Abstract
Parkinson's disease is a common neurodegenerative disorder with both motor symptoms and cognitive deficits such as executive dysfunction. Over the past 100 years, a growing body of literature has suggested that patients with Parkinson's disease have characteristic personality traits such as industriousness, seriousness and inflexibility. They have also been described as ‘honest’, indicating that they have a tendency not to deceive others. However, these personality traits may actually be associated with dysfunction of specific brain regions affected by the disease. In the present study, we show that patients with Parkinson's disease are indeed ‘honest’, and that this personality trait might be derived from dysfunction of the prefrontal cortex. Using a novel cognitive task, we confirmed that patients with Parkinson's disease (n = 32) had difficulty making deceptive responses relative to healthy controls (n = 20). Also, using resting-state 18F-fluorodeoxyglucose PET, we showed that this difficulty was significantly correlated with prefrontal hypometabolism. Our results are the first to demonstrate that the ostensible honesty found in patients with Parkinson's disease has a neurobiological basis, and they provide direct neuropsychological evidence of the brain mechanisms crucial for human deceptive behaviour.
Keywords: Parkinson's disease, prefrontal cortex, neuropsychology, PET, executive function
Introduction
Parkinson's disease, or paralysis agitans, was first described in 1817 by James Parkinson as ‘shaking palsy’ (Parkinson, 1817). It is a neurodegenerative disease characterized by clinical symptoms that include bradykinesia, rigidity, resting tremor and postural instability. In addition, it has been acknowledged that Parkinson's disease patients have impairments in cognitive functions (e.g. frontal executive dysfunction), which have a profound impact on quality of life for some of them (Pillon et al., 2001).
Certain personality traits have long been noted as being characteristic of Parkinson's disease patients. In 1913, Carl Camp wrote ‘It would seem that paralysis agitans affected mostly those persons whose lives had been devoted to hard work … The people who take their work to bed with them and who never come under the inhibiting influences of tobacco or alcohol are the kind that are most frequently affected. In this respect, the disease may be almost regarded as a badge of respectable endeavor’ (Camp, 1913). Since the publication of Camp's report, many researchers have investigated the association of Parkinson's disease with personality or behavioural traits, and have consistently shown that Parkinson's disease patients have characteristic personality traits such as industriousness, seriousness and inflexibility (Ishihara and Brayne, 2006).
Parkinson's disease patients have also been described as ‘honest’ (Menza, 2000), in the sense that they tend not to tell lies. Although the possibility that honest people are particularly vulnerable to this disease cannot be ruled out, insidious neuropathological changes in the course of the illness might underlie this specific trait. In relation to this idea, a previous study reported that the personality change in Parkinson's disease patients was primarily the result of the disease rather than aging (Mendelsohn et al., 1995), and some researchers have suggested the possibility that the personality traits are associated with Parkinson's disease-specific brain damage (Menza, 2000; Ishihara and Brayne, 2006). However, it may not be the case that Parkinson's disease patients choose not to tell lies, but rather that they actually have difficulty lying due to cognitive deficits resulting from pathological changes in certain brain regions.
One potentially critical contender for the role of mediator in complex cognitive processes such as deception is the prefrontal cortex, a structure known to support executive function. In particular, it is widely assumed that the lateral (especially dorsolateral) prefrontal cortex supports cognitive processes requiring executive function such as response inhibition and cognitive control (Mesulam, 2000; Anderson and Tranel, 2002). Some clinical studies have already implicated the prefrontal cortex as being responsible for executive dysfunction in Parkinson's disease patients (Carbon and Marie, 2003; Zgaljardic et al., 2003; Owen, 2004). Impairment in the prefrontal executive system can prevent people exhibiting flexible and goal-directed behaviours, which are regarded as essential features of human deceptive behaviour. In support of the clinical findings mentioned above, recent neuroimaging studies involving healthy individuals have provided substantial evidence that the prefrontal cortex is consistently active during the making of deceptive responses relative to honest responses (Spence et al., 2001; Langleben et al., 2002, 2005; Lee et al., 2002, 2005; Ganis et al., 2003; Kozel et al., 2004a, b, 2005, 2009; Davatzikos et al., 2005; Nunez et al., 2005; Phan et al., 2005; Abe et al., 2006; Mohamed et al., 2006; Abe et al., 2007, 2008; Gamer et al., 2007; Browndyke et al., 2008; Hakun et al., 2008; Lissek et al., 2008; Spence et al., 2008; Bhatt et al., 2009; Ganis et al., in press; Hakun et al., in press; Kozel et al., in press; Lee et al., 2009; Monteleone et al., in press).
The available evidence allows us to hypothesize that Parkinson's disease patients have difficulty making deceptive responses due to dysfunction of the prefrontal executive system, and that this is the reason why they seem to be relatively honest compared with healthy individuals. To test our hypothesis, we developed a novel cognitive task for measuring the ability of Parkinson's disease patients to give deceptive responses, and assessed the correlation between their ability to tell a lie and their resting brain metabolism using PET with 18F-fluorodeoxyglucose (FDG). Unlike the activation paradigm with normal participants, which assesses neural response during the actual performance of a task, resting-state studies of metabolic rate with FDG-PET in brain-damaged patients can reveal regional dysfunction associated with their cognitive impairments. Resting-state FDG-PET is considered to be especially useful in the context of the neuropsychological investigation of patients with neurodegenerative disease (Desgranges et al., 2002; Mentis et al., 2002; Eustache et al., 2004; Lozza et al., 2004; Piolino et al., 2007), because regional metabolic rate is a marker of integrated local synaptic activity and is sensitive to both direct neuronal/synaptic damage and secondary functional disruption at synapses distant from the primary site of pathology (Magistretti et al., 1999). To our knowledge, the present study is the first to provide direct neuropsychological evidence that the prefrontal cortex plays a critical role in human deceptive behaviour.
