Precisely controlling spatio-temporal environmental parameters has become increasingly important in the field of biotechnology. For this reason, biomedical research has increasingly moved toward the design and implementation of microfabricated systems to efficiently improve technologies such as drug delivery,[1] diagnostics,[2] and tissue engineering.[3] Technologies featuring micron length-scales tailored specifically for biomedical applications, termed Bio-microelectrical mechanical systems (BioMEMS), are able to interact with biological systems such as cells[4] or even single biomolecules.[5] Strategies for developing BioMEMS typically involve adapting traditional microfabrication materials and processes resulting in systems fabricated using non-degradable materials including silicon[6] and polydimethylsiloxane (PDMS).[7] Using biodegradable polymers allows for implantable Bio-MEMS to satisfy the growing demand for in vivo applications such systems for drug delivery systems[8] or tissue engineering. To address these potential applications, BioMEMS devices have been fabricated using biopolymers, both natural and synthetic, including gelatin,[9] alginate,[10] poly(l-lactic acid) (PLA),[8] poly(l-lactic-co-glycolic) acid (PLGA),[11] and poly(glycerol-co-sebacate) (PGS).[12]
An ideal biomaterial for BioMEMS fabrication from a material properties standpoint is one that; 1) can be processed using mild conditions to facilitate protein or growth factor incorporation; 2) naturally promotes adhesion and normal function of seeded cells; 3) contains moieties for potential chemical modification of the surface; 4) exhibits slow and predictable degradation rates to maximize duration of functional implanted devices; 5) has robust, yet flexible mechanical properties; 6) is relatively inexpensive. One class of natural biomaterials that could potentially meet these material requirements is silk fibroin.[13–20] Silk fibroin protein from the Bombyx mori silkworm is FDA approved and has been used in medicine for a wide variety of applications including surgical, drug delivery,[14] and tissue engineering.[13] Silk fibroin exhibits in vitro and in vivo biocompatibility,[13,15] robust mechanical properties including high mechanical modulus and toughness,[16] and relatively slow proteolytic biodegradation.[17]
In this report, we describe techniques and materials processing strategies utilized in the fabrication of cell-seeded silk fibroin microfluidic devices. We have developed material-specific processes for silk fibroin micromolding and device assembly that is analogous to soft-lithographic techniques. By implementing aqueous casting of regenerated aqueous silk fibroin solutions to produce microfabricated silk films, we avoid the use of toxic solvents and harsh processing conditions. Micro-fluidic devices were produced by laminating water-stable micromolded silk fibroin membranes, which were modified with macroscopic fluidic connections. Biocompatibility and functionality of patent devices with cells was studied by seeding and perfusion of a model human hepatocarcinoma cell line for up to five days. Hepatocytes cultured in silk fibroin-based microfluidic devices exhibited similar morphology and cell functions to those grown on other widely used biomaterials.
Silk films derived from regenerated aqueous silk solutions exhibited FT-IR absorbance peaks that are characteristic of amorphous silk I structure, such as the amide I peak at 1656.6 cm−1 and the amide II peak at 1541.5 cm−1. Treatment with aqueous-methanol solution shifted the peaks from silk I configuration to the crystalline silk II configuration, as amide I and amide II peaks shifted to 1616.3 cm−1 and 1515.6 cm−1 for post-methanol treated films, respectively (Supporting Information, Fig. 1). These peak shifts suggest an increase in the percentage of crystalline structure within the bulk, which has been demonstrated by others.[21] Fully hydrated water-stable films processed in this manner have been shown to increase β-sheet formation,[22] which result in increased stiffness, as determined by tensile Young’s modulus, increased toughness modulus, and an increased ultimate tensile strength over thermally crosslinked PGS films (Supporting Information, Fig. 1; Table 1).
Table 1.
