Abstract

A concise non-aldol approach for the stereoselective construction of all-anti polypropionate fragments was developed. The iterative epoxide-based methodology consists of the syn-selective epoxidation of cis homoallylic alcohols using the VO(acac)2 catalyzed conditions followed by epoxide cleavage with a propynyl aluminum reagent as key steps. The methodology was applied to the synthesis of the all-anti C6-C10 fragment of streptovaricin U.
Introduction
Polypropionate chains are structural motifs consisting of alternating methyl and hydroxy groups within an aliphatic framework. These structures are found in many natural products having a wide range of biological and medicinal activity.1 Their synthesis has attracted great interest among synthetic chemists due to the challenge represented by the stereoselective assembly of the contiguous stereogenic centers.2 Among the diverse approaches that have been developed for the synthesis of polypropionates, aldol and aldol-related strategies are most prevalent, since these processes are well understood and their intricacies are well documented.3 Interestingly, the anti,anti,anti-stereotetrad 1 (Figure 1), found in many important natural products such as ionomycin, zincophorin, the streptovaricins, and others, has been the most difficult to construct when employing enantioselective aldol or crotylmetalation strategies.4 This is because the anti,anti relationship must arise from disfavored anti-Felkin transition states.5 This challenge has produced a number of ingenious anti selective aldol and crotylmetal strategies that have been very useful in polyketide synthesis.6,7
Figure 1.
The all-anti stereotetrad 1, -pentad 2, and -hexad 3
While the alternative approaches present high diastereo- and enantioselectivities, efforts to develop efficient stereoselective non-aldol methods for the construction of anti,anti polypropionate subunits continue.8 These efforts become more evident when longer all-anti stereotetrad (1) or -pentad (2) subunits (such as the C18–C21 and the C6–C10 segments of ionomycin,9 and streptovaricin U,10 respectively) need to be elaborated.
Epoxide-based approaches have also been used for the synthesis of polypropionates, albeit to a lesser extent. These versatile intermediates, which can be prepared stereoselectively by several methods,11 were first considered for polypropionate synthesis by Kishi,12 Corey13 and Bartlett,14 in studies related the synthesis of the rifamycin S ansa chain. During the mid eighties, Lipshutz developed a reiterative route for the preparation of all syn-1,3-polyols and polyketides, reacting optically active epoxides with higher order cis- or trans-propenyl cuprates.15 Later, Miyashita and co-workers developed a reiterative entry to polypropionates by means of a regio- and stereoselective methylation of γ,δ-epoxy acrylates with trimethylalane.16
Recently, we reported an epoxide-based methodology for the stereoselective construction of polypropionates (Scheme 1).17 Our reiterative approach includes a sequence of three reactions: stereoselective epoxidation of unsaturated alcohols, regioselective alkynyl alane cleavage of the epoxide, and cis or trans reduction of the incorporated alkyne to produce a new homoallylic alcohol. Each sequence incorporates a propionate fragment into the growing chain. The propionate unit formed during the epoxide cleavage step will have a syn relationship if the epoxide geometry is trans or an anti arrangement if a cis epoxide is used. The configuration of the resulting hydroxy groups is determined by the absolute configuration of the epoxide precursor, which results from a diastereoselective epoxidation of the alkenol precursor.18
Scheme 1.
General reiterative epoxide-based approach for polypropionate synthesis
The application of our method to the preparation and cleavage of a series of diastereomeric 3,4-epoxy alcohols produced the corresponding stereotetrads with different degrees of regioselectivity and yield. Remarkably, the “arduously accessible19 all-anti stereotetrad 9 was readily attained using this approach (Scheme 2). For this, the TIPS protected cis epoxy alcohol 6 was regioselectively cleaved with diethyl propynyl alane to yield the first anti unit. Hydrogenation of the incorporated alkyne produced alkenol 7 in good yield. An alternative copper catalyzed cis-propenylmagnesium bromide cleavage of epoxide 6 produced 7 in similar yield and regioselectivity with the convenient exclusion of the alkyne reduction step.20 Alkenol 7 was converted to epoxide 8 using the VO(acac)2 catalyzed epoxidation under microwave irradiation, which produced exclusively the desired syn epoxy alcohol in higher yield than the established iodocarbonation/methanolysis method.11b,c,18a, Finally, the propynyl alane cleavage of epoxide 8 produced the anti,anti,anti alkynyl diol 9 as the only regioisomer. These results prompted us to further elaborate on this approach with the intent to develop an effective methodology for the elaboration of all-anti polypropionate fragments, which could be incorporated as synthetic modules for the preparation of longer polypropionate chains. Herein, we report a highly stereo- and regioselective epoxide-based methodology for the synthesis of all-anti polypropionate units and its application to the synthesis of the all-anti C5–C10 segments of streptovaricin U.