Materials and Methods
Participants
The participants were 32 idiopathic Parkinson's disease patients and 20 normal controls matched for age, sex and score on the Mini-Mental State Examination (MMSE). The demographics of the Parkinson's disease patients and normal controls are shown in Table 1. All the patients were recruited from the Tohoku University Hospital. Normal controls with no history of neurological or psychiatric disease were recruited from local communities via an advertisement. The diagnosis of Parkinson's disease was made by board-certified neurologists according to the UK Parkinson's Disease Society Brain Bank criteria (Gibb and Lees, 1988). The patients’ motor symptoms were evaluated using Hoehn-Yahr staging (Hoehn and Yahr, 1967) and the Unified Parkinson's Disease Rating Scale (UPDRS) part III (Fahn and Elton, 1987). The scores of UPDRS part III were recorded while the patients were ‘on’ medication. The inclusion criteria for patients in this study were as follows: age between 50 and 75 years, age at onset above 40 years, Hoehn-Yahr stage from 1 to 3, and a score of 24 or higher on the MMSE. The exclusion criteria were: a medical history of disease of the central nervous system not directly related to Parkinson's disease (e.g. stroke, head injury, epilepsy), concurrent psychiatric illness such as schizophrenia or manic depression, a documented or suspected history of drug abuse and/or alcoholism, diabetes mellitus and major abnormalities on brain MRI scans such as cerebral infarction or tumour. Of the 32 patients with Parkinson's disease, 14 were taking drugs for Parkinson's disease (i.e. levodopa and/or dopamine agonists), and they were asked not to take these drugs for at least 5 h before PET scanning.
Table 1.
Demographic and neuropsychological data (mean ± SD) of the Parkinson's disease patients and controls
| Variable | Parkinson's disease patients (n = 32) | Controls (n = 20) | P-values | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 65.9 (6.7) | 65.5 (4.8) | 0.807 | |
| Sex (Female/Male) | 19/13 | 11/9 | 0.756 | |
| Education | 11.7 (2.1) | 12.7 (2.4) | 0.127 | |
| Duration of Parkinson's disease | 4.1 (4.6) | – | – | |
| Levodopa equivalent dose (mg/day) | 507.0 (825.6) | – | – | |
| UPDRS part III (motor part)a | 18.1 (7.2) | – | – | |
| Hoehn-Yahr stage (median/range) | 2.5/1.0-3.0 | – | – | |
| Cognitive function | ||||
| MMSE | 28.3 (1.7) | 28.6 (1.1) | 0.386 | |
| Digit span | ||||
| Forward | 5.7 (0.9) | 5.6 (1.0) | 0.693 | |
| Backward | 4.1 (1.0) | 4.8 (1.0) | 0.035 | |
| Spatial span | ||||
| Forward | 5.7 (0.9) | 5.6 (1.1) | 0.546 | |
| Backward | 5.0 (1.0) | 4.9 (0.9) | 0.588 | |
| ADAS word recall | ||||
| Total score | 19.3 (4.3) | 21.3 (3.5) | 0.089 | |
| Verbal fluency | ||||
| Category: animal | 16.4 (5.3) | 22.1 (5.8) | 0.001 | |
| Syllables: ‘fu’, ‘a’, ‘ni’ | 22.1 (8.2) | 29.4 (8.7) | 0.004 | |
| Trail-making test (time required)b | ||||
| Part B - Part A | 83.0 (41.0) | 59.1 (33.7) | 0.035 | |
| Stroop task (accuracy) | ||||
| Congruent | 98.0 (11.0) | 100.0 (0.0) | 0.424 | |
| Incongruent | 93.5 (14.9) | 95.6 (8.8) | 0.565 | |
| Go/No-go task (accuracy) | ||||
| Go condition | 99.9 (0.5) | 100 (0) | 0.184 | |
| No-go condition | 99.6 (1.2) | 99.6 (1.3) | 0.942 |
Chi-square test was used for sex ratio, and t-test was used for the remaining variables.
a One patient was not assessed (n = 31).
b Two patients could not complete this test (n = 30).
ADAS = Alzheimer's Disease Assessment Scale.
Because we intended to conduct correlation analysis between the ability to tell lies and resting regional glucose metabolism within the group of patients, none of the control subjects who participated in the neuropsychological assessments was included in the PET study. However, even if correlation analysis within the group of patients identified the specific regions responsible for disability to tell lies, this would not prove that these findings were caused by the disease. To draw a definite conclusion, we needed to demonstrate explicitly that the regions identified in the correlation analysis were hypometabolic in the patients relative to the normal controls, i.e. lesioned. Therefore, we obtained PET data from another group consisting of 14 healthy participants without psychiatric or neurological disease (seven women, seven men; mean age 64 years; mean education 12.3 years; mean MMSE score 29.1). There was no significant difference in age, sex, education and MMSE score between the patients and these healthy participants (all P > 0.1). The PET data obtained from our sample of 32 patients were contrasted with those obtained from this normative group, and a resulting mask image was used in the correlation analysis in order to confine our analysis to regions showing hypometabolism in the Parkinson's disease patients. All the PET images were obtained with the same machine (see below).
After being given a detailed description of the study, written informed consent was obtained from all participants in accordance with guidelines approved by the Ethical Committee of Tohoku University and the Declaration of Helsinki.
Standard neuropsychological tests
For all the patients and controls, in addition to the MMSE, a set of standard neuropsychological tests was used to identify any explicit cognitive deficits. Attention was assessed by digit span and spatial span subtests from the Wechsler Memory Scale-Revised (WMS-R). Memory function was assessed by a word recall task from the Alzheimer's Disease Assessment Scale (ADAS). Frontal lobe function was assessed by verbal fluency tasks, the trail-making test and computerized versions of the Stroop task and the Go/No-go task.
In the computerized version of the Stroop task, the subjects were required to name the colour of the ink in which single words were printed, as each word was shown on the screen. Four ink colours were used, and all the words in the test were the names of these four colours. Therefore, trials were either congruent (colour and word the same) or incongruent (colour and word different). Each stimulus was presented for 2000 ms, with 2000-ms interstimulus intervals. The entire session consisted of 72 congruent and 24 incongruent trials. The verbal response was recorded on a digital sound-recording machine.
In the computerized version of the Go/No-go task, when a single digit appeared with the illustration of a dog in the centre of the screen, the subjects were required to read the digit aloud. When the number appeared with the illustration of a cat, the subjects were required to make no response. Each stimulus was displayed for 2000 ms, with 2000-ms interstimulus intervals. The entire session consisted of 72 Go trials and 24 No-go trials. The verbal response was recorded on a digital sound-recording machine.
