Abstract
Spatial updating is the means by which we keep track of the locations of objects in space even as we move. Four decades of research have shown that humans and non-human primates can take the amplitude and direction of intervening movements into account, including saccades (both head-fixed and head-free), pursuit, whole-body rotations and translations. At the neuronal level, spatial updating is thought to be maintained by receptive field locations that shift with changes in gaze and evidence for such shifts have been shown in several cortical areas. These regions receive information about the intervening movement from several sources including motor efference copies when a voluntary movement is made and vestibular/somatosensory signals when the body is in motion. Many of these updating signals arise from brainstem regions that monitor our ongoing movements and subsequently transmit this information to the cortex via pathways that likely include the thalamus. Several issues of debate include (1) the relative contribution of extra-retinal sensory and efference copy signals to spatial updating, (2) the source of an updating signal for real life, three-dimensional motion that cannot arise from brain areas encoding only two-dimensional commands, and (3) the reference frames used by the brain to integrate updating signals from various sources. This review highlights the relevant spatial updating studies and provides a summary of the field today. We find that spatial constancy is maintained by a highly evolved neural mechanism that keeps track of our movements, transmits this information to relevant brain regions, and then uses this information to change the way in which single neurons respond. In this way, we are able to keep track of relevant objects in the outside world and interact with them in meaningful ways.
Keywords: oculomotor, efference copy, reference frame, pursuit, vestibular, gaze
Introduction
The world beyond our body is comprised of objects that are moving and objects that are stationary. The majority fall in the latter category, including the chairs, tables and walls that occupy the space around us. But we as humans are constantly moving, and this motion causes the visual representation of these stationary objects to move across our retinas. And yet, despite this almost constant motion, we seem extremely well able to interact with objects in our environment, picking up cups and pens with great accuracy. Our ability to do this depends on maintaining spatial constancy. That is, we can keep track of the location of objects in the environment even as we move. Without this ability, we would misperceive that objects shift with us every time we move, resulting in a world that is constantly in motion and leaving us virtually helpless if we desire to interact with it. This spatial constancy, in turn, depends on the neural computations that underlie spatial updating, a process that is critical for the day-to-day tasks and the survival of all animals.
Definition
The term updating refers to the re-examination of a set of conditions after an event or a number of events have taken place. So, if a system has a known set of conditions at time 1, and one wishes to know the set of conditions of this same system at some later time (1+n), then one requires two pieces of information in order to update correctly: (1) the initial set of conditions of the system at time 1, and (2) information about any intervening event(s) that took place in the interim.
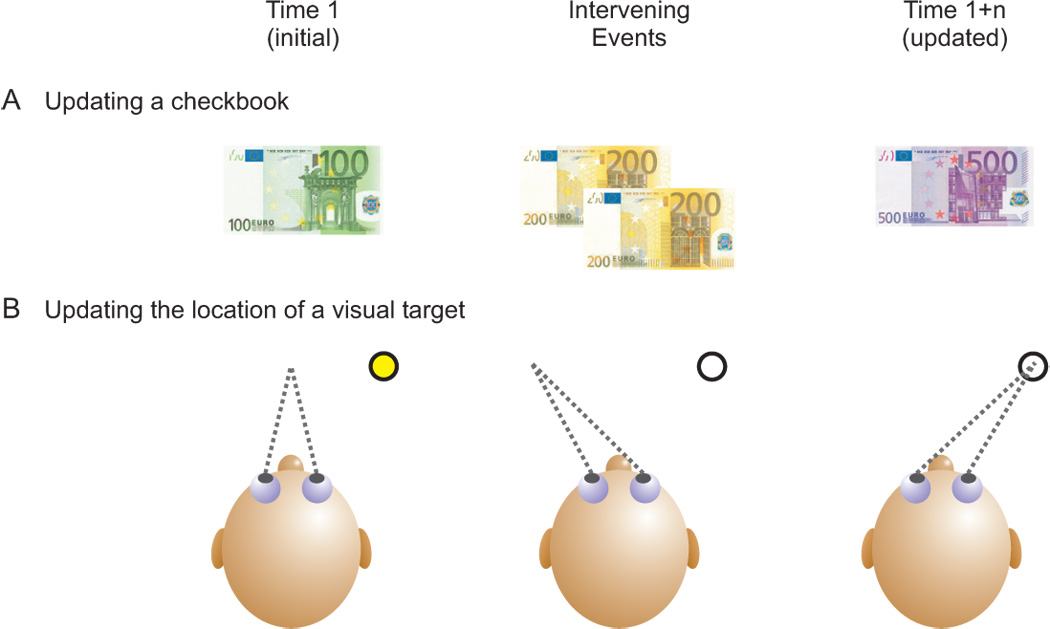
A familiar, everyday example of such a phenomenon is the process of updating a checkbook (figure 1A). If we know that last week we had €100 in our bank account (initial condition) and we would like to know how much money is currently available, we must take into consideration any credits or debits that have occurred in the intervening time period (intervening events). If, for example, a check was deposited in the amount of €400, then by combining these two pieces of information (i.e., the original balance and the credit), we can determine that there are now €500 in the account (updated condition). Here, a non-spatial entity has been updated in which the final value accurately represents the current conditions and so appropriate future (financial) decisions can be made based on this new information.
Figure 1.
Updating examples. A. Non-spatial example of updating a checkbook. An initial amount of €100 is updated to a value of €500 after a deposit of €400. B. Spatial example of updating the location of a visual target. A target initially seen 15° to the right, is updated to a location 25° to the right after an intervening eye movement of 10° to the left.
Similarly, one can also update spatial information like the location of a visual target in space (figure 1B). For example, if a person is fixating a central target and a peripheral target is briefly flashed at a location 15° to the right, then that target causes a retinal error of 15° (distance from the fovea to the loci of retinal stimulation). Executing an accurate saccade to that remembered target location is trivial. The retinal error caused by the target simply needs to be minimized to zero, as is always the case when one wishes to foveate an object in space, and thus the eyes must simply move 15° to the right (i.e., motor error = retinal error). However, what would happen if in between the time the target is flashed and the eye movement to the remembered location of the target, the subject makes a 10° movement to the left? Then a direct transformation of the original retinal error into an equivalent motor error is no longer valid. Instead, the subject must combine information about the original retinal error of the flashed target (initial condition) and the amplitude and direction of the intervening movement (intervening events) in order to determine that the flashed target is now located 25° to the right and thus necessitates a 25° eye movement (updated condition).
As with many other topics, spatial updating has been loosely defined and has thus taken on a variety of meanings. Here, we define spatial updating as the process by which a retinal signal (i.e., retinal error caused by an external object) is combined with extra-retinal information about the amplitude and direction of an intervening movement in order to produce a suitable motor error that directs the animal to the correct spatial location of an object. This process thus maintains spatial constancy (i.e., provides a continuous representation of visual space). Note that neither one of these two building blocks alone – either determining the object’s retinal error or determining the size of the intervening movement – is sufficient for this definition of spatial updating. Without intervening movements a motor error equivalent to the initial retinal error would suffice. On the other hand, information regarding intervening movements alone, without a retinal error to remap, simply shows how accurately we estimate the amplitude and direction of the these movements and not about how we use that information to determine the space-fixed location of a target. Therefore, the brain must not only determine these two independent factors, but must also combine them appropriately in order for spatial updating to take place.
Although this review focuses on spatial updating for the purpose of accurate motor control, it is important to note that several recent human studies have found accurate updating for perceptual tasks. For example, it has been shown that motion signals can be integrated after an intervening saccade as long as the motion signals are presented either in the same retinal location or the same spatial position before and after the eye movement (Melcher and Morrone 2003). In addition, transsaccadic integration studies have shown that individual visual targets presented across several saccades can be successfully integrated to form a more complex representation (Hayhoe et al. 1991), and that both visual orientation and spatial location information can be accurately retained and integrated across saccadic eye movements (Prime et al. 2006). Whereas these examples also represent important manifestations of spatial updating, here we focus on spatial updating for motor control (i.e., for the purpose of interacting with the environment)
Spatial Updating for Saccadic Eye Movements
The first studies on spatial updating were conducted by Hallett and Lightstone (1976a, 1976b) in which they designed the now classic double-step saccade task (figure 2). Here, subjects fixate a central target while two peripheral targets are briefly flashed in sequence (T1 followed by T2). The subject’s task is to first make a saccade to the first target (T1) and then make a second saccade to the second target (T2). As in the target localization example above, the saccade to the first target is simple since the motor error (ME1) must simply be equivalent to the retinal error caused by T1 (RE1). In contrast, the retinal error caused by T2 is oblique, up and to the right (RE2), however, once the eye is at T1, an equal motor error (i.e., up and to the right) will not bring the eyes to the correct location of T2 (red ME2). Instead, the brain must somehow combine the initial retinal error with the amplitude and direction of the intervening eye movement to T1 (i.e., ME1) and thereby generate an accurate eye movement that is purely vertical in direction (green ME2). Hallett and Lightstone (1976a, 1976b) found that humans could accurately perform this double-step saccade task and thus they showed that humans could update for horizontal and vertical movements of the eye.
Figure 2.
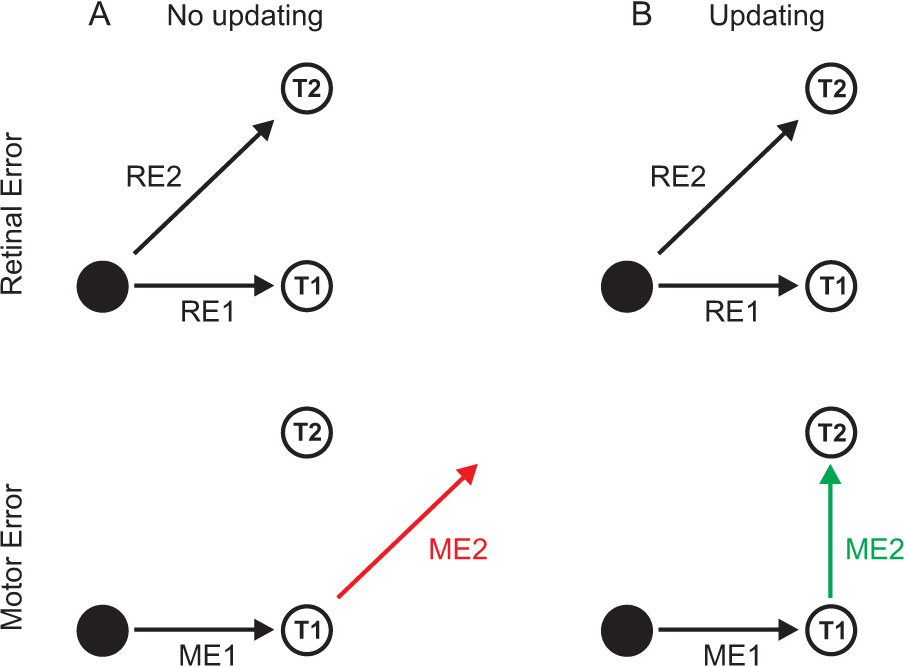
Spatial updating in a classic double-step saccade paradigm. When fixating a target (●), the retinal errors (RE1, RE2) caused by two other targets (T1, T2) are the same in both the no updating (A) and updating (B) conditions. A. Without updating, the movement to the first target (ME1) is made based on RE1, and the movement to the second target (red ME2) is made based on RE2. This leads to a mislocalization of T2. B. With proper updating, the movement to the first target (ME1) is based on RE1, but the movement to the second target (green ME2) is based both on RE2 and ME1. Taking the amplitude and direction of the first movement (ME1) into account leads to the correct localization of T2.
