regions not covered here. In particular, there is a need for additional information on the fluxes of nutrients (not only concentrations), the role of varying nutrient composition in HAB proliferation, and the interactions between nutrient loads and the complexity of other factors contributing to HABs.
Acknowledgements
We thank L. Brand for helping with issues relating to Karenia brevis blooms in Florida and M. Thomas for analysis of nutrient data presented in Fig. 1, and for the helpful comments of two anonymous reviewers. Support was provided through the Woods Hole Center for Oceans and Human Health (to DMA), National Science Foundation (NSF) grants OCE-9808173 and OCE-0430724 (to DMA), OCE-0234587 (to WPC), OCE04-32479 (to MLP), OCE-0138544 (to RMK), OCE-9981617 (to PMG); National Institute of Environmental Health Sciences (NIEHS) grants P50ES012742-01 (to DMA) and P50ES012740 (to MLP); NOAA Grants NA96OP0099 (to DMA), NA16OP1450 (to VLT), NA96P00084 (to GAV and CAH), NA160C2936 and NA108H-C (to RMK), NA860P0493 and NA04NOS4780241 (to PMG), NA04NOS4780239-02 (to RMK), NA06NOS4780245 (to DWT). Support was also provided from the West Coast Center for Oceans and Human Health (to VLT and WPC), USEPA Grant CR826792-01-0 (to GAV and CAH), and the State of Florida Grant S7701617826 (to GAV and CAH). This is ECOHAB contribution # 288 and # 4204 from the University of Maryland Center for Environmental Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Alexandrium tamarense and A. fundyense frequently co-occur and are considered to be varieties of the same species (Anderson et al., 1994). The name A. fundyense will be used to refer to both forms.
References
- Adolf JE, Bachvaroff T, Place AR. Does cryptophyte abundance trigger toxic Karlodinium veneficum blooms in eutrophic environments? Harmful Algae. 2008 this issue. [Google Scholar]
- Adolf JE, Stoecker DK, Harding LW. The balance of autotrophy and heterotrophy during mixotrophic growth of Karlodinium micrum (Dinophyceae) J.Plank. Res. 2006;28:737–751. [Google Scholar]
- Anderson CR, Brzenzinski MA, Washburn L, Kudela R. Circulation and environmental conditions during a toxigenic Pseudo-nitzschia australis bloom in the Santa Barbara Channel, California. Mar. Ecol. Prog. Ser. 2006;327:119–133. [Google Scholar]
- Anderson DM. Toxic algal blooms and red tides: A global perspective. In: Okaichi T, Anderson DM, Nemoto T, editors. Red Tides: Biology, Environmental Science and Toxicology. Elsevier; 1989. pp. 11–16. [Google Scholar]
- Anderson DM. Bloom dynamics of toxic Alexandrium species in the northeastern United States. Limnol. Oceanogr. 1997;42:1009–1022. [Google Scholar]
- Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries. 2002;25(4b):562–584. [Google Scholar]
- Anderson DM, Keafer BA, Kulis DM, Waters RM, Nuzzi R. An immunofluorescent survey of the brown tide chrysophyte Aureococcus anophagefferens along the northeast coast of the United States. J. Plank. Res. 1993;15:563–580. [Google Scholar]
- Anderson DM, Keafer BA, McGillicuddy DJ, Mickelson MJ, Keay KE, Libby PS, Manning JP, Mayo CA, Whittaker DK, Hickey JM, He R, Lynch DR, Smith KW. Initial observations of the 2005 Alexandrium fundyense bloom in southern New England: General patterns and mechanisms. Deep-Sea Res. II. 2005a;52(19–21):2856–2876. [Google Scholar]
- Anderson DM, Kulis DM, Doucette GJ, Gallager JC, Balech E. Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeast United States and Canada as determined by morphology, bioluminescence, toxin composition, and mating compatibility. Mar. Biol. 1994;120:467–478. [Google Scholar]
- Anderson DM, Stock CA, Keafer BA, Bronzino Nelson A, Thompson B, McGillicuddy DJ, Keller M, Matrai PA, Martin J. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep-Sea Res. II. 2005b;52(19–21):2522–2542. [Google Scholar]
- Anderson DM, Townsend DW, McGillicuddy DJ, Turner JT, editors. The ecology and oceanography of toxic Alexandrium fundyense blooms in the Gulf of Maine. Deep-Sea Res. II. 2005c;52(19–21):2365–2876. [Google Scholar]
- Aneja VP, Nelson DR, Roelle PA, Walker JT. Agricultural ammonia emissions and ammonium concentrations associated with aerosols and precipitation in the southeast United States. J. Geophys. Res. 2003;108(D4):ACD12-1–ACH12-11. [Google Scholar]
- Auro ME. M.S. Thesis. San Francisco, California, USA: San Francisco State University; 2007. Nitrogen dynamics and toxicity of the pennate diatom Pseudo-nitzschia cuspidata: A field and laboratory study; p. 91. [Google Scholar]
- Auro ME, Cochlan WP, Trainer VL. Nitrogen dynamics of Pseudo-nitzschia cuspidata from the U.S. Pacific Northwest. Twelfth International Conference on Harmful Algae; September 2006; Copenhagen, Denmark. 2006. (Abstract) [Google Scholar]
- Bagnis R. Natural versus anthropogenic disturbances to coral reefs: comparison in epidemiological patterns of ciguatera. Mem. Queensland Mus. 1994;34:455–460. [Google Scholar]
- Bates SS. Ecophysiology and metabolism of ASP toxin production. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological ecology of harmful algal blooms. Heidelberg: Springer-Verlag; 1998. pp. 405–426. [Google Scholar]
- Bates SS, Garrison DL, Horner RA. Bloom dynamics and physiology of domoic-acid-producing Pseudo-nitzschia species. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological ecology of harmful algal blooms. Heidelberg: Springer-Verlag; 1998. pp. 267–292. [Google Scholar]
- Berg GM, Glibert PM, Lomas MW, Burford M. Organic nitrogen uptake and growth by the Chrysophyte Aureococcus anophagefferens during a brown tide event. Mar. Biol. 1997;129:377–387. [Google Scholar]
- Berg GM, Repeta DJ, LaRoche J. Dissolved organic nitrogen hydrolysis rates in axenic cultures of Aureococcus anophagefferens (Pelagophyceae): comparison with heterotrophic bacteria. Appl. Environ. Microbiol. 2002;68:401–404. doi: 10.1128/AEM.68.1.401-404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg GM, Repeta DJ, LaRoche J. The role of the picoeukaryote Aureococcus anophagefferens in cycling of marine high-molecular weight dissolved organic nitrogen. Limnol. Oceanogr. 2003;48:1825–1830. [Google Scholar]
