Abstract
Surveillance for chronic kidney disease (CKD) using nationally representative samples of the US population is central in providing information on the magnitude and trends in CKD burden that will guide disease management and prevention planning for clinicians and public health authorities. We used a cross-sectional study design to estimate the change in prevalence of CKD over time using National Health and Nutrition Examination Survey (NHANES) data. NHANES III (1988-1994) included 15,488 participants and NHANES rounds 1999-2004 included 13,233 participants over the age of 20 years with serum creatinine measurements who were examined in a mobile examination center. Early stages of CKD were defined by glomerular filtration rate (GFR) as estimated by the Modification of Diet in Renal Disease (MDRD) Study equation and urinary albumin-to-creatinine ratio (ACR) following the classification system established by the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative. Moderately reduced GFR increased in prevalence from 5.4% to 7.7% (P<0.001) and severely reduced GFR increased from 0.21% to 0.35% (P=0.02) from 1988-1994 to 1999-2004. Within CKD stage 3, 18.6 % (SE 1.6%) of individuals should be referred to a nephrologist following a proposed set of criteria for referral; referral rates were highest for individuals with diabetes and lower among whites compared to other race-ethnicity groups. These survey data suggest that the prevalence of CKD has increased between the years of 1988-1994 and 1999-2004. Surveillance for early stages of CKD (CKD stages 1-4) should monitor these and other trends.
Index words: Surveillance, kidney, NHANES, national surveys, GFR
Introduction
Chronic kidney disease (CKD) has become a global public health problem and is a common condition in the U.S..1, 2 CKD is associated with a wide range of complications in addition to the risk of progression to kidney failure, or end-stage renal disease (ESRD), requiring dialysis or transplantation and being a marker of high cardiovascular disease (CVD) risk.3 During the years of 1991-2001, the incidence of treated ESRD adjusted for age, race, and sex increased by 43%; and in 2004 there were approximately 472,000 patients with treated ESRD, the final stage of CKD.4 In the last two decades, the prevalence of obesity, diabetes, and hypertension has also increased.5-8 Given the increase in incidence of ESRD and prevalence of major CKD risk factors, it is important to track trends over time in the prevalence of all stages of CKD.
Surveillance for CKD is central in providing information on the magnitude and trends in burden of CKD that will guide disease management and prevention planning for both clinicians and public health authorities. Surveillance, defined as “the continuing scrutiny of all aspects of occurrence and spread of a disease that are pertinent to effective control”9, can take many forms and can focus on diagnosed, or undiagnosed, disease. For kidney disease, surveillance can monitor high risk subgroups for incident CKD in order to take clinical action, or can quantify changes in incidence or prevalence of CKD in populations to guide public health activities. In contrast to treated ESRD where both prevalence and incidence trends are available, incidence data for CKD are more limited and trends in incidence are largely unavailable. Here we focus on quantifying the total prevalence of CKD including both diagnosed and undiagnosed disease in the U.S. population. This requires a representative sample of the population and the means to determine whether individuals in the sample have CKD, regardless of previous diagnosis. Unlike ESRD, marked by clinical symptoms indicating a need for renal replacement therapy, earlier stages of CKD are mostly asymptomatic. Thus, diagnosis of CKD relies on laboratory values of markers for kidney damage, quantified by persistent albuminuria, and decreased kidney function, classified as glomerular filtration rate (GFR) < 60 ml/min/1.73 m2.1 GFR can be measured directly using urine and serum concentrations of exogenous substances such as inulin, 125I-iothalamate, or Iohexol.10 Estimating GFR from serum creatinine, however, is the presently recommended method for CKD staging and is more feasible in the large studies that are required for accurate estimation of total CKD burden in the US.1, 2
The National Health and Nutrition Examination Surveys (NHANES), conducted by the National Center for Health Statistics (NCHS), represent an ideal sample for estimating the total burden of CKD and have been used extensively for estimating CKD prevalence in the broader US population. We summarize data from a recent publication updating trends data from 1988-200411 and expand on the characteristics of subgroups within CKD Stage 3 which contains the largest number of individuals compared to any other CKD stage. The NHANES surveys include laboratory measurement of both albuminuria and serum creatinine thus allowing for the staging of CKD in a nationally representative sample, regardless of the participants' awareness, or history of diagnosis, of CKD. Surveillance requires the ability to evaluate estimates of incidence or prevalence from current data against similar estimates from comparable past data. As such, initial estimates of prevalence of CKD stages from the NHANES III (1988-1994) survey have provided a benchmark for kidney disease studies, prevention efforts, and healthcare planning.2, 12 Studies have compared estimates from NHANES 1999-2000 to the baseline estimates provided by NHANES III finding increased prevalence of albuminuria but no significant increase in overall prevalence of CKD. The precision of these trend estimates were constrained by the relatively small sample size of the 1999-2000 survey and may have been biased due to the limited data to establish consistent calibration of the creatinine assays over time.12 Proper assay calibration is necessary when comparing estimates derived from laboratory data to ensure correct and unbiased comparisons, especially when assays are performed years apart and/or in different laboratories. A recent study calibrating serum creatinine in all NHANES surveys from 1988 to 2004 permitted a more rigorous examination, with less bias, of the trends in the prevalence of CKD using standardized creatinine.13
We compare the prevalence of CKD in 1988-1994 to 1999-2004 and describe the distribution of CKD stages and severity. In particular CKD Stage 3 is described in detail and the impact of proposed criteria for referral to a nephrologist is explored. The impact of the rising prevalence of diabetes and changes in hypertension and obesity are examined as explanatory variables for changes in CKD prevalence in the general US adult population.
