Abstract
When B-lymphocytes differentiate into plasma cells, immunoglobulin (Ig) heavy and light chain synthesis escalates and the entire secretory apparatus expands to support high-rate antibody secretion. These same events occur when murine B-cells are stimulated with lipopolysaccharide (LPS), providing an in vitro model in which to investigate the differentiation process. The unfolded protein response (UPR), a multi-pathway signaling response emanating from the endoplasmic reticulum (ER) membrane, allows cells to adapt to increasing demands on the protein folding capacity of the ER. As such, the UPR plays a pivotal role in the differentiation of antibody-secreting cells. Three specific stress sensors, IRE1, PERK/PEK and ATF6, are central to the recognition of ER stress and induction of the UPR. IRE1 triggers splicing of Xbp-1 mRNA, yielding a transcriptional activator of the UPR termed XBP-1(S), and activation of the IRE1/XBP-1 pathway has been reported to be required for expansion of the ER and antibody secretion. Here, we provide evidence that PERK is not activated in LPS-stimulated splenic B-cells, whereas XBP-1(S) and the UPR transcriptional activator ATF6 are both induced. We further demonstrate that Perk-/- B-cells develop and are fully competent for induction of Ig synthesis and antibody secretion when stimulated with LPS. These data provide clear evidence for differential activation and utilization of distinct UPR components as activated B-lymphocytes increase Ig synthesis and differentiate into specialized secretory cells.
Keywords: B-lymphocytes, plasma cells, antibody secretion, unfolded protein response, PERK
Introduction
Nascent immunoglobulin (Ig) heavy and light chains are co-translationally translocated into the lumen of the endoplasmic reticulum (ER), a calcium-rich, oxidizing environment containing many resident molecular chaperones and folding enzymes (van Anken and Braakman, 2005b). In this specialized compartment, Ig chains fold and assemble into functional, antigen-binding H2L2 molecules (Hendershot and Sitia, 2004). As Ig synthesis escalates and the abundance of nascent Ig chains in the ER rises during the differentiation of antibody-secreting plasma cells (Wiest et al., 1990), it follows that the load of client proteins for the ER folding machinery increases. The mechanisms that allow differentiating plasma cells (Brewer and Hendershot, 2005; Calame et al., 2003) to accommodate increased demands on the ER are not fully defined.
A major mechanism by which cells cope with challenges to the ER environment is activation of the unfolded protein response (UPR) (van Anken and Braakman, 2005a; Schroder and Kaufman, 2005). When unfolded proteins accumulate in the ER, one branch of the UPR is initiated by an ER transmembrane kinase/endoribonuclease, IRE1 (α and β isoforms; first identified in yeast as inositol requiring 1) (Tirasophon et al., 1998; Wang et al., 1998). Upon activation, IRE1 initiates site-specific splicing of transcripts encoding the X-box-binding protein-1 (XBP-1) transcription factor to generate XBP-1(S) (Calfon et al., 2002; Shen et al., 2001; Yoshida et al., 2001). The Xbp-1 gene and UPR-mediated splicing of Xbp-1 mRNA are essential for the differentiation of antibody-secreting cells (Iwakoshi et al., 2003; Reimold et al., 2001). XBP-1 is required for increased expression of many secretory pathway genes and for expansion of the ER during the differentiation process (Shaffer et al., 2004). Another branch of the UPR that can augment the protein folding capacity of the ER is mediated by activating transcription factor 6 (ATF6, α and β isoforms), an ER membrane-bound transcription factor (Haze et al., 2001; Haze et al., 1999). ER stress triggers the ATF6 proteins to traffic to the Golgi where they undergo proteolytic cleavage, a process that liberates their cytosolic domains as soluble transcription factors that up-regulate expression of various ER chaperones and folding enzymes (Haze et al., 1999; Okada et al., 2002; Yoshida et al., 1998). A role for ATF6 in plasma cell differentiation has not been established, but its activation has been observed during lipopolysaccharide (LPS)-induced differentiation of the CH12 B-cell lymphoma (Gass et al., 2002).
The third branch of the UPR is directed by the ER transmembrane kinase, PKR-like ER kinase (PERK; also known as PEK and EIF2AK3) (Harding et al., 1999; Shi et al., 1998). Upon activation, PERK phoshorylates the α subunit of eucaryotic translation initiation factor-2 (eIF-2) on serine 51, thereby efficiently down-regulating protein synthesis by inhibiting formation of 43S translation initiation complexes (Harding et al., 2000b; Harding et al., 1999; Shi et al., 1998). This global repression of protein synthesis reduces the flow of nascent polypeptides into the ER and, at the same time, facilitates the preferential translation of Atf4 mRNA, encoding a transcription factor that increases expression of genes involved in regulating amino acid availability, resistance to oxidative stress, as well as the UPR (Harding et al., 2000a; Harding et al., 2003). PERK has also been shown to phosphorylate an additional substrate NF-E2-related factor 2 (NRF2) (Cullinan et al., 2003), activating this transcription factor to induce genes involved in maintaining redox homeostasis (Cullinan and Diehl, 2004). Two UPR-responsive gene products, growth arrest DNA damage gene 34 (GADD34) and p58 inhibitor of protein kinase (p58IPK), have been implicated in feedback regulation of the PERK pathway. GADD34, a transcriptional target of ATF4, facilitates dephosphorylation of eIF-2α, allowing translation to resume within a few hours of UPR activation (Ma and Hendershot, 2003; Novoa et al., 2001). XBP-1(S) up-regulates expression of p58IPK (Lee et al., 2003; Shaffer et al., 2004), a chaperone that has the ability to negatively regulate PERK activity (van Huizen et al., 2003; Yan et al., 2002).