Scheme 2.
Preparation of the anti,anti,anti stereotetrad 9
Results and Discussion
For the elaboration of longer all-anti polypropionate fragments, we envisaged a linear progression that comprises a reiteration of our three-step reaction cycles (or a two-step sequence if the Grignard method is used) starting from alkynediol 9. An advantage of employing epoxide chemistry in this fashion is the inherent SN2 behavior of epoxides toward nucleophilic attack, providing stereodefined products. In addition, contrary to the generally more efficient convergent methodologies, a linear approach circumvents the setbacks associated with the coupling of advanced fragments that already contain stereogenic centers and are prone to mismatched interactions.21
In this regard, alkyne 9 was reduced to the cis-alkenediol 10 and subjected to an epoxidation using the microwave assisted VO(acac)2 conditions, producing epoxide 11 as a single diastereomer in 88% yield (Scheme 3). Epoxy diol 11 was protected as the acetonide 12 and reacted with the propynylalane reagent to produce the all-anti,cis epoxide 12.
Scheme 3.
Synthesis of the all-anti stereohexads 13, 14 and the streptovaricin U C5–C10 fragment 15
To evaluate the alternate epoxide cleavage manifold, epoxide 8 was subjected to the copper catalyzed cis-propenyl Grignard reaction conditions, with the expectation that diol 10 could be produced in one step. Instead of the alkenol product, a 1:1 mixture of ethyl ketone 17 and furan 18 were obtained as the major products (Scheme 4). The formation of ketone 17 is consistent with a MgBr2 promoted epoxide to ketone rearrangement under the Grignard reaction conditions (Schlenk equilibrium).22 Furan 18 results from the intramolecular attack of the magnesium alkoxide to the external epoxide carbon. Both reactions occur because of the low reactivity of disubstituted epoxide 8 under the copper catalyzed Grignard reaction conditions. To test this assumption and suppress the formation of these side products, epoxy alcohol 9 was protected as the benzyl ether 19. Although this strategy did curtail the formation of the unwanted products, only the starting benzyl ether 19 was recovered. The epoxidation of alkenediol 10 was also evaluated using the complementary iodocarbonation/methanolysis sequence. While the iodocarbonation reaction proceeded as expected, the methanolysis step produced epoxide 11, together with a mixture of methyl carbonate products. The results establish the vanadium catalyzed epoxidation and the propynyl alane mediated epoxide cleavage, as the preferred reactions for the elaboration of anti propionate units.
Scheme 4.
Epoxide cleavage attempts using the copper catalyzed cis-propenylmagnesium bromide conditions
To further extend the all-anti polypropionate fragment, another iteration was implemented. The reaction of epoxide 12 with diethylpropynylalane provided exclusively the desired external cleavage product in a 53% yield, however, a 2:1 mixture of acetonide 13 with the deprotected triol 14 was obtained. Thus, to complete the reaction sequence, compounds 13 and 14 were easily separated by chromatography and acetonide 13 was hydrogenated to produce alkenol 15. Compounds 13 and 14 represent termini-differentiated all-anti stereohexad elaborated in a straightforward and highly stereoselective fashion. The all-anti stereohexad 15 corresponds to the C5–C10 fragment of streptovaricin U containing the five chiral centers (C6–C10) with the correct relative configuration, a homoallylic alkene for further epoxidation (an anti epoxidation is required at this point), and a masked aldehyde at C5.
With alkenol 15 on hand, it was further syn epoxidized under the microwave assisted VO(acac)2 catalyzed conditions to efficiently furnish the more advanced epoxide 16. The resulting polypropionate chain has seven consecutive all-anti stereogenic centers assembled in a simple and highly stereoselective iterative fashion. In principle, another anti subunit could be further generated by cleavage of the epoxide moiety. Therefore, this epoxidation/cleavage sequence can be successfully applied for the preparation of advanced all-anti polypropionate fragments, which could be used as modules for chain elongation allowing further manipulations in both directions.
