Abstract
Some recent studies suggest that in progressive multiple sclerosis, neurodegeneration may occur independently from inflammation. The aim of our study was to analyse the interdependence of inflammation, neurodegeneration and disease progression in various multiple sclerosis stages in relation to lesional activity and clinical course, with a particular focus on progressive multiple sclerosis. The study is based on detailed quantification of different inflammatory cells in relation to axonal injury in 67 multiple sclerosis autopsies from different disease stages and 28 controls without neurological disease or brain lesions. We found that pronounced inflammation in the brain is not only present in acute and relapsing multiple sclerosis but also in the secondary and primary progressive disease. T- and B-cell infiltrates correlated with the activity of demyelinating lesions, while plasma cell infiltrates were most pronounced in patients with secondary progressive multiple sclerosis (SPMS) and primary progressive multiple sclerosis (PPMS) and even persisted, when T- and B-cell infiltrates declined to levels seen in age matched controls. A highly significant association between inflammation and axonal injury was seen in the global multiple sclerosis population as well as in progressive multiple sclerosis alone. In older patients (median 76 years) with long-disease duration (median 372 months), inflammatory infiltrates declined to levels similar to those found in age-matched controls and the extent of axonal injury, too, was comparable with that in age-matched controls. Ongoing neurodegeneration in these patients, which exceeded the extent found in normal controls, could be attributed to confounding pathologies such as Alzheimer's or vascular disease. Our study suggests a close association between inflammation and neurodegeneration in all lesions and disease stages of multiple sclerosis. It further indicates that the disease processes of multiple sclerosis may die out in aged patients with long-standing disease.
Keywords: multiple sclerosis, T cells, B cells, plasma cells, axonal injury
Introduction
Multiple sclerosis is traditionally seen as an inflammatory demyelinating disease of the central nervous system (Charcot, 1880). It was noticed in the earliest studies on multiple sclerosis pathology (Kornek and Lassmann, 1999) that axonal injury and loss occur in the disease lesions; that the extent of axonal injury correlates with the degree of inflammation (Doinikow, 1915; Ferguson et al., 1997; Kuhlmann et al., 2002; Trapp et al., 1998) and that axonal loss is a major correlate of permanent disability (Charcot, 1880; Bjartmar et al., 2000). The traditional view that inflammation is the cause of axonal and neuronal degeneration in multiple sclerosis brain has recently been challenged. Most importantly, current anti-inflammatory or immunomodulatory treatments, while partially effective in the relapsing stage of the disease, have only modest or no effect on the development of neurodegeneration and clinical disability in the progressive disease phase and particularly in patients with primary progressive disease (Coles et al., 1999; Filippi et al., 2000, 2004; Molyneux et al., 2000). Magnetic resonance imaging and spectroscopy studies show a very modest correlation between inflammation, depicted by Gd-enhancement (a marker of inflammation), and progressive brain and spinal cord atrophy (markers for neurodegeneration) (Bielekova et al., 2005; Filippi and Rocca, 2005; Anderson et al., 2006; Zivadinov and Cox, 2007). Neuropathology reveals profound axonal loss in the normal appearing white matter (NAWM), which seems to develop independently from axonal injury in demyelinated lesions (Lovas et al., 2000; Evangelou et al., 2005; DeLuca et al., 2006). Furthermore, axonal injury is also seen in inactive plaques in multiple sclerosis patients (Kornek et al., 2000), suggesting that chronic demyelination may render axons particularly vulnerable to progressive injury and destruction. Finally, ongoing myelin destruction, associated with axonal and neuronal degeneration, is seen in the cerebral cortex of chronic multiple sclerosis patients in the absence of parenchymal inflammatory infiltration by T- or B-lymphocytes (Peterson et al., 2001; Bo et al., 2003). Based on these observations, it has been suggested that neurodegeneration in multiple sclerosis may occur independently of inflammation or may even be the primary cause of CNS damage in this disease (Trapp and Nave, 2008). The above mentioned studies do not exclude the possibility that a diffuse inflammatory process in inactive lesions, the NAWM, the cortex or the meninges may ultimately drive neurodegeneration at these sites (Kornek et al., 2000; Kutzelnigg et al., 2005; Magliozzi et al., 2007). In addition, if demyelination and neurodegeneration in multiple sclerosis progress independently from inflammation, neurodegeneration should be seen in lesions and NAWM even when inflammation is absent. So far, no systematic study has correlated axonal injury and destruction in multiple sclerosis tissue, including the NAWM, with inflammation. In particular, it is unknown, whether disease progression and neurodegeneration may occur in multiple sclerosis in the absence of inflammation.
Patients and Methods
Sample characterization
This study was performed on paraffin embedded archival autopsy material from 67 multiple sclerosis cases and 28 control cases without neurological disease or brain lesions. The control cohort included 18 normal controls (young and aged controls) and 10 patients, who died under septic conditions (Table 1). The clinical course was defined by retrospective chart review according to established criteria, before and blinded to the pathological analysis (Lublin and Reingold, 1996). The multiple sclerosis cohort included nine acute cases, who died within 1 year of disease onset (Table 1) (Marburg, 1906). Furthermore, five cases of relapsing/remitting multiple sclerosis (RRMS), 35 cases of secondary progressive multiple sclerosis (SPMS) and 13 cases of primary progressive multiple sclerosis (PPMS) were included. We also evaluated five cases of benign or asymptomatic multiple sclerosis. Asymptomatic disease (n = 4) was defined when routine autopsy revealed multiple sclerosis pathology, but the patients had no clinical disease history at all. Benign multiple sclerosis (n = 1) was defined when, after 10 years of disease, the EDSS value was below or equal to 3. Cases were grouped into the three main clinical groups: acute/relapsing, SPMS and PPMS. Among pooled progressive (SPMS and PPMS) patients, we identified two distinct pathological subgroups: pathologically active progressive disease and pathologically inactive disease: Pathologically active progressive cases had active lesions or slowly expanding lesions in white matter or cortex. The categorization of patients with pathologically active and inactive disease was based on the analysis of an average of 18 lesions per case. Although, a single actively demyelinating lesion per case was sufficient to classify a case as pathologically active, such a scenario was exceptionally rare, seen for instance in cases with benign or asymptomatic multiple sclerosis. In average, in patients with pathologically active disease 59% of all lesions were either classical active or slowly expanding (for detailed definition of lesional activity see below). Pathologically inactive cases only had inactive lesions in white matter and cortex. For all cases an accurate clinical work up including retrospectively evaluated EDSS (expanded disability status scale) levels was performed (Kurtzke, 1983). Pathologically inactive disease patients were significantly older, showed longer disease duration and longer duration of the progressive phase compared with the pathologically active progressive multiple sclerosis patients, but they did not differ in their EDSS scores 6 to 24 months before death (Table 1). We also evaluated treatments (steroids or other immunosuppressant drugs) administered during the last month pre-mortem (Table 1).
Table 1.
