Abstract
Cognitive processing biases toward smoking-related and affective cues may play a role in tobacco dependence. Because processing biases may occur outside conscious awareness, the current study examined processing of smoking-related and affective stimuli presented at subliminal conditions. A pictorial subliminal repetition priming task was administered to three groups: (1) Nonsmokers (n = 56); (2) Smokers (≥10 cigarettes/day) who had been deprived from smoking for 12 h (n = 47); and (3) Nondeprived smokers (n = 66). Prime stimuli were presented briefly (17 ms) and were followed by a mask (to render them unavailable to conscious awareness) and then a target. Participants were required to make a speeded classification to the target. A posttask awareness check was administered to ensure that participants could not consciously perceive the briefly presented primes (i.e., smoking paraphernalia, neutral office supplies, and happy, angry, and neutral facial expressions). The groups differed in the degree to which they exhibited a processing bias for smoking-related stimuli, F(2, 166) = 4.99, p = .008. Deprived smokers exhibited a bias toward processing smoking (vs. neutral office supply) stimuli, F(1, 46) = 5.67, p = .02, whereas nondeprived smokers and nonsmokers did not (ps > .22). The three groups did not differ in the degree to which they exhibited a subliminal processing bias for affective stimuli. Tobacco deprivation appears to increase smokers’ subliminal processing of smoking-related (vs. neutral) stimuli but does not influence subliminal processing of affective stimuli. Future research should investigate whether subliminal biases toward smoking-related stimuli influence relapse.
Keywords: tobacco addiction, subliminal processing, smoking-related stimuli, affective stimuli, incentive salience
In recent years, information processing approaches have been used to understand drug use behavior (Franken, 2003; Ryan, 2002; Tiffany & Conklin, 2000). This approach often utilizes computerized reaction time (RT) tasks, derived from experimental cognitive psychology. A major advantage of this approach is that cognitive tasks are thought to tap into psychological processes that are unavailable to conscious introspection and immeasurable by self-report (i.e., “automatic cognitive processes”). This is significant because automatic processes may play an important role in psychological models of drug use motivation (Wiers & Stacy, 2005).
For example, Robinson and Berridge (2003) proposed that chronic drug administration sensitizes the brain’s motivational processing system, such that habitual drug users attribute a high degree of incentive salience to drug-related stimuli. The attribution of incentive salience is considered to operate automatically and below the level of conscious awareness. As part of this process drug-related stimuli may have the power to automatically “grab” users’ attention and promote drug-seeking behavior. This theory predicts that incentive sensitization operates during all periods of the addictive process; however, reexposure to drug-related cues during abstinence is thought to induce exaggerated incentive motivational states characterized by hyperactive automatic processing of drug-related cues (Curtin, McCarthy, Piper, & Baker, 2006), which could promote relapse.
In a somewhat different vein, Koob, Caine, Parsons, Markou, and Weiss (1997) propose that opponent processes become activated during withdrawal. They note that acute drug use typically amplifies reactions to nonpharmacological rewards. In addition, they argue that—in opposition to the process occurring during states of acute drug use—drug deprivation desensitizes the brain’s motivational processing system to nonpharmacological rewards, rendering them less reinforcing. As a result, users are motivated to seek out drugs during withdrawal states because, in contrast with nonpharmacological rewards, drugs remain capable of producing rewarding effects. This theory would predict that automatic processing of nonpharmacological rewarding cues should be reduced during drug-deprived states because of their diminished motivational salience.
Following from these theories, researchers have used RT-based cognitive assessments, such as the modified Stroop task, to examine automatic processing of smoking-related (e.g., “nicotine”) and affective (e.g., “pleasure”) stimuli. In the modified Stroop task, the participant is presented with words printed in color and asked to quickly name the color of each stimulus. Slowness in naming a color suggests interference in color naming because of attention being automatically “captured” by the meaning of the stimulus word. Consistent with incentive salience theories of addiction, smokers take longer to name the color of smoking-related words than neutral words, which indicates a processing bias toward smoking-related stimuli (Waters & Sayette, 2005). Studies that have examined the effects of nicotine deprivation on the modified Stroop task and related tasks have yielded mixed findings, with some showing that processing biases toward smoking stimuli are potentiated by deprivation (e.g., Gross, Jarvik, & Rosenblatt, 1993; Jarvik, Gross, Rosenblatt, & Stein, 1995; Waters & Feyerabend, 2000), and others reporting little or no effect of deprivation (e.g., Dawkins, Powell, West, Powell, & Pickering, 2006; Hendricks, Ditre, Drobes, & Brandon, 2006; Mogg & Bradley, 2002; Munafò, Mogg, Roberts, Bradley, & Murphy, 2003). Consistent with opponent process theories of addiction, Powell, Tait, and Lessiter (2002) reported that nondeprived smokers exhibited an automatic processing bias toward nonpharmacological positive and negative affective words (vs. neutral words), but deprived smokers did not (Powell et al., 2002). Two follow-up reports demonstrated that acute nicotine administration potentiated attentional bias to positive (but not negative) words in deprived smokers (Dawkins et al., 2006; Powell, Pickering, Dawkins, West, & Powell, 2004).
Although these findings are interesting, under certain conditions participants can use conscious strategies (nonautomatic or “controlled” processes) to counteract the ability of cognitive assessments to measure only automatic processes (Amir, McNally, Riemann, & Burns, 1996), which can complicate interpretation of results. For example, participants may increase their attentional effort to effectively suppress the interference produced in the Stroop task (Amir et al., 1996). To reduce the likelihood that controlled processes influence performance, subliminal stimulus presentations, that preclude conscious perception, can be used. Cognitive tasks using subliminal exposure conditions are useful because: (a) they might reveal underlying processing tendencies uncontaminated by conscious top-down processes (Öhman, 1997); and (b) they may detect automatic processing biases at the earliest stage of processing, which may indicate whether smokers shift their attention to salient stimuli before those stimuli have become available to consciousness (Field, 2006).
Subliminal processing tasks are also interesting from a neuropsychological perspective. With subliminal presentation, cues cannot be processed at cortical areas in the brain where conscious processing occurs (Breitmeyer & Ogmen, 2000). Rather, stimuli are processed through subcortical routes in which information bypasses areas of the sensory cortex and travels directly from sensory organs to the amygdala, a region of the limbic system involved in emotional and motivational processing (Morris & Dolan, 2001; Morris, Öhman, & Dolan, 1998). Therefore, tasks using subliminal presentation may tap into neural processing by limbic structures, which are thought to play a major role in mediating drug use motivation (Curtin et al., 2006; Koob et al., 1997; Robinson & Berridge, 2003).
Despite compelling evidence that drug users exhibit processing biases for supraliminally presented drug-related cues (see Field, Mogg, & Bradley, 2006), there is hitherto little evidence that they exhibit processing biases for subliminally presented cues (Bradley et al., 2004; Franken, Kroon, Wiers, & Jansen, 2000; Mogg & Bradley, 2002; Munafò et al., 2003). If such a dissociation between subliminal and supraliminal processing biases indeed exists, it would have important implications for cognitive models of addiction (Field et al., 2006). Most research showing null findings has used subliminal versions of the addiction Stroop and dot probe tasks (Bradley et al., 2004; Franken et al., 2000; Mogg & Bradley, 2002; Munafò et al., 2003). However, other studies have reported that recently abstinent alcoholics exhibit greater physiological reactivity to subliminally presented alcohol cues than neutral cues (Ingjaldsson, Thayer, & Laberg, 2003a, 2003b). Thus, in order to gather additional evidence on subliminal processing of drug stimuli, it seems important to use a variety of tasks.