Evidence for spatial updating has also come from neurophysiological studies in non-human primates. Among the first were a series of studies by Mays and Sparks (Mays and Sparks, 1980, 1981; Sparks and Mays, 1983; Sparks et al., 1987) in which a monkey fixated a central target while a peripheral target was briefly flashed. The monkey’s task was simply to generate an eye movement to the remembered location of the flash, but before they could do so, the experimenters delivered a train of electrical stimulation to various brainstem nuclei that control eye movements, including the superior colliculus (SC), the horizontal burst neurons in the paramedian pontine reticular formation (PPRF) and the trochlear nerve (cranial nerve IV). This stimulation drove the animal’s eyes away from the fixation target so that now, in order to generate an accurate saccade to the remembered target location, the animal had to take into account the amplitude and direction of the stimulation-induced eye movement. They found that updating was always accurate if the stimulation was delivered to the SC (Mays and Sparks, 1980, 1981; Sparks and Mays, 1983), sometimes accurate when delivered to the PPRF (Mays and Sparks, 1981; Sparks et al., 1987) and never accurate when delivered to the nerve (Mays and Sparks, 1981). These studies were later supplemented by others with similar paradigms showing that stimulation of the frontal eye fields (FEF) (Schiller and Sandell, 1983) and the dorsomedial frontal cortex (Tehovnik and Sommer, 1996) also produced accurate saccades, but that stimulation of the abducens nucleus did not (Schiller and Sandell, 1983). Thus, the spatial updating signal for eye-only movements may arise from various cortical regions as well as from brainstem areas as close to the final motor output as the burst neurons, but not from the motoneurons or nerves that directly innervate the eye. These findings thus exclude extraocular muscle proprioception (e.g., Wang et al. 2007) and leave efference copies as the main source of the updating signal (inadvertently ignoring other potential sources as will be discussed below).
The Neural Basis of Spatial Updating for Saccadic Eye Movements
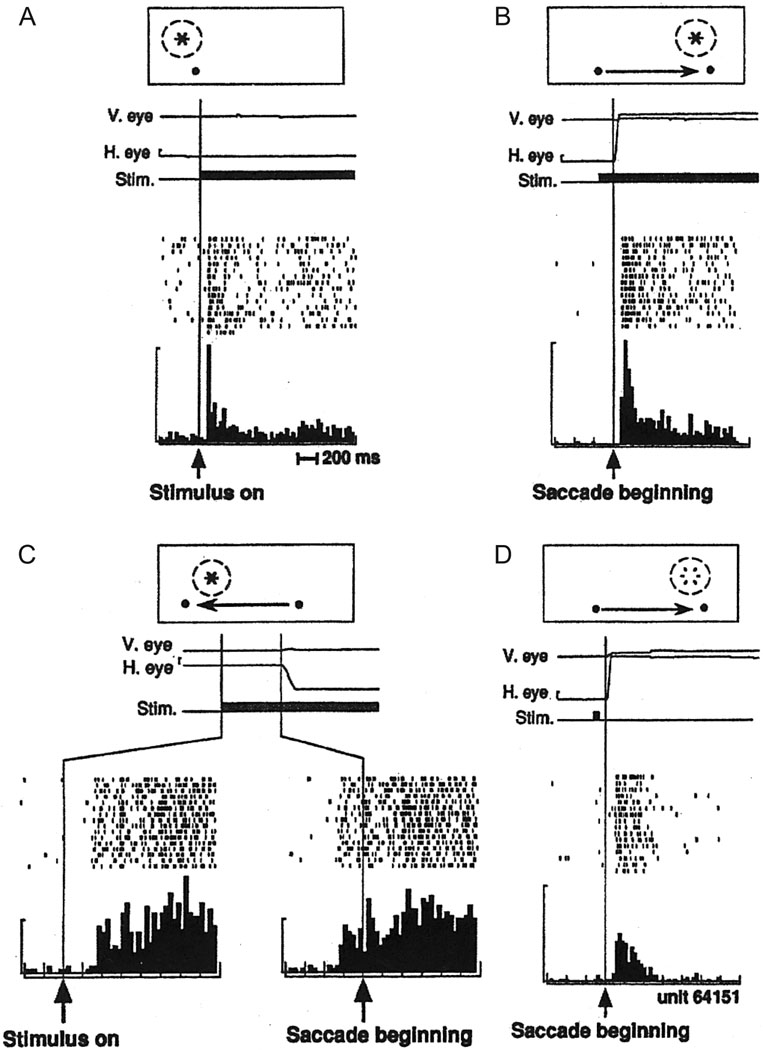
The neural substrate for spatial updating was first identified by a single-unit recording study examining this phenomenon in posterior parietal cortex area LIP (Duhamel et al., 1992a; reviewed in Colby et al., 1995). Here, the receptive field of each neuron was first identified by illuminating visual stimuli at various spatial locations. The cell would respond whenever the stimulus was turned on in this particular spatial location (figure 3A). In a subsequent set of trials, the monkey looked at a fixation point but nothing was illuminated in its receptive field. Instead, a second fixation point was turned on in the periphery and the monkeys had to make a saccade from the current fixation point to the new one. Importantly, they illuminated a visual stimulus in the cell’s future receptive field location while the animal was still looking at the first fixation point. This was possible since they knew what the amplitude and direction of the intervening saccade would be. Thus when the animal moved its eyes to the second fixation point, the receptive field location shifted such that it now encompassed the illuminated stimulus and, as expected, the cell began to fire (figure 3B). Interestingly, approximately one third of these cells began to fire even before the saccade had been initiated (figure 3C). This predictive updating could only happen if the cell had a priori knowledge of the amplitude and direction of the proceeding eye movement so that it could shift its receptive field before the eye movement took place. This phenomenon may reflect attentional mechanisms that are at work in the parietal cortex and/or a means by which to integrate visual information and maintain spatial constancy across eye movements.
Figure 3.
Neural evidence for spatial updating in LIP. A. A cell in LIP responds when a visual target falls within its receptive field. Data aligned on visual stimulus onset. B. A cell in LIP responds when a saccadic eye movement brings the cell’s receptive field onto an illuminated target. Data aligned on saccade onset. C. Some cells begin to respond to an impending shift in the cell’s receptive field even before the eye movement begins. Bottom-right raster plot and histogram are aligned on the onset of the saccade. D. Some cells respond when their receptive field shifts to a location in which a visual target was previously illuminated. Open flash symbol indicates that the stimulus was extinguished before the eyes moved. Data aligned on saccade onset. Replotted with permission from Duhamel et al., 1992a.
Finally, in the third version of their experiment (figure 3D), the monkey fixated on the first fixation point while the experimenters briefly flashed a visual stimulus in the cell’s future receptive field location. With the stimulus now extinguished, the animal made an eye movement to the second fixation point, which in turn brought the spatial location of the now extinguished flashed stimulus into the cell’s new receptive field location. The question was, would the cell respond? Logically, one would think that it should not, since the visual stimulus was no longer in the cell’s receptive field. However, the cell did respond to the memory trace of the stimulus. This could only be possible if the spatial location that was once represented in the receptive field of one cell had been transferred (or remapped) to another cell that now takes over the task of representing that particular spatial location. Such remapping could function as the neural basis of the accurate performance seen in the double step saccade task in which the spatial location of a briefly flashed target is tracked even though it is no longer present and even though intervening eye movements have occurred. Thus, the authors concluded that the brain can “…maintain a continuously accurate representation of visual space.”. Since this original study, this type of updating, at the level of single cells, has also been demonstrated in various other cortical areas (see Pathways section) and in the superior colliculus (Walker et al., 1995).
Spatial Updating during Pursuit Eye Movements
Spatial updating has also been investigated for intervening pursuit eye movements. In these paradigms, subjects are asked to pursue a moving target until a second target is briefly flashed in the periphery. The subject’s task is to make a saccade to the remembered, space-fixed location of the flashed target. But note that for a short period of time after the flash, the subject’s eyes continue to pursue the moving target, even though it is no longer present. The subject can either make an incorrect eye movement, based on the retinal error of the second target when it was flashed, or the subject can take into account the amplitude and direction of the pursuit eye movement that occurred between the flash and the saccade to the remembered target location. In the first study of this kind, it was found that monkeys were not spatially accurate, generating motor errors equivalent to the retinal errors induced by the flash (McKenzie and Lisberger, 1986). These results were later repeated with similar results in humans (Gellman and Fletcher, 1992), and so it initially seemed that spatial updating was not possible for pursuit.
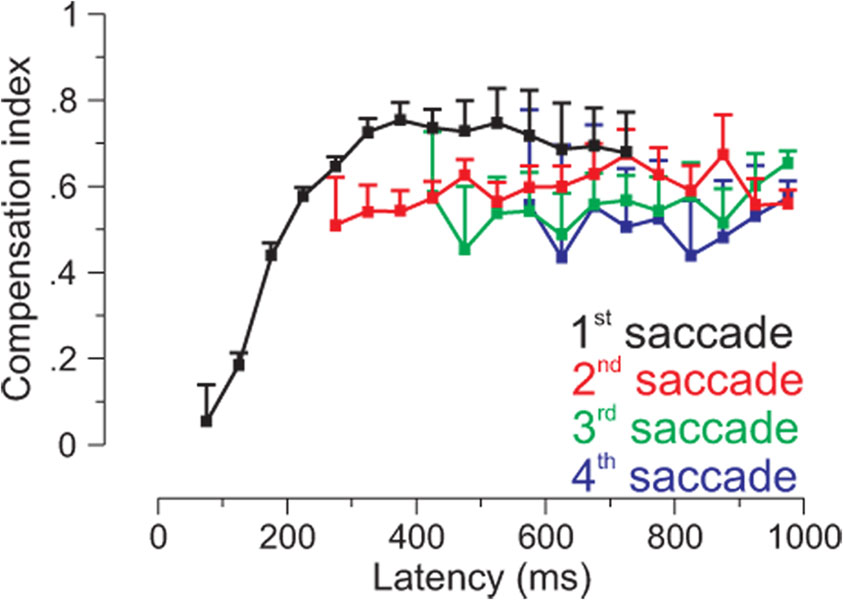
Subsequently, this issue received renewed attention, when it was discovered that monkeys could at least partially update the location of the target, especially when the duration of the flash was increased (Schlag et al., 1990). Once this was realized, several studies demonstrated accurate updating after intervening pursuit movements both with the head restrained (Baker et al. 2003; Herter and Guitton 1998; Ohtsuka 1994; Zivotofsky et al. 1996) and with the head free to move (Herter and Guitton 1998). In addition, it was found that humans generated not one, but multiple saccades to the remembered location of a flashed target after pursuit. And while the first saccade was generally in the direction of the retinal error signal, subsequent saccades did compensate for approximately 70% of the intervening pursuit (Blohm et al., 2003). Furthermore, modeling the observed updating behavior required a gain parameter close to 1 (meaning that 100% of the smooth eye displacement is accounted for), which would result in perfect updating if even more corrective saccades were to be made (Blohm et al., 2006). The fact that the first saccade could not correctly update, while later saccades could, implied that a time delay must exist before pursuit signals can be used for updating (figure 4). Further analysis indicated that short latency saccades executed <175 ms after the flash were made in the wrong direction, while those executed >175 ms after the flash could use extraretinal information to program accurate saccades (Blohm et al., 2005). The authors argued that two pathways could account for these observations, a fast, direct striatal-collicular path that only uses retinal information and a slower, indirect striatal-parietal-collicular path that could also process extraretinal information via delayed integration of efferent smooth eye velocity commands (Blohm et al., 2006). This delay for spatial updating during pursuit agrees with the timing of event-related potentials associated with updating signals in the double-step saccade task (Bellebaum et al., 2005) and with the peak in LIP activity during the single step remapping task (Heiser and Colby 2006).
Figure 4.
Updating of saccades to targets presented during pursuit. The first saccade to a visual target flashed during pursuit is inaccurate if generated with short latency, but becomes increasingly accurate with longer duration latencies. Second, third and fourth saccades are generally accurate. A compensation index of 1 indicates perfect updating. Modified with permission from Blohm et al., 1xxx.
Are Efference Copies the Source of the Extra-retinal Signal for Spatial Updating?
For saccades and pursuit, the source of the extra-retinal spatial updating signal is likely a motor efference copy [also known as corollary discharge (Sperry, 1950; Von Holst and Mittlestead, 1950)]. These signals are copies of voluntary, outgoing motor commands that are generated whenever we make a movement. For example, in order to move one’s eyes, one must generate a neural command that is transmitted down to motoneurons in the brainstem that controls the eye muscles. One can simply take a copy of this motor command and use it elsewhere in the brain for a variety of different goals, one of them being spatial updating. The stimulation studies cited above attempted to determine at what level of the saccadic pathway spatial updating efference copies can be derived. They concluded that such copies do not necessarily arise from the cortex, but can also be generated from areas much closer to the final motor output, like the superior colliculus and burst neurons (but not from the motoneurons themselves). In addition, a similar stimulation experiment to those of Mays and Sparks (see above) was conducted after the ophthalmic nerves were cut bilaterally, thereby eliminating any proprioceptive feedback from the eyes themselves. The resultant eye movements, made after electrical microstimulation to the superior colliculus, were still accurate, thus pointing to efference copies as the source of the updating signals (Guthrie et al., 1983).