Sample characterization
| AC/RRMS n=14 | SPMS n=35 | PPMS n=13 | Benign/asymptomatic multiple sclerosis n=5 | Septic control n=10 | Normal control n=18 | Pathalogically active progressive n=35 | Pathologically inactive disease n=12 | |
|---|---|---|---|---|---|---|---|---|
| Age (in years) | 52 (20–84) | 56 (28–84) | 58 (34–83) | 68 (61–72) | 77 (42–95) | 68 (30–97) | 53 (28–82) | 76 (64–84) |
| Female/male ratio | 7:7 | 28:7 | 8:5 | 4:1 | 6:4 | 12:6 | 25:10 | 11:1 |
| Disease duration (in months) | 3.5 (0.2–492) | 240.5 (84–540) | 168.0 (60–432) | — | — | — | 192.0 (60–444) | 372.0 (96–540) |
| Duration of progressive phase (in months) | — | 144 (14–312) | 168 (60–432) | — | — | — | 138(14–264) | 189(24–432) |
| EDSS | 10.0 (3.0–10.0) | 8.5 (4.0–9.5) | 8.0 (5.0–9.0) | 0.0 (0.0–3.0) | — | — | 8.5(5.0–9.5) | 8.5 (4.0–9.0) |
| Confounding pathology | ||||||||
| Alzheimer's disease | 1 (7.1%) | 4 (11.8%) | 2 (15.4%) | 1 (20.0%) | 1 (10.0%) | — | 1 (2.9%) | 5 (41.7%) |
| Vascular pathology | — | 3 (8.8%) | 0 | 1 (20.0%) | — | 3 (8.6%) | 1 (8.3%) | |
| Combined confounding patholgy | — | 2 (5.9%) | 1 (7.7%) | 1 (20.0%) | — | 1 (2.9%) | 1 (8.3%) | |
| Steroids or other IS last month | ||||||||
| Yes | 6 (42.9%) | 12 (34.3%) | 1 (7.7%) | — | — | — | 10 (28.6%) | 3 (25.0%) |
| No | 4 (28.6%) | 13 (37.1%) | 9 (69.2%) | 4 (80.0%) | — | — | 17 (48.6%) | 4 (33.3%) |
| Unknown | 4 (28.6%) | 10 (28.6%) | 3 (23.1%) | 1 (20.0%) | — | — | 8 (22.9%) | 5 (41.7%) |
| Lesion distribution and selection | ||||||||
| Active L Total/selected | 221/47 | 101/26 | 55/11 | 1/1 | — | — | 156/37 | 0/0 |
| SEL Total/selected | 0/0 | 136/35 | 88/12 | 5/3 | — | — | 217/47 | 0/0 |
| Inactive Total/selected | 55/8 | 356/56 | 177/25 | 19/4 | — | — | 370/46 | 104/34 |
| Pathological classification | ||||||||
| Active progressive disease | — | 25 (71.4%) | 10 (76.9%) | — | — | — | ||
| Inactive disease | — | 9 (25.7%) | 3 (23.1%) | — | — | — |
Table 1 gives an overview of the main sample characteristics grouping multiple sclerosis patients first into the three main clinical groups: acute–relapsing multiple sclerosis, secondary progressive multiple sclerosis (SPMS) and primary progressive multiple sclerosis (PPMS). In the right part of the table, we distinguish between pathologically active progressive and pathologically inactive disease: Pathologically active progressive multiple sclerosis cases (25 SPMS and 10 PPMS cases) have active lesions or slowly expanding lesions in white matter or cortex. Pathologically inactive multiple sclerosis cases (nine SPMS and three PPMS cases) only present with inactive lesions in white matter and cortex. Pathologically inactive disease patients are significantly older, show longer disease duration and longer duration of the progressive phase compared with pathologically active progressive multiple sclerosis patients, but they do not differ in their EDSS scores 6–24 months before death. We have also evaluated treatments (steroids or other immunosuppressant drugs) administered during the last month pre-mortem. Due to insufficient autopsy material, one SPMS case could not be classified as pathologically active progressive or pathologically inactive disease and is not included in the right part of the table. Values are median value and range or total number and percentage. Acute multiple sclerosis cases with disease durations of 0.2–0.5 months are evaluated as EDSS 10. If not stated explicitly in the clinical record, EDSS has been evaluated retrospectively using all available data in the clinical records. For three multiple sclerosis patients EDSS values could not be evaluated. Combined confounding pathology patients have both, Alzheimer's disease and vascular pathology. IS = immunosuppressant. Asym = asymptomatic multiple sclerosis.
Neuropathological techniques and immunohistochemistry
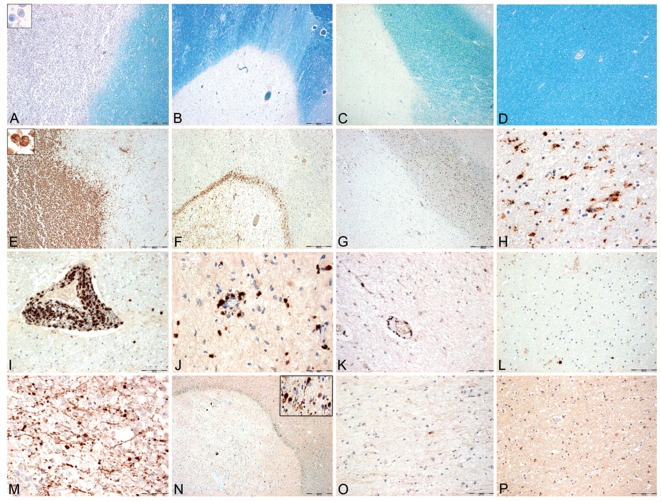
All cases underwent detailed neuropathological examination on multiple tissue blocks (median 7; 1–43 large blocks or routine blocks) from various brain regions, dependent upon the number of lesions seen by macroscopic inspection. In 17/67 multiple sclerosis cases large hemispheric or double hemispheric sections were additionally available. For precise classification of white matter lesions sections were stained with haematoxylin–eosin (HE), luxol fast blue myelin stain (LFB), and by immunohistochemistry for CD68 (cluster of differentiation) (macrophages), myelin oligodendrocyte glycoprotein (MOG), cyclic nucleotide phosphodiesterase (CNPase) and proteolipid protein (PLP; Fig. 1, Table 2). Active lesions were classified on the basis of myelin degradation products within macrophages (Bruck et al., 1995; Kutzelnigg et al., 2005). Active lesions were densely infiltrated by macrophages containing LFB and PLP reactive myelin degradation products. These lesions included early-active and late-active stages (Bruck et al., 1995). Among those lesions two different types could be distinguished: Acute lesions presented with a dense infiltration of macrophages with myelin degradation products throughout the entire lesion (Fig. 1A and E). Chronically active lesions contained a broad rim of macrophages with myelin degradation products at the edge and macrophages with later stages of myelin degradation products in the lesion centre. Since in a first subset analysis of these different types of active lesions, no significant differences regarding inflammation and axonal injury were seen (see also Kornek et al., 2000), we pooled all the active lesions (early active, late active, acute and chronically active lesions) into one group. Different from the group of active lesions are the slowly expanding lesions. The latter show an inactive centre, surrounded by a rim of activated microglia with some macrophages at the lesion margin (Fig. 1B and F). Only few of these macrophages or microglia cells contained early LFB reactive myelin degradation products. Inactive lesions revealed a sharp lesion border without macrophage infiltration or microglia activation (Fig. 1C and G). NAWM was defined as a region of the white matter, which was at least 1 cm apart from demyelinated plaques (Fig. 1D and H).
Figure 1.
Inflammation, demyelination and axonal injury in different multiple sclerosis lesions and the normal appearing white matter. (A, E, I and M) Panels show an active lesion from a patient with acute multiple sclerosis with active demyelination (A), profound macrophage (E) and T-cell infiltration (I) and extensive acute axonal injury (M), visualized with APP. (B, F, J and N): Panels show a slowly expanding lesion from a patient with pathologically active progressive multiple sclerosis (SPMS), with focal demyelination (B), a small but dense rim of CD 68 positive macrophages and microglia at the edge (F). Some of them contain early myelin degradation products. There is T-cell infiltration within the lesion (J) and profound acute axonal injury at the lesion's edge (N and insert). (C, G, K and O) Panels show an inactive lesion from a patient with pathologically active progressive multiple sclerosis (SPMS; i.e. presence of active or slowly expanding lesions at other locations). The focal demyelination is sharply demarcated from the surrounding NAWM (C). Some CD68 positive macrophages or microglia cells (G), mild tissue infiltration with T cells (K) and some APP positive axonal profiles (O) are seen within the inactive lesion. (D, H, L and P) Panels show the NAWM from a case with acute multiple sclerosis. There is no demyelination (D). Some CD68 positive microglia cells (H), few infiltrating T cells (L) and no APP positive axons are present (P). (A–D) Luxol fast blue myelin staining with PAS reaction (A–D ×13) (E–H) Immunohistochemistry for CD 68 (E–G ×13, H ×130) (I–L) Immunohistochemistry for CD3 (I, K, L ×65 and J ×130) (M–P): Immunohistochemistry for APP (M ×130, N ×13, insert ×130, O, P ×65).
Table 2.
