Abstract
We have found natural products exhibiting lipolysis-promoting activity in subcutaneous adipocytes, which are less sensitive to hormones than visceral adipocytes. The activities and a action mechanisms of a novel plant extract of Cirsium oligophyllum (CE) were investigated in isolated adipocytes from rat subcutaneous fat, and its fat-reducing effects by peroral administration and topical application were evaluated in vivo. CE-induced lipolysis was synergistically enhanced by caffeine, a phosphodiesterase inhibitor, and was reduced by propranolol, a β adrenergic antagonist. The peroral administration of 10% CE solution to Wistar rats for 32 days reduced body weight gain, subcutaneous, and visceral fat weights by 6.6, 26.2, and 3.0%, respectively, as compared to the control group. By the topical application of 2% of this extract to rats for 7 days, weight of subcutaneous fat in the treated skin was reduced by 23.2%. This fat mass reduction was accompanied by the up-regulation of uncoupling protein 1 (UCP), a principal thermogenic mitochondrial molecule related to energy dissipating, in subcutaneous fat and UCP3 in skin except for the fat layer. These results indicate that CE promotes lipolysis via a mechanism involving the β adrenergic receptor, and affects the body fat mass. This fat reduction may be partially due to UCP up-regulation in the skin including subcutaneous fat. This is the first report showing that repeated lipolysis promotion through CE administration may be beneficial for the systematic suppression of body fat accumulation or the control of fat distribution in obesity.
Keywords: β adrenergic receptor, lipolysis, fat reduction, uncoupling protein, plant extract
Introduction
The excessive accumulation of body fat causes problems in terms of health, such as metabolic syndrome, as well as esthetics. One of the representative measures against excessive body fat accumulation is promotion of the lipolysis of neutral lipids accumulated in adipocytes, reducing adipocyte size.
The lipolytic reaction in adipocytes is mainly explained by the intracellular signaling mechanism using cAMP as a second messenger 1, 2, 3. That is, adrenaline and noradrenaline stimulate at least three subtypes of β adrenergic receptor (βAR) on the adipocyte cell surface and sequentially induce the activation of a stimulatory G protein (Gs), increase cAMP due to the activation of adenylate cyclase, activate cAMP-dependent protein kinase A (PKA), and activate hormone-sensitive lipase (HSL), resulting in the hydrolysis of triglycerides in intracellular lipid droplets. Precise mechanisms involving the modification of HSL activity or location implicating perilipin, novel adipose triglyceride lipase, triglyceride hydrolase, and other cofactors, such as CGI-58, have been suggested 4, 5.
β adrenergic agonists, adenylate cyclase activators, and phosphodiesterase (PDE) inhibitors can promote lipolysis; however, obesity reduces adipose tissue responsiveness to hormones 6. Further, subcutaneous fat shows lower lipolytic responses to hormones than intra-abdominal (visceral) fat, and the above drugs with effects on visceral fat do not always have similar, adequate effects on subcutaneous fat 7, 8.
Uncoupling protein (UCP) is a mitochondrial membrane protein that uncouples oxidative phosphorylation by bypassing the electrochemical gradient across the inner membrane from F1-ATPase, thereby dissipating energy as heat (thermogenesis) 9. UCP1 is expressed exclusively in brown adipose tissue (BAT), which is the major site of regulatory thermogenesis of small rodents during cold acclimation, arousal from hibernation, and recovery from anesthetic hyperthermia 10. Since UCP1 is also activated during voluntary overfeeding, this isoform is believed to be a key molecule for diet- as well as cold-induced thermogenesis, and thereby for the autonomic control of energy expenditure and total body energy balance 11, 12, 13, 14. UCP2, expressed ubiquitously in various tissues, and UCP3, expressed abundantly in skeletal muscle and BAT, have been suggested to be involved in the regulation of energy dissipating 15, 16, 17. In non-adipose tissue and skeletal muscle, we identified UCP2 and UCP3 in the human epidermis and dermis, and also UCP1 in the epidermis 18. The expression of skin-UCP was affected by nutritional factors and hormones in in vitro experiments; however, the physiological role of skin-UCP and regulatory interactions between skin components has never been investigated.
We have discovered a thistle extract exhibiting a remarkable lipolysis-promoting activity in isolated subcutaneous adipocytes. In this study, we investigated its mechanism of action, and the in vivo effects of its application on the body fat mass and skin-UCP expression were evaluated.
Materials and methods
Preparation of plant extract
In this study, we selected Cirsium oligophyllum as a commercial availabile crude drug with lipolysis-promoting activity. Dried plants of Cirsium oligophyllum within Compositae, a type of thistle purchased from Tochimoto Tenkaido (Osaka, Japan) were subjected to extraction with a 30% ethanol aqueous solution at room temperature, followed by heating and concentration, and an extract (CE) was obtained. A part of the extract was frozen and dried, and the concentration of the extracted dry matter of the extract was obtained. In this experiment, the extract concentration was expressed as the extracted dry matter concentration. For experimental repeatability, we restricted to one species of plant, identified several active compounds, and confirmed the stability of its quality.