Methods
The methods utilized here have been published in detail recently.11 Briefly, the NHANES are cross-sectional, multi-stage, stratified, clustered probability samples of the US civilian non-institutionalized population conducted by the NCHS.14 We analyzed data from NHANES III and combined the NHANES 1999-2000, 2001-2002, and 2003-2004 (NHANES 1999-2004) following NCHS recommendations.15, 16 All surveys oversampled certain subgroups of the US population, including non-Hispanic Blacks, Mexican Americans, and the elderly in order to obtain adequate sample sizes for these groups in subsequent analyses.
Urinary albumin and creatinine concentrations were assayed in the same laboratory for all surveys. Urinary albumin-to-creatinine ratio (ACR, mg/g) was computed and forms the basis for the definition of albuminuria. Microalbuminuria is defined as ACR of 30 to < 300 mg/g, and macroalbuminuria is defined as ACR ≥ 300 mg/g. All albuminuria analyses excluded women who were pregnant or were in menses. Serum creatinine was measured using a kinetic rate Jaffe method. All serum creatinine measurements were re-calibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, Ohio).13 This re-calibration is necessary for appropriate estimation of GFR and accurate assessment of trends in prevalence over time using the different NHANES surveys.
GFR was estimated from re-calibrated serum creatinine using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation.17 The MDRD Study equation was originally developed using serum creatinine measured by a kinetic rate Jaffe method; here we use the IDMS-traceable MDRD Study equation that uses standardized creatinine18: GFR = 175 × (standardized serum creatinine)-1.154 × (age)-0.203 × 0.742(if the subject is a woman) × 1.212(if the subject is black). Estimated GFR is reported in ml/min/1.73 m2. GFR, estimated from standardized serum creatinine, and ACR were used to define early stages of CKD according to the classification system established by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative2 as follows: stage 1, persistent albuminuria with estimated GFR ≥ 90 ml/min/1.73 m2; stage 2, persistent albuminuria with estimated GFR of 60-89 ml/min/1.73 m2; stage 3, estimated GFR 30-59 ml/min/1.73 m2; stage 4, estimated GFR 15-29 ml/min/1.73 m2.
Estimation of the proportion of individuals with CKD Stage 3 eligible for referral to a nephrologist followed the criteria proposed in the KDOQI hypertension guidelines.19 Using these guidelines, all individuals with eGFR < 30 ml/min/1.73 m2 would be recommended for referral. Individuals with eGFR ≥ 30 ml/min/1.73 m2 would be referred based on any of the following indications of increased risk for CKD progression: 1) presence of macroalbuminuria; 2) type II diabetes with microalbuminuria; 3) diabetic retinopathy; 4) hyperkalemia (serum potassium > 5.5 mEq/L); 5) resistant hypertension. For this analysis resistant hypertension was defined as systolic blood pressure > 130 mmHg or diastolic blood pressure > 80 mmHg, while taking three or more anti-hypertensive medications. This analysis excluded women who were pregnant or in menses, and was limited to NHANES III since this was the only survey with data on retinopathy.
Given the complex survey design, and oversampling of certain subgroups, statistical analyses were performed using sampling weights to obtain unbiased estimates of CKD prevalence using Stata Version 8.2 (StataCorp, College Station, TX). Standard errors for all estimates were obtained using the Taylor series (linearization) method following NHANES recommended procedures and weights.14-16 Multi-variable logistic regression was used to compare albuminuria and estimated GFR < 60 ml/min/1.73 m2 in NHANES 1999-2004 to NHANES III. Demographic variables (age, sex, and race), body mass index (BMI), and diagnosed diabetes and hypertension were entered in the model to assess the amount that changes in these CKD risk factors would account for any increase in CKD prevalence. Information on age, sex, race/ethnicity, and smoking was based on self-report during the survey interview. Hypertension and diabetes were defined by self-report of physician diagnosis. Height and weight were measured during NHANES examinations and were used to calculate BMI (kg/m2). A sensitivity analysis (“conservative trends analysis”) was performed by adding 0.04 mg/dl to serum creatinine levels in NHANES III so its mean level in a young healthy subgroup was identical to NHANES 1999-2004. The aim of this analysis was to determine whether a difference in mean serum creatinine levels between the surveys, potentially due to residual laboratory calibration difference, might account for the changes in prevalence of CKD.
