Abstract
Background
Transcranial magnetic stimulation (TMS) is a relatively noninvasive brain stimulation technology that can focally stimulate the human cortex. One significant limitation of much of the TMS research to date concerns the nature of the placebo or sham conditions employed. When TMS pulses are delivered repetitively (especially prefrontal TMS) it is often experienced as painful. Most sham TMS techniques produce identical sounds to active TMS, but they do not cause much, if any, scalp or facial sensation or discomfort. This is a serious problem when investigators are attempting to evaluate the effects of TMS using traditional sham techniques because of unintended systematic differences between real and sham TMS groups (i.e., confounds). As long as traditional approaches to sham TMS are employed, the validity of the inferences regarding the efficacy of TMS will be limited. While some other sophisticated systems have been developed to address these concerns, they tend to be expensive and lack portability. Portability will likely become more and more important as TMS applications expand into different clinical areas (e.g., TMS in the post-anesthesia care unit following surgery)
Methods
The present study describes a portable electrical TMS sham system (eSham system) modeled after the James Long System that was designed to produce similar scalp sensations as real TMS. Preliminary results are presented on 9 healthy adults that received both real and eSham 10Hz rTMS (at 80%, 100% and 120% of resting motor threshold) over the prefrontal cortex and rated the sensation quality (pain, tingling, sharpness, piercing, electric, tugging, pinching), tolerability, and location. Results: Real TMS and eSham TMS were rated similarly across all 7 sensory dimensions examined. Real and eSham TMS were also rated similarly with respect to tolerability and perceived location of the TMS-induced sensations.
Conclusions
The eSham system may be a simple, affordable, and portable approach to providing convincing sham TMS for future clinical trials. The present study provides preliminary evidence supporting the use of the eSham system. Future larger-scale studies are warranted.
Introduction
Transcranial magnetic stimulation (TMS) is a noninvasive brain stimulation technology that can focally stimulate the human cortex.1,2 Several studies have found that rTMS delivered over motor cortex can affect the perception of laboratory-induced pain in healthy adults as well as chronic neuropathic pain in clinical samples. 3–12 Additionally, a few studies have demonstrated anti-nociceptive effects with TMS over the prefrontal cortex TMS.13–17
One significant limitation of much of the research on the effects of TMS on pain perception to date concerns the nature of the placebo or sham conditions employed. When TMS pulses are delivered repetitively (especially prefrontal TMS) it is often experienced as painful (and at a minimum it produces noticeable scalp and/or facial sensations; see Borckardt18). Most sham TMS techniques (whether they involve tilting the coil away from the scalp or whether a specially designed sham TMS coil is used) produce identical sounds to active TMS, but they do not cause much, if any, scalp or facial sensation or discomfort. This is a serious problem when investigators are attempting to evaluate the effects of TMS using traditional sham techniques for several reasons including: 1) introduction of unintended systematic differences between real and sham TMS groups (i.e., confounds), 2) participants may be able to correctly guess the condition to which they have been randomized (introducing demand characteristics), and 3) real TMS may lead to changes in pain perception independent of the intended cortical stimulation. A typical TMS session lasts 20-minutes, and it is possible that the painfulness of the experience triggers pain modulatory activity in research subjects (e.g., endogenous opioid activity, cognitive changes, activation of other descending pain inhibitory mechanisms). Thus, when comparing the effects of real TMS to sham TMS on pain perception, any observed antinociceptive effects of real TMS may be simply due to exposing subjects to a 20-minute painful procedure. These effects may have little or nothing to due with changes in cortical activation. Until a simple, affordable sham TMS system is available that produces facial/scalp sensations comparable to real TMS, valid inferences about the effects of TMS on pain perception will be limited.