Animals
Male Wistar rats aged 9 weeks (body weight, 180-220 g) were obtained from Japan SLC, Inc. (Shizuoka, Japan) and maintained at 22±1 Cº under a 12-h light-dark cycle (lights on from 7:00 AM to 7:00 PM). The rats were fed laboratory chow, CE-2 obtained from CLEA Japan, Inc. (Tokyo, Japan), and allowed ad libitum access to water for a week to stabilize the metabolic conditions.
We were certain that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during this research. All animal experiments were conducted in the Experimental Animal Facility of Kao Tochigi Institute. The Animal Care Committee of Kao Tochigi Institute approved the present study. All experiments strictly followed the guidelines of that committee.
Isolation of adipocytes
Isolated adipocytes were prepared from the abdominal-inguinal subcutaneous fat pads of three Wistar male rats aged 9 weeks and weighing about 180-220 g, according to the method reported by Rodbell 19. Briefly, the excised adipose tissue was cut into small pieces using ophthalmologic scissors, suspended and washed with Hank's buffer containing 2% bovine serum albumin fraction V, and digested with collagenase Type I in the presence of 1mg/ml glucose for about 60 minutes at 37°C. The digested tissue was filtered through a nylon mesh (mesh size: 40 μm), and floating cells were collected and gently rinsed 3 times with Hank's buffer, and suspended in the same buffer.
Treatment of isolated adipocytes
The isolated adipocyte suspension was incubated with various concentrations of NA, CE, propranorol, as an β adrenergic antagonist, or caffeine, as a PDE inhibitor, in Hank's buffer at 37°C for 2 hours. The glycerol released into the buffer after incubation was measured using the enzyme method 20. Briefly, the buffer after stimulation was mixed with glycerol-detecting solution and absorbance was measured at 480 nm. The composition of the glycerol-detecting solution was 2.7 mmol/l p-chlorophenol, 0.04% Triton X-100, 2 mmol/l magnesium sulfate, 2 mmol/l ATP, 0.05 mmol/l 4-amino antipiline, 1 mmol/l EDTA, 0.5 U glycerokinase, 4 U glycerol-3-phosphate oxidase and 2 U peroxidase. The quantified glycerol concentration was normalized by the number of isolated adipocytes, calculated using a hematocytometer. All determined data are presented as the mean ± S.E.M. of triplicate incubation tubes for each condition.
Peroral administration
Male Wistar rats were divided into three groups (8 rats in each group) to standardized the body weight among the groups, and they orally received water (control group) or 3 or 10% CE aqueous solution (0.5 ml) twice daily by gavage. Animals were maintained on the treatment for 32 days.
Topical application
Male Wistar rats were divided into six groups (8 rats in each group) to standardized the body weight among groups. The hair on their back was shaved once every 2-3 days, and water (control group) or 0.05, 0.2, 0.5, 1, or 2% CE (0.2 ml) was applied to the shaved area (about 5 x 5 cm) twice daily. The animals were maintained on the treatment for 7 days.
Food intake, body weight, adipose tissue weight, and tissue samples
Food intake represented the total weight of consumed chow per animal. The body weight was measured weekly throughout the study. After the application, adipose tissue comprising subcutaneous and intra-abdominal (visceral) fat was dissected from each animal according to defined anatomical landmarks and weighed. Dissected portions were the interscapular fat pad of the back as subcutaneous fat, as well as epididymal, retroperitoneal, and perirenal adipose tissue as visceral fat.
For RNA analysis, whole skin, excluding subcutaneous fat, was obtained from the back, and cutaneous muscle was separated. This superficial layer of skin, consisting of the epidermis and dermis, without subcutaneous components was used as the “superficial skin” sample in this study. White adipose tissue (WAT) of the superficial area of the interscapular fat pad was used as the subcutaneous WAT. BAT from the deep brown area of the interscapular fat pad was used as a positive control tissue expressing UCP.