Results
NHANES III included 15,488 participants and NHANES rounds 1999-2004 included 13,233 participants over the age of 20 years with serum creatinine measurements. During the time period between the surveys the US population became older and included a smaller proportion of Non-Hispanic Whites (Table 1). The shift in age distribution was less pronounced above 60 years where CKD is more common. At the same time, the prevalence of self reported diabetes and hypertension increased as did the mean body mass index and proportion of the population that is overweight and obese, all risk factors for CKD. Mean albuminuria increased across the surveys but mean ACR was not different among young healthy individuals (12.2 mg/g in 1988-1994 and 12.3 in 1999-2004). The mean serum creatinine was higher in 1999-2004 compared to 1988-1994 corresponding to a lower mean estimated GFR in 1999-2004. The conservative trends analysis which added 0.04 mg/dl to the serum creatinine in NHANES III resulted in nearly identical mean serum creatinine and mean estimated GFR across surveys.
Table 1.
Population Characteristics of U.S. Adults Age 20 Years or Older Based on NHANES 1988-1994 and NHANES 1999-2004
| NHANES 1988-1994 | NHANES 1999-2004 | |||||
|---|---|---|---|---|---|---|
| N | Mean or % | SE | N | Mean or % | SE | |
| Mean age, y | 15,488 | 44.8 | 0.5 | 13,233 | 46.2 | 0.3 |
| Age group, y | ||||||
| 20-39 | 6,367 | 45.7% | 1.0% | 4,714 | 39.4% | 0.8% |
| 40-59 | 4,194 | 31.7% | 0.6% | 3,921 | 38.3% | 0.7% |
| 60-69 | 2,174 | 11.4% | 0.5% | 2,015 | 10.5% | 0.4% |
| ≥70 | 2,753 | 11.2% | 0.7% | 2,583 | 11.9% | 0.4% |
| Sex | ||||||
| Women | 8,214 | 52.2% | 0.5% | 6,925 | 51.8% | 0.4% |
| Men | 7,274 | 47.9% | 0.5% | 6,308 | 48.2% | 0.4% |
| Race | ||||||
| Non-Hispanic White | 6,450 | 76.9% | 1.3% | 6,764 | 72.6% | 1.7% |
| Non-Hispanic Black | 4,168 | 10.3% | 0.6% | 2,477 | 10.5% | 1.0% |
| Mexican American | 4,250 | 5.1% | 0.4% | 3,009 | 7.3% | 0.9% |
| Other | 620 | 7.7% | 0.8% | 983 | 9.6% | 1.2% |
| Diabetes, self report | 1,266 | 5.4% | 0.3% | 1,278 | 6.8% | 0.3% |
| Hypertension, diagnosed | 4,211 | 23.8% | 0.7% | 4,120 | 27.1% | 0.8% |
| Mean BMI, kg/m2 | 15,453 | 26.6 | 0.1 | 12,857 | 28.1 | 0.1 |
| BMI group, kg/m2 | ||||||
| < 25 | 6,073 | 44.5% | 0.9% | 4,083 | 34.4% | 0.6% |
| 25-29.99 | 5,435 | 33.1% | 0.6% | 4,640 | 34.8% | 0.7% |
| ≥ 30 | 3,945 | 22.3% | 0.7% | 4,134 | 30.8% | 0.7% |
| Kidney function (GFR), ml/min/1.73 m2 | ||||||
| Normal, ≥90 | 8600 | 51.9% | 1.1% | 5891 | 40.7%*** | 1.0% |
| Mildly reduced, 60-89 | 5751 | 42.4% | 1.0% | 5946 | 51.2%*** | 0.8% |
| Moderately reduced, 30-59 | 1088 | 5.4% | 0.3% | 1316 | 7.7%*** | 0.3% |
| Severely reduced, 15-29 | 49 | 0.21% | 0.03% | 80 | 0.35%* | 0.05% |
| Albuminuria (ACR), mg/g† | ||||||
| Normal, ACR <30 | 12655 | 91.8% | 0.4% | 10636 | 90.5%* | 0.3% |
| Microalbuminuria, ACR 30-299 | 1353 | 7.1% | 0.4% | 1315 | 8.2%* | 0.3% |
| Macroalbuminuria, ACR ≥300 | 311 | 1.1% | 0.1% | 265 | 1.3% | 0.1% |
Abbreviations: BMI, body mass index; ACR, albumin to creatinine ratio; GFR, glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey.
p<0.05 ***p<0.001 compared to 1988-1994
Women who were pregnant or in menses were excluded.
Note: Conversion factors for units: ACR in mg/g to mg/mmol, ×0.113; GFR in ml/min/1.73 m2 to ml/s/1.73 m2, ×0.01667.
Age adjusted prevalence estimates for microalbuminuria and macroalbuminuria in 1988-1994 adjusted to the 1999-2004 age distribution in Table 1 are 7.2% and 1.2%, respectively.