A few studies have begun to address the limitations associated with traditional approaches to sham TMS. Okabe et al (2003) implemented an electrical sham system in a controlled trial of rTMS for Parkinson’s Disease. A 0.2 ms electrical pulse was delivered in-time with TMS coil clicks at twice the intensity of participants’ sensory thresholds for skin stimulation. However, it is unclear based on the material presented in this paper, what kind of equipment was used to produce the electrical stimulation, what methods were implemented to sync the electrical pulses with the TMS coil noises, what TMS coils the system would work with, and what costs were associated with building and implementing the system. Very recently, Rossi et al (2007) has introduced a sophisticated sham system (real electro-magnetic placebo; REMP) in which a compact wood component (contoured to the shape of a Magstim figure-8 coil) is attached to the active surface of a real Magstim coil with Velcro strips. The thickness of the component (3 cm) is likely sufficient to attenuate most of the induced electrical currents by the TMS coil. An electric stimulator is connected to round copper metal disks embedded in the surface of the REMP attachment. Electrical pulses are then delivered in sync with the TMS pulses. While it is likely possible to develop REMP attachments for different coil types and shapes, at present, it appears that this device has only been developed and tested for the 70mm figure-eight shaped Magstim coil. One potential problem that might arise with this otherwise very elegant system, is that it would be difficult to truly blind the TMS administrator as to whether a participant was receiving real or sham TMS because the REMP device changes the weight and appearance of the handheld coil. Thus, the present REMP design would permit the implementation of excellent single-blind research methods but double-blind studies would prove considerably more difficult.
One emerging issue regarding TMS systems in general is related to portability. Recently, Borckardt et al (2006a) has found that 20-minutes of 10Hz prefrontal TMS (at 100% of resting motor threshold) is associated with a reduction in patient-controlled analgesia use post-operatively (a total of 4000 pulses were delivered in the 20-minute session). In this study, a Neuronetics TMS machine was wheeled into the post-anesthesia care unit (PACU) and TMS was delivered to patients immediately following bariatric surgery. The available Magstim coils are not be capable of stimulating at a frequency of 10Hz for 20 minutes (4000 pulses) without problems related to coil overheating. Thus, the sham systems described above (for use with Magstim coils) would not work for this type of TMS application. In order to have provided sham stimulation that matched real TMS with respect to facial and scalp sensations with currently available technology using our Neuronetics machine, we would have had to transport an expensive CPU along with a monitor, and large electric pulse-generator to the PACU along with the TMS machine (as per the James-Long sham TMS system described in the Methods section). Additionally, we would have had to set-up this rather bulky and elaborate system for each participant in the study, which would have added considerable time and effort.
In this paper, we first describe the development of a simple, portable, and relatively inexpensive sham TMS system designed to mimic real TMS with respect to perceived facial/scalp sensations, and painfulness. This system is designed to work with any TMS machine that has a TTL output port. It is small enough to sit atop the TMS machine, and is inexpensive enough to implement in trials that have limited funding. Additionally, we present data from a small pilot trial in which the sensations and location (scalp and/or facial) produced by the sham system are compared to those produced by real TMS.
Methods
Current Sham TMS System Technology
In a current multi-site NIH sponsored trial of left prefrontal TMS for depression, the James Long sham TMS system is being employed (James Long Company; NY; USA). This system integrates a Mecta (Mecta Corp. Lake Oswego, OR) system with a Neuronetics (Neuronetics Inc, Malvern, PA) TMS machine. Two electrodes from the Mecta system are placed on the subjects forehead anterior to the TMS coil and the Mecta system is attached to the Neuronectics TMS machine. Every time a sham TMS pulse is delivered, a TTL pulse is sent from the TMS machine to the Mecta triggering a brief, mild electrical pulse that is delivered through the electrodes to the subject’s scalp. This system also employs an auditory masking system so that neither the subject or the TMS operator can hear the TMS pulses being delivered thereby reducing the chances of identifying whether real or sham TMS is being delivered. The James Long system provides an extremely high quality method for conducting double-blind TMS trials. However, it is quite expensive and requires the use of a lot of bulky equipment (2 separate computers plus the Mecta machine and digital display).
eSham System Development
With TMS research expanding into different hospital settings13, there is a need for a portable, convincing sham TMS system. Using James Long’s basic idea, the authors sought to develop a system that would be light, portable, inexpensive, and that produced scalp sensations similar to real TMS. Additionally, the system was designed to be able to work with any TMS machine that has a TTL output port. This system, just like the James Long system, delivers a brief, mild electrical stimulus to the scalp in sync with the sham TMS pulses (referred to as the eSham system in this paper).
