Abstract
Human African trypanosomiasis, or sleeping sickness, is caused by the protozoan parasites Trypanosoma brucei rhodesiense or Trypanosoma brucei gambiense, and is a major cause of systemic and neurological disability throughout sub-Saharan Africa. Following early-stage disease, the trypanosomes cross the blood–brain barrier to invade the central nervous system leading to the encephalitic, or late stage, infection. Treatment of human African trypanosomiasis currently relies on a limited number of highly toxic drugs, but untreated, is invariably fatal. Melarsoprol, a trivalent arsenical, is the only drug that can be used to cure both forms of the infection once the central nervous system has become involved, but unfortunately, this drug induces an extremely severe post-treatment reactive encephalopathy (PTRE) in up to 10% of treated patients, half of whom die from this complication. Since it is unlikely that any new and less toxic drug will be developed for treatment of human African trypanosomiasis in the near future, increasing attention is now being focussed on the potential use of existing compounds, either alone or in combination chemotherapy, for improved efficacy and safety. The kynurenine pathway is the major pathway in the metabolism of tryptophan. A number of the catabolites produced along this pathway show neurotoxic or neuroprotective activities, and their role in the generation of central nervous system inflammation is well documented. In the current study, Ro-61-8048, a high affinity kynurenine-3-monooxygenase inhibitor, was used to determine the effect of manipulating the kynurenine pathway in a highly reproducible mouse model of human African trypanosomiasis. It was found that Ro-61-8048 treatment had no significant effect (P = 0.4445) on the severity of the neuroinflammatory pathology in mice during the early central nervous system stage of the disease when only a low level of inflammation was present. However, a significant (P = 0.0284) reduction in the severity of the neuroinflammatory response was detected when the inhibitor was administered in animals exhibiting the more severe, late central nervous system stage, of the infection. In vitro assays showed that Ro-61-8048 had no direct effect on trypanosome proliferation suggesting that the anti-inflammatory action is due to a direct effect of the inhibitor on the host cells and not a secondary response to parasite destruction. These findings demonstrate that kynurenine pathway catabolites are involved in the generation of the more severe inflammatory reaction associated with the late central nervous system stages of the disease and suggest that Ro-61-8048 or a similar drug may prove to be beneficial in preventing or ameliorating the PTRE when administered as an adjunct to conventional trypanocidal chemotherapy.
Keywords: trypanosomiasis, brain, kynurenine pathway, mice, Ro-61-8048
Introduction
Human African trypanosomiasis (HAT), or sleeping sickness, is a major threat to the health of 60 million people inhabiting 36 countries in sub-Saharan Africa (WHO, 1998). The disease is caused by infection with the protozoan parasites Trypanosoma brucei gambiense (T. b. gambiense) or T. b. rhodesiense, causing West African and East African sleeping sickness respectively (Kennedy, 2004). Both forms of the disease are invariably fatal if not effectively treated. HAT develops in two stages, the early, or haemolymphatic, stage when the parasites multiply and spread in the blood and lymph nodes, followed by the late, or encephalitic, stage when the parasites become established within the central nervous system (CNS). Neurological features of late-stage HAT include the characteristic sleep disorder with alteration of sleep structure, a variety of neuropsychiatric symptoms, both motor and sensory disturbances, and progressive cerebral oedema, incontinence and coma leading to death (Kennedy, 2008) Early-stage infections are treated with either pentamidine (for T. b. gambiense) or suramin (for T. b. rhodesiense). However, the only drug that is effective against both forms of the disease once the CNS has become involved is the trivalent arsenical melarsoprol (Kennedy, 2004). Unfortunately, melarsoprol is highly toxic and treatment can result in the development of an extremely severe post-treatment reactive encephalopathy (PTRE) in about 10% of patients with a 50% mortality rate (Pepin and Milord, 1991, 1994). The PTRE is characterized neuropathologically by the development of a meningoencephalitis with diffuse astrocytosis and the presence of macrophages, lymphocytes and plasma cells in cerebral white matter as well as perivascular cuffing (Adams et al., 1986; Kennedy, 2006). Despite the unacceptable toxicity of current drugs for late-stage HAT, it is highly unlikely that any new and effective drugs will become available for use within the next 5 years (Kennedy, 2008). Therefore, the prospects for improved safety and efficacy are more likely to come from combination chemotherapy approaches utilizing contemporary trypanocidal drugs with the inclusion of existing or newly developed compounds as adjuncts to help prevent the adverse side-effects of the trypanocidal treatment.