Modified from JAMA. 2007;298(17):2038-2047
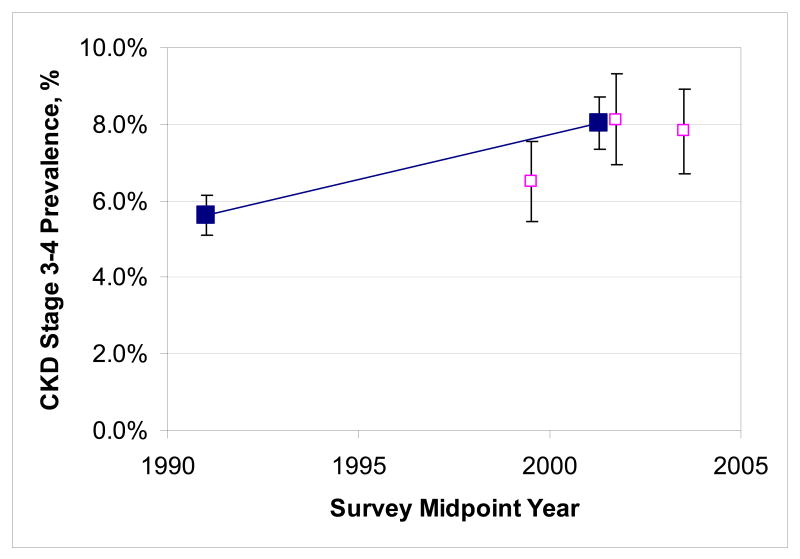
The proportion of the US population with mild, moderate or severely reduced estimated GFR increased from 1988-1994 to 1999-2004. The combined prevalence estimate for 1999-2004 had similar precision to the 1988-1994 estimate while prevalence estimates from each of the three two year surveys had relatively wide confidence intervals (Figure 1). Moderately reduced GFR increased in prevalence from 5.4% to 7.7% (P<0.001) and severely reduced GFR increased from 0.21% to 0.35% (P=0.02). Similarly, the proportion of the overall population with microalbuminuria on a single occasion increased from 7.1% to 8.2% (P=0.01). The prevalence of macroalbuminuria rose somewhat from 1.1% to 1.3% but this difference was well within the limits of random variation (P=0.4). Subdividing the prevalence of albuminuria by different levels of estimated GFR showed that the prevalence of microalbuminuria rose significantly among individuals with normal estimated GFR while all other subgroups showed no significant rise or fall in albuminuria.11
Figure 1.
Trends in the prevalence of CKD stages 3 and 4 between NHANES 1988-1994 and 1999-2004 (full squares) as well as the three component surveys for the later NHANES (empty squares for 1999-2000, 2001-2002 and 2003-2004). Error bars denote 95% confidence intervals. Abbreviations: CKD, chronic kidney disease; NHANES, National Health and Nutrition Examination Survey.
The prevalence estimate for each stage of CKD was higher in 1999-2004 than in 1988-1994 with the difference being statistically significant for CKD stages 2 to 4 and overall (Table 2). Stratified analyses by sex and race showed similar trends. The overall prevalences of CKD in 1988-1994 and 1999-2004 among men were 8.2% and 11.1%. Among women they were 12.1% and 15.0%. By ethnicity the change was from 10.5% to 13.8% among Non-Hispanic whites, 10.2% to 11.7% among non-Hispanic Blacks, and from 6.3% to 8.0% among Mexican Americans. After age adjustment prevalence odds ratios for estimated GFR < 60 ml/min/1.73 m2 between 1999-2004 and 1988-1994 were of similar magnitude (between 1.4 and 1.5) and statistically significant in men, women, non-Hispanic whites and non-Hispanic blacks and Mexican-Americans. The association was somewhat weaker in the smaller number of individuals of other ethnicity. Trends over time were also similar within age categories indicating the trends were not due to age differences in the population (data not shown11).
Table 2.
Prevalence of CKD Stages in US Adults Age 20 Years or Older Based on NHANES 1988-1994 and NHANES 1999-2004
| CKD Stage | NHANES | NHANES | Prevalence Ratio | Estimated Number |
|---|---|---|---|---|
| 1988-1994 | 1999-2004 | 1999-2004/1988-1994 | US in 2000 | |
| Prevalence, % (95% CI) |
Prevalence, % (95% CI) |
Ratio (95% CI) |
N, Millions (95% CI) |
|
| 1 | 1.71 (1.28, 2.18) | 1.78 (1.35, 2.25) | 1.05 (0.85-1.30) | 3.6 (2.7-4.5) |
| 2 | 2.70 (2.17, 3.24) | 3.24 (2.61, 3.88) | 1.21 (1.03-1.41) | 6.5 (5.2-7.8) |
| 3 | 5.42 (4.89, 5.95) | 7.69 (7.02, 8.36) | 1.42 (1.25, 1.62) | 15.5 (14.1-16.8) |
| 4 | 0.21 (0.15, 0.27) | 0.35 (0.25, 0.45) | 1.70 (1.11, 2.51) | 0.7 (0.5-0.9) |
| 5 | NA | NA | NA | NA |
| Total | 10.0 (9.2, 10.9) | 13.1 (12.4, 14.1) | 1.30 (1.19, 1.43) | 26.3 (24.2-28.3) |
CKD stages are defined based on standard criteria1 as follows: stage 1, persistent albuminuria with GFR >90 ml/min/1.73 m2; stage 2, persistent albuminuria with GFR 60-89 ml/min/1.73 m2; stage 3, GFR 30-59 ml/min/1.73 m2; stage 4, GFR 15-29 ml/min/1.73 m2. The age adjusted prevalence rates for CKD stages 1 to 4 in 1988-1994 adjusted to the 1999-2004 age distribution in Table 1 are 1.7%, 2.8%, 5.6%, and 0.2% for a total of 10.3%.