Comparison of Mechanical Properties of Regenerated Silk Fibroin and PGS Films. The value n represents the number of samples in each dataset. Data reported as mean ± s.d.
| Young’s Modulus [MPa] |
UTS [MPa] |
Elongation at Break [%] |
Toughness Modulus [MJ/m3] |
||
|---|---|---|---|---|---|
| PGS | (n=8) | 1.72 ± 0.79 | 0.281 ± 0.13 | 19.8 ± 1.11 | 0.0294 ± 0.0146 |
| Silk Fibroin | (n=5) | 107.63 ± 18.29 | 7.60 ± 0.51 | 20.9 ± 0.16 | 1.21 ± 0.0118 |
Devices were fabricated from regenerated silk fibroin films that measured approximately 200 microns in thickness, which was controlled by the volume to surface area ratio during casting. The lamination strategy utilized aqueous silk fibroin solution to bond replica-molded water-stable silk films (Fig. 1). The average and root-mean-squared surface roughness of silk films after the lamination process was 216.6 nm and 267.4 nm, respectively (Supporting Information, Fig. 2). Replica-molded silk fibroin films cast on PDMS negative molds could be produced in rapid succession while maintaining a high degree of feature fidelity. Features as small as 400 nm could be produced using this method (Fig. 2a). Micro-molded films (Fig. 2b) were bonded to flat films to produce microfludic devices (Fig. 2c and d) that could support flow (Fig. 2e and f). Occlusion of microchannels from excess aqueous silk fibroin solution during the bonding process at the inlet/outlet both contributed to reduced device yield. The microfluidic layout used in this study has been designed to produce a constant maximum wall shear stress within all microchannels in the device, given a steady volumetric flow rate.[23] This device geometry assisted in initial cell seeding by allowing cells to be evenly distributed throughout the device during attachment. Furthermore, the constant maximum wall shear stress design facilitates rapid perfusion, while minimizing the potential detachment of seeded cells from shear forces. The high cell seeding density resulted in the formation of HepG2 aggregates, which increased the opportunity for adhesion of cells to the microchannels. Suitable perfusion rates were characterized for the perfusion culture of HepG2 cells cultured in PGS microfluidic devices with similar length scales.[12] The morphology of HepG2 cells seeded and perfused in silk fibroin microdevices (Fig. 3) was similar to that of HepG2 cells cultured on other biomaterials including PGS[12] (Supporting Information, Fig. 3). Viability and liver-specific function of HepG2 cells cultured on silk fibroin were also determined to be similar across static and dynamic cultures. HepG2 cells cultured on silk fibroin films exhibited similar albumin secretion rates as those cultured on other typical biomaterials. Additionally, HepG2 cells cultured statically on films had similar albumin secretion levels to those cultured in dynamically perfused silk fibroin microfluidic devices (Supporting Information, Fig. 2). An increase in albumin secretion levels was observed from day 3 through day 5, which was likely due to increasing cell densities within the microchannels.
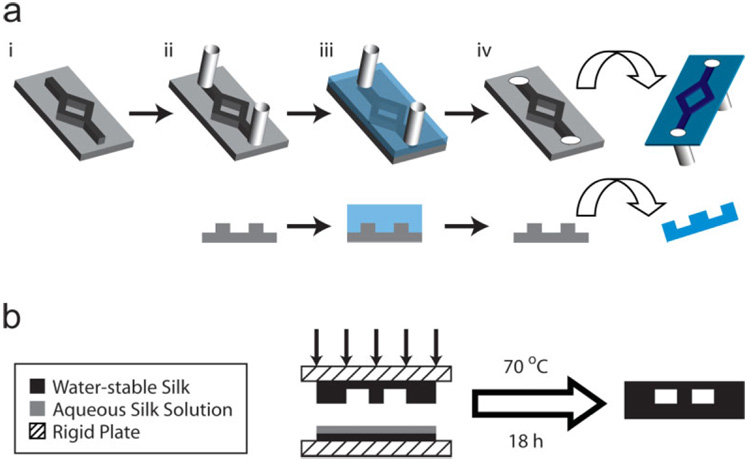
Figure 1. Fabrication Strategy for Silk Fibroin Microfluidic Devices.
a) The process flow is diagrammed in both an isometric view and a cross-sectional view through the midline (i) through the successive steps of the process. PDMS negative molds (i) are fabricated using traditional soft lithography techniques (not shown). PDMS molds are modified with silicone tubing (ii) prior to solvent casting of aqueous silk fibroin solution (iii). Upon water evaporation, micromolded films are delaminated with integrated macroscopic fluidic connections (iv). Both micromolded and flat silk films are treated with aqueous methanol solutions (see text). b) Final assembly of silk microfluidic devices was performed by bonding appropriate water-stable silk fibroin layers using additional regenerated aqueous silk fibroin solution. Layers are bound between rigid plates under mechanical pressure at 70 °C for 18 h to produce a water-insoluble silk fibroin interface with increased β-sheet content (see text).