Subliminal priming tasks are perhaps the most widely used methodology for assessing subliminal processing (Breitmeyer, Ogmen, & Chen, 2004; Greenwald, Abrams, Naccache, & Dehaene, 2003). A briefly presented stimulus (called the “prime”) is first presented. A meaningless mask is then presented after the prime. The mask ensures that the prime cannot be consciously perceived and is processed only at unconscious levels. Finally, another stimulus, called the “target,” is presented. Participants are required to classify the target stimulus on a given dimension as quickly as possible after the target is displayed. In a repetition priming task, the prime is systematically varied so that it is either identical to the target (“repetition-primed”) or unrelated to the target (“control-primed”). The degree of subliminal priming is indicated by the difference in RTs between repetition-primed and control-primed trials. Subliminal priming tasks have indeed been useful in studying subliminal processing biases underlying other behavioral disorders, such as depression (Bradley, Mogg, & Williams, 1994, 1995; Scott, Mogg, & Bradley, 2001).
Accordingly, the current study utilized a pictorial repetition priming task to examine subliminal processing of smoking-related and affective stimuli. Pictorial rather than word stimuli were used as the former may have better ecological validity. We examined subliminal processing in three groups: (1) never smokers; (2) smokers deprived from nicotine for 12 h; and (3) nondeprived smokers. Based on theories of addiction (Koob et al., 1997; Robinson & Berridge, 2003), we formulated several hypotheses about the extent of subliminal processing biases toward specific stimuli in the three groups. Evidence of processing biases would be indicated if subliminal priming effects (differences in RT on control- vs. repetition-primed trials) are greater for one category of stimuli in comparison to another (e.g., smoking-related vs. neutral).
Based on incentive sensitization theory, we hypothesized that deprived and nondeprived smokers would show greater subliminal priming for smoking-related stimuli as compared to neutral control stimuli (i.e., pictures of office supplies), which would be indicative of a processing bias. In contrast, we expected nonsmokers would show no differences in the extent of priming for smoking and office stimuli (i.e., no subliminal processing bias). Given that nicotine deprivation may potentiate the motivational salience of smoking-related stimuli (Curtin et al., 2006), we expected that subliminal processing biases toward smoking-related (vs. office) stimuli would be greater in deprived than nondeprived smokers. Based on opponent-process theory, we hypothesized that nondeprived smokers would exhibit greater subliminal processing of affective versus neutral stimuli, but this effect would be reduced in deprived smokers.
Method
Participants
Participants were 169 students enrolled at the University of Houston recruited through the placement of flyers and class announcements. The sample was predominately female (66.7%), with an average age of 24.2 (SD = 6.1) years; 14.3% self-identified as African American, 15.0% as Asian or Pacific Islander, 52.1% as Caucasian, 12.0% as Hispanic, and 6.6% as Middle-Eastern. The inclusion criteria were: (1) report normal or corrected-to-normal vision; (2) 18+ years of age; (3) report smoking 10+ cigarettes per day on average for the past 2 years (smokers), or report smoking <100 cigarettes in their lifetime (nonsmokers). Individuals were excluded if they could read or speak Chinese (one of the tasks not reported here required participants to rate Chinese ideographs). Smokers were excluded if they (1) planned to quit in the next 30 days; (2) were currently cutting down substantially; or (3) were currently using some form of nicotine replacement therapy. Participants received a $15 voucher redeemable at a department store and course credit for completing the study. The study was approved by the institutional review boards of the University of Texas–M. D. Anderson Cancer Center, and the University of Houston.
Procedure
Participants attended an orientation session in which they were informed about the study’s procedures, provided written inform consent, and completed the orientation session questionnaires (detailed in Measures section).
Smokers who completed the orientation session were randomized to be either deprived or nondeprived during their experimental session. Participants randomized to the deprived condition were asked to abstain from smoking for at least 12 h before the experimental session. Nondeprived participants were asked to smoke freely before the session and to smoke one cigarette within 30 min of the session. Breath carbon monoxide (CO) levels were assessed for all participants at the beginning of the experimental session. All participants then completed the subliminal priming task, an additional cognitive task (not reported in this article; analyses revealed no significant order effects for the two tasks), and experimental session questionnaires (detailed later). At the experimental session, the experimenters were blind to the smoking status and deprivation condition of the participants.
In total, 254 participants attended the orientation session (188 smokers, 66 nonsmokers). Of the 236 participants who subsequently completed the experimental session, 50 did not meet criteria for biochemical confirmation of either smoking or abstinence and were excluded from the primary analyses (see Data Reduction section). Of the remaining 186 participants, 17 performed above chance on the awareness check (see below) and were excluded from analyses. The final sample consisted of 56 nonsmokers, 66 nondeprived smokers, and 47 deprived smokers.
Questionnaire Measures
During the orientation session, we administered the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), a widely used and well-validated measure of nicotine dependence, to smokers. Smokers also completed an author-constructed Smoking History Questionnaire that assessed basic information such as number of years of smoking and number of cigarettes smoked per day.
To assess psychosocial characteristics, we administered the Beck Anxiety Inventory (BAI; Steer, Clark, Beck, & Ranieri, 1995), which assesses self-reported levels of anxiety in the past week, and the Center for Epidemiological Studies Depression Scale (CESD; Radloff, 1977), which measures depressive symptomatology in the past week, to all participants.
The following questionnaires, which have all been shown to be sensitive to deprivation state (Leventhal et al., 2007), were administered to all participants at the end of the experimental session:
The 28-item Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) assesses acute withdrawal symptoms, including anger, anxiety, sadness, hunger, cigarette craving, and concentration difficulty. Consistent with Leventhal et al. (2007), the wording was modified so that participants responded according to how they felt “so far today.”
The 10-item Questionnaire for Smoking Urges–Brief (QSU; Cox, Tiffany, & Christen, 2001) was administered to assess cigarette craving. Participants were asked to respond according to how they felt “right now.”
The 20-item Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) was used to assess state affect.
Subliminal Priming Task
The task was programmed using E-prime software (Version 1.1.; Schneider, Eschman, & Zuccolotto, 2002), and administered on a Pentium-IV computer using the Windows XP operating system. All instructions were presented on a 15″ CRT monitor, and all responses were recorded by the computer using the E-Prime Time Audit Function (with millisecond accuracy). Responses were entered directly on the computer’s keyboard. All peripheral connections were disabled during the experiment.