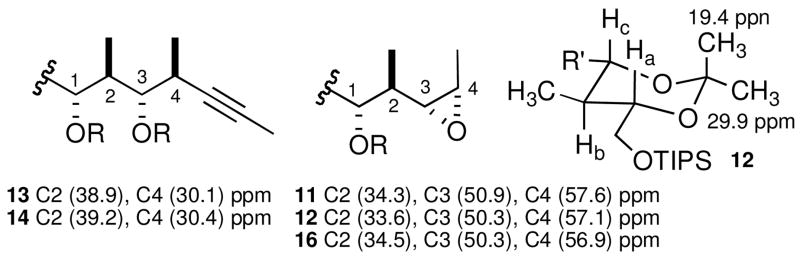
The regioselectivity of the epoxide cleavage reactions to produce the 1,3-diols resulting from the attack at the external epoxide carbon (C4) was confirmed by 13C NMR. For example, compound 13 showed diagnostic peaks at 38.9 ppm for the C2 methine and 30.1 ppm for the C4 propargyl carbon, compared to the 1,4-diols peaks at 34–37 and 41–46 ppm for the C2 and C3 carbon atoms, respectively (Figure 3). This was corroborated by the COSY spectra of 13 and 14, showing cross peaks between the C4 methyl protons and the propargylic methines.
Figure 3.
Selected 13C NMR data for the regio- and stereoselectivity determination
The syn 1,3-diol relationship in 11 was confirmed by its conversion to the corresponding 1,3-acetonide 12, where the 13C NMR revealed peaks at 19.4 and 29.9 ppm for the gem-dimethyl carbon atoms.17,23 In addition, 12 showed coupling constants of Jab = 10.3 Hz and Jbc = 10.5 Hz for the 6-membered ring vicinal protons corresponding to an axial-axial relationship, confirming the anti,anti stereotriad.24 The syn/anti stereoselectivity of the epoxidation reaction was again established by 13C NMR using the diagnostic C2 methine and C3,C4 epoxide carbons. As previously reported,18 when an anti 2-methyl-3-epoxy relationship is present, the epoxide C3 and C4 carbons show signals near 52 and 58 ppm respectively, while a syn relationship displays higher values between 54 and 60 ppm. This tendency holds for the cis-2-methyl-3,4-epoxy alcohols 11, 12 and 16, thus establishing the required syn hydroxy-epoxide selectivity (Figure 3).
Conclusion
In summary, we have successfully devised a concise method for the stereo- and regioselective construction of all-anti termini-differentiated polypropionate modules. The microwave-assisted VO(acac)2 catalyzed epoxidation of the cis-homoallylic alcohol precursors produced better yields than the iodocarbonation sequence and excellent syn diastereoselectivity. The diethylpropynylalane cleavage of the anti,anti,cis-3,4-epoxy alcohol systems showed to be more reliable than the copper catalyzed cis-propenyl Grignard conditions for the more advance all-anti systems. The simplicity of the chemical transformations and iterativity of the reaction sequence makes this method advantageous and practical. The methodology was applied to the synthesis of the all-anti C6–C10 fragment (15) of streptovaricin U.
Experimental Section
(±)-(2S*, 3S*, 4S*, 5S*, Z)-3,5-Dimethyl-1-[(triisopropylsilyl)oxy]-6-octene-2,4-diol. (10)
The alkyne diol 9 (3.0 g, 8.9 mmol) was hydrogenated18a using 0.59 mL of freshly distilled dry quinoline, 0.58 g of Pd/C catalyst (10% Pd, 0.066 g/mmol) and 18.8 mL of dry hexane. After workup and solvent evaporation, 2.88 g (94%) of neat crude was obtained. The alkenediol 10 was used for the next step without further purification. 1H NMR: ™ 5.52 (dd, J = 10.5, 5.0 Hz, 1H), 5.50 (dd, J = 10.5, 7.1 Hz, 1H), 3.84 (dd, J = 9.7, 3.4 Hz, 1H), 3.70 (ddd, J = 7.8, 7.8, 3.4 Hz, 1H), 3.57 (dd, J = 9.7, 7.8 Hz, 1H), 3.47 (dd, J = 8.1, 3.6 Hz, 1H), 2.72 (ddq, J = 7.1, 6.6, 3.6 Hz, 1H), 1.66 (ddq, J = 8.1, 7.8, 6.9 Hz, 1H), 1.62 (d, J = 5.0 Hz, 3H), 1.06 (m, 21H), 1.06 (d, J = 6.6 Hz, 3H), 0.79 (d, J = 6.9 Hz, 3H). 13C NMR: δ 131.4, 124.1, 79.4, 76.2, 65.9, 38.6, 34.2, 18.1, 17.9, 13.0, 13.0, 12.1.