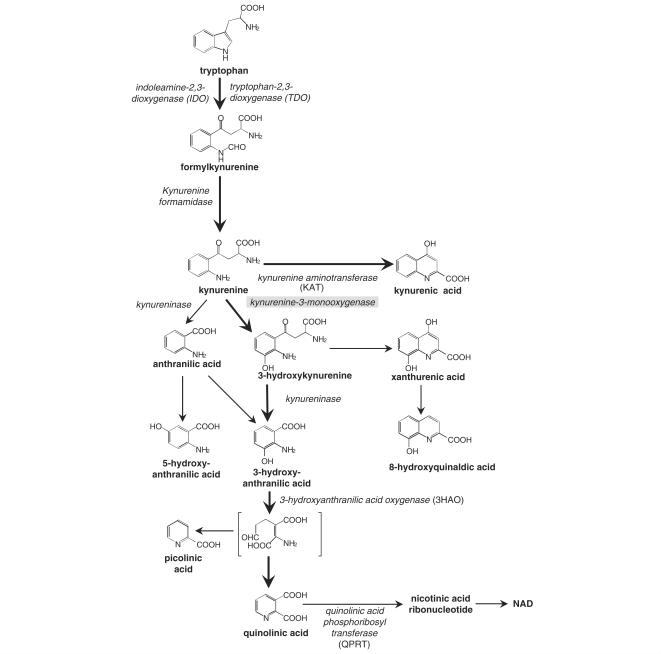
One major metabolic pathway which has been little explored in relation to trypanosomiasis is the oxidative conversion of tryptophan to kynurenine compounds (Fig. 1). In most tissues this pathway accounts for the majority of non-protein tryptophan metabolism, and in the CNS several components of the pathway have significant effects on neuronal activity. Quinolinic acid can activate a subpopulation of neuronal glutamate receptors specifically sensitive to N-methyl-d-aspartate (NMDA) producing depolarization (Stone and Perkins, 1981; Stone, 2001) and excitotoxicity (Schwarcz et al., 1983), while kynurenic acid is an antagonist at several subtypes of glutamate receptor (Perkins and Stone, 1982; Stone and Darlington, 2002). In addition, the pathway includes 3-hydroxykynurenine and 3-hydroxyanthranilic acid, both of which are redox active and able to produce neuronal damage via the generation of reactive oxygen species (Eastman and Guilarte, 1989, 1990; Okuda et al., 1996, 1998; Guidetti and Schwarcz, 1999; Giles et al., 2003). The kynurenine pathway is also an important regulator in both the innate and adaptive immune response (Gonzalez et al., 2008).
Figure 1.
A schematic representation illustrating the main components in the kynurenine pathway of tryptophan metabolism. The enzymes (italics) and catabolites present along the pathway are shown. Ro-61-8048 inhibits the enzyme KMO (grey highlight).
The first and rate limiting enzymes in the kynurenine pathway are; tryptophan-2,3-dioxygenase (TDO) within the liver, and indoleamine-2,3-dioxygenase (IDO), which is widely expressed in a wide range of cell types including dendritic cells, macrophages, monocytes, and T-cells in the immune system. The expression of IDO can be induced by cytokines or the presence of bacterial lipopolysaccharides leading to a well documented immunosuppressive effect (Gonzalez et al., 2008). The activation of the pathway by infection, with the generation of neuroactive products, raises the possibility that it could play a role in the development of the neuroinflammatory response associated with trypanosome infection and drug treatment. The current study arose out of our interest in identifying novel pathways that might be important in CNS HAT, and their potential for identifying possible inhibitors to ameliorate CNS disease. In this study we have used a highly reproducible mouse model of early CNS and late CNS stage HAT in conjunction with Ro-61-8048, a kynurenine-3-monooxygenase (KMO) inhibitor (Fig. 1), to investigate the effect of manipulating the production of kynurenine pathway catabolites on the neural consequences of trypanosome infection.
Materials and Methods
Inhibitor
The high affinity KMO inhibitor 3,4-dimethoxy-N-[4-(3-nitrophenyl) thiazol-2-yl]benzenesulfonamide (Ro-61-8048; synthesized by F. Hoffmann-La Roche Ltd.) was dissolved in DMSO and diluted in sterile 0.9% saline to a concentration of 10 mg/ml and the pH adjusted to 7.5.
