Abstract
The past 50 years have seen rapid development of new building materials, furnishings, and consumer products and a corresponding explosion in new chemicals in the built environment. While exposure levels are largely undocumented, they are likely to have increased as a wider variety of chemicals came into use, people began spending more time indoors, and air exchange rates decreased to improve energy efficiency. As a result of weak regulatory requirements for chemical safety testing, only limited toxicity data are available for these chemicals. Over the past 15 years, some chemical classes commonly used in building materials, furnishings, and consumer products have been shown to be endocrine disrupting chemicals—that is they interfere with the action of endogenous hormones. These include PCBs, used in electrical equipment, caulking, paints and surface coatings; chlorinated and brominated flame retardants, used in electronics, furniture, and textiles; pesticides, used to control insects, weeds, and other pests in agriculture, lawn maintenance, and the built environment; phthalates, used in vinyl, plastics, fragrances, and other products; alkylphenols, used in detergents, pesticide formulations, and polystyrene plastics; and parabens, used to preserve products like lotions and sunscreens. This paper summarizes reported indoor and outdoor air concentrations, chemical use and sources, and toxicity data for each of these chemical classes. While industrial and transportation-related pollutants have been shown to migrate indoors from outdoor sources, it is expected that indoor sources predominate for these consumer product chemicals; and some studies have identified indoor sources as the predominant factor influencing outdoor ambient air concentrations in densely populated areas. Mechanisms of action, adverse effects, and dose-response relationships for many of these chemicals are poorly understood and no systematic screening of common chemicals for endocrine disrupting effects is currently underway, so questions remain as to the health impacts of these exposures.
Keywords: indoor environment, flame retardants, plastics, exposure, toxicology
1. Background
The rapid development of new building materials, furnishings, and consumer products over the past 50 years has resulted in a corresponding increase in new chemicals in the built environment (Weschler, Under review). Indoor concentrations are largely uncharacterized, but they have likely increased over time as a wider variety of chemicals are used and air exchange rates in buildings decrease to improve energy efficiency (Weschler, Under review). Chemical concentrations are often highest indoors because many of the sources are indoors and because of limited degradation indoors compared with outdoors. Furthermore, people spend about 90% of their time indoors (Klepeis et al., 2001; Schweizer et al., 2007; US General Accounting Office, 1999), so indoor concentrations can be more relevant to human exposure assessment than ambient concentrations.
In this article we review six classes of semivolatile chemicals in indoor and outdoor air, including polychlorinated biphenyls (PCBs), brominated flame retardants (e.g. PBDEs), pesticides, phthalates, alkylphenols (e.g. nonylphenol, alkylphenol ethoxylates), and parabens (see Figure 1 for example chemical structures). We summarize available data on major sources and indoor and outdoor concentrations, describe the relative importance of inhalation and dust as routes of exposure, and provide a brief discussion of potential health effects.
Figure 1.
In general, as a result of weak regulatory requirements for chemical safety testing, only limited toxicity data are available for many common commercial chemicals (United States Government Accountability Office, 2005). For example, US EPA found that of the almost 3000 chemicals produced at more than 1 million pounds per year (high production-volume chemicals (HPV)), fewer than 7% had a complete set of six basic toxicity tests and 43% had none of the six tests (United States Government Accountability Office, 2005). Data on exposure to the HPV chemicals is similarly sparse. In light of these data gaps, the European Union recently initiated a regulatory program that requires chemical manufacturers to produce toxicity and exposure information on commercial high production chemicals – the Registration, Evaluation and Authorization of Chemicals (REACH) program (Lahl and Hawxwell, 2006).