Antibodies used for immunocytochemistry
| Primary antibodies |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Antigen | Pre- treatment | Dilution | Antibody type | Target | Source | ||||||
| 1 | CD3 | ST, E | 1:1000 CSA | Ab; rabbit | T-cell receptor | LabVision, Freemont, CA, USA | ||||||
| 2 | CD 20 | ST, E | 1:100 | mAb; mouse | B-cell receptor | LabVision, Freemont, CA, USA | ||||||
| 3 | HLA-DR | ST, C | 1:100 | mAb; mouse | Human leucocyte antigen, MHC Class II antigen, microglia, macrophages | DAKO, Glostrup, Denmark | ||||||
| 4 | Ig | P | 1:200 | Ab; sheep | Human immunglobulin––plasma cells | Amersham Pharmacia Biotech, Uppsala, Sweden | ||||||
| 5 | NF 150 kD | ST, E | 1:2000 | Ab; rabbit | Phosphorylated neurofilament chain M | Chemicon, Temecula, CA, USA | ||||||
| 6 | APP | ST, C | 1:1000 | mAb; mouse | APP; beta/A4 | Chemicon, Temecula, CA, USA | ||||||
| 7 | SPY | ST, E | 1: 200 | Ab; rabbit | Synaptic vesicle protein, C term | Dako, Glostrup, Denmark | ||||||
| 8 | SMI 312 | ST, E | 1: 18 000 | mAb; mouse | Phosphorylated pan-neurofilament | Abcam, Cambridge, UK | ||||||
| 9 | CD4 | ST, E | 1: 100 | mAb; mouse | T-helper cells | LabVision, Freemont, CA, USA | ||||||
| 10 | CD8 | ST, E | 1:250 CSA | mAb mouse | T-cytotoxic cells | Dako, Glostrup, Denmark | ||||||
| 11 | CD138 | ST, E | 1:500 | mAb; mouse | Plasma cells | Serotec, Raleigh, USA | ||||||
| 12 | CD 68 | ST, E | 1:100 | mAb; mouse | Sialomucin microglia, macrophages | Dako, Glostrup, Denmark | ||||||
| 13 | PLP | ST, E | 1:1000 | mAb; mouse | Proteolipid protein myelin | Serotec, Oxford, UK | ||||||
| 14 | MOG | ST, C | 1:1000 | mAb; mouse | Myelin oligodendrocyte glycoprotein | Kindly provided by Sara Piddlesden (Piddlesden et al., 1993) | ||||||
| 15 | CNPase | ST, E | 1:2000 | mAb; mouse | Myelin 2′3′ cyclic nucleotide 3′ phosphodiesterase | Sternberger Monoclonals Inc., Lutherville, MD | ||||||
1–6 were stained and evaluated quantitatively on all multiple sclerosis cases and all controls. Antibody # 7 was stained and evaluated quantitatively on progressive multiple sclerosis cases and all controls. Antibody # 8 was stained and evaluated quantitatively on a subset of cases (Fig. 5). Antibodies # 9–11 were applied on a selected sample for validation of our data. Antibodies # 12, 13 were used on all sections available per case for lesion classification. Antibodies # 14, 15 were used on sections with active plaques in order to distinguish between early active and late active plaques. Ab = polyclonal antibody; C = Citrate buffer (10 mM, pH 6.0); CSA = catalysed signal amplification; E = EDTA (EDTA 1 mM, Tris 10 mM), pH 9.0; mAb = monoclonal antibody; P = 0.03% Protease; SPY = synaptophysin; ST = Steamer.
Analysing the entire sample of multiple sclerosis tissue, we identified 1148 lesions, including 378 active lesions, 222 slowly expanding lesions and 548 inactive lesions. Out of these lesions, we selected a representative sample for detailed quantitative analysis, depending upon the presence of different lesion types per case. The aim of the selection was to include at least two samples of each lesion type, if present. In patients with inactive lesions only, two samples were taken, whereas in patients with different lesion types (active lesions, slowly expanding lesions and inactive lesions) the sample was accordingly larger. Thus immunocytochemistry and quantitative evaluation was done on 228 multiple sclerosis lesions (85 active, 50 slowly expanding and 93 inactive). Further, 139 areas of NAWM, 121 areas of meninges and 120 areas of cortex were analysed in multiple sclerosis cases. Cortical lesions were categorized slightly differently than white matter lesions (Peterson et al., 2001; Dal-Bianco et al., 2008). Active lesions in the cortex showed a high-density rim of HLA-D and CD68 positive microglia––macrophages at the lesion margins. Some of them contained early myelin degradation products. Inactive cortical plaques were demyelinated lesions without increased numbers of microglia–macrophages at their borders and without myelin degradation products in macrophages or microglia. In controls, areas of normal white matter (n = 41), meninges (n = 37) and normal cortex (n = 33) were included in the sample (Table 1).
Immunohistochemistry was performed on paraffin sections as described previously (Bauer et al., 2007; King et al., 1997). For a detailed list of primary antibodies, dilutions and the corresponding pre-treatment see Table 2.
Quantitative analysis
All sections were digitally scanned and specific lesions and regions of interest were manually outlined, and lesional activity was defined as described above. Each lesion type and randomly sampled areas of NAWM, meninges and cortex were quantitatively analysed. Perivascular and parenchymal inflammatory cells were counted separately, but later pooled for statistical analysis. If more lesions from a given activity stage were found in a selected slide, all lesions were counted and the values were pooled. This resulted in up to three distinct lesion types per patient with different stages of activity: active lesions, slowly expanding lesions and inactive lesions. Additionally, values per patient were acquired for the NAWM, the meninges and the cortex. For quantification, the immunostained sections were overlaid by a morphometric grid and inflammatory cell numbers or acutely injured or transected axons were manually counted in 5–100 fields per tissue area. The number of fields, selected per lesion and the applied magnification depended upon the overall density of cells or pathological axons and upon their even or uneven distribution within the tissue. For example, due to their low numbers within the tissue, more microscopic fields had to be analysed for B cells than T cells. In addition, perivascular accumulation of inflammatory cells in perivascular cuffs in active lesions required counting in large numbers of fields to obtain reliable values for global cell density. Thus we analysed 5 to 30 fields per lesion for CD3 and HLA-D (0.3–1.9 mm2); 20–40 fields per lesion for amyloid precursor protein (APP) (5–10 mm2), neurofilament-M (1.25–2.5 mm2), synaptophysin (SPY) (1.25–2.5 mm2) and SMI 312 (1.25–2.5 mm2), whereas 50–100 fields were counted per lesion for B cells and plasma cells (3.12–6.25 mm2). In normal controls, randomly sampled areas of NAWM were quantified in the same way. Cortical values were obtained differently: For T cells, B cells, plasma cells and axonal injury the total area of cortex per slide was quantified. Similarly, inflammation in the meninges was quantified over the entire cortex per slide. The area of meninges was determined using a morphometric grid as described in detail before (Dal-Bianco et al., 2008). For HLA-D, the total area of active lesions, inactive lesions and normal appearing grey matter (NAGM) was measured. Five to twenty fields (0.3–1.25 mm2) per lesion and NAGM were quantified as described for the white matter. An overall cortical value was calculated by multiplication of the normalized lesional and NAGM values with the respective area size in square millimetre. These values were added and the sum was divided with the total cortical area per slide.
All values are expressed as cell counts per square millimetre. The total sample was quantified for CD3, CD20, Ig and HLA-D positive cells. In order to verify our own data, we also performed a quantitative analysis of CD4, CD8 and CD138 positive cells on a subset of cases. Axonal injury was assessed and quantified using antibodies directed against APP, SPY, neurofilament M (Table 2). Quantitative analysis was carried out for APP, SPY, neurofilament M, and SMI312. An inter-rater agreement was assessed between three independent investigators (J.F., S.B. and H. Lassmann) for the quantitative analysis on a selection of randomly sampled slides and markers.
