Abstract
Streptozotocin (STZ), a glucose analogue known to induce diabetes in experimental animals, causes DNA strand breaks and subsequent activation of poly(ADPribose) polymerase (Parp). Because Parp uses NAD as a substrate, extensive DNA damage will result in reduction of cellular NAD level. In fact, STZ induces NAD depletion and cell death in isolated pancreatic islets in vitro. Activation of Parp therefore is thought to play an important role in STZ-induced diabetes. In the present study, we established Parp-deficient (Parp−/−) mice by disrupting Parp exon 1 by using the homologous recombination technique. These mice were used to examine the possible involvement of Parp in STZ-induced β-cell damage in vivo. The wild-type (Parp+/+) mice showed significant increases in blood glucose concentration from 129 mg/dl to 218, 370, 477, and 452 mg/dl on experimental days 1, 7, 21, and 60, respectively, after a single injection of 180 mg STZ/kg body weight. In contrast, the concentration of blood glucose in Parp−/− mice remained normal up to day 7, slightly increased on day 21, but returned to normal levels on day 60. STZ injection caused extensive necrosis in the islets of Parp+/+ mice on day 1, with subsequent progressive islet atrophy and loss of functional β cells from day 7. In contrast, the extent of islet β-cell death and dysfunction was markedly less in Parp−/− mice. Our findings clearly implicate Parp activation in islet β-cell damage and glucose intolerance induced by STZ in vivo.
Keywords: gene targeting, islet β cell, NAD, insulin-dependent diabetes mellitus
Various types of DNA damage produced by many environmental chemicals or reactive oxygen species generated by inflammatory reactions contribute to insulin-dependent diabetes mellitus (IDDM) through the induction of β-cell death in pancreatic islets (1–3). Acute exposure to streptozotocin [2-deoxy-2-(3-methyl-3-nitrosourea)l-d-glucopyranose, STZ] induces massive β-cell death and diabetes mellitus in experimental animals (4, 5). STZ also causes a rapid depletion of cellular NAD in islets (6–9), but this depletion is prevented by injection of nicotinamide immediately before or soon after the administration of STZ (10). Okamoto and colleagues (2, 11) demonstrated that STZ induces DNA strand breaks and activation of poly(ADP-ribose) polymerase (Parp) with subsequent reduction of NAD levels in the isolated pancreatic islets in vitro. These findings suggest the involvement of Parp as a key molecule in STZ-induced β-cell death and diabetes through extensive poly(ADP-ribose) formation and NAD depletion, leading to reduction of ATP level and cell death. In agreement with this hypothesis, Parp inhibitors such as 3-aminobenzamide or nicotinamide prevent the depletion of NAD and induction of STZ-induced β-cell death (12, 13). However, because Parp inhibitors possess other effects, such as scavenging hydrogen peroxide (14), it is not clear whether and how Parp activity contributes to β-cell death and the development of diabetes in vivo. Thus, engineering of a Parp-deficient animal model would be useful for investigating the role of Parp in STZ-induced diabetes. In the present study, we established Parp-deficient (Parp−/−) mice by disrupting Parp exon 1 (15) and compared the sensitivity of these mice to STZ-induced diabetes to that of wild-type (Parp+/+) mice. Our results showed that blood glucose reached a peak level at day 21 after STZ injection and remained elevated through the observation period in Parp+/+ animals. In Parp−/− mice, however, glucose concentration remained at normal levels, except for a mild and transient elevation after STZ administration. The data presented herein indicate that Parp is a major cellular factor responsible for STZ-induced diabetes.
MATERIALS AND METHODS
Establishment of Parp-Deficient Mice.
The establishment of Parp+/− embryonic stem (ES) cells has been described (15, 16). We constructed a targeting vector containing a Parp genomic fragment interrupted in exon 1 by a neomycin resistance gene (neor) cassette with a flanking diphtheria toxin A fragment gene (DT-A) cassette. The construct was electroporated into J1 ES cells (17) and G418-resistant clones were selected. The ES cell clones were confirmed to contain a Parp-disrupted allele by Southern blot analysis using a 5′ and 3′ recombination junction probe (see Fig. 1A). The cell clones were cocultured with ICR mouse embryos (CLEA, Tokyo, Japan) at eight-cell to morula stage, and aggregates that developed to a blastocyst or morula stage then were transferred into the uteri of pseudopregnant ICR mice as described (18). Chimeric mice were identified by their mosaic coat color and eye pigmentation. These chimeras subsequently were mated to ICR mice for germ-line transmission of the Parp replacement. Southern blot analysis of genomic DNA from the tip of the tail was used to identify mice heterozygous for Parp mutation (Parp+/−). Brother-sister mating of Parp+/− mice was carried out to generate both Parp−/− and Parp+/+ mice. All animals were maintained in a room controlled for light/dark (lights on from 5:00 to 19:00), temperature (24°C), and humidity (50 ± 10%). The animals used in this study were treated in accordance with the Guiding Principles for the Care and Use of Research Animals promulgated by Chugai Pharmaceutical, Shizuoka, Japan.