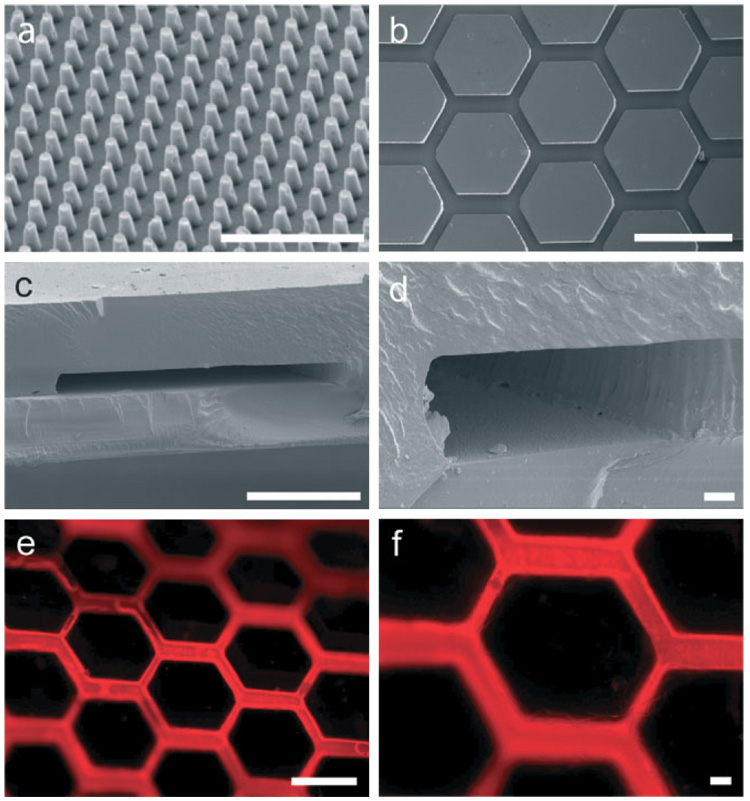
Figure 2. Silk Fibroin-based Microfluidic Devices.
Replica molded silk fibroin films produce high-fidelity features including a) nanometer-scale posts with minimum widths of approximately 400 nm and b) micrometer- scale fluidic channels, which were used in subsequent experiments (scale bars are 5 µm and 500 µm in (a) and (b), respectively). c,d) SEM images of the cross-sections of devices fabricated from the strategy outlined in Figure 1 demonstrate retention of feature geometries in thin films with microchannel widths of approximately c) 240 µm and d) 90 µm (scale bars are 200 µm and 10 µm in (c) and (d), respectively). e,f) Patent microfluidic devices are demonstrated by fluorescent micrographs of devices perfused with rhodamine solution. Retention of the perfusate within the microchannels suggests robust bonding at the interface (scale bars are 500 µm and 50 µm in (e) and (f), respectively).
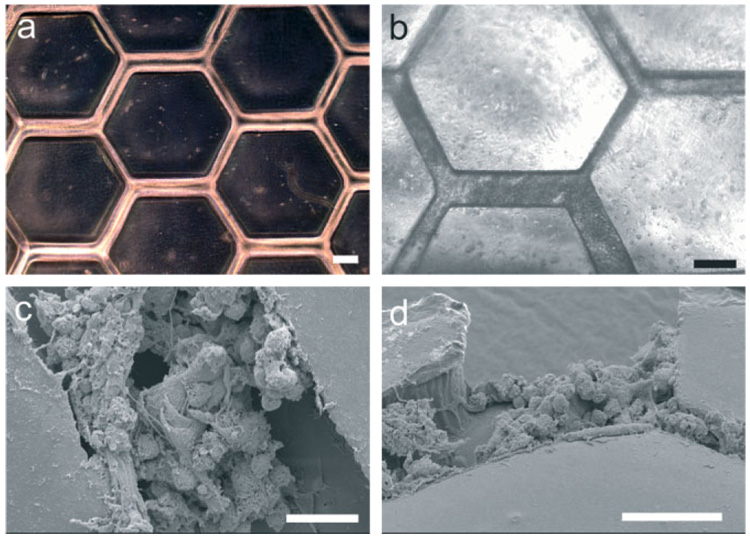
Figure 3. Cell-Seeded Silk Microfluidic Devices.