(±)-(2S*, 3S*, 4R*, 5R*, 6R*, 7S*)-6,7-Epoxy-3,5-dimethyl-1-[(triisopropylsilyl) oxy]-2,4-octanediol. (11)
The alkene diol 10 (5.37 g, 15.60 mmol) was subjected to the vanadium catalyzed epoxidation under MW irradiation condition18b using 0.058 g (0.014 equiv) of VO(acac)2, 60 mL of toluene, and 4.1 mL of t-butyl hydroperoxide (4.24 M in toluene, 1.1 equiv). After workup and solvent evaporation, 5.63 g (100%) of epoxide 11 was obtained. The crude was used without further purification. 1H NMR: δ 4.61 (s, 1H), 3.87 (dd, J = 9.6, 3.3 Hz, 1H), 3.68 (ddd, J = 8.3, 5.1, 3.3 Hz, 1H), 3.61 (dd, J = 8.3, 3.1 Hz, 1H), 3.57 (dd, J = 9.6, 8.3 Hz, 1H), 3.30 (s, 1H), 3.10 (dd, J = 9.2, 4.5 Hz, 1H), 2.99 (dq, J = 5.6, 4.5 Hz, 1H), 1.95 (ddq, J = 8.3, 6.8, 5.1 Hz, 1H), 1.70 (ddq, J = 9.3, 7.0, 3.1 Hz, 1H), 1.29 (d, J = 5.6 Hz, 3H), 1.06 (m, 21H), 1.06 (d, J = 7.0 Hz, 3H), 0.95 (d, J = 6.8 Hz, 3H). 13C NMR: δ 80.0, 76.7, 66.0, 57.1, 50.6, 38.6, 34.3, 17.9, 14.3, 13.4, 13.3, 11.8. Anal. Calcd for C19H40O4Si: C, 63.28; H, 11.18. Found: C, 63.49; H, 11.32.
(±)-(4R*,5S*,6S*)-4-[(1R*,2R*,3S*)-(2,3-Epoxy-1-methyl butyl)]-2,2,5-trimethyl-6-[(triisopropyl silyl oxy) methyl]-1,3-dioxane. (12)
The crude epoxy diol 11 (2.0 g, 5.55 mmol) was converted to the acetonide by adding 0.083 g of PPTS and 0.266 mL of 2-methoxypropene in 52.0 mL of CH2Cl2 at 0 °C. Workup, solvent evaporation and column chromatography (100:1 hexane/ether) yielded 1.31 g (59 % for the three-step sequence) of the expected product 12. 1H NMR: δ 3.82 (dd, J = 10.8, 3.5 Hz, 1H), 3.71 (dd, J = 10.8, 5.1 Hz, 1H), 3.52 (ddd, J = 10.3, 5.3, 3.5 Hz, 1H), 3.48 (dd, J = 10.5, 2.1 Hz, 1H), 3.09 (dd, J = 9.2, 4.5 Hz, 1H), 2.93 (dq, J = 5.5, 4.5 Hz, 1H), 1.86 (ddq, J = 10.5, 10.3, 6.5 Hz, 1H), 1.70 (ddq, J = 9.2, 7.1, 2.1 Hz, 1H), 1.41 (s, 3H), 1.36 (s, 3H), 1.27 (d, J = 5.5 Hz, 3H), 1.08 (m, 21H), 0.96 (d, J = 7.1 Hz, 3H), 0.85 (d, J = 6.5 Hz, 3H). 13C NMR: δ 97.8, 77.4, 76.1, 66.3, 56.4, 50.3, 33.6, 33.0 29.9, 19.4, 17.9, 13.9, 13.4, 12.1, 12.0. Anal. Calcd for C22H44O4Si: C, 65.95; H, 11.07. Found: C, 65.87; H, 11.14.