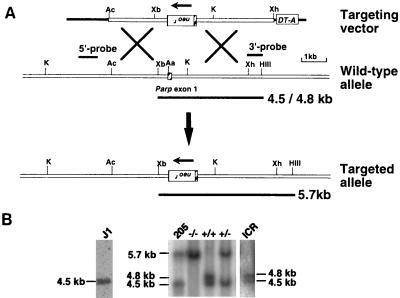
Figure 1.
Gene targeting in mice. (A) The targeting construct of the mouse Parp and predicted structure of the targeted Parp locus. The targeting vector contains a 1.1-kb pMC1-neor-poly(A) cassette inserted in the 6-kb fragment of an AccI–XhoI Parp genomic fragment and a 0.8-kb DT-A fragment at the 3′ terminus. In the targeted allele, the reversely oriented pMC1-neor-poly(A) cassette was inserted into AatI site in exon 1, 10 bases downstream of the first ATG. The length of diagnostic fragments and probes for Southern blot analysis are shown. The targeted allele yielded a 5.7-kb band, and the intact allele yielded a 4.5- or 4.8-kb band, the result of the polymorphism between ICR and 129SvJ. The hatched box represents Parp exon 1. The restriction enzyme sites are shown: Aa, AatI; Ac, AccI; HIII, HindIII; K, KpnI; Xh, XhoI; Xb, XbaI. (B) Southern blot analysis of genomic DNA from offspring derived from Parp+/− mice intercrosses. A representative result is shown. Genomic DNA was isolated from a tail biopsy and digested with HindIII and XbaI, and hybridization was carried out with the 3′ probe (0.5 kb of XhoI–HindIII fragment as shown in A). The 5.7-kb fragment indicates a homologous recombinant allele. Genomic DNAs from J1 and clone 205 ES cells, ICR mice were prepared and used for Southern blot analysis in parallel.
In-Gel Activity and Western Blot Analysis of Parp.
Cell extracts were obtained by homogenizing tissues in an extraction buffer containing 50 mM Tris⋅C1 (pH 8.0), 0.6 M NaCl, 0.1% Triton-X100, 1 mM EDTA, 10 mM NaHSO3, 0.5 mM DTT, 1 mM 3-aminobenzamide, 1 mM phenylmethylsulfonyl fluoride, and 5 μg/ml leupeptin, then rotated at 4°C for 30 min and centrifuged at 108,000 × g at 4°C for 15 min. The resultant supernatant was analyzed for protein concentration by using the Protein Assay Kit (Bio-Rad). In-gel activity analysis of Parp was performed with cell extracts separated by 6% SDS/PAGE, as described (19). Briefly, proteins were renaturated in the gel and incubated with 32P-NAD to measure the auto-poly(ADP-ribosyl)ation activity of Parp. For Western blot analysis, cell extracts were separated by 5–20% gradient SDS/PAGE, and proteins were blotted on Immobilon membrane (Millipore). After blocking with Blockace (Dainippon Pharmaceutical, Osaka, Japan), the membrane was incubated with a polyclonal antibody raised against 99-kDa C-terminal fragment of human Parp recombinant protein at a dilution of 1:200 and subsequently incubated with a horseradish peroxidase-linked protein A (Amersham Pharmacia) and detected with enhanced chemiluminescence reaction (ECL Kit, Amersham Pharmacia).
STZ Treatment of Mice.
Parp+/+ (n = 21) and Parp−/− (n = 22) male mice, 2–3 months of age, received a single i.p. injection of 180 mg STZ (Sigma)/kg body weight. The STZ solution was prepared in 0.9% NaCl after mixing with citric acid buffer (pH 4.5) and injected within 5 min of preparation.
Determination of Blood Glucose Concentration.