The experimental deception task
The experimental task consisted of an incidental study phase and a recognition memory test phase during which the participants were asked to tell the truth or a lie. First, we prepared colour photographs of 51 common living things and 51 common inanimate objects. Three of each type of these photographs were used as study buffers (three at the beginning and three at the end of a study list) to exclude primacy and recency effects on memory performance. The remaining 96 photographs were divided into two sets of equal numbers of animate and inanimate stimuli. One set was used as study items in the study phase and as target items to be recognized later in the test phase, and the other set was used as distracters in the test phase. These two sets of photographs were matched for visual complexity, familiarity and arousal (all P > 0.1), as rated by a separate group of 20 normal adults (10 women, 10 men; mean age 32.9 years), who did not participate in the present experimental deception task. Each set of 48 stimuli was further divided into four lists of 12 stimuli each. Then, four lists of photographs were compiled by combining 12 stimuli from one set and 12 stimuli from another. These four lists consisting of 24 stimuli were again matched for visual complexity, familiarity and arousal (all P > 0.1).
For the recognition memory phase, four actors (two men and two women) were videotaped over 96 trials. In each scene (lasting 6 s with a 1-s interval between scenes), one of the actors randomly showed a colour photograph of stimuli, while asking, in Japanese, ‘Did you see this photograph?’ Each actor showed 24 stimuli, one by one in randomized order, except that the same actor did not appear sequentially.
During the study phase, the participants viewed 48 study stimuli and six buffer stimuli, presented one at a time for 5 s on a computer screen. All the stimuli were presented visually in white squares on a black background. The interstimulus interval was 1 s, during which cross-fixation was presented. To ensure that the participants paid attention to the stimuli, they were instructed to indicate verbally whether each photograph represented an animate or an inanimate object.
During the test phase (the main part of the present study), the participants viewed a video consisting of 96 scenes. In total, 48 studied and 48 unstudied stimuli were presented by the four actors. The participants were asked to say whether each photograph was familiar (i.e. ‘I saw’) or not (i.e. ‘I didn’t see’) after the actor had asked the question, ‘Did you see this photograph?’ In addition, participants were also requested to tell the truth in response to three actors (Truth condition) and to tell a lie in response to the remaining actor (Lie condition). We used unequal stimulus classes (25% lie and 75% truth) on the assumption that truthful responses are frequent and ordinary, whereas deceptive responses should be infrequent and extraordinary. In fact, previous studies of executive function, such as the Stroop effect, have suggested that a lower proportion of incongruent trials (homologous with deceptive responses in the present study) increases the cognitive conflict associated with responding to the stimuli (Carter et al., 2000; Swick and Jovanovic, 2002; Fellows and Farah, 2005). The actor to whom a lie was to be told was counterbalanced across the participants.
The experiment yielded four types of responses: true responses for the studied items, true responses for the unstudied items, deceptive responses for the studied items, and deceptive responses for the unstudied items. In this study, collapsing across item type (i.e. studied or unstudied items), the data were analysed for honest and deceptive responses in the Parkinson's disease patients and normal controls. Mathematically, the effect of cognitive demand on deception was expressed by a deception task index (i.e. the percent of correct responses in the Truth condition minus that in the Lie condition). The deception task index reflected the difficulty making deceptive responses regardless of the participant's basic recognition memory performance, and was therefore used for correlation analyses.
To investigate the possibility that the Parkinson's disease patients’ apparent impaired ability to lie was due to forgetting to which actor they had to give deceptive responses, after the task was completed, both the patients and the controls were asked whether or not they had forgotten the target person they had to deceive throughout the task. They were also presented with face photographs of the four actors, and were asked to indicate the actor to whom they had been instructed to tell a lie. Throughout the entire task session, all the verbal responses made by the patients and the normal controls were recorded on a digital sound-recording machine. These data were subsequently used for the evaluation of performance accuracy and error pattern.
PET data acquisition and voxel-based analysis
After a fasting period of at least 5 h, PET images were obtained using 185–218 MBq FDG. Dynamic PET scans were performed in three-dimensional mode using a Siemens Biograph DUO PET scanner (Siemens Medical System, Inc., USA). Subjects were scanned under resting conditions with their eyes closed and ears unplugged. To minimize the effects of external stimuli during the FDG-uptake period of 1 h, the subjects stayed in a quiet room wearing eye masks. In-plane and axial resolutions of the scanner were 3.38 mm and 3.38 mm, respectively. An attenuation correction was performed with a CT scan. The data obtained were reconstructed using ordered subset expectation maximization (OSEM) algorithms (16 subsets × 6 iterations) with Gaussian filter with FWHM = 2.0 mm in 256 × 256 matrix, pixel size of 1.33 × 1.33 mm and a slice thickness of 2.0 mm. PET images and the values of arterial input function measurements were converted to cerebral metabolic rate of glucose images according to a model based on the autoradiographic technique (Phelps et al., 1979). The interval between the neuropsychological tests and PET scanning was <4 weeks.
The PET data were analysed with SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). All the PET images were normalized to the FDG-template based on the MNI reference brain (re-sampled voxel size 2 × 2 × 2 mm3). Then, all the images were smoothed using an isotropic Gaussian kernel of 10 mm to increase the signal-to-noise ratio and to compensate for differences in gyral anatomy between individuals. To reduce between-subject variation in global metabolic rates, the count of each voxel was normalized to the total count of the brain using proportional scaling.
The deception task indices were entered as covariates of interest in the analysis of the Parkinson's disease patients, with the aim of identifying regions showing decreased metabolism associated with low performance. The threshold of significance was set at P < 0.001 at the voxel level (uncorrected), with a significance of P < 0.05 at the cluster level (corrected). To confine our analysis to regions showing hypometabolism in the patients relative to the normal participants, the PET data obtained from our sample of 32 patients were contrasted with those obtained from a group of 14 healthy participants (who did not participate in the present experimental deception task), and a resulting map with a liberal statistical threshold (P < 0.05, uncorrected) was used for masking in the correlation analysis. In addition, possible confounding effects of age and sex (i.e. biological factors) were controlled by entering these variables into the model. Then, in separate analyses, the duration of Parkinson's disease, the effect of medication (i.e. levodopa equivalent dose), the scores of UPDRS part III (motor part), and the scores of MMSE—all of which are possible confounding factors for regional metabolism—were controlled by entering these variables into the model.
