SUMMARY
Normal vasculature development of the central nervous system is extremely important because patients with vascular malformations are at life-threatening risk of intracranial hemorrhage or cerebral ischemia. The etiology and pathogenesis of abnormal vasculature development in the central nervous system are unknown, and progress is hampered by the lack of animal models of the human cerebrovascular diseases. Here, we reported our current study on cerebral microvascular dysplasia (CMVD) development. Using vascular endothelial growth factor hyper-stimulation, we demonstrated that aberrant microvessels could be developed in the rodent brain in certain conditions (such as genetic deficient background, local cytokine and chemokine release, or exogenous vessel dilating stimulation) that may speed up focal angiogenesis and lead to cerebral vascular dysplasia.
Keywords: cerebral, dysplasia, microvascular, mice, VEGF
INTRODUCTION
Brain microvascular dysplasia is one of the most critical situations because these aberrant angiogenesis cause epilepsy, focal ischemia and even intracranial hemorrhage. During the disease process such as brain trauma, ischemia, or inflammation, local angiogenic factors (e.g., growth factors such as vascular endothelial growth factor [VEGF], platelet derived growth factor [PDGF]; cytokines such as interleukin-1 beta [IL-1β], interleukin-6 [IL-6], transforming growth factor-beta [TGF-β], tumor necrosis factor-alpha [TNFα]; chemokines such as monocyte chemotactic protein-1 [MCP-1], macrophage inflammatory protein 1 [MIP-1]; and others such as netrin-1; matrix metalloproteinases [MMPs], and integrin, etc.) are greatly increased [7]. These angiogenic mediators initially activate focal angiogenesis in the body, including brain tissue. Under certain conditions, such as genetic deficiency (endoglin and ALK mutation), vessel dilation stimulation (eNOS, hydralazine or nicardipine), or micro-environmental changes (increased VEGF, MMP, integrin, Homeobox gene expression), normal angiogenesis then turns to abnormal growth and develops to form cerebral microvascular dysplasia (CMVD). When this process takes place, increased BBB permeability and focal hemodynamic changes exacerbate existing dysplasia, enhance the dysplasia lesion region, or even cause focal hemorrhage. Currently, several angiogenic mediators are used to stimulate CMVD formation in the experimental animal brain. This strategy has provided a unique model for mechanistic studies.
Development CMVD animal models that phenocopy human disease
Experimental animal models for AVMs were first developed by surgeons seeking to understand the complex hemodynamics of arteriovenous shunting and its relationship to “normal perfusion pressure breakthrough,” an infrequent but morbid complication of AVM resection [29, 30]. Further model development included attempts to model aspects of the disease related to potential therapy development, such as a target for radiosurgery or embolic agents. A wide variety of models, generally extracranial arteriovenous fistulas between the common or internal carotid artery and the external jugular vein [1, 3, 6, 13, 16, 22] and/or dural fistulas [32, 33], has been described in a number of species, including rats, swine, cats, sheep, chicken, rabbits, pigs, and goats [15, 19–21, 23, 24]. With few exceptions, they are extradural in nature [26]. Of particular note is that they do not display the clinical syndrome of recurrent hemorrhage into the brain parenchyma or cerebral spinal fluid (CSF) spaces. Therefore, a parenchymal nidus is not formed; nidus growth and hemorrhage mimicking the human disease do not occur. To optimally study pathogenesis and disease progression, a model system is needed to test mechanistic questions. An ideal model system would have the following attributes by phenocopying the clinical cerebrovascular disease: 1) possess a nidus of abnormal vessels of varying sizes, encompassing both micro and macro-circulatory levels; 2) display spontaneous hemorrhage into the brain parenchyma or CSF spaces (but not preferentially subarachnoid and in basal cisterns); 3) display A-V shunting including early visualization of efferent veins relative to normal venous circulation; 4) possess flow rates that are sufficient to reproducibly lower proximal feeding artery pressure [9, 27]; 5) display high angiogenic and inflammatory signal expression consistent with human surgical specimens. [11, 12]
The transforming growth factor-beta (TGF-β) signaling is of particular interest (see companion article by Kim et al). Hereditary hemorrhagic telangiectasia (HHT; Osler-Weber-Rendu disease) is an autosomal dominant syndrome of mucocutanous fragility that is strongly associated with both pulmonary and brain AVMs. The two common subtypes of HHT (HHT1 and 2) are caused by well-characterized loss-of-function mutations in two genes [18]: endoglin (ENG), which codes for an accessory protein of TGF-β receptor complexes; and activin-like kinase 1 (ALK-1, or ACVLR1), a transmembrane kinase that participates with TGF-β receptor II in TGF-β signaling. Recently, two reports indicate that ALK1 can signal through bone morphogenetic protein 9 (BMP9), and that endoglin can potentiate the signal. These newer data suggest that BMP9 may represent the most physiologically relevant endothelial signaling pathway for HHT pathogenesis.[5, 28] The downstream target effecter for both TGF-β and BMP signaling for AVM pathogenesis is the mothers against decapentaplegic homolog 4 (SMAD4) gene, which is associated with juvenile polyposis and has recently been described for SMAD. This gene is mutated in a combined syndrome of juvenile polyposis and HHT.[8]
Approaches to CMVD model development
Based on the above attributes of the human lesional tissue, we aimed our model development towards establishing the means by which we simulated relevant aspects of the human disease, either by phenocopying aspects of the vascular phenotype or by genetic manipulation to provide an intermediate phenotype.