Perk-/- mice exhibit defects in both the endocrine and exocrine pancreas as well as in the major secretory cells of the skeletal system (Harding et al., 2001; Zhang et al., 2002; Zhang et al., 2006), suggesting that PERK plays a critical regulatory role in the development and function of specialized secretory cell types. However, in contrast to the UPR branches initiated by IRE1 and ATF6, there is currently no evidence for PERK activation during the differentiation of antibody-secreting cells (Gass et al., 2002; Zhang et al., 2005). Furthermore, Rag2-/- mice reconstituted with fetal hematopoietic liver cells homozygous for a non-phosphorylatable point mutant of eIF-2α (eIF-2αS51A) exhibited mature splenic B-cells and near normal levels of serum Ig (Zhang et al., 2005), suggesting that eIF-2α phosphorylation can be dispensable for B-cell development and antibody secretion in vivo. To directly address whether PERK is required for B-cell function, we monitored activation of PERK during the differentiation of primary B-lymphocytes into antibody-secreting cells and assessed the ability of Perk-/- B-cells to synthesize and secrete antibody.
Materials and Methods
Animals
C57BL/6 female mice were purchased (Jackson Laboratory, Bar Harbor, ME) and used experimentally at 8-12 weeks of age. Perk+/- mice, backcrossed into the outbred Swiss Webster strain, were generated and described by Dr. David Ron and colleagues (New York University, New York, NY) (Harding et al., 2001). Perk+/- breeders were kindly provided by Dr. Brian Popko (University of Chicago, Chicago, IL). Perk+/+, +/- and -/- littermates were genotyped as previously described (Harding et al., 2001) and used experimentally at 3-4 weeks of age. All animal work was performed according to protocols approved by the Loyola University Medical Center Institutional Animal Care and Use Committee.
Cell culture
Splenic B-cells were isolated from mice using red blood cell (RBC) depletion and negative selection with the MACS B-cell isolation kit (Miltenyi Biotec, Auburn, CA). Alternatively, RBC-depleted splenocytes were incubated with culture supernatant from the 30-H12 anti-Thy1.2-secreting hybridoma (provided by Dr. D. G. Quinn, University of Ulster, Ireland), followed by incubation with Low-Tox-M Rabbit Complement (Cedarlane, Ontario, Canada), and viable B-cells were isolated via a Lympholyte-M (Cedarlane) gradient. Cells were cultured in RPMI-1640 supplemented as described (Gass et al., 2002) at 1×106c/ml and stimulated with 10μg/ml LPS (E. coli 055:B5, Sigma, St. Louis, MO) for various intervals. To induce ER stress, cells were treated for various intervals with either tunicamycin (Sigma) or thapsigargin (Sigma) dissolved in dimethysulfoxide at the concentrations indicated in the figure legends. Cells were counted using trypan blue dye exclusion to determine viability.
Flow cytometry
Biotin-conjugated mouse anti-mouse IgDa (Becton Dickinson, Palo Alto, CA), APC-conjugated streptavidin (eBioscience, San Diego, CA) and FITC-conjugated rat anti-mouse CD45R/B220 (Southern Biotechnology Associates, Birmingham, AL) were purchased. Data from stained cell samples were acquired using a FACSCalibur flow cytometer (Becton Dickinson) and Flowjo software (Tree Star, Ashland, OR).
Analysis of secreted IgM
In some experiments, the amount of IgM that accumulated in culture supernatants over the course of LPS stimulation was determined. In other experiments, on day 3 of LPS stimulation B-cells were counted, washed twice in warm media and then replated at 5×105c/ml for 4 h. Culture supernatants were then harvested and assessed for IgM content. IgM was quantified by ELISA as previously described (Gass et al., 2002).