Animals, infections and treatments
Female CD1 mice were infected by intraperitoneal injection (i.p.) with 1 × 104 T. b. brucei parasites of cloned stabilate GVR35/C1.9. The infection was allowed to progress naturally until Day 12 post-infection. At this point the animals were divided into six groups of six mice. Groups 1–3 were employed to study the early CNS response while the late CNS stage was investigated in Groups 4–6. Group 1 was treated with Ro-61-8048 at a dose rate of 100 mg/kg (i.p.) every second day from Day 12 until the end point of the experiment on Day 28 post-infection. Group 2 was treated at the same time points with vehicle (0.9% NaCl–NaOH pH 7.5) only, whereas the infection was allowed to progress with no intervention in Group 3. Group 4 mice were given Ro-61-8048 in an identical manner to Group 1 animals but, on Day 21 post-infection, a time when the parasites are established within the CNS, the mice were given diminazene aceturate (Berenil®; Hoechst) 40 mg/kg i.p. This treatment is subcurative when administered during the CNS stage of the disease and induces a severe neuroinflammatory reaction in the mice. Group 5 was treated with vehicle and diminazene aceturate while Group 6 received diminazene aceturate only. Control groups comprising uninfected inhibitor treated and uninfected inhibitor and diminazene aceturate treated animals were run in parallel with the infected groups. A schematic representation of these treatment regimens is detailed in Fig. 2. Parasitaemia was monitored throughout the experiment in all infected groups of mice by microscopic examination of fresh blood smears. At Day 28 post-infection the mice were killed, the brain excised, fixed in 4% neutral buffered formalin and paraffin wax processed for histological analyses of H&E stained sections.
Figure 2.
Schematic representation of the treatment regimens used to investigate the effects of Ro-61-8048 (Ro) or vehicle (V) administration in T. b. brucei infected (I) mice during the early CNS stage of the infection and in animals treated with diminazene aceturate (D) to induce the late CNS stage of the disease. Uninfected (U) animals were included as controls. The number of days post-infection is indicated below the regimens. All mice were killed on Day 28 post-infection.
All animal procedures were authorized under the Animals (Scientific Procedures) Act 1986 and approved by the University of Glasgow Ethical Review Committee.
Neuropathological grading
The severity of the inflammatory reaction in each group of mice was assessed using a neuropathological grading scale implemented in previous studies (Kennedy et al., 1997). Briefly, slides were examined by two independent assessors in a blinded fashion. The severity of the reaction was graded on a scale of 0 to 4 where 0 represents a normal brain with no sign of pathological change, Grade 1 shows a mild meningitis, Grade 2 demonstrates a moderate meningitis with some degree of perivascular cuffing, Grade 3 shows a more severe meningitis with cuffing and some infiltration of the neuropil by inflammatory cells and Grade 4 is characterized by a severe meningoencephalitis with the presence of inflammatory cells in the brain parenchyma.
In vitro experiments
Bloodstream form T. brucei brucei (strain 427) were cultivated in HMI-9 medium (BioSera Ltd., UK) (Hirumi and Hirumi, 1989) supplemented with 2 mM β-mercaptoethanol (Sigma-Aldrich, UK) and 10% foetal calf serum (BioSera Ltd., UK) at 37°C in a humidified 5% CO2 environment. Trypanotoxicity was determined using an adapted version of the Alamar Blue assay (Raz et al., 1997). Cells (100 µl of 1 × 104 cells/ml) were added to wells of 96-well plates containing doubling dilutions of Ro-61-8048 (100 µl) ranging in concentration from 50 μM to 12 pM and incubated for 48 h. Alamar Blue reagent (20 µl, 0.49 mM in PBS, pH 7.4; Sigma-Aldrich, UK) was added to each well. After 24 h, fluorescence was measured using a LS 55 luminescence spectrophotometer (PerkinElmer Life and Analytical Sciences, USA) set at excitation and emission wavelengths of 530 and 590 nm, respectively. Data were analysed and IC50 values determined with Prism 5.0 (GraphPad Software, USA) software. The assay was repeated using a known trypanocidal drug, pentamidine, to act as a control. The experiment was performed in duplicate on three independent occasions.
Statistical analyses
Comparisons between groups of animals were carried out to assess the severity of the neuropathological reaction. The scores were based on an average of the independent assessor grades measured as described above. To confirm that the assessors graded the response in a uniform manner a Wilcoxon signed rank test was employed. Significant differences at the 5% level were detected using ANOVA and Tukey's multiple range test. Means, standard errors (SE) and 95% CI's are given as summary statistics.
Results
Early CNS stage
In our HAT model, mice infected with T. b. brucei normally survive to ∼35 days post-infection without drug intervention. All infected mice in the groups used to investigate the early CNS reaction remained parasitaemic throughout the experimental procedure with trypanosomes demonstrable within the brain sections (Fig. 3). All animals in the Ro-61-8048 treated group survived until the end point of the experiment, however, two mice died before Day 28 post-infection from both the infected untreated and the infected vehicle treated groups. Analysis of the neuropathology scores (Table 1) from the mice exhibiting the early CNS stage of the infection showed that treatment with Ro-61-8048 failed to reduce the neuropathological reaction [mean ± SE (1.000 ± 0.214)] significantly compared with either the non-treated (P = 0.4445; 1.5 ± 0.204) or the vehicle treated (P = 0.773, 1.313 ± 0.344) groups. The uninfected animals showed no pathological changes (0.00 ± 0.00) and this score was significantly lower than that seen in the infected untreated or the infected vehicle treated group (P = 0.0087, P = 0.0211, respectively). This difference was not reflected when the uninfected animals were compared with the infected mice treated with Ro-61-8048 (P = 0.0626) (Fig. 4).