Over the past 15 years, some chemicals used in building materials, furnishings, and consumer products have been shown to be endocrine disrupting chemicals (EDCs) -- that is they interfere with the action of endogenous hormones. The greatest concern associated with exposure to EDCs is the potential for adverse effects on reproduction and development, because they have been shown to disrupt normal endocrine signaling in in vitro and in vivo animal studies (Colborn et al., 1993). For example, nonylphenol and methyl paraben bind to the estrogen receptor and initiate transcription of estrogen-responsive genes (Bonefeld-Jorgensen et al., 2007; Routledge et al., 1998; Soni et al., 2005); PCBs and PBDEs have been shown to interfere with thyroid hormone homeostatis and as a consequence delay in utero neurological development in humans and rodents (Arisawa et al., 2005; Longnecker et al., 2003; Morse et al., 1996; Turyk et al., 2007; Winneke et al., 2002; Zhou et al., 2002), and dibutyl phthalate interferes with androgen production in utero (Gray et al., 1998). Exposure during development (in utero, infants and children) is of special concern, because developing tissues are exquisitely sensitive to endocrine signals and disruption of these signaling pathways can result in permanent alterations in tissue structure and function (Colborn et al., 1993; Henley and Korach, 2006; Markey et al., 2003; National Research Council, 1999; Rogan and Ragan, 2007). For example, in utero exposure to phthalates such as dibutyl phthalate has been shown to affect masculinization of the male rat fetus at doses of 100 mg/kg-day (LOAEL) (Mylchreest et al., 2000), to affect testicular testosterone levels at 50 mg/kg-day (LOAEL) (Lehmann et al., 2004), and to affect mammary gland and spermatocyte development at 1.5–3.0 mg/kg-day (LOAEL) in maternal diet (Lee et al., 2004); while testicular toxicity in adult animals is observed only at much higher doses (e.g. LOAEL ~700 mg/kg-day) (European Union, 2004; Marsman, 1995; National Toxicology Program (NTP), 2000).
While laboratory data on biological effects of EDCs raise concerns, they also leave many questions unanswered. Major uncertainties in understanding health risks of EDCs include the following:
No routine chemical screening identifies chemicals that act by known endocrine mechanisms.
Mechanisms by which chemicals may interfere with biological signaling are still incompletely described.
The implications of disrupting these biological signals are not well understood and so the toxicological implications are unknown.
Major exposure sources and pathways are not characterized.
Thus, while human data to support environmental endocrine disruption is extremely limited at this time, this lack of evidence is likely attributable to the broadness and complexity of the subject. In the meantime, information on chemical uses and air concentrations can aid in understanding major exposure sources and pathways in order to suggest opportunities for exposure reduction and aid in the design of human studies.
2. Sources, measured air concentrations, and potential health effects
a. Polychlorinated biphenyls
Polychlorinated biphenyl (PCB) use in the United States began in 1929 and peaked in the 1970s (Agency for Toxic Substances and Disease Registry, 2000; Vorhees, 2001). The mixtures of 209 possible PCB congeners based on the number and position of chlorine atoms were valued for their low flammability and vapor pressure and used extensively through the 1970s in a wide range of consumer products such as flame retardants, paints, plastics, adhesives, lubricants, sealants, hydraulic and heat transfer fluids, capacitors, transformers, vacuum pumps and gas transition turbines (Agency for Toxic Substances and Disease Registry, 2000; Jamshidi et al., 2007; Vorhees, 2001). Restriction of their use in the US began in the late 1970s through the US EPA Toxic Substance Control Act (Environmental Protection Agency, ; Vorhees, 2001)though they remain major global pollutants detected in the atmosphere as well as in human blood and breast milk (Arisawa et al., 2005; Centers for Disease Control and Prevention, 2005; Longnecker et al., 2003; Turyk et al., 2007).
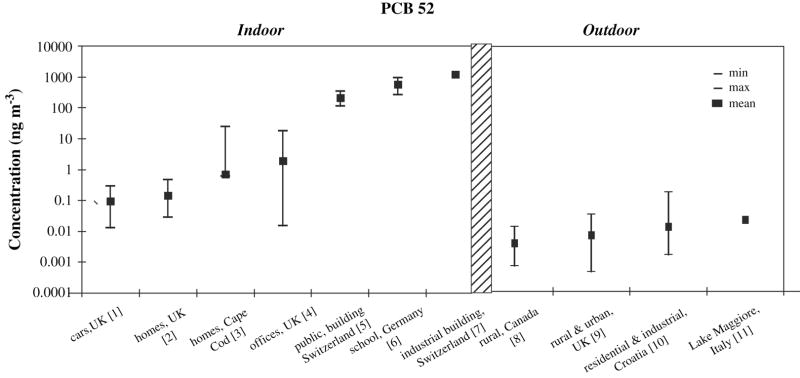
PCB concentrations in air tend to be highest in urban areas and lower in rural or remote areas (Harner et al., 2004; Jamshidi et al., 2007; Motelay-Massei et al., 2005; Shen et al., 2006), with congener composition varying from city to city, indicating that local sources may have a substantial influence on regional values (Shen et al., 2006) (see Figure 2). There is evidence of geographic variation in PCB homolog compositions according to latitude, where relative contributions of tetra-CBs seem to increase with increasing latitude, while penta-CB and hexa-CB contributions decrease with increase in latitude (Shen et al., 2006).