Results
Standard neuropsychological tests
Table 1 lists the results of the standard neuropsychological tests and statistical comparison between the Parkinson's disease patients and normal controls, as well as the demographic data. The t-test was used to assess the statistical significance for all the variables between the two groups except for sex ratio, for which the chi-squared test was used. The patients performed significantly worse than the controls on the digit span test (backward), the verbal fluency task related to syllables and category, and the trail-making test, indicating that Parkinson's disease patients had executive dysfunction. The patients also performed marginally worse than controls on the ADAS word recall test. No significant difference was found between the two groups in the Stroop task and the Go/No-go task, possibly due to ceiling effects resulting from the level of difficulty of these tests, which were specifically designed for the present study. Also, no difference was found between the patients and controls in the digit span test (forward) and the spatial span tests (forward and backward).
The experimental deception task
During the encoding phase, animate–inanimate judgment was virtually 100% correct for all the Parkinson's disease patients and normal controls, indicating that the participants paid sufficient attention to the stimuli.
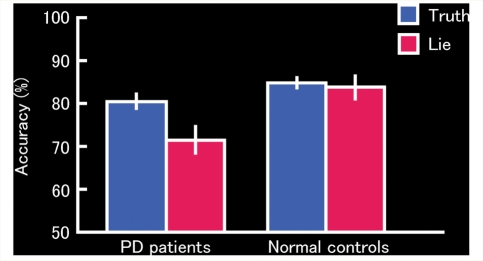
For the retrieval session, collapsing across item type (i.e. studied and unstudied items), the data related to mean accuracy were analysed. For the patients, mean accuracies were 80.4% (SD = 9.5) for the Truth condition and 71.5% (SD = 17.1) for the Lie condition. For the normal controls, mean accuracies were 84.8% (SD = 5.1) for the Truth condition and 83.8% (SD = 11.9) for the Lie condition. A 2 (Group: Parkinson's disease patients, normal controls) × 2 (Task: Truth, Lie) analysis of variance (ANOVA) revealed a significant main effect of Group [F(1,50) = 7.25, P = 0.010], a significant main effect of Task [F(1,50) = 9.22, P = 0.004] and a significant Group × Task interaction [F(1,50) = 5.77, P = 0.020]. Post hoc tests revealed the reason for this interaction: the Parkinson's disease patients showed a decreased number of correct responses in the Lie condition relative to the Truth condition [t(31) = 4.06, P = 0.0003], whereas the controls showed no difference in scores between these two conditions [t(19) = 0.47, P = 0.641]. The results are shown in Fig. 1.
Figure 1.
Proportion of correct honest (Truth condition) and deceptive (Lie condition) responses during the deception task in the Parkinson's disease patients and normal controls. Error bars represent standard error. PD = Parkinson's disease.
Although one patient stated in the middle of the task that she was not sure of the target person to deceive, the remaining patients stated with confidence after the experiment that they could easily and immediately recognize the target person to deceive throughout the task. However, in the forced-choice recognition test, all the patients, including the patient who had expressed uncertainty, correctly chose the target person to deceive. This indicates that the patients’ impaired ability to lie cannot be attributable to forgetting who to deceive. In addition, analysis of error pattern during the Lie condition in Parkinson's disease patients revealed that they often made errors by telling the truth (91.8% of all the error responses, but note that this rate includes errors for basic recognition memory performance). More importantly, there were few errors of no response (0.9%) and dual response (7.3%). The extremely low rate for these types of errors indicates that the patients understood sufficiently and performed the task without any difficulty resulting from motor dysfunction. Together, these findings support the view that the patients’ deteriorated performance was definitely derived from a failure to inhibit true responses and make deceptive responses.
To clarify the effect of set shifting on the deception task in Parkinson's disease patients, we also compared the accuracy of Truth trials that were preceded by Lie trials with that of the remaining Truth trials that were not preceded by Lie trials in Parkinson's disease patients. If the set-shifting deficits affected the deception task performance, the patients should show worse performance for the Truth trials preceded by Lie trials than for those not preceded by Lie trials. Mean accuracies were 79.1% (SD = 10.3) for the Truth trials preceded by Lie trials and 81.1% (SD = 10.3) for the Truth trials not preceded by Lie trials. We found that there was no significant difference between the two types of trials [t(31) = 1.32, P = 0.198], suggesting that there was no effect of set-shifting deficits on the deception task.
We further conducted correlation analyses to investigate the relationship between performance of the deception task and cognitive dysfunctions detected by the standard neuropsychological tests in Parkinson's disease patients (i.e. the backward digit span task, the verbal fluency for category and syllables, and the trail-making test). The deception task index was significantly correlated with the performance of verbal fluency for syllables (r = –0.429, P = 0.013) and with the performance (i.e. time required) of the trail-making test (n = 30, because of missing data for two patients, r = 0.372, P = 0.042). We also found a trend between the deception task index and the performance of verbal fluency for category (r = –0.303, P = 0.092). However, there was no significant correlation between the deception task index and performance of the digit span (backward) task (r = –0.245, P = 0.179).
Cognitive-metabolic correlations
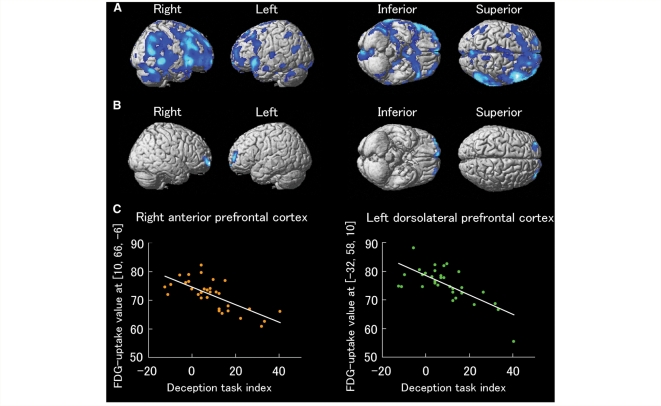
The results are shown in Table 2 and Fig. 2. Significant negative correlations were found between the deception task index and the metabolic rates of the right anterior prefrontal cortex (BA10) and the left dorsolateral prefrontal cortex (BA10/46). Note that the results were masked with the contrast of normal controls versus Parkinson's disease patients, indicating that these two regions were found within the regions showing hypometabolism in the patients relative to the normal participants. Furthermore, the confounding effects of age and sex were also controlled. If the effect of disease duration was further controlled, the results remained virtually unchanged, suggesting that they are not affected by duration of the disease. Similarly, if the effect of medication (i.e. levodopa equivalent dose) was further controlled, the results again remained virtually unchanged, suggesting that they are not affected by Parkinson's disease medication. If the UPDRS scores part III (motor part) were further controlled (n = 31, because of missing data for one patient), the results again remained virtually unchanged, suggesting that they are not affected by severity of motor symptoms. If the MMSE scores were further controlled, the results for these two regions remained significant (P < 0.001 at the voxel level, uncorrected, but with smaller cluster size; 102 voxels for the right anterior prefrontal cortex and 23 voxels for the left dorsolateral prefrontal cortex), suggesting that the main findings of this study cannot simply be explained in terms of the severity of general cognitive deficits.
