Abstract
Objective
Muscle triglyceride can be assessed in vivo using computed tomography (CT) and 1H magnetic resonance spectroscopy (MRS), two techniques that are based on entirely different biophysical principles. Little is known, however, about the cross-correlation between these techniques and their test—retest reliability.
Research Methods and Procedures
We compared mean muscle attenuation (MA) in soleus and tibialis anterior (TA) muscles measured by CT with intra- and extramyocellular lipids (IMCL and EMCL, respectively) measured by MRS in 51 volunteers (26 to 72 years of age, BMI = 25.5 to 39.3 kg/m2). MA of midthighs was also measured in a subset (n = 19). Test—retest measurements were performed by CT (n = 6) and MRS (n = 10) in separate sets of volunteers.
Results
MA of soleus was significantly associated with IMCL (r = −0.64) and EMCL, which by multiple regression analysis was explained mostly by IMCL (p < 0.001) rather than EMCL (β = −0.010, p = 0.94). Muscle triglyceride was lower in TA than in soleus, and MA of TA was significantly correlated with EMCL (r = −0.40) but not IMCL (r = −0.16). By CT, MA of midthighs was correlated with MA in soleus (r = 0.40, p = 0.07) and whole calf (r = 0.62, p < 0.05). Finally, both MA and IMCL were highly reliable in soleus (coefficient of variation = <2% and 6.7%, respectively) and less reliable in TA (4% and 10%, respectively).
Discussion
These results support the use of both CT and MRS as reliable methods for assessing skeletal muscle lipid.
Keywords: test—retest repeatability/reliability, 1H magnetic resonance spectroscopy normalization, intramyocellular lipid, extramyocellular lipid, computed tomography attenuation
Introduction
The quantity of fat or lipid droplets stored within skeletal muscle is becoming of considerable interest to researchers in the fields of diabetes, obesity, and exercise physiology (1,2). An increasing number of studies have shown that excess fat storage in or in association with skeletal muscle is correlated with insulin resistance, at least among sedentary individuals. These studies have assessed lipid content in a number of muscle groups, including the vastus lateralis (3,4), whole midthigh (5–9), soleus (10–14), and tibialis anterior (TA)1 (11,13), using a variety of techniques including biochemical extraction (3), oil red O histochemical staining (4), and electron microscopy morphometry (15) of needle biopsy samples, and computed tomography (CT) (5–9) and 1H magnetic resonance spectroscopy (MRS) (10–14,16) of intact muscle. CT and MRS offer several advantages over invasive needle biopsy procedures in that they are performed in vivo, allow for repeated (or serial) measurements, and allow for measurement of a larger sample area.
Kelley et al. and Simoneau et al. (17,18) were the first to use CT to assess muscle-associated fat in obesity and observe a positive relationship with insulin resistance (18). CT is an X-ray imaging technique that is capable of distinguishing fat from muscle tissue based on tissue attenuation characteristics that are a function of tissue density and chemical composition (19). Attenuation values are expressed in Hounsfield units (HUs; on the basis of a linear scale using water = 0 HU as the reference) and are positive for muscle and negative for fat. The mean attenuation of muscle measured by CT reflects the lipid content determined by biochemical and histological analysis, such that a lower mean muscle attenuation (MA) reflects a higher lipid content (6).
1H MRS, on the other hand, was used to assess skeletal muscle lipid and insulin resistance a few years later by different laboratories (10–13,20). MRS uses pulses of radiofrequency energy to probe molecules based on their interaction with an external magnetic field (21). 1H MRS is a spectroscopic technique that identifies the proton resonances of the methylene (CH2) and methyl (CH3) groups of extramyocellular lipid (EMCL) stored in adipocytes and intramyocellular lipid (IMCL) stored in spherical droplets in the myoplasm (22,23). These resonances appear as multiple peaks on the proton spectrum of skeletal muscle, which are shifted in frequency by 0.2 ppm (Figure 1) because of bulk susceptibility factors (24,25). Typically, the CH2 rather than the CH3 resonance peaks are used to represent muscle lipid because of their larger signal intensity. MRS may be advantageous over CT because it distinguishes between IMCL and EMCL and does not involve ionizing radiation. The ability to distinguish fat storage inside compared with outside the muscle may be an important advantage, because recent MRS studies have suggested that IMCL rather than EMCL influences insulin resistance (11,20). By CT imaging, however, adipose tissue adjacent to muscle and beneath the fascia lata in the thigh has been found to correlate with insulin resistance (8).
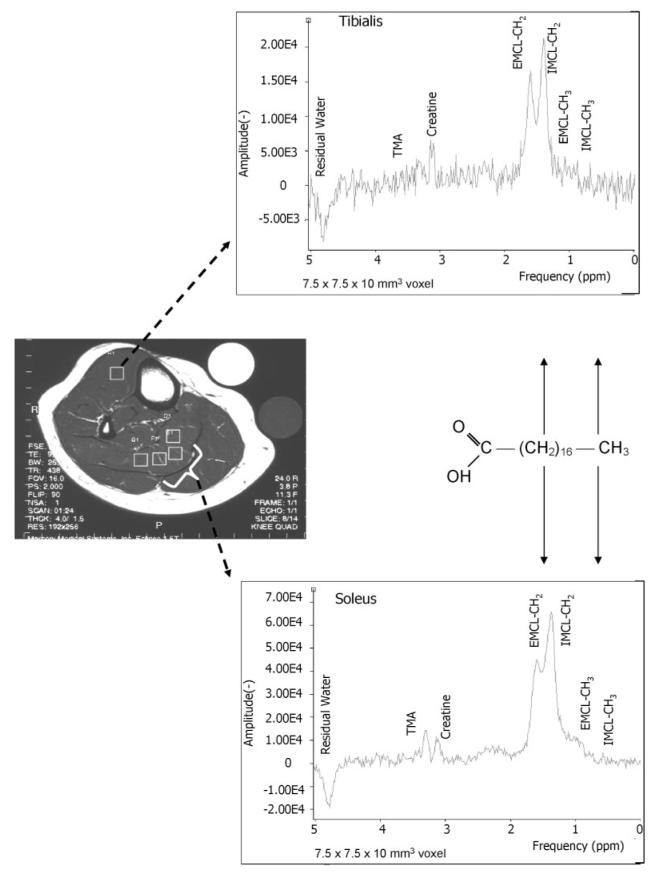
Figure 1.
Water-suppressed PRESS boxes (7.5 × 7.5 × 10 mm3 voxels) were collected from the largest volume of the calf muscle (TE = 35 ms; TR = 2 seconds). The figure shows four possible positions in the soleus and one position in the TA muscle in a cross-sectional scout image (TE = 9.7 ms; TR = 500 ms; field of view = 10 × 7.5 mm). IMCL and EMCL were determined from the average of the sum of three PRESS boxes in the soleus and one to two PRESS boxes in the TA muscles. Typical 1H MRS obtained from a single PRESS box in the soleus and TA muscles is shown. The spectrum shows the CH2 and CH3 peaks of EMCL and IMCL that are shifted in frequency by 0.2 ppm and the residual water trimethyl amine (A) and creatine peaks.
