Abstract
Here we report on the reaction of rhenate anions (ReO3−) with multiply protonated peptide cations in a quadrupole linear ion trap mass spectrometer. These reactions effect the formation of an anion–cation complex that, upon collisional activation, dissociates along the peptide backbone rather than by displacement of the anion. Cleavage of the peptide backbone, with anion retention, leads us to conclude the anion–cationcomplexmust be tightly bound, most probably through coordination chemistry. We describe this chemistry and detail the possible application of such ion attachment reactions to the characterization of intact proteins.
Keywords: Ion/ion reaction, Mass spectrometry, Ion attachment, Ion trap
1. Introduction
Ion/ion chemical reactions have become important tools for mass spectrometry-based proteomics and are broadly classified into three categories: (1) proton transfer, (2) electron transfer, or (3) anion attachment [1–6]. Anion composition is a major factor in determining which of these pathways is followed. Most anionic reagents proceed exclusively via the proton transfer (PT) pathway; however, radical anions of polyaromatic hydrocarbons are one subset of anions that show the ability to transfer electrons to peptide cations (electron transfer dissociation)—a method that offers electron capture-like peptide fragmentation on radio frequency-type ion trapping mass spectrometers [7–12]. The third reaction, anion attachment, has received much less attention, but has latent analytical utility. Phosphorus hexafluoride, I−, and certain metal-containing anions can form long-lived complexes with peptide cations; however, these complexes are easily dissociated upon collisional activation (i.e., they are intermediates of the proton transfer reaction) [13–15]. Anions that covalently bind to a peptide/protein cation have potential use for inducing selective gas-phase cleavage of peptide bonds. So far this chemistry has remained elusive. Glish and Payne, however, have provided some evidence of this chemistry in their work detailing the reaction of FeCO2− and peptide cations, where they observed backbone cleavage products that contained the reagent [16]. Further evidence for this chemistry comes from the work of Gunawardena et al. in the reaction of AuCl2− anions with peptide cations for selective disul.de bond cleavage [17]. These works, along with our own, lead us to conclude that anionic composition is the main driver of ion/ion chemistry and that continued exploration is certain to reveal new classes of anions that form more tightly bound complexes.
Through peptide–metal chelation, metal ions serve as required catalysts for nearly 1/3 of all protein enzymes. Coordinated metal ions ensure proper enzyme–substrate orientation, activate bonds, facilitate nucleophilic attack, and stabilize charge (i.e., lower activation barriers) [18]. Metallopeptidases, for example, utilize Ca or Zn ions to catalyze hydrolysis of peptide bonds, in the cell [19]. Given the propensity of metal ions, or their complexes, to associate with proteins in the condensed-phase, this class of compounds is obvious to investigate. As representatives of this class, we have generated anionic oxides of rhenium (ReO3− and ReO4−, rhenate and perrhenate, respectively) and studied their reaction phenomenology with peptide cations. These species are easily generated by regulation of a small oxygen leak into the chemical ionization source region of our mass spectrometer’s anion source as Re atoms are released by the filament and oxidized in the presence of oxygen. Here we demonstrate that the reaction of rhenate anions with peptide cations can result in the loss of two molecules of water and an attachment product that remains intact, even upon collisional activation. The presence of the bound rhenate also affects the preferred peptide dissociation channels.
2. Experimental
Multiply-protonated peptides were generated by electrospray ionization (ESI) using an Advion Nanomate ESI device (Advion, Ithaca, NY). A 40% aqueous acetonitrile solution (with 0.1% acetic acid) containing peptides at 1 pmol/µL. The studied peptides were either purchased or in-house synthesized (Sigma–Aldrich, St. Louis, MO, USA). A Finnigan LTQ linear ion trap mass spectrometer (Thermo Scientific, San Jose, CA, USA) was adapted to accept a chemical ionization source, which was mounted on the rear side of the device, opposing the factory nanospray source. Negative chemical ionization (NICI), with methane buffer gas (MG Industries, Malvern, PA, USA), was used to produce anions. Rhenium-containing anions were generated by a regulated leak of oxygen, sometimes 18O2 (Sigma, St. Louis, MO). Ion/ion reactions were conducted in the linear ion trap by use of charge-sign-independent trapping, i.e., the electronics were modified to allow superposition of a secondary RF trapping voltage to the end lenses of the ion trap [9,10]. This provided axial containment to complement the radial containment provided by the main RF “quadrupole” trapping field, allowing simultaneous trapping of both anions and cations.
