Abstract
Members of the Mycobacterium avium-Mycobacterium intracellulare (MAI) complex are typeable because each serovar is characterized by its own specific antigenic glycolipid. By means of an enzyme-linked immunosorbent assay, we studied serum specimens obtained from 148 healthy college students for antibodies to these glycopeptidolipids. Ninety-two (61.5%) of the serum specimens were positive to the specific glycolipid antigen from MAI serovar 8. In a study of a pediatric population, antibodies appeared to develop during adolescence. Individuals with overt mycobacterial disease had a significantly lower incidence (tuberculosis patients, 34.5%; leprosy patients, 25%). We found a lower incidence of positive results in a survey of 96 Japanese serum specimens (29.1%), but the results from a survey of sera obtained from Bombay, India, indicated a large degree of reactivity (55.5%). Antibodies to other MAI serovars (serovars 2, 4, and 11) were not found, except antibodies to MAI serovar 21 were seen in the same individuals with antibodies to serovar 8. The dominant epitope of the MAI serovar 8-specific glycopeptidolipid is a terminal pyruvylated 3-O-methylglucose residue [4,6-(1'-carboxyethylidene)-3-O-methyl-alpha-D-glucopyranosyl] unit, whereas that of the MAI serovar 21 has the same terminal pyruvylated glucose devoid of the 3-methoxy group. Thus the antibodies appear specific for the pyruvylated glucose. It is unclear whether the high prevalence of antibodies to these epitopes reflects a high incidence of subclinical colonization or infection with certain MAI serovars or whether they are acquired through contact with some other related antigen source.
Full text
PDF

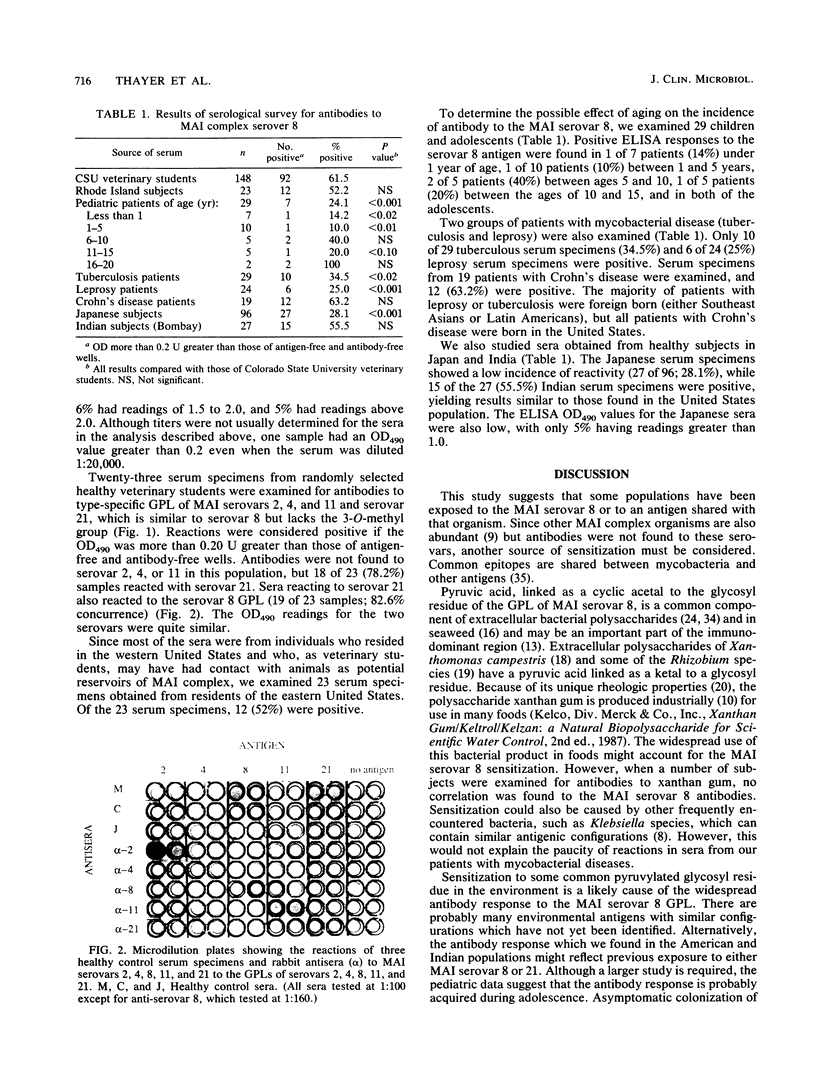


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATWELL R. J., PRATT P. C. Unclassified mycobacteria in the gastric contents of healthy personnel and of patients of a tuberculosis hospital. Am Rev Respir Dis. 1960 Jun;81:888–892. doi: 10.1164/arrd.1960.81.6.888. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Mayer H., Aspinall G. O., Nam Shin J. E. Structures of the glycopeptidolipid antigens from serovars in the Mycobacterium avium/Mycobacterium intracellulare/Mycobacterium scrofulaceum serocomplex. Eur J Biochem. 1981 Mar 16;115(1):7–15. doi: 10.1111/j.1432-1033.1981.tb06190.x. [DOI] [PubMed] [Google Scholar]
- Brennan P. J. Structures of the typing antigens of atypical mycobacteria: a brief review of present knowledge. Rev Infect Dis. 1981 Sep-Oct;3(5):905–913. doi: 10.1093/clinids/3.5.905. [DOI] [PubMed] [Google Scholar]
- Brooks R. W., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. V. Numbers in eastern United States soils and correlation with soil characteristics. Am Rev Respir Dis. 1984 Oct;130(4):630–633. doi: 10.1164/arrd.1984.130.4.630. [DOI] [PubMed] [Google Scholar]
- Brown J. A., Stone M. M., Sutherland I. B.C.G. vaccination of children against leprosy in Uganda: results at end of second follow-up. Br Med J. 1968 Jan 6;1(5583):24–27. doi: 10.1136/bmj.1.5583.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN J. S., BERNARD J. S. The tolerances of unclassified mycobacteria. I. Limits of pH tolerance. Am Rev Respir Dis. 1962 Oct;86:582–583. doi: 10.1164/arrd.1962.86.4.582. [DOI] [PubMed] [Google Scholar]