Blood samples (20 μl each) were obtained from the caudal veins of fed animals for determination of blood glucose level. The samples were obtained 1 day before STZ treatment and on experimental days 1, 7, 21, and 60 after STZ injection. Blood glucose concentrations were measured with a New Blood Sugar Test Kit (Boehringer Mannheim) by using the protocol provided by the manufacturer. Differences in glucose concentrations between groups were analyzed statistically by using Tukey’s Studentized Range test and Student’s t test, and differences were considered significant when the P value was less than 0.05.
Histological Analysis of Islets of STZ-Treated Mice.
Animals were sacrificed by cervical dislocation at 8 hr and on experimental days 1, 7, 21, and 60 after STZ injection. Pancreatic tissues were resected and fixed with 20% neutral-buffered formalin solution, and then were embedded in paraffin blocks according to the standard procedure, and sectioned at 4 μm thickness and stained with hematoxylin/eosin. After deparaffination, some sections subsequently were treated with Bouin’s fixative, stained for functional pancreatic β cell with Gomori’s aldehyde fuchsin (GAF) (20), and counterstained with fuchsin-ponceau and light green.
RESULTS
Generation of Mice Homozygously Null for the Parp Locus.
Two of four Parp+/− ES clones (205 and 226) contributed to produce chimeric mice. Two and one male chimeras from ES clones 205 and 226, respectively, transmitted the disrupted Parp allele through the germ line. Brother-sister mating of Parp+/− animals produced viable Parp−/− offspring. As shown in Fig. 1B, Southern blot analysis of genomic DNA using the 3′ probe, flanking to the targeted 6-kb region, detected 4.5- and 4.8-kb HindIII/XbaI restriction fragments for Parp+/+ mice. The appearance of two hybridized fragments was caused by the presence of polymorphic Parp alleles in ICR mice. A 5.7-kb fragment was detected for the targeted locus. Parp deficiency was transmitted in a mendelian fashion (12 Parp−/− of 39 offspring). Parp−/− mice, both males and females, were normal in growth and fertile, and litter sizes were similar to those of Parp+/+ mice. Previously, exon 2-disrupted Parp−/− mice with mixed genetic background of C57BL6/129SvJ showed skin hyperplasia and obesity (21), but this result was not seen in exon 4-disrupted Parp−/− mice (22). In the present study, exon 1-disrupted Parp−/− mice had no skin hyperplasia or obesity.
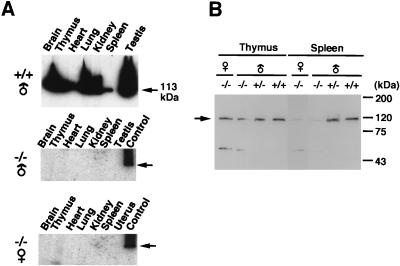
Parp activity was detected in various tissues of Parp+/+ mice, but not in Parp−/− mice as demonstrated by in-gel activity analysis (Fig. 2A). Western blot analysis using a Parp polyclonal antibody detected a crossreactive band at around 113 kDa in tissues of Parp−/− mice (Fig. 2B). This protein is either a mutated form of Parp or some other protein that crossreacted to the antibody. The mutated form of Parp would be translated from a downstream start codon within nucleotide positions 109–114, numbered from the first ATG in Parp cDNA, containing the Kozak consensus sequence (23). The truncated form of Parp, being shorter by only 35 aa, would be indistinguishable from intact Parp by SDS/PAGE. The truncated form of Parp would be inactive, because it lacks a critical N-terminal zinc finger (24).
Figure 2.
Parp activity and protein level. (A) In-gel activity analysis of Parp. Mouse tissue extracts containing 100 μg of protein were applied onto 6% SDS polyacrylamide gel containing 100 μg/ml of sonicated calf thymus DNA. After electrophoresis and renaturation of proteins in the gel, the gel was incubated with 32P-NAD containing reaction mixture, and the incorporated radioactivity was detected by an image analyzer. Brain extract from Parp+/+ male mice was applied as a positive control (right lanes) for Parp−/− male (Middle) and female (Bottom) mice. The bands at 113-kDa Parp (indicated by arrows) were observed with tailing, which probably is caused by various levels of automodification on Parp. (B) Western blot analysis of Parp (an arrow) with anti-Parp polyclonal antibody.
Resistance of Parp−/− Mice to STZ-Induced Hyperglycemia.