3. Results and discussion
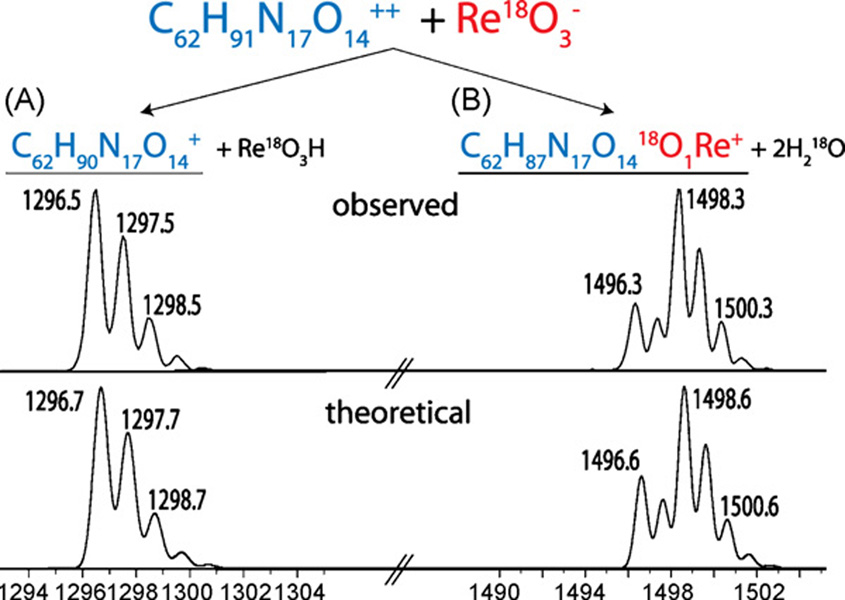
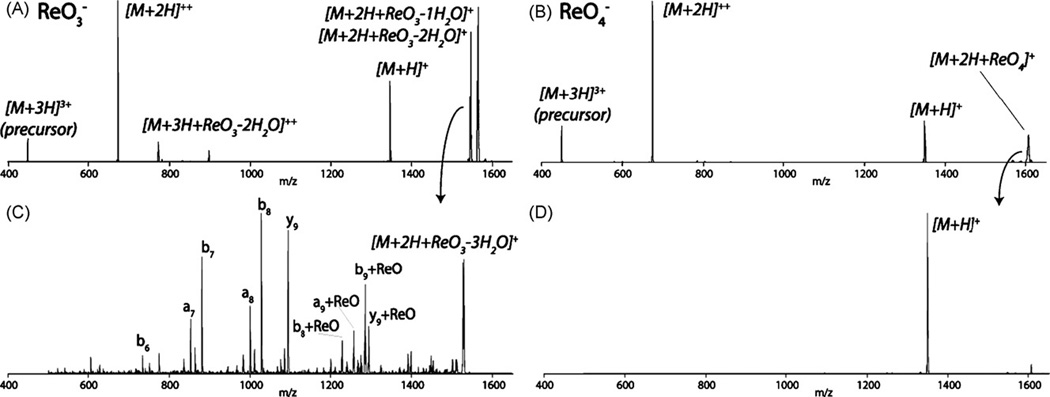
Fig. 1 displays the products of a reaction involving a doubly protonated peptide cation with 18O-labeled rhenate anions. Two reaction pathways were observed—proton transfer and ion attachment (Fig. 1 pathways A and B, respectively). We note rhenate attachment results in the concomitant elimination of 1 or 2 molecules of water; both of the displaced oxygen atoms come from those originally present in the rhenate. Also shown in Fig. 1 is the theoretical isotopic distribution of the rhenate attachment product (C62H87N17O1418O1Re+), which exactly matches that of the observed reaction product. Reaction of numerous other peptide cations with 18O-labeled rhenate anions produced results identical to those shown in Fig. 1—that is, two molecules of water were released, each containing an 18O from the labeled rhenate anion (data not shown). This observation leads us to conclude that rhenate attachment likely results in the formation of peptide–rhenium coordination complex. Next the triply protonated cation of substance P (RPKPQQFFGLM) was reacted with rhenate anions (Fig. 2A), followed by collisional activated dissociation (CAD) of the attachment product ([M+2H + ReO−2H2O]+, Fig. 2C). Fig. 2C displays that the rhenate attachment product dissociates along the peptide backbone to create N- and C-terminal fragment ions (b- and y-type, respectively) with no detectable anion loss. Cleavage of the peptide backbone, with anion retention, following CAD suggests the anion–cationcomplex is tightly bound, probably through a covalent interaction.