Table 2.
Brain regions showing a significant correlation between deception task performance and regional metabolism
| Regions (Brodmann's Area) | Coordinates |
Z-value | Cluster size | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Controlling for age and sex (shown in Figure 2) | |||||
| Right anterior prefrontal cortex (10) | 10 | 66 | −6 | 4.03 | 426 |
| Left dorsolateral prefrontal cortex (10/46) | −32 | 58 | 10 | 3.99 | 261 |
| Controlling for age, sex and disease duration | |||||
| Right anterior prefrontal cortex (10) | 8 | 68 | −6 | 4.00 | 396 |
| Left dorsolateral prefrontal cortex (10/46) | −32 | 58 | 10 | 3.84 | 198 |
| Controlling for age, sex and levodopa equivalent dose | |||||
| Right anterior prefrontal cortex (10) | 8 | 68 | −6 | 4.14 | 455 |
| Left dorsolateral prefrontal cortex (10/46) | −18 | 58 | 12 | 3.91 | 225 |
| Controlling for age, sex and UPDRS motor scores | |||||
| Right anterior prefrontal cortex (10) | 10 | 68 | −6 | 4.06 | 588 |
| Left dorsolateral prefrontal cortex (10/46) | −32 | 60 | 10 | 3.91 | 214 |
| Controlling for age, sex and MMSE scores | |||||
| Right anterior prefrontal cortex (10) | 8 | 66 | −4 | 3.56 | 102 |
| Left dorsolateral prefrontal cortex (10/46) | −32 | 58 | 10 | 3.40 | 23 |
The results were masked with the contrast of normal controls versus Parkinson's disease patients.
Figure 2.
(A) Brain regions showing hypometabolism in the Parkinson's disease patients compared with the normal controls. Note that the statistical threshold was relatively liberal in this group comparison (P < 0.05, uncorrected), since this analysis was done only for generating a mask image included in the cognitive-metabolic correlation analysis within the group of Parkinson's disease patients. The regions are displayed on a surface-rendered standard brain. (B) Brain regions showing a significant correlation between performance in the deception task and regional cerebral glucose metabolism in the Parkinson's disease patients (P < 0.001, uncorrected). Note that the results were masked with the above contrast of the normal controls versus the Parkinson's disease patients to confine our analysis to the regions showing hypometabolism in the Parkinson's disease patients. The possible confounding effects of age and sex were also controlled. (C) Scatter plots of the correlations between the deception task indices and the FDG-uptake values in the right anterior prefrontal cortex (r = –0.719, P < 0.001) and the left dorsolateral prefrontal cortex (r = –0.709, P < 0.001). FDG = fluorodeoxyglucose; PD = Parkinson's disease.
Discussion
In the present study, we tested our hypothesis that patients with Parkinson's disease have difficulty making deceptive responses due to dysfunction of the prefrontal cortex. As predicted, the patients could not successfully make deceptive responses compared with the healthy controls. Furthermore, consistent with previous neuroimaging studies with healthy individuals that have indicated an association between deception and the prefrontal cortex, FDG-PET imaging revealed that the patients’ failure in the deception task was significantly correlated with hypometabolism in the prefrontal cortex, regardless of age, sex and other possible confounding factors. To our knowledge, this is the first neuropsychological evidence that dysfunction of the prefrontal cortex is involved in the inability to inhibit true responses and produce deceptive responses in Parkinson's disease patients.
The results of the present study raise two important points. First, certain personality traits of Parkinson's disease patients (Menza, 2000; Ishihara and Brayne, 2006) might be at least partly explained by neuropsychological deficits. In other words, the cognitive deficits may have an influence on ostensible personality traits in Parkinson's disease patients. More specifically, the present results indicate that honesty in Parkinson's disease patients might result from impairment of the executive functions necessary for the processes involved in telling lies. Indeed, the patients showed worse performance in the verbal fluency task and the trail-making test (generally used as measures of executive function) compared with the normal controls. Although these tests are different from the deception task in terms of how the subjects respond (e.g. open-ended responses in verbal fluency and forced-choice responses in the deception task), and therefore are not likely to have direct impact on deception task performance, there is still a possibility that these tests partially share the cognitive and neural mechanisms of deception in terms of higher-order cognitive processes including executive function. In line with this idea, these task performances were significantly correlated with deception task performance. Future studies using an approach similar to that of the present study might further clarify the relationships between cognitive dysfunction and characteristic personality and behavioural traits in Parkinson's disease patients.
Second, the results reveal a direct association between a cognitive control system subserving deception and function of the prefrontal cortex. It is known that brain imaging of healthy people cannot provide direct evidence that a certain brain region is necessary for the performance of a specific cognitive task (Frackowiak et al., 1997). That is, some activation in functional brain imaging studies may reflect brain activity that is not essential for the function of interest. Therefore, direct evidence is derived from loss-of-function studies. In the present study, we revealed that the right anterior prefrontal cortex and left dorsolateral prefrontal cortex, which have been activated during deception in a number of carefully designed imaging studies (for reviews, see Spence et al., 2004; Sip et al., 2008; Christ et al., in press), are associated with making deceptive responses. In line with our results, a recent study using transcranial direct current stimulation provided evidence that manipulation of functions in the dorsolateral prefrontal cortex altered the speed and efficiency of deceptive responses (Priori et al., 2008). Furthermore, the association between deception and the left dorsolateral prefrontal cortex in the present study is highly consistent with the findings of a series of neuroimaging studies that we have conducted with healthy individuals (Abe et al., 2006, 2007, 2008).