Procedure
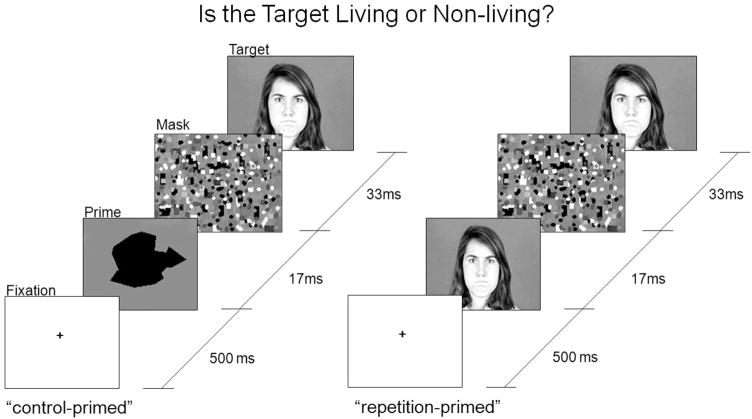
The task was based on previously used subliminal priming paradigms (e.g., Bradley et al., 1994; Breitmeyer et al., 2004). On each trial, a fixation cross was presented for 500 ms, immediately followed by a prime for 16.7 ms (1 monitor refresh at 60 Hz), immediately followed by a brief multicolored pattern mask presentation of 33.3 ms (2 refreshes), which was immediately followed by a target. The target remained on the screen until the participant responded. There were no interstimulus intervals between the fixation, prime, mask, and target; however, there was a 1000-ms intertrial interval. Participants were informed that the task assessed detection speed and accuracy. They were asked to categorize target pictures as living versus nonliving by pressing a corresponding keyboard button as quickly and accurately as possible (assignment of buttons was counterbalanced across participants). The temporal parameters of the task are depicted in Figure 1.
Figure 1.
Temporal sequence of example control-primed and repetition-primed trials for an Angry stimulus on the subliminal priming task.
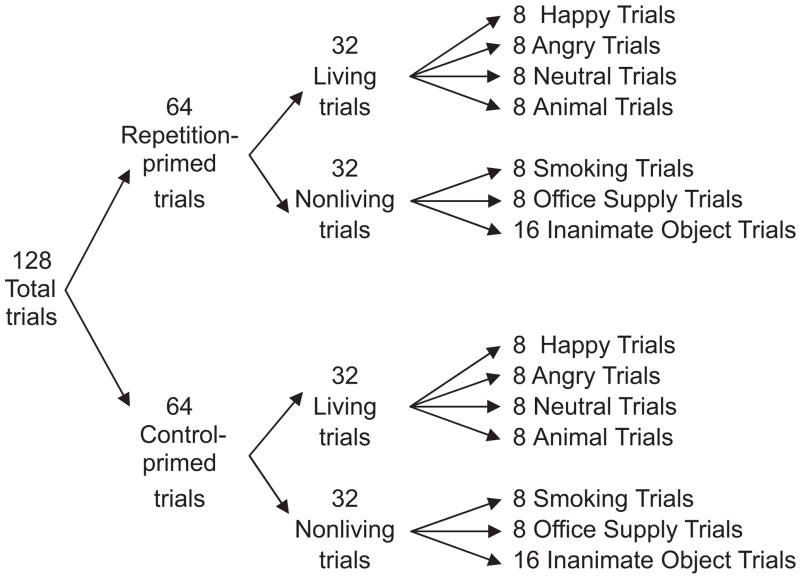
In the entire task, there were 64 different pictures from seven categories: pictures of smoking-related paraphernalia (8), pictures of office supplies (8), pictures of happy (8), angry (8), and neutral (8) facial expressions, pictures of animals (8), and 16 pictures of inanimate objects (e.g., umbrella, book). Facial expressions and animals comprised the living stimuli (32 pictures). Smoking-related, office supply, and inanimate objects comprised the nonliving stimuli (32 pictures). Each of the 64 pictures were presented in two separate trials, once on (a) “repetition-primed” trials, in which prime and target stimuli were identical except in size (prime was 10% smaller than the target) (64 trials), and once on (b) “control-primed” trials, in which a meaningless control prime (random black-colored polygons, used by Murphy & Zajonc, 1993) preceded a target (64 trials). The order of the 128 trials was randomized for each participant. The structure of the task is depicted in Figure 2.
Figure 2.
Structure of subliminal priming task.
The primary dependent variable included the mean RTs in each stimulus category type (i.e., smoking, office, angry, neutral, happy), with separate mean RTs for control-primed and repetition-primed trials. For example, the mean control-primed smoking RT was the mean RT on the eight trials in which the polygons served as primes and the smoking pictures served as targets.
Visibility check
To confirm that participants could not consciously identify the briefly presented primes, a visibility check was performed following the subliminal priming task. The visibility check was administered after (rather than before) the priming task in order to: (a) avoid potential changes in cognitive strategy caused by completing the visibility check (Dagenbach, Carr, & Wilhelmsen, 1989); and (b) limit habituation effects caused by previous subliminal exposure of focal stimuli (Wong & Root, 2003). The visibility check consisted of 64 trials in which all living and nonliving stimuli were displayed as primes and targets. The pairing of primes and targets was randomly assigned. Participants were encouraged to ignore the target and mask and to focus on the prime. They were informed that the prime would be either living or nonliving, and were asked to classify the prime on the keyboard. Concordant with previous research (Breitmeyer et al., 2004), a living versus nonliving discrimination was the classification task used in the visibility check in order to maintain consistency across both the priming experiment and visibility check. They were also instructed that if they could not see the prime they must make their best guess. Given N = 64 trials, and an a priori guessing probability of p = .5, the expected frequency of correct responses based on chance performance is Np = 32; and the standard deviation is (Np[1 − p]).5 = 4 (Breitmeyer et al., 2004). We accepted RT data from participants as long as they made correct responses on no more than 40 trials, which is within the 95% confidence interval (≤2 SDs above the expected accuracy based on chance performance). A pilot study (n = 16) revealed that the stimulus parameters (i.e., 16.7-ms prime, 33.3-ms mask) produced subliminal conditions: no participants identified primes correctly on more than 40 trials. In the actual study, 17 (9.0%) participants made correct classifications on more than 40 trials. There is a reasonable chance that the prime was, in fact, consciously available to these participants. Their data were therefore excluded.
Stimuli
The office supply and smoking-related stimuli were created with a digital camera. The office supply pictures matched the smoking pictures in background color, stimulus complexity, and luminance (pictures available upon request). The facial stimuli were selected from the Japanese and Caucasian Facial Expressions of Emotion system (JACFEE-NEUF: Matsumoto & Ekman, 2004).1 Angry (rather than sad) expressions were chosen because prior studies have shown angry faces to be more effective than sad faces for producing subliminal processing (Morris & Dolan, 2001; Morris, Öhman, & Dolan, 1998;Wong & Root, 2003). All of the JACFEE-NEUF stimuli were matched in background color. Affectively neutral inanimate and animal pictures were selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2001) as filler stimuli in order to balance the number of living and nonliving stimuli.2 Eight pattern-style, high-contrast masks were constructed using the Adobe Photoshop program to mask the primes and match the background color of the five stimulus types (mask luminance: M = 1.60 cd/m2, SD = .16). Example stimuli from each of the picture categories as well as masks are depicted in Figure 3. All target stimuli and masks were 5 cm by 3.5 cm (147 × 110 pixels) and were presented in the center of the screen. Subjects sat at eye-level with the screen center at a viewing distance of 45 cm, making the visual angle of the stimuli 6.3 deg by 4.4 deg.