- Biegalski SR, Villareal T. Correlations between atmospheric aerosol trace element concentrations and red tide at Port Aransas, Texas, on the Gulf of Mexico. J. Radioanal. Nucl. Chem. 2005;263:997–1005. [Google Scholar]
- Bill BD, Cox FH, Horner RA, Borchert JA, Trainer VL. The first closure of shellfish harvesting due to domoic acid in Puget Sound, Washington, USA. Afr. J. Mar. Sci. 2006;28(2):437–442. [Google Scholar]
- Bodeanu N, Ruta G. Development of the planktonic algae in the Romanian Black Sea sector in 1981–1996. In: Reguera B, Blanco J, Fernandez ML, Wyatt T, editors. Harmful Algae. Paris, France: Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO; 1998. pp. 188–191. [Google Scholar]
- Bomber JW. Ph.D. dissertation. Melbourne, Fl: Department of Oceanography and Ocean Engineering, Florida Institute of Technology; 1987. Ecology, genetic variability, and physiology of the ciguatera-causing dinoflagellate Gambierdiscus toxicus Adachi et Fukuyo; p. 147. [Google Scholar]
- Brand LE. The transport of terrestrial nutrients to south Florida coastal waters. In: Porter JW, Porter KG, editors. The Everglades, Florida Bay and Coral Reefs of the Florida Keys, An Ecosystem Sourcebook. Boca Raton, Fl: CRC Press; 2002. pp. 361–413. [Google Scholar]
- Brand LE, Compton A. Long-term increase in Karenia brevis abundance along the Southwest Florida Coast. Harmful Algae. 2007;6(2):232–252. doi: 10.1016/j.hal.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett JR, Zala CA. Daily patterns of nitrogen excretion and oxygen consumption of sockeye salmon (Oncorhynchus nerka) under controlled conditions. J. Fish. Res. Board Can. 1975;32:2479–2486. [Google Scholar]
- Bricker SB, Longstaff B, Dennison W, Jones A, Boicourt K, Wicks C, Woerner J. A decade of change. NOAA Coastal Ocean Program Decision Analysis Series No. 26. Silver Spring, MD: National Centers for Coastal Ocean Service; 2007. Effects of nutrient enrichment in the Nation’s estuaries: A decade of change; p. 328. [Google Scholar]
- Bricker SB, Longstaff B, Dennison W, Jones A, Boicourt K, Wicks C, Woerner J. Effects of nutrient enrichment in the Nation’s estuaries: A decade of change. Harmful Algae. 2008 this issue. [Google Scholar]
- Bronk DA, Sanderson MP, Mulholland MR, Heil CA, O’Neil JM. Organic and inorganic nitrogen uptake kinetics in field populations dominated by Karenia brevis. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002. St. Petersburg, Florida: Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commissionof UNESCO; 2004. pp. 80–82. [Google Scholar]
- Burkholder JM. Implications of harmful microalgae and heterotrophic dinoflagellates in management of sustainable marine fisheries. Ecol. Appl. 1998;8:S37–S62. [Google Scholar]
- Burkholder JM, Glasgow HB. Pfiesteria piscicida and other Pfiesteria-like dinoflagellates: behavior, impacts and environmental controls. Limnol. Oceanogr. 1997;42:1052–1075. [Google Scholar]
- Burkholder JM, Dickey DA, Kinder C, Reed RE, Mallin MA, Melia G, McIver MR, Cahoon LB, Brownie C, Deamer N, Springer J, Glasgow H, Toms D, Smith J. Comprehensive trend analysis of nutrients and related variables in a large eutrophic estuary: A decadal study of anthropogenic and climatic influences. Limnol. Oceanogr. 2006;51:463–487. [Google Scholar]
- Burkholder JM, Glasgow HB, Deamer-Melia NJ. Overview and present status of the toxic Pfiesteria complex. Phycologia. 2001;40:186–214. [Google Scholar]
- Burkholder JM, Glibert PM, Skelton HM. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae. 2008 this issue. [Google Scholar]
- Burkholder JM, Gordon AS, Moeller PD, Law JM, Coyne KJ, Lewitus AJ, Ramsdell JS, Marshall HG, Deamer NJ, Cary SC, Kempton JW, Morton SL, Rublee PA. Demonstration of toxicity to fish and to mammalian cells by Pfiesteria species: Comparison of assay methods and multiple strains. Proc. Natl. Acad. Sci. (U.S.A) 2005;102:3471–3476. doi: 10.1073/pnas.0500168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder JM, Mallin MA, Glasgow HB, Jr, Larsen LM, McIver MR, Shank GC, Deamer-Melia N, Briley DS, Springer J, Touchette BW, Hannon EK. Impacts to a coastal river and estuary from rupture of a large swine waste holding lagoon. J. Env. Qual. 1997;26:1451–1466. [Google Scholar]
- Buskey EJ, Liu H, Collumb C, Bersano JGF. The decline and recovery of a persistent Texas brown tide algal bloom in the Laguna Madre (Texas, USA) Estuaries. 2001;24:337–346. [Google Scholar]
- Buskey EJ, Stewart J, Peterson J, Collumb C. Tex. Nat. Resour. Conserv. Comm. Austin: Rep. CCBNEP-07; 1996. Current status and historical trends of brown tide and red tide phytoplankton blooms in the Corpus Christi Bay National Estuary Program study area; p. 174. [Google Scholar]
- Busse LB, Venrick EL, Antrobus R, Miller PE, Vigilant V, Silver MW, Mengelt C, Mydlarz L, Prezelin BB. Domoic acid in phytoplankton and fish in San Diego, CA, USA. Harmful Algae. 2006;5:91–101. [Google Scholar]
- Carlson RD, Tindall DR. Distribution and periodicity of toxic dinoflagellates in the Virgin Islands. In: Anderson DM, White AW, Baden DG, editors. Toxic Dinoflagellates. New York: Elsevier Science Publishing Co., Inc.; 1985. pp. 171–177. [Google Scholar]
- Cloern JE. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 2001;210:223–253. [Google Scholar]
- Cochlan WP, Herndon J, Kudela RM. Inorganic and organic nitrogen uptake by the toxigenic diatom Pseudo-nitzschia australis (Bacillariophyceae) Harmful Algae. 2008 this issue. [Google Scholar]
- Collos Y, Gagne C, Laabir M, Vaquer A, Cecchi P, Souchu P. Nitrogenous nutrition of Alexandrium catenella (Dinophyceae) in cultures and in Thau lagoon, southern France. J. Phycol. 2004;40:96–103. [Google Scholar]