a) Silk microfluidic devices prior to cell seeding are optically clear to permit observation via light microscopy. Hepatocytes were statically seeded for four hours at which time perfusion commenced. b) Devices were partially confluent with cells exhibiting native morphology after 24 h (scale bars are 100 µlm in (a) and (b)). c,d) Viable cells remained attached and retained function within devices (see text) for up to 5 d of perfusion as shown in SEM images of sectioned devices (scale bars are 50 µm in (C) and (D)).
The device fabrication strategy in this study allows for the rapid and scaleable production of silk-fibroin-based microfluidic devices without the need for harsh processing conditions or cytotoxic compounds. The techniques employed in this strategy are scalable by designing systems with increased surface area and lamination of multiple layers. Although the device yield for patent devices in this study was relatively low, the success rate could be increased by designing flow layouts with redundant microchannel connectivity and by employing additional covalent bonding agents such as (1-ethyl-3-[3-dimehtylaminopropyl] carbodiimide hydrochloride) (EDC) and N-hydroxysuccinimide (NHS), an established chemical route for bioconjugation of amines to carboxylates.[13,24] Similar techniques could be used to covalently link peptides or other bioactive molecules both on the surface and throughout the bulk of the material of the device as previously shown for cell binding peptides and morphogens.[19,28] The aspects of device scalability and incorporation of biomolecules may be important in the design and fabrication of tissue engineering scaffolds for highly vascularized tissue. These biodegradable microfluidic systems can also be integrated with existing biomaterial systems and technologies for tissue-specific applications and increased functionality. For example, drug delivery systems,[25] cell patterning techniques,[26,27] contact guidance cues,[28] and co-culture systems for hepatocytes[29] can be integrated within the microchannels to promote the organization of seeded cells into complex tissues. The robust properties including a high toughness modulus and ultimate tensile strength could permit the use of silk fibroin-based devices in dynamic mechanical environments associated with in vivo applications. In addition to the fabrication of microfluidic systems, strategies for micromolding silk fibroin could be potentially useful in other Bio- MEMS devices including biodegradable drug delivery devices, scaffolds, or biosensors.[8,11–12] The techniques in this report are general and could be used for advancement of the field of implantable and resorbable microfabricated systems, further expanding the impact of related technologies in biomedical applications.
Experimental
Preparation of Micromolded Silk Fibroin Films
Microfluidic networks with a constant shear design were chosen for device fabrication [23]. The finalized mask layouts were converted to DXF files using AutoCAD 2000 and printed onto 1/20-mil transparencies (International Phototool, Colorado Springs, CO). Standard photolithographic and soft-lithography techniques were used in a mold-transfer process. Briefly, 100 mm diameter silicon wafers were patterned with SU-82 000 photoresist (Microchem, Newton, MA) according to manufacturer’s recommendations to produce a “positive mold”. PDMS (Sylgard Elastomer Kit, Essex Group, Edison, NJ) was cast on patterned SU-8 masters using a 10:1 (w/w) ratio of polymer to curing agent and cured for at least 3 h at 65 °C. Masters were passivated with a fluorocarbon treatment, and PDMS “negative molds” were delaminated and used for subsequent replica molding of silk fibroin films. Aqueous silk fibroin solutions derived from B. mori cocoons were prepared using a slight modification to previously published procedures [30]. Briefly, cocoons were boiled for 30 min in an aqueous solution of 0.2 M Na2CO3 to extract sericin proteins. The purified silk fibroin was dissolved in aqueous 9.3M LiBr at 60 °C for 3 h to produce a 20% (w/w) solution. The concentrated silk fibroin solution was dialyzed against water in a Slide-a-lyzer cassette with a 3500 MWCO (Pierce Biotechnology, Rockford, IL) for 48 h. Final aqueous silk fibroin solutions were estimated to be 8% (w/w). Silk solutions were cast on both microfabricated PDMS negative molds and flat PDMS substrates through water evaporation at room temperature and ambient humidity for 72 h. Silk fibroin films were delaminated and treated with a methanol-water solution (50%v/v) for 4 h to produce water-stable films, which were then washed in ddH20 for 24 h. Samples for attenuated total reflectance FT-IR (ATR FT-IR) were prepared by first drying the film and mounting it on a crystal for film analysis. Spectra were recorded using a Nicolet Magna 550 Series II IR Spectrometer equipped with OMNIC Software using 32 scans across the wavenumbers 4000-400 cm−1 at a resolution of 2 cm−1. Surface roughness measurements were performed with aWyko optical profiler (Veeco, Fremont, CA).