Unfortunately, despite the widespread use of these two techniques in clinical research, little is known about the correlation between skeletal muscle lipid assessed by CT and MRS. To our knowledge, all previous studies that have assessed skeletal muscle lipid stores have used only one in vivo method (MRS or CT). Similarly, a number of important methodological and biological questions concerning both MRS and CT remain either unanswered or scantly reported. Thus, the aim of this study was to compare IMCL and EMCL measured by MRS with MA measured by CT in the soleus and TA muscles. Other aims were to 1) assess the variation in lipid content measured by both MRS and CT across commonly assessed muscle groups (soleus and TA by MRS and soleus, TA, whole calf, and whole thigh by CT); 2) ascertain the use of an external peanut oil phantom vs. an internal water peak or creatine peak to semiquantify IMCL and EMCL peak areas by MRS; and 3) determine the methodological variances in measures of muscle lipid by MRS and CT.
Research Methods and Procedures
The population for this set of studies was comprised of healthy participants from several clinical trials. These studies were approved by the Institutional Review Board of the Pennington Biomedical Research Center, Baton Rouge, LA, and the University of Pittsburgh, Pittsburgh, PA, and all participants gave their written informed consent.
Comparison between 1H MRS and CT in the Calf Muscle
The subjects included 51 men and women between the ages of 26 and 74 years. Thirty-one were overweight individuals who were enrolled in a study assessing the effect of calorie restriction on markers of aging (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy Study) (26), and 20 were obese type 2 diabetic (n = 9) and non-diabetic control (n = 11) subjects participating in an ancillary study of the Look AHEAD trial (27). All of the subjects were sedentary. Look AHEAD is a randomized multicenter controlled trial of a lifestyle intervention for weight loss in overweight or obese adults (45 to 75 years of age) with type 2 diabetes. The non-diabetic subjects in the ancillary study were recruited separately. Participants were recruited from only one site (Baton Rouge) of the four Adipose Tissue Study Group sites. As part of their participation in these studies, participants had MRS and CT scans of the midcalf performed on the same day, as described below. Body composition was also assessed by DXA (Hologic QDR4500, Hologic, Bedford, MA) and analyzed using QDR for Windows software (Version 11.1, SPSS, Inc., Chicago, IL). The characteristics of the subjects are shown in Table 1.
Table 1.
Physical characteristics of 51 subjects
| Mean ± SD | Range | |
|---|---|---|
| Sex (males/females) | 21/30 | |
| Age (years) | 46 ± 11 | 26 to 74 |
| Height (cm) | 170 ± 10 | 152 to 189 |
| Weight (kg) | 86.1 ± 13.0 | 63.9 to 121.2 |
| BMI (kg/m2) | 29.7 ± 3.3 | 25.5 to 39.3 |
| Body fat (%) | 34.1 ± 7.5 | 20.3 to 46.2 |
| Fasting glucose (mg/dL) | 102.9 ± 24.8 | 80 to 190 |
1H MRS
IMCL was measured in the soleus and TA muscles by a 1H-MRS technique on a 1.5-T whole body imaging and spectroscopy system (Picker Edge Eclipse, Picker International, Cleveland, OH) using the PRESS box (Point RESolved Spectroscopy) technique. Measurements were obtained from the right calf with the volunteer lying in the supine position within the spectrometer. The volunteer’s leg was positioned inside a commercially made radiofrequency 1H knee coil with the knee in extension and the ankle in a neutral position. The orientation of the leg in reference to the magnetic field (B0) and the coil position was determined from our earlier work (28) and was selected to provide optimal splitting of the IMCL and EMCL resonances in the soleus and TA muscles. Separate water-suppressed PRESS boxes (7.5 × 7.5 × 10-mm3 voxels) were collected from the largest volume of the calf muscle [echo time (TE) = 35 ms; repetition time (TR) = 2 seconds; number of averages = 64 plus 8 reference averages]. For each separate voxel, the water-suppressed collection was immediately followed by a non—water-suppressed collection for a total scan time of 2.4 min/voxel. Our choices of acquisition parameters were selected to optimize 1) acceptable spectral quality and signal-to-noise ratio; 2) representative sampling of lipid content; and 3) total scan time relative to patient burden (i.e., shorter scan times decrease patient discomfort and increase patient compliance). Boxes were positioned to avoid fascia, vascular structures, and gross marbling as determined using the cross-sectional images (TR = 500 ms; TE = 9.7 ms; 10 × 7.5 mm field of view; 4-mm slice thickness with a slice separation of 1.5 mm; Figure 1).
IMCL and EMCL contents were determined from the average of the sum of three PRESS boxes in the soleus and one to two PRESS boxes in the TA. Summing the signals increases the signal-to-noise ratio but was not always possible in the TA muscle because of muscle size limitations. The approximate location of the boxes was documented on the cross-sectional image so that the position could be kept consistent for follow-up measurements. Peak positions and areas of interest (EMCL-CH2, IMCL-CH2, EMCL-CH3, IMCL-CH3, and creatine) were determined by time domain fitting using jMRUI (Java-Based Magnetic Resonance User Interface) (29) and a set of prior knowledge files (30). In brief, all water-suppressed free induction decays (FIDs; metabolite FID) were deconvoluted with the water unsuppressed FID (water FID) acquired from the same voxel to correct for zero-order phasing and removal of eddy-current induced artifacts (31). The resulting metabolite FIDs were analyzed with AMARES, a non-linear least square fitting algorithm operating in the time domain (32). The time domain model function is composed of four exponentially decaying sinusoids corresponding to the four Lorentzian peaks in the frequency domain assigned to the resonances of interest [i.e., EMCL and IMCL -(CH2)n- and -CH3 peaks]. Lorentzian decaying sinusoids were fit to represent the additional lipid resonances, and two Gaussian decaying sinusoids were used to represent the total creatine and trimethyl amine (A) resonances. This combination (of using both Lorentzian and Gaussian peak fits) proved to be the most reproducible and provided the least amount of residual signal after fitting. While the CH2 peak of EMCL and IMCL is most commonly reported, the CH3 peaks are measured as additional markers by some laboratories and should correlate strongly with the CH2 peak. Area of all peaks were normalized by referencing to an external oil phantom (peanut oil, one 7.5 × 7.5 × 10-mm3 voxel, TE = 35 ms, TR = 2 seconds) using the methodology described by Perseghin et al. (13) and, for comparison purposes, were also set relative to the corresponding internal water peak as described by Krssak et al. (12). Because the relaxation times of signals from lipid and water are intra- and inter-individually constant (23) and the applied echo time was short, results were not corrected for relaxation effects.
CT
CT imaging was conducted using a GE Lightspeed Plus (Milwaukee, WI). With the volunteer supine, four 2.5-mm cross-sectional scans were obtained at the largest portion of the calf. Scans were obtained at 120 kv (peak), 310 mA, and a scanning time of 1 second to measure muscle lean and fat tissue compositions. The field of view was 40 cm, and the matrix was 512 × 512 pixels, which achieved a pixel resolution of 0.78 mm. MA of the soleus and TA muscles was measured separately for each subject from a single slice (first in the order of four) by windowing the area of the soleus or TA, respectively (Figure 2), using commercially available CT software (Analyze; AnalyzeDirect, Lenexa, KS). MA was expressed as the mean attenuation value from all pixels within an individually set range between 100 HU at the upper end and the difference between the average attenuation of adipose tissue and average attenuation of soleus (or TA) muscle at the lower end (i.e., at approximately −30 to −35 HU). This method allows for exclusion of most of the intermuscular or “marbled” adipose tissue in the analysis.