Fig. 1.
Stoichiometry of rhenate anion attachment. Rhenate attachment results in the loss of two molecules of water, of which the oxygen atoms are supplied by rhenate. The observed and theoretical product ion isotope distributions are shown.
Fig. 2.
Ion attachment of ReO3− and ReO4− anions to a triply protonated peptide following a 100ms reaction (panels A and B, respectively). Panels C and D display product ions spectra following collisional activation of the rhenate and perrhenate attachment products. Peptide backbone bonds are broken, with no detectable anion loss following collisional activation of the rhenate attachment product (panel C).
Perrhenate (ReO4−) also binds to peptide cations following an ion/ion reaction; however, unlike rhenate, no water losses are observed (Fig. 2B). Fig. 2D displays that upon CAD, the perrhenate attachment product dissociates exclusively to form the deprotonated peptide ([M+H]+)—indicating that perrhenate can attach, but forms a long-lived proton transfer intermediate rather than a coordination complex (Fig. 2D). These differences in behavior of rhenate and perrhenate are reflected in their respective electronic states—the rhenate anion (Re+5, d2, 14 electron) can be described as coordinatively unsaturated, while perrhenate is not (18 electron) [20]. Rhenium prefers an 18 electron environment and, thus, it is not surprising that rhenate readily attaches to peptide cations through bond formation (i.e., the activation barrier for bond formation is lowered because of rhenate’s electronic state). These experiments strengthen our hypothesis that the rhenate species forms a covalently bound complex upon reaction with peptide cations.
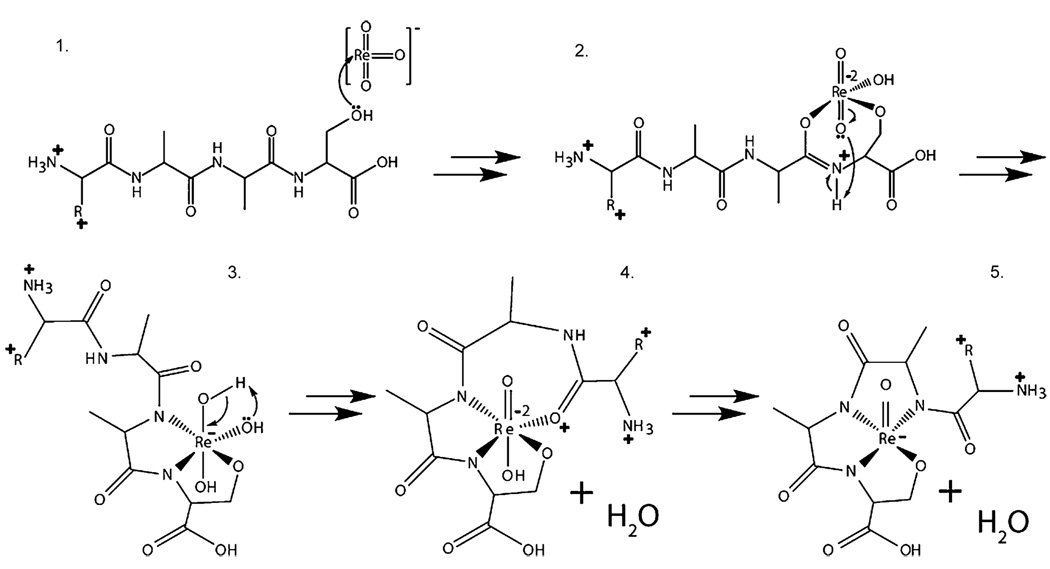
Fig. 3 presents a possible mechanism for the reaction of rhenate with peptide cations. Ion/ion reactions result from the formation of a long-lived cation/anion-orbiting complex [21–26]. During this time, an anion may come into close proximity to the peptide cation so that the Lewis basic lone pairs of the peptide attack the electrophilic coordinatively unsaturated electron rhenate anion, thus, forming an initial linkage (Fig. 3, structure 1). Failure to locate a suitable ligand would result in a proton transfer from the cation to the anion, which is also observed (Fig. 1A). We postulate that through a series of nucleophilic attacks from backbone carbonyls and subsequent ring rearrangements, the Re atom is highly coordinated through four linkages (either amidate, or side-chain) and bears only one of the three O atoms from rhenate. Rhenium(V) OXO complexes are known to react with acidic protons to lose water via hydroxyl complexes in the condensed-phase [27].We note that the gas-phase attachment (coordination) chemistry displayed by rhenium is consistent with condensed-phase observations [27]. Oxidative cleavage of peptide bonds through chelation of metal complexes in the condensed-phase has been described extensively [28]. Platinum and palladium complexes, for example, can initiate regioselective cleavage, each with their own specificity [29–35]. And Meares and co-workers have described iron–EDTA complexes as a cleaving reagent to characterize the subunits of RNA polymerase [36], while copper and nickel peptidases have also been described [19].
