Abstract
Brain arteriovenous malformations (AVM) cause intracranial hemorrhage (ICH). Molecular characterization of lesional tissue implicates angiogenic (VEGF, ANG-2, MMP-9) and inflammatory (cytokines and chemokines) pathways, but the pathogenesis remain obscure and medical therapy is lacking. Macrophage and neutrophil invasion has also been observed in the absence of prior ICH. Single nucleotide polymorphisms (SNPs) in interleukin-1β (IL-1β) and activin receptor-like kinase-1 (ALK-1) are associated with AVM susceptibility, and SNPs in IL-1β, IL-6, TNF-α and APOE are associated with AVM rupture. These observations suggest that even without a complete understanding of the determinants of AVM development, the recent discoveries of downstream derangements in vascular function and integrity may offer potential targets for therapy development. Further, biomarkers can be established for assessing ICH risk. Finally, these data will aid in development of model systems for mechanistic testing, by development of surrogate phenotypes (microvascular dysplasia) and/or models recapitulating the clinical syndrome of recurrent spontaneous ICH.
Keywords: angiogenesis, inflammation, vascular malformations
Brain arteriovenous malformations (AVM) are a cause of intracranial hemorrhage (ICH). The basic morphology is a tangle of abnormal, dilated channels with intervening gliosis, called the nidus, that directly shunts blood between the arterial and venous circulations without a true capillary bed. Prevention of new or recurrent ICH is the primary rationale to treat AVMs. The risk of spontaneous ICH varies widely, depending on clinical and angioarchitectural risk factors. Mean estimates are 2–4% per year,1 but range from 1–30% per year. Other than non-specific control of symptoms, e.g., headache and seizures, primary medical therapy is lacking.
Etiology and pathogenesis
There are no known environmental risk factors for AVMs, and, despite direct evidence, they are usually described as congenital. However, there are multiple reports of AVMs that grow or regress, including de novo AVM formation, hinting that an appreciable fraction may in fact have a post-natal genesis. Although unproven, inciting event(s) might include subclinical trauma, tissue hypoxia, infection, inflammation, irradiation, or compression, perhaps involving localized venous hypertension, a potent angiogenic stimulus. Scarce available data on longitudinal assessment of AVM growth suggest that roughly 50% of cases display interval growth. 2
AVM tissue assays suggest an active angiogenic and inflammatory lesion rather than a static congenital anomaly, e.g., increased endothelial proliferation, overexpression of VEGF-A, Ang-2,2 myeloperoxidase (MPO) IL-6, and MMP-9; even in unruptured, non-embolized AVMs, neutrophils and macrophages/microglia are evident in both vascular wall and intervening stroma.3
Genetic considerations
Although rare, familial cases of AVM outside the context of HHT have been reported, but linkage studies are underpowered.4 Using these reported data, one can derive modest estimates of recurrence risk ratio of disease in siblings, λs, suggesting a genetic influence.
Hereditary hemorrhagic telangiectasia (HHT), an autosomal dominant disorder with a high prevalence of brain AVM, may be a natural model for sporadic disease insofar as it suggests the pathways, if not the genes involved. The majority of HHT cases involve loss-of-function mutations in two genes originally implicated in TGF-β signaling pathways: (1) endoglin (ENG), encoding an accessory protein of TGF-β receptor complexes; and (2) activin receptor-like kinase 1 (ALK-1), encoding a transmembrane kinase.5 In endothelium, ALK-1 may signal through BMP-9 enhanced by ENG; ablation of murine Alk-1 causes embryonic A-V fistula formation.6
As a class, AVMs in HHT are characterized by small size, single hole fistulas, cortical location and multiplicity, but are generally similar to the sporadic lesions and cannot be distinguished individually on the basis of their angioarchitecture. Brain AVMs are approximately ten times more common in HHT1/ENG (~20%) than HHT2/ALK-1 (~2%).5 Compared to sporadic lesions, presence of an ENG or ALK-1 mutation results in a 10,000 and 1,000-fold increased risk, respectively, of developing a brain AVM. The greatly elevated risk of brain AVM development in HHT raises the hypothesis that germline sequence variants of genes in this pathway may likewise pose a significant risk for sporadic brain AVM development. The growth and behavior of AVMs are likely under genetic influence from modifier pathways, e.g., there are multiple genetic loci that control VEGF-induced angiogenesis.7
The first common genetic variant associated with sporadic AVM susceptibility, thought to result in alternative splicing, is an intronic variant of ALK-1, which was recently replicated.8 Common promoter polymorphisms in IL-1β (−31T>C and −511C>T) also are associated with AVM susceptibility.9 The GG genotype of the IL-6 -174G>C promoter SNP was associated with clinical presentation of ICH; the G allele correlated with IL-6 mRNA and protein levels in resected tissue.10 A promoter SNP in TNF-α and APOE ε2 genotype were associated with new ICH after diagnosis.11, 12 These genetic association studies are in need of replication and large cooperative studies will be needed.