Figure 2.

MA was measured from a single slice cross-sectional CT image (120 kv, 310 mA, 40-cm field of view, 512 × 512 pixel matrix) by windowing the area of the desired muscle. This figure shows windowing of the soleus muscle from a scan obtained at midcalf.
Comparison between MA in Different Muscle Groups by CT
The MA of both the thigh(s) and whole calf measured in a subset of volunteers (7 men, 12 women; 57 ± 8 years; 91.5 ± 15.0 kg; BMI = 32.4 ± 2.6 kg/m2) was analyzed in Dr. Kelley’s laboratory at the University of Pittsburgh as previously described (7). For this analysis, a 10-mm cross-sectional scan of both legs in each subject was obtained at the midpoint between the anterior superior iliac crest and the patella. Scans were obtained by using 170 mA, 48-cm field of view, and a 512 × 512 matrix. Skeletal MA was measured as the mean attenuation value from all pixels within the range of 0 (lower end) and 100 (upper end) HU in both thighs (7). Thus, the lower cut-off point for eliminating intermuscular adipose tissue was slightly different between our laboratory and Kelley’s laboratory.
Use of an External Oil Phantom and the Internal Water Peak as a Reference in MRS
To account for day-to-day variation in system performance, IMCL and EMCL peak areas must be expressed either relative to the peak area of an external phantom of known constant concentration (13,33) or relative to an internal peak assumed to be of constant concentration [i.e., water peak of non—water-suppressed signal (11,12,14,20) or creatine peak (10,16)]. To determined how these different normalizing methods compare, we compared IMCL and EMCL peak areas normalized in reference to the external peanut oil phantom (13) to IMCL and EMCL peak areas normalized relative to the corresponding internal water peak (12) in both the soleus and TA muscles in the whole group of 51 volunteers.
Test—Retest Reliability of MRS and CT Measures of Muscle-associated Lipid
To test the reliability of our MRS and CT protocols (including both the methodological and biological variability), we recruited two groups of volunteers who were not participating in the aforementioned trials. For the MRS reproducibility analysis, 10 volunteers (5 men and 5 women; 31 ± 13 years; 70.5 ± 16.2 kg; BMI = 24.9 ± 4.9 kg/m2) had two back-to-back MRS measurements performed using the exact procedures described above. The group of 10 was mostly sedentary (n = 9) and included 2 individuals who were obese and 2 who were overweight. IMCL and EMCL contents were determined from the average of the sum of three PRESS boxes in the soleus and one PRESS box in the TA by an investigator (B.R.N.) who was blinded to subject identity and test. The approximate location of the boxes was documented relative to its crosssectional orientation. The reliability and variability of each voxel relative to the other voxels, however, was not assessed because of signal-to-noise considerations.
After the initial scan, volunteers were asked to exit the magnet and stretch while all equipment was completely removed and reestablished. Volunteers were repositioned for the second scan. This maximized any errors associated with set-up variations and leg and/or foot placement. PRESS box positions during the second scan were positioned as close as possible to the cross-sectional orientation of the first scan.
For the CT reliability analysis, six volunteers (two men and four women; 43 ± 11 years; 68.4 ± 13.6 kg; BMI = 24.4 ± 4.9 kg/m2) had three repeated single-slice scans of the midcalf performed according to protocol. The first two were performed back-to-back, and the third was performed at the same time of day 2 days later. Between the back-to-back measurements, volunteers were asked to rise off the patient bed and were repositioned for the second scan. This maximized errors associated with leg and/or foot positioning. Between the day 1 and day 3 scans, volunteers were asked not to exercise and to maintain a similar diet. The group of six was mostly sedentary (n = 4) and included one individual who was obese and one who was overweight. There were no statistical differences in the physical characteristics (weight, age, and BMI) between the 6 volunteers participating in the CT reliability and the 10 volunteers participating in the MRS reliability study.
Statistical Considerations
All data are reported as means ± SD. Relationships between muscle lipid measurements and descriptive variables were tested using Pearson or Spearman’s ρ correlations (as appropriate) and regression procedures. Test—retest reproducibility was assessed by using random effects statistical models. The variance components and the population mean for each variable were estimated using the method of restricted maximum likelihood. For the CT variables, the models included both subject and time (i.e., day) as random effects; for the MRS variables, there was no time effect. The resulting parameter estimates were used to obtain point estimates of both coefficients of variation (CVs) and the intraclass correlation (ICC). Between-subject CV was taken to be 100 times the ratio of the square root of the between-subject variance component to the population mean. To maintain consistency between the CT and MRS measures, within-subject variance was taken to be total variance minus between-subject variance in the definition and computation of the within-subject CV.
Results
MRS
Figure 3 summarizes the EMCL and IMCL peak areas (mean ± SD) normalized to both the external peanut oil phantom (Figure 3A) and the internal water peak (Figure 3B) for the soleus and TA muscles. TA spectra from three of the volunteers were unusable because of excessive noise relative to signal (n = 2) or dominance by an EMCL peak of large amplitude and no splitting (n = 1); thus, they could not be fit. Therefore, soleus data are presented for all 51 volunteers, and TA data are presented for 48. As can be seen in Figure 3, IMCL was roughly six to eight times higher in the soleus compared with the TA muscle (p < 0.001 between soleus and TA IMCLs peaks). EMCL was slightly higher in the soleus compared with the TA muscle, but this difference was significant only for the EMCL-CH2 peak (p < 0.05).
Figure 3.

Muscle-associated lipid storage measured by 1H MRS (A and B) and CT (C) in the soleus (n = 51) and TA (n = 48) muscles. EMCL and IMCL measured by MRS are in arbitrary units relative to a peanut oil phantom positioned external to the calf (A) or relative to the internal water peak (B). CT data are the mean attenuation values in HUs. Values are means ± SD.
CT
Figure 3C summarizes the mean MA measured in the soleus and TA muscles. CT data are consistent with MRS data, indicating higher lipid stores in the soleus than in the TA muscle (i.e., mean MA was lower in soleus compared with TA muscle).
Comparison between 1H MRS and CT in the Calf Muscle
Table 2 summarizes the correlations between EMCL and IMCL measures by MRS (normalized to the oil phantom) and mean MA measured by CT for the soleus and TA muscles. In the soleus muscle, mean MA was significantly associated with IMCL, EMCL, and total muscle lipid (EMCL + IMCL peak areas) measured by both the CH2 and CH3 peaks. The relationship between mean MA in the soleus and IMCL-CH2 and EMCL-CH2 is shown in Figure 4. Using a multiple regression model of the CH2 peaks, further analysis found that EMCL did not add significantly (β = −0.010, p = 0.94) once IMCL (β = −0.636, p < 0.001) was in the regression equation. In the TA muscle, mean MA was similarly correlated with EMCL (as in soleus muscle) but less associated overall with IMCL and total muscle lipid than in the soleus (Table 2). There was a trend, however, for TA MA to be correlated with TA EMCL peak areas. Correlations between MRS and CT measurements were almost identical for soleus and TA muscles when EMCL and IMCL peak areas were normalized to the internal water peak rather than the oil phantom (data not presented).
Table 2.
