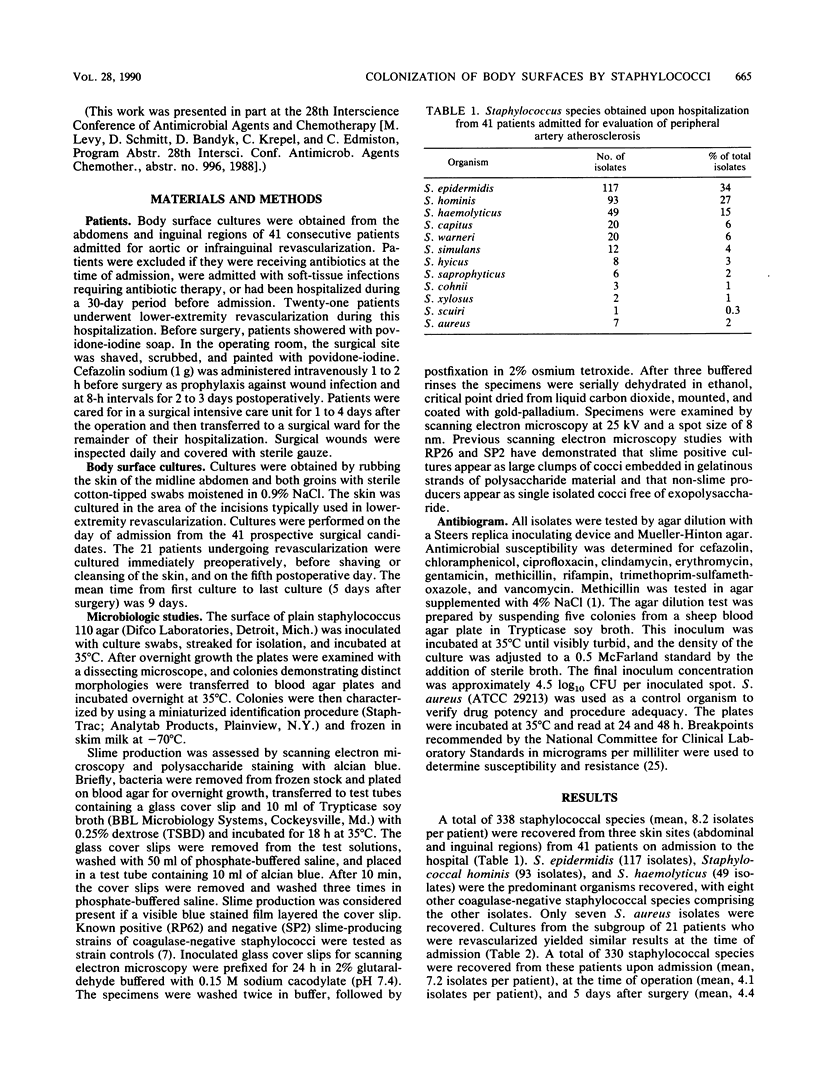
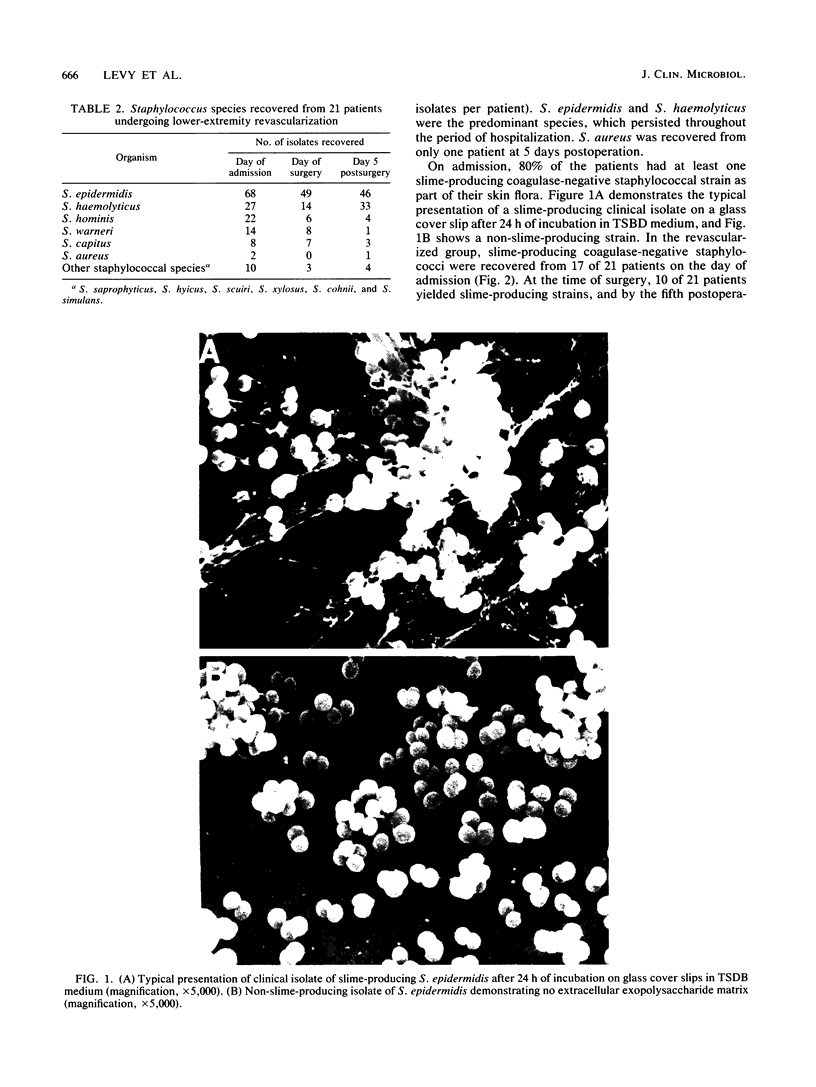
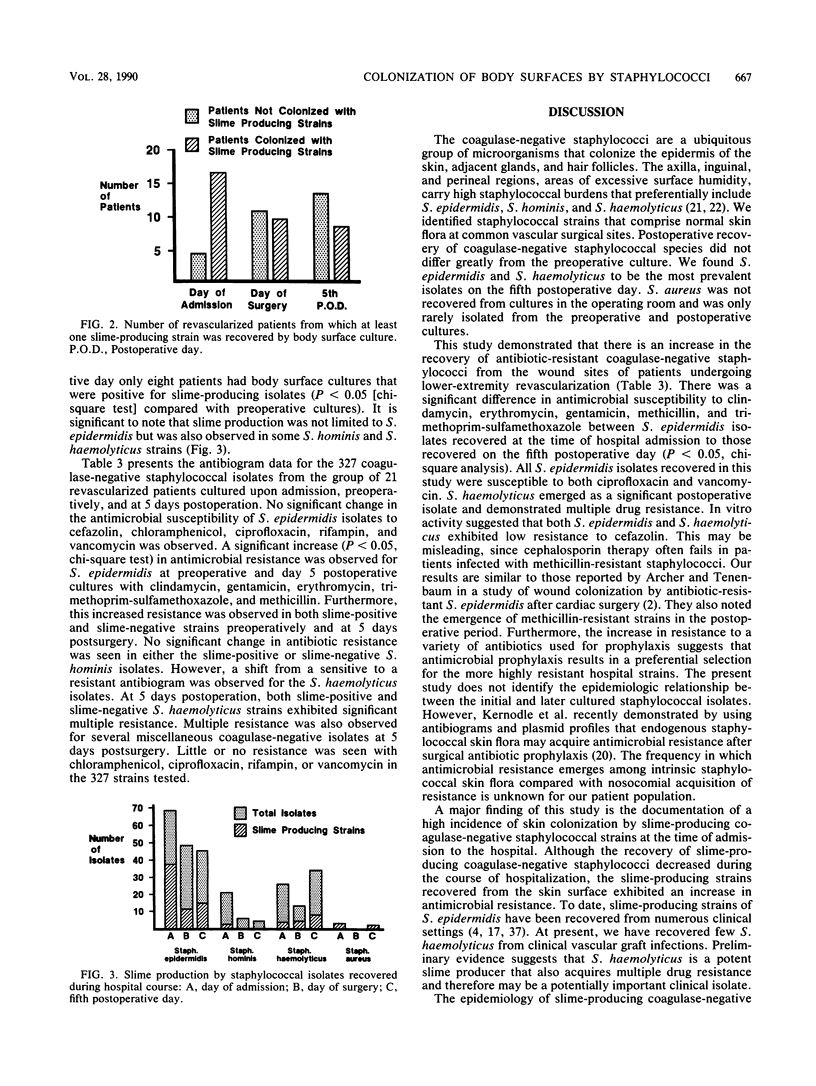
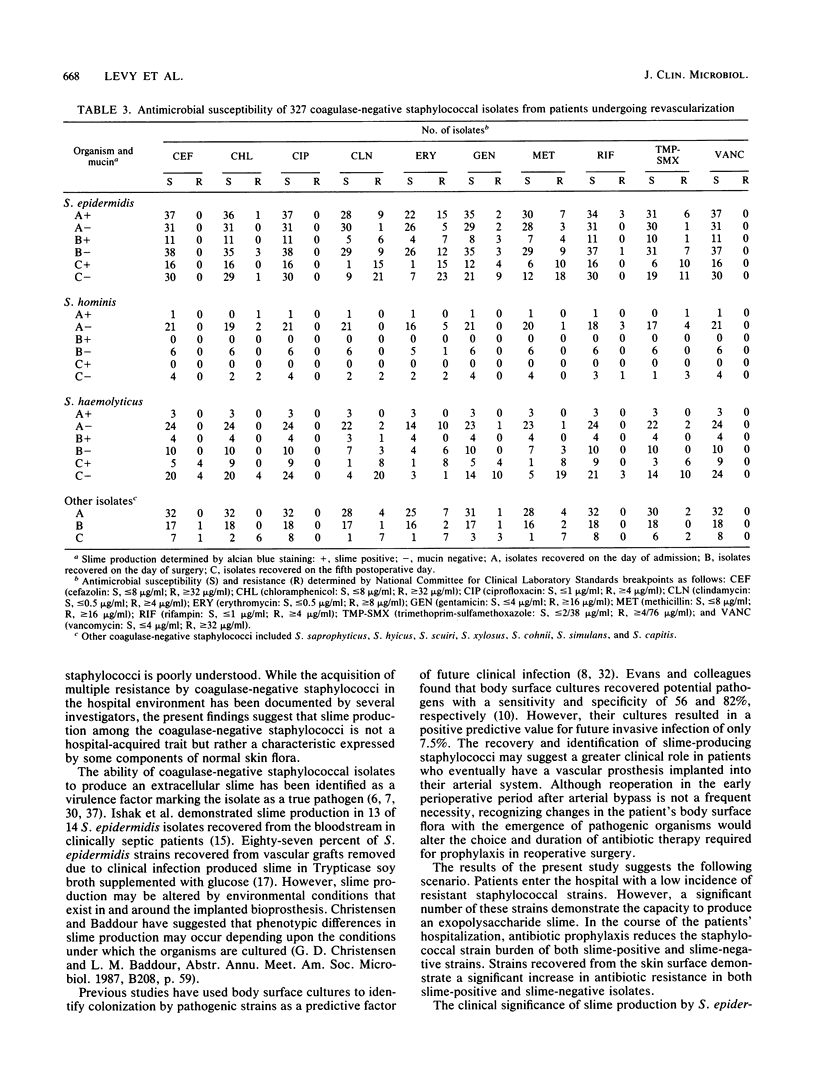
Abstract
Slime-producing coagulase-negative staphylococci are pathogens in vascular surgery by virtue of their ability to adhere to and persist on prosthetic graft material. Inguinal and abdominal skin sites were cultured in 41 patients upon hospitalization, and slime production and antimicrobial susceptibility were assessed in all recovered staphylococcal isolates. Twenty-one patients eventually underwent lower-extremity revascularization. In the operative population, cultures were also obtained on the day of surgery and fifth postoperative day. All 21 patients received perioperative cefazolin. Of 327 coagulase-negative staphylococci recovered, Staphylococcus epidermidis (47%), S. haemolyticus (21%), and S. hominis (10%) were the predominant isolates. Slime-producing coagulase-negative staphylococci were recovered from 17 of 21 patients at admission but only from 8 of 21 patients on day 5 postoperation (P less than 0.05). S. epidermidis isolates demonstrated increasing multiple resistance from admission to 5 days postoperation to methicillin, gentamicin, clindamycin, erythromycin, and trimethoprim-sulfamethoxazole (P less than 0.05). All coagulase-negative staphylococcal isolates were susceptible to ciprofloxacin and vancomycin. Slime-producing capability was not associated with increased methicillin resistance for the recovered isolates. The data demonstrate that patients enter the hospital colonized with slime-producing strains of coagulase-negative staphylococci and