Based on the previous findings and the present results, we propose that the left dorsolateral prefrontal cortex, the region implicated in a wide range of higher-level cognitive operations such as working memory (D’Esposito et al., 1995; Salmon et al., 1996) and resolution of response conflict (MacDonald et al., 2000; Badre and Wagner, 2004), plays a pivotal role in telling lies. The right anterior prefrontal cortex is also likely to play a critical role in integrating the multiple cognitive processes (Ramnani and Owen, 2004) in deception. One might think that set-shifting deficits, one of the well-known cognitive deficits in Parkinson's disease (Ravizza and Ciranni, 2002; Monchi et al., 2004; Moustafa et al., 2008; Nagano-Saito et al., 2008), affect the results. However, our analysis of set-shifting effect on the response accuracy in Truth trials did not support this interpretation. We believe that our task does not simply measure set shifting, and that dysfunction of the left dorsolateral and right anterior prefrontal cortices specifically prevents Parkinson's disease patients from inhibiting true responses and producing deceptive responses.
It is important to determine how frontal executive dysfunction, possibly disrupting deceptive behaviour, is derived from the neuropathological changes observed in Parkinson's disease patients. One possibility is that prefrontal hypometabolism in Parkinson's disease patients results from degeneration of the substantia nigra pars compacta with subsequent depletion of dopamine in the striatum. A recent study suggests that the dorsolateral prefrontal circuit consisting of the dorsolateral prefrontal cortex, caudate nucleus, globus pallidus, substantia nigra, and thalamus (Cummings, 1993; McPherson and Cummings, 2002) is specifically associated with executive dysfunction in Parkinson's disease patients (Zgaljardic et al., 2006). Alternatively, the executive dysfunction may reflect a functional disturbance of the frontal cortex itself caused by locally impaired mesocortical dopaminergic transmission (Mattay et al., 2002). Although these two models are not mutually exclusive, there is controversy in the recent literature in that some researchers have argued that both the nigrostriatal and mesocortical pathways are disrupted in Parkinson's disease (Monchi et al., 2007), whereas others have shown impaired nigrostriatal dopaminergic function with preserved mesocortical dopaminergic transmission in early Parkinson's disease (Sawamoto et al., 2008). As for dopaminergic transmission, a study in which the ‘on’ and ‘off’ medication states are directly compared would also be useful. We can predict that dopaminergic medication would have a beneficial effect on the regions affected by depletion of dopamine, such as the caudate nucleus and thereby its connections to the dorsolateral prefrontal cortex, and that the ability to make deceptive responses would improve in Parkinson's disease patients. In fact, some previous studies have reported the beneficial effects of levodopa on cognitive performance, although it should be noted that the effects depend on the nature of the task (Gotham et al., 1988; Cools et al., 2001; Lewis et al., 2005).
In conclusion, our results provide new evidence that damage to the prefrontal cortex disrupts the processes involved in making deceptive responses in Parkinson's disease patients. It appears that the ‘honesty’ of patients is caused by an impaired ability to deceive others that results from brain dysfunction caused by the disease. However, there are some limitations of the present study that should be borne in mind for future studies. First, the present study examined only the processes associated with executive control during deception. The participants were instructed to tell a lie, which cannot be viewed as being the same as deception in real life. The neural bases of genuine deception or immoral lying should be investigated further in both healthy individuals and brain-damaged patients. Second, it remains a possibility that the association between difficulty deceiving others and prefrontal dysfunction may not be specific to Parkinson's disease patients, and further studies are needed to examine whether patients with other neurological disorders affecting the prefrontal cortex show similar deficits (see Spence and Kaylor-Hughes, 2008). Third, the present study investigated only patients with mild Parkinson's disease of short duration. Whether our claim is true of patients in general is an important issue to be pursued. Finally, it is also important to determine how (and when) the brain pathology derived from Parkinson's disease causes specific personality traits together with explicit cognitive deficits. A longitudinal assessment with detailed neuropsychological assessment and multimodal neuroimaging in Parkinson's disease patients is required.
Funding
Japan Society for the Promotion of Science for Young Scientists Research Fellowships (05J04930 to N.A.); Grant-in-Aid for Scientific Research on Priority Areas—System study on higher-order brain functions—from the Ministry of Education, Culture, Sports, Science and Technology of Japan (18‱020‱003 to E.M.). Funding to pay the Open Access publication charges for this article will be provided by ‘Grant-in-Aid for Young Scientists (Start-up) from the Japan Society for the Promotion of Science (20‱800‱006 to N.A.)’.
Acknowledgements
We are grateful to Takafumi Hasegawa, Akio Kikuchi, Shigenori Kanno, Yoichi Sawada, Kazue Okada, and Kaori Tachibana for their constant support and insightful comments. We also thank the patients and their families for sparing their valuable time to participate in this study.