Figure 3.
Example stimuli and masks.
The seven stimuli types were evaluated on five dimensions: (1) background color; (2) orientation; (3) stimulus size; (4) stimulus frequency/complexity; and (5) luminance. The first four dimensions were rated by the first author (AML). Luminance was measured by a 1 degree narrow angle luminance probe and digital photometer (Tektronix Inc.). χ2 tests and analyses of variance (ANOVAs) revealed that there were no significant differences between office supply and smoking stimuli on any of the five dimensions, and the same was true for the facial stimuli.
The prime was 90% of the size of the target. This was to ensure that repetition priming effects were not solely due to perceptual priming effects that occur because the prime and target are the same stimulus and thus have identical perceptual properties.
Data reduction
Consistent with Breitmeyer et al. (2004), only RTs from correct responses were used. The average number of participants’ incorrect classifications out of the 128 total trials was 2.23 leading to an error rate of 1.8% (range = 0–15.7). Trials for which primes/masks were presented at inaccurate durations were excluded from analyses. Primes/masks were presented at the correct duration on every trial for 97.9% of subjects. For five subjects, a prime or mask was presented for too long a duration on at least one of the trials. To remove outliers, RTs that were more than two SDs away from each subject’s mean RT were excluded.
Because of missing data (due to discarding trials with incorrect responses by the participant, inaccurate presentation by the computer, or outlier status), there were several cases in which there were fewer than four (out of eight) RTs available to compute a mean RT for a specific stimulus type. These mean RT scores were excluded from analyses because they would likely have poor reliability (Angry, n = 1, Smoking, n = 2, Office n = 1; Happy, n = 0, Neutral, n = 0).
Data from nondeprived smokers with CO levels <9 ppm at the experimental session were excluded from the primary analyses as there was significant doubt as to whether these participants complied with instructions to smoke a cigarette within 30 minutes of the experimental session (n = 14, 16%). Recent evidence indicates that withdrawal effects can emerge within a few hours of abstinence (Hendricks et al., 2006). It therefore seems important to exclude participants if there is any suspicion that they have not been smoking normally. Consistent with Powell et al. (2002), data from deprived smokers with a CO level ≥9 ppm were excluded from the primary analyses as there was significant doubt as to whether they complied with instructions to abstain on the day of the test (n = 34, 40%). Small doses of nicotine administration during deprivation can modulate affective processes (e.g., Duka, Tasker, Russell, & Stephens, 1998); any smoking, however modest, might alter the responses of deprived smokers. Data from nonsmokers who had CO levels ≥9 ppm were excluded from analyses as there was significant doubt about their smoking status (n = 2, 3%).
Multivariate analyses of variance(MANOVAs) indicated that there were between-exclusion (excluded vs. included on the basis of CO level) differences on smoking variables in the deprived, F(5, 88) = 2.66, p = .03, but not the nondeprived group, F(5, 88) = 1.95, p =.10. Excluded deprived smokers were heavier, more chronic, and more dependent smokers than included deprived smokers. Excluded deprived smokers were also more likely to be male than included deprived smokers, χ2 (1, N = 85) = 6.89, p =.009. There were no other between-exclusion differences in demographics for any group.
Data Analysis
Preliminary analyses examined group differences in demographic, psychosocial, and smoking variables. Group differences in self-report measures administered during the experimental session were examined to confirm that deprivation altered self-reported withdrawal, craving, and affect in the expected direction.
To examine group differences in subliminal biases toward smoking (vs. office) stimuli, a three-way mixed factorial ANOVA with Group (Deprived smokers vs. Non-deprived smokers vs. Nonsmokers) as a between-subjects factor, and Target Type (Smoking vs. Office) and Prime Type (Control vs. Repetition) as within-subject variables was conducted with mean RT serving as the dependent variable. If a significant three-way interaction were found, additional analyses would assess for three-way interactions in a subsample that restricted the Group factor to each of the possible two-group comparisons (deprived vs. nondeprived; nonsmoker vs. deprived; nonsmoker vs. nondeprived; consistent with Munafò et al., 2003 and Maxwell & Delaney, 1990). In addition, follow up within-subject ANOVAs that determined, for each group separately, whether priming scores were greater for smoking versus office stimuli were performed using priming scores as the dependent variable (defined as the difference between RTs on control- vs. repetition-primed trials). Priming scores index the degree of subliminal priming for each target type (Bradley et al., 1994).
To examine group differences in subliminal biases toward affective (vs. neutral) facial stimuli, a three-way mixed factorial ANOVA with Group (Deprived smokers vs. Nondeprived smokers vs. Nonsmokers) as a between-subjects factor, and Target Type (Angry vs. Neutral vs. Happy) and Prime Type (Control vs. Repetition) as within-subject variables was conducted with mean RT serving as the dependent variable. If a significant three-way interaction were found, additional analyses would be conducted in the manner described above for the analyses of smoking/office stimuli.
All analyses were conducted both unadjusted and adjusted for demographic, psychosocial, and baseline smoking variables for which there were statistically significant group differences. Alpha-level was set at .05 and all tests were two-tailed. All AN(C)OVAs were tested in SAS using PROC GLM for unbalanced cell sizes (SAS Institute Inc., 2003).
Results
Participant Characteristics
Nondeprived smokers were significantly older than deprived smokers and nonsmokers (see Table 1). Both smoking groups had a higher prevalence of Caucasians than the nonsmokers. Both smoking groups had significantly higher BAI scores than nonsmokers; deprived smokers had significantly higher CESD scores than nonsmokers. Deprived smokers had lower FTND scores, smoked fewer cigarettes per day, and had been smoking for a shorter period of time than nondeprived smokers (see Table 1). It is likely that differences between groups were influenced by the exclusion of data from participants whose experimental session CO levels indicated noncompliance with smoking/abstinence instructions.
Table 1.
Sample Characteristics, by Group
| Nonsmokers (n = 56) | Nondeprived smokers (n = 66) | Deprived smokers (n = 47) | F/χ2 | p | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, M (SD) | 23.4 (6.0)a | 26.3 (7.5)b | 22.4 (4.6)a | 6.04 | .003 |
| Female, n (%) | 43 (78.2)a | 37 (56.1)b | 32 (68.1)ab | 6.67 | .04 |
| Ethnicity, n (%) | 32.19 | <.0001 | |||
| African American | 13 (23.2)a | 10 (15.3)b | 1 (2.2)b | ||
| Asian or Pacific Islander | 9 (16.1) | 11 (16.9) | 5 (10.9) | ||
| Caucasian | 15 (26.8) | 40 (61.5) | 32 (69.6) | ||
| Hispanic | 13 (23.2) | 2 (3.1) | 5 (10.9) | ||
| Middle Eastern | 6 (10.7) | 2 (3.1) | 3 (6.5) | ||
| Psychosocial characteristics | |||||
| CESD, M (SD) | 13.0 (8.2)a | 16.1 (10.6)ab | 18.6 (12.7)b | 3.70 | .03 |
| BAI, M (SD) | 9.7 (8.0)a | 15.1 (11.6)b | 15.0 (11.2)b | 4.98 | .008 |
| Smoking characteristics | |||||
| FTND, M (SD) | — | 4.3 (2.0) | 3.2 (1.8) | 8.17 | .005 |
| Cigarettes per day, M (SD) | — | 15.5 (5.7) | 13.6 (3.3) | 4.14 | .04 |
| Age started regular smoking, M (SD) | — | 17.6 (3.5) | 17.6 (2.0) | 0.00 | .95 |
| Years of regular smoking, M (SD) | — | 8.6 (7.4) | 4.8 (4.4) | 10.13 | .002 |
Note. CESD = Center for Epidemiological Studies Depression Scale; BAI = Beck Anxiety Inventory; FTND = Fagerström Test of Nicotine Dependence. Groups with different superscript letters are significantly different in pairwise post-hoc tests. Because of missing data, sample size varies across analyses N = 167 to 169.