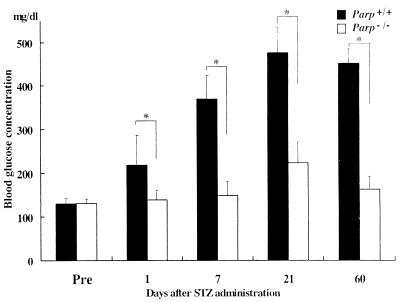
A diabetogenic dose of 180 mg STZ/kg body weight was administered into Parp+/+ and Parp−/− mice, and their blood glucose concentrations were measured. As shown in Fig. 3, there was a gradual increase in blood glucose concentration in Parp+/+ mice at day 1 after injection to day 21, and the values were significantly higher than those of untreated control (P < 0.01). Furthermore, high levels of blood glucose were persistently observed at day 60 in Parp+/+ mice. Parp−/− mice did not develop such hyperglycemia during the first 7 days of observation. On day 21, a transient 1.7-fold increase in blood glucose was noted in Parp−/− mice, relative to untreated control mice (P < 0.01), although blood glucose returned to the control level on day 60. Urinalysis on day 60 showed glycosuria in Parp+/+ mice, whereas no such change was observed in Parp−/− mice (data not shown).
Figure 3.
Changes in blood glucose concentrations in Parp+/+ and Parp−/− mice after STZ treatment. Filled and empty bars indicate, respectively, the mean ± SD concentrations of blood glucose of Parp+/+ and Parp−/− mice. ∗, P < 0.01. Pre, untreated mice (control) 1 day before STZ injection. Blood glucose concentrations on days 1, 7, 21, and 60 were significantly higher in Parp+/+ mice than the untreated control (P < 0.01), whereas in Parp−/− mice, a significant elevation was observed only on day 21 (P < 0.01).
Parp Deficiency Prevents β-Cell Disruption.
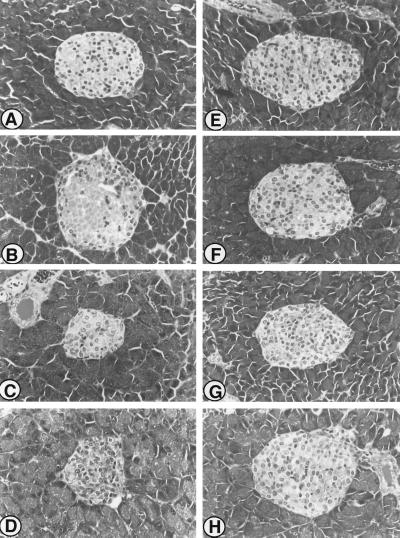
Histopathological examination of the islets in STZ-treated Parp+/+ mice showed extensive changes, including β-cell death and atrophy, during the 60-day observation period. As shown in Fig. 4, a massive β-cell death in the islets of Parp+/+ mice was evident even on day 1 (Fig. 4B). Furthermore, the majority of β cells contained pyknotic nuclei with eosinophilic cytoplasm at 8 hr after STZ injection. Atrophy of the islets frequently was observed in Parp+/+ mice from day 7 and throughout the remainder of the 60-day observation period (Fig. 4 C and D). In contrast to Parp+/+ mice, islet cells of Parp−/− mice were near normal after STZ treatment (Fig. 4 F–H). β-Cell death was observed only occasionally between 8 hr and 60 days after STZ injection, and atrophy was not detected during the observation period. As shown in Fig. 5, the extent of β-cell damage was further estimated by specific staining for functional β cells with GAF (19). In both untreated Parp+/+ and Parp−/− mice, GAF-positive cells were present throughout the islets (Fig. 5 A and D). However, STZ caused a marked reduction in GAF-positive cells in the islets of Parp+/+ mice from day 1 (Fig. 5B) and only a few GAF-positive cells were observed in the islets of Parp+/+ animals on day 60 (Fig. 5C). In contrast, the number of GAF-positive cells in the islets of Parp−/− mice on day 7 was only slightly reduced, relative to baseline. Furthermore, more than half of islet cells were positive for GAF staining throughout the 60-day observation period (Fig. 5 E and F). These histopathological changes in Parp+/+ and Parp−/− mice paralleled changes in blood glucose concentrations after injection of STZ.
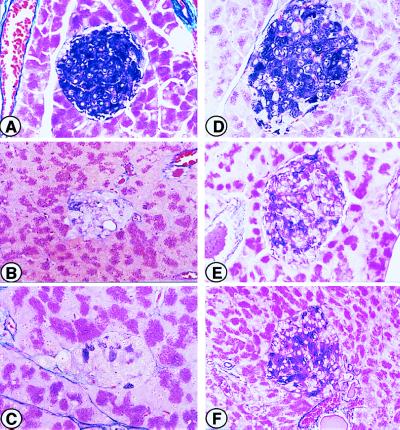
Figure 4.