Correlations between EMCL and IMCL stores measured by MRS and mean MA measured by CT of the soleus (n = 51) and TA muscles (n = 48)
| Mean MA |
||
|---|---|---|
| Pearson correlation |
Spearman’s ρ | |
| Soleus (relative to oil phantom) |
||
| EMCL-CH2 | −0.37† | −0.20 |
| IMCL-CH2 | −0.64† | −0.64† |
| Total lipid (EMCL + IMCL-CH2) |
−0.61† | −0.60† |
| EMCL-CH3 | −0.33* | −0.20 |
| IMCL-CH3 | −0.55† | −0.61† |
| Total lipid (EMCL + IMCL-CH3) |
−0.57† | −0.57† |
| TA (relative to oil phantom) |
||
| EMCL-CH2 | −0.41† | −0.32* |
| IMCL-CH2 | −0.16 | −0.29* |
| Total lipid (EMCL + IMCL-CH2) |
−0.40† | −0.36* |
| EMCL-CH3 | −0.46† | −0.35* |
| IMCL-CH3 | −0.27‡ | −0.25‡ |
| Total lipid (EMCL + IMCL-CH3) |
−0.38* | −0.34* |
EMCL, extramyocellular; IMCL, intramyocellular; MRS, magnetic resonance spectroscopy; MA, muscle attenuation; CT, computed tomography; TA, tibialis muscle; CH2, methylene; CH3, methyl.
Significant at 0.05 level.
Significant at 0.01 level.
0.05 < p < 0.10.
Figure 4.

Relationship between IMCL and EMCL CH2 peak measured by 1H MRS and mean MA determined by CT in the soleus muscle (r = −0.64 for IMCL and −0.37 for EMCL; p < 0.01; n = 0.51). IMCL and EMCL are expressed in arbitrary units relative to a peanut oil phantom that is positioned external to the calf. A low mean MA reflects a higher fat content.
Comparison of MA in Different Muscle Groups by CT
Table 3 summarizes the mean MA values for the subset of 19 volunteers who had CT measurements performed in the soleus, TA, whole-calf, thigh of the dominant leg, and both thighs. Interestingly, much higher mean MA, which is reflective of less muscle-associated lipid, was noted in the thigh measurement of the dominant leg as well as in the TA muscle. Table 4 shows the Pearson correlations among mean MA values measured across the various muscle groups. Correlations varied by the muscle groups compared and ranged from highly (soleus muscle vs. whole calf, r = 0.87) to poorly (soleus muscle vs. dominant thigh, r = 0.28) correlated. Correlations using Spearman’s ρ were similar or nearly identical for all variables.
Table 3.
Muscle-associated lipid storage in the soleus, TA, calf, and thigh muscles measured by CT in a subset of 19 subjects
| Mean ± SD | Range | |
|---|---|---|
| Soleus MA (HU) | 46.5 ± 4.6†‡§¶ | 37.5 to 54.0 |
| TA MA (HU) | 58.8 ± 5.1*‡¶ | 45.6 to 67.1 |
| Calf MA (HU) | 48.9 ± 3.2*†§ | 41.2 to 53.2 |
| Dominant thigh MA (HU) | 58.3 ± 2.5*‡¶ | 55.1 to 63.0 |
| Both thighs MA (HU) | 49.2 ± 2.3*†§ | 44.7 to 53.2 |
TA, tibialis muscle; CT, computed tomography; SD, standard deviation; MA, muscle attenuation; HU, Hounsfield unit.
Significantly different from soleus MA.
Significantly different from TA MA.
Significantly different from calf MA.
Significantly different from dominant thigh MA.
Significantly different from both thighs MA.
Table 4.
Pearson correlation matrix of mean MA measured by CT in various muscle groups in a subset of 19 volunteers
| Variable | TA MA | Calf MA | Thigh MA |
Both thighs MA |
|---|---|---|---|---|
| Soleus MA | 0.25 | 0.87† | 0.28 | 0.40‡ |
| TA MA | 0.54* | 0.10 | 0.36 | |
| Calf MA | 0.42‡ | 0.62† | ||
| Thigh MA | 0.64† |
MA, muscle attenuation; CT, computed tomography; TA, tibialis muscle; HU, Hounsfield unit. All MA values are expressed in HU.
Significant at 0.05 level.
Significant at 0.01 level.
0.05 < p < 0.10.
Use of an External Oil Phantom and the Internal Water Peak as a Reference in MRS
Overall, high cross-correlations were found among all lipid peaks when the oil phantom and the internal water peak were used as the normalization reference. For the CH2 peaks, the cross-correlations between the two normalization references were 0.995 for EMCL and 0.981 for IMCL in the soleus (Figure 5) and 0.999 for EMCL and 0.990 for IMCL in the TA. For the smaller CH3 peaks, the cross-correlations were 0.999 for EMCL and 0.992 for IMCL in the soleus and 0.998 for EMCL and 0.994 for IMCL in the TA (p < 0.01 for all).
Figure 5.

The cross-correlation between the IMCL CH2 peak set relative to an external oil phantom and the internal water peak (r = 0.98; p < 0.01; n = 0.51).
Comparison between EMCL and IMCL Measured by MRS in the Soleus and TA Muscles
Table 5 presents the relationship between EMCL and IMCL in the soleus and TA muscles for both the CH2 and CH3 peaks normalized to the oil phantom. For both muscle groups, the IMCL-CH2 peak was highly correlated with the IMCL-CH3 peak, and the EMCL and IMCL peaks (for both CH2 and CH3 peaks) were moderately correlated. Quite interestingly, however, muscle lipid (EMCL or IMCL) measured in the soleus was not correlated with muscle lipid measured in TA, similarly to what was observed for MA in the two muscles as measured by CT. Correlations using Spearman’s ρ were similar or nearly identical to Pearson correlations for all variables.
Table 5.
Pearson correlation matrix among EMCL and IMCL CH2 and CH3 peaks set relative to an external oil phantom
| Variable | Soleus IMCL-CH2 |
Soleus EMCL-CH3 |
Soleus IMCL-CH3 |
TA EMCL-CH2 |
TA IMCL-CH2 |
TA EMCL-CH3 |
TA IMCL-CH3 |
|---|---|---|---|---|---|---|---|
| Soleus | 0.57† | 0.40† | 0.61† | 0.34* | 0.03 | 0.34* | 0.17 |
| EMCL-CH2 | (51) | (51) | (51) | (48) | (48) | (48) | (48) |
| Soleus | 0.36* | 0.91† | 0.33 | 0.13 | 0.37 | 0.00 | |
| IMCL-CH2 | (51) | (51) | (48) | (48) | (48) | (48) | |
| Soleus | 0.30* | −0.02 | −0.05 | −0.03 | −0.14 | ||
| EMCL-CH3 | (51) | (48) | (48) | (48) | (48) | ||
| Soleus | 0.35* | −0.09 | 0.39† | 0.06 | |||
| IMCL-CH3 | (48) | (48) | (48) | (48) | |||
| TA | 0.31* | 0.94† | 0.68† | ||||
| EMCL-CH2 | (48) | (48) | (48) | ||||
| TA | 0.13 | 0.79† | |||||
| IMCL-CH2 | (48) | (48) | |||||
| TA | 0.47† | ||||||
| EMCL-CH3 | (48) |
EMCL, extramyocellular; IMCL, intramyocellular; CH2, methylene; CH3, methyl; TA, tibialis muscle.nis shown in parentheses.
Significant at 0.05 level.
Significant at 0.01 level.