RNA extraction and RT-PCR analysis
Total RNA was extracted with guanidine-isothiocyanate using Isogen with proteinase K and DNase I treatment according to the manufacturer's instructions. The mRNA of each UCP subtype was analyzed with the reverse transcriptase polymerase chain reaction (RT-PCR). Briefly, cDNA was synthesized from total RNA (5-20ng) using TaqMan Reverse Transcription Reagents, and quantified by real-time PCR with a TaqMan PCR kit using an ABI Prism 7700 (Applied Biosystems Japan, Tokyo, Japan) according to the manufacturer's instructions. Sequences (5' to 3' end) of primer sets and fluorescence-labeled probe for real-time PCR were TGCACCACCAACTGCTTAGC, GGCATGGACTGTGGTCATGA and TCCATGACAACTTTGGCATCGTGGAA (front primer, reverse primer and probe, respectively) for rat GAPDH(GenBank No.NM017008), ATCATCACCTTCCCGCTGG, CCTGGCCTTCACCTTGGA and CACCGCCAAAGTCCGCCTTCA for rat UCP1 (No.X03894), CCTCCTGAAAGCCAACCTCA, CCTTTGGGGCAGGCTTCT and ACAGACGACCTCCCTTGCCACTTCACTTCT for rat UCP2 (No.AB006613), GGGCCCCACAGCCTTCTAC, TCACGTTCCAGGATCCCAGA and AAAGGATTCATGCCCTCCTTTCTGCG for rat UCP3 (No.U92069).
Chemicals
NA, propranolol, and caffeine were obtained from Wako Pure Chemical Industries (Osaka, Japan). Bovine serum albumin fraction V purchased from Sigma (MO, USA), and collagenase type I from Worthington Biochemical (NJ, USA), Isogen from Nippon Gene (Tokyo, Japan), proteinase K and DNase I treatment from Qiagen (Hilden, Germany), TaqMan Reverse Transcription Reagents and TaqMan PCR kit from Applied Biosystems Japan (Tokyo, Japan) were used.
Statistical analysis
All determined data are presented as the mean ± S.E.M. of each condition. The data were analyzed by ANOVA followed by the Bonferroni method for multiple comparisons between pairs. Each experiment was repeated at least three times with similar results.
Results
Lipolysis-promoting activity in adipocytes
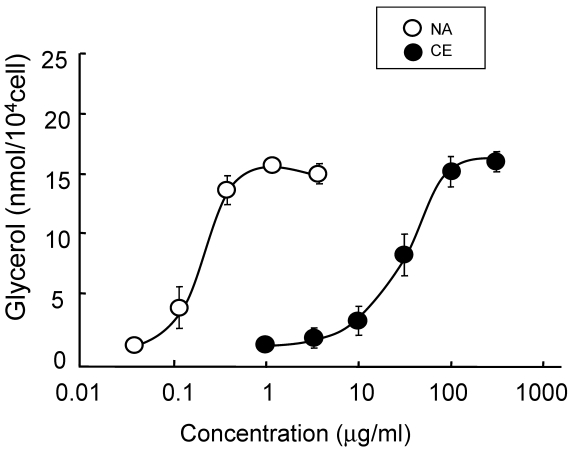
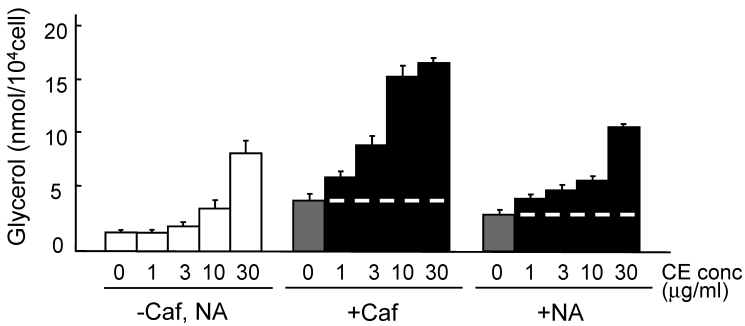
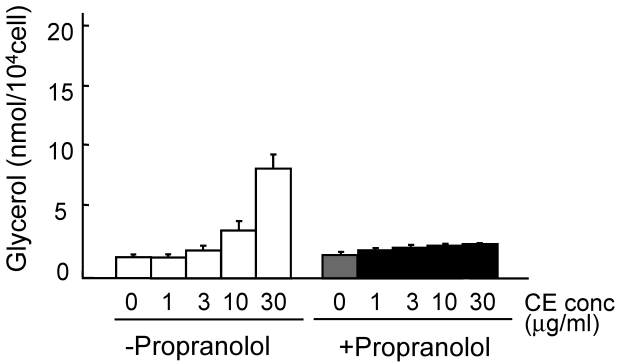
The lipolysis-promoting activity of CE in isolated subcutaneous adipocytes was compared to NA. As can be seen in Fig. 1, CE showed marked lipolysis-promoting activity, and its EC50 was about 35 μg/ml. The maximum response to CE was the same as that to NA. The synergism of CE and other pharmacological agents is shown in Fig. 2. Caffeine synergistically enhanced CE-induced lipolysis; the maximum response to this combination was the same as that to CE alone, but the EC50 of the former was about 4.2 μg/ml, 1/10-fold that of the latter. Co-treatment of CE with NA barely exerted an additional effect. Propranolol markedly reduced the lipolysis-promoting activity of CE (Fig. 3), and the degree of reduction depended on the concentration of propranolol added (data not shown).