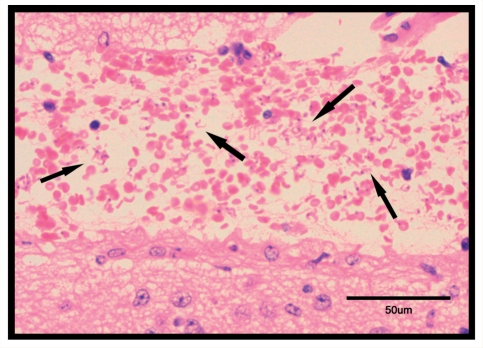
Figure 3.
H&E stained section through the vascularized space situated between the hippocampus and the thalamus adjacent to the third ventricle, taken from a T. b. brucei infected mouse killed on Day 28 post-infection following treatment with Ro-61-8048. Note the presence of high numbers of trypanosomes (→) throughout the area occupied by the red blood cells.
Table 1.
Assessment of the influence of Ro-61-8048 treatment on the severity of the early CNS stage neuropathological response
| I + Ro | I | I + V | U + Ro | |
|---|---|---|---|---|
| I | P=0.4445 (−0.454, 1.454) | |||
| I + V | P=0.7731 (−0.641, 1.266) | P=0.9511 (−1.232, 0.858) | ||
| U + Ro | P=0.0626 (−2.045, 0.045) | P=0.0087 (0.371, 2.629) | P=0.0211 (0.184, 2.441) | |
| Mean±SE | 1.000±0.214 | 1.500±0.204 | 1.313±0.344 | 0.000±0.000 |
| Number | 6 | 4 | 4 | 3 |
The neuropathology scores were measured in mice infected (I) with T. b. brucei and treated with Ro-61-8048 (Ro) or vehicle (V). Uninfected (U), Ro-61-8048 treated mice were assessed in parallel with the infected animals. The mean score and standard error (Mean ± SE) together with the number of animals in each group are detailed. The figures in the body of the table demonstrate the comparisons, in terms of statistical significance, between the groups shown in the row and column headings. The 95% CIs for the differences between the group means are given along with the P-values.
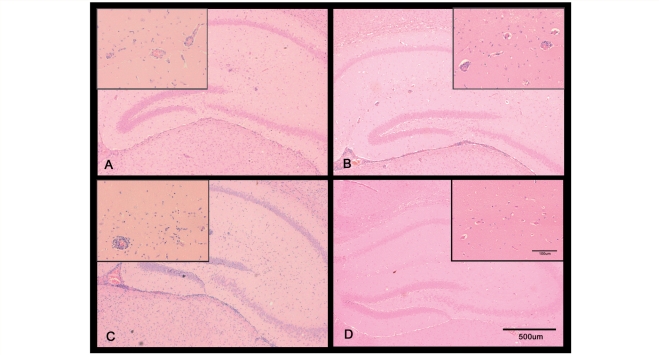
Figure 4.
Coronal sections through the hippocampal brain region of T. b. brucei-infected mice exhibiting the early CNS stage of the disease. The animals were treated with Ro-61-8048 (100 mg/kg i.p.) (A) untreated animals (B) or vehicle only (C). Uninfected mice treated with Ro-61-8048 (D) are included as a control. All treatments were administered every second day beginning on Day 12 post-infection until the end point of the experiment on Day 28 post-infection. Note the presence of mild inflammation in sections A–C with a few inflammatory cells present around the blood vessels. No inflammatory changes are apparent in the uninfected animals (D).
Late CNS stage
Parasites were detected in all animals used to investigate the late CNS stage response prior to administration of trypanocide on Day 21 post-infection. This treatment is subcurative and although it clears the trypanosomes from the blood stream parasites within the CNS are protected from the drug since it cannot cross the blood–brain barrier efficiently (Jennings et al., 1979; Sternberg et al., 2005). All animals in the infected diminazene aceturate, Ro-61-8048 treated and the infected diminazene aceturate treated groups reached the end point in the experiment, however, two animals died in the infected diminazene aceturate treated group receiving vehicle.