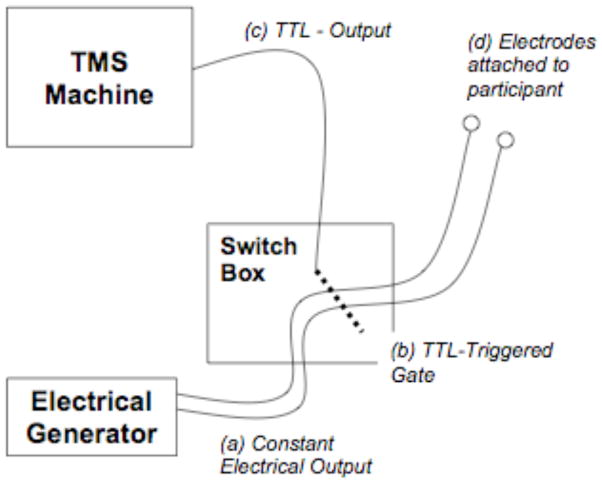
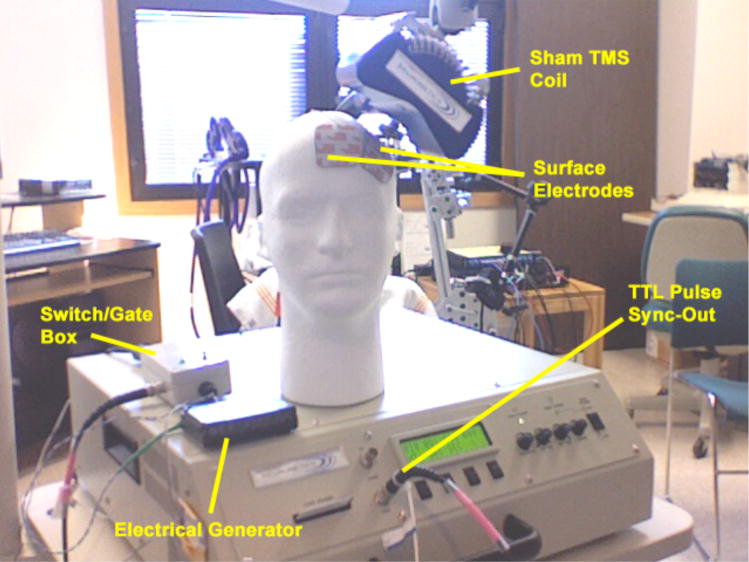
A portable electrical stimulus generator (Epix VT; Empi, St. Paul, MN; USA) powered by a 9-volt battery is used to delivery a constant stimulus (150 pulses per second) to a custom developed switch-box that blocks the continuous electrical stimulus from reaching the participant. Two 1/2-inch, round, metal electrodes (or flat Thymapad ® Stimulus Electrodes, Somatics, LLC; Lake Bluff, IL, USA) are attached from the switch box to the subject’s forehead immediately anterior to the TMS coil (or directly underneath the TMS coil if Thymapad ® Stimulus Electrodes are used). A BNC cable connects the TMS machine to the switch-box and every time the TMS machine delivers a pulse, a TTL signal is sent via the BNC cable to the switch-box. Upon receiving the TTL pulse, the switch box opens a gate for ~250 μs allowing the electrical stimulus through to the subject’s scalp. Thus, participants experience a brief (~250 μs) electrical pulse every time the sham TMS coil clicks. The intensity of the stimulus is adjustable at the electrical generator (1 to 60 mA) and the time that the gate is let open after each TTL trigger is adjustable on the switch-box as well. See figure 1 for a diagram of the sham system and see figure 2 for a photo of the actual system attached to a TMS machine.
Figure 1.