(±)-(4S*, 5S*, 6S*)-4-[(1S*, 2S*, 3S*)-1,3-Dimethyl-2-hydroxy-4-hexynyl]-6-[(triisopropyl silyl oxy)-methyl]-2,2,5-trimethyl-1,3-dioxane. (13)
Epoxide 12 (0.50 g, 1.25 mmol) was subjected to the propynylalane cleavage procedure11 using 9.6 mL of dry toluene (0.13 M), 2.8 mL (6.2 mmol, 5 equiv) of n-BuLi (2.27 M in hexane), an excess of propyne gas, and 3.4 mL (6.2 mmol, 5 equiv) of diethylaluminum chloride (1.8 M in toluene). Work up, solvent evaporation and flash chromatography (12:1 Hex/EtOAc), yielded 0.195 g (35%) of pure product 13 and 0.089 g (18%) of the expected product 14 without the acetonide moiety. 1H NMR: δ 3.79 (dd, J = 10.8, 3.5 Hz, 1H), 3.72 (dd, J = 10.8, 5.1 Hz, 1H), 3.55 (dd, J = 10.6, 1.7 Hz, 1H), 3.52 (ddd, J = 10.3, 5.1, 3.5 Hz, 1H), 3.43 (ddd, J = 8.5, 6.0, 2.4 Hz, 1H), 2.72 (d, J = 6.0 Hz, 1H), 2.67 (dqq, J = 7.0, 2.4, 2.4 Hz, 1H), 2.06 (ddq, J = 8.5, 7.0, 1.7 Hz, 1H), 1.94 (ddq, J = 10.6, 10.3, 6.5 Hz, 1H), 1.82 (d, J = 2.4, 3H), 1.42 (s, 3H), 1.38 (s, 3H), 1.25 (d, J = 7.0 Hz, 3H), 1.06 (m, 21H), 0.96 (d, J = 7.0 Hz, 3H), 0.90 (d, J = 6.6 Hz, 3H). 13C NMR: δ 98.0, 79.6, 78.8, 78.1, 76.4, 76.3, 66.1, 38.9, 34.4, 30.4, 30.1, 19.2, 18.9, 18.0, 16.2, 12.5, 12.0, 3.7. Anal. Calcd for C25H48O4Si: C, 68.13; H, 10.98. Found: C, 67.94; H, 11.08.
(±)-(3S*,4S*,5S*,6S*,7S*,8S*,Z)-3,5,7-trimethyl-1-[(triisopropylsilyl)oxy]-dec-8-ene-2,4,6-triol (14)
1H NMR: δ 3.86 (dd, J = 9.6, 3.8 Hz, 1H), 3.76 (ddd, J = 8.3, 7.4, 3.8 Hz, 1H), 3.86 (dd, J = 9.6, 3.6 Hz, 1H), 3.58 (dd, J = 5.4, 5.4 Hz, 1H), 3.41 (dd, J = 8.4 2.2 Hz, 1H), 2.72 (dqq, J= 7.8, 2.2, 1.8 Hz, 1H), 2.08 (ddq, J = 8.4, 6.9, 5.4 Hz, 1H), 2.05 (ddq, J = 7.4, 6.9. 5.4 Hz, 1H), 1.81 (d, J = 1.8, 3H), 1.23 (d, J = 7.8 Hz, 3H), 1.06 (m, 21H), 0.95 (d, J = 6.9 Hz, 3H) and (d, J = 6.9 Hz, 3H). 13C NMR: δ 81.6, 79.1, 78.6, 78.5, 75.2, 65.8, 40.3, 39.2, 30.4, 18.7, 17.9, 15.5, 14.3, 11.9, 3.6.