The idea of efference copies also finds support in the aforementioned study by Duhamel et al. (1992a), as LIP neurons showed updating activity even before the eyes had moved to the new target (i.e., neural responses in figure 3C). Such predictive updating could only be possible if the brain had knowledge about the movement it was about to make before actually making it. And such knowledge could only be available via efference copies of the intended motor command.
The richness and versatility of the extra-retinal signal needed for updating is further elucidated in a study involving active eye-head gaze shifts during a dynamic version of the double-step saccade task (Vliegen et al., 2005). Here, subjects were instructed to make a gaze shift to a flashed target and, during that movement, a second target was briefly flashed. Once the first gaze shift was completed, they were required to make a second gaze shift to the second target. Since the second target was only visualized midway through the first gaze shift, this study was uniquely able to differentiate between inaccurate predictive updating (which must use motor efference copies available before the movement began) and more precise online updating (that uses feedback signals available only once the movement is in progress). The subjects in this study performed accurately, indicating that the information used for updating was not merely the motor efference prior to the movement, but a command appropriately modified by feedback. This updating ability was preserved in a visual-auditory dynamic double-step paradigm in which an auditory target was the final target to which subjects had to orient (Vliegen et al., 2004).
Two additional studies have attempted to make similar distinctions using an adaptation paradigm in which the first saccade of a double-step task was adapted by jumping the first target by 25% of the amplitude. Thus a target flashed at 20° became associated with a 15° saccade. Subsequently, the accuracy of the second saccade was monitored to determine if it was accurate (implying that the actual movement amplitude of 15° was used for updating) or inaccurate (implying that the initial 20° target amplitude was used). Preliminary results report that both humans (Tsotsos et al., 2007) and monkeys (Phillips et al., 2007) are able to accurately reach the location of the second target, indicating that information about the movement that was actually executed is used to update the retinal error of the second target. The apparently conflicting results of these studies begin to point to the fact that the brain maybe capable of utilizing signals form a variety of sources, including online mechanisms, for spatial updating and that it does not rely on efference copies alone. This point is strengthened in the following section.
Updating for Self-motion
While versatile efference copies may work as an updating signal for eye movements made with the head and body fixed in space (e.g., saccades and pursuit), our everyday movements typically also involve movements of the head and body. For such movements, the vestibular system, which measures the body’s inertial motion, is likely also involved. Specifically, three semicircular canals measure how the body rotates in three-dimensional space (i.e., yaw, pitch and roll), and two otolith organs (the utricle and saccule) measure how the body translates in space and how it is positioned relative to gravity.
One group of spatial updating studies involved active head and/or body movements. First, Medendorp et al. (2002) investigated whether humans can update for torsional movements of the head (i.e., rotation about the naso-occipital axis) (figure 5A). Briefly, subjects first rotated their heads on their body in either a right ear down (clockwise) or a left ear down (counterclockwise) direction. In this torted position, a peripheral target briefly flashed and subjects had to remember its space-fixed location. Once extinguished, the subjects brought their heads back to an upright orientation, at which time they attempted to foveate the remembered location of the space-fixed target. The subjects performed this task relatively well and thus it was shown that humans can update for torsional movements of the head. Subsequently, a similar study found that subjects are also quite accurate at updating after intervening lateral translational motion (i.e., side-to-side movements) (Medendorp et al., 2003). These results were also extended to pointing movements (Admiraal et al. 2004; Medendorp et al. 1999; Van Pelt and Medendorp 2007).
Figure 5.
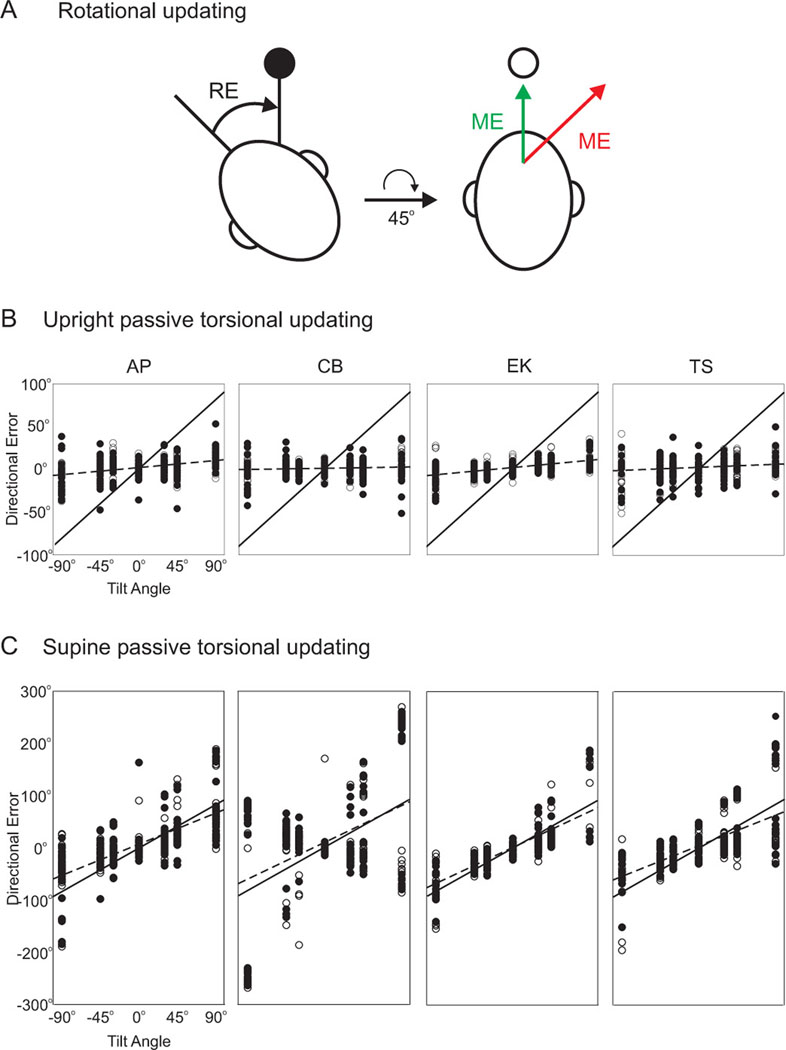
Updating for roll rotations. A. A subject in a static roll position is shown a briefly flashed target (●), and is then rotated to an upright orientations. If the motor error to the location of the remembered target is based only on the retinal error caused by the target (red ME = RE), then the target is mislocalized. If the motor error is based on both the retinal error (RE) and the amplitude and direction of the intervening roll rotation (i.e., 45° clockwise), then the target is correctly localized (green ME). B. When subjects are rolled about the naso-occipital axis from an upright orientation, saccades to remembered target locations are accurate, independent of the body tilt. The average slope across subjects was 0.07. Best fit slopes for each subject are indicated by a dashed line. A slope of 0 indicates perfect updating, while a slope of 1 (solid line) indicates no updating. Replotted with permission from Klier et al., 1995. C. When subjects are rolled about the naso-occipital axis in a supine orientation, saccades are much less accurate. The average slope across subjects was 0.70. Dashed lines and solid lines as in B. Replotted with permission from Klier et al., 1995.
These active studies could not detect which signals – vestibular or efference copy – were used as the source of the updating information because both are present during self-generated movements. Thus, to uncover if sensory (i.e., vestibular) signals can also provide the information used for updating during whole-body movements, passive movements would be particularly revealing. To this end, a number of updating experiments were conducted in which the intervening movements were passive in nature. This was possible with the use of specialized motion platforms that could either rotate or translate the subjects in space. Here, the subjects did not generate any movement on their own (in fact the amplitude and direction of the motion was randomly chosen in every trial), and thus no motor efference copies were available for spatial updating. Subjects should perform poorly in these passive updating tasks if motor efference copies are the only source of the updating signals. On the other hand, subjects should perform well if other sensory signals, like those arising from the vestibular system, are also available for maintaining spatial constancy.
An initial group of experiments examined updating after intervening yaw rotations (Blouin et al., 1995a, 1995b, 1998). They found poor updating ability which was later determined not to be due to an underestimation of the yaw rotation itself, but rather due to an inability to integrate signals from various modalities (e.g., retinal signals about target location and vestibular signals about body motion) (Blouin et al. 1995c). In fact, many experiments consistently report accurately perceived amplitudes of rotations (Bloomberg et al., 1988, 1991; Blouin et al., 1995c; Israël et al., 1995; Glasauer and Brandt, 2007) and translations (Israël et al., 1993, 1997; Berthoz et al., 1995; Siegler et al., 2000). Furthermore, Bresciani et al. (2002) found that subjects could compensate for their body rotation online by changing their arm position as they moved, implying that vestibular signals can be processed during the movement. But subjects were less accurate when pointing after the yaw movement was completed. Thus they concluded that while the brain is quite capable of monitoring ongoing whole-body movements, it might not be as good at using these monitoring signals to update internal representations of visual space for pointing.
A subsequent experiment involving intervening passive rotations about the torsional, roll axis of the head showed that, despite inter-trial variability, on average subjects are able to localize remembered, space-fixed targets quite well (Klier et al., 2005). Plots of direction errors as a function of actual body tilt indicated an average slope across subjects of 0.07, indicated good updating ability (a slope of 0 represents perfect updating, while a slope of 1 represents no updating) (figure 5B).
Notably, the fact that humans can update for torsional movements (Medendorp et al., 2002; Klier et al., 2005) highlights the underlying complexity of spatial updating. While some researchers believe that updating for horizontal and vertical motion simply involves “subtraction” of the intervening eye movement vector (figure 2 – ME1) from the retinal error vector of the remembered target (figure 2 – RE2), updating for torsion cannot be explained by such a simple mechanism. This is because a single, unique vector cannot represent this difference across all visual space when motion occurs around the line of sight (Medendorp et al., 2002). To illustrate this further, a study examined if spatial updating is still accurate after two non-commutative rotations (Klier et al., 2007). Here, subjects ended up in different final orientations relative to the remembered space-fixed target depending on the sequence of the two rotations (Tweed and Vilis, 1987), such that they were required to generate different saccades in order to correctly reach the target. Most subjects could update quite well, indicating that the brain can also take the complexities of non-commutativity into account.
These studies show that even in the absence of motor efference copies, subjects can still update relatively well for roll using only sensory cues. To further probe whether static (i.e., gravitational) or dynamic (i.e., motion) cues are critical for updating, the passive, torsional updating experiment (Klier et al., 2005) was repeated with the subjects supine rather than upright. In the supine condition, roll rotations about the same naso-occipital axis only cause dynamic changes that are detected by the semicircular canals, while the gravity vector now remains fixed (relative to the head) throughout the rotation. The results showed a ten-fold decrease in the subjects’ abilities to update. Plots of directional errors versus body tilt now indicated an average slope of 0.70 (Klier et al., 2005) (figure 5C), which implicates static, gravity-related cues as being critical for whole-body spatial updating. Note that gravity information in this task can arise from either the otolith organs or proprioceptive/somatosensory cues, which could not be dissociated in these human experiments (but see below).
However, this influence of gravity did not generalize to yaw rotations. Here, updating after passive yaw rotations was examined at various body tilt positions where the gravity vector during the rotation changed along a continuum (upright – gravity vector most static; supine – gravity vector most dynamic) (Klier et al., 2006) (figure 6A). First, as summarized earlier, yaw updating was not as good as updating for roll (average updating ratio of 0.71 for yaw versus 0.92 for roll; where a value of 1 indicates perfect updating). Second, updating ability did not depend on tilt angle (e.g., figure 6B compares yaw updating in upright versus supine conditions). Thus, while gravity cues seem to play an important role in updating when passive motion occurs about an axis that typically moves the body relative to gravity (i.e., roll), these same cues do not seem to matter when motion occurs about an axis that does not (i.e., yaw). Thus, in summary, for whole-body rotations, updating performance seems to be dependent on both the axis of rotation and the ability of each axis to integrate the available updating signals. Passive updating maybe better for roll than yaw because the roll axis can better utilize gravitational cues. In contrast, yaw rotations may rely more heavily on efference copies available during active movements.