Light microscopic examination of pancreatic islets of Parp+/+ and Parp−/− mice stained with hematoxylin/eosin. (A) Pancreatic islet of a representative untreated control Parp+/+ mice. (B) Pancreatic islet of Parp+/+ animal sacrificed on day 1 after STZ injection. Note the presence of dead β cells with distinct eosinophilic cytoplasm and loss of nuclei in the center of islet. (C) Pancreatic islet of Parp+/+ mice sacrificed on day 7. Note the well-established atrophy of the islet, together with increased cellularity and pleomorphic nuclei. (D) Pancreatic islet of Parp+/+ mice sacrificed on day 21 after STZ injection. (E) Pancreatic islet of untreated control Parp−/−. Morphological abnormality caused by Parp deficiency is not seen. (F–H) Pancreatic islet of Parp−/− mice sacrificed on day 1 (F), day 7 (G), and day 21 (H) after STZ injection. Note that the islet cells remain nearly normal, though apoptotic cells and increased cellularity occasionally are seen. Atrophy of the pancreatic islets is not seen in Parp−/− animals (F–H). Magnification: ×120.
Figure 5.
Light microscopic examination of typical pancreatic islets of Parp+/+ and Parp−/− mice stained with GAF as described in Materials and Methods. β cells are stained dark purple. More than 80% of the area of the islet is positive for GAF in both untreated Parp+/+ (A) and Parp−/− (D) mice. Note that GAF-positive cells were markedly reduced in Parp+/+ mice at day 7 after injection of 180 mg STZ/kg (B), whereas about 60% of the area in the islet is positive for GAF in Parp−/− mice (E). On day 60 after STZ injection, less than 20% of the area of the islet is stained with GAF in Parp+/+ mice (C). In contrast, GAF-positive cells constitute about 80% of the area in the islet on day 60 in Parp−/− animals (F). Magnification: ×120.
DISCUSSION
We have demonstrated in the present study the establishment of Parp-deficient mice by disrupting Parp exon 1. These mice were less sensitive to STZ-induced pancreatic β-cell death and consequently demonstrated resistance to STZ-induced diabetes. Our results indicate that Parp is one of the major factors involved in STZ-induced diabetes. They support the previous model proposed by Okamoto (2) that poly(ADP-ribose) formation induced by extensive DNA damage causes β-cell dysfunction and death. Our data are also in agreement with the previous observation that inhibition of Parp activity by 3-aminobenzamide or nicotinamide leads to the prevention of β-cell death and development of diabetes (6, 7). STZ-treated Parp−/− mice showed a delayed onset of mild but transient hyperglycemia. Because exposure of isolated islets to STZ in vitro also results in a decrease of glucose oxidation to CO2 (9) and insulin synthesis (25), in addition to the direct DNA damage, these effects may contribute to the delayed and slight β-cell dysfunction also seen in Parp−/− mice.
Blood insulin levels (data not shown) and glucose concentrations were similar in untreated Parp−/− and Parp+/+ mice. Thus, it seems that the basal function of the pancreas in Parp−/− mice in the absence of DNA damages is similar to that of Parp+/+ mice. A lack of Parp-dependent DNA damage response is likely to be responsible for the resistance of Parp−/− mice to diabetes. Because STZ causes a rapid depletion of NAD in pancreatic islets, it is considered that a substantial NAD depletion through massive poly(ADP-ribose) formation is prevented in Parp−/− mice.
IDDM is caused by destruction of insulin-secreting β cells of the islets. Genetic studies have indicated that several genes, including those in the HLA regions, could be implicated in IDDM as genetic susceptibility factors (1). However, the majority of patients with these susceptible genes do not develop IDDM, and epidemiological studies have shown that nongenetic factors are also important (1). It is likely that environmental factors such as viral infection and exposure to chemicals that induce chronic immune disorders leading to β-cell death may determine the development of IDDM. Our data indicate that Parp inhibitors might be clinically useful in preventing the development of IDDM in high-risk groups, such as individuals carrying susceptible genes for IDDM or with chronic inflammation in the pancreas like autoimmune disease.
Acknowledgments
We thank S. Uchida, T. Iwata, E. Arai, and M. Kinoshita for technical assistance. We also thank R. Dashwood for the careful reading of the manuscript. This work was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health and Welfare, a Grant-in-Aid from the Sankyo Foundation for Life Science, a Research Grant of the Princess Takamatsu Cancer Research Fund (97-22909) and a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture of Japan (08280102).