- Cooper MJ. Ciguatera and other marine poisoning in the Gilbert Islands. Pac. Sci. 1964;18:411–440. [Google Scholar]
- Cosper EM, Dennison W, Milligan A. An examination of environmental factors important to initiating and sustaining "brown tide" blooms. In: Cosper EM, Bricelj VM, Carpenter EJ, editors. Novel phytoplankton blooms: Causes and impacts of recurrnet brown tides and other unusual blooms. Berlin: Springer-Verlag; 1989. pp. 317–340. [Google Scholar]
- Deeds JR, Terlizzi DE, Adolf JE, Stoecker DK, Place AR. Toxic activity from cultures of Karlodinium micrum (=Gyrodinium galatheanum) (Dinophyceae) - a dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae. 2002;1:169–189. [Google Scholar]
- del Amor FM, Navarro J, Aparicio PM. Isotopic discrimination as a tool for organic farming certification in sweet pepper. J. Env. Qual. 2008;37:182–185. doi: 10.2134/jeq2007.0329. [DOI] [PubMed] [Google Scholar]
- Denton W, Contreras C. Water Qual. Tech. Ser. WQTS-2004-01. Austin: Tex. Parks and Wildlife Dept.; 2004. The red tide (Karenia brevis) bloom of 2000. [Google Scholar]
- Deonarine SN, Gobler CJ, Lonsdale DJ, Caron DA. The role of zooplankton the occurrence of harmful brown tide blooms (Aureococcus anophagefferens) in US mid-Atlantic estuaries. Aquat. Microb. Ecol. 2006;44:181–195. [Google Scholar]
- Durand-Clement M. A study of toxin production by Gambierdiscus toxicus in culture. Toxicon. 1986;24:1153–1157. doi: 10.1016/0041-0101(86)90141-8. [DOI] [PubMed] [Google Scholar]
- [EPA] U.S. Environmental Protection Agency. Environmental impacts of animal feeding operations; EPA Office of Water, Standards, and Applied Sciences Division; 1998. [Google Scholar]
- [EPA] U.S. Environmental Protection Agency. Deposition of air pollutants to the Great Waters. 3rd report to the U.S. Congress, (1) Section A, Chapter 4- water quality issues related to multiple watersheds in the Neuse River Basin. 2000
- Fournier RO, Marra J, Bohrer R, Van Det M. Plankton dynamics and nutrient enrichment of the Scotian Shelf. J. Fish. Res. Board Can. 1977;34:1004–1018. [Google Scholar]
- Gastrich MD, Bell JL, Gobler CJ, Anderson OR, Wilhelm SW. Viruses as potential regulators of regional brown tide blooms caused by the alga, Aureococcus anophagefferens: a comparison of bloom years 1999–2000 and 2002. Estuaries. 2004;27:112–119. [Google Scholar]
- Giblin AE, Gaines AG. Nitrogen inputs to a marine embayment: the importance of groundwater. Biogeochem. 1990;10:309–328. [Google Scholar]
- Glasgow HB, Burkholder JM, Mallin MA, Deamer-Melia NJ, Reed RE. Field ecology of toxic Pfiesteria complex species, and a conservative analysis of their role in estuarine fish kills. Env. Health Perspect. 2001;109:715–730. doi: 10.1289/ehp.01109s5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glibert P, editor. HABs in Eutrophic Systems. Paris and Baltimore: IOC and SCOR; 2006. GEOHAB, Global Ecology and Oceanography of Harmful Algal Blooms Programme; p. 74. [Google Scholar]
- Glibert PM, Alexander J, Trice TM, Michael B, Magnien RE, Lane L, Oldach D, Bowers H. Chronic urea nitrogen loading: A correlate of Pfiesteria spp. in the Chesapeake and Coastal Bays of Maryland, USA. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002; Proceedings of the Xth International Conference on Harmful Algae; Florida Fish and Wildlife Conservation Commission and Intergovernmental Oceanographic Commission of UNESCO; 2004a. pp. 74–76. [Google Scholar]
- Glibert PM, Burkholder JM. The complex relationships between increasing fertilization of the earth, coastal eutrophication and proliferation of harmful algal blooms. In: Granéli E, Turner J, editors. Ecology of Harmful Algae. Springer; 2006. pp. 354–354. [Google Scholar]
- Glibert PM, Burkholder JM, Kana TM, Alexander J, Skelton H, Shilling C. Grazing by Karenia brevis on Synechococcus enhances its growth rate and may help to sustain blooms. Aquat. Microb. Ecol. submitted. [Google Scholar]
- Glibert PM, Burkholder JM, Parrow MW, Lewitus AJ, Gustafson D. Rates of direct uptake of nitrogen and nitrogen nutritional preferences by functional types of Pfiesteria piscicida and Pfiesteria shumwayae. Harmful Algae. 2006a;5:380–394. [Google Scholar]
- Glibert PM, Harrison J, Heil C, Seitzinger S. Escalating worldwide use of urea – a global change contributing to coastal eutrophication. Biogeochem. 2006b;77:441–463. [Google Scholar]
- Glibert PM, Heil CA, Hollander D, Revilla M, Hoare A, Alexander J, Murasko S. Evidence for dissolved organic nitrogen and phosphorus uptake during a cyanobacterial bloom in Florida Bay. Mar. Ecol. Prog. Ser. 2004a;280:73–83. [Google Scholar]
- Glibert PM, Magnien R, Lomas MW, Alexander J, Fan C, Haramoto E, Trice M, Kana TM. Harmful algal blooms in the Chesapeake and Coastal Bays of Maryland, USA: Comparisons of 1997, 1998, and 1999 events. Estuaries. 2001;24:875–883. [Google Scholar]
- Glibert PM, Mayorga E, Seitzinger S. Prorocentrum minimum tracks anthropogenic nitrogen and phosphorus inputs on a global basis: application of spatially explicit nutrient export models. Harmful Algae. 2008 this issue. [Google Scholar]
- Glibert PM, Seitzinger S, Heil CA, Burkholder JM, Parrow MW, Codispoti LA, Kelly V. The role of eutrophication in the global proliferation of harmful algal blooms. Oceanography. 2005a:198–209. [Google Scholar]
- Glibert PM, Trice TM, Michael B, Lane L. Urea in the tributaries of the Chesapeake and Coastal Bays of Maryland. Water, Air and Soil Poll. 2005b;160:229–243. [Google Scholar]
- Glibert PM, Wazniak CE, Hall M, Sturgis B. Seasonal and interannual trends in nitrogen in Maryland’s Coastal Bays and relationships with brown tide. Ecol. Appl. 2007;17(5):S79–S87. [Google Scholar]
- Gobler CJ, Boneillo GE, Debenham C, Caron DA. Nutrient limitation, organic matter cycling, and plankton dynamics during an Aureococcus anophagefferens bloom in Great South Bay, NY. Aquat. Microb. Ecol. 2004;35:31–43. [Google Scholar]