Figure 6.
Updating for passive, body-fixed, yaw rotations at various pitch angles. A. As the pitch angle increases (from left to right), the gravity cue available for updating increases. In the upright orientation, the gravity vector remains constant during yaw rotation, while in the supine condition, the gravity vector changes maximally during yaw rotation. Solid lines on head indicate axis of rotation. B. Updating ability was equally good, for all subjects (different symbols), in both the upright and supine orientations. A value of 1 indicates perfect updating, while a value of 0 indicates no updating. Modified and replotted with permission from Klier et al., 2006.
Finally, spatial updating is also possible after passive translational motion in three-dimensional space (i.e., lateral, vertical and fore-aft translations) (Klier et al., 2008). Spatial updating for translations is more complex than updating for rotations because translations change both the direction and the distance of an object from the observer. In addition, translational updating for targets in depth requires changes in the relative positions of the two eyes, therefore updating accuracy was measured by ocular vergence during fore-aft motion trials. Although the results showed considerable intra- and inter-subject variability, subjects could update quite well for rightward, leftward, upward, forward and backward passive translations, with slightly poorer performance for downward movements. Again, this updating could not have been accomplished via motor efference copy cues as the motion was passive.
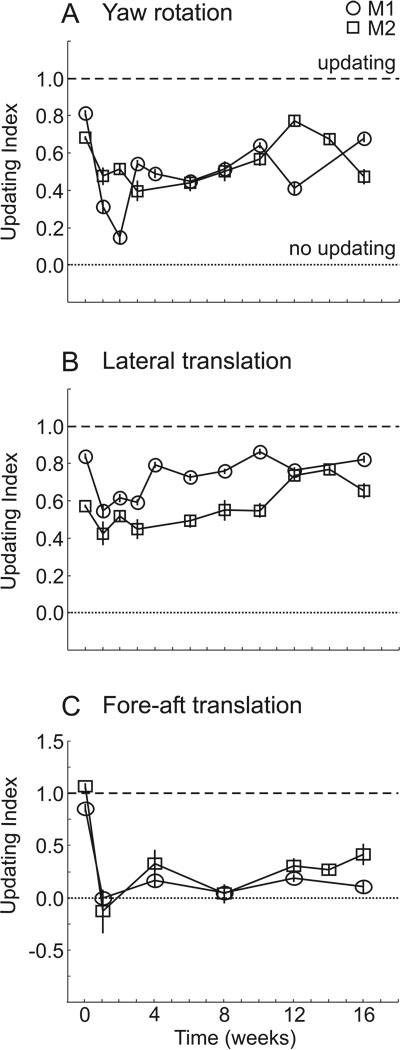
Similar conclusions have also been reached in non-human primates. Rhesus monkeys can spatially update for yaw rotations (Baker et al., 2003; Li et al., 2005) as well as for lateral (Li et al., 2005) and fore-aft (Li and Angelaki, 2005) translations. As with humans, these animals also show considerable variability in their responses. Interestingly, these experiments were taken a step further by bilaterally labyrinthectomizing the animals and quantifying updating abilities after vestibular loss (Li and Angelaki, 2005; Wei et al., 2006). Immediately after the lesion, the animals’ updating abilities were significantly compromised for both rotations and translations (figure 7). However, while updating ability slowly improved over time to near normal levels for yaw rotations and lateral translations, there was no improvement for fore-aft motion, even after four months (Wei et al., 2006). Thus, vestibular signals may play a more important role for updating movements that require a vergence response (i.e., fore-aft motion) than for those that require a version response.
Figure 7.
Updating before and after labyrinthine lesions. Updating ability is evaluated, for two animals, by an updating index (1 indicates perfect updating; 0 indicates no updating) before (time = 0) and up to 16 weeks after lesions of the labyrinths. A. Updating performance for yaw rotations. B. Updating for lateral translations. C. Updating for fore-aft translations. Replotted with permission from Wei et al., 2006.
In all these passive movement experiments, the subjects were always fixating a central target during the motion. This is necessary so that the rotational/translational VOR is turned off during the movement. If the VOR were to be active, then the retinal error of the flashed target would remain in a fixed position relative to the fovea, and no updating would be required at the end of the movement (i.e., a motor error equivalent to the retinal error could be used to accurately reach the target). Since the VOR must be cancelled during these spatial updating experiments, a feedback signal proportional to the cancelled VOR eye movement may be available for updating. Note that although this feedback signal is also a type of efference copy, it is different from the motor efference copies discussed above as it is only present during the movement itself and can therefore not be used for predictive updating.
Taken together with the previous section, both sensory cues and motor signals can be used for spatial updating when they are available. Likely the brain has become optimized to use a variety of different signal types to maintain spatial constancy, and which cues it uses probably depends on the availability and reliability of the individual cues (Ernst and Banks, 2002). For example, in the roll updating experiments that were performed both actively (Medendorp et al., 2002) and passively (Klier et al., 2005), subjects’ performances were slightly better when the task was active. This improvement maybe due to the availability of neck proprioception signals that have been shown to improve updating ability after intervening whole-body rotations (Mergner et al., 1998).
Pathways
Although all possible sensory and motor sources of updating signals have not yet been examined, it is becoming obvious that many originate in the brainstem, like vestibular signals and efference copies from the superior colliculus and oculomotor burst neurons. But evidence for neurons that perform spatial updating lies mainly in cortical areas including LIP (Duhamel et al., 1992a; Kusunoki and Goldberg, 2003; Heiser and Colby, 2006), FEF (Goldberg and Bruce, 1990; Umeno and Goldberg, 1997, 2001) and even in early visual areas like V2 and V3 (Nakamura and Colby, 2002; Merriam et al., 2007). fMRI studies, comparing BOLD activity during the double-step saccade task with activity during visually guided saccades, implicate a parieto-frontal cortical network in spatial updating that includes area LIP, precuneus, insula, inferior frontal gyrus and anterior cingulum (Tobler and Müri, 2001). A similar study using triple-step saccades showed that the right intraparietal sulcus and parts of the frontal and supplementary eye fields are vital for spatial updating (Heide et al., 2001).
Cortical areas have also been implicated in spatial updating based on inactivation and patient studies. When area LIP was inactivated in monkeys, the accuracy of the second saccade was adversely affected when the first saccade was directed into the contralateral visual field (Li and Andersen, 2001). Similar results have also been reported in humans with lesions in parietal cortex (Duhamel et al., 1992b; Heide et al., 1995). In addition, transcranial magnetic stimulation has been used to disrupt posterior parietal cortex processing at various times during a double-step saccade task, leading to mislocalizations of the updated targets (Van Donkelaar and Müri, 2001). Thus a plethora of evidence indicates that updating occurs in the cortex. But if the signals used for updating originate in the brainstem, then how do these signals travel from the brainstem to the cortex?
A thalamic pathway for spatial updating was first suggested by Schlag-Rey and Schlag (1989). They proposed the intralaminar nuclei as a possible transmission site since (1) its neurons have a firing rate that is related to the position of the target in space, (2) it receives inputs from brainstem areas associated with eye movements like the superior colliculus, and (3) it projects to frontal and parietal cortices where updating has been observed. Furthermore, the intralaminar nuclei and surrounding areas (collectively known as the oculomotor thalamus) have delay-period activity (Wyder et al., 2003, 2004), which may carry information regarding the amplitude and direction of the intervening movement.
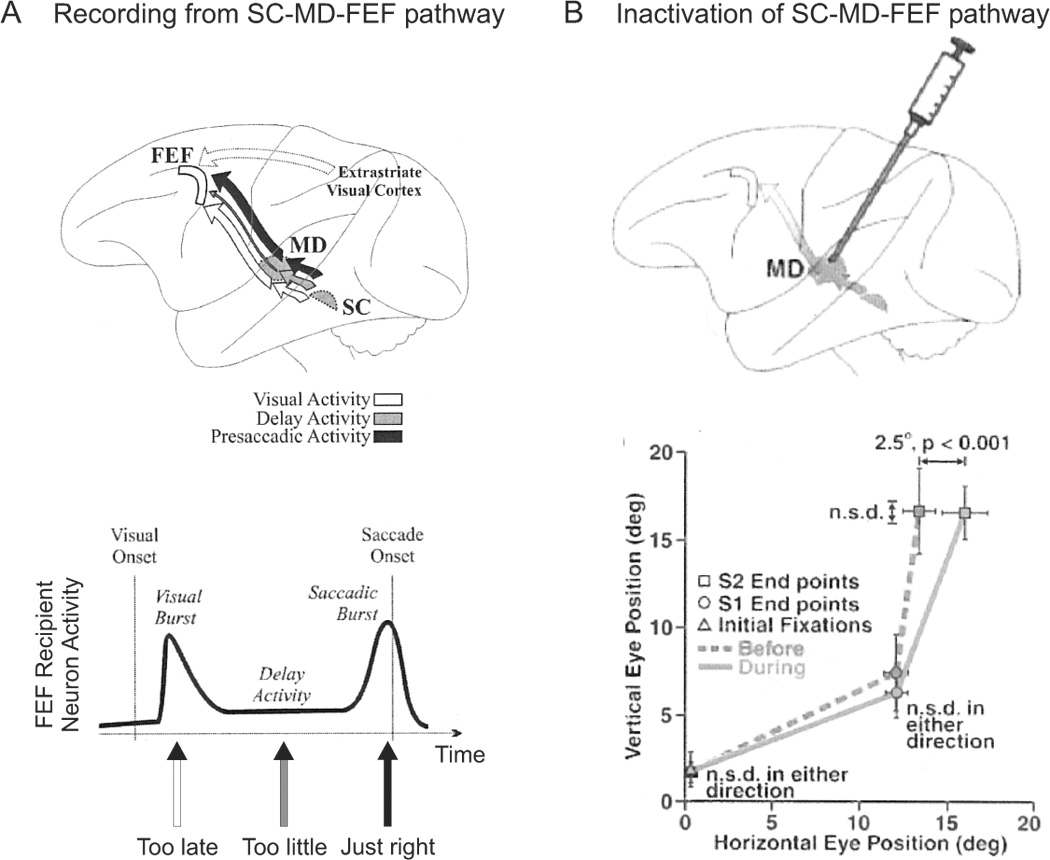
In a series of elegant experiments, Sommer and Wurtz (2002, 2004a, 2004b, 2006) investigated the role of the medial-dorsal (MD) thalamus in transmitting motor efference copies for saccades from the SC to the FEF. Using orthodromic and antidromic stimulation, they localized cells in MD that both receive projections from the SC and project to the FEF. They found that the SC transmits visual, delay and presaccadic activity to MD and FEF in a topographical manner, but that only the presaccadic activity is used for updating (Sommer and Wurtz, 2004a) (figure 8A). Then they went on to show that if one knows the spatial and temporal properties of the updating signal, one can easily predict the changes in visual processing in the FEF whenever a saccade is made (Sommer and Wurtz, 2006).
Figure 8.
The SC-MD-FEF pathway for updating signals. A. Recording studies delineate a pathway for updating signals from the SC to the FEF via the MD thalamus. Of the three signal types received by the FEF through this pathway, the visual burst arrives too late to be utilized for spatial updating, the delay period activity is too small to account for spatial updating, but the saccadic burst activity is appropriate for spatial updating. Replotted with permission from Sommer and Wurtz, 2004a. B. Inactivation of area MD leads to horizontal mislocalization of the second target in a double-step saccade task. Replotted with permission from Sommer and Wurtz, 2002.
Importantly, inactivation of MD by muscimol caused modest, yet significant deficits in the accuracy of the second saccade in the classic double-step saccade task, whenever the first saccade was directed into the contralateral visual field. Rather than being perfectly accurate, the animals’ saccades now had an accuracy of approximately 80% (Sommer and Wurtz, 2002) (figure 8B). Specifically, errors in the endpoint of the second saccade were due to (1) judgement errors about the amplitude of the first saccade and (2) an inability to detect variations in the amplitude of the first saccade across trials. Thus inactivation of MD led to deficits in both the accuracy and precision of the updating signal (Sommer and Wurtz, 2004b). Finally, these injections were also shown to negatively affect the spatiotemporal properties of visual processing in FEF neurons that received projections from MD (Sommer and Wurtz, 2006). These results in monkeys are consistent with reported spatial updating deficits in patients with thalamic lesions (Gaymard et al., 1994; Versino et al., 2000; Bellebaum et al., 2006).