Fig. 3.
Possible mechanism for the attachment of rhenate to a doubly protonated peptide.
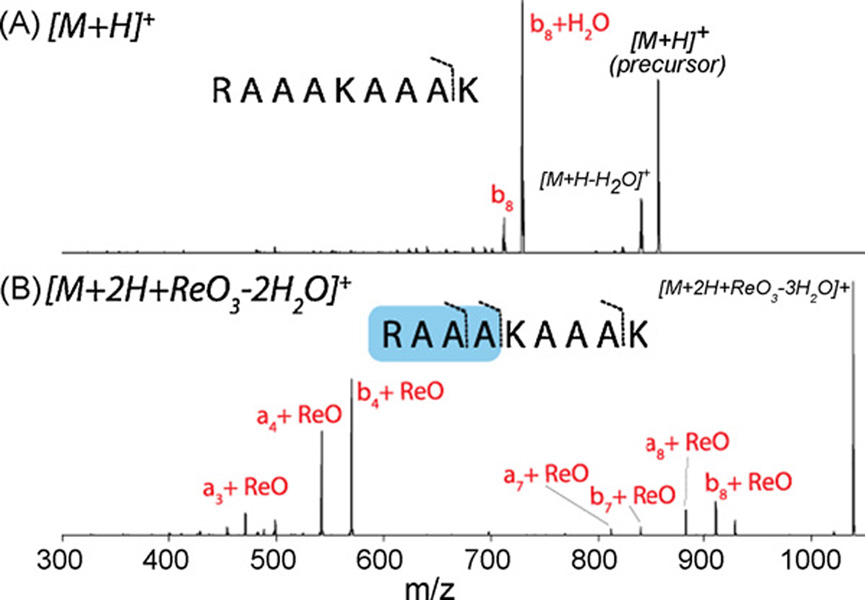
Next we tested whether the presence of the bound rhenate altered the preferred peptide fragmentation pathways. CAD of the singly protonated RAAAKAAAK peptide (unmodified, Fig. 4A) generates one major backbone bond cleavage—b8 product (a fragment carrying the first eight residues from the N-terminus). Dissociation of the singly protonated RAAAKAAAK + rhenate attachment product ([M+2H + ReO3−2H2O]+, Fig. 4B), on the other hand, induces cleavage between the fourth and fifth residues (a4 and b4); both of these products contain the rhenium atom. Some cleavage is also observed at the eighth residue as before, but these ions also contain the anion. From these data we postulate that the rhenium complex preferentially binds at the N-terminal four residues of this peptide and, upon CAD, its presence alters which dissociation pathways are favored. This trend is also observed among the other synthetic peptides tested (data not shown).
Fig. 4.
Collision-activated dissociation (CAD) of the singly protonated peptide RAAAKAAAK (A) and the same peptide following attachment of the rhenate anion (B). Note the preferred dissociation pathways change.