Statistical analysis
Due to the uneven distribution of our data, statistical analysis was performed with non-parametric tests. Descriptive analysis included median value and range. Differences between two groups were assessed with Wilcoxon–Mann–Whitney U-test. Differences between more than two groups were assessed with Kruskal–Wallis test, followed by pairwise Wilcoxon–Mann–Whitney U-tests. In case of multiple testing (comparison of more than two groups), significant values were corrected with Bonferroni–Holm or Shaffer's procedure as appropriate. Interdependence of variables was evaluated by Spearman non-parametric correlation test. Differences in the incidence of confounding pathology were assessed by comparison of observed and expected counts with Fisher's exact test. SPSS 14.0 statistical software system (SPSS Inc., Chicago, IL) was used for calculations. The reported P-values are results of two-sided tests. A P-value smaller or equal to 0.05 is considered statistically significant after Bonferroni–Holm or Shaffer's correction for multiple testing. For all statistical analysis, distinct values per patient for each present lesion type, NAWM, meninges and cortex were used. If necessary for certain statistical analysis, lesions were pooled in one group. Differences between multiple sclerosis patients and controls were first assessed by comparing different types of lesions pooled from all types of this disease, to normal control NAWM and septic control NAWM. Patient NAWM was compared to control NAWM. Multiple sclerosis meningeal and cortical values were equally compared to respective control areas. Differences between pathologically active progressive and pathologically inactive disease were first assessed by pooling all lesional, normal white matter, meningeal and cortical values. In order to achieve a more accurate comparison of pathologically active progressive and pathologically inactive disease, we also compared those disease courses separately in lesions of the same stage of activity (lesion types), in the NAWM, the meninges and the cortex. The interdependences of inflammatory infiltrates and axonal injury with age and disease duration were also analysed separately in pooled lesions, NAWM and cortex. The interdependence of inflammatory infiltrates and axonal injury was analysed separately in pooled lesions, the NAWM, meninges and cortex.
Results
Inflammation reflects the activity of the disease process in demyelinating lesions and in different disease stages
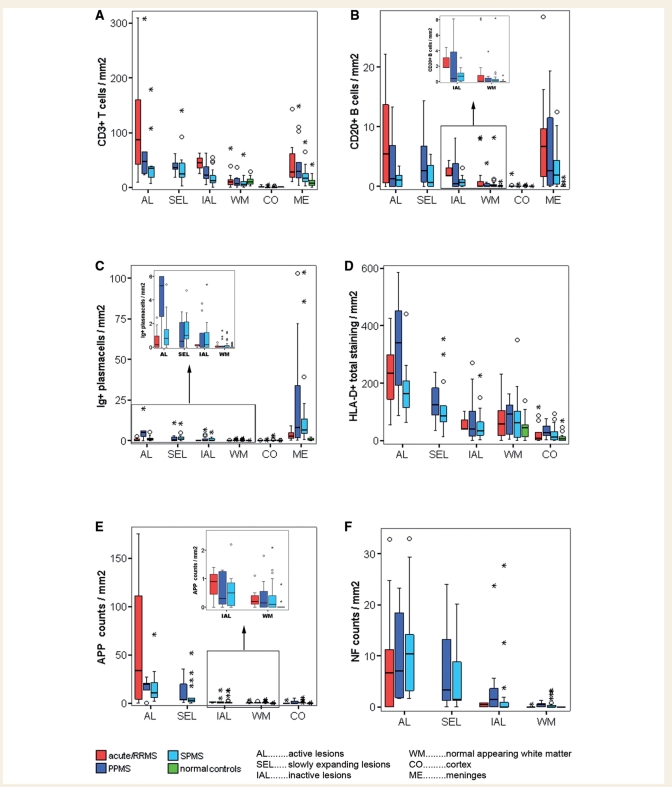
In a first step, we analysed the density of inflammatory infiltrates in relation to lesional activity (Fig. 2, Supplementary Table 1). As expected, the most pronounced inflammation by T cells was seen in active lesions (Figs 2A and 1I), which are most frequent in patients with acute or RRMS (Table 1). Those were followed by slowly expanding lesions, which were only found in the progressive stage of the disease (Figs 2A and 1J). Much lower numbers of T cells were seen in inactive lesions and in the NAWM (Figs 2A, 1K and L). T cells were rare or absent in the cortical parenchyma, but there was pronounced infiltration in the meninges (Fig. 2A). Consistent with prior studies (Gay et al., 1997; Babbe et al., 2000), a sub-analysis of CD4 and CD8 positive T cells revealed that the majority of T-cell infiltration in multiple sclerosis lesions consisted of CD8 positive T cells (data not shown).
Figure 2.
Density of inflammatory infiltrates and axonal injury in relation to lesional activity and disease course. Graphs display densities of T cells (A), B cells (B), plasma cells (C), microglia/macrophages (D) as well as APP positive (E) and neurofilament positive axonal injury (F) in different lesion types, NAWM, meninges and cortex separated for the three main disease courses: acute–relapsing multiple sclerosis, SPMS and PPMS. Values of normal control white matter, cortex and meninges are additionally shown. The graph depicts the density of inflammatory infiltrates and axonal injury in relation to lesional activity and disease course. Inserts show the densities of inflammatory infiltrates in inactive lesions and the NAWM in more detail. Box plots represent median value (50th percentile) and range of lesional densities. Outliers (values that are between 1.5 and 3 times the interquartile range) are marked with a circle. Extreme values (values that are more than three times the interquartile range) are marked with an asterisk. Values are mean values for each present lesion type or area per patient. The densities of T-cells and microglia/macrophages are highest in active lesions, followed by slowly expanding lesions and inactive lesions (A and D). B-cell infiltration is mainly seen in active lesions and meninges followed by slowly expanding lesions (B), while plasma cells are predominantly found in meninges (C). Equally, axonal injury (E and F) is mainly seen in active lesions and slowly expanding lesions. Active lesions and slowly expanding lesions separated for acute–relapsing, SPMS or PPMS cases all display significantly higher levels compared to normal control WM for all inflammatory and neurodegenerative markers (A–F). Meningeal infiltrates of T cells (A), B cells (B) and plasma cells (C) in acute–relapsing, SPMS and PPMS cases equally display significantly higher levels than pooled control meninges. The same situation applies when inflammatory infiltrates and axonal injury of active lesions and slowly expanding lesions or meningeal inflammatory infiltrates, separated for acute–relapsing, SPMS and PPMS, are compared to pooled control WM (with septic controls). Inactive lesions in acute–relapsing multiple sclerosis cases display significantly higher T cell (A) and B cell (B insert) infiltrates and significantly more APP positive (E) and NF-M positive (F) axonal injury compared to normal control white matter. Inactive lesions in PPMS and SPMS cases show a heterogeneous pattern of differences compared to normal or pooled controls (see Results section and separation of SPMS and PPMS cases into the categories of pathologically active progressive and pathologically inactive). We do not observe significant differences between SPMS and PPMS cases. (F) In order to simplify graphical presentation three acute–relapsing multiple sclerosis active lesional extreme values have been cut.
A similar pattern of inflammation was seen for B cells (Fig. 2B) and HLA-D-positive macrophages and microglia cells (Fig. 2D). In comparison to T cells, the number of B cells was on average 10 times lower and these cells were predominantly seen in perivascular cuffs or meninges and only few of them dispersed into the parenchyma. HLA-D positive cells revealed a dominant macrophage phenotype in all active lesions (Fig. 1A and E). In slowly expanding lesions, most HLA-D positive cells displayed a microglia phenotype with only few intermingled macrophages. A ramified microglia like phenotype dominated in inactive lesions, cortex and NAWM (Fig. 1G, I and H). Plasma cells (Fig. 2C) were sparse in the parenchyma of lesions, NAWM and cortex. They mainly accumulated in the perivascular and meningeal connective tissue spaces of the brain and revealed only little relation to lesional activity. As described before by Magliozzi et al., (2007) lymph follicle-like structures were found in the meninges in 15/67 multiple sclerosis cases. In patients with SPMS and PPMS, such follicles were only present in cases with active progressive disease (for definition see below).
In relation to disease stage, we found most profound inflammation in patients with acute/relapsing disease followed by those with progressive disease. This was similar for T and B cells. In contrast, plasma cells were mainly seen in lesions, NAWM and meninges in patients with progressive disease, but were less abundant in patients, who died during the acute/relapsing stage. When individual lesion types (active lesions, slowly expanding lesions and inactive lesions) were compared no significant differences in the density of inflammatory infiltrates were noted between SPMS and PPMS.
Given the archival nature of our tissue resources, the vast majority of our patients died before modern immunomodulatory treatments were available. Nevertheless, we analysed the potential impact of steroid or other immunosuppressive treatment on our findings. Comparing those with and those without steroid treatment, there were no significant differences observed in the extent of inflammation in lesions, NAWM, meninges and cortex when pooling all multiple sclerosis types.