Diagram of the eSham system. A constant electrical stimulus (a) is delivered to a specially designed switch box (b) where it is gated and prevented from continuing-on to the surface electrodes attached to the participant. Whenever a TTL pulse is (c) is delivered to the switch box (i.e., when the TMS machine fires), the gate is opened for ~250 μs allowing a very brief electrical pulse through to the participant’s scalp in sync with the audible TMS sham-coil pulse..
Figure 2.
Photo of the eSham system attached to a TMS machine equipped with a sham TMS coil.
Subjects
Nine non-depressed adults (3 female) with no history of chronic pain disorders volunteered to participate in this study approved by the Institutional Review Board for the Protection of Human Subjects at the Medical University of South Carolina. All subjects were free of medications known to lower seizure threshold, had no implanted medical devices, and had no history of stroke or seizure. None reported a history of any chronic medical conditions including psychiatric disorders and chronic pain conditions.
Motor Threshold Assessment and Coil Placement
After providing written informed consent, resting motor threshold was estimated. Two Neuronetics 2100 TMS machines were used (one with an active coil and one with sham), and were placed behind the participants, out of site. The active machine was set to 40% of maximum output. The TMS coil was positioned over each subject’s motor cortex and pulses were delivered at the rate of 1 per 4 seconds. The intensity and location of the stimuli delivered were systematically adjusted until the area of the motor cortex that controls the Abductor Pollicus Brevis muscle (APB) was located. Next, a parameter estimation by sequential testing (PEST) algorithm was used to determine the amount of machine output necessary to produce visual thumb movement 50% of the time (resting motor threshold; rMT19). After motor threshold was assessed, the prefrontal cortex was located by moving the coil 5 cm anterior along a parasagital line. The coil position was marked on the subject’s scalp using a non-toxic felt-tipped marker.
Titrating the eSham TMS system
Next, metal electrodes from the portable eSham system were attached to each subject’s forehead immediately anterior to the TMS coil, and held in place by a rubber strap. The cathode was placed medially. Redux gel was used to ensure good contact between the electrodes and the subject’s scalp.
Subjects were administered 1-second-trains of real TMS over the prefrontal cortex (10 Hz) at 80%, 100%, and 120% of rMT (randomly ordered) and they rated the painfulness of each sensation using a numeric rating scale (0=no pain at all to 10=worst pain imaginable). These ratings were recorded on the clinical research form for future reference. Next, the sham TMS coil was placed over the subject’s prefrontal cortex and the eSham system was set to deliver electrical stimuli starting at 1mA (in sync with the audible TMS pulses at 10Hz) in trains lasting 1-second. Subjects were asked to rate the painfulness of each 1-second train using the same numeric rating scale. The intensity of the electrical pulses were adjusted and a PEST algorithm was used to match the subjective pain rating of the electrical stimulation to the rating of the real TMS at 100%. A minimum of 30 secs elapsed between all of the 1-second pulse trains. The entire sham titration procedure takes between 3 and 5 minutes to complete.
Study Design
To compare real TMS with the eSham system, participants received a total of 12 4-sec stimulus trains. Half of the trains were delivered using the real TMS coil at 80%, 100% or 120% of rMT (2 trials each). The other 6 trains were delivered using the sham coil and eSham system at 80%, 100% or 120% (2 trains each) of the mA setting that was matched to real TMS (at 100% of rMT) during the titration process. The order of stimuli was randomized. Subjects were blind to whether the stimuli were real or sham TMS and they were not told the intensity of each stimulus.