Reproduced from JAMA. 2007;298(17):2038-2047
Abbreviations: CKD, chronic kidney disease; GFR, glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey; NA, ***.[EF3]
Note: Conversion factors for units: GFR in ml/min/1.73 m2 to ml/s/1.73 m2, ×0.01667.
Differences in prevalence of decreased GFR and albuminuria between 1988-1994 and 1999-2004 remain substantial after adjustment for changes in the age, sex and race/ethnic composition of the US population over this time period (Table 3). The higher prevalence of diagnosed diabetes, hypertension, and higher body mass index explained some of the higher prevalence. For albuminuria trends, the higher prevalence was partly explained by the older age and high proportion of minority groups (odds ratio declined from 1.18 to 1.12 after adjustment). Further adjustment for the higher prevalence of diagnosed diabetes and hypertension and higher body mass index explained practically all of the difference (odds ratio declined to 1.03). In the fully adjusted models, the prevalence of albuminuria was strongly associated with diagnosed diabetes (OR 3.58; 95% CI 3.12-4.12) and hypertension (OR 1.70; 95% CI 1.1-1.92) as well as older age, and all race-ethnicity groups other than non-Hispanic whites (P<0.001) but not higher body mass index (P=0.1). The prevalence odds ratio of estimated GFR less than 60 ml/min/1.73 m2 in 1999-2004 compared to 1988-1994 was 1.47. Age adjustment had little impact, likely because the increase in the number of older individuals was offset by a similar increase in the number of younger individuals leading the percentage of individuals aged 60+ years to remain relatively unchanged (Table 1). The prevalence odds ratio increased further to 1.53 after adjustment for age, sex and race due to the lower prevalence of decreased GFR among minority groups. The odds ratio decreased to 1.43 with additional adjustment for diagnosed diabetes and hypertension and body mass index. In the fully adjusted model, the prevalence of low GFR was strongly associated with diagnosed diabetes (OR 1.54; 95% CI 1.28-1.80) and hypertension (OR 1.98; 95% CI 1.73-2.67) as well as higher body mass index (OR 1.08; 95% CI 1.02-1.15 per 5 kg/m2) and older age but was lower among men, non-Hispanic blacks, and Mexican-Americans compared to non-Hispanic whites (P<0.001).
Table 3.
Logistic Regression of Albuminuria and Decreased Estimated GFR comparing 1999-2004 to 1988-1994 Before and After Adjustment
| Trends | ||||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P[EF4] | ||||
| Albuminuria in 1999-2004 vs. 1988-1994 | ||||||
| Unadjusted | 1.18 | 1.03-1.34 | 0.01 | |||
| Adjusted for age | 1.15 | 1.00-1.32 | 0.05 | |||
| + sex and race | 1.12 | 0.99-1.28 | 0.08 | |||
| + diagnosed diabetes and hypertension | 1.06 | 0.93-1.21 | 0.4 | |||
| + body mass index | 1.03 | 0.90-1.18 | 0.6 | |||
| Conservative Trends Analysis* | ||||||
| Estimated GFR< 60 ml/min/1.73m2 in 1999-2004 vs. 1988-1994 | OR | 95% CI | P | |||
| Unadjusted | 1.47 | 1.27-1.69 | 0.000 | 1.17 | 1.02-1.34 | 0.03 |
| Adjusted for age | 1.50 | 1.31-1.73 | 0.000 | 1.13 | 0.99-1.30 | 0.07 |
| + sex and race | 1.53 | 1.33-1.76 | 0.000 | 1.15 | 1.00-1.32 | 0.05 |
| + diagnosed diabetes and hypertension | 1.45 | 1.27-1.67 | 0.000 | 1.10 | 0.96-1.26 | 0.2 |
| + body mass index | 1.43 | 1.24-1.63 | 0.000 | 1.08 | 0.94-1.24 | 0.3 |
Abbreviations: OR, odds ratio. GFR, glomerular filtration rate.
+ indicates addition of variables to the model in the previous row.
Serum creatinine among young healthy participants (age 20-39 without diabetes and hypertension) was adjusted to be identical across surveys by adding 0.04 mg/dl to serum creatinine in NHANES 1988-1994.
Reproduced from JAMA. 2007;298(17):2038-2047
Note: Conversion factors for units: GFR in ml/min/1.73 m2 to ml/s/1.73 m2, ×0.01667.
The conservative trends analysis showed that the difference in mean serum creatinine between surveys accounts for much but possibly not all of the higher prevalence of lower GFR in 1999-2004. In this analysis, the prevalence of CKD in 1988-1994 was higher (1.5%, 2.8%, 6.7%, and 0.23% for CKD stages 1 to 4 for a total of 11.3%). The prevalence odds ratio of estimated GFR less than 60 ml/min/1.73 m2 comparing 1999-2004 to 1988-1994 was 1.17 (95% CI 1.02-1.34). After full adjustment in the conservative trends analysis the prevalence odds ratio of decreased GFR between surveys was 1.08 (0.94-1.24) indicating that the differences in mean serum creatinine, demographics, diagnosed diabetes, hypertension, and body mass index between surveys explain nearly all of the difference in prevalence of low GFR between 1988-1994 and 1999-2004 (P=0.3).