ABBREVIATIONS
- ES
embryonic stem
- GAF
Gomori’s aldehyde fuchsin
- IDDM
insulin-dependent diabetes mellitus
- Parp
poly(ADP-ribose) polymerase
- STZ
streptozotocin
References
- 1.Leslie R D G, Elliott R B. Diabetes. 1994;43:843–850. doi: 10.2337/diab.43.7.843. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto H. Mol Cell Biochem. 1981;37:43–61. doi: 10.1007/BF02355886. [DOI] [PubMed] [Google Scholar]
- 3.Eisenbarth G S. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 4.Rakieten N, Rakieten M L, Nadkarni M V. Cancer Chemother Rep. 1963;29:91–98. [PubMed] [Google Scholar]
- 5.Dunn J S, McLetchie N G B. Lancet. 1943;2:384–387. [Google Scholar]
- 6.Hinz M, Katsilambros N, Majer V, Schatz H, Pfeiffer E F. FEBS Lett. 1973;30:225–228. doi: 10.1016/0014-5793(73)80656-8. [DOI] [PubMed] [Google Scholar]
- 7.Ho C-K, Hashim S A. Diabetes. 1972;21:789–793. doi: 10.2337/diab.21.7.789. [DOI] [PubMed] [Google Scholar]
- 8.Shein P S, Cooney D A, McMenamin M G, Anderson T. Biochem Pharmacol. 1973;22:2625–2631. doi: 10.1016/0006-2952(73)90071-3. [DOI] [PubMed] [Google Scholar]
- 9.Gunnarsson R, Berne C, Hellerström C. Biochem J. 1974;140:487–494. doi: 10.1042/bj1400487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shein P S, Cooney D A, Vernon M L. Cancer Res. 1967;27:2324–2332. [PubMed] [Google Scholar]
- 11.Yamamoto H, Uchigata Y, Okamoto H. Nature (London) 1981;294:284–286. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto H, Okamoto H. Biochem Biophys Res Commun. 1980;95:474–481. doi: 10.1016/0006-291x(80)90762-7. [DOI] [PubMed] [Google Scholar]
- 13.Uchigata Y, Yamamoto H, Kawamura A, Okamoto H. J Biol Chem. 1982;257:6084–6088. [PubMed] [Google Scholar]
- 14.Szabó C, Virág L, Cuzzocrea S, Scott G S, Hake P, O’Connor M P, Zingarelli B, Salzman A, Kun E. Proc Natl Acad Sci USA. 1998;95:3867–3872. doi: 10.1073/pnas.95.7.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masutani, M., Nozaki, T., Nishiyama, E., Shimokawa, T., Tachi, Y., Suzuki, H., Nakagama, H., Wakabayashi, K. & Sugimura, T. (1999) Mol. Cell. Biochem., in press. [PubMed]
- 16.Masutani M, Nozaki T, Nishiyama E, Ochiya T, Nakagama H, Wakabayashi K, Suzuki H, Sugimura T. Proc Japan Acad Ser B. 1998;74:233–236. [Google Scholar]
- 17.Li E, Bestor T H, JaeNisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Kamada N, Ueda O, Jishage K, Kurihara H, Kurihara Y, Kodama T, Yazaki Y, Azuma S, Toyoda Y. J Reprod Dev. 1994;40:361–365. doi: 10.1538/expanim.46.17. [DOI] [PubMed] [Google Scholar]
- 19.Masutani M, Nozaki T, Hitomi Y, Ikejima M, Nagasaki K, de Prati A C, Kurata S, Natori S, Sugimura T, Esumi H. Eur J Biochem. 1994;220:607–614. doi: 10.1111/j.1432-1033.1994.tb18662.x. [DOI] [PubMed] [Google Scholar]
- 20.Schweisthal M R, Frost C C, Brinn J E. Stain Technol. 1975;50:161–170. doi: 10.3109/10520297509117053. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z-Q, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M, Wagner E F. Genes Dev. 1995;9:509–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- 22.de Murcia J M, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver F J, Masson M, Dierich A, LeMeur M, et al. Proc Natl Acad Sci USA. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozak M. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikejima M, Noguchi S, Yamashita R, Ogura T, Sugimura T, Gill D M, Miwa M. J Biol Chem. 1990;265:21907–21913. [PubMed] [Google Scholar]
- 25.Maldonato A, Trueheart P A, Renold A E, Sharp G W G. Diabetologia. 1976;12:471–481. doi: 10.1007/BF01219511. [DOI] [PubMed] [Google Scholar]