Measuring pain location, quality and intensity
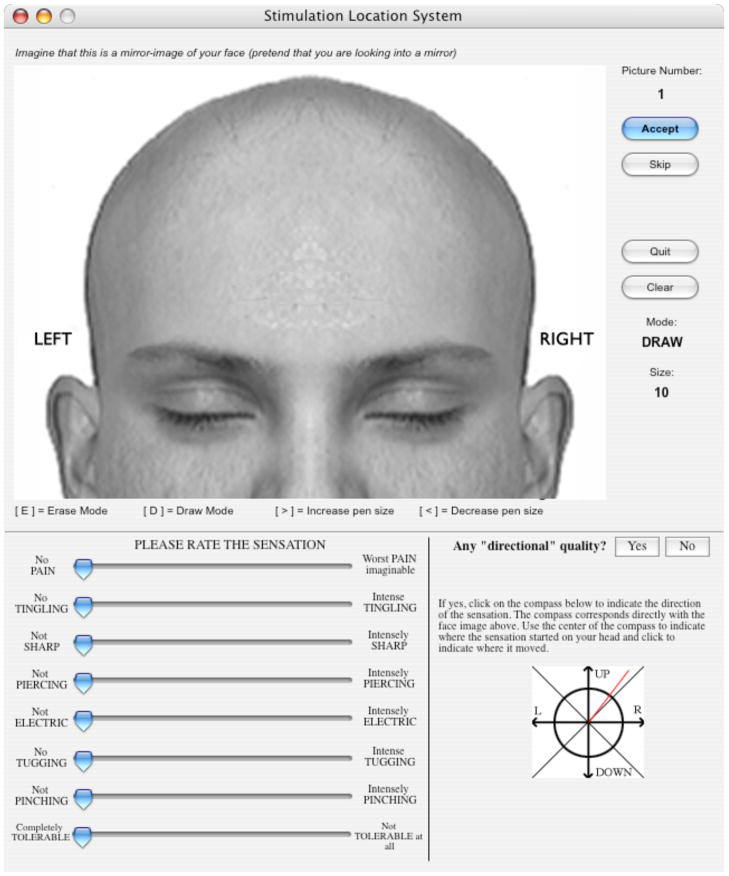
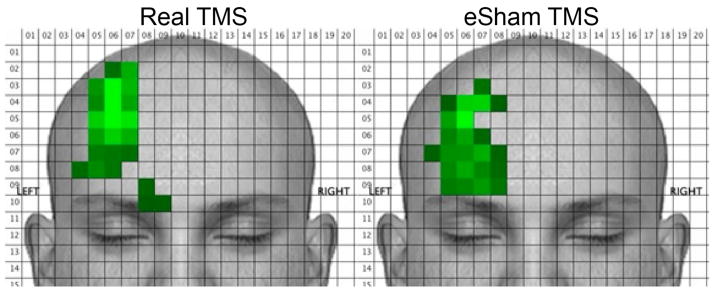
After each stimulus was delivered, subjects used a custom-developed computer program with several visual analogue scales (ranging from 0 to 100) to rate the sensation (pain, tingling, sharp, piercing, electric, tugging, pinching, and overall tolerability). These sensory dimensions were selected as they were thought to cover a broad range of possible scalp sensations associated with TMS and sham stimulation. They also used the computer mouse to draw on a picture of a human face to indicate where the sensation(s) were felt. Lastly, subjects indicated whether the sensation had a directional quality (i.e., whether it felt like the sensation “moved” across their skin) and, if so, they indicated the direction that the sensation moved using an on screen “compass.” The computerized drawings of the facial/scalp sensation locations were compiled and common areas of activation were determined as the mean number of colored pixels across subjects within 20 by 20 pixel squares (2-dimensional voxels). Brighter green voxels on the summary figures indicate that participants colored more pixels on average within that voxel. Figure 3 shows a screen-shot of the program.
Figure 3.
Screen shot of the software used to collect sensation ratings and to assess the areas of the face and scalp where the sensations were felt.
Results
Both real and eSham rTMS were experienced as mildly to moderately painful. Real TMS at 80% of rMT was rated, on average, 19.28 (StdDev=17.52) out of 100 while the eSham system was rated as 29.22 (StdDev=25.61). Real TMS at 100% of rMT was rated as 37.06 (StdDev=27.60) and sham TMS was rated on average as 34.61 (StdDev=19.86). Real TMS at 120% rMT was rated as 55.28 (StdDev=31.68) while sham TMS at 120% was rated 39.72 (StdDev=27.56).