Despite the importance of these findings, of all the muscimol inactivations performed, the average deficits observed were only in the order of about 19% (Sommer and Wurtz, 2002, 2004b). The authors speculated that perhaps they only inactivated a small region of MD, or that the monkeys began to compensate for their deficit using proprioceptive cues, or that additional updating pathways may also exist. This latter point is important because implicating the SC as the sole source of the updating signal is problematic for a number of reasons. First, as shown above, updating can occur for passive movements and not solely for active movements. Since the SC is part of the motor pathway for saccades, it could only provide the updating signal for active, self-generated movements. Second, the motor map of the SC has been shown to be two-dimensional in nature (Van Opstal, 1991; Klier et al., 2002). Thus it only outputs the horizontal and vertical components of the desired motor output. But the eyes move in three-dimensions, and since it has been shown that updating also occurs for torsion (Medendorp et al., 2003; Klier et al., 2005), the source of these torsional signals cannot be the SC. Thus, while the SC-MD-FEF pathway may constitute one pathway, updating signals likely arise from many sources, both motor and sensory, both two-dimensional and three-dimensional.
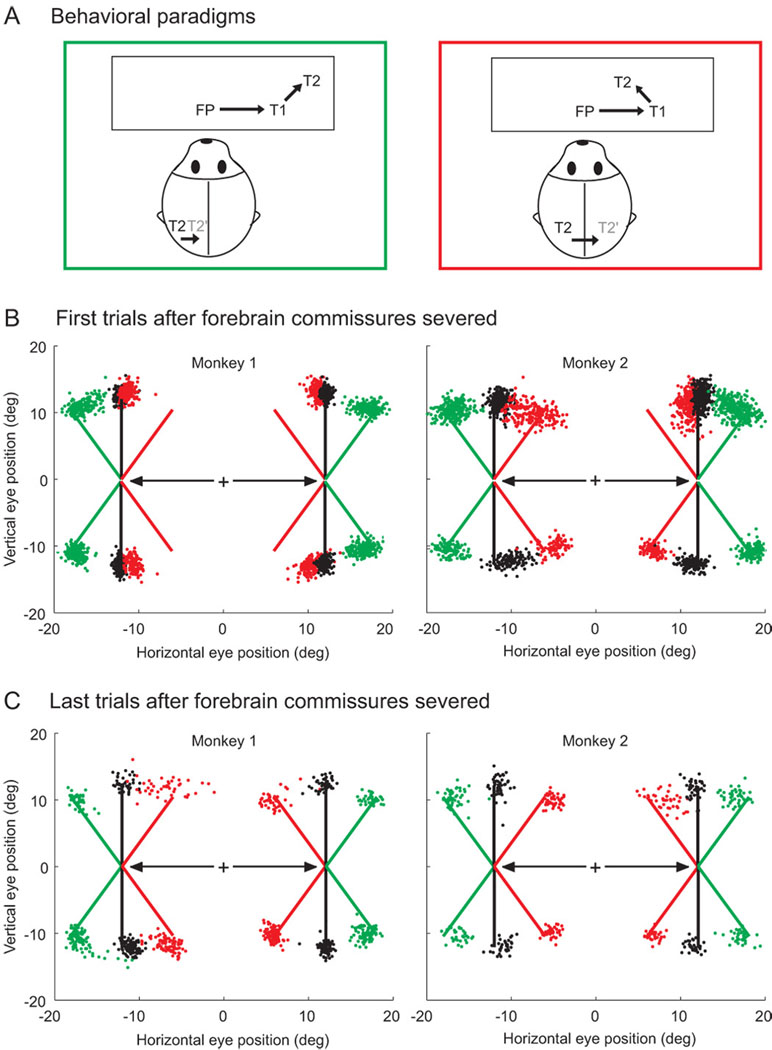
In some updating paradigms, changes in gaze cause the representation of the remembered target location to shift form one hemisphere to the other (i.e., a target flashed 10° in the right hemifield will be represented in the left hemisphere until a 20° rightward movement causes its representation to be shifted into the left hemisphere). Since visual representations are extremely lateralized (Trevarthen 1990), then a pathway for inter-hemispheric transfer of updating signals may exist via the forebrain commissures (i.e., the corpus callosum and anterior commisure). These structures serve as the main connection between the left and right cerebral hemispheres. Colby and colleagues have examined the transmission of such updating signals in animals trained on two versions of the double-step saccade task: one in which updating occurs entirely within one hemifield (i.e., the intervening eye movement to the first target maintains the location of the second target in the same hemifield in which it was originally seen), and one in which the representation of the target to be updated jumps from one hemifield to the other (i.e., the intervening eye movement to the first target causes the location of the second target to be moved into the opposite hemifield) (figure 9A). Behaviorally, they found extremely poor updating performance in across-hemifield updating after the forebrain commissures were severed (figure 9B). But remarkably, although these strong connections between hemispheres were destroyed, the animals regained most of their ability to perform accurate double-step saccades (Berman et al., 2005) (figure 9C). Perhaps even more surprising was the fact that LIP neurons, which typically show updating activity in the intact animal (figure 3), continued to show visual remapping after the inter-hemispheric connections were damaged (although remapping activity in across-hemispheric updating was weaker then within-hemispheric updating) (Heiser et al., 2005; Berman et al., 2007). Therefore, while cortico-cortical connections play some role in spatial updating, other subcortico-cortical pathways must also be involved and likely compensate for any damage that may occur at the level of the cortex.
Figure 9.
Updating after split-brain experiments. A. Two versions of the double-step saccade task. In both versions, a monkey fixates on FP and two targets (T1, T2) are briefly flashed in the right visual hemifield. Thus both targets are represented in the left hemisphere. Green square: An eye movement to T1, causes the representation of T2 to remain in left hemisphere. Red square: An eye movement to T1, causes T2 to shift into the right hemisphere. B. After the forebrain commissure is severed, monkeys have difficulty localizing T2 when its representation crosses form one hemisphere to the other (red stippling). C. After some time, the monkeys recover their cross-hemisphere updating ability (red stippling). Replotted with permission from Berman et al., 2005.
Again, these cortico-cortical circuits cannot explain torsional updating since torsional signals are only added on to the motor command after the SC (Klier et al., 2003). Also, updating can occur for passive rotations and translations, which occur via sensory cues derived from brainstem regions like the vestibular nuclei. Thus alternative pathways must still be identified. For example, anatomical studies show that the vestibular nuclei project to the intralaminar nuclei of the thalamus (Lang et al., 1979; Klier et al., 2004), and neurophysiological studies have found rotational and translational vestibular signals in thalamic regions more lateral to MD (e.g., ventral posterior and ventral lateral nuclei) (Meng et al., 2007). Further experiments must be carried out to determine if these regions are involved in visuospatial updating.
Theoretical Models of Spatial Updating
The mathematical computations that underlie spatial updating have also been described. As can be observed in figure 2, the correct updated motor error to T2 in a double-step saccade task could theoretically be computed by subtracting the motor error to T1 from the retinal error caused by T2 (i.e., green ME2 = RE2 – ME1). This formula is called vector subtraction and modeling it requires information pertaining to (1) the eye-centered representation of the visual target and (2) the metrics of the intervening movement. Paradoxically, vector subtraction has been recently modeled using gain fields (i.e., neural responses that are modulated by eye position; [Zipser and Andersen 1988]), a concept that is typically used for implementing non-linear (e.g., multiplicative) operations (Pouget and Sejnowski, 1997). It has been argued that a multiplicative gain field mechanism can perform the neural equivalent of vector subtraction, and that the output of these cells is equivalent to the eye-centered amplitude of the saccade to the updated target (Cassanello and Ferrera, 2007a, b). This vector-subtraction-by-gain field model requires that the eye position inputs are inversely correlated with retinal position sensitivity (i.e., cells with central receptive fields are weakly modulated by eye position, while cells with peripheral receptive fields are strongly modulated by eye position). These authors went on to show that cells in FEF support these findings at both the single-unit and population levels.
However, updating using vector subtraction across many saccades leads to predicted errors that are not observed in humans (Smith and Crawford, 2001). In addition, vector subtraction models are not geometrically correct because rotations are non-commutative. So in non-linear, three-dimensional geometry, the vector coding scheme gets into trouble and now the use of gain fields is crucial in order to achieve reference frame transformations. And it is the non-linear aspect that requires the use of gain-fields (Salinas and Abbott, 1995; Pouget and Sejnowski, 1997).
A neural network model, using two-dimensional retinal error but three-dimensional eye position and three-dimensional motor error as inputs, learned to update accurately in three-dimensions (Keith and Crawford, 2008; Keith et al., 2007a). In this model, hidden layer units were found that fell along a continuum between units that implemented linear vector subtraction and units that implemented the eye-position dependent, non-linear aspects of spatial updating. Moreover, the temporal aspects of updating on topographic maps (like the SC and FEF) were found to depend on the nature of the efference copy (Keith et al., 2007b). A two-dimensional, spatially coded motor error command produced scattered activity that was reconstructed at the right location at the end of the intervening movement, whereas three-dimensional, temporally coded eye velocity signals produced a suppressed hill of activity that moved across the map toward its updated location. Ultimately, a combination of these signals best matched the observed single-unit data to date.
In another study, a neural network was programmed to generate saccades both with (i.e., space-fixed targets) and without (i.e., gaze-fixed targets) spatial updating (White and Snyder, 2004). Unlike the extremely accurate updating performance observed with intervening saccadic eye movements, the model performed more poorly when the intervening motion was a slow phase movement (i.e., pursuit or whole-body rotation). Interestingly, accuracy was improved when using velocity, rather than position, signals for updating. Gain fields were seldomly observed and only present when position signals were used for updating. The behavior of these hidden units was found to be similar to that of LIP neurons (White and Snyder, 2007).
In summary, existing models generally predict many features of experimental results. Although many of them utilize similar principles, a controversy remains as to whether linear vector subtraction approximations or non-linear, three-dimensional computations are implemented by neural populations for the maintenance of spatial constancy.
Reference Frames
Early behavioral evidence suggested that spatial constancy is partially maintained by a central representation of visual space in an allocentric (i.e., world-centered) reference frame (Dassonville et al., 1995; Karn et al., 1997; reviewed in Burgess 2006). However, as summarized above, subsequent single unit recording experiments (e.g., figure 3), pointed toward a retinotopic (i.e., eye-centered) reference frame for spatial updating (Batista et al., 1999; Kusunoki and Goldberg, 2003; Heiser and Colby, 2006; reviewed in Colby et al., 1995). This is because the receptive fields in area LIP shift the area of space to which they respond in the same direction and by the same amplitude as the associated eye movements. Thus, spatial constancy could be maintained without creating an explicit map of the world in either body-, space- or object-centered reference frames. Notably, the gain fields described in the previous section have been implicated in forming head-centered target representations by combining retinal information with eye position signals (Zipser and Andersen 1988). To this end, some studies have demonstrated the existence of eye and head position signals in areas LIP and 7a that could at least theoretically produce body-centered and world-centered reference frames, respectively (Brotchie et al., 1995; Snyder et al., 1998). Yet, at present, a world-centered reference frame has never been explicitly demonstrated in the activity of single neurons. In fact, several researchers now believe that that the brain likely utilizes multiple reference frames simultaneously, each suited to solve a specific task (Andersen et al. 1997; Boussaoud and Bremmer, 1999; Colby, 1998). This is partly because neural networks often use implicit codes that are not confined to any particular frame (Avilac et al., 2005; Pouget and Sejnowski, 1997). Notably, different cell responses (e.g., visual vs. motor) and methodologies (e.g., single-unit vs. stimulation) often point to different frames of reference (Blohm et al. 2006; Salinas and Abbott 1995).