Immunoblotting
Cells were washed twice with cold PBS, pelleted by centrifugation and frozen. For immunoblot analysis of ATF6α(N), cells were solubilized in SDS-PAGE sample buffer (0.0625 M Tris-HCl pH 6.8, 10% glycerol, 5% 2-ME, 2% SDS), sonicated briefly, and boiled. For all other immunoblotting, cells were lysed on ice in Nonidet P-40 (NP-40)/deoxycholate (DOC) lysis buffer (0.5% NP-40, 0.5% DOC, 50mM Tris-HCL pH 7.5, 150mM NaCl, 20 μg/ml leupeptin, 40 μg/ml aprotinin, 100 μg/ml PMSF). Lysates from an equal number of cells were resolved on 10% polyacrylamide gels using standard SDS-PAGE under reducing conditions. Chemiluminescent immunoblotting of Ig μ and κ chains, BiP/GRP78, TRAPα, XBP-1(S), ATF6α(N) and β actin was performed as described (Gass et al., 2002; Gunn and Brewer, 2006). Immunoblot analysis of PERK was performed using an affinity purified rabbit polyclonal antibody raised against recombinant protein containing the carboxy terminus of PERK. The rabbit anti-GADD34 antibody was purchased (Santa Cruz Biotechnology, Santa Cruz, CA), and the mouse monoclonal anti-p58IPK antibody was generously provided by Dr. Michael Katze (University of Washington, Seattle, WA). Peroxidase-conjugated donkey anti-mouse IgG and peroxidase-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) were used as secondary antibodies.
Metabolic labeling and immunoprecipitation
B-cells were washed twice with warm PBS and then cultured at 1.5×106 cells/ml in warm media lacking methionine and cysteine (Gibco Invitrogen, Carlsbad, CA) for 20 min. Cells were then labeled for 10 min with 75-100 μCi/ml [35S]methionine and cysteine using Tran35S-label (MP Biomedicals, Irvine, CA) as described in the figure legends, harvested by centrifugation, and washed twice with cold PBS. Cells were solubilized on ice in NP-40/DOC lysis buffer containing protease inhibitors as described above. Ig μ and κ chains were immunoprecipitated, resolved by SDS-PAGE under reducing conditions and assessed by phosphorimaging as previously described (Gass et al., 2002). To measure the incorporation of radiolabeled methionine and cysteine into proteins, lysates from metabolically labeled cells were normalized to cell number and equivalent amounts spotted on Whatman filter paper in triplicate. Filters were boiled in 10% tricholoroacetic acid (TCA) and analyzed by scintillation spectroscopy. Lysates of labeled cells were also resolved by SDS-PAGE under reducing conditions and assessed by phosphorimaging.
Results
Induction of Ig synthesis and expansion of the secretory machinery in LPS-stimulated B-cells
The bacterial component LPS signals through Toll-like receptor complexes (Poltorak et al., 1998) and activates ∼ 30% of murine splenic B-cells in vitro to proliferate, up-regulate Ig expression, expand the secretory apparatus and secrete antibody (Andersson et al., 1972; Rush et al., 1991; Shohat et al., 1973). Importantly, splenic B-cells, the vast majority (90-95%) of which are follicular B-cells (IgMlowIgDhighCD21intCD23high), undergo these LPS-induced changes over the course of a few days, reaching maximal antibody production within 3 to 4 days (Gunn and Brewer, 2006; Oliver et al., 1999). Thus, we used LPS stimulation of splenic B-cells in vitro to study the development of antibody-secreting cells.
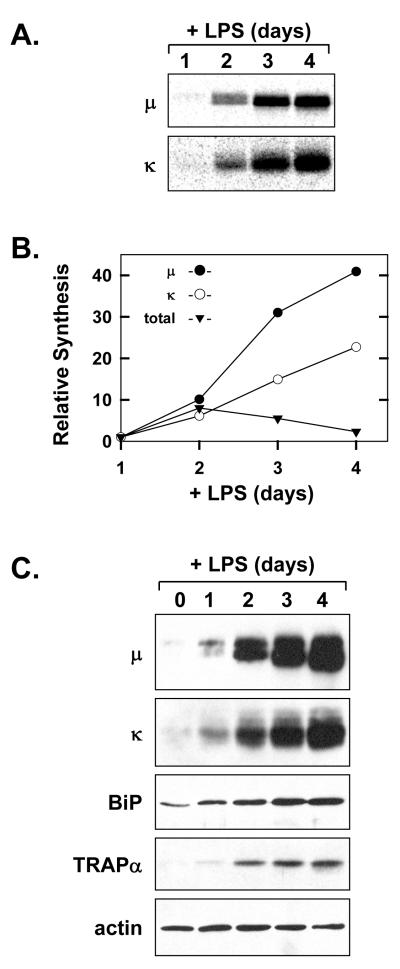
As expected, metabolic labeling with [35S]methionine and cysteine revealed large increases in the level of synthesis for both Ig μ heavy chains and κ light chains by day 2 of LPS stimulation, with even greater enhancement by days 3 and 4 (Fig. 1A). In contrast to the steady escalation of Ig synthesis, total protein synthesis increased by day 2 of stimulation and then gradually declined (Fig. 1B). This profile of total protein synthesis in B-cells over the course of LPS stimulation has been previously reported for splenic B-cells (Rush et al., 1991) as well as B-cell lines (van Anken et al., 2003). Immunoblot analysis revealed that the intracellular load of Ig μ heavy chains and κ light chains steadily increased over the course of LPS stimulation (Fig. 1C) and was accompanied by increases in components of the secretory machinery (Fig. 1C). For example, the level of the soluble ER resident molecular chaperone BiP (immunoglobulin binding protein; also known as glucose-regulated protein 78) and the ER translocon component TRAPα (translocon associated protein α) elevated substantially during the course of LPS stimulation (Fig. 1C), providing a biochemical correlate for expansion of the rough ER. These data illustrate that LPS elicits the differentiation of B-lymphocytes into specialized secretory cells dedicated to high-rate Ig production.