When the effect of Ro-61-8048 on the development of CNS inflammation in these animals was investigated (Table 2) a significant reduction in the severity of the neuroinflammation (2.792 ± 0.100) was detected compared with infected animals treated with diminazene aceturate alone (P = 0.0284, 3.458 ± 0.187) and those given diminazene aceturate and vehicle (P = 0.0385, 3.500 ± 0.228). All infected groups of animals showed a significantly (P < 0.000) higher neuropathology score than that of the uninfected mice treated with Ro-61-8048 and diminazene aceturate (0.000 ± 0.000) (Fig. 5).
Table 2.
Assessment of the influence of Ro-61-8048 treatment on the severity of the late CNS stage neuropathological response
| I − D + Ro | I − D | I − D + V | U − D + Ro | |
|---|---|---|---|---|
| I – D | P=0.0284 (0.062, 1.272) | |||
| I – D + V | P=0.0385 (0.032, 1.385) | P=0.9979 (−0.635, 0.718) | ||
| U − D + Ro | P<0.0001 (−3.533, −2.051) | P<0.0001 (2.717, 4.199) | P<0.0001 (2.700, 4.300) | |
| Mean±SE | 2.792±0.100 | 3.458±0.187 | 3.500±0.228 | 0.000±0.000 |
| Number | 6 | 6 | 4 | 3 |
The neuropathology scores were measured in mice infected (I) with T. b. brucei and treated with Ro-61-8048 (Ro) or vehicle (V). Uninfected (U), Ro-61-8048 treated mice were assessed in parallel with the infected animals. All animals were treated with diminazene aceturate (D) on Day 21 post-infection to induce a late-stage response. The mean score and standard error (Mean ± SE) together with the number of animals in each group are detailed. The figures in the body of the table demonstrate the comparisons, in terms of statistical significance, between the groups shown in the row and column headings. The 95% CIs for the differences between the group means are given along with the P-values.
Figure 5.
Coronal sections through the hippocampal brain region of T. b. brucei-infected mice exhibiting the late CNS stage of the disease. The animals were treated with Ro-61-8048 (100 mg/kg i.p.) (A) untreated animals (B) or vehicle only (C). Uninfected mice treated with Ro-61-8048 (D) are included as a control. All treatments were administered every second day beginning on Day 12 post-infection until the end-point of the experiment on Day 28 post-infection. All mice were treated with diminazene aceturate (40 mg/kg i.p.) on Day 21 post-infection to exacerbate the inflammatory reaction. Note the reduced numbers of inflammatory cells present around the blood vessels and within the ventricle in infected animals that were treated with Ro-61-8048 (A) compared to animals that were not given the inhibitor (B, C). No inflammatory changes are apparent in the uninfected animals (D).
In vitro experiments
In vitro experiments to investigate the effects of Ro-61-8048 on T. b. brucei (427) showed that addition of the inhibitor to the culture medium in concentrations ranging from 50 μM to 12 pM had no demonstrable effect of trypanosome growth in culture. When the control, pentamidine, was added to the medium trypanosome growth was rapidly inhibited with the trypanocidal drug showing an IC50 of 0.2 nM and confirming the validity of the negative result gained from inclusion of the inhibitor (Fig. 6).
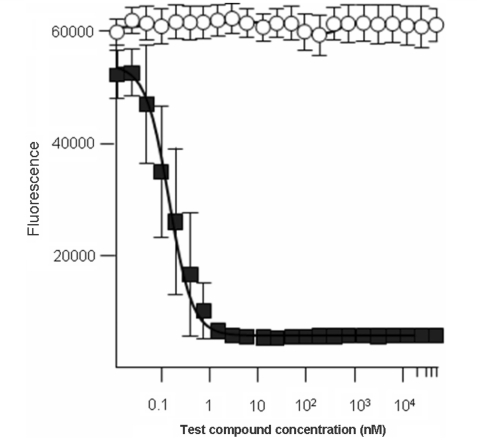
Figure 6.
Trypanosoma brucei (427) bloodstream form trypanosomes were treated in vitro with Ro-61-8048 (open circle) or pentamidine control (closed square) and the fluorescence emission measured using the Alamar blue test. Ro-61-8048 appeared to have no influence on trypanosome growth and proliferation while pentamidine showed an IC50 of 0.2 nM.
Discussion
Inhibition of KMO diverts the kynurenine pathway towards the production of neuroprotective kynurenic acid and inhibits the production of catabolites with neurotoxic properties such as 3-hydroxykynurenine, 3-hydroxyanthranilic acid and quinolinic acid. Manipulation of the pathway in this manner should provide a more neuroprotective environment, reducing neurotoxicity and inflammation and producing an overall beneficial effect.