that during hospitalization the staphylococcal skin burden shifts from a predominately susceptible to a resistant microbial population, which may enhance the importance of slime production as a risk factor in lower-extremity revascularization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Tenenbaum M. J. Antibiotic-resistant Staphylococcus epidermidis in patients undergoing cardiac surgery. Antimicrob Agents Chemother. 1980 Feb;17(2):269–272. doi: 10.1128/aac.17.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyk D. F., Berni G. A., Thiele B. L., Towne J. B. Aortofemoral graft infection due to Staphylococcus epidermidis. Arch Surg. 1984 Jan;119(1):102–108. doi: 10.1001/archsurg.1984.01390130084015. [DOI] [PubMed] [Google Scholar]
- Bayston R., Penny S. R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- Bunt T. J., Mohr J. D. Incidence of positive inguinal lymph node cultures during peripheral revascularization. Am Surg. 1984 Oct;50(10):522–523. [PubMed] [Google Scholar]
- COVENTRY K. J., ISBISTER C. A bacteriological and clinical study of infection in newborn babies in a maternity hospital nursery. Med J Aust. 1951 Sep 22;2(12):394–396. doi: 10.5694/j.1326-5377.1951.tb68387.x. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Younger J. J., Baddour L. M., Barrett F. F., Melton D. M., Beachey E. H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985 Dec;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston C. E., Jr, Schmitt D. D., Seabrook G. R. Coagulase-negative staphylococcal infections in vascular surgery: epidemiology and pathogenesis. Infect Control Hosp Epidemiol. 1989 Mar;10(3):111–117. doi: 10.1086/645977. [DOI] [PubMed] [Google Scholar]