Figure 1.
Induction of Ig synthesis in LPS-stimulated splenic B-cells. Splenic B-cells were cultured in the presence of LPS. A, At the indicated intervals of LPS stimulation, 2×106 viable cells were metabolically labeled with 75μCi/ml [35S]methionine and cysteine for 10 min. Ig μ and κ chains were immunoprecipitated from the cell lysates and resolved by SDS-PAGE under reducing conditions. Signals from the labeled Ig chains were captured by phosphorimaging. B, Synthesis of Ig μ and κ chains at each interval of LPS stimulation was assessed by quantification of the signals shown in A. Total protein synthesis was assessed by TCA precipitation of cell lysates (2.5×105 cells at each interval) from the experiment in A followed by scintillation spectroscopy. The relative level of synthesis is plotted for μ(●), κ(○), and total protein (▼) at each interval as compared to day 1 (set at 1). C, Cell lysates were prepared at the indicated intervals and equal cell equivalents were assessed by immunoblotting for Ig μ and κ chains, the soluble ER chaperone BiP, the ER translocon component TRAPα, and actin as a loading control.
Activation status of UPR transducers in LPS-stimulated B-cells
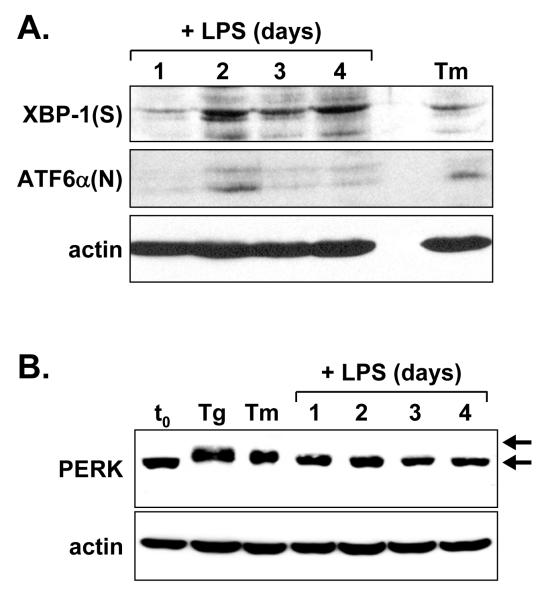
We then determined whether the large increase in Ig synthesis in LPS-stimulated splenic B-cells is accompanied by activation of the UPR transducer PERK. As controls, we assessed the activation status of the IRE1/XBP-1 and ATF6 branches of the UPR. Consistent with previous studies of LPS-stimulated splenic B-cells (Gunn and Brewer, 2006; Iwakoshi et al., 2003), we detected the XBP-1(S) transcription factor as early as day 1 of LPS stimulation, but it was more strongly expressed by day 2 of stimulation and was maintained through day 4 (Fig. 2A). Given the short half-life of XBP-1(S) (Calfon et al., 2002), our data and that of others indicate that IRE1 activation occurs early during LPS stimulation and persists thereafter. Immunoblotting also revealed an increase in the level of the active form of ATF6α, termed ATF6α(N), most prominently on day 2 of LPS stimulation (Fig. 2A). These data indicating transient induction of ATF6α(N) in LPS-stimulated splenic B-cells are the first evidence for ATF6 activation during differentiation of primary antibody-secreting B-cells. Upon activation, PERK undergoes autophosphorylation, a modification that slows its electrophoretic mobility (Harding et al., 1999; Shi et al., 1998). In B-cells treated with standard ER stress-inducing agents thapsigargin (Tg, inhibitor of the ER calcium ATPase) and tunicamycin (Tm, inhibitor of N-linked glycosylation), PERK showed reduced gel mobility as judged by immunoblot analysis (Fig. 2B). However, the shift in PERK mobility was not observed in splenic B-cells through 4 days of LPS stimulation (Fig. 2B). These data support the idea that the type of physiologic UPR triggered during the differentiation of antibody-secreting B-cells includes activation of the IRE1 and ATF6 transducers, but not of PERK.
Figure 2.
Immunoblot analysis of UPR components in LPS-stimulated splenic B-cells. Splenic B-cells were cultured in the presence of LPS for the indicated intervals. At day 3, a portion of the cells was treated either for 5 h with 1 μg/ml tunicamycin (Tm) or 1 h with 0.4 μM thapsigargin (Tg) to provide positive controls for UPR activation. Cell lysates were prepared and equal cell equivalents were assessed by immunoblotting for A, XBP-1(S) and ATF6α(N) and B, PERK. Phosphorylated and nonphosphorylated forms of PERK are denoted by upper and lower arrows, respectively. Actin was assessed as a loading control.