Conceptually, dysplasia represents enlarged, dysmorphic vascular structures that have structural instability. Our model was initially based on growth factor hyper-stimulation of murine brain by viral transfection. Although many exciting studies have been conducted on the initiation, formation and growth of angiogenesis, the nature of vascular signaling pathways in the abnormal cerebral vascular phenotypes in both human disease and experimental animals is still unclear [17]. The main causes of pathologic angiogenesis in the CNS include vascular malformations, tissue ischemia, traumatic damage and neoplasm. Angiogenesis is a rapidly growing field with some 4477 publications listed in PubMed for the year 2007, six times the number from the previous 10 years [2]. Development of a non-tumor brain angiogenesis model is therefore timely.
Hyper-stimulation of angiogenesis can induce abnormal vessel formation in a variety of tissues including the brain [25, 34]. We began our modeling efforts by introducing VEGF into a normal adult circulatory bed by an adenoviral (Ad) or adeno-associated viral (AAV) vector to hyper-stimulate naive tissue, thus deregulating paracrine homeostasis. Although viral vector-delivered hyper-stimulation of VEGF can cause some degree of morphological changes [31], and other kinds of VEGF delivery cause dysmorphic vessels, a combination of the VEGF delivery system with altered genetic background may more reliably produce vascular dysplasia.
To develop a feasible and reproducible focal brain microvessel dysplasia model, we performed the following experiment based on the understanding of multi-systemic focal vascular lesions in HHT disease [10, 17]. Following induction of anesthesia, AAVVEGF was stereotactically injected into the right hemisphere of ENG+/− or ALK1+/− or ENG/ALK1+/− mice. Animals were sacrificed following 3 to 6 weeks of AAVVEGF gene transfer. Lectin staining was performed for microvessel counting. We found much more abnormal microvessel patterns in the ENG+/−, ALK1+/− or ENG/ALK1+/− mice brain following VEGF hyper-stimulation. Our result thus indicated that VEGF hyper-stimulation could induce microvessel dysplasia in the genetic deficient situation.
To develop a CMVD model, we tested the effect of flow-augmentation in the VEGF-transduced mice. Using micro-osmotic pump infusion technique, we observed the effect of hydralazine on microvessel dysplasia formation. We counted microvessel numbers in AAVVEGF-transduced ALK1+/− mice with hydralazine or nicardipine or saline treatment. We found that microvessel counts were greatly increased in the AAVVEGF-transduced mice compared to the AAVlacZ plus saline infusion group; hydralazine or nicardipine did not further increase microvessel counts in hydralazine-treated AAVVEGF or AAVlacZ-transduced ALK1+/− mice. We then counted the number of dilated microvessels (>8 µm in diameter), which increased after treatment with hydralazine or nicardipine in both AAVlacZ and AAVVEGF-transduced mice compared to the saline infusion group mice (p<0.05). BrdU staining showed that CD31 positive cells were well merged with the BrdU positive staining, indicating active proliferation of endothelial cells in hydralazine or nicardipine infusion in AAVVEGF-transduced ALK1+/− mice. We further examined the microvascular morphologic changes. Interestingly, we detected much more abnormal microvasculature, such as a massive or single enlarged node, or clustered, twisted, or spiral microvessels, following hydralazine or nicardipine micro-pump infusion in the AAVVEGF-transduced ALK+/− mice than in the saline infused mice. These abnormal microvessels usually developed in the ipsilateral cortex and caudate putamen adjacent to the needle track. Confocal microscope demonstrated that these abnormal microvessels were not overlapped, confirming the abnormal microvessel morphology.