Fig 1.
Promotion of lipolysis by CE and NA. Adipocytes were isolated from subcutaneous fat pads and treated with CE or NA for 120 min, and released glycerol was measured. Each value represents the mean ± S.E.M. of three incubation tubes for each condition.
Fig 2.
Promotion of lipolysis by CE with caffeine or NA. Adipocytes were isolated from subcutaneous fat pads and treated with CE and caffeine (0.1 mM) or NA (0.1μg/ml) for 120 min, and released glycerol was measured. Short dashed line represents level of Caf or NA treatment. Each value represents the mean ± S.E.M. of three incubation tubes for each condition.
Fig 3.
Promotion of lipolysis by CE with propranolol. Adipocytes were isolated from subcutaneous fat pads and treated with CE and propranolol (0.1 μM) for 120 min, and released glycerol was measured. Each value represents the mean ± S.E.M. of three incubation tubes for each condition.
Changes of body weight and fat mass by peroral administration
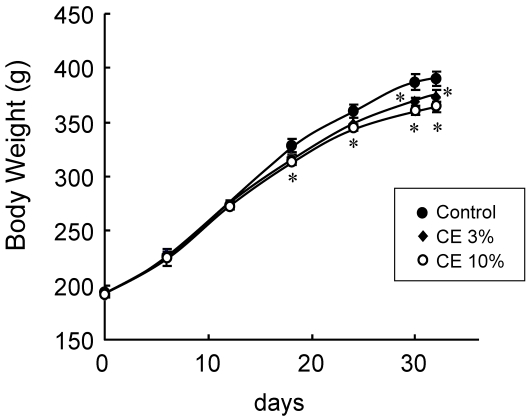
As CE exhibited a transient lipolysis-promoting activity, the effects of the daily oral administration of 3 or 10% CE aqueous solution on the body fat were evaluated in vivo. As Fig. 4 shows, body weight gain was gradually reduced, and the final value after administration for 32 days was significantly lower in the CE groups than in the control group. While food intake during the administration period did not differ among the groups (Table. 1), the final body weight was reduced by 6.6% on average in the group given 10% CE, and the degree of reduction depended on the CE concentration. Both the subcutaneous and visceral fat weights were significantly lower in the CE than in the control group. Mean reduction rates were 26.2% for subcutaneous fat and 3.0% for visceral fat, confirming the body fat-reducing effects of the peroral administration of this extract.
Fig 4.
Body weight changes by the peroral administration of CE. Wistar rats perorally received water (control group) or 3 or 10% CE solution twice daily for 32 days. Each value represents the mean ± S.E.M. of eight animals for each group. *: P<0.05, compared with the value of the control group.
Table 1.
Changes in food intake, body weight, and adipose tissue weight by the peroral administration of CE. Wistar rats perorally received water (control group) or 3 or 10% CE solution twice daily for 32 days. Each value represents the mean ± S.E.M. of eight animals for each group. *: P<0.05, compared with the value of the control.
| Total Food Intake (g) | Final Body Weight (g) | Subcutaneous Fat Weight (g) | Visceral Fat Weight (g) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (%) | Mean | SEM | Relative Value | Mean | SEM | Relative Value | Mean | SEM | Relative Value | Mean | SEM | Relative Value | ||||
| 0 (Control) | 847.4 | 33.3 | 100.0 | 389.8 | 6.4 | 100.0 | 8.000 | 0.440 | 100.0 | 8.30 | 0.35 | 100.0 | ||||
| 3 | 850.1 | 33.0 | 100.3 | 372.3 | 7.7 | 95.5 | * | 7.100 | 0.350 | 88.8 | * | 8.18 | 0.36 | 98.6 | * | |
| 10 | 829.8 | 34.1 | 97.9 | 364.0 | 4.4 | 93.4 | * | 5.900 | 0.370 | 73.8 | * | 8.05 | 0.40 | 97.0 | * | |
Changes in body fat mass and UCP expression on topical application
The effects of a daily topical application of 0.05, 0.2, 0.5, 1, or 2% CE aqueous solution to the skin on body fat were evaluated. As shown in Table 2, during the topical application period for 7 days, neither the food intake nor final body weight differed among groups. However, the subcutaneous fat weight on the back, the area of agent application, was significantly lower in the groups given CE at concentrations ≥ 0.5% as compared to the control group; the 2% CE group showed a mean reduction of 23.2%. The extent of reduction depended on the CE concentration. The visceral fat weight in the CE groups was not significantly reduced.
Table 2.