4. Conclusions
Here we report that rhenate anions engage in ion attachment chemistry when reacted with peptide cations in an RF-linear ion trap. The reaction results in the formation of coordination complexes that affect downstream peptide dissociation pathways. From these experiments we conclude that further anion exploration will likely reveal other metal-containing anionic reagents that coordinate to peptide and protein cations with high sequence specificity. There are many potential applications of these chemistries, in one exciting scenario the bound anionic reagents serve as scaffolds to harbor site-specific proteolytic cleavage, with enzyme-like specificity. Today the mass of whole protein molecules can be determined on a sub-second time-scale with remarkable precision and accuracy using mass spectrometry. Molecular weight alone, however, cannot uniquely identify a protein’s primary sequence. For sequence identification, a population of gas-phase protein ions is dissociated, within the mass spectrometer, and the resulting fragment masses recorded. Such a strategy represents a ‘top-down’ sequencing method, one that is steadily gaining favor because it captures the most biological information [37]. However, as protein mass increases (~>25 kDa), its efficacy diminishes. Simply put, increasing protein size elevates the number of possible dissociation channels—translating to increased spectral complexity and decreased signal-to-noise. One approach to remove the mass limitation of the ‘top-down’ method is to develop chemistries for the systematic disassembly of a GP protein cation-utilizing reagent ions that catalyze residue-specific peptide hydrolysis. By facilitating the sequencing of high mass proteins, such reactions would significantly advance the rapidly growing field of proteomics.
The data presented here demonstrates that ion/ion reactions can result in covalently bound complexes – a solid first step; however, the overall goal defined above demands sequence-specific anion binding – an ambition not yet fulfilled. Our proposed mechanism suggests that rhenate should attach to certain peptide sequences with higher propensity than others—that is, certain amino acid side chains are likely to be more effective nucleophiles than others and, thus, should bind rhenate more effectively. Along with reagent anion discovery, systematic studies of the effect of amino acid side chain and secondary structure on anion binding is a critical component of future work in this area.
Acknowledgments
We thank Charles Casey, Judith Burstyn, Jeffrey Shabanowitz, John Syka, Lloyd Smith, and Willard Harrison for helpful discussions Thermo Scientific, the Beckman Foundation, the American Society of Mass Spectrometry, Eli Lilly, the National Science Foundation (0701846; 0747990), and the NIH (1R01GM080148) provided financial support for this work. GCM gratefully acknowledges support from an NIH pre-doctoral fellowships (Biotechnology Training Program, NIH 5T32GM08349).
References
- 1.Loo RRO, Udseth HR, Smith RD. Journal of the American Society for Mass Spectrometry. 1992;3:695. doi: 10.1016/1044-0305(92)87082-A. [DOI] [PubMed] [Google Scholar]
- 2.McLuckey SA, Stephenson JL. Mass Spectrometry Reviews. 1998;17:369. doi: 10.1002/(SICI)1098-2787(1998)17:6<369::AID-MAS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Scalf M, Westphall MS, Krause J, Kaufman SL, Smith LM. Science. 1999;283:194. doi: 10.1126/science.283.5399.194. [DOI] [PubMed] [Google Scholar]
- 4.He M, McLuckey SA. Journal of Mass Spectrometry. 2004;39:1231. doi: 10.1002/jms.629. [DOI] [PubMed] [Google Scholar]
- 5.Coon JJ, Syka JE, Shabanowitz J, Hunt DF. Biotechniques. 2005;38:519. doi: 10.2144/05384TE01. [DOI] [PubMed] [Google Scholar]
- 6.Good DM, Coon JJ. Biotechniques. 2006;40:783. doi: 10.2144/000112194. [DOI] [PubMed] [Google Scholar]