- Evans M. E., Schaffner W., Federspiel C. F., Cotton R. B., McKee K. T., Jr, Stratton C. W. Sensitivity, specificity, and predictive value of body surface cultures in a neonatal intensive care unit. JAMA. 1988 Jan 8;259(2):248–252. [PubMed] [Google Scholar]
- Fitzgerald R. H., Jr, Nolan D. R., Ilstrup D. M., Van Scoy R. E., Washington J. A., 2nd, Coventry M. B. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977 Oct;59(7):847–855. [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Ishak M. A., Gröschel D. H., Mandell G. L., Wenzel R. P. Association of slime with pathogenicity of coagulase-negative staphylococci causing nosocomial septicemia. J Clin Microbiol. 1985 Dec;22(6):1025–1029. doi: 10.1128/jcm.22.6.1025-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. M., Lee D. A., Regelmann W. E., Gray E. D., Peters G., Quie P. G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986 Oct;54(1):13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaebnick H. W., Bandyk D. F., Bergamini T. W., Towne J. B. The microbiology of explanted vascular prostheses. Surgery. 1987 Oct;102(4):756–762. [PubMed] [Google Scholar]
- Kaiser A. B., Clayson K. R., Mulherin J. L., Jr, Roach A. C., Allen T. R., Edwards W. H., Dale W. A. Antibiotic prophylaxis in vascular surgery. Ann Surg. 1978 Sep;188(3):283–289. doi: 10.1097/00000658-197809000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernodle D. S., Barg N. L., Kaiser A. B. Low-level colonization of hospitalized patients with methicillin-resistant coagulase-negative staphylococci and emergence of the organisms during surgical antimicrobial prophylaxis. Antimicrob Agents Chemother. 