Test—Retest Reliability of MRS and CT Measures of Muscle-associated Lipid
Test—retest results of muscle-associated lipid storage measured by MRS and CT in the soleus and TA muscles are presented in Table 6. Overall, CT seems to be more repeatable than MRS. By MRS, 1) the CH2 peak of IMCL was more reproducible than CH3 peak in both muscle groups; 2) IMCL measured in the soleus using our protocol was more reproducible than IMCL measured in TA; and 3) IMCL normalized to either the oil phantom or the internal water peak was considerably more reproducible than IMCL normalized to the internal creatine peak. It should be noted, however, that TA data were not compared using the creatine peak as a normalization reference, because this peak was missing (undetectable) in 4 of 10 repeat studies. The test—retest results for both attenuation and IMCL-CH2 measured in the soleus are shown in Figure 6.
Table 6.
Test—retest results of muscle-associated lipid storage measured by MRS (n = 10) and CT (n = 6)
| Variable | Test (mean ± SD) |
Retest (mean ± SD) |
Between-subject CV% |
Within-subject CV% |
ICC |
|---|---|---|---|---|---|
| MRS (relative to oil phantom) | |||||
| Oil phantom (AU) | 17,842 ± 2314 | 17,565 ± 2342 | 12.87 | 2.72 | 0.96 |
| Soleus EMCL-CH2 (AU × 10-2) | 2.166 ± 1.00 | 1.83 ± 0.71 | 40.98 | 18.86 | 0.83 |
| Soleus IMCL-CH2 (AU × 10-2) | 2.59 ± 1.43 | 2.72 ± 1.47 | 54.10 | 7.25 | 0.98 |
| Soleus EMCL-CH3 (AU × 10-2) | 0.13 ± 0.09 | 0.13 ± 0.08 | 38.70 | 46.54 | 0.41 |
| Soleus IMCL-CH3 (AU × 10-2) | 0.45 ± 0.26 | 0.47 ± 0.24 | 53.17 | 10.05 | 0.97 |
| TA EMCL-CH2 (AU × 10-2) | 1.10 ± 0.70 | 1.20 ± 0.95 | 66.56 | 28.51 | 0.84 |
| TA IMCL-CH2 (AU × 10-2) | 0.38 ± 0.15 | 0.41 ± 0.18 | 40.58 | 10.86 | 0.93 |
| TA EMCL-CH3 (AU × 10-2) (n = 9) | 0.13 ± 0.06 | 0.16 ± 10 | 41.78 | 42.77 | 0.49 |
| TA IMCL-CH3 (AU × 10-2) (n = 9) | 0.08 ± 0.03 | 0.08 ± 0.03 | 44.89 | 20.02 | 0.83 |
| MRS (relative to internal water peak) | |||||
| Soleus internal water peak (AU) | 12,329.9 ± 1253.1 | 12,415.9 ± 1058.7 | 9.03 | 2.49 | 0.93 |
| Soleus EMCL-CH2 (AU × 10-2) | 2.84 ± 1.29 | 2.57 ± 1.03 | 39.37 | 17.87 | 0.82 |
| Soleus IMCL-CH2 (AU × 10-2) | 3.81 ± 2.32 | 3.85 ± 2.23 | 59.00 | 6.30 | 0.99 |
| Soleus EMCL-CH3 (AU × 10-2) | 0.18 ± 0.11 | 0.19 ± 0.14 | 51.77 | 41.74 | 0.61 |
| Soleus IMCL-CH3 (AU × 10-2) | 0.66 ± 0.42 | 0.67 ± 0.35 | 57.29 | 10.53 | 0.97 |
| TA internal water peak (AU) | 10,318.2 ± 1232.3 | 10,515.6 ± 998.7 | 10.58 | 2.19 | 0.96 |
| TA EMCL-CH2 (AU × 10-2) | 1.87 ± 1.22 | 1.95 ± 1.61 | 69.75 | 26.83 | 0.87 |
| TA IMCL-CH2 (AU × 10-2) | 0.67 ± 0.29 | 0.68 ± 0.31 | 43.86 | 10.26 | 0.95 |
| TA EMCL-CH3 (AU × 10-2) (n = 9) | 0.20 ± 0.12 | 0.24 ± 0.16 | 49.28 | 42.74 | 0.57 |
| TA IMCL-CH3 (AU × 10-2) (n = 9) | 0.13 ± 0.07 | 0.12 ± 0.07 | 50.97 | 19.03 | 0.88 |
| MRS (relative to internal Cr peak) | |||||
| Soleus internal Cr peak (AU) | 66.21 ± 14.73 | 67.12 ± 14.84 | 18.74 | 11.57 | 0.72 |
| Soleus EMCL-CH2 (AU × 10-2) | 17.40 ± 10.51 | 15.86 ± 9.44 | 54.20 | 25.65 | 0.82 |
| Soleus IMCL-CH2 (AU × 10-2) | 23.80 ± 18.91 | 24.00 ± 17.51 | 74.59 | 15.46 | 0.96 |
| Soleus EMCL-CH3 (AU × 10-2) | 0.98 ± 0.54 | 1.08 ± 0.86 | 44.64 | 52.64 | 0.42 |
| Soleus IMCL-CH3 (AU × 10-2) | 4.14 ± 3.33 | 4.15 ± 2.77 | 70.96 | 20.0 | 0.93 |
| CT | |||||
| Soleus mean MA (HU) | 49.6 ± 5.2 | 50.0 ± 5.4 | 10.22 | 1.96 | 0.96 |
| TA mean MA (HU) | 57.5 ± 2.7 | 57.6 ± 2.4 | 3.4 | 4.05 | 0.41 |
MRS, magnetic resonance spectroscopy; CT, computed tomography; SD, standard deviation; CV, coefficient of variation; ICC, intraclass correlation; AU, arbitrary unit; EMCL, extramyocellular lipid; IMCL, intramyocellular lipid; CH2, methylene; CH3, methyl; TA, tibialis muscle; Cr, creatine; HU, Hounsfield unit.
Figure 6.

Test—retest results of muscle lipid storage measured by 1H MRS and CT in the soleus muscle. (Top) Test—retest data for mean MA measured by CT in six individuals (ICC = 0.96; CV = 1.96%). (Bottom) Test—retest data for IMCL measured by MRS (sum of three voxels) in 10 individuals (ICC = 0.98; CV = 7.25%). In both cases, volunteers exited the patient bed after scan 1 and were repositioned for scan 2.
Discussion
1H MRS and CT are commonly used techniques in obesity and diabetes research that allow measurement of skeletal muscle lipid stores in vivo. To our knowledge, this study is the first to report that lipid stores measured by both techniques are correlated and that, in the soleus but not the TA muscle, intrarather than extramyocellular lipid stores measured by MRS better reflect muscle attenuation measured by CT. The study also found that the methodological variability of both techniques is quite small. For MRS, it was observed that both an oil phantom and the internal non—water-suppressed water peak are reliable and highly correlated methods for normalizing MRS lipid peaks (as long as the intra- and extracellular water compartment are unchanging) but that normalizing using the creatine peak produces greater methodological error. Furthermore, mean muscle attenuation was found to track well among different muscle groups of the leg.