Changes in food intake, body weight, and adipose tissue weight by the topical application of CE. Wistar rats were externally applied with water (control group) or CE solution twice daily for 7 days. Each value represents the mean ± S.E.M. of eight animals for each group. *: P<0.05, compared with the value of the control.
| Total Food Intake (g) | Final Body Weight (g) | Subcutaneous Fat Weight (g) | Visceral Fat Weight (g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc (%) | Mean | SEM | Relative % | Mean | SEM | Relative % | Mean | SEM | Relative % | Mean | SEM | Relative % | |||
| 0 (Control) | 190.2 | 20.5 | 100.0 | 161.0 | 9.1 | 100.0 | 0.322 | 0.076 | 100.0 | 1.031 | 0.108 | 100.0 | |||
| 0.05 | 189.1 | 22.3 | 99.4 | 160.7 | 7.5 | 99.8 | 0.314 | 0.034 | 97.5 | 1.031 | 0.180 | 100.0 | |||
| 0.2 | 192.4 | 18.9 | 101.2 | 162.5 | 5.8 | 100.9 | 0.261 | 0.040 | 81.3 | 1.018 | 0.193 | 98.8 | |||
| 0.5 | 185.8 | 20.1 | 97.7 | 164.6 | 4.6 | 102.2 | 0.257 | 0.018 | 80.0 | * | 0.997 | 0.155 | 96.8 | ||
| 1 | 191.9 | 22.0 | 100.9 | 161.8 | 8.7 | 100.5 | 0.241 | 0.009 | 75.0 | * | 1.005 | 0.129 | 97.5 | ||
| 2 | 190.7 | 17.8 | 100.3 | 164.2 | 2.6 | 102.0 | 0.247 | 0.019 | 76.8 | * | 0.967 | 0.155 | 93.8 | ||
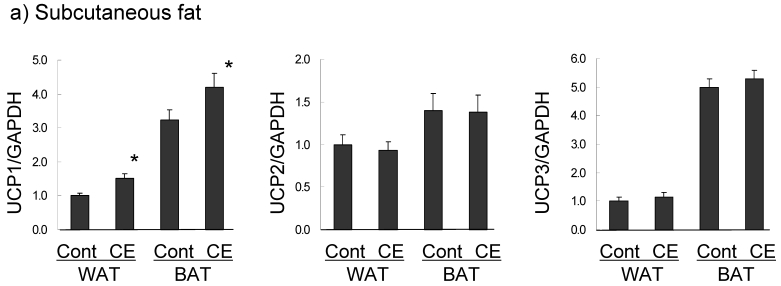
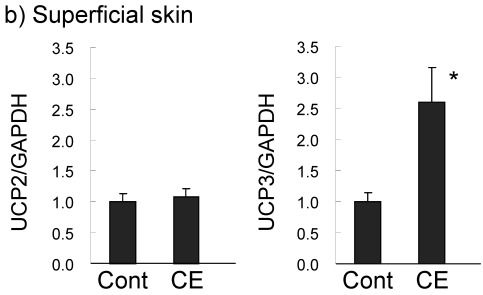
UCP expression in subcutaneous fat and the superficial skin of the treated area were compared in the control and 0.5% CE group. In subcutaneous WAT (Fig. 5a), UCP1 was barely expressed in the control group, but the level was significantly increased (1.5-fold compared to the control group) in the CE group to half of that of BAT in the control group. UCP1 expression in BAT was slightly up-regulated (1.3-fold). However, UCP2 and UCP3 mRNAs in WAT and BAT were not altered by CE application. In contrast to adipose tissue, only UCP3 was markedly increased (2.6-fold) by CE application in the superficial skin (Fig. 5b). No significant expression of UCP1 was observed, and UCP1 and UCP2 were not up-regulated in this tissue.
Fig 5.
Changes in UCP expression in subcutaneous fat (a) and the superficial skin (b) by the topical application of CE. Wistar rats were externally applied with water (control group) or 0.5% CE solution twice daily for 7 days. WAT represents white adipose tissue of subcutaneous fat, compared to BAT. Each value represents the mean ± S.E.M. of eight animals for each group relative to the control group. *: P<0.05, compared with the value of the control group.
Discussion
The promotion of lipolysis in adipocytes is one of the main strategies to prevent fat accumulation; however, obesity-related diabetes was found to be accompanied by catecholamine resistance, showing low-level metabolic responsiveness 6, 21, 22. Further, there have been many reports showing regional differences in adipose metabolism, including responsiveness to dietary treatments or exercise and lipolysis-promoting hormones 23, 24, 25, 26. Since enlarged adipocytes in obesity and subcutaneous adipocytes were less sensitive to lipolytic hormones, our hypothesis was that substances exhibiting a lipolysis-promoting activity in enlarged subcutaneous adipocytes could promote in vivo fat mobilization.