- Gobler CJ, Lonsdale DJ, Boyer GL. A synthesis and review of causes and impact of harmful brown tide blooms caused by the alga, Aureococcus anophagefferens. Estuaries. 2005;28:726–749. [Google Scholar]
- Gobler CJ, Renaghan MJ, Buck NJ. Impacts of nutrients and grazing mortality on the abundance of Aureococcus anophagefferens during a New York brown tide bloom. Limnol. Oceanogr. 2002;47:129–141. [Google Scholar]
- Gobler CJ, Sañudo-Wilhelmy SA. Effects of organic carbon, organic nitrogen, inorganic nutrients, and iron additions on the growth of phytoplankton and bacteria during a brown tide bloom. Mar. Ecol. Prog. Ser. 2001a;209:19–34. [Google Scholar]
- Gobler CJ, Sañudo-Wilhelmy SA. Temporal variability of groundwater seepage and brown tide blooms in a Long Island embayment. Mar. Ecol. Prog. Ser. 2001b;217:299–309. [Google Scholar]
- Goshorn D, Deeds J, Tango P, Poukish C, Place AR, McGinty M, Butler W, Luckett C, Magnien R. Occurrence of Karlodinium micrum and its association with fish kills in Maryland estuaries. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002; Proceedings of the Xth International Conference on Harmful Algae; Florida Fish and Wildlife Conservation Commission and Intergovernmental Oceanographic Commission of UNESCO; 2004. pp. 361–363. [Google Scholar]
- Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99. [Google Scholar]
- Harrison JH, Caraco NF, Seitzinger SP. Global patterns and sources of dissolved organic matter export to the coastal zone: results from a spatially explicit, global model. Global Biogeochem. Cycles. 2005;19:GBS406. [Google Scholar]
- Havens JJ, Heil CA, Hollander D, Vargo GA, Ault D, Murasko S, Walsh JJ. Isotopic constraints on nutrient sources supporting the 2001 Karenia brevis bloom. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002; Proceedings of the Xth International Conference on Harmful Algae; Paris. Florida Fish and Wildlife Conservation Commission and Intergovernmental Oceanographic Commission of UNESCO; 2004. pp. 32–34. [Google Scholar]
- Haywood AJ, Steidinger KA, Truby EW, Bergquist PR, Bergquist PL, Adamson J, MacKenzie L. Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. J. Phycol. 2004;40:165–179. [Google Scholar]
- Heil CA, Glibert PM, Fan C. Prorocentrum minimum (Pavillard) Schiller –A review of a harmful algal bloom species of growing worldwide importance. Harmful Algae. 2005;4:449–470. [Google Scholar]
- Heil CA, Revilla M, Glibert PM, Murasko S. Nutrient quality drives differential phytoplankton community composition on the southwest Florida shelf. Limnol. Oceanogr. 2007;52:1067–1078. [Google Scholar]
- Heil C, Truby E, Wolny J, Pigg R, Richardson B, Garrett M, Haywood A, Petrik K, Flewelling L, Stone E, Cook S, Scott P, Steidinger K, Landsberg J. The multi-species nature of the 2005 Karenia bloom in the eastern Gulf of Mexico. Proceedings 12th International Conference on Harmful Algae; Sept 4–9, 2006; Copenhagen. In press. [Google Scholar]
- Heisler J, Glibert P, Burkholder J, Anderson D, Cochlan W, Dennison W, Gobler C, Dortch Q, Heil C, Humphries E, Lewitus A, Magnien R, Marshall H, Sellner K, Stockwell D, Stoecker D, Suddleson M. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae. 2008 doi: 10.1016/j.hal.2008.08.006. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon J, Cochlan WP. Nitrogen utilization by the raphidophyte Heterosigma akashiwo: growth and uptake kinetics in laboratory cultures. Harmful Algae. 2007;6:260–270. [Google Scholar]
- Herndon J, Cochlan WP, Horner R. Heterosigma akashiwo blooms in San Francisco Bay. Interagency Ecological Program for the San Francisco Estuary Newsletter. 2003;16:46–48. [Google Scholar]
- Hood RR, Neuer S, Cowles TJ. Autotrophic production, biomass and species composition at two stations across an upwelling front. Mar. Ecol. Prog. Ser. 1992;83:221–232. [Google Scholar]
- Horner RA. Identification of some Pseudo-nitzschia species from western Washington waters. In: Bates SS, editor. Proceedings of the Eighth Canadian Workshop on Harmful Marine Algae Can. Vol. 2498. Tech. Rep. Fish. Aq. Sci.; 2003. pp. 77–79. [Google Scholar]
- Howard M, Cochlan WP, Ladizinsky N, Kudela RM. Evaluation of California isolates of Lingulodinium polyedrum for the production of yessotoxin. Afr. J. Mar. Sci. 2006;28(2):399–402. [Google Scholar]
- Howarth RW. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae. 2008 this issue. [Google Scholar]
- Howes BL, Ramsey JS, Kelley SW, Cote JM. Progress report from the 2001 and 2002 field season: water quality monitoring and hydrodynamic modeling. Massachusetts: Final Report to the Town of Orleans; 2003. Water quality and habitat health of the embayment systems of Orleans, Massachusetts; p. 50. [Google Scholar]
- Hu C, Muller-Karger FE, Swarzenski PW. Hurricanes, submarine groundwater discharge, and Florida’s red tides. Geophys. Res. Lett. 2006;33:L11601. [Google Scholar]
- Hurst JW., Jr History of paralytic shellfish poisoning on the Maine coast. In: LoCicero VR, editor. Toxic dinoflagellate blooms; Proceedings of the International Conference (lst) Massachusetts Science and Technology Foundation; 1975. pp. 525–528. [Google Scholar]
- Jaworski N. Retrospective of the water quality issues of the upper Potomac estuary. Aquatic Science. 1990;3:11–40. [Google Scholar]
- Jeong HJ, Park JY, Nho JH, Park MO, Ha JH, Seong KA, Jeng C, Seong CN, Lee KY, Yih WH. Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. Aq. Microb. Ecol. 2005a;41:1331-143. [Google Scholar]
- Jeong HJ, Yoo YD, Park JY, Song JY, Kim ST, Lee SH, Kim KY, Yih WH. Feeding by the phototrophic red-tide dinoflagellates: 5 species newly revealed and 6 species previously known to be mixotrophic. Aq. Microb. Ecol. 2005b;40:133–150. [Google Scholar]
- Johnson KS, Chavez FP, Elrod VA, Fitzwater SE, Pennington JT, Buck KR, Walz PM. The annual cycle of iron and the biological response in central California coastal waters. Geophysical Research Letters. 2001;28:1247–1250. [Google Scholar]