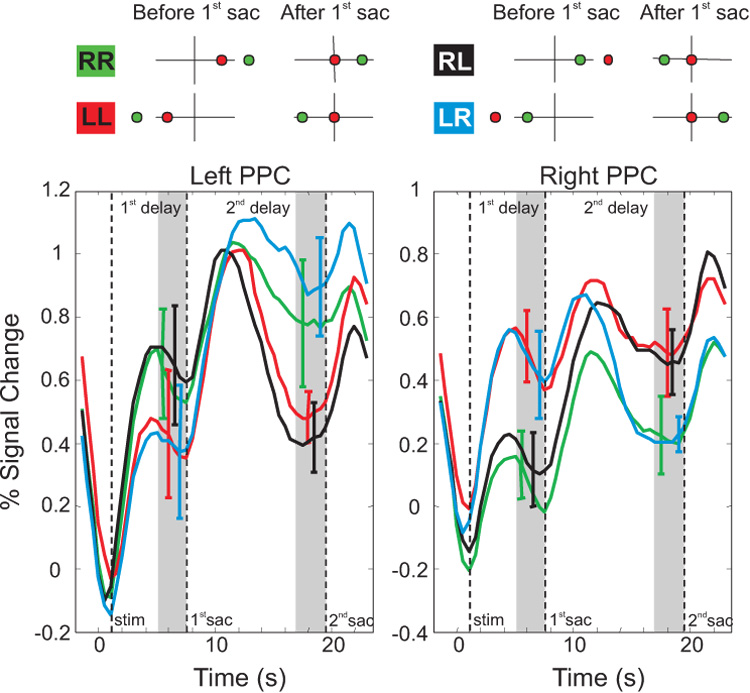
Interestingly, unlike single unit results, human fMRI experiments more clearly point to eye-centered representations for spatial updating. In a series of fMRI experiments, subjects were trained on a modified version of the double-step saccade task (Medendorp et al., 2003) (figure 10). Subjects fixated a central target and were then showed two peripheral targets in sequence (green circle then red circle). The task was to make a first saccade to the red target and then a second saccade to the green target. As in the split-brain experiments, some trials kept both targets in one visual hemifield (RR and LL), while in other trials the intervening eye movement to the red target caused the location of the green target to jump from one hemifield to the other (RL and LR). fMRI activity was observed in an area of posterior parietal cortex that is thought to be the human equivalent of monkey area LIP. In the delay period after both targets were presented but before any saccade was made, a buildup of activity was seen in the hemifield contralateral to the targets’ locations (e.g., left hemisphere activation when the targets were presented in the right hemifield). If the first saccade left the second target in the same hemifield, then activity remained in the same hemisphere (green and red traces). However, if the first saccade caused the second target to switch from one hemifield to the other, then the BOLD response also shifted to the opposite hemisphere (black and blue traces). This imaging experiment, which demonstrates spatial constancy for motor control, and a similar one showing spatial constancy for visual perceptions (Merriam et al. 2003), both highlight the gaze-dependent behavior of spatial updating in humans.
Figure 10.
Eye-centered reference frame revealed by fMRI. A subject fixates the origin while two targets are briefly flashed (before 1st saccade column). The final goal (green target) is flashed first, followed by the first fixation point (red target). The subject first makes a saccade to the red target (after 1st saccade column) and subsequently makes a saccade to the green target (not shown). In some conditions, the representation of the final goal (green target) is kept in the same hemisphere (e.g., goal stays in right hemisphere in the green RR condition and goal stays in the left hemisphere in the red LL condition). In other conditions, the saccade to the first fixation point (red target) causes the final goal (green target) to switch its location from one hemisphere to the other (e.g., from right to left in black RL condition and from left to right in blue LR condition). B. Activity that stays in the right hemisphere is shown by the green trace (RR condition), while activity in the left hemisphere is shown by the red trace (LL condition). Activity can be seen jumping from one hemisphere to the other with the black (RL) and blue (LR) conditions. For example, in the left PPC, the black trace follows the green trace during 1st delay period, but follow the red trace during the 2nd delay period. Replotted with permission from Medendorp et al., 2003a.
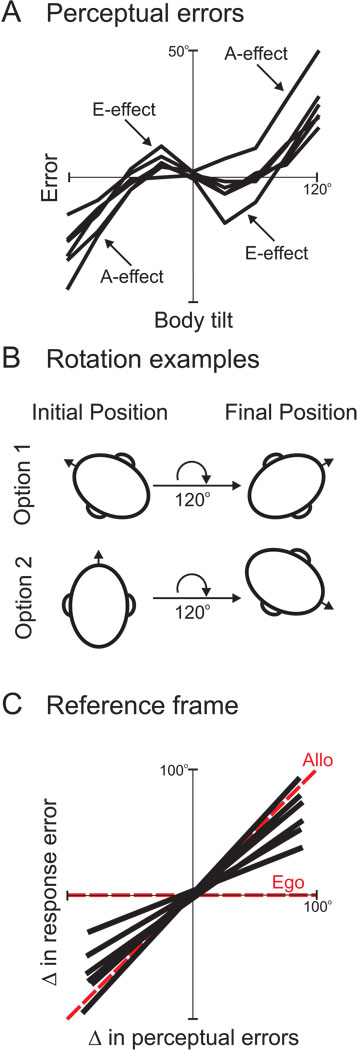
But if gravity and inertial cues play a role in updating (Klier et al., 2005), is it not possible that other allocentric reference frames are also used? This idea was tested (Van Pelt et al., 2005) by exploiting two psychophysical phenomena – the A-effect and the E-effect – in which subjects misestimate the gravity vector when placed in different static roll positions (figure 11A). In the former, subjects misestimate the gravity vector in the direction of their body tilt when they are tilted >60° (Aubert, 1861; Kaptein and Van Gisbergen, 2004). In the latter, subjects misestimate the gravity vector in the direction opposite to their body tilt when they are tilted <30° (Howard, 1982). In this experiment, subjects were first roll-tilted to one initial position and then a peripheral target briefly flashed and the subjects were required to remember the location of the flash. Next, subjects were rotated by a particular roll angle, say 120° (either clockwise or counterclockwise) to a different final roll tilt position. However, while the rotation amplitude was held constant, the initial and final static roll positions differed. For example, subjects may have started at 60° left ear down and rotated 120° clockwise to a position 60° right ear down, or they may have started from upright (0°) and rotated 120° clockwise to a position 120° right ear down (figure 11B). At the final orientation, the subjects had to make a saccade to the remembered flash location. They discovered that subjects made errors in accuracy that reflected the A- and E-effects, which in turn depend on their final orientation relative to gravity (figure 11C). Thus gravity had a strong effect on their updating abilities, pointing to another potential reference frame based on an allocentric variable (i.e., gravity). This finding is supported by studies that demonstrate an effect of gravito-inertial forces on spatial localization (Klier et al., 2005; Prieur et al., 2005).
Figure 11.
Allocentric reference frame depends on gravity cues. A. Subjects show errors in perceiving the orientations of vertical lines in space depending on their tilted roll angle. With small body tilts, subjects misperceive the orientations of vertical lines in a direction opposite to that of their body tilt (E-effect). With larger body tilts, subjects misperceive the orientations of vertical lines in the same direction as their body tilt (A-effect). Replotted with permission from Van Pelt et al., 2005. B. Subjects were rotated by a fixed roll angle (e.g., 120°) from different initial orientations (e.g., option 1 = 60° counterclockwise; option 2 = 0°) to different final orientations (e.g., option 1 = 60° clockwise; option 2 = 120° clockwise). C. Updating ability more closely follows the predictions of an allocentric model (dashed red unity slope) that was based on the perceptual errors in the different final orientations, than the predictions of an egocentric model that is independent of these perceptual errors. Replotted with permission from Van Pelt et al., 2005.
Conclusions
Spatial updating, the neural process underlying spatial constancy, is truly a complex and intriguing phenomenon. It combines retinal error signals with a variety of sensory and motor commands that describe the amplitude and direction of movements that occur between the time we see an object and when we decide to generate an action toward it. These intervening movements not only include eye movements such as saccades and pursuit, but also rotations of the head as well as whole-body rotations and translations. Rotational movements can be updated for all three directions of rotation – yaw, pitch and roll – and translational movements can be updated for all three directions of translations – fore/aft, lateral and vertical (although the degree to which updating occurs may vary across directions and sometimes shows large variability). Even the non-commutativity of rotations is taken into account in the computations that generate the final updated motor command. Recent studies have begun to delineate the pathways responsible for the transmission of certain updating signal and others have identified potential reference frames used for spatial updating. Delineating the means by which we maintain a stable representation of the outside world is important not only for spatial constancy, but also more generally for determining how incoming sensory information is processed into appropriate outgoing motor commands that control behavior.
Future Directions
While an impressive amount of work has been conducted on spatial updating over the last 15 years, many more issues remain to be addressed. First, while behavioral updating studies have looked at a variety of intervening eye movements including saccades, pursuit and VOR suppression, single-unit studies have focused solely on saccadic eye movements (except Powell and Goldberg, 1997). If we are to explain why updating is found is so many cortical and subcortical areas, the repertoire of single-unit studies must be expanded not only into different eye movement types, but also to include updating for whole-body rotations/translations and head-free movements. Perhaps some brain regions specialize in fast eye movements (i.e., saccades), while others are dedicated to updating for slow eye movements (i.e., pursuit/VOR). Some areas may exhibit predictive remapping while others may not, and some may show updating for perception across eye movements while others concern themselves with motor-related tasks.
Second, because the real world provides us with a plethora of cues whenever we move (i.e., visual, vestibular, auditory, somatosensory, etc.), it is necessary to understand how these multi-modal cues are integrated to provide a coherent measure of the metrics of our intervening movements. Is each modality updated independently and then combined with measures from other modalities, or is information from the individual modalities first combined into a single measure of the motion parameters? This questions is especially interesting when considering how information from different modalities, which are coded in different reference frames, are properly combined.
Third, the pathways carrying updating signals from various sources to the brain regions that exhibit updating must be characterized. The deficits caused by eliminating the pathways examined to date have either been small (e.g., inactivation of MD in the SC-MD-FEF pathway) or surmountable via alternate pathways (e.g., anterior commisure decussation). And no studies have yet addressed the paths traveled by brainstem vestibular signals or other three-dimensional position/velocity commands to the cortex. All these issues remain to be addressed and quantified in future studies.
Acknowledgements
The authors would like to thank the following people for providing modified versions of their figures to this paper: Rebecca A. Berman, Gunnar Blohm, W. Pieter Medendorp and Min Wei. Support was provided by National Institutes of Health grant DC04260 to DEA.