Figure 2.
[1] (Harrad et al., 2006; Harrad. personal communication), n = 24, gas phase only
[2] (Harrad et al., 2006; Harrad, personal communication), n = 31, gas phase only
[3] (Rudel et al.. 2003: Rudel, personal communication), n = 120, median (not mean) plotted
[4] (Harrad et al., 2006; Harrad, personal communication), n = 32, gas phase only
[5] (Kohler et al., 2002), n = 4
[6] (Gabrio et al., 2000), n = 5
[7] (Kohler et al., 2002), n =1
[8] (Su et al.. 2007), n = 67, gas phase only
[9] (Jamshidi et al., 2007), n = 110, gas phase only
[10] (Romanic and Krauthacker, 2007), n = 80, median (not mean) plotted
[11] (Vives et al., 2007), n = 1
In Figure 2, concentrations of a single PCB congener (IUPAC PCB 52) are plotted to enhance comparability across studies. PCB 52 is among the more volatile of the congeners, so it tends to be readily detected in air. PCB 52 is estimated to comprise 5% to 10% of the sum of major PCB congeners in two sets of air sampling data (personal communication, D. Vorhees, S. Harrad).
Indoor air exposures to PCBs are considered to be more significant than outdoor exposures as studies have shown indoor air concentrations to be at least ten and up to 100,000 times higher than outdoor air concentrations (Figure 2). Buildings constructed between 1950 and 1979 generally have higher concentrations of PCBs in indoor air (Hazrati and Harrad, 2006). Caulking in older buildings (pre 1977) is another important source and its presence in schools is of particular concern. For example, Figure 2 shows elevated PCB concentrations in several schools and public buildings in Switzerland and Germany where PCB-containing caulk was used (Gabrio et al., 2000; Kohler et al., 2005; Kohler et al., 2002). Furthermore, Liebl et al showed that higher air concentrations of lower chlorinated PCB congeners (as found in PCB contamined schools) corresponded to increased blood levels of the same PCB congeners (Liebl et al., 2004). In addition, certain floor finishes that were commercially available for residential use during the 1950s and 1960s contained PCBs. Rudel et al. (Rudel et al., 2008) reported elevated PCBs in indoor air, house dust, and residents’ blood in two homes where the PCB-containing wood finish Fabulon or “bowling alley wax” was reportedly used in the 1960s. Sanding of floors to refinish them increased exposures substantially (Rudel et al., 2008). PCBs may also leach from old electronic products, and so in some cases indoor air concentrations can be reduced by properly disposing of old appliances and fluorescent lighting that is not marked ANo PCB (typically installed prior to 1978) (U.S. Environmental Protection Agency Region 10, 2007; Vorhees, 2001)
Researchers have hypothesized that PCBs enter the atmosphere from environmental media such as soil (Vorhees, 2001), although recent consideration of chiral signatures indicates that volatization from soil is not a major source of outdoor air PCB concentrations (Jamshidi et al., 2007). Rather, outdoor air concentrations in urban areas seem to be due to venting of indoor air (Jamshidi et al., 2007).
Although most PCB uses were banned many years ago, PCBs can still be found in the environment today due to their thermal stability, resistance to microbial degradation, and chemical inertness (Vorhees, 2001). PCBs tend to bioaccumulate, leading to dietary exposures through fish, meat, dairy and processed foods (La Rocca and Mantovani, 2006). As PCBs become less prevalent, PCB levels in blood seem to be decreasing (Link et al., 2005; Sjodin et al., 2004). A US study found median blood serum levels of PCB-153 of 90 ng/g lipid in 1985–1989 and 35 ng/g lipid in 2000–2002, with median values steadily decreasing over the years in between (Sjodin et al., 2004). Some studies have shown a slow decline in ambient PCB concentrations from the 1980s to 1990s (Vorhees, 2001) though others show no significant decline from 1997 to 2005 (Harrad et al., 2006). While diet is considered to be the major source of exposure, dietary levels seem to be decreasing faster than indoor air concentrations, making inhalation an increasingly important route of exposure (Harrad et al., 2006; Vorhees, 2001). A study of several North Carolina day care centers concluded that inhalation of PCBs was the major route of exposure to the children, compared with dietary and non-dietary ingestion (Wilson et al., 2001), and in homes with elevated PCBs in indoor air and house dust, possibly due to Fabulon wood floor finish, residents had blood PCB concentrations in the highest 5% of a representative sample of adults in the US (Rudel et al., 2008).