Endothelial nitric oxide synthase (eNOS) is critical for vascular remodeling, mural cell recruitment, and blood flow reserve [36]. eNOS-derived NO can serve as a vasodilator to reduce vascular resistance, improve blood flow, and maintain proportional remodeling of blood vessels during changes in blood flow. In addition, eNOS exerts a second messenger role in VEGF signaling and is necessary for many of the actions of VEGF in cultured endothelial cells or in postnatal mice. PI3K mediates a critical pathway in NO- and VEGF-mediated angiogenesis. In vivo results demonstrate that elevated eNOS expression or activity is sufficient to activate the PI3K/Akt signaling pathway via PKG, leading to neovascularization in ischemic tissues [14].
Using adenoviral vector delivering eNOS gene (AdeNOS) transfer, AdeNOS was injected into basal ganglia region in the mouse brain. The result demonstrated that dilated microvessels are increased in the AdeNOS-transduced mouse brain compared to the control (AdlacZ), and eNOS positive staining is mainly located in the vessel wall. In addition, less eNOS positive staining was found in the lowest dose group, suggesting that eNOS expression is in a dose-dependent manner. We also examined microvessel morphology by lectin staining. Dilated and abnormal microvessels were identified under the hyper-stimulation of eNOS.
Functional consequences of vascular dysplasia
Morphological characterizations are the most visible and widely accepted evidence for vascular dysplasia. However, the functional consequence of vascular dysplasia is important evidence supporting dysplasia vasculature as being detrimental. Within this functional determination, we decided to examine BBB permeability and micro-hemorrhage because these are often detected in vascular dysplasia.
The main dilemma of pathologic angiogenesis is consistent BBB leakage, which exacerbates brain edema and tissue damage. We demonstrated that BBB disruption occurred during active angiogenesis. BBB permeability was determined using albumin staining, as previously described [35]. The BBB permeability also can be determined quantitatively using a measurement of Evans blue with similar results. Micro-hemorrhage is another complication during active angiogenesis. We also found that micro-hemorrhage occurred in the AdVEGF-transduced mouse brain. The extent of cerebral hemorrhage can be quantified using a spectrophotometric assay with Drabkin’s reagent [4]. We demonstrated that hemorrhage was in the mouse brain after injection of AdVEGF, but not in the viral vector control group after AdFc injection. The pattern of ICH induced by focal VEGF hyper-stimulation in the brain is illustrated using hemotoxylin-eosin staining.
Conclusions and further considerations for model development
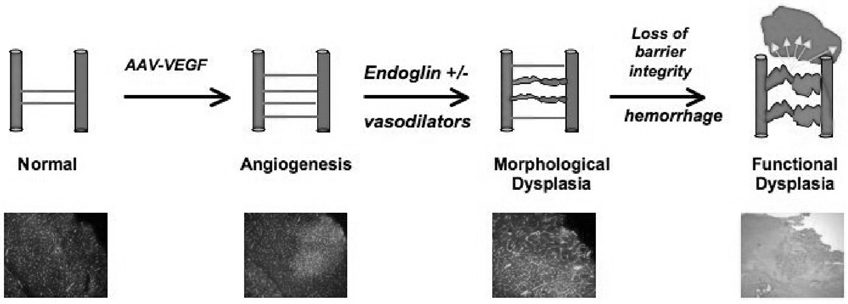
Our primary approach to creating our intermediate brain AVM phenotype has been to combine viral hyper-stimulation with manipulation of genetic background (e.g., endoglin deficient mice), or by manipulation of local hemodynamics (e.g., increased tissue perfusion by local vasodilator infusion), as illustrated in Figure 1. We hypothesize that with further development of these experimental conditions to achieve a focal vascular lesion, we will detect direct arteriovenous shunting and spontaneous ruptures, which mimic human disease. Continually integrating hemodynamic influences on vascular remodeling is also important. Furthermore, currently developed dysplastic abnormalities are present in the microcirculation and need to be brought to the level of macrocirculation, as seen in human disease. These in vivo animal models will provide us with an opportunity to discern causal relationships in the formation of an abnormal vascular phenotype and to comprehensively investigate the pathways involved. The relationship of vascular dysplasia to the disease remains to be clarified, but it is a first step toward generating a morphological correlate of the human phenotype.