1988 Feb;32(2):202–208. doi: 10.1128/aac.32.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos W. E., Musselwhite M. S. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol. 1975 Sep;30(3):381–385. doi: 10.1128/am.30.3.381-395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liekweg W. G., Jr, Greenfield L. J. Vascular prosthetic infections: collected experience and results of treatment. Surgery. 1977 Mar;81(3):335–342. [PubMed] [Google Scholar]
- Lowy F. D., Hammer S. M. Staphylococcus epidermidis infections. Ann Intern Med. 1983 Dec;99(6):834–839. doi: 10.7326/0003-4819-99-6-834. [DOI] [PubMed] [Google Scholar]
- Morgenstern L. Postoperative jaundice. An approach to a diagnostic dilemma. Am J Surg. 1974 Aug;128(2):255–261. [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Quie P. G., Belani K. K. Coagulase-negative staphylococcal adherence and persistence. J Infect Dis. 1987 Oct;156(4):543–547. doi: 10.1093/infdis/156.4.543. [DOI] [PubMed] [Google Scholar]
- Rather P. N., Davis A. P., Wilkinson B. J. Slime production by bovine milk Staphylococcus aureus and identification of coagulase-negative staphylococcal isolates. J Clin Microbiol. 1986 May;23(5):858–862. doi: 10.1128/jcm.23.5.858-862.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J., Rogers W. A., Taylor H. M., Everett E. D., Prowant B. F., Fruto L. V., Nolph K. D. Peritonitis during continuous ambulatory peritoneal dialysis. Ann Intern Med. 1980 Jan;92(1):7–13. doi: 10.7326/0003-4819-92-1-7. [DOI] [PubMed] [Google Scholar]
- SIMON H. J., YAFFE S. J., GLUCK L. Effective control of staphylococci in a nursery. N Engl J Med. 1961 Dec 14;265:1171–1176. doi: 10.1056/NEJM196112142652401. [DOI] [PubMed] [Google Scholar]