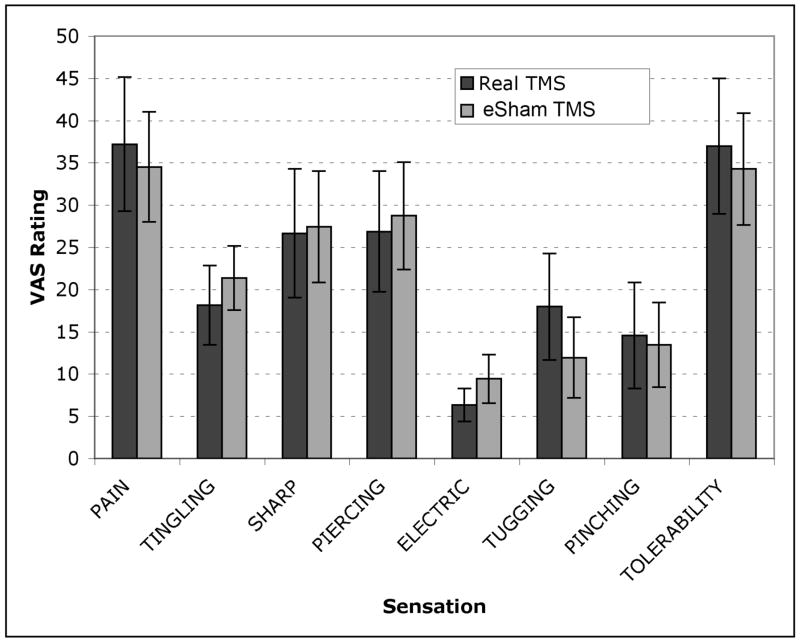
Means (and 95% confidence intervals) for the all of the sensation ratings are shown in figure 4 for real and eSham TMS conditions. No significant differences were found between real and eSham TMS for any of the sensation dimensions. Break-down of the sensation ratings by stimulus intensity (80%, 100%, 120%) did not reveal any differences on any of the sensation dimensions between real and sham TMS. Figure 5 shows the face and scalp areas that produced sensations under both real and eSham TMS conditions. The eSham system produced sensations in the same general facial/scalp areas as real TMS. The left, right, top and bottom edges of the sensations in each condition were captured via the Face Locator program as well as the center of the each sensation across both the x and y axes. Independent t-tests were conducted on each of these dependent measures for real versus eSham TMS, and no statistically significant differences were emerged with respect to the location of the sensations.
Figure 4.
Mean (and 95% CI) visual analogue scale ratings for each of the sensory dimensions assessed during real and eSham TMS.
Figure 5.
Mean face and scalp areas of activation during both real and eSham TMS
The sensations were not more likely to be perceived as having a directional quality as a function of the real or eSham system and there were no differences in directionality of the sensations between conditions.
Discussion
The eSham system appears to produce face and scalp sensations that are comparable to real TMS. Additionally, the location of the sensations appears to be comparable between the two conditions (real and sham). The eSham system produced sensations slightly lower on the forehead, which may be due to the fact that the electrodes employed in this trial (metal electrodes) were placed immediately anterior to the TMS coil. As briefly mentioned in the methods section, different electrodes can be used with the eSham system. Thymapad Stimulus Electrodes appear to work well with the system. These pads have a greater contact area and are flat allowing for placement directly beneath the TMS coil (which may eliminate the slight difference in sensation location observed in this pilot).
Repetitive TMS over the left-prefrontal cortex appears to be mildly to moderately painful (Borckardt et al, 2006b). Typical rTMS clinical and research settings involve repeated stimulation at 100% or 120% of rMT. The average pain intensity ratings of such stimulation in this pilot were 37.06 and 55.28 out of 100, respectively. This degree of pain intensity is substantial enough that it should not be overlooked in future trials of TMS for pain (or for any other disorders or conditions). If sham TMS systems that produce no physical sensations continue to be used, it will continue to be difficult to discern whether any observed analgesic TMS effects are due to cortical stimulation or are just the result of having subjects undergo a mildly to moderately painful 20-minute procedure.