(±)-(4S*, 5S*, 6S*)-4-[(1S*, 2S*, 3S*, Z)-1,3-Dimethyl-2-hydroxy-4-hexenyl]-6-[(triisopropylsilyloxy)-methyl]-2,2,5-trimethyl-1,3-dioxane (15)
Alkynol 13 (0.34 g, 0.77 mmol) was hydrogenated18a using 0.05 mL of freshly distilled dry quinoline, 0.05 g of Pd/C catalyst (10% Pd, 0.066 g/mmol) and dry hexane. After workup and solvent evaporation, column chromatography (50:1 HexEtAc), yielded 0.24 g (71%) of pure product 15. 1H NMR: δ 5.52 (dq, J = 11.0, 5.0 Hz, 1 H), 5.49 (dd, J = 11.0, 8.9 Hz, 1H), 3.77 (dd, J = 10.9, 3.2 Hz, 1H), 3.72 (dd, J = 10.9, 5.0 Hz, 1H), 3.49 (ddd, J = 10.5, 5.0, 3.2 Hz, 1H), 3.49 (dd, J = 8.3, 3.4 Hz, 1H), 3.48 (dd, J = 10.5, 1.7 Hz, 1H), 3.10 (bs, 1H), 2.70 (ddq, J = 8.9, 6.8, 3.4 Hz, 1H), 1.93 (ddq, J = 10.5, 10.5, 6.5 Hz, 1H), 1.83 (ddq, J = 8.3, 7.1, 1.7 Hz, 1H), 1.62 (d, J = 5.0, 3H), 1.42 (s, 3H), 1.38 (s, 3H), 1.06 (m, 21H), 1.05 (d, J = 6.8 Hz, 3H), 0.94 (d, J = 7.1 Hz, 3H), 0.86 (d, J = 6.5 Hz, 3H). 13C NMR: δ 132.1, 123.7, 98.1, 80.0, 78.7, 76.4, 65.9, 36.9, 34.6, 34.2, 30.2, 19.1, 18.4, 18.0, 13.0, 12.6, 12.0. Anal. Calcd. for C25H50O4Si: C, 67.82; H 11.38. Found: C, 67.55; H, 11.42.
(±)-(4S*,5S*,6S*)-4-[(1S*,2S*,3R*,4R*,5S*)-1,3-Dimethyl-2-hydroxy-4,5-epoxyhexyl]-6-[(triisopropylsilyloxy)-methyl]-2,2,5-trimethyl-1,3-dioxane. (16)
Alkenol 15 (0.21 g, 0.47 mmol) was subjected to the vanadium catalyzed epoxidation under MW irradiation conditions18b using 0.0017 g (0.014 equiv) of VO(acac)2, 10 mL of toluene, and 0.13 mL of t-butyl hydroperoxide (3.85 M in toluene, 1.1 equiv). After column chromatography (9:1 hexane/ethyl acetate), 0.176 g (87%) of the epoxide 16 was obtained. 1H NMR: δ 3.77 (dd, J = 10.9, 3.3 Hz, 1H), 3.71 (dd, J = 10.9, 5.1 Hz, 1H), 3.60 (ddd, J = 6.3, 2.6, 2.7 Hz, 1H), 3.51 (ddd, J = 10.3, 5.1, 3.3 Hz, 1H), 3.48 (dd, J = 10.8, 1.0 Hz, 1H), 3.38 (d, J = 2.7 H, 1H), 3.11 (dd, J = 9.2, 4.6 Hz, 1H), 2.98 (dq, J = 5.4, 4.6 Hz, 1H), 2.28 (ddq, J = 7.0, 6.3, 1.0 Hz, 1H), 1.95 (ddq, J = 10.8, 10.3, 6.5 Hz, 1H), 1.61 (ddq, J = 9.2, 6.9, 2.6 Hz, 1H), 1.42 (s, 3H), 1.39 (s, 3H), 1.28 (d, J = 5.4 Hz, 3H), 1.05 (m, 24H), 0.96 (d, J = 7.0 Hz, 3H) 0.91 (d, J = 6.5 Hz, 3H). 13C NMR: δ 98.1, 80.2, 78.3, 76.5, 65.8, 56.9, 50.3, 36.6, 35.1, 34.5, 30.2, 19.0, 18.2, 17.9, 14.7, 13.4, 12.6, 12.0. Anal. Calcd for C25H50O5Si: C, 65.45; H, 10.99. Found: C, 65.72; H, 11.22.
Supplementary Material
Figure 2.
Ionomycin (4) and streptovaricin U (5) and their all-anti,anti C18–C21 and C6–C10 stereotetrad and -pentad
Acknowledgments
This work was supported by NIH RISE (1R25-GM-61151-01A1) and NIH SCORE (2S06GM-08102-29) programs.
Footnotes
Supporting Information Available
NMR spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Omura S, editor. Macrolide Antibiotics. Chemistry, Biology, and Practice. 2. Academic Press; New York: 2002. [Google Scholar]; (b) Davies-Coleman MT, Garson MJ. Nat Prod Rep. 1998;15:477–493. doi: 10.1039/a815477y. [DOI] [PubMed] [Google Scholar]; (c) O’Hagan D. The Polyketide Metabolites. Ellis Harwood; Chichester: 1991. [Google Scholar]; (d) O’Hagan D. Nat Prod Rep. 1995;12:1–32. [Google Scholar]
- 2.For recent reviews see: Koskinen AMP, Karisalmi K. Chem Soc Rev. 2005;34:677–690. doi: 10.1039/b417466f.Faul MM, Huff BE. Chem Rev. 2000;100:2407–2474. doi: 10.1021/cr940210s.