Axonal degeneration in multiple sclerosis brains
For analysis of acute axonal injury, we used markers of fast axonal transport, APP and SPY as well as the presence of focal axonal swellings and end-bulbs (axonal spheroids) that stained for neurofilament epitopes. Active multiple sclerosis lesions demonstrated a high number of acutely injured axons as evidenced by focal accumulations of APP or SPY. Only a fraction of those axons with disturbed axonal transport also stained as axonal end-bulbs positive for neurofilament. In contrast, in inactive lesions, axonal spheroids, reactive for neurofilament markers, were present, which did not contain APP or SPY. This suggests that disturbance of axonal transport (APP––or SPY––positive axons) is likely an initial but transient phenomenon of acute axonal injury, whereas neurofilament positive axonal spheroids may be retained within lesions for a more prolonged period of time.
As expected, the most extensive acute axonal injury was seen in active plaques, followed in the order of magnitude by slowly expanding lesions, inactive plaques, NAWM and cortex (Fig. 2E and F). This was similar for APP positive axons (Fig. 2E) and neurofilament positive axonal end-bulbs (Fig. 2F), although the absolute numbers of immunoreactive profiles were different. Since classical active lesions were enriched in patients with acute and relapsing disease, and these lesions showed the highest density of acutely injured axons; it was not surprising that more acute axonal injury (APP positive) was seen in these patients compared with those with progressive disease. However, when we compared lesions of the same stage of activity, there were no significant differences in the extent of axonal injury between acute–relapsing, SPMS and PPMS patients. Thus, the density of acutely injured axonal profiles was similar in active or inactive lesions, respectively, regardless whether they were present in acute/relapsing or progressive multiple sclerosis. The same applied to NAWM, with one exception: Neurofilament-M positive axonal spheroids were present in a significantly higher amount in the NAWM of progressive disease patients (pooled PPMS and SPMS; P < 0.05) than in acute–relapsing disease patients (Fig. 2F). As with inflammation no significant differences were seen regarding axonal damage, when individual lesions types (active lesions, slowly expanding lesions and inactive lesions) or NAWM were compared between SPMS and PPMS. No differences were seen in the extent of axonal injury between male and female patients (data not shown).
Axonal injury is associated with inflammation not only in all multiple sclerosis stages but also in progressive multiple sclerosis alone
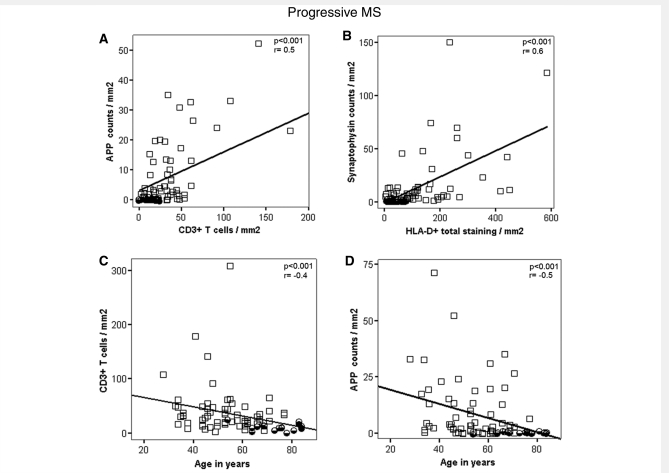
When all lesions included in this study were pooled, we found a significant correlation between the extent of acute axonal injury with the number of inflammatory cells, quantified in consecutive sections (T cells: P < 0.001, r = 0.6; B cells: P < 0.01; r = 0.3; Plasma cells P < 0.05; r = 0.2; HLA-D: P < 0.001; r = 0.7). Similar results were obtained when the extent of neurofilament reactive end-bulbs was correlated with inflammation (T cells: P < 0.001; r = 0.5; B cells: P < 0.01; r = 0.3; Plasma cells P < 0.01; r = 0.3; HLA-D: P < 0.001, r = 0.6).
Since, it has been suggested that in progressive multiple sclerosis (SPMS and PPMS) axonal injury may develop independently from inflammation we performed similar correlative studies only in patients with progressive multiple sclerosis, excluding cases with acute and relapsing disease. Even in this analysis we found very similar correlations between inflammation and axonal damage (Fig. 3A, Supplementary Table 2). The correlations for APP reactive axonal profiles were P < 0.001, r = 0.5 for T cells, P < 0.05, r = 0.2 for B cells, P < 0.05, r = 0.3 for plasma cells and P < 0.001, r = 0.6 for HLA-D positive macrophages and microglia. Very similar significance levels and correlation coefficients were seen when neurofilament reactive end-bulbs were used as a marker for axonal degeneration. Furthermore, the data were confirmed by using another marker for acute disturbance of axonal transport (SPY) (Fig. 3B). A correlation between axonal injury and inflammation was not only seen in lesions in the progressive stage of the disease, but also in the NAWM. There, SPY reactive axonal profiles correlated with the number of T cells (P < 0.01, r = 0.4) and B cells (P < 0.01, r = 0.5).
Figure 3.
(A and B) Graphs show examples of the interdependence of inflammation and neurodegeneration among progressive multiple sclerosis patients (SPMS and PPMS). Multiple sclerosis values represent lesional mean values for all lesion types per patient. We observe highly significant positive correlations between acute axonal injuries and inflammatory infiltrates (A and B) among progressive multiple sclerosis patients. We also find those correlations when analysed in all multiple sclerosis cases (acute–relapsing, SPMS and PPMS) (see Results section). (C and D) Graphs express examples of the relation between age and densities of inflammatory infiltrates (C) or axonal injury (D) in progressive multiple sclerosis (SPMS and PPMS) lesions. Multiple sclerosis values represent lesional values for all lesion types per patient. We find highly significant negative correlations between inflammation and age at death (C) and age at death and axonal injury (D) among progressive multiple sclerosis patients. Those negative correlations also persist when analysed in all multiple sclerosis patients (see Results section). For more detailed information see Supplementary Table 2.
These data show that not only in the acute and relapsing stage of the disease but also in progressive multiple sclerosis (SPMS and PPMS), axonal injury is associated with inflammation. This is different from what we found in control brains: There, acute axonal injury, depicted by the focal expression of APP, is only associated with HLA-D positive macrophages and microglia (P < 0.01; r = 0.3), but not with the numbers of T cells, B cells and plasma cells within the tissue.
Inflammation and axonal injury declines to levels seen in age-matched controls in aged multiple sclerosis patients with pathologically inactive disease
In patients with PPMS and SPMS, the density of inflammatory infiltrates as well as of dystrophic axons in lesions and NAWM was highly variable between patients. Furthermore, we found significant negative correlations between age (Fig. 3C and D; Supplementary Table 2) or disease duration and inflammatory infiltration or axonal injury. In particular, in a similar proportion of SPMS and PPMS patients, very low numbers of inflammatory cells and dystrophic axons were seen.
To assess this point we divided the patients, who died in the late progressive stage (all SPMS and PPMS patients) of the disease into two categories: Patients with pathologically active progressive disease, where global neuropathological analysis revealed the persistence of classical active or slowly expanding lesions, and patients with pathologically inactive disease, who showed inactive plaques only. In comparison to those with pathologically active progressive disease, patients with pathologically inactive disease were significantly older and had significantly longer disease duration, while their extent of clinical deficit at the time of death was similar (Table 1). The percentages of patients with pathologically active progressive and pathologically inactive disease were similar among SPMS and PPMS (Table 1).