Experimental AVM models
A model system for studying AVM is needed. With few exceptions, most “AVM” models are either extradural fistulas, i.e., creation of a carotid jugular fistula, or result in morphological microvascular changes that are probably best termed “vascular dysplasia.” A parenchymal nidus is not formed; nidus growth and hemorrhage mimicking the human disease do not occur. An ideal model would feature: 1) a nidus of abnormal vessels of varying sizes, encompassing both micro and macro-circulatory levels; 2) spontaneous ICH into the brain parenchyma or CSF spaces; 3) A-V shunting; 4) flow rates sufficient to lower proximal feeding artery pressure; and 5) high angiogenic and inflammatory signal expression consistent with human surgical specimens.
Virally-mediated VEGF gene transfer in Eng-deficient mice enhances vascular abnormalities, suggesting synergy between TGFβ and VEGF signaling pathways in development of abnormal or “dysplastic” vessels.13 Coupled with conditional deletion of Eng or Alk-1,6 stimulation techniques show promise for development of a true AVM model. Success of this strategy in phenocopying the clinical disorder would lend strength to the approach of controlling pathological angiogenesis and/or inflammation14 to halt disease progression and decrease risk of spontaneous hemorrhage.15
Conclusions
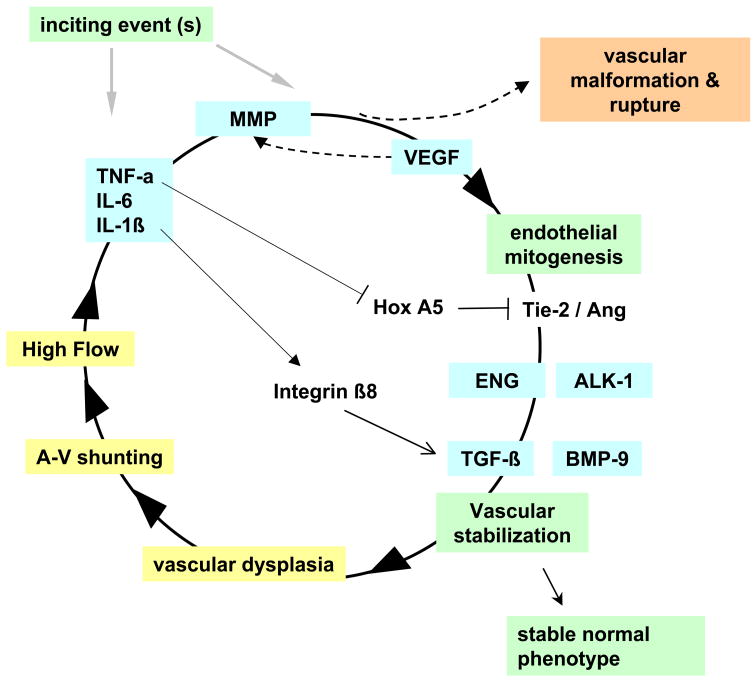
The prevailing hypothesis is that AVM pathophysiology is governed to a large extent by chronic hemodynamic derangements imposed on a congenital lesion. Recent findings suggest an alternative hypothesis where angiogenic and inflammatory pathways synergize with underlying defects or hemodynamic injury to result in the clinical phenotype, perhaps in conjunction with as yet undetermined genetic or environmental influences (Figure 1). Elucidating these mechanisms offers promise for developing innovative treatments and better risk stratification, and may provide insights into mechanisms of vascular biology.
Figure 1. AVM pathogenesis: speculative synthesis of observations.