Glossary
Abbreviations
- ADAS = Alzheimer's Disease Assessment Scale
- FDG = 18F-fluorodeoxyglucose
- MMSE = Mini-Mental State Examination
- OSEM = ordered subset expectation maximization
- WMS-R = Wechsler Memory Scale-Revised
References
- Abe N, Okuda J, Suzuki M, Sasaki H, Matsuda T, Mori E, et al. Neural correlates of true memory, false memory, and deception. Cereb Cortex. 2008;18:2811–9. doi: 10.1093/cercor/bhn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe N, Suzuki M, Mori E, Itoh M, Fujii T. Deceiving others: distinct neural responses of the prefrontal cortex and amygdala in simple fabrication and deception with social interactions. J Cogn Neurosci. 2007;19:287–95. doi: 10.1162/jocn.2007.19.2.287. [DOI] [PubMed] [Google Scholar]
- Abe N, Suzuki M, Tsukiura T, Mori E, Yamaguchi K, Itoh M, et al. Dissociable roles of prefrontal and anterior cingulate cortices in deception. Cereb Cortex. 2006;16:192–9. doi: 10.1093/cercor/bhi097. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Tranel D. Handbook of neuropsychology. 2nd. Vol. 7. Amsterdam: Elsevier; 2002. Neuropsychological consequences of dysfunction in human dorsolateral prefrontal cortex; pp. 145–56. [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–87. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Mbwana J, Adeyemo A, Sawyer A, Hailu A, Vanmeter J. Lying about facial recognition: an fMRI study. Brain Cogn. 2009;69:382–90. doi: 10.1016/j.bandc.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Browndyke JN, Paskavitz J, Sweet LH, Cohen RA, Tucker KA, Welsh-Bohmer KA, et al. Neuroanatomical correlates of malingered memory impairment: event-related fMRI of deception on a recognition memory task. Brain Inj. 2008;22:481–9. doi: 10.1080/02699050802084894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp CD. Paralysis agitans, multiple sclerosis and their treatment. In: White WA, Jelliffe SE, Kimpton H, editors. Modern treatment of nervous and mental disease. Philadelphia: Lea & Febiger; 1913. pp. 651–7. [Google Scholar]
- Carbon M, Marie RM. Functional imaging of cognition in Parkinson's disease. Curr Opin Neurol. 2003;16:475–80. doi: 10.1097/01.wco.0000084225.82329.3c. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2000;97:1944–8. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ SE, Van Essen DC, Watson JM, Brubaker LE, McDermott KB. Cereb Cortex (in press) The contributions of prefrontal cortex and executive control to deception: evidence from activation likelihood estimate meta-analyses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–43. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–80. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–81. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Ruparel K, Fan Y, Shen DG, Acharyya M, Loughead JW, et al. Classifying spatial patterns of brain activity with machine learning methods: application to lie detection. Neuroimage. 2005;28:663–8. doi: 10.1016/j.neuroimage.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, Lalevee C, Giffard B, Viader F, de La Sayette V, et al. The neural substrates of episodic memory impairment in Alzheimer's disease as revealed by FDG-PET: relationship to degree of deterioration. Brain. 2002;125:1116–24. doi: 10.1093/brain/awf097. [DOI] [PubMed] [Google Scholar]
- Eustache F, Piolino P, Giffard B, Viader F, De La Sayette V, Baron JC, et al. ‘In the course of time’: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer's disease. Brain. 2004;127:1549–60. doi: 10.1093/brain/awh166. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Unified Parkinson's Disease Rating Scale. Fluhum Park, NJ: Macmillan Healthcare Information; 1987. Vol 2. [Google Scholar]
- Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005;128:788–96. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- Frackowiak RS, Friston KJ, Frith CD, Dolan RJ, Mazziotta J, editors. Human brain function. San Diego: Academic Press; 1997. [Google Scholar]
- Gamer M, Bauermann T, Stoeter P, Vossel G. Covariations among fMRI, skin conductance, and behavioral data during processing of concealed information. Hum Brain Mapp. 2007;28:1287–301. doi: 10.1002/hbm.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganis G, Kosslyn SM, Stose S, Thompson WL, Yurgelun-Todd DA. Neural correlates of different types of deception: an fMRI investigation. Cereb Cortex. 2003;13:830–6. doi: 10.1093/cercor/13.8.830. [DOI] [PubMed] [Google Scholar]
- Ganis G, Morris RR, Kosslyn SM. Soc Neurosci (in press) Neural processes underlying self- and other-related lies: an individual difference approach using fMRI. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. ‘Frontal’ cognitive function in patients with Parkinson's disease ‘on’ and ‘off’ levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Hakun JG, Ruparel K, Seelig D, Busch E, Loughead JW, Gur RC, et al. Soc Neurosci (in press) Towards clinical trials of lie detection with fMRI. [DOI] [PubMed] [Google Scholar]
- Hakun JG, Seelig D, Ruparel K, Loughead JW, Busch E, Gur RC, et al. fMRI investigation of the cognitive structure of the Concealed Information Test. Neurocase. 2008;14:59–67. doi: 10.1080/13554790801992792. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Ishihara L, Brayne C. What is the evidence for a premorbid parkinsonian personality: a systematic review. Mov Disord. 2006;21:1066–72. doi: 10.1002/mds.20980. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Johnson KA, Grenesko EL, Laken SJ, Kose S, Lu X, et al. Functional MRI detection of deception after committing a mock sabotage crime. J Forensic Sci. 2009;54:220–31. doi: 10.1111/j.1556-4029.2008.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel FA, Johnson KA, Laken SJ, Grenesko EL, Smith JA, Walker J, et al. Soc Neurosci (in press) Can simultaneously acquired electrodermal activity improve accuracy of fMRI detection of deception? [DOI] [PubMed] [Google Scholar]
- Kozel FA, Johnson KA, Mu Q, Grenesko EL, Laken SJ, George MS. Detecting deception using functional magnetic resonance imaging. Biol Psychiatry. 2005;58:605–13. doi: 10.1016/j.biopsych.2005.07.040. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Padgett TM, George MS. A replication study of the neural correlates of deception. Behav Neurosci. 2004a;118:852–6. doi: 10.1037/0735-7044.118.4.852. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Revell LJ, Lorberbaum JP, Shastri A, Elhai JD, Horner MD, et al. A pilot study of functional magnetic resonance imaging brain correlates of deception in healthy young men. J Neuropsychiatry Clin Neurosci. 2004b;16:295–305. doi: 10.1176/jnp.16.3.295. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Loughead JW, Bilker WB, Ruparel K, Childress AR, Busch SI, et al. Telling truth from lie in individual subjects with fast event-related fMRI. Hum Brain Mapp. 2005;26:262–72. doi: 10.1002/hbm.20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben DD, Schroeder L, Maldjian JA, Gur RC, McDonald S, Ragland JD, et al. Brain activity during simulated deception: an event-related functional magnetic resonance study. Neuroimage. 2002;15:727–32. doi: 10.1006/nimg.2001.1003. [DOI] [PubMed] [Google Scholar]