- Kana TM, Lomas MW, MacIntyre HL, Cornwell JC, Gobler CJ. Stimulation of the brown tide organism, Aureococcus anophagefferens, by selective nutrient additions to in situ mesocosms. Harmful Algae. 2004;3:377–388. [Google Scholar]
- Keller AA, Rice RL. Effects of nutrient enrichment on natural populations of the brown tide phytoplankton Aureococcus anophagefferens (Chrysophyceae) J. Phycol. 1989;25:636–646. [Google Scholar]
- Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC, Fisher TR, Glibert PM, Hagy JD, Harding LW, Houde ED, Kimmel DG, Miller WD, Newell RIE, Roman MR, Smith EM, Stevenson JC. Eutrophication in Chesapeake Bay: Historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 2005;303:1–29. [Google Scholar]
- Kudela RM, Cochlan WP. The kinetics of nitrogen and carbon uptake and the influence of irradiance for a natural population of Lingulodinium polyedrum (Pyrrophyta) off southern California. Aquatic. Microbial Ecol. 2000;21:31–47. [Google Scholar]
- Kudela RM, Cochlan WP, Roberts A. Spatial and temporal patterns of Pseudo-nitzschia spp. in central California related to regional oceanography. Harmful Algal Blooms 2002. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002; Proceedings of the Xth International Conference on Harmful Algae. Florida Fish and Wildlife Conservation Commission and Intergovernmental Oceanographic Commission of UNESCO; 2004. pp. 347–349. [Google Scholar]
- Kudela RM, Lane JQ, Cochlan WP. The potential role of anthropogenically derived nitrogen in the growth of harmful algae in California, USA. Harmful Algae. 2008 this issue. [Google Scholar]
- Ladizinsky N. Master’s Thesis. Monterey Bay, CA, USA: California State University; 2003. The influence of dissolved copper on the production of domoic acid by Pseudo-nitzschia species in Monterey Bay, California: laboratory experiments and field observations; p. 68. [Google Scholar]
- Lange CB, Reid FMH, Vernet M. Temporal distribution of the potentially toxic diatom Pseudo-nitzschia australis at a coastal site in Southern California. Mar. Ecol. Prog. Ser. 1994;104:309–312. [Google Scholar]
- Langlois G. Marine biotoxin monitoring in California, 1927–1999. In: RaLonde R, editor. Harmful Algae Blooms on the North American West Coast. Univ. Alaska Sea Grant College Program Report No. AK-SG-01-05. 2001. pp. 31–34. [Google Scholar]
- Langlois G, Smith P. Phytoplankton. In: Karl HA, Chin JL, Ueber E, Stauffer PH, Hendley JW III, editors. Beyond the Golden Gate—Oceanography, geology, biology and environmental issues in the Gulf of the Farallones. Vol. 1198. U.S. Geological Survey Circular; 2001. pp. 123–132. [Google Scholar]
- Lapointe BE. Simultaneous top-down and bottom-up forces control macroalgal blooms on coral reefs. Limnol. Oceanogr. 1999;44:1586–1592. [Google Scholar]
- Lapointe BE, Barile PJ, Yentsch CS, Littler MM, Littler DS, Kakuk B. The relative importance of nutrient enrichment and herbivory on macroalgal communities near Norman’s Pond Cay, Exumas Cays, Bahamas: a “natural” enrichment experiment. J. Exp. Mar. Biol. Ecol. 2004;298:275–301. [Google Scholar]
- LaRoche J, Nuzzi R, Waters R, Wyman K, Falkowski PG, Wallace DWR. Brown tide blooms in Long Island's coastal waters linked to interannual variability in groundwater flow. Glob. Change. Biol. 1997;3:397–410. [Google Scholar]
- Lechat I, Partenski F, Chungue E. Gambierdiscus toxicus: Culture and toxin production. In: Delesalle B, Galzin R, Salvat B, editors. Proceedings of the Fifth International Coral Reef Congress, Tahiti, Antenne Museum-EPHE, Moorea (French Polynesia) 1985. pp. 443–448. [Google Scholar]
- Lehman PW, Boyer G, Hall C, Waller S, Gehrts K. Distribution and toxicity of a new colonial Microcystis aeruginosa bloom in San Francisco Bay estuary, California. Hydrobiol. 2005;541:87–99. [Google Scholar]
- Lewitus A, Brock LM, Burke MK, DeMattio KAS, Wilde SB. Lagoonal stormwater detention ponds as promoters of harmful algal blooms and eutrophication along the South Carolina coast. Harmful Algae. 2008 this issue. [Google Scholar]
- Lewitus AJ, Burkholder JM, Glasgow HB, Jr, Glibert PM, Willis BM, Hayes KC. Mixotrophy and nitrogen uptake by Pfiesteria piscicida (Dinophyceae) J. Phycol. 1999a;35:1430–1437. [Google Scholar]
- Lewitus AJ, Glasgow HB, Burkholder JM. Kleptoplastidy in the toxic dinoflagellate, Pfiesteria piscicida. J. Phycol. 1999b;35:303–312. [Google Scholar]
- Lewitus AJ, Schmidt LB, Mason LJ, Kempton JW, Wilde SB, Wolny JL, Williams BJ, Hayes KC, Hymel SN, Keppler CJ, Ringwood AH. Harmful algal blooms in South Carolina residential and golf course ponds. Population Environ. 2003;24:387–413. [Google Scholar]
- Li Y, Smayda TJ. Heterosigma akashiwo (Raphidophyceae): On prediction of the week of bloom initiation and maximum during the initial pulse of its bimodal bloom cycle in Narragansett Bay. Plank. Biol. Ecol. 2000;47:80–84. [Google Scholar]
- Lomas MW, Glibert PM, Berg GM. Characterization of nitrogen uptake by natural populations of Aureococcus anophagefferens (Chrysophyceae) as a function of incubation duration, substrate concentration, light, and temperature. J. Phycol. 1996;32:907–916. [Google Scholar]
- Lomas MW, Kana TM, MacIntyre HL, Cornwell JC, Nuzzi R, Waters R. Inter-annual variability of Aureococcus anophagefferens in Quantuck Bay, Long Island: natural test of the DON hypothesis. Harmful Algae. 2004;3:389–402. [Google Scholar]
- Love RC, Loder TC, Keafer BA. Nutrient conditions during Alexandrium fundyense blooms in the western Gulf of Maine, USA. Deep-Sea Res. II. 2005;52:2450–2466. [Google Scholar]
- Lund EJ. Annual Report of the Texas Game, Fish, and Oyster Commission (1934–35) Austin: 1936. Some facts relating to the occurrences of dead and dying fish on the Texas coast during June, July, and August 1935; pp. 47–50. [Google Scholar]