- 3.For reviews see: Schetter B, Mahrwald R. Angew Chem Int Ed. 2006;45:7506–7525. doi: 10.1002/anie.200602780.Mahrwald R, editor. Modern Aldol Reactions. Wiley-VCH; New York: 2004. Paterson I, Cowden CJ, Wallace DJ. In: Modern Carbonyl Chemistry. Otera J, editor. Wiley-VCH; New York: 2000. p. 249.Carreira EM. In: Modern Carbonyl Chemistry. Otera J, editor. Wiley-VCH; New York: 2000. p. 279.
- 4.For reviews see: Chemler SR, Roush WR. In: Modern Carbonyl Chemistry. Otera J, editor. Wiley-VCH; New York: 2000. p. 403.Denmark SE, Almstead MG. In: Modern Carbonyl Chemistry. Otera J, editor. Wiley-VCH; New York: 2000. p. 299.
- 5.(a) Roush WR, Palkowitz AD, Ando K. J Am Chem Soc. 1990;112:6348–6359. [Google Scholar]; (b) Roush WR. J Org Chem. 1991;56:4151–4157. [Google Scholar]; (c) Hoffmann RW, Dahmann G, Andersen MW. Synthesis. 1994:629–638. [Google Scholar]
- 6.For reviews see: Ramachandran PV. Aldrichimica Acta. 2002;35:23–35.Mahrwald R. Chem Rev. 1999;99:1095–1120. doi: 10.1021/cr980415r.Brown HC, Ramachandran PV. J Organometal Chem. 1995;500:1–19.Duthaler RO, Hafner A. Chem Rev. 1992;92:807–832.Hoffmann RW. Angew Chem Int Ed Engl. 1982;21:555–566.
- 7.For recent examples: Karisalmi K, Koskinen AMP. Synthesis. 2004:1331–1342.Calter MA, Song W, Zhou JG. J Org Chem. 2004;69:1270–1275. doi: 10.1021/jo035668l.Kiyooka S. Tetrahedron: Asymm. 2003;14:2897–2910.Guindon Y, Brazeau JF. Organic Lett. 2004;6:2599–2602. doi: 10.1021/ol049086h.Chemler SR, Roush WR. J Org Chem. 2002;68:1319–1333. doi: 10.1021/jo0267908.Hassfeld J, Christmann M, Kalesse M. Org Lett. 2001;3:3561–3564. doi: 10.1021/ol016677o.
- 8.For recent non-aldol methods see Sarabia F, Martín-Gálvez F, García-Castro M, Chammaa S, Sánchez-Ruiz A, Tejón-Blanco JF. J Org Chem. 2008;73:8979–8986. doi: 10.1021/jo801728s.Parker K, Xie Q. Org Lett. 2008;10:1349–1352. doi: 10.1021/ol702989g.El-Awa A, du Jourdin XM, Fuchs PL. J Am Chem Soc. 2007;129:9086–9093. doi: 10.1021/ja071217x.Defosseux M, Blanchard N, Meyer C, Cossy J. Tetrahedron. 2005;61:7632–7653.Breit B, Zahn SK. J Org Chem. 2001;66:4870–4877. doi: 10.1021/jo015634i.Arjona O, Menchaca R, Plumet J. J Org Chem. 2001;66:2400–2413. doi: 10.1021/jo001660p.
- 9.Structure: Toeplitz BK, Cohen AI, Funke PT, Parker WL, Gougoutas JZ. J Am Chem Soc. 1979;101:3344–3353.Total synthesis: Evans DA, Dow RL, Shih TL, Takacs JM, Zahler R. J Am Chem Soc. 1990;112:5290–5313.Hanessian S, Cooke NG, Dehoff B, Sakito Y. J Am Chem Soc. 1990;112:5276–5290.Lautens M, Colucci JT, Hiebert S, Smith ND, Bouchain G. Org Lett. 2002;4:1879–1882. doi: 10.1021/ol025872f.