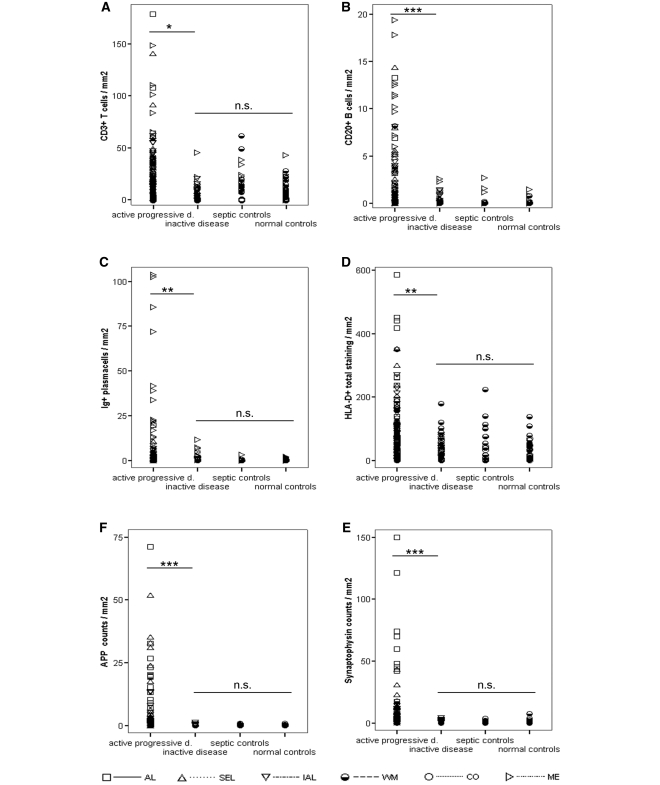
In contrast to those with pathologically active progressive disease, inflammatory infiltrates of pathologically inactive disease patients were significantly lower and declined to levels seen in age matched or septic controls (Fig. 4A–D; Supplementary Table 3). However, among pathologically inactive patients, a modest but significantly higher amount of meningeal plasma cell and B-cell densities (P < 0.05) were observed compared with normal controls. When analysing neurodegeneration in these patients, we found that they also had significantly less acute axonal injury as compared to those, who died during the pathologically active progressive phase (Fig. 4E and F). Furthermore, pathologically inactive patients displayed densities of acutely injured axons or of neurofilament reactive axonal end-bulbs, similar to those seen in age-matched controls (Fig. 4E and F). Additionally to the data shown in Fig. 4, we also assessed the differences between pathologically active progressive and inactive disease separately in inactive lesions, the NAWM and meninges (i.e. comparable lesions types; see Supplementary Table 3). Remarkably, even when comparing any of these relatively silent lesions or areas, we found significantly higher densities of inflammatory infiltrates and axonal injury among pathologically active than inactive patients (see Supplementary Table 3). These data suggest that multiple sclerosis-related neurodegeneration ceases in parallel to the inflammatory reaction.
Figure 4.
The graph displays the differences between pathologically active progressive multiple sclerosis, pathologically inactive multiple sclerosis and controls (normal and septic controls). Among pooled progressive multiple sclerosis (SPMS and PPMS) patients, we have identified two distinct pathological subgroups: Pathologically active progressive multiple sclerosis cases had active lesions or slowly expanding lesions in white matter or cortex. Pathologically inactive multiple sclerosis cases only had inactive lesions in white matter and cortex. Apart from significant differences in their clinical presentation (Table 1), those entities also differ in their pathological presentation. Overall, pathologically active progressive disease cases display significantly higher densities of inflammatory infiltrates (A–D) and axonal injury (E and F) than pathologically inactive disease cases. Importantly, these differences remained when analysing inactive lesions or NAWM only (see Supplementary Table 3). In addition, pathologically active progressive disease cases also display significantly higher densities of inflammatory infiltrates (A–D) and axonal injury (E and F) than pooled controls. These differences were consistent using pooled data or values from either inactive lesions or NAWM only (the latter two not shown). Remarkably, even when using pooled data, both inflammatory infiltrates (A, C and D) and axonal injury (E and F) of pathologically inactive disease cases were in the range of normal or pooled control values. Areas of confounding pathology are not included. Multiple sclerosis values are lesional, normal appearing white matter, cortical and meningeal densities of inflammatory infiltrates (A–D) and lesional, NAWM and cortical densities of axonal injury (E and F). Control values are white matter, cortical and meningeal densities of inflammatory infiltrates (A–D) and white matter and cortical densities of axonal injury (E and F). Differences between pathologically active progressive, pathologically inactive disease and pooled controls have been assessed with Mann–Whitney U-tests by pooling all values (lesional, white matter, cortical and meningeal values). P-values are corrected with Shaffer's procedure. AL = active lesions; SEL = slowly expanding lesions; IAL = inactive lesions; WM, white matter; CO, cortex; ME, meninges; NS = not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
One can argue that such patients do not reflect the normal course of the disease, but represent a selected sample of patients with benign multiple sclerosis. This is not the case, since neurological disability (median EDSS 8.5) in these patients was similar to that seen in patients, who died during the pathologically active progressive phase of the disease (Table 1). To further address this issue, we analysed a separate tissue collection from five patients with asymptomatic or benign multiple sclerosis. These patients were either diagnosed at autopsy without preceding clinical disease or only had a few bouts at the onset of the disease without further deterioration. In their brains, only few lesions were seen. In contrast to pathologically inactive disease cases, in asymptomatic or benign multiple sclerosis, classical active or slowly expanding lesions were found as well as inactive lesions. Lesions in these patients displayed a similar extent of inflammation and axonal injury, as did lesions of the same stage of activity in pathologically active progressive patients (data not shown).
Additional neurodegenerative changes in pathologically inactive multiple sclerosis
Three different additional pathologies were encountered in the brains of patients, who died in the pathologically inactive stage of multiple sclerosis) (Fig. 8): unusually thick demyelinated axons within inactive plaques (Fig. 5A), synaptic patterns of SPY immunoreactivity within lesions and the NAWM (Fig. 5B and C) and axonal or neuronal injury due to confounding pathology (Fig. 5D).
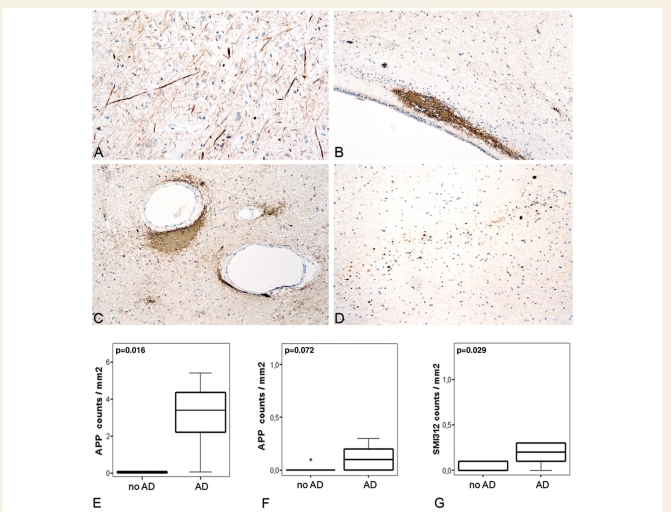
Figure 5.
Additional CNS pathologies in multiple sclerosis patients with pathologically inactive disease. In the brains of patients with late stage inactive multiple sclerosis, different additional pathologies are observed: (A) Staining for phosphorylated neurofilament (SMI312, ×140) revealed unusually thick demyelinated axons in inactive plaques. Clusters of synaptophysin reactivity (B and C ×70) are located periventricularly (B) and perivascularly (C) within inactive lesions with severe axonal loss. In a patient with concomitant vascular pathology, axonal tract degeneration is visualized by APP staining (D ×70). Since Alzheimer's disease was most frequent in pathologically inactive disease patients, we compared the extent of axonal injury in cortex (E) and NAWM (F and G) of inactive multiple sclerosis patients (n = 12) with and without concomitant Alzheimer's disease. The graphs express the density of axons positive for APP (E and F) or phosphorylated neurofilament (SMI312, G). Values represent cortical mean values and NAWM mean values per patient. Box plots represent median value (50th percentile) and range. Extreme values (values that are more than three times the interquartile range) are marked with an asterisk. Those patients with concomitant Alzheimer's disease pathology (n = 5) show significantly more cortical neurodegeneration (E) than those without (n = 7). In the NAWM patients with concomitant Alzheimer's disease pathology show significantly more chronic axonal injury (G) but only a trend to more acute axonal injury (F).
As described before (Shintaku et al., 1988; Lovas et al., 2000; Bergers et al., 2002; Fisher et al., 2007), unusually thick demyelinated axons with high reactivity for phosphorylated neurofilament (Fig. 5A) were present in variable numbers in all chronically demyelinated plaques in patients with progressive disease. These axonal changes may represent a chronic axonal reaction to demyelination (Waxman, 2008; Young et al., 2008; Mahad et al., 2009).