The sham system employed in this pilot appears to be safe and there were no reports of side effects. We do not believe that there is any theoretical or empirical evidence to suggest that the electrical stimulation at the levels used in this study (ranging from 2 mA to 7 mA) delivered to the scalp would reach the cortex and result in any unintended cortical or subcortical effects.
One unique feature of the present study involves the implementation of a titration procedure designed to match the sham sensations with real. Titration may be important to conduct from participant to participant and from TMS-session to TMS-session. Participants will likely differ with respect to their subjective experiences of TMS-related discomfort, and with respect to their experiences of the electrical stimuli. Additionally, variability from session to session with respect to electrode contact (i.e., impedance) and placement may impact subjective experiences of unpleasantness. Thus, titration should probably be performed before each TMS session. In controlled rTMS trials employing the eSham system, it is important to attach the eSham system and titrate it even if participants are scheduled to receive real TMS. This would help to minimize procedural differences between real and sham TMS conditions.
There are several limitations to the present study that should be addressed in future research. First, the sample employed is small. It is possible that with larger samples, differences may emerge between the experience of real versus eSham TMS. Larger trials may permit identification of subtle differences and then efforts can be made to further improve the eSham system. Also, participants were not asked to guess whether each train delivered was real or sham. While we believe that the methods employed permit conclusions regarding the similarity of real and eSham TMS, future studies should specifically ask participants to guess whether they had received real or sham TMS. It is unclear whether prior experience with TMS would influence the experience of eSham TMS. This issue should be addressed in future studies as well.
The eSham system was built for about $1000 and the components sit on top of the TMS machine allowing for good portability. The system can be used with any TMS machine that has a TTL output port. If additional personnel are used to configure the TMS machine (i.e., change the TMS coil from real to sham if necessary, set the eSham electrical generator output) outside the treatment room before rTMS stimulation is started with a research participant, it is possible for the TMS administrator and the participant to be blinded as to whether real or sham TMS is being delivered, thus permitting true double-blind, placebo-controlled rTMS studies.
Table 1.
Mean (and standard deviation) painfulness ratings of real and eSham TMS at 3 different TMS intensities expressed as a percentage of resting motor threshold.
| Intensity | Condition Mean | Pain Rating | Std. Deviation | t-value | p-value |
|---|---|---|---|---|---|
| 80% | Real | 19.28 | 17.522 | ||
| Sham | 29.22 | 25.607 | 1.36 | .18 | |
| 100% | Real | 37.06 | 27.603 | ||
| Sham | 34.61 | 19.856 | 0.31 | .76 | |
| 120% | Real | 55.28 | 31.676 | ||
| Sham | 39.72 | 27.563 | 1.57 | .13 |
References
- 1.George MS, Nahas Z, Kozel FA, et al. Mechanisms and the current state of transcranial magnetic stimulation. CNS Spectrums. 2003;8(7):496–514. doi: 10.1017/s1092852900018976. [DOI] [PubMed] [Google Scholar]
- 2.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of the human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 3.Migita K, Uozumi T, Arita K, Monden S. Transcranial magnetic coil stimulation of motor cortex in patients with central pain. Neurosurgery. 1995;36:1037–9. doi: 10.1227/00006123-199505000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Rollnik JD, Wustefeld S, Dauper J, Karst M, Fink M, Kossev A, Dengler R. Repetitive transcranial magnetic stimulation for the treatment of chronic pain-a pilot study. Eur Neurol. 2002;48:6–10. doi: 10.1159/000064950. [DOI] [PubMed] [Google Scholar]