Since CKD Stage 3 includes a large number of individuals we examined the distribution of individuals in this stage across different characteristics (Table 4). As expected, the majority of individuals had a higher estimated GFR (45-59 ml/min/1.73 m2 included 78.8% of all CKD stage 3 individuals adding the percentages across sex, age and albuminuria categories). Further, 61.5% of all individuals in CKD stage 3 had an estimated GFR between 45-59 ml/min/1.73 m2 with no evidence of albuminuria. The table also shows the distribution of albuminuria, with 25.7% of CKD stage 3 individuals with and 74.3% without albuminuria, and age, where 22.1%, 22.7% and 55.2% were age <60, 60-69 and 70+ years. A total of 16.6% of individuals in CKD stage 3 had previously diagnosed diabetes, and slightly less than half of these had evidence of albuminuria (7.5% of all individuals in stage 3).
Table 4.
Distribution of individuals with CKD stage 3 by estimated GFR < 45 ml/min/1.73 m2, age, sex, presence of albuminuria, and diabetes: Combined NHANES 1988-1994 and 1999-2004
| Age < 60 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Diagnosed Diabetes | Total | ||||||||
| Women | Men | Yes | No | |||||||
| % | SE | % | SE | % | SE | % | SE | % | SE | |
| GFR 45-59 ml/min/1.73 m2 | ||||||||||
| Normal, ACR<30 | 10.6 | 1.1 | 6.1 | 1.0 | 1.2† | 0.4 | 15.5 | 1.5 | 16.7 | 1.6 |
| Albuminuria, ACR>=30 | 1.7 | 0.3 | 1.5† | 0.5 | 0.8† | 0.4 | 2.4† | 0.5 | 3.2 | 0.6 |
| GFR 30-44 ml/min/1.73 m2 | ||||||||||
| Normal, ACR<30 | 0.9† | 0.3 | 0.2† | 0.1 | 0.3† | 0.2 | 0.8† | 0.3 | 1.1† | 0.4 |
| Albuminuria, ACR>=30 | 0.5† | 0.3 | 0.7† | 0.2 | 0.5† | 0.2 | 0.7† | 0.2 | 1.2† | 0.3 |
| Age 60-69 | ||||||||||
| GFR 45-59 ml/min/1.73 m2 | ||||||||||
| Normal, ACR<30 | 9.6 | 0.8 | 5.9 | 0.6 | 2.5 | 0.4 | 13.0 | 0.8 | 15.6 | 0.9 |
| Albuminuria, ACR>=30 | 2.3† | 0.5 | 1.3† | 0.3 | 1.4† | 0.4 | 2.2 | 0.4 | 3.6 | 0.6 |
| GFR 30-44 ml/min/1.73 m2 | ||||||||||
| Normal, ACR<30 | 1.2 | 0.2 | 0.9† | 0.3 | 0.3† | 0.1 | 1.8† | 0.4 | 2.1 | 0.3 |
| Albuminuria, ACR>=30 | 0.7† | 0.2 | 0.7† | 0.2 | 0.7† | 0.2 | 0.8† | 0.2 | 1.4† | 0.3 |
| Age 70+ | ||||||||||
| GFR 45-59 ml/min/1.73 m2 | ||||||||||
| Normal, ACR<30 | 19.0 | 1.2 | 10.4 | 0.6 | 3.4 | 0.4 | 26.0 | 1.2 | 29.4 | 1.2 |
| Albuminuria, ACR>=30 | 5.7 | 0.5 | 4.7 | 0.5 | 2.6 | 0.4 | 7.8 | 0.7 | 10.4 | 0.7 |
| GFR 30-44 ml/min/1.73 m2 | ||||||||||
| Normal, ACR<30 | 6.6 | 0.7 | 2.8 | 0.3 | 1.4† | 0.4 | 8.1 | 0.6 | 9.5 | 0.7 |
| Albuminuria, ACR>=30 | 3.6 | 0.5 | 2.3 | 0.3 | 1.5 | 0.3 | 4.3 | 0.5 | 5.9 | 0.6 |
| Total | 62.4 | 1.3 | 37.6 | 1.3 | 16.6 | 1.2 | 83.4 | 1.2 | 100 | |
Percentages were calculated using survey weights and add up to 100% across all the cells in the main columns (N=2272 individuals with CKD Stage 3).
Abbreviations: GFR, glomerular filtration rate; ACR, albumin to creatinine ratio; NHANES, National Health and Nutrition Examination Survey.
Women who were pregnant or in menses were excluded
Note: Conversion factors for units: ACR in mg/g to mg/mmol, ×0.113; GFR in ml/min/1.73 m2 to ml/s/1.73 m2, ×0.01667.