In sections stained for SPY, we frequently encountered dense clusters of small immunoreactive profiles, closely similar to SPY positive synapses in the grey matter. They were mainly located in periventricular or perivascular areas in chronic demyelinated plaques with severe axonal loss (Fig. 5B and C), but were also found in the NAWM. They may represent synaptic sprouts, originating from transsected axons.
In our multiple sclerosis sample, 16 patients were identified who had confounding pathology (Table 1). This was more frequently seen among aged multiple sclerosis patients with pathologically inactive disease than among acute–relapsing or pathologically active progressive multiple sclerosis patients (P < 0.01). Eleven patients revealed cortical lesions consisting of amyloid plaques and neurofibrillary tangles, fulfiling the CERAD and Braak criteria for neuropathological diagnosis of Alzheimer's disease (Dal-Bianco et al., 2008). As Alzheimer's disease was most frequent among pathologically inactive patients (P < 0.01), we compared within this subgroup those with and without Alzheimer's disease pathology: In patients with concomitant Alzheimer's disease pathology, we found significantly more acute axonal injury in the cortex and in the white matter (P < 0.05) compared to multiple sclerosis patients with pathologically inactive disease and the absence of Alzheimer type pathology (Fig. 5E–G). Acute axonal injury in the white matter of Alzheimer's disease patients most likely reflects Wallerian degeneration due to loss of cortical neurons. In addition, eight patients showed small focal ischaemic lesions in the white or grey matter. Not surprisingly, tract degeneration was seen within and around these vascular lesions (Fig. 5D). These data underline that in progressive multiple sclerosis patients, neurodegeneration may progress due to age-related concurrent diseases, even when multiple sclerosis related inflammation and axonal injury have ceased.
Discussion
One of the key results of our study is that in the progressive stage of the disease, active demyelination and neurodegeneration are only seen in patients with pronounced inflammation in the brain. Further, we also show that in aged patients at the late stage of the disease, the inflammatory process may die out and inflammation declines to levels seen in age-matched controls. In this situation, ongoing neurodegeneration is reduced to the levels seen in controls, provided there is no confounding age-related disease, such as Alzheimer's or vascular disease.
Thus, there is no doubt that the pathology of progressive multiple sclerosis is fully consistent with that of a classical inflammatory disease. This contrasts with MRI findings that show only rare Gadolinium (Gd)-enhancing lesions in comparable patients, clearly documenting a dissociation between Gd-enhancement and progressive damage of the grey and white matter (Bielekova et al., 2005; Filippi and Rocca, 2005; Anderson et al., 2006; Zivadinov and Cox, 2007; Waxman, 2008; Young et al., 2008). Furthermore, the question arises, why anti-inflammatory therapies are largely ineffective in the progressive disease stage and in particular in patients with PPMS (Coles et al., 1999; Molyneux et al., 2000). One possible explanation is that in the progressive stage of multiple sclerosis, inflammation becomes trapped within the brain compartment behind a closed or repaired blood–brain barrier. In fact, dissociation between inflammation and blood–brain barrier disturbance in multiple sclerosis patients has been described by using a specific marker, which selectively stained leaky endothelial cells in brain vessels (Hochmeister et al., 2006). Most importantly many vessels with profound perivascular inflammation in the absence of leaky endothelial cells were seen in patients with progressive multiple sclerosis. Furthermore, in the progressive stage of multiple sclerosis, lymph follicle-like structures are formed in the meninges and in the large perivascular spaces. Those lymph-follicle-like structures are associated with rapid disease progression and profound brain damage (Magliozzi et al., 2007). It has been suggested that chronic inflammation within the brains of multiple sclerosis patients creates a microenvironment, which favours homing and retention of inflammatory cells within this compartment (Krumbholz et al., 2005; Meinl et al., 2008).
Another interesting aspect of inflammation in multiple sclerosis is the difference in the pattern of T-cell, B-cell and plasma cell infiltration. T cells, and to a lesser degree B cells are markers for the activity of the disease process and tissue damage. The more active lesions are, the more of these cells are seen within the tissue. However, there are compartmental differences in the distribution of these cells. T cells are seen in large perivascular cuffs especially in active lesions in the acute and relapsing disease stage but they also infiltrate the CNS parenchyma in high numbers. This is opposite for B cells and even more so for plasma cells. Those cells tend to accumulate predominantly in the connective tissue spaces of the brain, such as the perivascular spaces and the meninges. Furthermore, plasma cells accumulate later in the CNS in relation to disease stage and persist within the brain even at time points, when T-cell and B-cell inflammation is cleared. This may be explained by the existence of a population of long-lived plasma cells, which may accumulate in the CNS in chronic inflammatory conditions. It may also explain the persistence of oligoclonal bands in the CSF in chronic brain inflammation, which may be seen for a prolonged time after recovery (Meinl et al., 2006).
Regarding neurodegeneration, our study focused on markers for the disturbances of fast axonal transport and the formation of axonal swellings and end-bulbs. Disturbance of fast axonal transport is currently the most accurate and sensitive marker for acute axonal injury, occurring at a short-time window before axonal death, whereas axonal swellings and end-bulbs may persist at sites of damage for an extended period (Li et al., 1995). Thus, the disturbance of fast axonal transport seems to be a suitable marker to identify the acute or progressive damage, which takes place at the time of axonal death. The detection of dying neuronal cell bodies is much more difficult. Neuronal loss has previously been shown to occur in active cortical and deep grey matter lesions (Peterson et al., 2001; Cifelli et al., 2002; Wegner et al., 2006). In some cases with active cortical lesions, associated with profound meningeal inflammation, neuronal apoptosis has been observed (Peterson et al., 2001; Magliozzi et al., 2007). However, in more slowly developing lesions, acute nerve cell destruction is rare or absent, similar to what has been described in the cortex of Alzheimer's disease patients (Stadelmann et al., 1998). Thus, neuronal loss in grey matter may contribute to neurodegeneration in multiple sclerosis.
Understanding the relation between inflammation and neurodegeneration is of key importance for future therapeutic strategies in multiple sclerosis. If inflammation drives subsequent neurodegeneration, proper anti-inflammatory therapies are the best choice to stop the disease and to prevent further clinical deterioration of the patients. If there is a neurodegenerative component, which leads to brain damage independent from inflammation, the effect of anti-inflammatory therapies will be limited and neuroprotective strategies will become the prime target. Clinical experience and magnetic resonance imaging studies suggest that neurodegeneration may become independent from inflammation in the progressive disease (Trapp and Nave, 2008). We show for the first time in a pathological study that axonal injury is invariably associated with inflammation, especially in progressive multiple sclerosis. Further, in late stages of the disease, inflammation declines in a considerable proportion of the patients to an extent, which is similar to that seen in age-matched controls. This indicates that the inflammatory reaction may die out at late stages of the disease. If there is a neurodegenerative component, which progresses independently from inflammation, one would predict that in such patients axonal injury continues. This was not the case in our study. In contrast, axonal injury in such patients was similar in extent, compared with age-matched controls.
This, however, does not mean that there is no further brain damage in multiple sclerosis patients, when the inflammatory process has stopped. In such patients acute axonal injury does not return to zero, but rather to the levels seen in age-matched controls. Furthermore, confounding pathology is frequently seen in aged multiple sclerosis patients. We have previously shown that Alzheimer's disease lesions develop in multiple sclerosis patients at similar rates compared with those present in a normal aging cohort (Dal-Bianco et al., 2008). In our present study, we could also show that in patients with concomitant multiple sclerosis and Alzheimer's disease, acute axonal injury is more pronounced compared to that in non-Alzheimer's multiple sclerosis patients. The same holds true for patients with concomitant vascular lesions. Thus, in patients with profound multiple sclerosis related brain damage, which exceeds the functional reserve capacity of the nervous tissue, such age related or concomitant brain damage may give rise to further progression of functional deficits, even when the multiple sclerosis related disease process has stopped.
Supplementary material
Supplementary material is available at Brain online.
Funding
European Union (LSHM-CT2005-018637); National MS-Society, USA (NMSS RG 3185-07).