- 5.Lefaucheur J-P, Drouot X, Keravel Y, Nguyen J-P. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. NeuroReport. 2001;12:2963–2965. doi: 10.1097/00001756-200109170-00041. [DOI] [PubMed] [Google Scholar]
- 6.Topper R, Hfoltys H, Meister IG, Sparing R, Boroojerdi B. Repetitive transcranial magnetic stimulation of the parietal cortex transiently ameliorates phantom limb pain-like syndrome. Clinical Neurophysiology. 2003;114:1521–1530. doi: 10.1016/s1388-2457(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 7.Pleger B, Janssen F, Schwenkreis P, et al. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neuroscience Letters. 2004;356:87–90. doi: 10.1016/j.neulet.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Tamura Y, Okabe S, Ohnishi T, et al. Effects of 1-Hz repetitive transcranial magnetic stimulation on acute pain induced by capsaicin. Pain. 2004;107:107–115. doi: 10.1016/j.pain.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Summers J, Johnson S, Pridemore S, Oberoi G. Changes to cold detection and pain thresholds following low and high frequency transcranial magnetic stimulation of the motor cortex. Neuroscience Letters. 2004;368:197–200. doi: 10.1016/j.neulet.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Lefaucheur JP, Drouot X, Menard-Lefaucher I, Nguyen JP. Neuropathic pain controlled for more than a year by monthly sessions of repetitive transcranial magnetic stimulation of the motor cortex. Neurophys Clin. 2004;34(2):91–95. doi: 10.1016/j.neucli.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Canavero S, Bonicalzi V, Dotta M, et al. Transcranial magnetic cortical stimulation relieves central pain. Stereo Funct Neurosurg. 2002;78(3–4):192–196. doi: 10.1159/000068965. [DOI] [PubMed] [Google Scholar]
- 12.Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76:833–838. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR, Byrne K, Morgan K, George M. Post-Operative Left Prefrontal Repetitive Transcranial Magnetic Stimulation Reduces Patient-Controlled Analgesia Use. Anesthesiology. 2006;105(3):1–6. doi: 10.1097/00000542-200609000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Reid P, Pridmore S. Improvement in chronic pain with transcranial magnetic stimulation. Australian & New Zealand Journal of Psychiatry. 2001;35(2):252. doi: 10.1046/j.1440-1614.2001.0884e.x. [DOI] [PubMed] [Google Scholar]
- 15.Graff-Guerrero A, Gonzalez-Olivera J, Fresan A, Gomez-Martin D, Mendez-Nunez JC, Pellicer F. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Cognitive Brain Research. 2005;21(1):153–160. doi: 10.1016/j.cogbrainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Sampson SM, Rome JD, Rummans TA. Slow-frequency rTMS reduces fibromyalgia pain. Pain Medicine. 2006;7(2):115–118. doi: 10.1111/j.1526-4637.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 17.Avery DH, Holtzheimer PE, Fawaz W, Russo J, Neumaier J, Dunner DL, Haynor DR, Claypoole KH, Wajdik C, Roy-Byrne P. Transcranial Magnetic Stimulation Reduces Pain in Patients With Major Depression: A Sham-Controlled Study. Journal of Nervous and Mental Disease. 2007;195(5):378–381. doi: 10.1097/NMD.0b013e31802f58d1. [DOI] [PubMed] [Google Scholar]
- 18.Borckardt JJ, Smith AR, Hutcheson K, Johnson K, Nahas Z, Anderson B, Schneider MB, Reeves ST, George MS. Reducing pain and unpleasantness during repetitive transcranial magnetic stimulation. Journal of ECT. 2006;22(4):259–264. doi: 10.1097/01.yct.0000244248.40662.9a. [DOI] [PubMed] [Google Scholar]
- 19.Borckardt JJ, Nahas Z, Koola J, George MS. Estimating Resting Motor Thresholds In TMS Research And Practice: A computer simulation evaluation of best methods. Journal of ECT. 2006;22(3):169–175. doi: 10.1097/01.yct.0000235923.52741.72. [DOI] [PubMed] [Google Scholar]
- 20.Rossi S, Ferro M, Cincotta M, Ulivelli M, Bartalini S, Miniussi C, Giovannelli F, Passero S. A real electro-magnetic placebo (REMP) device for sham transcranial magnetic stimulation. Clinical Neurophysiology. 2007;118:709–716. doi: 10.1016/j.clinph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Okabe S, Ugawa Y, Kanazawa I. 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic sham stimulation in Parkinson’s Disease. Movement Disorders. 2002;18(4):382–388. doi: 10.1002/mds.10370. [DOI] [PubMed] [Google Scholar]