There has been a recent increase in the use of 1H MRS to non-invasively measure IMCL in human skeletal muscle. This technique measures the resonances from the CH2 and CH3 protons of triglycerides, which appear as multiple peaks on the proton spectrum of skeletal muscle (21) (see Figure 1). A number of lines of evidence support the notion that the CH2 resonance peak at 1.4 is representative of lipid droplets stored within the muscle fiber, whereas the CH2 peak at 1.6 is representative of adipocytes stored external to the myofibril. For example, in muscle tissue devoid of adipose cells, a single resonance is observed at 1.3 to 1.4 ppm (22), whereas a single resonance at 1.5 to 1.6 ppm is observed in adipose tissue (34). Patients with congenital generalized lipodystrophy, a condition characterized by almost complete absence of adipose tissue, also have no resonance peak at 1.6 ppm (22). In addition, MRS has recently been validated against electron microscopy morphometry for measuring IMCL, which supports the suitability of MRS as a replacement for invasive techniques (35). Disadvantages of MRS include the unavailability of MRS systems for research purposes, the unavailability of spectroscopy acquisition software on clinical systems, the need for highly trained support staff, the dependence of signal on muscle fiber orientation, the inability to detect adequate splitting between IMCL and EMCL peaks in 100% of data sets, and the overall experimental cost.
CT, on the other hand, allows for distinction of the fat within muscle tissue based on the tissue attenuation characteristics that are negative for fat and positive for muscle based on a linear scale with water assigned to zero (19). The fat content of muscle is typically discerned from the mean tissue attenuation (6,7,17), such that lower mean attenuation reflects higher fat tissue content (6), but has also been estimated by calculating the low density lean tissue area (0 to 29 or 34 HUs) (17,18,36,37). Experimental studies have shown that CT is able to detect differences in lipid emulsion phantoms of varying concentrations (from 0 to 10 g/100 mL, representing an attenuation of ∼60 to 52 HU) (6), and that mean MA correlates with muscle triglyceride content measured in biopsy samples using both biochemical extraction (r = −0.58) and histochemical staining (r = −0.43) (6). The disadvantages of CT include that subjects are exposed to ionizing radiation and that CT cannot directly “measure” lipid content or detect the location of fat storage within or surrounding the muscle cell. However, its relatively low cost, acquisition speed, and availability make it a popular method for large-scale clinical studies (38). Furthermore, while CT cannot directly quantify lipid content, experiments using the lipid emulsion phantoms (6) suggest that it may be valuable for quantifying larger changes with weight loss over time such that a change in muscle attenuation by ∼1 HU represents a lipid concentration change of ∼1 g/100 mL.
This study found that the results obtained from both in vivo techniques are moderately correlated, despite their being based on different biophysical principles. Furthermore, intrarather than extramyocellular lipid stores measured by MRS better reflect muscle attenuation measured by CT. Overall, these findings are somewhat expected, given that clinical trials have found a consistent relationship between muscle lipid accumulation and insulin resistance that seems to be independent of whether CT or MRS was used to assess muscle lipid stores (3–14). The finding that IMCL rather than EMCL more closely explains attenuation in the soleus is a novel and important observation and central to the main goal of this study. These results help confirm the methodological assumption of CT that most of the intermuscular or “marbled” adipose tissue can be excluded from the CT analysis by eliminating pixels that are highly negative [e.g., <0 HU in Kelley’s laboratory (6) and less than approximately −30 to −35 HUs in our laboratory]. Conversely, these findings should be interpreted with some caution because of the limitations of spectroscopy to truly measure EMCL and our rather inconsistent findings in the TA. During 1H MRS experiments, voxels are specifically positioned in areas that are free from gross marbling (i.e., areas without visible adipose tissue storage in the extramyocellular region) to prevent a large EMCL signal that can dominate the 1H spectrum and prohibit both detection and fitting of the IMCL signal (39). The extramyocellular bulk fat signal is also dependent on muscle fiber orientation and voxel size (factors that do not influence the intramyocellular lipid droplet signal), which prohibit detection of a technically reliable EMCL peak that is proportional to the EMCL content of muscle. Furthermore, it is also imperative to mention, particularly for non-MRS users, that it is common for a certain percentage of MRS data (for example 2 of 51 TA scans) to be unusable because the EMCL and IMCL peak signals cannot be separated and fit. This limitation is noted in all fitting routines, whether time or frequency based, and is evident even at higher field strengths (i.e., 4.1 T). Future studies in different muscle groups using CT and high-resolution magnetic resonance imaging, a novel technique that allows for accurate quantification of intra- and perimuscular adipose tissue (40,41), would provide an interesting analysis to help resolve this methodological and practical issue. Our findings in the TA muscle may be explained by the interesting observation that, while both muscles have similar EMCL stores that are similarly correlated with muscle attenuation (r = 0.37 in the soleus and 0.40 in the TA), the IMCL stores in the TA are roughly one-sixth to one-eight those in the soleus. Thus, lower overall correlation with IMCL and total muscle lipid in the TA muscle may be driven by extremely low IMCL stores in this muscle.
A second important purpose of this study was to assess the variation in lipid content measured by both MRS and CT across commonly assessed muscle groups. Generally, research studies using CT have followed the lead of Kelley’s group (17,18) and assessed the attenuation value of the midthigh region (36,37,42,43), whereas MRS studies have assessed lipid storage primarily in the soleus and TA muscles of the calf (10–14,44). The latter is the case because of methodological restraints related to the dimensions of the MRS coil (which would need to be larger for thigh vs. calf muscle) and the requirement that the muscle fibers and surrounding lipid layers be in near parallel alignment with respect to the static magnetic field for optimal signal detection and splitting (39). Comfortable positioning of the human body within the magnetic field can easily be accomplished for measuring the soleus (10–14,44,45) and TA muscles (11–13,35,39) but is more challenging for thigh muscles (16) because of their pennation angles. Results from this study indicate that, while good correlations were noted for attenuation measured in a variety of individual muscles and muscle groups in the leg, no correlation was apparent for IMCL (or EMCL) measured in the soleus and TA muscles. In agreement with the former, Goodpaster et al. (6) also found good correlations between attenuation in the midthigh and attenuation of other distinct muscle groups that included the midcalf (r = 0.60), psoas (r = 0.65), and erector spinae (r = 0.77). The discrepancy between CT and MRS studies, however, may be explained by the fiber composition of muscles analyzed rather than methodological differences. For example, the mean attenuation values of midthigh and midcalf are mixtures of attenuation values of different muscle groups (i.e., the vastus lateralis, vastus intermedius, rectus femoris, biceps femoris, semitendinosus, semimembranosus, abductor magnus, abductor longus and sartorius for midthigh and soleus, tibialis anterior, tibialis posterior, gastrocnemius, peroneus longus, and flexor digitorium longus for midcalf) and are, therefore, reflective of mixed muscle. In contrast, both MA and IMCL measured in soleus, a predominantly slow-twitch oxidative fiber with a high capacity for storage of lipid droplets, may not be expected to correlate with MA or IMCL measured in the TA muscle, a predominantly fast-twitch muscle with a lower relative capacity for storage of lipid droplets (46). Indeed, our finding that IMCL in the soleus muscle was approximately six times greater than in the TA muscle is in agreement with the fiber type distribution hypothesis and similar to reports of other groups who have reported IMCL values of ∼3- to 5-fold higher in the soleus compared with the TA muscle (11,44,45). Overall, these results suggest that the muscle group(s) selected should be carefully considered. With respect to published studies on insulin resistance and muscle lipid accumulation, however, it is interesting to note that the muscle group most commonly measured by CT (midthigh of both legs) was correlated with the muscle most commonly measured by MRS (soleus; r = −0.55 for IMCL, n = 19, data not reported).