Effects of Tobacco Deprivation
Nondeprived smokers exhibited higher breath CO levels than deprived smokers and nonsmokers (see Table 2). Deprived smokers gave higher ratings than both nondeprived smokers and nonsmokers on the WSWS and the PANAS negative affect scale, and lower ratings on the PANAS positive affect scale. On the QSU, all three groups were significantly different from one another; deprived smokers gave the highest QSU ratings. Between group (deprived vs. nondeprived smokers) comparisons on questionnaire measures revealed moderate to large effects (Cohen’s ds ranged from .61 to .94, not shown in Table 2). Thus, the tobacco deprivation manipulation was effective. The results were unchanged when controlling for demographic and smoking characteristics that differed between groups.
Table 2.
Subjective and Biochemical Measures of Withdrawal, by Group
| Nonsmokers (n = 56) | Nondeprived smokers (n = 66) | Deprived smokers (n = 47) | F | p | |
|---|---|---|---|---|---|
| Carbon monoxide (ppm), M (SD) | 2.3 (1.2)a | 21.1 (8.9)b | 4.9 (2.1)a | 190.39 | <.0001 |
| WSWS, M (SD) | 32.6 (12.0)a | 38.7 (15.9)a | 47.5 (17.5)b | 12.26 | <.0001 |
| QSU, M (SD) | 0.0 (0.0)a | 20.9 (10.7)b | 31.6 (12.9)c | 150.59 | <.0001 |
| PANAS-Negative Affect, M (SD) | 15.9 (5.2)a | 16.3 (5.8)a | 20.8 (8.6)b | 8.74 | .0002 |
| PANAS-Positive Affect, M (SD) | 31.4 (9.3)a | 30.2 (8.8)a | 24.9 (9.8)b | 7.05 | .001 |
Note. WSWS = Wisconsin Smoking Withdrawal Scale; QSU = Questionnaire of Smoking Urges–Brief; PANAS = Positive and Negative Affect Schedule. Groups with different superscript letters are significantly different in pairwise post-hoc tests.
RT Data
Mean RT data are summarized in Table 3. Priming scores are also presented.
Table 3.
Reaction Time Data For The Subliminal Priming Task, M (SD)
| Nonsmokers (n = 56) | Nondeprived smokers (n = 66) | Deprived smokers (n = 47) | All groups | |
|---|---|---|---|---|
| Smoking | ||||
| Repetition-primed | 562.7 (94.8) | 594.0 (105.1) | 597.4 (107.2) | 584.6 (103.0) |
| Control-primed | 572.0 (99.7) | 587.2 (115.8) | 615.9 (156.9) | 590.2 (124.4) |
| Priming scorea | 9.33 (70.8) | −6.79 (93.1) | 18.50 (106.3) | 5.59 (90.5) |
| Office | ||||
| Repetition-primed | 551.2 (81.5) | 546.7 (95.3) | 587.1 (171.7) | 559.4 (118.6) |
| Control-primed | 557.5 (94.5) | 555.8 (85.2) | 551.2 (95.3) | 555.1 (90.7) |
| Priming scorea | 6.34 (59.2) | 9.09 (58.9) | −35.90 (122.3) | −4.34 (83.5) |
| Happy | ||||
| Repetition-primed | 504.7 (105.4) | 490.3 (83.1) | 503.3 (139.4) | 498.7 (108.0) |
| Control-primed | 500.8 (78.5) | 504.8 (85.0) | 506.3 (101.4) | 503.9 (87.4) |
| Priming scorea | −3.90 (65.2) | 14.58 (53.3) | 3.07 (106.2) | 5.25 (75.1) |
| Angry | ||||
| Repetition-primed | 503.3 (88.2) | 487.3 (85.6) | 514.5 (153.7) | 500.2 (109.4) |
| Control-primed | 505.9 (71.0) | 503.4 (78.9) | 527.9 (138.5) | 511.0 (97.1) |
| Priming scorea | 2.61 (66.0) | 16.12 (57.8) | 13.39 (86.6) | 10.9 (69.3) |
| Neutral | ||||
| Repetition-primed | 496.2 (87.5) | 502.7 (94.2) | 509.5 (97.2) | 502.4 (92.5) |
| Control-primed | 504.9 (83.2) | 511.8 (95.4) | 510.1 (103.4) | 509.0 (93.4) |
| Priming scorea | 8.66 (36.9) | 9.08 (65.6) | 0.64 (62.1) | 6.59 (56.4) |
| All stimulib | ||||
| Repetition-primed | 546.3 (85.8) | 542.9 (81.5) | 559.4 (126.3) | 548.6 (96.9) |
| Control-primed | 549.6 (80.5) | 550.9 (85.6) | 564.0 (119.4) | 554.1 (94.3) |
| Priming scorea | 3.35 (22.0) | 7.96 (31.4) | 4.65 (52.7) | 5.51 (36.1) |
Note. Data includes correct responses only for participants who did not perform above chance on the awareness check.
Priming Score = reaction time (RT) on Control-Primed–RT on Repetition-Primed trials.
Includes animal and inanimate object stimuli.
Subliminal processing of smoking-related stimuli
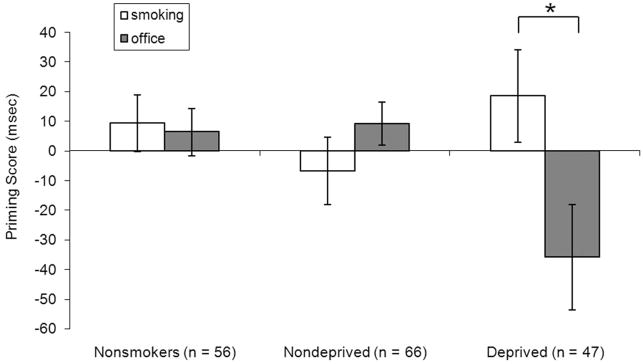
A Group (Deprived smokers vs. Nondeprived smokers vs. Nonsmokers) × Target Type (Smoking vs. Office) × Prime Type (Control vs. Repetition) ANOVA yielded a significant main effect of Target Type, F(1, 166) = 47.71, η2 =.22, p < .0001, and a significant Target Type × Group interaction, F(2, 166) = 3.96, partial η2 =.03, p = .02. Most important, there was a significant Group × Target Type × Prime Type interaction, F(2, 166) = 4.99, partial η2 =.03, p = .008 (see Figure 4). No other effects in the ANOVA were significant. Follow up two-group interaction contrast effects revealed that the three-way interaction was significant for the deprived versus non-deprived, F(1, 111) = 8.04, partial η2 = .04, p = .005, and the deprived versus nonsmoker comparisons, F(1, 101) = 4.31, partial η2 = .02, p = .04, but not for the nondeprived versus nonsmoker comparison, F(1, 120) = 1.08, partial η2 = .001, p = .30. Follow-up single-group ANOVAs on priming scores revealed that the priming scores were significantly larger for smoking-related versus office stimuli for the deprived smokers, F(1, 46) = 5.67, p = .02, but not for the nondeprived smokers, F(1, 46) = 1.46, p = .23, or the nonsmokers, F(1, 55) = .06, p = .81 (see Figure 4).