List of Abbreviations
- BOLD
Blood Oxygen Level Dependent
- FEF
Frontal Eye Fields
- fMRI
functional Magnetic Resonance Imaging
- LIP
Lateral Intra-parietal Cortex
- MD
Medial-dorsal Thalamus
- ME
Motor Error
- PPRF
Paramedian Pontine Reticular Formation
- RE
Retinal Error
- SC
Superior Colliculus
- T
Target
- VOR
Vestibulo-ocular Reflex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Admiraal MA, Keijsers NL, Gielen CC. Gaze affects pointing toward remembered visual targets after a self-initiated step. J Neurophysiol. 2004;92:2380–2393. doi: 10.1152/jn.01046.2003. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Aubert H. Eine scheinbare bedeutende Drehung von Objekten bei Neigung des Kopfes nach rechts oder links. Virchows Arch. 1861;20:381–393. [Google Scholar]
- Avillac M, Deneve S, Olilvier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neuro. 2005;8:941–949. doi: 10.1038/nn1480. [DOI] [PubMed] [Google Scholar]
- Baker JT, Harper TM, Snyder LH. Spatial memory following shifts of gaze. I. Saccades to memorized world-fixed and gaze-fixed targets. J Neurophysiol. 2003;89:2564–2576. doi: 10.1152/jn.00610.2002. [DOI] [PubMed] [Google Scholar]
- Batista AP, Buneo CH, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science. 1999;285:257–260. doi: 10.1126/science.285.5425.257. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Hoffmann KP, Daum I. Post-saccadic updating of visual space in the posterior parietal cortex in humans. Behav Brain Res. 2005;163:194–203. doi: 10.1016/j.bbr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Hoffmann KP, Koch B, Schwarz M, Daum I. Altered processing of corollary discharge in thalamic lesion patients. Eur J Neurosci. 2006;24:2375–2388. doi: 10.1111/j.1460-9568.2006.05114.x. [DOI] [PubMed] [Google Scholar]
- Berman RA, Heiser LM, Saunders RC, Colby CL. Dynamic circuitry for updating spatial representations. I. Behavioral evidence for interhemispheric transfer in the split-brain macaque. J Neurophysiol. 2005;94:3228–3248. doi: 10.1152/jn.00028.2005. [DOI] [PubMed] [Google Scholar]
- Berman RA, Heiser LM, Dunn CA, Saunders RC, Colby CL. Dynamic circuitry for updating spatial representations. III. From neurons to behavior. J Neurophysiol. 2007;98:105–121. doi: 10.1152/jn.00330.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoz A, Israel I, Georges-Francois P, Grasso R, Tsuzuku T. Spatial memory of body linear displacement: what is being stored? Science. 1995;269:95–98. doi: 10.1126/science.7604286. [DOI] [PubMed] [Google Scholar]
- Blohm G, Missal M, Lefèvre P. Smooth anticipatory eye movements alter the memorized position of flashed targets. J Vis. 2003;3:761–770. doi: 10.1167/3.11.10. [DOI] [PubMed] [Google Scholar]
- Blohm G, Missal M, Lefèvre P. Processing of retinal and extraretinal signals for memory-guided saccades during smooth pursuit. J Neurophysiol. 2005;93:1510–1522. doi: 10.1152/jn.00543.2004. [DOI] [PubMed] [Google Scholar]
- Blohm G, Optican L, Lefèvre P. A model that integrates eye velocity commands to keep track of smooth eye displacements. J Comput Neurosci. 2006;21:51–70. doi: 10.1007/s10827-006-7199-6. [DOI] [PubMed] [Google Scholar]
- Bloomberg J, Jones GM, Segal B, Mcfarlane S, Soul J. Vestibular-contingent voluntary saccades based on cognitive estimates of remembered vestibular information. Adv Otorhinolaryngol. 1988;41:71–75. doi: 10.1159/000416034. [DOI] [PubMed] [Google Scholar]
- Bloomberg J, Melvill Jones G, Segal B. Adaptive modification of vestibularly perceived rotation. Exp. Brain Res. 1991;84:47–56. doi: 10.1007/BF00231761. [DOI] [PubMed] [Google Scholar]
- Blouin J, Bridgeman B, Teasdale N, Bard C, Fleury M. Visual stability with goal-directed eye and arm movements toward a target displaced during saccadic suppression. Psychol Res. 1995a;58:169–176. doi: 10.1007/BF00419632. [DOI] [PubMed] [Google Scholar]
- Blouin J, Gauthier GM, Vercher JL. Failure to update the egocentric representation of the visual space through labyrinthine signal. Brain Cogn. 1995b;29:1–22. doi: 10.1006/brcg.1995.1264. [DOI] [PubMed] [Google Scholar]
- Blouin J, Labrousse L, Simoneau M, Vercher JL, Gauthier GM. Updating visual space during passive and voluntary head-in-space movements. Exp Brain Res. 1998;122:93–100. doi: 10.1007/s002210050495. [DOI] [PubMed] [Google Scholar]
- Blouin J, Teasdale N, Bard C, Fleury M. Control of rapid arm movements when target position is altered during saccadic suppression. J Mot Behav. 1995c;27:114–122. doi: 10.1080/00222895.1995.9941704. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Bremmer F. Gaze effects in the cerebral cortex: reference frames for space coding and action. Exp Brain Res. 1999;128:170–180. doi: 10.1007/s002210050832. [DOI] [PubMed] [Google Scholar]
- Bresciani JP, Blouin J, Popov K, Bourdin C, Sarlegna F, Vercher JL, Gauthier GM. Galvanic vestibular stimulation in humans produces online arm movement deviations when reaching towards memorized visual targets. Neurosci Lett. 2002;318:34–38. doi: 10.1016/s0304-3940(01)02462-4. [DOI] [PubMed] [Google Scholar]
- Bresciani JP, Gauthier GM, Vercher JL, Blouin J. On the nature of the vestibular control of arm-reaching movements during whole-body rotations. Exp Brain Res. 2005;164:431–441. doi: 10.1007/s00221-005-2263-4. [DOI] [PubMed] [Google Scholar]
- Brotchie PR, Andersen RA, Snyder LH, Goodman SJ. Head position signals used by parietal neurons to encode locations of visual stimuli. Nature. 1995;375:232–235. doi: 10.1038/375232a0. [DOI] [PubMed] [Google Scholar]
- Burgess N. Spatial memory: how egocentric and allocentric combine. Trends in Cognitive Neurosci. 2006;10:551–557. doi: 10.1016/j.tics.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Cassanello CR, Ferrera VP. Computing vector differences using a gain field-like mechanism in monkey frontal eye field. J Physiol. 2007a;582:647–664. doi: 10.1113/jphysiol.2007.128801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassanello CR, Ferrera VP. Visual remapping by vector subtraction: analysis of multiplicative gain field models. Neural Comput. 2007b;19:2353–2386. doi: 10.1162/neco.2007.19.9.2353. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Oculocentric spatial representation in parietal cortex. Cereb Cortex. 1995;5:470–481. doi: 10.1093/cercor/5.5.470. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Schlag J, Schlag-Rey M. The use of egocentric and exocentric location cues in saccadic programming. Vision Res. 1995;35:2191–2199. doi: 10.1016/0042-6989(94)00317-3. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992a;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Goldberg ME, Fitzgibbon EJ, Sirigu A, Grafman J. Saccadic dysmetria in a patient with a right frontoparietal lesion. The importance of corollary discharge for accurate spatial behaviour. Brain. 1992b;115:1387–1402. doi: 10.1093/brain/115.5.1387. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415:429–433. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Rivaud S, Pierrot-Deseilligny C. Impairment of extraretinal eye position signals after central thalamic lesions in humans. Exp Brain Res. 1994;102:1–9. doi: 10.1007/BF00232433. [DOI] [PubMed] [Google Scholar]
- Gellman RS, Fletcher WA. Eye position signals in human saccadic processing. Exp Brain Res. 1992;89:425–434. doi: 10.1007/BF00228258. [DOI] [PubMed] [Google Scholar]
- Glasauer S, Brandt T. Noncummutative updating of perceived self-orientation in three dimensions. J Neurophysiol. 2007;97:2958–2964. doi: 10.1152/jn.00655.2006. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bruce CJ. Primate frontal eye fields. III. Maintenance of a spatially accurate saccade signal. J Neurophysiol. 1990;64:489–508. doi: 10.1152/jn.1990.64.2.489. [DOI] [PubMed] [Google Scholar]
- Guthrie BL, Porter JD, Sparks DL. Corollary discharge provides accurate eye position information to the oculomotor system. Science. 1983;221:1193–1195. doi: 10.1126/science.6612334. [DOI] [PubMed] [Google Scholar]
- Hallett PE, Lightstone AD. Saccadic eye movements towards stimuli triggered by prior saccades. Vision Res. 1976a;16:99–106. doi: 10.1016/0042-6989(76)90083-3. [DOI] [PubMed] [Google Scholar]
- Hallett PE, Lightstone AD. Saccadic eye movements to flashed targets. Vision Res. 1976b;16:107–114. doi: 10.1016/0042-6989(76)90084-5. [DOI] [PubMed] [Google Scholar]
- Hayhoe M, Lachter J, Feldman J. Integration of form across saccadic eye movements. Perception. 1991;20:393–402. doi: 10.1068/p200393. [DOI] [PubMed] [Google Scholar]
- Heide W, Binkofski F, Seitz RJ, Posse S, Nitschke MF, Freund JH, Kömpf D. Activation of frontoparietal cortices during memorized triple-step sequences of saccadic eye movements: an fMRI study. Eur J Neurosci. 2001;13:1177–1189. doi: 10.1046/j.0953-816x.2001.01472.x. [DOI] [PubMed] [Google Scholar]
- Heide W, Blankenburg M, Zimmermann E, Kömpf D. Cortical control of double-step saccades: implications for spatial orientation. Ann Neurol. 1995;38:739–748. doi: 10.1002/ana.410380508. [DOI] [PubMed] [Google Scholar]
- Heiser LM, Berman RA, Saunders RC, Colby CL. Dynamic circuitry for updating spatial representations. II. Physiological evidence for interhemispheric transfer in area LIP of the split-brain macaque. J Neurophysiol. 2005;94:3249–3258. doi: 10.1152/jn.00029.2005. [DOI] [PubMed] [Google Scholar]
- Heiser LM, Colby CL. Spatial updating in area LIP is independent of saccade direction. J Neurophysiol. 2006;95:2751–2767. doi: 10.1152/jn.00054.2005. [DOI] [PubMed] [Google Scholar]
- Herter TM, Guitton D. Human head-free gaze saccades to targets flashed before gaze-pursuit are spatially accurate. J Neurophysiol. 1998;80:2785–2789. doi: 10.1152/jn.1998.80.5.2785. [DOI] [PubMed] [Google Scholar]
- Howard IP. Human visual orientation. New York: Wiley; 1982. [Google Scholar]
- Israël I, Fetter M, Koenig E. Vestibular perception of passive whole-body rotation about horizontal and vertical axes in humans: goal-directed vestibulo-ocular reflex and vestibular memory-contingent saccades. Exp Brain Res. 1993;96:335–346. doi: 10.1007/BF00227113. [DOI] [PubMed] [Google Scholar]
- Israël I, Grasso R, Georges-Francois P, Tsuzuku T, Berthoz A. Spatial memory and path integration studied by self-driven passive linear displacement. I. Basic properties. J Neurophysiol. 1997;77:3180–3192. doi: 10.1152/jn.1997.77.6.3180. [DOI] [PubMed] [Google Scholar]
- Israël I, Sievering D, Koenig E. Self-rotation estimate about the vertical axis. Acta Otolaryngol. 1995;115:3–8. doi: 10.3109/00016489509133338. [DOI] [PubMed] [Google Scholar]
- Kaptein RG, Van Gisbergen JA. Interpretation of a discontinuity in the sense of verticality at large body tilt. J Neurophysiol. 2004;91:2205–2214. doi: 10.1152/jn.00804.2003. [DOI] [PubMed] [Google Scholar]
- Karn KS, Moller P, Hayhoe MM. Reference frames in saccadic targeting. Exp Brain Res. 1997;115:267–282. doi: 10.1007/pl00005696. [DOI] [PubMed] [Google Scholar]
- Keith GP, Blohm G, Crawford JD. When do hills of activation move and when do they jump? The dynamics of remapping during saccade, pursuit and combined eye movements. SFN abstract. 2007b 508.7. [Google Scholar]
- Keith GP, Crawford JD. Saccade-related remapping of target representations between topographic maps: a neural network study. J Comput Neurosci. 2008;24:157–178. doi: 10.1007/s10827-007-0046-6. [DOI] [PubMed] [Google Scholar]
- Keith GP, Smith MA, Crawford JD. Functional organization within a neural network trained to update target representations across 3-D saccades. J Comput Neurosci. 2007a;22:191–209. doi: 10.1007/s10827-006-0007-5. [DOI] [PubMed] [Google Scholar]
- Klier EM, Angelaki DE. Thalamic pathways for position and velocity signals during a spatial updating task. Soc Neurosci Abst . 2004;30 186.4. [Google Scholar]
- Klier EM, Angelaki DE, Hess BJM. Roles of gravitational cues and efference copy signals in the rotational updating of memory saccades. J Neurophysiol. 2005;94:468–478. doi: 10.1152/jn.00700.2004. 2005. [DOI] [PubMed] [Google Scholar]
- Klier EM, Angelaki DE, Hess BJM. Human visuospatial updating after non-commutative rotations. J Neurophysiol. 2007;98:537–544. doi: 10.1152/jn.01229.2006. [DOI] [PubMed] [Google Scholar]
- Klier EM, Hess BJM, Angelaki DE. Differences in the accuracy of human visuospatial memory after yaw and roll rotations. J Neurophysiol. 2006;95:2692–2697. doi: 10.1152/jn.01017.2005. [DOI] [PubMed] [Google Scholar]