Another important goal of this study was to assess the methodological variation of CT and MRS. This is important for determining whether both methods are capable of detecting small changes caused by biological variation or clinical intervention. We found that MA measured by CT was more reproducible overall than EMCL or IMCL measured by MRS but that the CVs for both MA (2% in soleus and 4% in TA) and IMCL (6% to 7% in soleus, 10% to 11% in TA) were perfectly acceptable for biological measures. Our CVs by CT, however, are slightly higher than those reported by Goodpaster et al. (6) in both the midthigh (CV = 0.51%) and calf (CV = 0.85%) muscle groups. This may be caused by the added methodological error of identifying and tracing the region of interest for the individual muscle groups from the CT image. This task was most difficult in leaner individuals with small facial layers and in the TA compared with soleus muscle. Our CVs for IMCL and EMCL, nevertheless, are in close agreement with reports from other laboratories, including our previously published results using spectroscopic imaging at a higher field strength (22,28,39,47). For example, using a PRESS sequence at 1.5 T, Szczepaniak et al. (22) reported a CV of 7.8% and 11.8% for IMCL and 22.6% and 52.5% for EMCL (12- to 15-mm3 voxel sizes) in repeated measures of lean and obese subjects, respectively. At 4.1 T, we found a CV of 6.4% for soleus IMCL and 18.3% for EMCL determined from the sum of nine contiguous pixels representing a muscle volume of 2.5 mL. Furthermore, research from Rico-Sanz et al. (47) suggested that much of the test—retest variability of MRS experiments may be accounted for by positioning (or repositioning) during follow-up scans (which is very difficult with most patient table and coil set-ups). Their studies found that the test—retest variability for IMCL and EMCL in soleus muscle was only ∼2% and 3%, respectively, when volunteers were not repositioned between follow-up PRESS sequences at 1.5 T (47). The slightly higher CV for TA compared with the soleus in this study is likely caused by a smaller signal-to-noise ratio in this primarily fast twitch fiber, which, because of its size in sedentary individuals, prohibits the same signal summing that is possible in the soleus muscle. The greater variability of MRS measurements is probably a combination of the smaller sampling area (lipid storage is not homogenous across the muscle) and greater sensitivity to positioning compared with CT, combined with the additional error associated with resonance peak fitting (which is partially dependent on adequacy of peak splitting between EMCL and IMCL signals). While this study presents important reliability data for a mixed group of normal weight, over-weight, and obese subjects, future studies should focus on whether reliability of both CT and MRS is different in groups varying in adiposity and fitness level. Certainly, the degree to which subcutaneous fat stores can influence these measurements may be in question, but, more importantly, contamination of IMCL by EMCL as a result of fiber heterogeneity in more obese sedentary subjects may influence reliability of MRS data.
A final purpose of the study was to address a few methodological questions related to MRS that remain unanswered. Most importantly, we studied whether an external oil phantom and the non—water-suppressed internal water peak could be used as a normalization reference in obesityrelated studies. Typically, to account for day-to-day variation in the MR system and external magnetic field, proton peak areas fit from MR spectra are normalized to either an external phantom of known constant concentration (13,33) or an internal peak that scales with muscle and is assumed to be of constant concentration [e.g., the water peak of non—water-suppressed signal (11,12,14,20) or the creatine peak (10,16)]. Our preference has been to develop an alternative to using muscle water as the reference peak because much of our interest is also in measuring skeletal muscle lipid before and after endurance exercise, where it is likely that the intra- and extracellular water compartments are changing (28). We had previously relegated the creatine peak as a normalization reference because of its high variability and resonance peak broadening noted during our spectroscopic imaging protocol (CV of raw creatine peak = 13% to 14%; unpublished observations). This study at 1.5 T confirmed this earlier finding, noting that the test—retest variability of the peanut oil phantom and the non—water-suppressed water peak (CVs <3%) was significantly less than that of the creatine peak (CV = 11.6% in soleus). More importantly, this study found that the peanut oil phantom and the water peak produced normalized results for all peak areas that were similar (CV for IMCL-CH2 = 7.26 and 6.3, respectively; Table 6), were more reliable than creatine normalized results (CV for IMCL-CH2 = 15.64%), and were strongly cross-correlated (i.e., R2 = 0.96 to 1.0; Figure 5). In addition, we also found that the fatty acid CH3 proton peak, which is often disregarded because of low signal intensity and presumed inadequate resolution at clinical field strength (22), is measurable, reproducible, and highly correlated with the CH2 peak (particularly in soleus muscle, r = 0.91). These results assure use of the CH3 peak as an additional marker of IMCL (30,48) and lend credence to its possible use as an independent spectroscopic measure of muscle lipid. The CH3 peak is independent of fatty chain length and saturation and, thus, could be important in quantification models, given adequate and consistent resolution. Our ability to reproducibly measure the CH3 peak may be because of our prior knowledge files (30), signal summing, and time domain fitting.
In conclusion, this set of clinical experiments supports the use of both CT and MRS as reliable in vivo methods for assessing skeletal muscle lipid stores in obesity and diabetes research. Although MRS is truly non-invasive (involves no exposure to ionizing radiation) and may be advantageous for specifically measuring IMCL or resolving differences between muscle groups, CT is an extremely reliable method that is more accessible and produces results in the soleus muscle that are moderately correlated with MRS. Further testing in other muscle groups, e.g., thigh muscle, however, is warranted.
Acknowledgments
This work was supported by KO1 Grant DK062018–01, UO1 Grant AG20478, NIDDK Grant DK60412–02, and Look AHEAD (DK056990–04, G.B.) grants at the Pennington Biomedical Research Center and the University of Pittsburgh (DK057002–06, MO1RR00056, D.K.). We are grateful to all of the volunteers, the staff, and the principal investigators (PIs) and study coordinators (SCs) of the Look AHEAD Site at Pennington and George Bray (PI), Allison Strate (Co-SC), and Kristi Rau (Co-SC) for providing Look AHEAD subjects for the study. We are also grateful to the Pennington CALERIE Group and Jana Ihrig (SC). The authors also thank Edward J. Robarge, Ravishankar Madduri, Julia St Amant, Tuong Nguyen, and Bret Goodpaster for technical assistance with collection and analysis of MRS and CT data and Stephen Redmann, PhD, for statistical assistance on the test—retest analysis.
Appendix: LookAHEAD Adipose Research Group
Clinical Sites
Pennington Biomedical Research Center: Eric Ravussin, Steven R. Smith, Leonie K. Heilbronn, Severine G. Dubois, Olga Sereda, Michele McNeil, Salman Balghian, and Mandy Shipp.
University of Pittsburgh: David Kelley, Therese McKolanis, Koichiro Azuma, and Carol Kelley.