- MacIntyre HL, Lomas MW, Cornwell J, Suggett DJ, Gobler CJ, Koch EW, Kana TM. Mediation of benthic-pelagic coupling by microphytobenthos: an energy- and material-based model for initiation of blooms of Aureococcus anophagefferens. Harmful Algae. 2004;3:403–437. [Google Scholar]
- Mackas DL, Harrison PJ. Nitrogenous nutrient sources and sinks in the Juan de Fuca Strait/Strait of Georgia/Puget Sound estuarine system: assessing the potential for eutrophication. Est. Coastal Shelf Sci. 1997;44:1–21. [Google Scholar]
- Magaña HA, Contreras C, Villareal TA. A historical assessment of Karenia brevis in the western Gulf of Mexico. Harmful Algae. 2003;2:163–171. [Google Scholar]
- Mallin MA. Impacts of industrial-scale swine and poultry production on rivers and estuaries. Am. Sci. 2000;88:26–37. [Google Scholar]
- Mallin MA, Cahoon LB. Industrialized animal production – a major source of nutrient and microbial pollution to aquatic ecosystems. Pop. Environ. 2003;24:369–385. [Google Scholar]
- Marshall HG, Burchardt L, Lacouture R. A review of phytoplankton composition within Chesapeake Bay and its tidal estuaries. J. Plankton Res. 2005a;27:1083–1102. [Google Scholar]
- Marshall HG, Egerton T, Burchardt L, Cerbin S, Kokocinski M. Long-term monitoring results of harmful algal populations in Chesapeake Bay and its major tributaries in Virginia, U.S.A. Oceanol. Hydrobiol. Studies. 2005b;34:35–41. [Google Scholar]
- Marshall HG, Hargraves PE, Burkholder JM, Parrow MW, Elbrächter M, Allen EH, Knowlton VM, Rublee PA, Hynes WL, Egerton TA, Remington DL, Wyatt KB, Lewitus AJ, Henrich VC. Taxonomy of Pfiesteria (Dinophyceae) Harmful Algae. 2006;5:481–496. [Google Scholar]
- Martorano CD. MS thesis, Institute for the Study of Earth, Oceans, and Space. Durham, NH: University of New Hampshire; 1997. Nutrient dynamics during blooms of Alexandrium spp. in the southwestern Gulf of Maine; p. 132. [Google Scholar]
- Moeller PDR, Beauchesne KR, Huncik KM, Davis WC, Christopher SJ, Riggs-Gelasco P, Gelasco A. Metal complexes and free radical toxins produced by Pfiesteria piscicida. Env. Sci. Technol. 2007;41:1166–1172. doi: 10.1021/es0617993. [DOI] [PubMed] [Google Scholar]
- Mulholland MR, Bernhardt PW, Heil CA, Bronk DA, O'Neil JM. Nitrogen fixation and rate of release of fixed nitrogen by Trichodesmium spp. in the Gulf of Mexico. Limnol. Oceanogr. 2006;51:1762–1776. [Google Scholar]
- Mulholland MR, Gobler CJ, Lee C. Peptide hydrolysis, amino acid oxidation, and nitrogen uptake in communities seasonally dominated by Aureococcus anophagefferens. Limnol. Oceanogr. 2002;47:1094–1108. [Google Scholar]
- Nishitani L, Chew K. PSP toxins in the Pacific coast states: monitoring programs and effects on bivalve industries. J. Shellfish Res. 1988;7:653–669. [Google Scholar]
- Nuzzi R, Waters RA. Long-term perspective on the dynamics of brown tide blooms in Long Island coastal bays. Harmful Algae. 2004;3:279–293. [Google Scholar]
- Okaichi T. Red tides in the Seto Inland Sea. In: Okaichi T, Yanagi Y, editors. Sustainable Development in the Seto Inland Sea, Japan - From the Viewpoint of Fisheries. Tokyo, Japan: Terra Scientific Publishing Company; 1997. pp. 251–304. [Google Scholar]
- Pan Y, Subba Rao DV, Mann KH. Changes in domoic acid production and cellular chemical composition of the toxigenic diatom Pseudo-nitzschia multiseries under phosphate limitation. J. Phycol. 1996a;32:371–381. [Google Scholar]
- Pan Y, Subba Rao DV, Mann KH, Brown RG, Pocklington R. Effects of silicate limitation on production of domoic acid, a neurotoxin, by the diatom Pseudonitzschia pungens f. multiseries (Hasle). I. Batch culture studies. Mar. Ecol. Prog. Ser. 1996b;131:225–233. [Google Scholar]
- Parrow MW, Burkholder JM, Deamer NJ, Zhang C. Vegetative and sexual reproduction in Pfiesteria spp. (Dinophyceae) cultured with algal prey, and inferences their classification. Harmful Algae. 2002;1:5–33. [Google Scholar]
- Parsons ML, Dortch Q, Turner RE. Sedimentological evidence of an increase Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal eutrophication. Limnol. Oceanogr. 2002;47:551–558. [Google Scholar]
- Parsons ML, Preskitt LB. A survey of epiphytic dinoflagellates from the coastal waters of the island of Hawai‘i. Harmful Algae. 2007;6:658–669. [Google Scholar]
- Parsons ML, Walsh WJ, Settlemier CJ, White DJ, Ballauer JM, Ayotte PM, Osada KM, Carman B. A multivariate assessment of the coral ecosystem health of two embayments on the lee of the island of Hawai‘i. Mar. Poll. Bull. 2008;56:1138–1149. doi: 10.1016/j.marpolbul.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Phlips EJ, Badylak S, Lynch TC. Blooms of the picoplanktonic cyanobacterium Synechococcus in Florida Bay, a subtropical inner-shelf lagoon. Limnol. Oceanogr. 1999;44:1166–1175. [Google Scholar]
- Popels LC, Coyne KJ, Forbes R, Pustizzi F, Gobler CJ, Cary SC, Hutchins DA. The use of quantitative polymerase chain reaction for the detection and enumeration of the harmful alga Aureococcus anophagefferens in environmental samples along the United States east coast. Limnol. Oceanogr. Methods. 2003;1:92–102. [Google Scholar]
- Portnoy JW, Nowicki BL, Roman CT, Urish DW. The discharge of nitrate-contaminated groundwater from developed shoreline to marsh-fringed estuary. Water Resources Res. 1998;34(11):3095–3104. [Google Scholar]
- Poulton NJ, Keafer BA, Anderson DM. Toxin variability in natural populations of Alexandrium fundyense in Casco Bay, Maine – evidence of nitrogen limitation. Deep-Sea Res. II. 2005;52(19–21):2501–2521. [Google Scholar]
- Price DW, Kizer KW, Hansgen KH. California’s paralytic shellfish poisoning prevention program, 1927–1989. J. Shellfish. Res. 1991;10:119–145. [Google Scholar]
- Randall JE. A review of ciguatera, tropical fish poisoning with a tentative explanation of its cause. Bull. Mar. Sci. Gulf Carib. 1958;8:236–267. [Google Scholar]