Figure 1.
The schematic illustrates the current approach to modeling vascular dysplasia in the adult brain. The main steps include initiation of angiogenesis with VEGF hyper-stimulation, and induction of morphological dysplastic changes (deficit endoglin in genetic background or local flow increases), which leads to loss of barrier integrity and micro-hemorrhage.
REFERENCES
- 1.Bederson JB, Wiestler OD, Brustle O, Roth P, Frick R, Yasargil MG. Intracranial venous hypertension and the effects of venous outflow obstruction in a rat model of arteriovenous fistula. Neurosurgery. 1991;29(3):341–350. doi: 10.1097/00006123-199109000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 3.Chaloupka JC, Vinuela F, Robert J, Duckwiler GR. An in vivo arteriovenous malformation model in swine: Preliminary feasibility and natural history study. AJNR Am J Neuroradiol. 1994;15(5):945–950. [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhri TF, Baker KZ, Winfree CJ, Hoh BL, Simon A, Solomon RA, Berman M, Connolly ES. Intraoperative mild hypothermia is not associated with increased craniotomy wound infection rate or length of hospitalization. Surg Forum. 1997;48:548–551. [Google Scholar]
- 5.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) endothelial cells. Blood. 2007;109(5):1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 6.De Mey JG, Schiffers PM, Hilgers RH, Sanders MM. Toward functional genomics of flow-induced outward remodeling of resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288(3):H1022–H1027. doi: 10.1152/ajpheart.00800.2004. [DOI] [PubMed] [Google Scholar]
- 7.Fan Y, Yang GY. Therapeutic angiogenesis for brain ischemia: a brief review. J Neuroimmune Pharmacol. 2007;2(3):284–289. doi: 10.1007/s11481-007-9073-3. [DOI] [PubMed] [Google Scholar]
- 8.Gallione CJ, Richards JA, Letteboer TG, Rushlow D, Prigoda NL, Leedom TP, Ganguly A, Castells A, Ploos van Amstel JK, Westermann CJ, Pyeritz RE, Marchuk DA. SMAD4 Mutations found in unselected HHT patients. J Med Genet. 2006;43(10):793–797. doi: 10.1136/jmg.2006.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao E, Young WL, Ornstein E, Pile-Spellman J, Ma Q. A theoretical model of cerebral hemodynamics: application to the study of arteriovenous malformations. J Cereb Blood Flow Metab. 1997;17(8):905–918. doi: 10.1097/00004647-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Guttmacher AE, Marchuk DA, White RI. Hereditary hemorrhagic telangiectasia. N Engl J Med. 1995;333(14):918–924. doi: 10.1056/NEJM199510053331407. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM, Young WL. Co-expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery. 2005;56(5):1058–1065. discussion 1058–1065. [PubMed] [Google Scholar]
- 12.Hashimoto T, Young WL. Roles of angiogenesis and vascular remodeling in brain vascular malformations. Seminars in Cerebrovascular Diseases and Stroke. 2004;4(4):217–225. [Google Scholar]
- 13.Herman JM, Spetzler RF, Bederson JB, Kurbat JM, Zabramski JM. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83(3):539–545. doi: 10.3171/jns.1995.83.3.0539. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki K, Smith RS, Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003;23(16):5726–5737. doi: 10.1128/MCB.23.16.5726-5737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutluk K, Schumacher M, Mironov A. The role of sinus thrombosis in occipital dural arteriovenous malformations--development and spontaneous closure. Neurochirurgia (Stuttg) 1991;34(5):144–147. doi: 10.1055/s-2008-1052075. [DOI] [PubMed] [Google Scholar]
- 16.Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87(2):267–274. doi: 10.3171/jns.1997.87.2.0267. [DOI] [PubMed] [Google Scholar]
- 17.Lim M, Cheshier S, Steinberg GK. New vessel formation in the central nervous system during tumor growth, vascular malformations, and Moyamoya. Curr Neurovasc Res. 2006;3(3):237–245. doi: 10.2174/156720206778018730. [DOI] [PubMed] [Google Scholar]