We have shown that inhibition of KMO in a murine model of HAT results in an amelioration of CNS inflammation that is highly dependent on the stage of the infection. When Ro-61-8048 was administered in mice which had been treated to induce the early CNS stage of the disease, and which therefore exhibited only a limited degree of CNS inflammation, no reduction in the severity of the neuroinflammatory response was detected. However, when animals were treated sub-curatively on Day 21 post-infection with diminazene aceturate to induce pathology mirroring the late CNS stage of the disease, a significant reduction in the CNS inflammatory reaction was apparent.
It is well documented that activation of the kynurenine pathway, specifically induction of indolamine-2,3-dioxygenase (IDO) expression, can lead to suppression of the immune response and this topic has been recently reviewed by Gonzalez et al. (2008). In brief, the ‘tryptophan depletion theory’ suggests that the upregulation of IDO that occurs in immune cells following stimulation with IFN-γ or lipopolysaccharides leads to a depletion of the essential amino acid tryptophan which is required for protein synthesis and cell division. This lack of tryptophan ‘starves’ any rapidly dividing pathogens of an essential amino acid consequently inhibiting their growth and proliferation. Evidence to support this theory has been found by many researchers mainly through in vitro studies. Pfefferkorn (1984) has shown that IFN-γ mediated IDO induction leads to reduced tryptophan concentrations in the culture medium that correlate with inhibition of Toxoplasma gondii growth (Pfefferkorn, 1984) and a similar result was found when staphyloccocal infections of vascular endothelial cell cultures were investigated (Schroten et al., 2001). However data from animal disease models investigating either pathogen proliferation or tryptophan levels following infection are limited. Studies using a murine model of cerebral malaria suggest that factors other than tryptophan depletion play a role in CNS protection following IDO induction. These investigations showed that Plasmodium berghei ANKA infection, strongly induced IDO expression in the brain leading to reduced tryptophan levels. However, it is likely that the reduced tryptophan levels were not a major limitation on the parasite growth since, when IDO expression was inhibited by dexamethasone treatment, no concomitant increase in parasitaemia was detected (Sanni et al., 1998). Furthermore, in vitro studies have shown that activated immune cells can be selectively inhibited by IDO catabolites and that this inhibition is enhanced following tryptophan depletion but not dependent upon it (Frumento et al., 2002; Terness et al., 2002). Therefore, a second hypothesis, the ‘tryptophan utilization theory’, was devised which takes into account the pharmacological actions of kynurenine pathway metabolites in the control of immune reactions in addition to the biostatic effect of tryptophan depletion (Moffett and Namboodiri, 2003). Alterations in the generation of tryptophan catabolites by Ro-61-8048 have been demonstrated previously in a murine model of cerebral malaria (Clark et al., 2005). Following Ro-61-8048 treatment dramatic increases in the levels of both kynurenic acid and anthranilic acid were detected in the brains of both control mice and animals infected with P. berghei ANKA. Anthranilic acid (Jhamandas et al., 1990) and kynurenic acid (Perkins and Stone, 1982; Stone and Darlington, 2002) have both been shown to act as antagonists to the neurotoxic actions of quinolinic acid and could thereby reduce the excitotoxicity of endogenous quinolinic acid or glutamate, resulting in the prolonged survival found in inhibitor treated P. berghei infected mice.
In our model of HAT, animals exhibiting early CNS stage disease show only mild inflammatory changes with the presence of a limited number of T-cells and macrophages in the brain accompanied by a diffuse astrogliosis whereas late CNS stage mice show higher numbers of T-cell and macrophages as well as the presence of B-cells and plasma cells in the inflammatory infiltrate and a more pronounced astroglial activation (Jennings et al., 1997). In addition, lower levels of IFN-γ are found in the brain of mice during the early CNS stage of the disease compared with the late CNS stage animals (Sternberg et al., 2005). When these two factors are considered in tandem it is possible that manipulation of the kynurenine pathway, towards the production of kynurenic acid through inhibition of KMO, could alter the ratio of quinolinic acid to kynurenic acid in this HAT model, producing a more favourable local environment and limiting the severity of the inflammatory neurotoxic reaction. This may be more pronounced in cases where increased levels of inflammation are found since the higher IFN-γ levels and abundance of inflammatory cells could result in elevated levels of IDO expression compared with the early CNS stage of the disease. This widespread activation of the kynurenine pathway could allow Ro-61-8048 to have a much greater effect on reducing the production of additional downstream catabolites, such as 3-hydroxykynurenine, 3-hydroxyanthranilic acid and 5-hydroxyanthranilic acid, which increase cell death (Smith et al., 2007). The overall effect of manipulating the pathway in this manner would manifest in a reduction in the neuroinflammation found in late CNS stage mice but with only minimal changes in early CNS stage animals. Vincendeau et al. (1999) found a striking reduction in tryptophan levels in the serum of mice following T. b. brucei infection, corresponding with the number of circulating parasites suggesting that trypanosomes utilize exogenous sources of tryptophan (Vincendeau et al., 1999). This was confirmed by in vitro investigations using T. b. gambiense parasites that demonstrated a depletion of tryptophan in the culture medium correlating with the number of trypanosomes present (Vincendeau et al., 1999). As the development of trypanosomiasis progresses in our model, very mild inflammatory changes in the CNS can be detected on microscopic examination from Day 14 post-infection with the reaction becoming more pronounced with time but never reaching the severity seen in sub-curatively treated mice. The numbers of parasites detected within the CNS also increases in parallel with the development of the pathology (author's unpublished observations). In this situation, the depletion of tryptophan would reduce the formation of toxic metabolites such as quinolinic acid and 3-hydroxykynurenine, generating a picture of mild inflammation without significant neurotoxicity. However, following sub-curative treatment, with diminazene aceturate, to induce the late CNS stage of the disease the parasitaemia within the brain decreases dramatically. This could allow the tryptophan levels to increase, permitting the activation of the kynurenine pathway and generating the associated cascade of catabolites capable of enhancing the inflammatory reaction and producing neurotoxicity. Under these circumstances, inhibition of KMO could result in the significant decrease in inflammation seen following treatment with Ro-61-8048 in our model.