- Rensel JE. Prepared by Parametrix, Battelle Northwest Laboratories and Rensel Associates for the State of Washington Departments of Ecology, Fisheries and Natural Resources. 1991. Phytoplankton and nutrient studies near salmon net-pens at Squaxin Island, Washington. Technical appendices of Programmatic Environmental Impact Statement: Fish culture in floating net-pens; p. 161. appendices. [Google Scholar]
- Rensel JE. Factors controlling Paralytic Shellfish Poisoning in Puget Sound. J. Shellfish Res. 1993;12(2):371–376. [Google Scholar]
- Rensel JE. Harmful algal blooms and finfish resources in Puget Sound. In: Robichaud E, editor. Puget Sound Research. Volume 1. Olympia, Washington: Puget Sound Water Quality Authority; 1995. pp. 442–429. [Google Scholar]
- Rensel JE. Prepared by Rensel Associates Aquatic Sciences for the National Oceanic and Atmospheric Administration Center for Sponsored Coastal Ocean Research (CSCOR) Washington, D.C.: 2007. Fish kills from the harmful alga Heterosigma akashiwo in Puget Sound: Recent blooms and review; p. 58. ( http://www.whoi.edu/fileserver.do?id=39383&pt=2&p=29109>http://www.whoi.edu/fileserver.do?id=39383&pt=2&p=29109) [Google Scholar]
- Riley GA. The plankton of estuaries. In: Lauff GH, editor. Estuaries. Vol. 83. Washington, D.C.: Amer. Assoc. Adv. Sci., Publ.; 1967. pp. 316–326. [Google Scholar]
- Rothschild BJ, Ault JS, Goulletquer P, Heral M. The decline of the Chesapeake Bay oyster population: A century of habitat destruction and overfishing. Mar. Ecol. Progr. Ser. 1994;111(1–2):29–39. [Google Scholar]
- Rothenberger MB. Ph.D. dissertation. Raleigh, NC: Department of Plant Biology, North Carolina State University; 2007. Long-term Impacts of Changing Land-use Practices on Water Quality and Phytoplankton Assemblages in the Neuse Estuary Ecosystem, North Carolina. [Google Scholar]
- Rothenberger MB, Burkholder JM, Brownie C. Long-term effects of changing land use practices on surface water quality in a coastal river and lagoonal estuary. Environ. Manag. doi: 10.1007/s00267-009-9330-8. Submitted. [DOI] [PubMed] [Google Scholar]
- Rue E, Bruland K. Domoic acid binds iron and copper: a possible role for the toxin produced by the marine diatom Pseudo-nitzschia. Mar. Chem. 2001;76:127–134. [Google Scholar]
- [SAIC] Science Applications International Corp. Recommended interim guidelines for the management of salmon net-pen culture in Puget Sound. Olympia WA: Washington Dept. Energy 87-5, Ecology No. C-0087110; 1986. p. 48. [Google Scholar]
- Schnetzer A, Miller PE, Schaffner RA, Stauffer BA, Jones BH, Weisberg SB, DiGiacomo PM, Berlson WM, Caron DA. Blooms of Pseudo-nitzschia and domoic acid in the San Pedro Channel and Los Angeles harbor areas of the Southern California Bight, 2003–2004. Harmful Algae. 2007;6:372–387. [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Van Dolah FM. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature. 2000;403:80–84. doi: 10.1038/47481. [DOI] [PubMed] [Google Scholar]
- Seitzinger SP, Harrison JA, Dumont E, Beusen AHW, Bouwman AF. Sources and delivery of carbon, nitrogen and phosphorous to the coastal zone: An overview of global nutrient export from watersheds (NEWS) models and their application. Global Biogeochem. Cycles. 2005;19:GB4S09. [Google Scholar]
- Smayda TJ. Primary production and the global epidemic of phytoplankton blooms in the sea: a linkage? In: Cosper EM, Carpenter EJ, Bricelj M, editors. Novel Phytoplankton Blooms: Causes and Impacts of Recurrent Brown Tide and Other Unusual Blooms. Berlin: Springer-Verlag; 1989. pp. 213–228. [Google Scholar]
- Smith JE, Smith CM, Hunter CL. An experimental analysis of the effects of herbivory and nutrient enrichment on benthic community dynamics on a Hawaiian reef. Coral Reefs. 2001;19:332–342. [Google Scholar]
- Smith RL. The physical processes of coastal ocean upwelling systems. In: Summerhayes CP, Emeis K-C, Angel MV, Smith RL, Zeitzschel B, editors. Upwelling in the ocean: Modern processes and ancient records. John Wiley and Sons Ltd.; 1992. pp. 39–64. [Google Scholar]
- Sperr AE, Doucette GJ. Variation in growth rate and ciguatera toxin production among geographically distinct isolates of Gambierdiscus toxicus. In: Yasumoto T, Oshima Y, Fukuyo Y, editors. Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO; 1996. pp. 309–312. [Google Scholar]
- Springer JJ, Burkholder JM, Glasgow HB, Glibert PM, Reed RE. Use of a real-time monitoring network (RTRM) and shipboard sampling to characterize a dinoflagellate bloom in the Neuse Estuary, North Carolina, U.S.A. Harmful Algae. 2005;4:553–574. [Google Scholar]
- Steel J, editor. Status and Trends Report of the Albemarle-Pamlico Estuarine Study. Raleigh: NC DEHNR and U.S. EPA National Estuarine Program; 1991. [Google Scholar]
- Stoecker DK. Mixotrophy among dinoflagellates. J. Eukaryot. Microbiol. 1999;46:397–401. [Google Scholar]
- Tango P, Butler W, Wazniak C. Assessment of harmful algae bloom species in the Maryland Coastal Bays. In: Wazniak C, Hall M, editors. Maryland’s Coastal Bays Ecosystem Health Assessment 2004. Annapolis, MD: Maryland Department of Natural Resources; 2004. http://www.dnr.state.md.us/coastalbays/sob_2004.html. [Google Scholar]
- Tango PJ, Magnien R, Butler W, Luckett C, Luckenbach M, Lacouture R, Poukish C. Impacts and Potential Effects due to Prorocentrum minimum blooms in Chesapeake Bay. Harmful Algae. 2005;4(3):525–531. [Google Scholar]
- Tester PA, Steidinger KA. Gymnodinium breve red tide blooms: Initiation, transport, and consequences of surface circulation. Limnol. Oceanogr. 1997;42:1039–1051. [Google Scholar]
- Thessen A, Stoecker D. Distribution, abundance, and domoic acid analysis of the toxic diatom genus Pseudo-nitzschia from the Chesapeake Bay. Estuaries and Coasts. 2008 [Google Scholar]
- Tosteson TR, Ballantine DL, Tosteson CG, Bardales AT, Durst HD, Higerd TB. Comparative toxicity of Gambierdiscus toxicus, Ostreopsis cf. lenticularis, and associated microflora. Mar. Fish. Rev. 1986;48:57–59. [Google Scholar]
- Townsend DW. Sources and cycling of nitrogen in the Gulf of Maine. J. Mar. Sys. 1998;16:283–295. [Google Scholar]