Footnotes
- TA
- tibialis anterior
- CT
- computed tomography
- MRS
- magnetic resonance spectroscopy
- HU
- Hounsfield unit
- MA
- muscle attenuation
- EMCL
- extramyocellular lipid
- IMCL
- intramyocellular lipid
- TE
- echo time
- TR
- repetition time
- FID
- free induction decay
- CV
- coefficient of variation
- ICC
- intraclass correlation
- CH2
- methylene
- CH3
- methyl
References
- 1.Goodpaster BH, Kelley DE. Role of muscle in triglyceride metabolism. Curr Opin Lipidol. 1998;9:231–6. doi: 10.1097/00041433-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24:933–41. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 3.Pan D, Lillioja S, Kriketos A, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–8. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–61. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–7. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–85. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–92. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–72. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 10.Forouhi NG, Jenkinson G, Thomas EL, et al. Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia. 1999;42:932–5. doi: 10.1007/s001250051250. [DOI] [PubMed] [Google Scholar]
- 11.Jacob S, Machann J, Rett K, et al. Association of increased intramyocellular lipid content with insulin resistance in lean non-diabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–9. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 12.Krssak M, Petersen F Falk, Dresner A, et al. Intramyocel-lular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study (rapid communication) Diabetologia. 1999;42:113–6. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 13.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–6. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 14.Sinha R, Dufour S, Petersen KF, et al. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51:1022–7. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 15.Levin K, Daa Schroeder H, Alford FP, Beck-Nielsen H. Morphometric documentation of abnormal intramyocellular fat storage and reduced glycogen in obese patients with type II diabetes. Diabetologia. 2001;44:824–33. doi: 10.1007/s001250100545. [DOI] [PubMed] [Google Scholar]
- 16.Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, et al. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 2001;50:2337–43. doi: 10.2337/diabetes.50.10.2337. [DOI] [PubMed] [Google Scholar]
- 17.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–15. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 18.Simoneau J-A, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9:273–8. [PubMed] [Google Scholar]
- 19.Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. Computed tomography. In: Passano WM, editor. The Essential Physics of Medical Imaging. Lippincott Williams & Wilkins; Philadelphia, PA: 1994. pp. 327–72. [Google Scholar]
- 20.Stein DT, Szczepaniak LS, Dobbins RL, Snell P, McGarry JD. Skeletal muscle triglyceride stores are increased in insulin resistant state. Proc Intl Soc Mag Reson Med. 1998:388. [Google Scholar]
- 21.Salibi N, Brown MA. Clinical MR Spectroscopy. First Principles. Wiley-Liss; New York: 1998. [Google Scholar]
- 22.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglycerice stores by 1H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–89. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 23.Schick F, Eismann B, Jung WI, et al. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: two lipid compartments in muscle tissue. NMR Biomed. 1993;29:158–67. doi: 10.1002/mrm.1910290203. [DOI] [PubMed] [Google Scholar]
- 24.Boesch C, Kreis R. Dipolar coupling and ordering effects observed in magnetic resonance spectra of skeletal muscle. NMR Biomed. 2001;14:140–8. doi: 10.1002/nbm.684. [DOI] [PubMed] [Google Scholar]
- 25.Szczepaniak LS, Dobbins RL, Stein DT, McGarry JD. Bulk magnetic susceptibility effects on the assessment of intra- and extramyocellular lipids in vivo. Magn Reson Med. 2002;47:607–10. doi: 10.1002/mrm.10086. [DOI] [PubMed] [Google Scholar]
- 26.Heilbronn LK, Delany J, De Jonge L, et al. Effect of 6-mo calorie restriction on 24h energy expenditure and core temperature in healthy subjects. Submitted for publication.
- 27.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 28.Larson-Meyer DE, Hunter GR, Newcomer BR. Influence of endurance running and recovery diet on intramyocellular lipid content in women: a 1H-NMR study. Am J Physiol. 2002;282:E95–106. doi: 10.1152/ajpendo.2002.282.1.E95. [DOI] [PubMed] [Google Scholar]
- 29.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–52. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 30.Rico-Sanz J, Thomas EL, Jenkinson G, et al. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J Appl Physiol. 1999;87:2068–72. doi: 10.1152/jappl.1999.87.6.2068. [DOI] [PubMed] [Google Scholar]
- 31.Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990;14:26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- 32.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 33.Perseghin G, Scifo P, Danna M, et al. Normal insulin sensitivity and IMCL content in overweight humans are associated with higher fasting lipid oxidation. Am J Physiol Endocrinol Metab. 2002;283:E556–64. doi: 10.1152/ajpendo.00127.2002. [DOI] [PubMed] [Google Scholar]
- 34.Boesch C, Decombaz J, Slotboom J, Kreis R. Observation of intramyocellular lipids by means of 1H magnetic resonance spectroscopy. Proc Nutr Soc. 1999;58:841–50. doi: 10.1017/s0029665199001147. [DOI] [PubMed] [Google Scholar]
- 35.Howald H, Boesch C, Kreis R, et al. Content of intramyocellular lipids derived by electron microscopy, biochemical assays, and (1)H-MR spectroscopy. J Appl Physiol. 2002;92:2264–72. doi: 10.1152/japplphysiol.01174.2001. [DOI] [PubMed] [Google Scholar]
- 36.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23:126–32. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 37.Munoz J, Gower BA. Relationship between serum leptin concentration and low-density muscle in postmenopausal women. J Clin Endocrinol Metab. 2003;88:1157–61. doi: 10.1210/jc.2002-020959. [DOI] [PubMed] [Google Scholar]
- 38.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 39.Boesch C, Slotboom J, Hoppeler H, Kreis R. In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magn Reson Med. 1997;37:484–93. doi: 10.1002/mrm.1910370403. [DOI] [PubMed] [Google Scholar]
- 40.Shen W, Punyanita M, Wu KE, et al. High resolution magnetic resonance imaging in quantification of intra-muscular and peri-muscular adipose tissue in leg region (abstract) Obes Res. 2003;11:A55. [Google Scholar]
- 41.Hwang JH, Pan JW, Heydari S, Hetherington HP, Stein DT. Regional differences in intramyocellular lipids in humans observed by in vivo 1H-MR spectroscopic imaging. J Appl Physiol. 2001;90:1267–74. doi: 10.1152/jappl.2001.90.4.1267. [DOI] [PubMed] [Google Scholar]
- 42.Deriaz O, Dumont M, Bergeron N, et al. Skeletal muscle low attenuation area and maximal fat oxidation rate during submaximal exercise in male obese individuals. Int J Obes Relat Metab Disord. 2001;25:1579–84. doi: 10.1038/sj.ijo.0801809. [DOI] [PubMed] [Google Scholar]
- 43.Cuff DJ, Meneilly GS, Martin A, et al. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–82. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 44.Thamer C, Machann J, Bachmann O, et al. Intramyo-cellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab. 2003;88:1785–91. doi: 10.1210/jc.2002-021674. [DOI] [PubMed] [Google Scholar]
- 45.Brechtel K, Dahl DB, Machann J, et al. Fast elevation of the intramyocellular lipid content in the presence of circulating free fatty acids and hyperinsulinemia: a dynamic 1H-MRS study. Magn Reson Med. 2001;45:179–83. doi: 10.1002/1522-2594(200102)45:2<179::aid-mrm1023>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 46.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111–29. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 47.Rico-Sanz J, Hajnal JV, Thomas EL, et al. Intracellular and extracellular skeletal muscle triglyceride metabolism during alternating intensity exercise in humans. J Physiol. 1998;510:615–22. doi: 10.1111/j.1469-7793.1998.615bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krssak M, Perersen KF, Bergeron R, et al. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnet resonance. J Clin Endocrinol Metab. 2000;85:748–54. doi: 10.1210/jcem.85.2.6354. [DOI] [PubMed] [Google Scholar]