Trypanosoma brucei has been shown to possess genes encoding orthologues of enzymes of the kynurenine pathway including a putative KMO (Accession number XP_827034), although to date, the enzyme has not been expressed and its function verified. Untargeted metabolomic analysis (Kamleh et al., 2008) revealed the presence of kynurenine in trypanosome extracts indicating that the parasites do possess a kynurenine pathway. However, in vitro testing of Ro-61-8048 showed that even at concentrations as high as 50 μM the drug was inactive against T. brucei bloodstream form, and early CNS stage mice remained parasitaemic throughout the Ro-61-8048 administration (Fig. 3). This indicates that the anti-inflammatory response seen following Ro-61-8048 treatment is due to effects on host cells and not secondary effects involving parasite destruction.
The evidence gained from this study demonstrates a reduction of the inflammatory reaction within the CNS following inhibition of KMO in late CNS stage trypanosome infection. This implies that Ro-61-8048, or a similar inhibitory compound, may be of use as an adjunct to current trypanocidal therapy in CNS-stage disease to help prevent and/or ameliorate the devastating PTRE that kills about 5% of all patients who are treated with melarsoprol. Further work will be necessary both to extend these findings and to unravel the complex biochemical mechanisms underlying them.
Funding
Wellcome Trust (082786).
Acknowledgements
The authors would like to thank Prof. Max Murray for his continual encouragement and guidance, and Dr L. G. Darlington for helpful comments on the manuscript. We are grateful to Dr Stephan Roever (F. Hoffmann-La Roche Ltd.) for the gift of Ro-61-8048 and Federica Giordani for in vitro trypanocide testing.
Glossary
Abbreviations
- HAT
human African trypanosomiasis
- IDO
indolamine-2,3-dioxygenase
- PTRE
post-treatment reactive encephalopathy
- KMO
kynurenine-3-monooxygenase
References
- Adams JH, Haller L, Boa FY, Doua F, Dago A, Konian K. Human African trypanosomiasis (T. b. gambiense): a study of 16 fatal cases of sleeping sickness with some observations on acute reactive arsenical encephalopathy. Neuropathol Appl Neurobiol. 1986;12:81–94. doi: 10.1111/j.1365-2990.1986.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Clark CJ, Mackay GM, Smythe GA, Bustamante S, Stone TW, Phillips RS. Prolonged survival of a murine model of cerebral malaria by kynurenine pathway inhibition. Infect Immun. 2005;73:5249–51. doi: 10.1128/IAI.73.8.5249-5251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CL, Guilarte TR. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain Res. 1989;495:225–31. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- Eastman CL, Guilarte TR. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem Res. 1990;15:1101–7. doi: 10.1007/BF01101711. [DOI] [PubMed] [Google Scholar]
- Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles GI, Collins CA, Stone TW, Jacob C. Electrochemical and in vitro evaluation of the redox-properties of kynurenine species. Biochem Biophys Res Commun. 2003;300:719–24. doi: 10.1016/s0006-291x(02)02917-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Varo N, Alegre E, Diaz A, Melero I. Immunosuppression routed via the kynurenine pathway: a biochemical and pathophysiologic approach. Adv Clin Chem. 2008;45:155–97. doi: 10.1016/s0065-2423(07)00007-8. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Schwarcz R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur J Neurosci. 1999;11:3857–63. doi: 10.1046/j.1460-9568.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75:985–9. [PubMed] [Google Scholar]
- Jennings FW, Gichuki CW, Kennedy PG, Rodgers J, Hunter CA, Murray M, et al. The role of the polyamine inhibitor eflornithine in the neuropathogenesis of experimental murine African trypanosomiasis. Neuropathol Appl Neurobiol. 1997;23:225–34. [PubMed] [Google Scholar]