Development and LPS-induced differentiation of Perk-/- B-cells
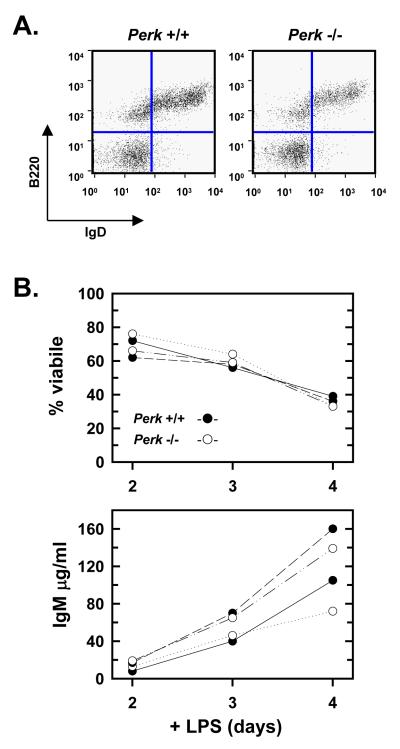
To directly determine the importance of PERK in the development of B-cells, we measured the levels of these lymphocytic cells in the spleens of Perk+/+ and Perk-/- mice. We found that B-cells developed normally in the absence of PERK as illustrated by the similar frequency of mature B220+IgD+ B-cells (Fig. 3A). Consistent with these data, flow cytometric analysis revealed similar profiles of pro-B, pre-B and immature B-cells in the bone marrow of Perk+/+, Perk+/-, and Perk-/- animals (data not shown). These data establish that B-cell development is not dependent on PERK function.
Figure 3.
Development and LPS response of PERK-deficient B-cells. Splenocytes and splenic B-cells were prepared from age- and sex-matched Perk+/+ and Perk-/- mice. A, Splenocytes were prepared, stained with FITC-conjugated anti-B220 and biotin-conjugated anti-IgD plus streptavidin-APC, and assessed by flow cytometry. Splenocytes scoring as B220+IgD+ mature B-cells appear in the upper right quadrants. Five mice for each genotype were analyzed and representative data are shown. B, Splenic B-cells from two separate Perk+/+ mice (●) and two separate Perk-/- mice (○) were cultured in the presence of LPS. At the indicated intervals, cell viability was assessed by trypan blue dye exclusion (upper panel) and the amounts of IgM present in the culture supernatants were determined by ELISA (lower panel).
The complex pathophysiology and limited lifespan of PERK-deficient mice (Harding et al., 2001; Zhang et al., 2002) complicates the analysis of immune function in vivo. Therefore, to assess the ability of PERK-deficient B-cells to differentiate into antibody-secreting cells, we cultured Perk+/+ and Perk-/- splenic B-cells in vitro in the presence of LPS for 4 days. The viability of wild-type and PERK-deficient B-cells was highly similar over the culture period (Fig. 3B, upper panel). Likewise, the amount of IgM that accumulated in the culture supernatant over 4 days of LPS stimulation was comparable for wild-type and PERK-deficient B-cells (Fig. 3B, lower panel). These results demonstrate that PERK is not required for normal lifespan or antibody secretion by B-cells stimulated with LPS.
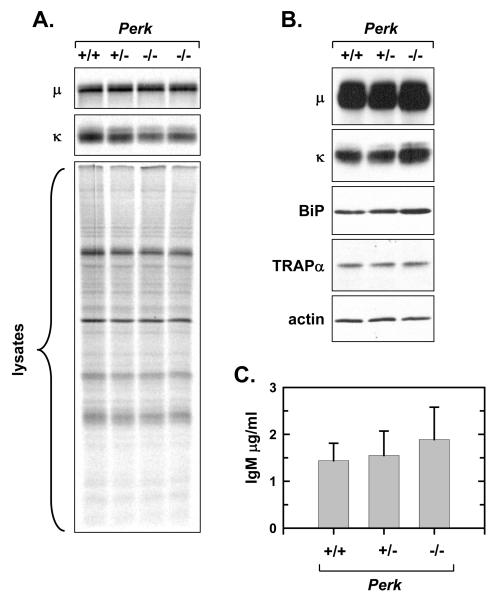
To further investigate the differentiation of PERK-deficient B-cells, we focused on the day 3 interval of LPS stimulation, a time at which the majority of splenic B-cells remain viable (Fig. 3B, upper panel) and the predominant population of splenic B-cells, the follicular B-cell subset, has initiated antibody secretion (Gunn and Brewer, 2006; Oliver et al., 1999). First, metabolic labeling with [35S]methionine and cysteine revealed that the level of synthesis for Ig μ heavy chains was similar for Perk+/+, Perk+/-, and Perk-/- splenic B-cells at day 3 of LPS stimulation (Fig. 4A, upper panel), while synthesis of Ig κ light chains was slightly reduced in Perk-/- cells (Fig. 4A, middle panel). Total protein synthesis and the profile of translated proteins were very similar in the three B-cell genotypes (Fig. 4A, lower panel). Next, immunoblot analysis showed that intracellular Ig μ heavy chains and κ light chains were comparably abundant in Perk+/+, Perk+/-, and Perk-/- splenic B-cells at day 3 of LPS stimulation (Fig. 4B). The ER proteins BiP and TRAPα were also readily detectable in each of the three B-cell genotypes after 3 days of stimulation (Fig. 4B), indicating comparable expansion of the ER and secretory machinery. Finally, we found that Perk+/+, Perk+/-, and Perk-/- B-cells secreted similar levels of IgM at the day 3 interval of stimulation (Fig. 4C). These data demonstrate that Ig synthesis, expression of key ER proteins and Ig secretion proceed normally in LPS-stimulated B-cells in the absence of PERK.