Mechanical Testing
PGS films for use in mechanical testing were prepared in a previously published procedure [12]. Briefly, a high molecular pre-polymer was thermally crosslinked onto sucrose-coated silicon wafers at 150 °C for 15 h at a pressure of less than 50 mTorr. Sol-free PGS films were cut into dog bone geometries with dimensions of 1 mm × 6.5 mm × 25 mm (T × W × L). Silk fibroin films dedicated for mechanical testing were cut into dog-bone geometries with dimensions of 0.15 mm × 6.5 mm × 25 mm (T × W × L). Tensile testing was performed using an Instron 5542 using a 50 N load cell equipped with Merlin software. Samples were extended at a constant rate of 50 mm min−1 and were elongated until failure. Young’s modulus, toughness modulus, ultimate tensile strength and elongation at break were calculated from tensile stress versus engineering strain curves.
Silk Fibroin Device Fabrication
The strategy employed for microfluidic device fabrication was similar to that implemented in PDMS microfluidics [31]. Briefly, silk fibroin sheets were trimmed and punched to achieve appropriate macroscopic fluidic connections between layers (Fig. 1). Devices were fabricated by laminating micromolded and flat silk fibroin layers. Microfluidic layers were stacked, aligned, and bonded together at 70 °C for 18 h under mechanical pressure using additional 8%aqueous silk fibroin solution at the interface. Silicone tubing (1/16” ID, 1/8” OD, Cole-Parmer, Vernon Hills, IL) was inserted into the devices in a sterile environment. Luer-Lok connections were inserted into the tubing and the base of the connections was sealed with epoxy (McMaster-Carr, New Brunswick, NJ). In some cases, additional PDMS structures were added to the inlet and outlet to prevent dissociation of the tubing from the device. Samples used from SEM were sputter-coated with Gold/Palladium using a Cressington 108 Auto sputter coater (Cressington Scientific Instruments Inc, Cranberry Twp, PA). Scanning electron micrographs were taken using a Hitachi S-3500N at 5 kV. Fluorescent micrographs to demonstrate patency were obtained by flowing 100 µg mL−1 rhodamine in PBS solutions (Sigma, St. Louis, MO) through single layer devices.