We selected CE as a natural product with marked lipolysis-promoting effects in isolated subcutaneous adipocytes, and investigated its in vitro activity and in vivo efficacy. Experiments using the PDE inhibitor and β antagonist showed that CE could directly promote adipose lipolysis, and strongly suggested that this activity is mediated mainly by βAR, similarly to the β agonist. We investigated Cirsium oligophyllum, a species of thistle, which has long been eaten as a vegetable in a particular area of Asia. However, it has never been clarified whether this plant exhibits some pharmacological effects or contains compounds modulating fat metabolism or adipose function. We identified several alkaloid derivatives, but further investigation is necessary to confirm precise active compounds within this plant.
After confirming transient lipolysis-promoting activity in vivo by peroral administration and the transdermal absorption of CE (data not shown), we investigated whether the repeated stimulation of lipolysis by CE can mobilize body fat. The peroral administration of CE inhibited body weight gain and fat accumulation, and its topical application also markedly reduced subcutaneous fat accompanying no change in the visceral fat weight. The efficacy of peroral administration suggested dietary applicability of CE to reduce the fat mass systemically. The fat reduction may have been due to the accumulation of effects promoting lipolysis; however, body weight reduction was possibly due to enhanced energy consumption, since CE intake showed no toxicity, such as a decrease in food intake or diarrhea. Previous studies have reported a reduction of body fat after the consecutive intake of CL316243, a β3 adrenergic agonist, in mice 27, 28, 29, 30, and after similar intake of methylxantines 31, 32, 33. Regarding the efficacy of β3 agonist treatment, not only transient lipolysis promotion, but also the role of UCP activity in energy dissipating was strongly suggested. From this viewpoint, Inokuma et al. 34 provided evidence that UCP activation is indispensable for fat reduction by β adrenergic stimulation using UCP1-knockout mice. Direct evidence for changes in energy balance brought about by CE administration has to be generated as the next step.
Contrary to a previous study 26, the effect on the fat mass of CE administration was larger in subcutaneous than visceral fat. It is possible that the topically applied region showed a stronger response portion to CE; however, further examinations in obese model rats or obese humans are necessary.
The efficacy of topical application suggested the applicability of CE to nutra-ceutical external agents that can alter regional fat accumulation. Since significant fat reduction was restricted to the applied region, being less effective for visceral and subcutaneous fat in other separated regions (data not shown), it was revealed that CE can be transdermaly absorbed and it directly affects the metabolism of subcutaneous fat. Interestingly, the repeated application of CE markedly up-regulated UCP3 in the superficial skin, and significantly increased UCP1 mRNA in subcutaneous WAT and BAT, suggesting that regional subcutaneous fat reduction was induced by CE due to not only transient lipolysis promotion but also the activation of UCP.
There have been few studies on the efficacy of the external application of agents on fat mobilization. Topical fat reduction by the external application of a preparation containing aminophylline, a PDE inhibitor, was reported 35; however, there are no reports of fat reduction due to external β agonistic application and no discussion on UCP activation in skin including subcutaneous fat. Contrary to our previous report on human skin 18, the superficial skin of rats showed no expression of UCP1 but expressed UCP3, which was up-regulated by β stimulation on CE treatment, suggesting that functional subtypes of skin-UCP differ between species, and possibly exhibit some physiological functions responding to hormonal signals. In the case of skeletal muscle and myocytes, the up-regulation of UCP3 caused by catecholamines, fasting, or exercise was reported 36. Since interactions between the metabolic pathway and the transcriptional regulation of skin components have not been revealed, these need to be elucidated in detail.
Another possible mechanism of the action of CE involves the activation of the autonomic nervous system. Lipolysis and UCP activation are also controlled by the autonomic nervous system, and olfactory stimulation by fruit or spices can stimulate nerve activity and promote lipolysis 37. Whether or not the in vivo efficacy of CE administration involves the activity of the autonomic nervoous system innervating adipose tissue or the skin also remains to be determined.
This study showed that CE could transiently promote lipolysis via a mechanism involving βAR, similar to β agonist, and that the chronic administration of this agent reduced the body fat mass systemically or regionally, accompanied by the up-regulation of skin-UCP. This is the first report showing the in vivo efficacy of a repeatedly applied β agonist to promote lipolysis leading to fat reduction or the modification of fat distribution and expression of skin-UCP. These results suggested that dietary and external applications could be beneficial methods for systematic fat reduction or the control of fat distribution, and that synergistic enhancement of efficacy could be expected in combination with other active ingredients. Further studies are necessary to confirm the action mechanism of this material, involving alteration of the energy balance, and to evaluate its possible application to health promotion and esthetics.
Acknowledgments
The authors wish to express gratitude to Professor Masayuki Saito at the Department of Nutrition, School of Nursing and Nutrition, Tenshi College (Sapporo, Japan).