- Jennings FW, Whitelaw DD, Holmes PH, Chizyuka HG, Urquhart GM. The brain as a source of relapsing Trypanosoma brucei infection in mice after chemotherapy. Int J Parasitol. 1979;9:381–4. doi: 10.1016/0020-7519(79)90089-4. [DOI] [PubMed] [Google Scholar]
- Jhamandas K, Boegman RJ, Beninger RJ, Bialik M. Quinolinate-induced cortical cholinergic damage: modulation by tryptophan metabolites. Brain Res. 1990;529:185–91. doi: 10.1016/0006-8993(90)90826-w. [DOI] [PubMed] [Google Scholar]
- Kamleh A, Barrett MP, Wildridge D, Burchmore RJ, Scheltema RA, Watson DG. Metabolomic profiling using Orbitrap Fourier transform mass spectrometry with hydrophilic interaction chromatography: a method with wide applicability to analysis of biomolecules. Rapid Commun Mass Spectrom. 2008;22:1912–8. doi: 10.1002/rcm.3564. [DOI] [PubMed] [Google Scholar]
- Kennedy PG. Human African trypanosomiasis of the CNS: current issues and challenges. J Clin Invest. 2004;113:496–504. doi: 10.1172/JCI21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PG. Diagnostic and neuropathogenesis issues in human African trypanosomiasis. Int J Parasitol. 2006;36:505–12. doi: 10.1016/j.ijpara.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Kennedy PG. The continuing problem of human African trypanosomiasis (sleeping sickness) Ann Neurol. 2008;64:116–126. doi: 10.1002/ana.21429. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Rodgers J, Jennings FW, Murray M, Leeman SE, Burke JM. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. Proc Natl Acad Sci USA. 1997;94:4167–70. doi: 10.1073/pnas.94.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–65. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci USA. 1996;93:12553–8. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- Pepin J, Milord F. African trypanosomiasis and drug-induced encephalopathy: risk factors and pathogenesis. Trans R Soc Trop Med Hyg. 1991;85:222–4. doi: 10.1016/0035-9203(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Pepin J, Milord F. The treatment of human African trypanosomiasis. Adv Parasitol. 1994;33:1–47. doi: 10.1016/s0065-308x(08)60410-8. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–7. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz B, Iten M, Grether-Buhler Y, Kaminsky R, Brun R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 1997;68:139–147. doi: 10.1016/s0001-706x(97)00079-x. [DOI] [PubMed] [Google Scholar]
- Sanni LA, Thomas SR, Tattam BN, Moore DE, Chaudhri G, Stocker R, et al. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am J Pathol. 1998;152:611–9. [PMC free article] [PubMed] [Google Scholar]
- Schroten H, Spors B, Hucke C, Stins M, Kim KS, Adam R, et al. Potential role of human brain microvascular endothelial cells in the pathogenesis of brain abscess: inhibition of Staphylococcus aureus by activation of indoleamine 2,3-dioxygenase. Neuropediatrics. 2001;32:206–10. doi: 10.1055/s-2001-17375. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–8. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Stone TW, Smith RA. Neurotoxicity of tryptophan metabolites. Biochem Soc Trans. 2007;35:1287–9. doi: 10.1042/BST0351287. [DOI] [PubMed] [Google Scholar]
- Sternberg JM, Rodgers J, Bradley B, MacLean L, Murray M, Kennedy PG. Meningoencephalitic African trypanosomiasis: brain IL-10 and IL-6 are associated with protection from neuro-inflammatory pathology. J Neuroimmunol. 2005;167:81–9. doi: 10.1016/j.jneuroim.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Stone TW. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog Neurobiol. 2001;64:185–218. doi: 10.1016/s0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–20. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981;72:411–2. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincendeau P, Lesthelle S, Bertazzo A, Okomo-Assoumou MC, Allegri G, Costa CV. Importance of L-tryptophan metabolism in trypanosomiasis. Adv Exp Med Biol. 1999;467:525–31. doi: 10.1007/978-1-4615-4709-9_65. [DOI] [PubMed] [Google Scholar]
- WHO. WHO technical report series. Vol. 881. Geneva: 1998. Control and surveillance of African trypanosomiaisis; pp. 1–113. [PubMed] [Google Scholar]