Figure 4.
Ig synthesis and secretion by LPS-stimulated PERK-deficient B-cells. Splenic B-cells were prepared from age- and sex-matched Perk+/+, Perk+/- and Perk-/- mice and cultured in the presence of LPS for 3 days. A, 2×106 viable cells were metabolically labeled with 75μCi/ml [35S]methionine and cysteine for 10 min. Ig μ and κ chains were immunoprecipitated from the cell lysates and resolved by SDS-PAGE under reducing conditions along with an equivalent amount of cell lysate (2×105 cells). Signals from the labeled Ig chains (upper and middle panels) and from labeled total proteins (lower panel) were captured and quantified by phosphorimaging. As compared to +/+ (set at 1), the mean ± SD for the μ signal: 1.04 ± 0.19 for +/-, n = 3; 0.90 ± 0.28 for -/-, n = 6; κ signal: 0.98 ± 0.14 for +/-, n = 3; 0.68 +/- 0.07 for -/-, n = 6. As compared to +/+ (set at 1), the mean ± SD for total protein synthesis was 0.94 +/- 0.16 for +/-, n = 3 and 0.89 +/- 0.08 for -/-, n = 4. B, Cell lysates were prepared and equal cell equivalents were assessed by immunoblotting for Ig μ and κ chains, the soluble ER chaperone BiP, the ER translocon component TRAPα, and actin as a loading control. C, Cells were harvested, washed and replated at 5×105 c/ml for 4 h. The amount of IgM secreted was determined by ELISA and plotted as mean ±SD (+/+ and +/-, n = 3; -/-, n = 4; p > 0.1).
Expression of PERK regulators and PERK responsiveness in LPS-stimulated B-cells
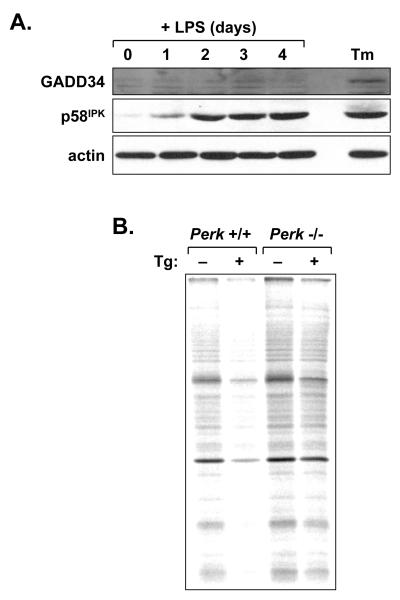
We previously proposed that PERK-mediated signaling might be negatively regulated during the differentiation of antibody-secreting B-cells so that its activity does not impede Ig translation (Brewer and Hendershot, 2005; Gass et al., 2002). This notion is consistent with the normal function of LPS-stimulated B-cells in Perk-/- mice (Figs. 3 and 4). To investigate this further, we assessed LPS-stimulated splenic B-cells for expression of the GADD34 and p58IPK proteins. Immunoblotting revealed minimal GADD34 protein over 4 days of LPS stimulation (Fig. 5A). In sharp contrast, p58IPK protein increased substantially by day 1 of stimulation and to higher levels thereafter (Fig. 5A). To assess PERK responsiveness in differentiating B-cells, we measured protein synthesis in LPS-stimulated B-cells exposed to thapsigargin, a potent inducer of ER stress and PERK-mediated repression of protein synthesis (Harding et al., 2000b). We cultured wild-type and Perk-/- splenic B-cells for 3 days in the presence of LPS and then treated the cells for 1 h with thapsigargin. Metabolic labeling with [35S]methionine and cysteine revealed a marked decrease in protein synthesis in thapsigargin-treated B-cells, and this effect was less pronounced in PERK-deficient cells (Fig. 5B). Therefore, while p58IPK is upregulated in B-cells differentiating in response to LPS, these cells remain competent for PERK-dependent repression of protein synthesis.
Figure 5.