Cell Culture
All cell culture products were obtained from Invitrogen Inc, Carlsbad, CA unless otherwise noted. Hepatocyte carcinoma cells (HepG2, ATCC, Manassas, VA) were cultured with Eaglei’s Modified Essential Medium supplemented with 10% fetal bovine serum, 25 mM HEPES, 100 µg mL−1 streptomycin, 100 U mL−1 penicillin, at 37 °C and 5% CO2. Cells were harvested using Trypsin 0.025 %/ EDTA 0.01% and quenched with an equal volume of medium to re-suspend the cells. Silk fibroin devices were prepared for seeding by incubating with medium for 4 h at 37 °C immediately prior to cell seeding. The devices were statically seeded for 4 h to allow for cell attachment using cellular concentrations of approximately 5 × 108 cells mL−1. After this period, the devices were set up in a linear perfusion circuit consisting of a syringe pump (New Era Pump System NE-1600, Farmingdale, NY), media reservoir, microfluidic scaffold, and media waste container. Fresh medium was perfused through single layer devices in a non-pulsatile manner with a volumetric flow rate of 150 µL h−1. Albumin samples were taken every 24 h by sampling the waste container and aspirating the remaining medium to ensure the accuracy of future sampling. Cells were fixed by injecting the device with Accustain fixative (Sigma, St. Louis, MO) manually with a syringe under high hydrostatic pressures. The sheets were sectioned and serially immersed in solutions of 25 %, 50 %, 75 %, and 90% (v/v) ethanol in PBS for 5 min each. The samples were then immersed in 100% ethanol followed by HMDS (Sigma, St. Louis, MO) each for 15 min. The samples were then allowed to air dry for 24 h prior to further SEM preparation as previously described. Optical imaging was performed using a Carl Zeiss inverted microscope with AxioCam software. Polymer substrates for HepG2 albumin production were produced as follows. Briefly, thermal crosslinking of PGS prepolymer on to glass slides and solvent casting of 5% (w/w) solution of PLGA (65-35 High IV, Lakeshore Biomaterials, Birmingham, AL) in hexafluoroisopropanol (Sigma, St. Louis, MO) was used to produce PGS and PLGA films respectively. HepG2 cells were seeded at a density of 25 000 cells cm−2. Albumin quantification was determined by a human ELISA kit (Bethyl Laboraratories, Montgomery, TX) with absorption measurements made at 450 nm wavelength using a SpectraMax Plus 384 (Moleculare Devices, Sunnyvale, CA) equipped with SOFTmax Pro 4.0 software. Albumin secretion rates were normalized by surface area of cell culture and volumetric flow rate, in the case of perfusion culture.
Footnotes
The authors would like to acknowledge the following: The MEMS Technology Group at the Draper Laboratory for direct funding and use of facilities; James Hsiao and Asish Misra for contributions in microfabrication and cell culture; The Tissue Engineering Resource Center at Tufts University for providing cocoons; Hyeon Joo Kim for technical discussions and assistance in silk purification. C.J.B. was funded through a Draper Fellowship. Additional funding provided through DL-H-550154, and by NIH R01DE-013023-06. Support from the Center for Integration of Medicine and Innovative Technology, US Army DAMD17-02-2-0006, is also gratefully acknowledged. The content of this paper does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred.
Supporting Information is available online from Wiley Inter-Science or from the authors.
Contributor Information
Christopher J. Bettinger, Biomedical Engineering Center, Charles Stark Draper Laboratory, 555 Technology Square, Cambridge, MA 02139 (USA) Department of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Room E25-342, Cambridge, MA 02139, (USA).
Kathleen M. Cyr, Department of Biomedical Engineering, Tufts University Science and Technology Center, 4 Colby Street, Medford, MA 02155 (USA)
Akira Matsumoto, Department of Biomedical Engineering, Tufts University Science and Technology Center, 4 Colby Street, Medford, MA 02155 (USA).
Robert Langer, Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Room E25-342, Cambridge, MA 02139 (USA)
Jeffrey T. Borenstein, Biomedical Engineering Center, Charles Stark Draper Laboratory, 555 Technology Square, Cambridge, MA 02139 (USA), E-mail jborenstein@draper.com