Supplementary Material
Acknowledgements
The authors thank Marianne Leisser, Ulrike Koeck, Angela Kury and Helene Breitschopf for expert technical assistance.
Glossary
Abbreviations
- APP
amyloid precursor protein
- EDSS
expanded disability status scale
- NAGM
normal appearing grey matter
- NAWM
normal appearing white matter
- PPMS
primary progressive multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
References
- Anderson VM, Fox NC, Miller DH. Magnetic resonance imaging measures of brain atrophy in multiple sclerosis. J Magn Reson Imaging. 2006;23:605–18. doi: 10.1002/jmri.20550. [DOI] [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Elger CE, Hans VH, Schramm J, Urbach H, Lassmann H, et al. Astrocytes are a specific immunological target in Rasmussen's encephalitis. Ann Neurol. 2007;62:67–80. doi: 10.1002/ana.21148. [DOI] [PubMed] [Google Scholar]
- Bergers E, Bot JC, De Groot CJ, Polman CH, Nijeholt GJ, Castelijns JA, et al. Axonal damage in the spinal cord of MS patients occurs largely independent of T2 MRI lesions. Neurology. 2002;59:1766–71. doi: 10.1212/01.wnl.0000036566.00866.26. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Kadom N, Fisher E, Jeffries N, Ohayon J, Richert N, et al. MRI as a marker for disease heterogeneity in multiple sclerosis. Neurology. 2005;65:1071–6. doi: 10.1212/01.wnl.0000178984.30534.f9. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. [PubMed] [Google Scholar]
- Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–31. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- Bruck W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–96. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- Charcot JM. Lecons sur les maladies du systeme nerveux faites a la Salpetriere. Paris: A. Delahaye; 1880. [Google Scholar]
- Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM. Thalamic neurodegeneration in multiple sclerosis. Ann Neurol. 2002;52:650–3. doi: 10.1002/ana.10326. [DOI] [PubMed] [Google Scholar]
- Coles AJ, Wing MG, Molyneux P, Paolillo A, Davie CM, Hale G, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol. 1999;46:296–304. doi: 10.1002/1531-8249(199909)46:3<296::aid-ana4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dal-Bianco A, Bradl M, Frischer J, Kutzelnigg A, Jellinger K, Lassmann H. Multiple sclerosis and Alzheimer's disease. Ann Neurol. 2008;63:174–83. doi: 10.1002/ana.21240. [DOI] [PubMed] [Google Scholar]
- DeLuca GC, Williams K, Evangelou N, Ebers GC, Esiri MM. The contribution of demyelination to axonal loss in multiple sclerosis. Brain. 2006;129:1507–16. doi: 10.1093/brain/awl074. [DOI] [PubMed] [Google Scholar]
- Doinikow B. Über De- und Regenerationserscheinungen an Achsenzylindern bei der multiplen Sklerose. Z ges Neurol Psych. 1915;27:151–78. [Google Scholar]
- Evangelou N, DeLuca GC, Owens T, Esiri MM. Pathological study of spinal cord atrophy in multiple sclerosis suggests limited role of local lesions. Brain. 2005;128:29–34. doi: 10.1093/brain/awh323. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120(Pt 3):393–9. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA. MRI evidence for multiple sclerosis as a diffuse disease of the central nervous system. J Neurol. 2005;252(Suppl 5):v16–24. doi: 10.1007/s00415-005-5004-5. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA, Pagani E, Iannucci G, Sormani MP, Fazekas F, et al. European study on intravenous immunoglobulin in multiple sclerosis: results of magnetization transfer magnetic resonance imaging analysis. Arch Neurol. 2004;61:1409–12. doi: 10.1001/archneur.61.9.1409. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rovaris M, Rice GP, Sormani MP, Iannucci G, Giacomotti L, et al. The effect of cladribine on T(1) ‘black hole’ changes in progressive MS. J Neurol Sci. 2000;176:42–4. doi: 10.1016/s0022-510x(00)00303-8. [DOI] [PubMed] [Google Scholar]
- Fisher E, Chang A, Fox RJ, Tkach JA, Svarovsky T, Nakamura K, et al. Imaging correlates of axonal swelling in chronic multiple sclerosis brains. Ann Neurol. 2007;62:219–28. doi: 10.1002/ana.21113. [DOI] [PubMed] [Google Scholar]
- Gay FW, Drye TJ, Dick GW, Esiri MM. The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesion. Brain. 1997;120(Pt 8):1461–83. doi: 10.1093/brain/120.8.1461. [DOI] [PubMed] [Google Scholar]
- Hochmeister S, Grundtner R, Bauer J, Engelhardt B, Lyck R, Gordon G, et al. Dysferlin is a new marker for leaky brain blood vessels in multiple sclerosis. J Neuropathol Exp Neurol. 2006;65:855–65. doi: 10.1097/01.jnen.0000235119.52311.16. [DOI] [PubMed] [Google Scholar]
- King G, Payne S, Walker F, Murray GI. A highly sensitive detection method for immunohistochemistry using biotinylated tyramine. J Pathol. 1997;183:237–41. doi: 10.1002/(SICI)1096-9896(199710)183:2<237::AID-PATH893>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kornek B, Lassmann H. Axonal pathology in multiple sclerosis. A historical note. Brain Pathol. 1999;9:651–6. doi: 10.1111/j.1750-3639.1999.tb00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–76. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Derfuss T, Rosenwald A, Schrader F, Monoranu CM, et al. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 2005;201:195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–12. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–12. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Li GL, Farooque M, Holtz A, Olsson Y. Changes of beta-amyloid precursor protein after compression trauma to the spinal cord: an experimental study in the rat using immunohistochemistry. J Neurotrauma. 1995;12:269–77. doi: 10.1089/neu.1995.12.269. [DOI] [PubMed] [Google Scholar]
- Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123(Pt 2):308–17. doi: 10.1093/brain/123.2.308. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Mahad D, Ziabreva I, Campbell G, Lax N, Hanson PS, Lassmann H, et al. Brain. Mitochondrial changes within axons in multiple sclerosis. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- Marburg O. Die sogenannte akute Multiple Sklerose. Jahrbuch Psychiatrie. 1906;27:211–312. [Google Scholar]
- Meinl E, Krumbholz M, Derfuss T, Junker A, Hohlfeld R. Compartmentalization of inflammation in the CNS: A major mechanism driving progressive multiple sclerosis. J Neurol Sci. 2008;274:42–4. doi: 10.1016/j.jns.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol. 2006;59:880–92. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- Molyneux PD, Kappos L, Polman C, Pozzilli C, Barkhof F, Filippi M, et al. The effect of interferon beta-1b treatment on MRI measures of cerebral atrophy in secondary progressive multiple sclerosis. European Study Group on Interferon beta-1b in secondary progressive multiple sclerosis. Brain. 2000;123(Pt 11):2256–63. doi: 10.1093/brain/123.11.2256. [DOI] [PubMed] [Google Scholar]
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol. 1993;143:555–64. [PMC free article] [PubMed] [Google Scholar]
- Shintaku M, Hirano A, Llena JF. Increased diameter of demyelinated axons in chronic multiple sclerosis of the spinal cord. Neuropathol Appl Neurobiol. 1988;14:505–10. doi: 10.1111/j.1365-2990.1988.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Bruck W, Bancher C, Jellinger K, Lassmann H. Alzheimer disease: DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis. J Neuropathol Exp Neurol. 1998;57:456–64. doi: 10.1097/00005072-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–69. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Axonal dysfunction in chronic multiple sclerosis: meltdown in the membrane. Ann Neurol. 2008;63:411–3. doi: 10.1002/ana.21361. [DOI] [PubMed] [Google Scholar]
- Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006;67:960–7. doi: 10.1212/01.wnl.0000237551.26858.39. [DOI] [PubMed] [Google Scholar]
- Young NP, Weinshenker BG, Lucchinetti CF. Acute disseminated encephalomyelitis: current understanding and controversies. Semin Neurol. 2008;28:84–94. doi: 10.1055/s-2007-1019130. [DOI] [PubMed] [Google Scholar]
- Zivadinov R, Cox JL. Neuroimaging in multiple sclerosis. Int Rev Neurobiol. 2007;79:449–74. doi: 10.1016/S0074-7742(07)79020-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.