References
- 1.Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, Strosberg AD. Molecular characterization of the human β3-adrenergic receptor. Science. 1989;245:1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- 2.Arner P. Regulation of lipolysis in fat cells. Diabetes Reviews. 1996;4:450–463. [Google Scholar]
- 3.Hoffstedt J, Arner P, Hellers G, Lonnqvist F. Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. J Lipid Res. 1997;38:795–804. [PubMed] [Google Scholar]
- 4.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–6. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Soni KG, Semache M, Casavant S, Fortier M, Pan L, Mitchell GA. Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab. 2008;95:117–26. doi: 10.1016/j.ymgme.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Mauriège P, Prud'homme D, Lemieux S, Tremblay A, Després JP. Regional differences in adipose tissue lipolysis from lean and obese women: existence of postreceptor alterations. Am J Physiol. 1995;269:E341–50. doi: 10.1152/ajpendo.1995.269.2.E341. [DOI] [PubMed] [Google Scholar]
- 7.Hellmer J, Marcus C, Sonnenfeld T, Arner P. Mechanisms for differences in lipolysis between human subcutaneous and omental fat cells. J Clin Endocrinol Metab. 1992;75:15–20. doi: 10.1210/jcem.75.1.1320047. [DOI] [PubMed] [Google Scholar]
- 8.Tavernier G, Galitzky J, Valet P, Remaury A, Bouloumie A, Lafontan M, Langin D. Molecular mechanisms underlying regional variations of catecholamine-induced lipolysis in rat adipocytes. Am J Physiol. 1995;268:E1135–E1142. doi: 10.1152/ajpendo.1995.268.6.E1135. [DOI] [PubMed] [Google Scholar]
- 9.Jezek P. Fatty acid interaction with mitochondrial uncoupling proteins. J Bioenerg Biomembr. 1999;31:457–466. doi: 10.1023/a:1005496306893. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu Y, Saito M. Activation of brown adipose tissue thermogenesis in recovery from anesthetic hypothermia in rats. Am Phys Sci. 1991;261:301–304. doi: 10.1152/ajpregu.1991.261.2.R301. [DOI] [PubMed] [Google Scholar]
- 11.Cannon B, Hedin A, Nedergaard D. Exclusive occurrence of thermogenin antigen in brown adipose tissue. FEBS Lett. 1982;150:129–132. doi: 10.1016/0014-5793(82)81319-7. [DOI] [PubMed] [Google Scholar]
- 12.Nedergaard J, Cannon B. 3H GDP binding and thermogenin amount in brown adipose tissue mitochondria from cold-exposed rats. Am J Physiol. 1985;248:C365–371. doi: 10.1152/ajpcell.1985.248.3.C365. [DOI] [PubMed] [Google Scholar]
- 13.Klaus S, Munzberg H, Truloff C, Heldmaier G. Physiology of transgenic mice with brown fat ablation: obesity is due to lowered body temperature. Am J Physiol. 1998;274:287–293. doi: 10.1152/ajpregu.1998.274.2.R287. [DOI] [PubMed] [Google Scholar]
- 14.Baumruk F, Flachs P, Horakova M, Floryk D, Kopecky J. Transgenic UCP1 in white adipocytes modulates mitochondrial membrane potential. FEBS Lett. 1999;444:206–210. doi: 10.1016/s0014-5793(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 15.Boss O, Samec A, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino J. Uncoupling protein-3: a new member of the mitochindrial carrier family with tissue-specific expression. FEBS Lett. 1997;408:39–47. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- 16.Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 17.Solanes G, Vidal-Puig AJ, Grujic D, Flier JS, Lowell BB. The human uncoupling protein-3 gene. Genomic structure, chromosomal localiztion, and genetic basis for shot and long form transcripts. J Biol Chem. 1997;272:25433–25436. doi: 10.1074/jbc.272.41.25433. [DOI] [PubMed] [Google Scholar]
- 18.Mori S, Yoshizuka N, Takizawa M, Takema Y, Murase T, Tokimitsu I, Saito M. Expression of uncoupling proteins in human skin and skin-derived cells. J Invest Dermatol. 2008;128:1894–1900. doi: 10.1038/jid.2008.20. [DOI] [PubMed] [Google Scholar]
- 19.Rodbell M. Metabolism of isolated fat cells I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 20.Kawano-Takahashi Y, Ohminaji H, Ubagai E, Okuda H. Mechanisms of action of mazindol in preventing onset and development of obesity induced by gold thioglucose injection. Int J Obes. 1984;8:655–664. [PubMed] [Google Scholar]