- Klier EM, Hess BJM, Angelaki DE. Human visuospatial updating after passive translations in three-dimensional space. J Neurophysiol. 2008 doi: 10.1152/jn.01091.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klier EM, Wang H, Crawford JD. The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci. 2001;4:627–632. doi: 10.1038/88450. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Crawford JD. Three-dimensional eye-head coordination is implemented downstream from the superior colliculus. J Neurophysiol. 2003;89:2839–2853. doi: 10.1152/jn.00763.2002. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Goldberg ME. The time course of perisaccadic receptive field shifts in the lateral intraparietal area of the monkey. J Neurophysiol. 2003;89:1519–1527. doi: 10.1152/jn.00519.2002. [DOI] [PubMed] [Google Scholar]
- Lang W, Buttner-Ennever JA, Buttner U. Vestibular projections to the monkey thalamus: an autoradiographic study. Brain Res. 1979;177:3–17. doi: 10.1016/0006-8993(79)90914-4. [DOI] [PubMed] [Google Scholar]
- Li CS, Andersen RA. Inactivation of macaque lateral intraparietal area delays initiation of the second saccade predominantly from contralesional eye positions in a double-saccade task. Exp Brain Res. 2001;137:45–57. doi: 10.1007/s002210000546. [DOI] [PubMed] [Google Scholar]
- Li N, Angelaki DE. Updating visual space during motion in depth. Neuron. 2005;48:149–158. doi: 10.1016/j.neuron.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Li N, Wei M, Angelaki DE. Primate memory saccade amplitude after intervened motion depends on target distance. J Neurophysiol. 2005;94:722–733. doi: 10.1152/jn.01339.2004. [DOI] [PubMed] [Google Scholar]
- Mays LE, Sparks DL. Saccades are spatially, not retinotopically, coded. Science. 1980;208:1163–1165. doi: 10.1126/science.6769161. [DOI] [PubMed] [Google Scholar]
- Mays L, Sparks D. The localization of saccade targets using a combination of retinal and eye position information. In: Fuchs A, Becker W, editors. Progress in oculomotor research. New York: Elsevier/North Holland; 1981. pp. 39–47. [Google Scholar]
- McKenzie A, Lisberger SG. Properties of signals that determine the amplitude and directrion of saccadic eye movements in monkeys. J Neurophysiol. 1986;56:196–207. doi: 10.1152/jn.1986.56.1.196. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Van Asselt S, Gielen CC. Pointing to remembered visual targets after active one-step self-displacements within reaching space. Exp Brain Res. 1999;125:50–60. doi: 10.1007/s002210050657. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Smith MA, Tweed DB, Crawford JD. Rotational remapping in human spatial memory during eye and head motion. J Neurosci. 2002;22:RC196. doi: 10.1523/JNEUROSCI.22-01-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T, Crawford JD. Gaze-centered updating of visual space in human parietal cortex. J Neurosci. 2003a;23:6209–6214. doi: 10.1523/JNEUROSCI.23-15-06209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp WP, Tweed DB, Crawford JD. Motion parallax is computed in the updating of human spatial memory. J Neurosci. 2003b;23:8135–8142. doi: 10.1523/JNEUROSCI.23-22-08135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher D, Morrone MC. Spatiotopic temporal integration of visual motion across saccadic eye movements. Nat Neurosci. 2003;6:877–881. doi: 10.1038/nn1098. [DOI] [PubMed] [Google Scholar]
- Meng H, May PJ, Dickman JD, Angelaki DE. Vestibular signals in primate thalamus: properties and origins. J Neurosci. 2008;27:13590–13602. doi: 10.1523/JNEUROSCI.3931-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergner T, Nasios G, Anastasopoulos D. Vestibular memory-contingent saccades involve somatosensory inut from the body support. Neuroreport. 1998;9:1469–1473. doi: 10.1097/00001756-199805110-00041. [DOI] [PubMed] [Google Scholar]
- Mergner T, Nasios G, Maurer C, Becker W. Visual object localisation in space. Interaction of retinal, eye position, vestibular and neck proprioceptive information. Exp Brain Res. 2001;141:33–51. doi: 10.1007/s002210100826. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL. Spatial updating in human parietal cortex. Neuron. 2003;39:361–373. doi: 10.1016/s0896-6273(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL. Remapping in human visual cortex. J Neurophysiol. 2007;97:1738–1755. doi: 10.1152/jn.00189.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Colby CL. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proc Natl Acad Sci. 2002;99:4026–4031. doi: 10.1073/pnas.052379899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K. Properties of memory-guided saccades toward targets flashed during smooth pursuit in human subjects. Invest Ophthal Vis Sci. 1994;35:509–514. [PubMed] [Google Scholar]
- Phillips MH, Steenrod SC, Goldberg ME. Predictive remapping of LIP receptive fields occurs in motor coordinates. Soc Neurosci Abst. 2007;33 19.5. [Google Scholar]
- Pouget A, Sejnowski TJ. A new view of hemineglect based on the response properties of parietal neurones. Philos Trans R Soc Lond B Biol Sci. 1997;352:1449–1459. doi: 10.1098/rstb.1997.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KD, Goldberg ME. Remapping of visual responses in primate parietal cortex during smooth changes in gaze. Soc Neurosci Abstract. 1997;1 14.11. [Google Scholar]
- Prieur JM, Bourdin C, Vercher JL, Sarès F, Blouin J, Gauthier GM. Accuracy of spatial localization dependiing on head posture in a perturbed gravitoinertial force field. Exp Brain Res. 2005;161:432–440. doi: 10.1007/s00221-004-2087-7. [DOI] [PubMed] [Google Scholar]
- Prime SL, Niemeier M, Crawford JD. Transsaccadic integration of visual features in a line intersection task. Exp Brain Res. 2006;169:532–548. doi: 10.1007/s00221-005-0164-1. [DOI] [PubMed] [Google Scholar]
- Salinas E, Abbott LF. Transfer of coded information from sensory to motor networks. J Neurosci. 1995;15:6461–6474. doi: 10.1523/JNEUROSCI.15-10-06461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH. Interactions between visually and electrically elilcited saccades before and after superior colliculus and frontal eye field ablations in the rhesus monkey. Exp Brain Res. 1983;49:381–392. doi: 10.1007/BF00238780. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M, Dassonville P. Saccades can be aimed at the spatial location of targets flashed during pursuit. J Neurophysiol. 1990;64:575–581. doi: 10.1152/jn.1990.64.2.575. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Schlag J. The central thalamus. Rev Oculomot Res. 1989;3:361–390. [PubMed] [Google Scholar]
- Siegler I, Viaud-Delmon I, Israel I, Berthoz A. Self-motion perception during a sequence of whole-body rotation in darkness. Exp Brain Res. 2000;134:66–73. doi: 10.1007/s002210000415. [DOI] [PubMed] [Google Scholar]
- Smith MA, Crawford JD. Implications of ocular kinematics for the internal updating of visual space. J Neurophysiol. 2001;86:2112–2117. doi: 10.1152/jn.2001.86.4.2112. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Grieve KL, Brotchie P, Andersen RA. Separate body- and world-referenced representations of visual space in parietal cortex. Nature. 1998;394:887–891. doi: 10.1038/29777. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A Pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol. 2004a;91:1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol. 2004b;91:1403–1423. doi: 10.1152/jn.00740.2003. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;222:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Spatial localization of saccade targets. I. Compensation for stimulation-induced perturbations in eye position. J Neurophysiol. 1983;49:45–63. doi: 10.1152/jn.1983.49.1.45. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE, Porter JD. Eye movements induced by pontine stimulation: interaction with visually triggered saccades. J Neurophysiol. 1987;58:300–318. doi: 10.1152/jn.1987.58.2.300. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of spontaneous optokinetic responses produced by visual inversion. J Comparative Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Compensatory saccades made to remembered targets following orbital displacement by electrically stimulating the dorsomedial frontal cortex or frontal eye fields of primates. Brain Res. 1996;727:221–224. doi: 10.1016/0006-8993(96)00475-1. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Müri RM. Role of human frontal and supplementary eye fields in double-step saccades. Neuroreport. 2001;13:253–255. doi: 10.1097/00001756-200202110-00016. [DOI] [PubMed] [Google Scholar]
- Trevarthen C. Integrative functions of the cerebral commissures. In: Boller FG, Grafman J, editors. Handbook of Neuropsychology. Vol. 4. Amsterdam: Elsevier; 1990. pp. 49–83. [Google Scholar]
- Tsostsos LE, Henriques DYP. Estimating the size of eye movements for updating spatial memory. Soc Neurosci Abst. 2007;33 508.14. [Google Scholar]
- Tweed D, Vilis T. Implications of rotational kinematics for the oculomotor system in three dimensions. J neurophysiol. 1987;58:832–849. doi: 10.1152/jn.1987.58.4.832. [DOI] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol. 1997;78:1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. II. Memory responses. J Neurophysiol. 2001;86:2344–2352. doi: 10.1152/jn.2001.86.5.2344. [DOI] [PubMed] [Google Scholar]
- Van Donkelaar P, Müri R. Craniotopic updating of visual space across saccades in the human posterior parietal cortex. Proc Biol Sci. 2001;269:735–739. doi: 10.1098/rspb.2001.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Opstal AJ, Hepp K, Hess BJ, Straumann D, Henn V. Two- rather than three-dimensional representation of saccades in monkey superior colliculus. Science. 1991;252:1313–1315. doi: 10.1126/science.1925545. [DOI] [PubMed] [Google Scholar]
- Van Pelt S, Medendorp WP. Gaze-centered updating of remembered visual space during active whole-body translations. J Neurophysiol. 2007;97:1209–1220. doi: 10.1152/jn.00882.2006. [DOI] [PubMed] [Google Scholar]
- Van Pelt S, Van Gisbergen JA, Medendorp WP. Visuospatial memory computations during whole-body rotations in roll. J Neurophysiol. 2005;94:1432–1442. doi: 10.1152/jn.00018.2005. [DOI] [PubMed] [Google Scholar]
- Versino M, Beltrami G, Uggetti C, Cosi V. Auditory saccade impairment after central thalamus lesions. J Neurol Neurosurg Psychiatry. 2000;68:234–237. doi: 10.1136/jnnp.68.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegen J, Van Grootel TJ, Van Opstal AJ. Dynamic sound localization during rapid eye-head gaze shifts. J Neurosci. 2004;24:9291–9302. doi: 10.1523/JNEUROSCI.2671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegen J, Van Grootel TJ, Van Opstal AJ. Gaze orienting in dynamic visual double steps. J Neurophysiol. 2005;94:4300–4313. doi: 10.1152/jn.00027.2005. [DOI] [PubMed] [Google Scholar]
- Von Holst E, Mittlesteadt H. Das reafferenzprinzip. Naturwissenschaffeten. 1950;37:464–476. [Google Scholar]
- Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat Neurosci. 2007;10:640–646. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]
- Wei M, Li N, Newlands D, Dickman JD, Angelaki DE. Deficits and recovery in visuospatial memory during head motion after bilateral labyrinthine lesion. J Neurophysiol. 2006;96:1676–1682. doi: 10.1152/jn.00012.2006. [DOI] [PubMed] [Google Scholar]
- White RL, Snyder LH. A neural network model of flexible spatial updating. J Neurophysiol. 2004;91:1608–1619. doi: 10.1152/jn.00277.2003. [DOI] [PubMed] [Google Scholar]
- White RL, Snyder LH. Spatial constancy and the brain: insights from neural networks. Philos Trans R Soc Lond B Biol Sci. 2007;362:375–382. doi: 10.1098/rstb.2006.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MF, Fitzgibbon EJ, Goldberg ME. Neurons in the monkey superior colliculus predict the visual result of impending saccadic eye movements. J Neurophysiol. 1995;73:1988–2003. doi: 10.1152/jn.1995.73.5.1988. [DOI] [PubMed] [Google Scholar]
- Wyder MT, Massoglia DP, Stanford TR. Quantitative assessment of the timing and tuning of visual-related, saccade-related, and delay period activity in primate central thalamus. J Neurophysiol. 2003;90:2029–2052. doi: 10.1152/jn.00064.2003. [DOI] [PubMed] [Google Scholar]
- Wyder MT, Massoglia DP, Stanford TR. Contextual modulation of central thalamic delay-period activity: representation of visual and saccadic goals. J neurophysiol. 2004;91:2628–2648. doi: 10.1152/jn.01221.2003. [DOI] [PubMed] [Google Scholar]
- Zivotofsky AZ, Rottach KG, Averbuch-Heller L, Kori AA, Thomas CW, Dell’Osso LF, Leigh RJ. Saccades to remembered targets: the effects of smooth pursuit and illusory stimulus motion. J Neurophysiol. 1996;76:3617–3632. doi: 10.1152/jn.1996.76.6.3617. [DOI] [PubMed] [Google Scholar]