- Codias E. K., Reinhardt D. J. Distribution of serotypes of the Mycobacterium avium-intracellulare-scrofulaceum complex in Georgia. Am Rev Respir Dis. 1979 Jun;119(6):965–970. doi: 10.1164/arrd.1979.119.6.965. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, Parker B. C., Gruft H. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am Rev Respir Dis. 1980 Jun;121(6):931–937. doi: 10.1164/arrd.1980.121.6.931. [DOI] [PubMed] [Google Scholar]
- Good R. C. Opportunistic pathogens in the genus Mycobacterium. Annu Rev Microbiol. 1985;39:347–369. doi: 10.1146/annurev.mi.39.100185.002023. [DOI] [PubMed] [Google Scholar]
- Gruft H., Falkinham J. O., 3rd, Parker B. C. Recent experience in the epidemiology of disease caused by atypical mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):990–996. doi: 10.1093/clinids/3.5.990. [DOI] [PubMed] [Google Scholar]
- Ichiyama S., Shimokata K., Tsukamura M. The isolation of Mycobacterium avium complex from soil, water, and dusts. Microbiol Immunol. 1988;32(7):733–739. doi: 10.1111/j.1348-0421.1988.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Kenne L., Lindberg B., Ljunggren H., Lönngren J., Rudén U., Svensson S. Demonstration of an octasaccharide repeating unit in the extracellular polysaccharide of Rhizobium meliloti by sequential degradation. J Am Chem Soc. 1977 May 25;99(11):3812–3815. doi: 10.1021/ja00453a049. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Kenne L., Lindberg B. Structure of extracellular polysaccharide from Xanthomonas campestris. Carbohydr Res. 1975 Dec;45:275–282. doi: 10.1016/s0008-6215(00)85885-1. [DOI] [PubMed] [Google Scholar]
- Kazda J. Die Bedeutung von Wasser für die Verbreitung von potentiell pathogenen Mykobakterien. II. Vermehrung der Mykobakterien in Gewässermodellen. Zentralbl Bakteriol Orig B. 1973 Oct;158(2):170–176. [PubMed] [Google Scholar]
- Kazda J. Vermehrung von Mykobakterien in der grauen Schicht der Sphagnum-Vegetation. Zentralbl Bakteriol Orig B. 1978 May;166(4-5):463–469. [PubMed] [Google Scholar]
- Kiehn T. E., Edwards F. F., Brannon P., Tsang A. Y., Maio M., Gold J. W., Whimbey E., Wong B., McClatchy J. K., Armstrong D. Infections caused by Mycobacterium avium complex in immunocompromised patients: diagnosis by blood culture and fecal examination, antimicrobial susceptibility tests, and morphological and seroagglutination characteristics. J Clin Microbiol. 1985 Feb;21(2):168–173. doi: 10.1128/jcm.21.2.168-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C. J., McCleary C. W., Nakada H. I., Rees D. A., Sutherland I. W., Wilkinson J. F. Structural analysis of colanic acid from Escherichia coli by using methylation and base-catalysed fragmentation. Comparison with polysaccharides from other bacterial sources. Biochem J. 1969 Dec;115(5):947–958. doi: 10.1042/bj1150947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapother M. E., Songer J. G. In vitro interaction of Mycobacterium avium with intestinal epithelial cells. Infect Immun. 