Estimates with low precision (SE > 20% of estimate)
An analysis estimating what proportion of individuals with CKD stage 3 would be eligible for referral to a nephrologist using one proposed referral recommendation suggested that 18.6% of individuals should be referred to a nephrologist while the other 81.4% could be managed by their internists (Table 5). Under this scenario, the largest proportion of referrals in CKD stage 3 was due to macroalbuminuria, type II diabetes with presence of albuminuria, or diabetic retinopathy. Independently of other criteria for referral, macroalbuminuria accounted for 20.5% (SE 4.0%) of referrals, type II diabetes with albuminuria for 32.9% (SE 5.6%), and diabetic retinopathy for 23.1% (SE 4.5%). Hyperkalemia (0.5%, SE 0.3%) and resistant hypertension (11.5%, SE 4.2%) accounted for less, and 11.5% of individuals would be referred for more than one criterion. The suggested referral rate was lower for Whites than other groups because of a lower rate of albuminuria and diabetes (Table 6). However, retinopathy data are limited to NHANES III making many of the specific referral groups relatively small and estimated rates imprecise.
Table 5.
Proportion of individuals with CKD stage 3 and whether they meet proposed criteria for referral to a nephrologist by age, sex, race, and diabetes status: NHANES 1988-1994
| CKD Stage 3 | Meet a Proposed Set of Criteria for Referral to a Nephrologist | ||||
|---|---|---|---|---|---|
| N | % | N | % | SE | |
| Overall | 1021 | 100.0% | 217 | 18.6% | 1.6 |
| Age | |||||
| < 60 | 119 | 18.5% | 30 | 16.9% | 3.5 |
| 60-69 | 235 | 27.1% | 67 | 21.5% | 3.6 |
| 70+ | 667 | 54.4% | 120 | 17.6% | 2.0 |
| Sex | |||||
| Women | 567 | 62.8% | 113 | 18.3% | 2.2 |
| Men | 454 | 37.2% | 104 | 19.0% | 2.9 |
| Race | |||||
| White | 708 | 87.2% | 112 | 16.9% | 1.6 |
| Black | 174 | 6.4% | 57 | 32.9% | 3.7 |
| Hispanic | 106 | 1.4% | 41 | 35.2% | 5.8 |
| Other | 33 | 5.0% | 7 | 23.6%† | 12.4 |
| Diabetes | |||||
| No | 833 | 84.8% | 100 | 10.4% | 1.5 |
| Yes | 187 | 15.2% | 117 | 64.6% | 5.1 |
Proposed criteria19 for referral include: ACR > 300 mg/g; type II diabetes w/ ACR > 30 mg/g; diabetic retinopathy; hyperkalemia; or resistant hypertension.
Only NHANES 1988-1994 used due to availability of data on retinopathy.
Women who were pregnant or in menses were excluded.
Estimates with low precision (SE > 20% of estimate)
Abbreviations: CKD, chronic kidney disease; NHANES, National Health and Nutrition Examination Survey.
Table 6.
Proportion of individuals with CKD Stage 3 according to proposed criteria for referral to a nephrologist by race: NHANES 1988-1994
| White | Black | Mexican-American | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | SE | N | % | SE | N | % | SE | |
| Not Referred | 596 | 83.1 | 1.65 | 117 | 67.1 | 3.7 | 65 | 64.8 | 5.8 |
| Referred | 112 | 16.9 | 1.65 | 57 | 32.9 | 3.7 | 41 | 35.2 | 5.8 |
| Reason for referral: | |||||||||
| Macroalbuminuria | 28 | 3.3† | 0.7 | 11 | 7.6† | 2.8 | 11 | 11.9† | 4.5 |
| Diabetes with Microalbuminuria | 34 | 5.1† | 1.3 | 21 | 11.9 | 2.3 | 9 | 6.7† | 3.2 |
| Retinopathy | 23 | 4.4† | 1.1 | 12 | 6.7† | 1.7 | 5 | 4.2† | 1.8 |
| Hyperkalemia | 2 | 0.1† | 0.1 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 |
| Resistant Hypertension | 12 | 2.3† | 1.0 | 4 | 2.2† | 1.1 | 0 | 0.0 | 0.0 |
| Diabetes with Microalbuminuria & Retinopathy | 10 | 1.7† | 0.4 | 5 | 3.0† | 1.4 | 11 | 6.3† | 2.1 |
| Macroalbuminuria & Retinopathy | 2 | 0.1† | 0.1 | 2 | 0.9† | 0.6 | 1 | 1.1† | 1.1 |
| Multiple Other | 1 | 0.1† | 0.1 | 2 | 0.6† | 0.5 | 4 | 5.0† | 3.4 |
Only NHANES 1988-1994 used due to availability of data on retinopathy.
Women who were pregnant or in menses were excluded.
Estimates with low precision (SE > 20% of estimate)
Abbreviations: CKD, chronic kidney disease; NHANES, National Health and Nutrition Examination Survey.
Discussion
In summary, the NHANES surveys provide an excellent source for tracking trends in the total prevalence of CKD including diagnosed and undiagnosed cases. The most recent data suggest a rising prevalence of all stages of CKD which is at least partly explained by a rise in the prevalence of obesity, diagnosed diabetes and treated hypertension. Estimating the magnitude of the rise is sensitive to small differences in the mean value of serum creatinine across surveys. Thus continued efforts to standardized serum creatinine and assess drift in calibration over time are central to any surveillance activity.