David L. Kaplan, Department of Biomedical Engineering, Tufts University Science and Technology Center, 4 Colby Street, Medford, MA 02155 (USA), E-mail: david.kaplan@tufts.edu
References
- 1.Santini JT, Cima MJ, Langer R. Nature. 1999;397:335. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 2.Gawad S, Schild L, Renaud P. Lab Chip. 2001;1:76. doi: 10.1039/b103933b. [DOI] [PubMed] [Google Scholar]
- 3.Pins GD, Toner M, Morgan JR. FASEB J. 2000;14:593. doi: 10.1096/fasebj.14.3.593. [DOI] [PubMed] [Google Scholar]
- 4.Bettinger CJ, Orrick B, Misra A, Langer R, Borenstein JT. Bio Biomaterials. 2006;27:2558. doi: 10.1016/j.biomaterials.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Hong JW, Studer V, Hang G, Anderson WF, Quake SR. Nat. Biotechnol. 2004;22:435. doi: 10.1038/nbt951. [DOI] [PubMed] [Google Scholar]
- 6.Kaihara S, Borenstein JT, Koka R, Lalan S, Ochoa ER, Ravens M, Pien H, Cunningham B, Vacanti JP. Tissue Eng. 2000;6:105. doi: 10.1089/107632700320739. [DOI] [PubMed] [Google Scholar]
- 7.Borenstein JT, Terai H, King KR, Weinberg EJ, Kaazempur-Mofrad MR, Vacanti JP. Biomed. Microdevices. 2002;4:167. doi: 10.1023/B:BMMD.0000048559.29932.27. [DOI] [PubMed] [Google Scholar]
- 8.Richards-Grayson AC, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Nat. Mater. 2003;2:767. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 9.Paguirigan A, Beebe DJ. Lab Chip. 2005;6:407. doi: 10.1039/b517524k. [DOI] [PubMed] [Google Scholar]
- 10.Cabodi M, Choi NW, Gleghorn JP, Lee CSD, Bonassar LJ, Stroock AD. J. Am. Chem. Soc. 2005;127:13788. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- 11.King KR, Wang CCJ, Kaazempur-Mofrad MR, Vacanti JP, Borenstein JT. Adv. Mater. 2004;16:2007. [Google Scholar]
- 12.Bettinger CJ, Weinberg EJ, Kulig KM, Vacanti JP, Wang Y, Borenstein JT, Langer R. Adv. Mater. 2006;18:165. doi: 10.1002/adma.200500438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. J. Biomed. Mater. Res. 2001;54:139. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Rujiravanit R, Kruaykitanon S, Jamieson A, Tokura S. Macromol. Biosci. 2003;3:604. [Google Scholar]
- 15.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Biomaterials. 2003;24:401. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Rigueiro J, Viney C, Llorca J, Elices M. J. Appl. Polym. Sci. 2000;75:1270. [Google Scholar]
- 17.Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. Biomaterials. 2005;26:3385. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Altman G, Horan R, Moreau J, Kaplan DL. J. Biomed. Mater. Res. 2003;67A:559. doi: 10.1002/jbm.a.10120. [DOI] [PubMed] [Google Scholar]
- 19.Vepari CP, Kaplan DL. Biotechnol. Bioeng. 2006;93:1130. doi: 10.1002/bit.20833. [DOI] [PubMed] [Google Scholar]
- 20.Jin H-J, Chen J, Karageorgiou V, Altman GH, Kaplan DL. Biomaterials. 2004;25:1039. doi: 10.1016/s0142-9612(03)00609-4. [DOI] [PubMed] [Google Scholar]
- 21.Tsukada M, Cotoh Y, Nacura M, Minoura N, Kasai N, Freddi C. J. Polym. Sci. Part B. 1994;32:961. [Google Scholar]
- 22.Park J. Ph.D. Thesis. Medford, MA: Tufts University; 2004. [Google Scholar]
- 23.Weinberg EJ, Borenstein JT, Kaazempur-Mofrad MR, Orrick B, Vacanti JP. Mater. Res. Soc. Symp. Proc. 2004;820R:121. [Google Scholar]
- 24.Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Schwob J, Kaplan DL. J. Biomed. Mater. Res. 2004;71A:528. doi: 10.1002/jbm.a.30186. [DOI] [PubMed] [Google Scholar]
- 25.Richardson DM, Peters MC, Ennet AB, Mooney DJ. Nat. Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 26.Suh KY, Seong J, Khademhosseini A, Laibinis PE, Langer R. Biomaterials. 2004;25:55. doi: 10.1016/s0142-9612(03)00543-x. [DOI] [PubMed] [Google Scholar]
- 27.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Leven-berg S, Lange R. Biomaterials. 2004;25:3583. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 28.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Biomaterials. 1999;20:573. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 29.Bhatia SN, Balis UJ, Yarmush ML, Toner M. FASEB J. 1999;13:1883. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 30.Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Cebe R, Kaplan DL. Adv. Funct. Mater. 2005;15:1241. [Google Scholar]
- 31.Duffy DC, McDonald JC, Schueller JA, Whitesides GM. Anal. Chem. 1998;70:4974. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]