- 21.Bairras C, Mauriege P, Bukowiecki L, Atgie C. Regulation of lypolysis in white adipose tissues of lean and obese Zucker rats. J Physiol Biochem. 2007;63:287–96. doi: 10.1007/BF03165760. [DOI] [PubMed] [Google Scholar]
- 22.Böttcher H, Fürst P. Decreased white fat cell thermogenesis in obese individuals. Int J Obes Relat Metab Disord. 1997;21:439–44. doi: 10.1038/sj.ijo.0800425. [DOI] [PubMed] [Google Scholar]
- 23.Hellmer J, Marcus C, Sonnenfeld T, Arner P. Mechanisms for differences in lipolysis between human subcutaneous and omental fat cells. J Clin Endocrinol Metab. 1992;75:15–20. doi: 10.1210/jcem.75.1.1320047. [DOI] [PubMed] [Google Scholar]
- 24.Tavernier G, Galitzky J, Valet P, Remaury A, Bouloumie A, Lafontan M, Langin D. Molecular mechanisms underlying regional variations of catecholamine-induced lipolysis in rat adipocytes. Am J Physiol. 1995;268:E1135–1142. doi: 10.1152/ajpendo.1995.268.6.E1135. [DOI] [PubMed] [Google Scholar]
- 25.Vidal-Puig A. Gene expression in visceral and subcutaneous adipose tissue. Ann Med. 2001;33:547–555. doi: 10.3109/07853890108995965. [DOI] [PubMed] [Google Scholar]
- 26.Doucet E, St-Pierre S, Alméras N, Imbeault P, Mauriège P, Pascot A, Després JP, Tremblay A. Reduction of visceral adipose tissue during weight loss. Eur J Clin Nutr. 2002;56:297–304. doi: 10.1038/sj.ejcn.1601334. [DOI] [PubMed] [Google Scholar]
- 27.Nagase I, Yoshida T, Kumamoto K, Umekawa T, Sakane N, Nikami H, Kawada T, Saito M. Expression of uncoupling protein in skeletal muscle and white fat of obese mice treated with thermogenic β3-adrenergic agonist. J Clin Invest. 1996;97:2898–2904. doi: 10.1172/JCI118748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas RF, Holt BD, Schwinn DA, Liggett SB. Long-term agonist exposure induces upregulation of β3-adrenergic receptor expression via multiple cAMP response elements. Proc Natl Acad Sci USA. 1992;89:4490–4494. doi: 10.1073/pnas.89.10.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atgie C, Faintrenie G, Carpene C, Bukowiecki LJ, Geloen A. Effects of chronic treatment with noradrenaline or a specific β3-adrenergic agonist, CL316243, on energy expenditure and epididymal adipocyte lipolytic activity in rat. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:629–636. doi: 10.1016/s1095-6433(97)00476-5. [DOI] [PubMed] [Google Scholar]
- 30.Langin D, Tavernier G, Lafontan M. Regulation of β3-adrenoceptor expression in white fat cells. Fundam Clin Pharmacol. 1995;9:97–106. doi: 10.1111/j.1472-8206.1995.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida T, Sakane N, Umekawa T, Kondo M. Relationship between basal metabolic rate, thermogenic response to caffeine, and body weight loss following combined low calorie and exercise treatment in obese women. Int J Obes Relat Metab Disord. 1994;18:345–350. [PubMed] [Google Scholar]
- 32.Lupi O, Semenovitch IJ, Treu C, Bottino D, Bouskela E. Evaluation of the effects of caffeine in the microcirculation and edema on thighs and buttocks using the orthogonal polarization spectral imaging and clinical parameters. J Cosmet Dermatol. 2007;6:102–107. doi: 10.1111/j.1473-2165.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 33.Acheson KJ, Zahorska-Markiewicz B, Pittet P, Anantharaman K, Jequier E. Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am J Clin Nutr. 1980;33:989–997. doi: 10.1093/ajcn/33.5.989. [DOI] [PubMed] [Google Scholar]
- 34.Inokuma K, Okamatsu-Ogura Y, Omachi A, Matsushita Y, Kimura K, Saito M. Indinpensable role of mitochondrial UCP1 for antiobesity effect of β3-adrenergic stimulation. Am. J. Physiol. Endocrinol Metab. 2006;290:1014–1021. doi: 10.1152/ajpendo.00105.2005. [DOI] [PubMed] [Google Scholar]
- 35.Greenway FL, Bray GA. Regional fat loss from the thigh in obese women after adrenergic modulation. Clin Ther. 1987;9:663–669. [PubMed] [Google Scholar]
- 36.Nagase I, Yoshida T, Saito M. Up-regulation of uncoupling proteins by β-adrenergic stimulation in L6 myotubes. FEBS Lett. 2001;494:175–180. doi: 10.1016/s0014-5793(01)02341-9. [DOI] [PubMed] [Google Scholar]
- 37.Shen J, Niijima A, Tanida M, Horii Y, Nakamura T, Nagai K. Mechanism of changes induced in plasma glycerol by scent stimulation with grapefruit and lavender essential oils. Neurosci Lett. 2007;416:241–246. doi: 10.1016/j.neulet.2006.12.063. [DOI] [PubMed] [Google Scholar]