Induction of PERK regulators and responsiveness of the PERK pathway in LPS-stimulated B-cells. A, Splenic B-cells were cultured in the presence of LPS for the indicated intervals. At day 3, a portion of the cells was treated for 5 h with 1 μg/ml tunicamycin (Tm) to provide a positive control for UPR activation. Cell lysates were prepared and equal cell equivalents were assessed by immunoblotting for GADD34, p58IPK and actin as a loading control. B, Splenic B-cells were prepared from age- and sex-matched Perk+/+ and Perk-/- mice and cultured in the presence of LPS for 3 days. Cells were then treated for 1 h with vehicle alone or 1 μM thapsigargin (Tg) and metabolically labeled with 100μCi/ml [35S]methionine and cysteine for 10 min. Equivalent amounts of cell lysate (2×105 cells) were resolved by SDS-PAGE under reducing conditions and signals from labeled proteins were captured by phosphorimaging. Thapsigargin treatment resulted in a 40% decrease in protein synthesis in +/+ cells and a 14% decrease in -/- cells.
Discussion
We have provided evidence that the differentiation of B-lymphocytes into antibody-secreting cells involves a type of UPR that is distinct from the UPR present in other specialized secretory cell types and in cells subjected to pharmacologically induced ER stress. First, when B-cells upregulated Ig synthesis and secreted antibody in response to LPS, activation of both the IRE1/XBP-1 and ATF6 branches of the UPR was observed (Fig. 2A), whereas phosphorylation of PERK was not detected (Fig. 2C). These data regarding PERK are consistent with the findings of Zhang and colleagues (Zhang et al., 2005); however, their study ended at the day 2 interval of LPS stimulation prior to optimal Ig synthesis and antibody secretion. Furthermore, we found that PERK is dispensable for the development of B-cells and their differentiation into antibody-secreting cells (Figs. 3 and 4). These results suggest that there are important biological conditions under which portions of the UPR are selectively activated. In the case of B-cell differentiation where synthesis and secretion of antibodies are paramount, there is selective activation of the IRE1/XBP-1 and ATF6 arms of the UPR that enhance transcription of genes involved in assembly and transport of secretory proteins. These events may initiate early after B-cell activation, thereby “preparing” for subsequent induction of maximal Ig biosynthesis (Gass et al., 2002; Skalet et al., 2005; van Anken et al., 2003). By contrast, the PERK portion of the UPR that features significant dampening of global protein synthesis is not engaged.
An important question concerns the mechanism(s) that directs selective activation of the IRE1 and ATF6 branches of the UPR, with PERK remaining inactive in differentiating B-cells. One possibility is that a negative regulator of PERK activation is induced during the differentiation process. Indeed, our data demonstrating a large increase in p58IPK protein by day 1 of LPS stimulation (Fig. 5A) raise the intriguing possibility that induction of p58IPK provides a mechanism for suppression of PERK activation during the differentiation process. Arguing against this idea is our observation that translation repression by PERK is readily activated when LPS-stimulated B-cells are treated with a pharmacological inducer of ER stress (Fig. 5B). Therefore, if a repressing factor like p58IPK influences PERK activity in differentiating B-cells, such interactions do not render PERK completely inert.
Another possible explanation for the lack of PERK activation during the differentiation process is that in B-cells the “threshold” of ER stress required for activating PERK is higher than that required for activating IRE1 and ATF6. The amino-terminal portions of the ER transmembrane proteins PERK and IRE1 are located in the ER lumen and bound to BiP. In response to accumulation of malfolded proteins during ER stress, BiP is proposed to be released from PERK and IRE1, leading to their autophosphorylation and activation (Bertolotti et al., 2000). According to this model, there would be differential release of BiP between PERK and IRE1 during B-cell differentiation, with BiP being readily discharged from IRE1, while remaining bound to PERK. Walter and colleagues proposed an alternative model for IRE1 activation based on the crystal structure for the ER lumenal domain of yeast IRE1 (Credle et al., 2005). In this model, a groove identified in the lumenal region of IRE1 is proposed to directly bind to unfolded protein, contributing to the intermolecular phosphorylation and activation of IRE1. Given the sequence similarity in the regulatory regions of IRE1 and PERK, this model would predict that differential activation of these two UPR sensors during B-cell differentiation could result from variations in their affinities for malfolded protein. However, Kaufman and colleagues recently analyzed the crystal structure for the ER lumenal domain of human IRE1 and concluded the groove to be too narrow for peptide binding (Zhou et al., 2006). A mechanistic basis for differential activation of IRE1 and PERK, therefore, remains to be delineated.
Conclusions
Terminally differentiating B-cells initiate, upregulate and maintain Ig synthesis and secretion until death, and there is currently no evidence that antibody-secreting cells modulate Ig production up or down during their lifespan. In contrast, pancreatic secretory cells must cope with episodic high-rate synthesis of secretory proteins according to the fluctuating metabolic needs of the organism. Clearly, PERK is critical for normal development and function of pancreatic secretory cells, whereas this UPR component is dispensable for the development of antibody-secreting B-cells. We propose that PERK activation in antibody-secreting B-cells would be counterproductive for humoral immunity as it would interfere with constitutive Ig synthesis, thereby restraining antibody output. In addition, recent data suggest that high-rate Ig synthesis coupled with reduced proteasomal activity may be linked to the apoptosis of LPS-stimulated B-cells (Cenci et al., 2006). Thus, if the absence of PERK activation contributes to maximal Ig synthesis, this might also facilitate mechanisms that limit the lifespan of antibody-secreting B-cells. We emphasize that the in vitro model of LPS stimulation is most applicable to the differentiation of short-lived plasma cells in vivo. The role of the various UPR components in the differentiation and function of long-lived plasma cells awaits further study.