- 18.Marchuk DA, Srinivasan S, Squire TL, Zawistowski JS. Vascular morphogenesis: tales of two syndromes. Hum Mol Genet. 2003;12 Suppl 1:R97–R112. doi: 10.1093/hmg/ddg103. [DOI] [PubMed] [Google Scholar]
- 19.Massoud TF, Ji C, Vinuela F, Guglielmi G, Robert J, Duckwiler GR, Gobin YP. An experimental arteriovenous malformation model in swine: anatomic basis and construction technique. AJNR Am J Neuroradiol. 1994;15(8):1537–1545. [PMC free article] [PubMed] [Google Scholar]
- 20.Massoud TF, Ji C, Vinuela F, Turjman F, Guglielmi G, Duckwiler GR, Gobin YP. Laboratory simulations and training in endovascular embolotherapy with a swine arteriovenous malformation model. AJNR Am J Neuroradiol. 1996;17(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan MK, Anderson RE, Sundt TM., Jr The effects of hyperventilation on cerebral blood flow in the rat with an open and closed carotid-jugular fistula. Neurosurgery. 1989;25(4):606–611. doi: 10.1097/00006123-198910000-00015. discussion 611–612. [DOI] [PubMed] [Google Scholar]
- 22.Morgan MK, Anderson RE, Sundt TM., Jr A model of the pathophysiology of cerebral arteriovenous malformations by a carotid-jugular fistula in the rat. Brain Res. 1989;496(1–2):241–250. doi: 10.1016/0006-8993(89)91071-8. [DOI] [PubMed] [Google Scholar]
- 23.Murayama Y, Massoud TF, Vinuela F. Hemodynamic changes in arterial feeders and draining veins during embolotherapy of arteriovenous malformations: an experimental study in a swine model. Neurosurgery. 1998;43(1):96–104. doi: 10.1097/00006123-199807000-00064. discussion 104–106. [DOI] [PubMed] [Google Scholar]
- 24.Nagasawa S, Kawanishi M, Kondoh S, Kajimoto S, Yamaguchi K, Ohta T. Hemodynamic simulation study of cerebral arteriovenous malformations. Part 2. Effects of impaired autoregulation and induced hypotension. J Cereb Blood Flow Metab. 1996;16(1):162–169. doi: 10.1097/00004647-199601000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietila TA, Zabramski JM, Thellier-Janko A, Duveneck K, Bichard WD, Brock M, Spetzler RF. Animal model for cerebral arteriovenous malformation. Acta Neurochir (Wien) 2000;142(11):1231–1240. doi: 10.1007/s007010070019. [DOI] [PubMed] [Google Scholar]
- 27.Quick CM, Leonard EF, Young WL. Adaptation of cerebral circulation to brain arteriovenous malformations increases feeding artery pressure and decreases regional hypotension. Neurosurgery. 2002;50(1):167–173. doi: 10.1097/00006123-200201000-00025. discussion 173–165. [DOI] [PubMed] [Google Scholar]
- 28.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, Ten Dijke P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120(Pt 6):964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 29.Scott BB, McGillicuddy JE, Seeger JF, Kindt GW, Giannotta SL. Vascular dynamics of an experimental cerebral arteriovenous shunt in the primate. Surg Neurol. 1978;10(1):34–38. [PubMed] [Google Scholar]
- 30.Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 31.Stiver SI, Tan X, Brown LF, Hedley-Whyte ET, Dvorak HF. VEGF-A angiogenesis induces a stable neovasculature in adult murine brain. J Neuropathol Exp Neurol. 2004;63(8):841–855. doi: 10.1093/jnen/63.8.841. [DOI] [PubMed] [Google Scholar]
- 32.Terada T, Higashida RT, Halbach VV, Dowd CF, Tsuura M, Komai N, Wilson CB, Hieshima GB. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80(5):884–889. doi: 10.3171/jns.1994.80.5.0884. [DOI] [PubMed] [Google Scholar]
- 33.TerBrugge KG, Lasjaunias P, Hallacq P. Experimental models in interventional neuroradiology. AJNR Am J Neuroradiol. 1991;12(6):1029–1033. [PMC free article] [PubMed] [Google Scholar]
- 34.Xu B, Wu YQ, Huey M, Arthur HM, Marchuk DA, Hashimoto T, Young WL, Yang GY. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab. 2004;24(2):237–244. doi: 10.1097/01.WCB.0000107730.66603.51. [DOI] [PubMed] [Google Scholar]
- 35.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25(8):1658–1664. doi: 10.1161/01.str.25.8.1658. discussion 1664–1665. [DOI] [PubMed] [Google Scholar]
- 36.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102(31):10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]