Surveillance for CKD should acknowledge that stages 1 to 5 differ markedly in their severity as well as prevalence. The most severe stages 4 and 5 have the lowest prevalence. However, since they have the highest rates of complications and medical costs, tracking them is vital. Given their low prevalence (∼0.3% each) individuals with these stages are not well represented in population based surveys. It is also a concern that these individuals may have clinical symptoms and may be less likely to volunteer or come to an examination. Thus, alternative methods of surveillance for CKD stages 4 and 5 should be considered. We should look beyond the established surveillance for treated ESRD which combines the presence of kidney failure with the patient being offered and deciding to accept renal replacement therapy. Large health care organization and laboratory chains could be a useful way to track individuals with severe CKD, although inferences will be complicated by the non-random nature of participation in these systems. Data from the Department of Veterans Affairs [EF2]on 2.6 million veterans age 18 to 100 with at least one outpatient serum creatinine measurement indicated that 5,300 (0.2%) had an estimated GFR of less than 15 without being on dialysis, while 14,637 (0.6%) were treated for ESRD.20 The amount of time spent in stage 5 CKD without treatment is relatively short since annual ESRD incidence is 70-80% for veterans aged less than 65 and decreased to 29% after age 85. In contrast, mortality rates increased from 3% below the age of 44 to 49% after the age of 85 years.21
Population based surveys can be very useful for CKD stages 1-3 and with large surveys CKD stage 4. However, within these stages it would be useful to also track the distribution of albuminuria and other markers of severity and likelihood of complications and progression. For example, while the prevalence of CKD stage 3 based on NHANES 1988-1994 and 1999-2004 increased from 5.4% to 7.7%, the estimated proportion of these individuals with albuminuria decreased somewhat such the proportion of the population with both moderately decreased GFR and albuminuria increased from 1.5% to 1.9% (calculated from Table 2 of Coresh et al.11).
It is useful to evaluate criteria for potential action as well as the evidence for these criteria. We evaluated a set of proposed criteria for referral to a nephrologist which suggest that while all CKD stage 4 and 5 patients (n ∼ 1.1 million) should see a nephrologist only 18.6% of individuals in stage 3 (n ∼ 2.9 million) should be referred. Concerning individuals with estimated GFR above 60 mg/ml/1.73 m2 and albuminuria 30.8% (n ∼ 3.0 million) should be referred, a majority of these due to presence of macroalbuminuria (69.2%, SE 2.3%), although these estimates do not incorporate persistence data. Further studies evaluating the evidence for such criteria and the impact of such referral are needed. However, initial estimates can be useful to assess the potential impact of recommendations, policies or ad-hoc practice patterns.
In summary, laboratory evaluation, combined with rigorous survey methodology is central to surveillance of CKD. While a flattening in the age adjusted rates of treated ESRD is reassuring, it is counteracted by an aging population and longer survival on dialysis which increase the number of individuals treated for ESRD. It is important to also track untreated kidney failure (CKD stage 5) as well as undiagnosed CKD. The most recent data suggest that the obesity epidemic is leading to a rise in the prevalence of albuminuria and decreased kidney function (CKD stages 1-4). Surveillance of these trends in the future is important to better understand this population at risk for a wide range of complications. Surveillance efforts should also focus on defining meaningful subgroups of CKD patients by their need for services (referral to specialists), risk of different complications, and potential benefit from different therapies.
Acknowledgments
This paper reproduces much of the information presented previously in a paper on “Prevalence of Chronic Kidney Disease in the United States” JAMA. 2007;298(17):2038-2047.
Support: The project is funded by grants UO1 DK 053869, UO1 DK 067651, and UO1 DK 35073 from the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK). Dr Castro was supported by National Institutes of Health/ National Heart, Lung, and Blood Institute grant T32HL007024
Footnotes
Financial Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: A position statement from kidney disease: Improving global outcomes (KDIGO) Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 3.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension. 2003;42(5):1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Renal Data Systems. USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 5.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the united states, 1988-2000. JAMA. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 6.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the united states 1999 to 2000: A rising tide. Hypertension. 2004;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 7.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293(15):1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 8.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Abramson JH, Last JM International Epidemiological Association. A dictionary of epidemiology. 3rd. New York: Oxford University Press; 1995. [Google Scholar]
- 10.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 11.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third national health and nutrition examination survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 13.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the national health and nutrition examination surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918–26. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services; Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. [May 18,2008]; http://www.cdc.gov/nchs/nhanes.htm.
- 15.National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) Analytic Guidelines. [May 18, 2008]; http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/analytical_guidelines.htm.
- 16.National Center for Health Statistics; Centers for Disease Control and Prevention. Analytic and reporting guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988-1994) http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf Accessibility verified May 20, 2008.
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 Suppl 1):S1–290. [PubMed] [Google Scholar]
- 20.O'Hare AM, Bertenthal D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: One size for all ages? J Am Soc Nephrol. 2006;17(3):846–853. doi: 10.1681/ASN.2005090986. [DOI] [PubMed] [Google Scholar]
- 21.O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]