- Schmitt D. D., Bandyk D. F., Pequet A. J., Malangoni M. A., Towne J. B. Mucin production by Staphylococcus epidermidis. A virulence factor promoting adherence to vascular grafts. Arch Surg. 1986 Jan;121(1):89–95. doi: 10.1001/archsurg.1986.01400010103013. [DOI] [PubMed] [Google Scholar]
- Schmitt D. D., Bandyk D. F., Pequet A. J., Towne J. B. Bacterial adherence to vascular prostheses. A determinant of graft infectivity. J Vasc Surg. 1986 May;3(5):732–740. [PubMed] [Google Scholar]
- Slaughter L., Morris J. E., Starr A. Prosthetic valvular endocarditis. A 12-year review. Circulation. 1973 Jun;47(6):1319–1326. doi: 10.1161/01.cir.47.6.1319. [DOI] [PubMed] [Google Scholar]
- Szilagyi D. E., Smith R. F., Elliott J. P., Vrandecic M. P. Infection in arterial reconstruction with synthetic grafts. Ann Surg. 1972 Sep;176(3):321–333. doi: 10.1097/00000658-197209000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Yamashita N., Goldmann D. A., Pier G. B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988 Apr;157(4):713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- Tollefson D. F., Bandyk D. F., Kaebnick H. W., Seabrook G. R., Towne J. B. Surface biofilm disruption. Enhanced recovery of microorganisms from vascular prostheses. Arch Surg. 1987 Jan;122(1):38–43. doi: 10.1001/archsurg.1987.01400130044006. [DOI] [PubMed] [Google Scholar]
- Younger J. J., Christensen G. D., Bartley D. L., Simmons J. C., Barrett F. F. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987 Oct;156(4):548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]