- Lee TM, Au RK, Liu HL, Ting KH, Huang CM, Chan CC. Are errors differentiable from deceptive responses when feigning memory impairment? An fMRI study. Brain Cogn. 2009;69:406–12. doi: 10.1016/j.bandc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Lee TM, Liu HL, Chan CC, Ng YB, Fox PT, Gao JH. Neural correlates of feigned memory impairment. Neuroimage. 2005;28:305–13. doi: 10.1016/j.neuroimage.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Lee TM, Liu HL, Tan LH, Chan CC, Mahankali S, Feng CM, et al. Lie detection by functional magnetic resonance imaging. Hum Brain Mapp. 2002;15:157–64. doi: 10.1002/hbm.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson's disease. Neuropsychologia. 2005;43:823–32. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Lissek S, Peters S, Fuchs N, Witthaus H, Nicolas V, Tegenthoff M, et al. Cooperation and deception recruit different subsets of the theory-of-mind network. PLoS ONE. 2008;3:e2023. doi: 10.1371/journal.pone.0002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozza C, Baron JC, Eidelberg D, Mentis MJ, Carbon M, Marie RM. Executive processes in Parkinson's disease: FDG-PET and network analysis. Hum Brain Mapp. 2004;22:236–45. doi: 10.1002/hbm.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–7. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, et al. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol. 2002;51:156–64. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- McPherson S, Cummings JL. The frontal lobes and frontal-subcortical circuits in neuropsychiatric disorders. In: Grafman J, editor. Handbook of neuropsychology. 2nd. Amsterdam: Elsevier; 2002. pp. 99–116. [Google Scholar]
- Mendelsohn GA, Dakof GA, Skaff M. Personality change in Parkinson's disease patients: chronic disease and aging. J Pers. 1995;63:233–57. doi: 10.1111/j.1467-6494.1995.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, McIntosh AR, Perrine K, Dhawan V, Berlin B, Feigin A, et al. Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson's disease. Am J Psychiatry. 2002;159:746–54. doi: 10.1176/appi.ajp.159.5.746. [DOI] [PubMed] [Google Scholar]
- Menza M. The personality associated with Parkinson's disease. Curr Psychiatry Rep. 2000;2:421–6. doi: 10.1007/s11920-000-0027-1. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Behavioral neuroanatomy: large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations. In: Mesulam MM, editor. Principles of behavioral and cognitive neurology. New York: Oxford University Press; 2000. pp. 1–120. [Google Scholar]
- Mohamed FB, Faro SH, Gordon NJ, Platek SM, Ahmad H, Williams JM. Brain mapping of deception and truth telling about an ecologically valid situation: functional MR imaging and polygraph investigation–initial experience. Radiology. 2006;238:679–88. doi: 10.1148/radiol.2382050237. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson's disease. J Neurosci. 2004;24:702–10. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson's disease during executive processing depends on striatal involvement. Brain. 2007;130:233–44. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone GT, Phan KL, Nusbaum HC, Fitzgerald D, Irick JS, Fienberg SE, et al. Soc Neurosci (in press) Detection of deception using fMRI: better than chance, but well below perfection. [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Sherman SJ, Frank MJ. A dopaminergic basis for working memory, learning and attentional shifting in Parkinsonism. Neuropsychologia. 2008;46:3144–56. doi: 10.1016/j.neuropsychologia.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28:3697–706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JM, Casey BJ, Egner T, Hare T, Hirsch J. Intentional false responding shares neural substrates with response conflict and cognitive control. Neuroimage. 2005;25:267–77. doi: 10.1016/j.neuroimage.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in Parkinson's disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525–37. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Parkinson J. An essay on the shaking palsy. London: Sherwood Neely & Jones; 1817. [Google Scholar]
- Phan KL, Magalhaes A, Ziemlewicz TJ, Fitzgerald DA, Green C, Smith W. Neural correlates of telling lies: a functional magnetic resonance imaging study at 4 Tesla. Acad Radiol. 2005;12:164–72. doi: 10.1016/j.acra.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979;6:371–88. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Pillon B, Boller F, Levy R, Dubois B. Cognitive deficits and dementia in Parkinson's disease. In: Boller F, Cappa SF, editors. Handbook of neuropsychology. 2nd. Vol. 6. Amsterdam: Elsevier; 2001. pp. 311–71. [Google Scholar]
- Piolino P, Chetelat G, Matuszewski V, Landeau B, Mezenge F, Viader F, et al. In search of autobiographical memories: a PET study in the frontal variant of frontotemporal dementia. Neuropsychologia. 2007;45:2730–43. doi: 10.1016/j.neuropsychologia.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Priori A, Mameli F, Cogiamanian F, Marceglia S, Tiriticco M, Mrakic-Sposta S, et al. Lie-specific involvement of dorsolateral prefrontal cortex in deception. Cereb Cortex. 2008;18:451–5. doi: 10.1093/cercor/bhm088. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–94. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Ciranni MA. Contributions of the prefrontal cortex and basal ganglia to set shifting. J Cogn Neurosci. 2002;14:472–83. doi: 10.1162/089892902317361985. [DOI] [PubMed] [Google Scholar]
- Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, et al. Regional brain activity during working memory tasks. Brain. 1996;119:1617–25. doi: 10.1093/brain/119.5.1617. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson's disease. Brain. 2008;131:1294–302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Sip KE, Roepstorff A, McGregor W, Frith CD. Detecting deception: the scope and limits. Trends Cogn Sci. 2008;12:48–53. doi: 10.1016/j.tics.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Spence SA, Farrow TF, Herford AE, Wilkinson ID, Zheng Y, Woodruff PW. Behavioural and functional anatomical correlates of deception in humans. Neuroreport. 2001;12:2849–53. doi: 10.1097/00001756-200109170-00019. [DOI] [PubMed] [Google Scholar]
- Spence SA, Hunter MD, Farrow TF, Green RD, Leung DH, Hughes CJ, et al. A cognitive neurobiological account of deception: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2004;359:1755–62. doi: 10.1098/rstb.2004.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SA, Kaylor-Hughes CJ. Looking for truth and finding lies: the prospects for a nascent neuroimaging of deception. Neurocase. 2008;14:68–81. doi: 10.1080/13554790801992776. [DOI] [PubMed] [Google Scholar]
- Spence SA, Kaylor-Hughes C, Farrow TF, Wilkinson ID. Speaking of secrets and lies: the contribution of ventrolateral prefrontal cortex to vocal deception. Neuroimage. 2008;40:1411–18. doi: 10.1016/j.neuroimage.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Swick D, Jovanovic J. Anterior cingulate cortex and the Stroop task: neuropsychological evidence for topographic specificity. Neuropsychologia. 2002;40:1240–53. doi: 10.1016/s0028-3932(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cogn Behav Neurol. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Feigin A, et al. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson's disease. J Clin Exp Neuropsychol. 2006;28:1127–44. doi: 10.1080/13803390500246910. [DOI] [PMC free article] [PubMed] [Google Scholar]