- 7.Coon JJ, Shabanowitz J, Hunt DF, Syka JEP. Journal of the American Society for Mass Spectrometry. 2005;16:880. doi: 10.1016/j.jasms.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Coon JJ, Ueberheide B, Syka JEP, Dryhurst DD, Ausio J, Shabanowitz J, Hunt DF. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9463. doi: 10.1073/pnas.0503189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coon JJ, Syka JEP, Schwartz JC, Shabanowitz J, Hunt DF. International Journal of Mass Spectrometry. 2004;236:33. [Google Scholar]
- 10.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9528. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chrisman PA, Pitteri SJ, Hogan JM, McLuckey SA. Journal of the American Society for Mass Spectrometry. 2005;16:1020. doi: 10.1016/j.jasms.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor PB, Cournoyer JJ, Pitteri SJ, Chrisman PA, McLuckey SA. Journal of the American Society for Mass Spectrometry. 2006;17:15. doi: 10.1016/j.jasms.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson JL, McLuckey SA. Journal of the American Chemical Society. 1997;119:1688. [Google Scholar]
- 14.Newton KA, McLuckey SA. Journal of the American Society for Mass Spectrometry. 2004;15:607. doi: 10.1016/j.jasms.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Newton KA, Amunugama R, McLuckey SA. Journal of Physical Chemistry A. 2005;109:3608. doi: 10.1021/jp04416i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payne AH, Glish GL. International Journal of Mass Spectrometry. 2001;204:47. [Google Scholar]
- 17.Gunawardena HP, O’Hair RAJ, McLuckey SA. Journal of Proteome Research. 2006;5:2087. doi: 10.1021/pr0602794. [DOI] [PubMed] [Google Scholar]
- 18.Voet D, Voet JG. Biochemistry. 2nd ed. John Wiley and Sons, Inc.; 1995. [Google Scholar]
- 19.Polzin GM, Burstyn JN. In: Metal Ions in Biological Systems. Sigel A, Sigel H, editors. vol. 38. New York: Marcel Dekker, Inc.; 2001. p. 104. [Google Scholar]
- 20.Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. 5th ed. New York: John Wiley and Sons; 1988. [Google Scholar]
- 21.Mcluckey SA, Glish GL, Kelley PE. Analytical Chemistry. 1987;59:1670. [Google Scholar]
- 22.Herron WJ, Goeringer DE, Mcluckey SA. Journal of the American Society for Mass Spectrometry. 1995;6:529. doi: 10.1016/1044-0305(95)00199-N. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson JL, McLuckey SA. Journal of the American Chemical Society. 1996;118:7390. [Google Scholar]
- 24.Stephenson JL, McLuckey SA. International Journal of Mass Spectrometry. 1997;165:419. [Google Scholar]
- 25.Stephenson JL, VanBerkel GJ, McLuckey SA. Journal of the American Society for Mass Spectrometry. 1997;8:637. [Google Scholar]
- 26.McLuckey SA, Wells JM. Chemical Reviews. 2001;101:571. doi: 10.1021/cr990087a. [DOI] [PubMed] [Google Scholar]
- 27.McCleverty JA, Meyer TJ. Comprehensive Coordination Chemistry. II. From Biology to Nanotechnology. Vol. 5. Amsterdam: Elsevier/Pergamon; 2004. [Google Scholar]
- 28.Grant KB, Kassai M. Current Organic Chemistry. 2006;10:1035. [Google Scholar]
- 29.Dutca LM, Ko KS, Pohl NL, Kostic NM. Inorganic Chemistry. 2005;44:5141. doi: 10.1021/ic050137w. [DOI] [PubMed] [Google Scholar]
- 30.Milovic NM, Dutca LM, Kostic NM. Chemistry—A European Journal. 2003;9:5097. doi: 10.1002/chem.200304772. [DOI] [PubMed] [Google Scholar]
- 31.Grove TZ, Kostic NM. Journal of the American Chemical Society. 2003;125:10598. doi: 10.1021/ja036009t. [DOI] [PubMed] [Google Scholar]
- 32.Milovic NM, Kostic NM. Journal of the American Chemical Society. 2003;125:781. doi: 10.1021/ja027408b. [DOI] [PubMed] [Google Scholar]
- 33.Milovic NM, Badjic JD, Kostic NM. Journal of the American Chemical Society. 2004;126:696. doi: 10.1021/ja038404p. [DOI] [PubMed] [Google Scholar]
- 34.Johnson TW, Kostic NM. Journal of the Serbian Chemical Society. 2004;69:887. [Google Scholar]
- 35.Stoffregen SA, Griffin AKK, Kostic NM. Inorganic Chemistry. 2005;44:8899. doi: 10.1021/ic0506613. [DOI] [PubMed] [Google Scholar]
- 36.Greiner DP, Hughes KA, Gunasekera AH, Meares CF. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:71. doi: 10.1073/pnas.93.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelleher NL. Analytical Chemistry. 2004;76:196A. [PubMed] [Google Scholar]