After an inciting event, inflammatory or angiogenic activity (MMP, VEGF) initiates microvascular growth and remodeling, which are stabilized through interplay of pathways including TIE-2/ANG and TGF-β or BMP-9 signaling through the ALK-1/ENG pathway. Lack of Integrin β8 and Hox A5, an anti-angiogenic transcription factor, may also play a role. Normal vessels stabilize, but an incipient AVM undergoes a dysplastic response. Arteriovenous (A-V) shunting and high flow rates synergize with the dysplastic response and with inflammatory signals, causing a vicious cycle in a localized area destined to become the nidus. Eventually, the human disease phenotype emerges. Genetic variation can influence any step of the cycle.
References
- 1.Kim H, Sidney S, McCulloch CE, Poon KY, Singh V, Johnston SC, Ko NU, Achrol AS, Lawton MT, Higashida RT, Young WL. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38:2430–2437. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- 2.Young WL, Yang GY. Are there genetic influences on sporadic brain arteriovenous malformations? Stroke. 2004;35:2740–2745. doi: 10.1161/01.STR.0000145054.35083.32. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, Hashimoto T, Yang GY, Young WL. Evidence for inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008 doi: 10.1227/01.neu.0000333306.64683.b5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue S, Liu W, Inoue K, Mineharu Y, Takenaka K, Yamakawa H, Abe M, Jafar JJ, Herzig R, Koizumi A. Combination of linkage and association studies for brain arteriovenous malformation. Stroke. 2007;38:1368–1370. doi: 10.1161/01.STR.0000260094.03782.59. [DOI] [PubMed] [Google Scholar]
- 5.Letteboer TG, Mager JJ, Snijder RJ, Koeleman BP, Lindhout D, Ploos van Amstel JK, Westermann CJ. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006;43:371–377. doi: 10.1136/jmg.2005.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, Oh SP. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2 (HHT2) Blood. 2008;111:633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers MS, D’Amato RJ. The effect of genetic diversity on angiogenesis. Exp Cell Res. 2006;312:561–574. doi: 10.1016/j.yexcr.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Young WL, Kwok PY, Pawlikowska L, Lawton MT, Kim H, Hysi PG, Marchuk DA. Arteriovenous malformation. J Neurosurg. 2007;106:731–732. doi: 10.3171/jns.2007.106.4.731. author reply 732–733. [DOI] [PubMed] [Google Scholar]
- 9.Hysi PG, Kim H, Pawlikowska L, McCulloch CE, Zaroff JG, Sidney S, Burchard EG, Marchuk DA, Lawton MT, Kwok PY, Young WL. Association of interleukin-1 beta (IL1B) gene and brain arteriovenous malformation in Caucasians (Abstract) Stroke. 2007;38:456. [Google Scholar]
- 10.Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, Lawton MT, Kwok PY, Yang GY, Young WL. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59:72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- 11.Achrol AS, Pawlikowska L, McCulloch CE, Poon KY, Ha C, Zaroff JG, Johnston SC, Lee C, Lawton MT, Sidney S, Marchuk D, Kwok PY, Young WL. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke. 2006;37:231–234. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 12.Pawlikowska L, Poon KY, Achrol AS, McCulloch CE, Ha C, Lum K, Zaroff J, Ko NU, Johnston SC, Sidney S, Marchuk DA, Lawton MT, Kwok PY, Young WL. Apoliprotein E epsilon2 is associated with new hemorrhage risk in brain arteriovenous malformation. Neurosurgery. 2006;58:838–843. doi: 10.1227/01.NEU.0000209605.18358.E5. discussion 838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Wu YQ, Huey M, Arthur HM, Marchuk DA, Hashimoto T, Young WL, Yang GY. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab. 2004;24:237–244. doi: 10.1097/01.WCB.0000107730.66603.51. [DOI] [PubMed] [Google Scholar]
- 14.Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracranial hemorrhage. Stroke. 2007;38:2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- 15.Frenzel T, Lee CZ, Kim H, Quinnine NJ, Hashimoto T, Lawton MT, Guglielmo BJ, McCulloch CE, Young WL. Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: pilot study of adverse events and tolerance. Cerebrovasc Dis. 2008;25:157–163. doi: 10.1159/000113733. [DOI] [PMC free article] [PubMed] [Google Scholar]