Figure 4.
Mean (SE) of priming scores (reaction time [RT] on Control Primed – RT on Repetition Primed trials) for smoking and office stimuli by group. There was a significant Prime Type (Smoking vs. Office) by Group (Nonsmoker vs. Nondeprived vs. Deprived) interaction (corresponding to the significant 3-way interaction reported in the text). There were significant differences between priming scores for office and smoking stimuli in the deprived group (*p = .02) but not in the nondeprived (p = .23) and nonsmokers (p = .81) groups.
ANCOVA, which adjusted for age, gender, ethnicity, CESD, and BAI scores, also revealed a significant Group × Target Type × Prime Type interaction, F(2, 155) = 6.09, partial η2 = .04, p = .003. Likewise, a follow-up two group interaction contrast ANCOVA involving the smoking groups, which adjusted for characteristics that significantly differed between deprived and nondeprived smokers (i.e., age, FTND, cig/day, years smoked), also revealed a significant three-way interaction, F(1, 106) = 6.34 partial η2 = .03, p = .01. In these models, neither cig/day nor FTND scores significantly moderated any effects involving Target Type. No other effects in the ANCOVA were significant.
Subliminal processing of affective stimuli
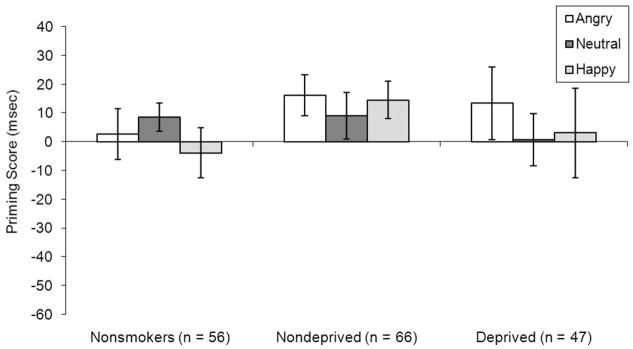
A Group (Deprived smokers vs. Nondeprived smokers vs. Nonsmokers) × Target Type (Angry vs. Neutral vs. Happy) × Prime Type (Control vs. Repetition) ANOVA revealed a significant main effect of Prime Type, F(1, 166) = 4.34, η2 = .03, p = .04, indicating that participants were faster to classify faces as living on repetition-primed (vs. control-primed) trials. The three-way interaction was not significant, F(4, 330) = .36, partial η2 = .001, p = .77. ANCOVA, which adjusted for age, gender, ethnicity, CESD, and BAI scores, also revealed that the three way interaction was not significant, F(4, 314) = .09, partial η2 < .001, p = .99. No other effects in the ANOVA or the ANCOVA were significant. There were no significant differences in priming scores by Target Type within any of the three groups (see Figure 5).3
Figure 5.
Mean (SE) of priming scores (reaction time [RT] on Control Primed – RT on Repetition Primed trials) for happy, angry, and neutral stimuli by group. There were no significant Target Type by Group interactions for priming scores. There were no significant differences in priming scores by Target Type within any of the three groups.
Discussion
The aim of this study was to examine whether deprived smokers, nondeprived smokers, and nonsmokers differed in the degree of subliminal processing of smoking-related and affective stimuli. The data suggested that between-groups differences in subliminal processing were observed (significant Group × Target Type × Prime Type interaction) for the comparison of smoking and office stimuli. Deprived smokers exhibited larger priming for smoking-related stimuli than office stimuli (i.e., a subliminal processing bias), whereas nondeprived smokers and nonsmokers did not. No group differences in subliminal biases toward affective stimuli were found.
Subliminal Processing of Smoking Stimuli
Based on the incentive sensitization theory of addiction (Robinson & Berridge, 2003), we hypothesized that smokers would exhibit a subliminal processing bias toward smoking-related stimuli and that this processing bias would be potentiated by deprivation. The data partially supported this prediction. Only the deprived smokers exhibited a processing bias, which suggests that this bias is only apparent under conditions of tobacco deprivation. Inspection of Figure 4 indicates that, in deprived smokers, the priming score tended in the positive direction for smoking stimuli and tended in the negative direction for office stimuli. Although the meaning of negative subliminal priming effects for comparison stimuli are not currently clear, this has been reported before in the experimental psychopathology literature (Bradley et al., 1994, 1995; Scott et al., 2001). For example, Scott et al. (2001) assessed subliminal priming effects for positive, negative, and neutral words in participants with low and high levels of depressive symptoms in two experiments. Similar to the current data, priming effects tended in the positive direction for depression words and tended in the negative direction for neutral comparison words for the high depression participants.
The current findings are most consistent with those of Jarvik and colleagues (1995), who compared smokers randomized to either nondeprived or deprived conditions, as was done in this study. Participants were required to identify smoking-related and neutral words, which were presented (not masked) for a very brief duration (35 ms). Deprived smokers correctly identified a greater proportion of smoking words than neutral words, whereas nondeprived smokers did not. The current study extends the results of Jarvik et al. (1995) by demonstrating that this processing bias can be observed under subliminal presentation conditions.
Consistent with the present findings, other investigations have also reported no evidence of subliminal processing biases toward smoking-related cues in nondeprived smokers as compared to nonsmokers (Mogg & Bradley, 2002; Munafò et al., 2003; also see Franken et al., 2000). Similarly, on measures that assess biases in the earlier stages of processing (e.g., visual orienting, latency of initial visual fixation), there are generally little or no differences between nondeprived smokers and nonsmokers (Bradley et al., 2003 Experiment 1; Bradley et al., 2004; Mogg, Bradley, Field, & de Houwer, 2003).