- Townsend DW, Pettigrew NR, Thomas AC. On the nature of Alexandrium fundyense blooms in the Gulf of Maine. Deep-Sea Res. II. 2005;52:2603–2630. [Google Scholar]
- Townsend DW, Thomas AC, Mayer LM, Thomas M, Quinlan J. Oceanography of the northwest Atlantic continental shelf. In: Robinson AR, Brink KH, editors. The Sea. Vol. 14A. Cambridge MA: Harvard University Press; 2006. pp. 119–168. [Google Scholar]
- Trainer VL, Adams NG, Bill BD, Anulacion BF, Wekell JC. Concentration and dispersal of a Pseudo-nitzschia bloom in Penn Cove, Washington, USA. Nat. Toxins. 1998;6:1–13. doi: 10.1002/(sici)1522-7189(199805/08)6:3/4<113::aid-nt14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Trainer VL, Bill BD. Characterization of a domoic acid binding site from Pacific razor clam. Aquat. Toxicol. 2004;69:125–132. doi: 10.1016/j.aquatox.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Trainer VL, Adams NG, Bill BD, Stehr CM, Wekell JC. Domoic acid production near California coastal upwelling zones, June 1998. Limnol. Oceanogr. 2000;45:1818–1833. [Google Scholar]
- Trainer VL, Cochlan WP, Erickson A, Bill BD, Cox FH, Borchert JA, Lefebvre KA. Recent domoic acid closures of shellfish harvest areas in Washington State inland waterways. Harmful Algae. 2007;6:449–459. [Google Scholar]
- Trainer VL, Eberhart B-TL, Wekell JC, Adams NA, Hanson L, Cox F, Dowell J. Paralytic shellfish toxins in Puget Sound, Washington State. J.Shellfish Res. 2003;22(1):213–224. [Google Scholar]
- Trainer VL, Hickey BM, Horner RA. Biological and physical dynamics of domoic acid production off the Washington U.S.A. coast. Limnol. Oceanogr. 2002;47:1438–1446. [Google Scholar]
- Trebatoski B. Rep. 88-02. Austin, TX: Tex Water Comm.; 1988. Observations on the 1986–1987 Texas red tides Ptychodiscus brevis; p. 48. [Google Scholar]
- Trice TM, Glibert PM, Lea C, Van Heukelem L. HPLC pigment records provide evidence of past blooms of Aureococcus anophagefferens in the Coastal Bays of Maryland and Virginia, USA. Harmful Algae. 2004;3:295–304. [Google Scholar]
- Turner RE, Rabalais NN. Coastal eutrophication near the Mississippi river delta. Nature. 1994;368:619–621. [Google Scholar]
- Turner RE, Rabalais NN, Fry B, Atilla N, Milan CS, Lee JM, Normandeau C, Oswald TA, Swenson EM, Tomasko DA. Paleo-indicators and water quality change in the Charlotte Harbor estuary (Florida) Limnol. Oceanogr. 2006;51:518–533. [Google Scholar]
- Tyler MA, Seliger HH. Annual subsurface transport of a red tide dinoflagellate to its bloom area: water circulation patterns and organism distributions in the Chesapeake Bay. Limnol. Oceanogr. 1978;23:227–246. [Google Scholar]
- Vargo GA, Shanley E. Alkaline phosphatase activity in the red tide dinoflagellate Ptychodiscus brevis. PSZN I: Mar. Ecol. 1985;6:251–262. [Google Scholar]
- Vargo GA, Heil CA, Ault DN, Neely MB, Murasko S, Havens J, Lester KM, Dixon LK, Merkt R, Walsh J, Weisberg R, Steidinger KA. Four Karenia brevis blooms: A comparative analysis. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002; Proceedings of the Xth International Conference on Harmful Algae; Paris. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography and Intergovernmental Oceanographic Commission of UNESCO; 2004. pp. 14–16. [Google Scholar]
- Vargo GA, Heil CA, Fanning KA, Dixon LK, Neely MB, Lester K, Ault D, Murasko S, Havens J, Walsh J, Bell S. Nutrient availability in support of Karenia brevis blooms on the central West Florida Shelf: What keeps Karenia blooming? Cont. Shelf Res. 2008;28:73–98. [Google Scholar]
- Villareal TA, Brainard MA, McEachron LW. Gymnodinium breve (Dinophyceae) in the western Gulf of Mexico: Resident versus advected populations as a seed stock for blooms. In: Hallegraeff GM, Blackburn SI, Bolch CJ, Lewis RJ, editors. Harmful Algal Blooms 2000. Paris: Intergovenrmental Oceanographic Commission of UNESCO; 2001. pp. 153–156. [Google Scholar]
- Walsh JJ, Steidinger KA. Saharan dust and Florida red tides: the cyanophyte connection. J. Geophys. Res. 2001;106:11597–11612. [Google Scholar]
- Walsh JJ, Jolliff JK, Darrow BP, Lenes JM, Milroy SP, Dieterle DA, Carder KL, Chen FR, Vargo GA, Weisberg RH, Fanning KA, Muller-Karger FE, Steidinger KA, Heil CA, Prospero JS, Lee TN, Kirkpatrick GJ, Whitledge TE, Stockwell DA, Tomas CR, Villareal TA, Jochens AE, Bontempi PS. Red tides in the Gulf of Mexico: where, when, and why? J. Geophys. Res. 2006;111:C11003. doi: 10.1029/2004JC002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tang D, He F, Fukuyo Y, Azanza RV. Occurrences of harmful algal blooms (HABs) associated with ocean environments in the South China Sea. Hydrobiol. 2008;596:79–93. [Google Scholar]
- Wazniak CE, Hall MR, Carruthers T, Sturgis R, Dennison WC. Linking water quality to living resources in a mid-Atlantic lagoon system USA. Ecol. Appl. 2007;17(5):S64–S78. [Google Scholar]
- Wells ML, Trick CG, Cochlan WP, Hughes MP, Trainer VL. Domoic acid: the synergy of iron, copper, and the toxicity of diatoms. Limnol. Oceanogr. 2005;50:1908–1917. [Google Scholar]
- Withers NW. Toxin production, nutrition, and distribution of Gambierdiscus toxicus (Hawaiian strain) In: Gomez ED, editor. Fourth International Coral Reef Symposium. Philippines: Manila; 1981. pp. 449–451. [Google Scholar]
- Yasumoto T, Inoue A, Ochi T, Fujimoto K, Oshima Y, Fukuyo Y, Adachi R, Bagnis R. Environmental studies on a toxic dinoflagellate responsible for ciguatera. Bull. Jpn. Soc. Sci. Fish. 1980;46:1397. [Google Scholar]
- Yasumoto T, Nakajima I, Bagnis R, Adachi R. Finding of a dinoflagellate as a likely culprit of ciguatera. Bull. Jpn. Soc. Sci. Fish. 1977;43:1021–1026. [Google Scholar]
- Zhang Y, Fu F-X, Whereat E, Coyne KJ, Hutchins DA. Bottom-up controls on a mixed-species HAB assemblage: A comparison of sympatric Chattonella subsalsa and Heterosigma akashiwo (Raphidophytes) isolates from the Delaware Inland Bays. Harmful Algae. 2006;5:310–320. [Google Scholar]