Acknowledgments
We thank Patricia Simms (Loyola University Medical Center Flow Cytometry Facility) and LeeTerry Moore for expert technical assistance. This work was supported in part by grants from the National Institutes of Health (J.N.G., T32AI007508; R.C.W., GM64350; J.W.B., GM61970).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson J, Sjoberg O, Moller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur. J. Immunol. 1972;2:349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat. Immunol. 2005;6:23–29. doi: 10.1038/ni1149. [DOI] [PubMed] [Google Scholar]
- Calame KL, Lin K-I, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu. Rev. Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Cenci S, Mezghrani A, Cascio P, Bianchi G, Cerruti F, Fra A, Lelouard H, Masciarelli S, Mattioli L, Oliva L, Orsi A, Pasqualetto E, Pierre P, Ruffato E, Tagliavacca L, Sitia R. Progressively impaired proteasomal capacity during terminal plasma cell differentiation. EMBO J. 2006;25:1104–1113. doi: 10.1038/sj.emboj.7601009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18773–18784. doi: 10.1073/pnas.0509487102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JN, Gifford NM, Brewer JW. Activation of an unfolded protein response during differentiation of antibody-secreting B-cells. J. Biol. Chem. 2002;277:49047–49054. doi: 10.1074/jbc.M205011200. [DOI] [PubMed] [Google Scholar]
- Gunn KE, Brewer JW. Evidence that marginal zone B-cells possess an enhanced secretory apparatus and exhibit superior secretory activity. J. Immunol. 2006;177:3791–3798. doi: 10.4049/jimmunol.177.6.3791. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000a;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol. Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000b;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettman T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Haze K, Okada T, Yoshida H, Yanagi H, Yura T, Negishi M, Mori K. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem. J. 2001;355:19–28. doi: 10.1042/0264-6021:3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot LM, Sitia R. Immunoglobulin assembly and secretion. In: Alt FW, Honjo T, Neuberger MS, editors. Molecular Biology of B-cells. Elsevier Science Ltd; London, UK: 2004. pp. 261–273. Chapter 17. [Google Scholar]
- Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. Delineation of the negative feedback regulatory loop that controls protein translation during ER stress. J. Biol. Chem. 2003;278:34864–34873. doi: 10.1074/jbc.M301107200. [DOI] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem. J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B-cells. J. Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- Rush JS, Sweitzer T, Kent C, Decker GL, Waechter CJ. Biogenesis of the endoplasmic reticulum in activated B-lymphocytes: temporal relationships between the induction of protein N-glycosylation activity and the biosynthesis of membrane protein and phospholipid. Arch. Biochem. Biophys. 1991;284:63–70. doi: 10.1016/0003-9861(91)90264-j. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Quian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, Hurt EM, Petroulakis E, Sonenberg N, Yewdell JW, Calame K, Glimcher LH, Staudt LM. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Shen X, Ellis R, Lee K, Liu C-Y, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, Kaufman RJ. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat M, Janossy G, Dourmashkin RR. Development of rough endoplasmic reticulum in mouse splenic lymphocytes stimulated by mitogens. Eur. J. Immunol. 1973;3:680–687. doi: 10.1002/eji.1830031106. [DOI] [PubMed] [Google Scholar]
- Skalet AH, Isler JA, King LB, Harding HP, Ron D, Monroe JG. Rapid B cell receptor-induced unfolded protein response in nonsecretory B cells correlates with pro-versus antiapoptotic cell fate. J. Biol. Chem. 2005;280:39762–39771. doi: 10.1074/jbc.M502640200. [DOI] [PubMed] [Google Scholar]
- Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anken E, Braakman I. Endoplasmic reticulum stress and the making of a professional secretory cell. Crit. Rev. Biochem. Molec. Biol. 2005a;40:269–283. doi: 10.1080/10409230500315352. [DOI] [PubMed] [Google Scholar]
- van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit. Rev. Biochem. Molec. Biol. 2005b;40:191–228. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJ. Sequential waves of functionally related proteins are expressed when B-cells prepare for antibody secretion. Immunity. 2003;18:243–253. doi: 10.1016/s1074-7613(03)00024-4. [DOI] [PubMed] [Google Scholar]
- van Huizen R, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2alpha signaling. J. Biol. Chem. 2003;278:15558–15564. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest DL, Burkhardt JK, Hester S, Hortsch M, Meyer DI, Argon Y. Membrane biogenesis during B cell differentiation: most endoplasmic reticulum proteins are expressed coordinately. J. Cell Biol. 1990;110:1501–1511. doi: 10.1083/jcb.110.5.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, Katze MG. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B-cell lymphopoiesis. J. Clin. Invest. 2005;115:268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14343–14348. doi: 10.1073/pnas.0606480103. [DOI] [PMC free article] [PubMed] [Google Scholar]