In contrast with the current findings, which showed evidence of a deprivation effect, two studies have reported no effect of deprivation on subliminal processing biases toward smoking words when using the subliminal version of the smoking Stroop task (Mogg & Bradley, 2002; Munafò et al., 2003). Conversely, two other investigations reported that subliminally presented alcohol-related cues resulted in greater physiological reactivity than subliminally presented neutral cues in recently abstinent alcoholics (Ingjaldsson et al., 2003a, 2003b). Taken together, these findings suggest deprivation-provoked subliminal processing biases toward drug-related cues may be present, but they may only be detectible using certain tasks. The subliminal modified Stroop task assesses the degree to which subliminally presented salient (vs. neutral) cues interfere with color naming of a subsequently presented supraliminal neutral stimulus. Studies using this task have shown consistent interference effects for aversive stimuli (e.g., Mogg, Bradley, Williams, & Mathews, 1993;Yovel & Mineka, 2005), but have generally not shown effects for drug-related (Mogg & Bradley, 2002; Munafò et al., 2003; Franken et al., 2000) or nondrug incentive stimuli (e.g., food-related words; Mogg, Bradley, Hyare, & Lee, 1998). Interference might be more robust on the subliminal Stroop task when using aversive cues because processing these cues could produce a fear response (e.g., behavioral freezing) that could interfere with the primary task (i.e., color naming). In contrast, it is unlikely that subliminal incentive stimuli would promote fear that would slow color naming responses. Thus, tasks that rely on interference effects, such as the subliminal Stroop task, may not be sensitive indicators of hyperactive incentive motivational states during drug deprivation.
Subliminal Processing of Affective Stimuli
Based on opponent process theories of addiction (Koob et al., 1997), we hypothesized that nicotine deprivation would attenuate subliminal processing biases toward affective stimuli. The data did not support these hypotheses; differences in priming for the affective (facial) stimuli did not vary as a function of deprivation. Although previous studies have reported evidence of altered processing of supraliminally presented affective stimuli during nicotine deprivation (e.g., Cinciripini et al., 2006; Dawkins et al., 2006; Gilbert et al., 2007; Powell et al., 2002, 2004), the current study shows that these effects are not observed on a subliminal repetition priming task.
One should note that in the supraliminal Stroop investigations, a processing bias toward positive (vs. neutral) stimuli was shown in nondeprived smokers, which was attenuated under conditions of deprivation (Dawkins et al., 2006; Powell et al., 2002, 2004). However, on the current subliminal priming task the nondeprived smokers showed no bias in processing affective (vs. neutral) stimuli. The absence of a baseline bias (i.e., a significant processing bias in nondeprived smokers) may have restricted our ability to show attenuation of this bias under conditions of deprivation. Future investigation using subliminal tasks that show robust affective biases in nondeprived smokers may be warranted to detect deprivation-provoked changes in subliminal processing of affective cues.
Limitations, Implications, and Conclusions
The current study had limitations. Data from a large number of smokers (n = 48) were excluded from the primary analyses because these smokers presented with high (deprived group) or low (nondeprived group) CO levels respectively. This procedure maximized the probability that the smokers in the final sample had indeed smoked normally (nondeprived) or remained abstinent (deprived group). However, the procedure also differentially influenced the composition of the two groups such that the nondeprived group was comprised of heavier, more severely dependent, smokers than the deprived group, presumably because heavy smokers in the latter group had failed to maintain abstinence. However, confidence in our findings is increased by the following observations. First, results remained robust when we adjusted for baseline variables that were significantly different between the groups (ANCOVAs). Second, the results remained robust when the data were reanalyzed using different CO cutoff criteria, and when no participants were excluded (see footnote 3). Third, neither smoking heaviness nor FTND scores interacted with Target Type (Office vs. Smoking) or Target Type × Prime Type effects, suggesting that these variables (which differed between the groups) were unlikely to significantly influence the findings.
Another limitation is that we do not know whether the deprivation effects observed in the study were due to the changes in nicotine levels or psychological factors associated with tobacco deprivation (e.g., expectations about nicotine withdrawal). The study was also limited to young adult smokers who were not attempting to quit, and it is unclear whether these findings will generalize to older smokers attempting to quit. Finally, even though the three-way interaction was robust, the pattern of effects underlying subliminal processing biases may be complex and the mechanisms accounting for these effects remain uncertain. Thus, further evidence is required before the existence of subliminal processing biases toward smoking-related stimuli in deprived smokers is confirmed.
The study also had strengths, including: (a) the use of a novel task to examine subliminal processing uncontaminated from the influence of strategic processing; (b) the use of a three-group design, which allowed an examination of the effects of deprivation and smoking status within the same study; (c) the inclusion of both smoking-related and affective stimuli within the same study, which permitted comparison of their relative effects; and (d) the use of pictorial stimuli, which enhanced the ecological validity of the task. In addition, to the best of our knowledge, this study is the first to document differences in subliminal processing biases between deprived and nondeprived smokers. Based on these results, future research should investigate whether subliminal biases toward smoking-related information influence relapse.
Acknowledgments
This project was funded by a National Cancer Institute predoctoral fellowship awarded to Adam M. Leventhal (R25 CA 57730-11; PI-Robert Chamberlain). The authors extend their gratitude to Maria Archila, Colleen Axtell, Illeana Garcia, and Lindsay Reid for being instrumental to collecting these data.
Footnotes
The facial stimuli selected from the JACFEE and JACNUEF system were as follows: Angry (E1 – E8), Happy (E33 – E40), Neutral (N1, N10, N16, N23, N30, N33, N43, N53). Each stimulus was a photograph of a different person. Within each of the three affective categories, there were two photographs of Caucasian females, two Caucasian males, two Japanese females, and two Japanese males.
The stimuli selected from the IAPS were as follows: animals (1440, 1450, 1500, 1510, 1540, 1600, 1670, 1740), inanimate objects (7004, 7006, 7009, 7010, 7020, 7035, 7040, 7050, 7060, 7080, 7090, 7150, 7175, 7190, 7211, 7950).
To evaluate the robustness of the primary analyses, we reran all analyses 1) using different cutoffs for CO levels at the experimental session (i.e., 8 ppm, N = 164; 10 ppm, N = 171), and 2) when including all participants who did not perform above chance on the awareness check (N = 217), regardless of their CO levels at the experimental session. Results from these secondary analyses were consistent with those from the primary analyses. All nonsignificant effects in the primary analyses remained so. All the three-way Group × Target Type (Smoking vs. Office) × Prime Type interaction effects remained significant for all three group comparisons and two-group interaction contrasts comparing deprived and nondeprived smokers. The single exception was the analysis using all participants, in which the three-way interaction became marginally significant, F(2, 214) = 2.81, partial η2 =.02, p = .06.
Contributor Information
Adam M. Leventhal, Center for Alcohol and Addiction Studies, Brown University
Andrew J. Waters, Department of Medical and Clinical Psychology, Uniformed Services University of the Health Sciences, Bethesda, Maryland
Bruno G. Breitmeyer, Department of Psychology, University of Houston
Evelina Tapia, Department of Psychology, University of Houston.
Elizabeth Miller, Department of Behavioral Science, University of Texas–M.D. Anderson Cancer Center.
Yisheng Li, Department of Biostatistics, University of Texas–M.D. Anderson Cancer Center.