- 10.Structure: Knoll WMJ, Rinehart KL, Wiley PF, Li LH. J Antibiot. 1980;33:249–251. doi: 10.7164/antibiotics.33.249. Total synthesis: Miyashita M, Yamasaki T, Shiratani T, Hatakeyama S, Miyazawa M, Irie H. Chem Commun. 1997:1787–1788.Miyashita M, Shiratani T, Kawamine K, Hatakeyama S, Irie H. Chem Commun. 1996:1027–1028.
- 11.(a) Rao AS, Paknicar SK, Kirtane JG. Tetrahedron. 1983;39:2367–2370. [Google Scholar]; (b) Cardillo G, Orena M. Tetrahedron. 1990;46:3321–3408. [Google Scholar]; (c) Bongini A, Cardillo G, Orena M, Porzi G, Sandri S. J Org Chem. 1982;47:4626–4633. [Google Scholar]; (d) Fleming PR, Sharpless KB. J Org Chem. 1991;56:2869–2875. [Google Scholar]; (e) Sharpless KB, Woodward SS, Finn MG. Pure and Appl Chem. 1983;55:1823–1836. [Google Scholar]
- 12.(a) Nagaoka H, Rutsch W, Schimid G, Lio H, Johnson MR, Kishi Y. J Am Chem Soc. 1980;102:7962–7965. [Google Scholar]; (b) Nagaoka H, Kishi Y. Tetrahedron. 1981;37:3873–3888. [Google Scholar]
- 13.Corey EJ, Hase T. Tetrahedron Lett. 1979;20:335–338. [Google Scholar]
- 14.(a) Bartlett PA, Meadows JD, Brown EG, Morimoto A, Junstedt KK. J Org Chem. 1982;47:4013–4018. [Google Scholar]; (b) Bartlett PA, Myerson J. J Am Chem Soc. 1978;100:3950–3952. [Google Scholar]
- 15.(a) Lipshutz BH, Kozlowski JA. J Org Chem. 1984;49:1147–1149. [Google Scholar]; (b) Lipshutz BH, Barton JC. J Org Chem. 1988;53:4495–4499. [Google Scholar]; (c) Lipshutz BH, Kotsuki H, Lew W. Tetrahedron Lett. 1986;27:4825–4828. [Google Scholar]; (d) Lipshutz BH, Moretti R, Crow R. Tetrahedron Lett. 1989;30:15–18. [Google Scholar]
- 16.(a) Miyashita M, Hoshino M, Yoshikoshi A. J Org Chem. 1991;56:6483–6485. [Google Scholar]; (b) Miyashita M, Yoshihara K, Kawamine K, Hoshino M, Irie H. Tetrahedron Lett. 1993;34:6285–6288. [Google Scholar]
- 17.Tirado R, Torres G, Torres W, Prieto JA. Tetrahedron Lett. 2005;46:797–801. [Google Scholar]
- 18.(a) Tirado R, Prieto JA. J Org Chem. 1993;58:5666–5673. [Google Scholar]; (b) Torres G, Torres W, Prieto JA. Tetrahedron. 2004;60:10245–10251. [Google Scholar]
- 19.Marshall JA, Perkins JF, Wolf MA. J Org Chem. 1995;60:5556–5559. [Google Scholar]
- 20.Rodríguez D, Mulero M, Prieto JA. J Org Chem. 2006;71:5826–5829. doi: 10.1021/jo060833t. [DOI] [PubMed] [Google Scholar]
- 21.(a) Hoffmann RW, Dresely S. Chem Ber. 1989;122:903–909. [Google Scholar]; (b) Short RP, Masamune S. Tetrahedron Lett. 1987;28:2841–2844. [Google Scholar]; (c) Tanimoto N, Gerritz SW, Sawabe A, Noda T, Filla SA, Masamune S. Angew Chem Int Ed. 1994;33:673–675. [Google Scholar]; (d) Andersen MW, Hildebrandt B, Dahmann G, Hoffmann RW. Chem Berichte. 1991;124:2127–2139. [Google Scholar]
- 22.Schlenk W, Schlenk W., Jr Ber Dtsch Chem Ges. 1929;62:920–924. [Google Scholar]
- 23.(a) Rychnovsky SD, Skalitzky DJ. Tetrahedron Lett. 1990;31:945. [Google Scholar]; (b) Evans DA, Rieger DL, Gage JR. Tetrahedron Lett. 1990;31:7099–7100. [Google Scholar]
- 24.Wang Z, Schreiber SL. Tetrahedron Lett. 1990;31:31–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.