1984 Jul;45(1):67–73. doi: 10.1128/iai.45.1.67-73.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchy J. K. The seroagglutination test in the study of nontuberculous mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):867–870. doi: 10.1093/clinids/3.5.867. [DOI] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- Meissner G., Anz W. Sources of Mycobacterium avium complex infection resulting in human diseases. Am Rev Respir Dis. 1977 Dec;116(6):1057–1064. doi: 10.1164/arrd.1977.116.6.1057. [DOI] [PubMed] [Google Scholar]
- Palmer C. E., Long M. W. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am Rev Respir Dis. 1966 Oct;94(4):553–568. doi: 10.1164/arrd.1966.94.4.553. [DOI] [PubMed] [Google Scholar]
- Rivoire B., Ranchoff B. J., Chatterjee D., Gaylord H., Tsang A. Y., Kolk A. H., Aspinall G. O., Brennan P. J. Generation of monoclonal antibodies to the specific sugar epitopes of Mycobacterium avium complex serovars. Infect Immun. 1989 Oct;57(10):3147–3158. doi: 10.1128/iai.57.10.3147-3158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tsukamura M. Mycobacterium intracellulare from public bath water. Jpn J Microbiol. 1976 Dec;20(6):561–563. doi: 10.1111/j.1348-0421.1976.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Singer E. Non-specific sensitization to old tuberculin: Asymptomatic infection with Mycobacteria. Tubercle. 1965 Sep;46(3):270–272. doi: 10.1016/s0041-3879(65)80021-6. [DOI] [PubMed] [Google Scholar]
- Songer J. G., Bicknell E. J., Thoen C. O. Epidemiological investigation of swine tuberculosis in Arizona. Can J Comp Med. 1980 Apr;44(2):115–120. [PMC free article] [PubMed] [Google Scholar]
- Thorns C. J., Morris J. A. Common epitopes between mycobacterial and certain host tissue antigens. Clin Exp Immunol. 1985 Aug;61(2):323–328. [PMC free article] [PubMed] [Google Scholar]
- Tsukamura M. Clinical significance of casual isolation of acid-fast organisms from sputum of tuberculous patients. Am Rev Respir Dis. 1973 Dec;108(6):1429–1430. doi: 10.1164/arrd.1973.108.6.1429. [DOI] [PubMed] [Google Scholar]
- Tsukamura M., Mizuno S., Toyama H. [Mycobacteria from dusts of Japanese houses]. Kekkaku. 1984 Dec;59(12):625–631. [PubMed] [Google Scholar]
- Wayne L. G., Anderson B., Chetty K., Light R. W. Antibodies to mycobacterial peptidoglycolipid and to crude protein antigens in sera from different categories of human subjects. J Clin Microbiol. 1988 Nov;26(11):2300–2306. doi: 10.1128/jcm.26.11.2300-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenthal A. M., Powell K. E., Kopp J., Spindler J. W. Increase in Mycobacterium avium complex isolations among patients admitted to a general hospital. Public Health Rep. 1982 Jan-Feb;97(1):61–65. [PMC free article] [PubMed] [Google Scholar]
- Winter S. M., Bernard E. M., Gold J. W., Armstrong D. Humoral response to disseminated infection by Mycobacterium avium-Mycobacterium intracellulare in acquired immunodeficiency syndrome and hairy cell leukemia. J Infect Dis. 1985 Mar;151(3):523–527. doi: 10.1093/infdis/151.3.523. [DOI] [PubMed] [Google Scholar]