References
- Amir N, McNally RJ, Riemann BC, Burns J. Suppression of the emotional Stroop effect by increased anxiety in patients with social phobia. Behaviour Research and Therapy. 1996;34:945–948. doi: 10.1016/s0005-7967(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Bradley B, Field M, Mogg K, de Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behavioural Pharmacology. 2004;15:29–36. doi: 10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Williams R. Implicit and explicit memory for emotional information in non-clinical subjects. Behaviour Research and Therapy. 1994;32:65–78. doi: 10.1016/0005-7967(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Williams R. Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behaviour Research and Therapy. 1995;33:755–770. doi: 10.1016/0005-7967(95)00029-w. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Wright T, Field M. Attentional bias in drug dependence: Vigilance for cigarette-related cues in smokers. Psychology of Addictive Behaviors. 2003;17:66–72. doi: 10.1037/0893-164x.17.1.66. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: A comparison, review, and update. Perception & Psychophysics. 2000;62:1572–1595. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ogmen H, Chen J. Unconscious priming by color and form: Different processes and levels. Consciousness and Cognition: An International Journal. 2004;13:138–157. doi: 10.1016/j.concog.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam C, Wu X, de Moor CA, et al. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine & Tobacco Research. 2006;8:379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and Explicit Drug Motivational Processes: A Model of Boundary Conditions. In: Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: Sage; 2006. pp. 233–250. [Google Scholar]
- Dagenbach D, Carr TH, Wilhelmsen A. Task-induced strategies and near-threshold priming: Conscious influences on unconscious perception. Journal of Memory & Language. 1989;28:412–443. [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I–effects on incentive motivation. Psychopharmacology. 2006;189:355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, Russell K, Stephens DN. Discriminative stimulus properties of nicotine at low doses: The effects of caffeine preload. Behavioural Pharmacology. 1998;9:219–229. [PubMed] [Google Scholar]
- Field M. Attentional biases in drug abuse and addiction: Cognitive mechanisms, causes, consequences and implications. In: Munafò M, Albery IP, editors. Cognition and addiction. New York: Oxford University Press; 2006. pp. 73–99. [Google Scholar]
- Field M, Mogg K, Bradley BP. Attention to drug-related cues in drug abuse and addiction: Component processes. In: Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: Sage; 2006. pp. 151–163. [Google Scholar]
- Franken IHA. Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Kroon LY, Wiers RW, Jansen A. Selective cognitive processing of drug cues in heroin dependence. Journal of Psychopharmacology. 2000;14:395–400. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Sugai C, Zuo Y, Rabinovich NE, McClernon FJ, Froeliger B. Brain indices of nicotine’s effects on attentional bias to smoking and emotional pictures and to task-relevant targets. Nicotine & Tobacco Research. 2007;9:351–363. doi: 10.1080/14622200701188810. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Abrams RL, Naccache L, Dehaene S. Long-term semantic memory versus contextual memory in unconscious number processing. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:235–247. doi: 10.1037/0278-7393.29.2.235. [DOI] [PubMed] [Google Scholar]
- Gross TM, Jarvik ME, Rosenblatt MR. Nicotine abstinence produces content-specific Stroop interference. Psychopharmacology. 1993;110:333–336. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Thayer JF, Laberg JC. Craving for alcohol and pre-attentive processing of alcohol stimuli. International Journal of Psychophysiology. 2003a;49:29–39. doi: 10.1016/s0167-8760(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Thayer JF, Laberg JC. Preattentive processing of alcohol stimuli. Scandinavian Journal of Psychology. 2003b;44:161–165. doi: 10.1111/1467-9450.00334. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Gross TM, Rosenblatt MR, Stein RE. Enhanced lexical processing of smoking stimuli during smoking abstinence. Psychopharmacology. 1995;118:136–141. doi: 10.1007/BF02245831. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacology, Biochemistry and Behavior. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: Effects on subjective, cognitive, and physiological measures. Experimental And Clinical Psychopharmacology. 2007;15:21–36. doi: 10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian Facial Expressions of Emotion (JACFEE) and Neutral Faces (JACNeuF) Berkeley, CA: Paul Ekman; 2004. [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison perspective. Mahwah, NJ: Erlbaum; 1990. [Google Scholar]
- Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: Manipulation of deprivation level and comparison of three measures of processing bias. Journal of Psychopharmacology. 2002;16:385–392. doi: 10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Field M, de Houwer J. Eye movements to smoking-related pictures in smokers: Relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98:825–836. doi: 10.1046/j.1360-0443.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Hyare H, Lee S. Selective attention to food-related stimuli in hunger: Are attentional biases specific to emotional and psychopathological states, or are they also found in normal drive states? Behaviour Research and Therapy. 1998;36:227–237. doi: 10.1016/s0005-7967(97)00062-4. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R, Mathews A. Subliminal processing of emotional information in anxiety and depression. Journal of Abnormal Psychology. 1993;102:304–311. doi: 10.1037//0021-843x.102.2.304. [DOI] [PubMed] [Google Scholar]
- Morris J, Dolan R. The amygdala and unconscious fear processing. In: De Gelder B, De Haan EHF, Heywood CA, editors. Out of mind: Varieties of unconscious processes. New York: Oxford University Press; 2001. pp. 185–204. [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Munafò M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified Stroop task. Journal of Psychopharmacology. 2003;17:310–316. doi: 10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- Murphy ST, Zajonc ST. Affect, cognition, and awareness: Affective priming with optimal and suboptimal stimulus exposures. Journal of Personality and Social Psychology. 1993;64:723–729. doi: 10.1037//0022-3514.64.5.723. [DOI] [PubMed] [Google Scholar]
- Öhman A. As fast as the blink of an eye: Evolutionary preparedness for preattentive processing of threat. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Mahwah, NJ: Erlbaum Publishers; 1997. pp. 165–184. [Google Scholar]
- Powell J, Tait S, Lessiter J. Cigarette smoking and attention to signals of reward and threat in the Stroop paradigm. Addiction. 2002;97:1163–1170. doi: 10.1046/j.1360-0443.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addictive Behaviors. 2004;29:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Ryan F. Detected, selected, and sometimes neglected: Cognitive processing of cues in addiction. Experimental and Clinical Psychopharmacology. 2002;10:67–76. doi: 10.1037//1064-1297.10.2.67. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows (Version 8.2) Cary, NC: SAS Institute Inc; 2003. [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh: Psychology Software Tools Inc; 2002. [Google Scholar]
- Scott KM, Mogg K, Bradley BP. Masked semantic priming of emotional information in subclinical depression. Cognitive Therapy and Research. 2001;25:505–524. [Google Scholar]
- Steer RA, Clark DA, Beck AT, Ranieri WF. Common and specific dimensions of self-reported anxiety and depression: A replication. Journal of Abnormal Psychology. 1995;104:542–545. doi: 10.1037//0021-843x.104.3.542. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95:S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Feyerabend C. Determinants and effects of attentional bias in smokers. Psychology of Addictive Behaviors. 2000;14:111–120. doi: 10.1037//0893-164x.14.2.111. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA. Implicit cognition and tobacco addiction. In: Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: Sage; 2005. pp. 309–338. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW. Handbook of implicit cognition and addiction. Thousand Oaks, CA: Sage; 2005. [Google Scholar]
- Wong PS, Root JC. Dynamic variations in affective priming. Consciousness & Cognition: An International Journal. 2003;12:147–168. doi: 10.1016/s1053-8100(03)00007-2. [DOI] [PubMed] [Google Scholar]