PCBs are established developmental neurotoxicants in humans, with elevated prenatal PCB concentrations being associated with cognitive deficits in children (Arisawa et al., 2005; Winneke et al., 2002). PCBs are also associated with thyroid toxicity, effects on immune, reproductive, nervous, and endocrine systems, and cancer effects including breast cancer (Agency for Toxic Substances and Disease Registry, 2000; Arisawa et al., 2005; Brody et al., 2007; Turyk et al., 2007; Winneke et al., 2002). Among the 209 PCB congeners, different congeners are associated with different types of effects; mono-ortho-substituted and co-planar congener structures show toxicological effects similar to dioxin (Schecter et al., 1994), while ortho-substituted congeners appear more neurotoxic (Giesy and Kannan, 1998).
b. Polybrominated Diphenyl Ethers
Polybrominated diphenyl ethers (PBDEs) are current use flame retardants found in a variety of consumer products such as plastics, upholstery, construction materials, and electrical appliances (Deng et al., 2007; Goel et al., 2006). PBDEs are lipophilic and hydrophobic compounds that tend to persist in the environment, bioaccumulate, and be globally transported due to their resistance to chemical, physical and biological transformations (Deng et al., 2007; Vives et al., 2007); thus, PBDEs can be considered persistent organic pollutants, or POPs, many of which are targeted for global elimination (Ballschmite et al., 2002). There are 209 PBDE congeners, named according to the total number of bromines off the phenyl rings. Generally the lower brominated congeners (mono through penta) are considered to be potentially more harmful to humans (Deng et al., 2007). While PBDEs are fairly evenly distributed between the particulate and the vapor phase in air, as the congeners get heavier (more bromine atoms) they are increasingly likely to be found in the particulate phase ( Vives et al., 2007).
PBDEs are sold as three commercial mixtures: penta-broma diphenyl ether (penta-BDE), octa-broma diphenyl ether (octa-BDE), and deca-broma diphenyl ether (deca-BDE) (Allen et al., 2007). The Bromine Science Environment Forum (BSEF) estimated that 67,000 tons of PBDEs were produced worldwide in 2001 (Deng et al., 2007). Deca-BDE is the most widely used, with an estimated 56,100 tons produced worldwide in 2001, compared to 7,500 tons of penta-BDE produced in the same year (Harrad et al., 2006). While penta-BDE and octa-BDE have recently gone out of use due to restrictions posed by certain governments and a voluntary production ban by some major manufacturers, many sources of exposure remain as these PBDEs are prevalent in a number of common commercial products (Allen et al., 2007; Cahill et al., 2007; Deng et al., 2007; Harrad et al., 2006). Traditionally, penta-BDE was dominated by the BDE 47 and BDE 99 congeners and used in polyurethane foam, foam products, bedding, vehicle interiors, and furniture (Allen et al., 2007; Harrad et al., 2006). Octa-BDE contained a mixture of hepta, octa and nona congeners while deca-BDE consists primarily of BDE 209 (Allen et al., 2007). Both octa- and deca-BDE can be found in electronics such as computers and televisions and deca-BDE is also often in textiles (Allen et al., 2007; Harrad et al., 2006). Because PBDEs are additives mixed into polymers, and are thus not chemically bound to plastics or textiles, they tend to leach off products and enter the air and dust (Deng et al., 2007).
PBDEs are considered to be a ubiquitous environmental contaminant found both in the environment (sediment, soil, sewage, air, and wildlife) and in people (tissue, blood, and breast milk) worldwide (Deng et al., 2007; Goel et al., 2006). Though body burdens show regional variation (the highest values are found in the United States), they are also increasing exponentially with approximately a 4–6 year doubling rate (Allen et al., 2007; Deng et al., 2007; Goel et al., 2006; Hites, 2004; Vives et al., 2007). PBDEs have been found in marine mammals and air in remote areas including the Baltic Sea and the northern Atlantic Sea, indicating that the atmosphere is a pathway for transport (Allen et al., 2007; Deng et al., 2007; Hites, 2004; Vives et al., 2007; Wang et al., 2005)
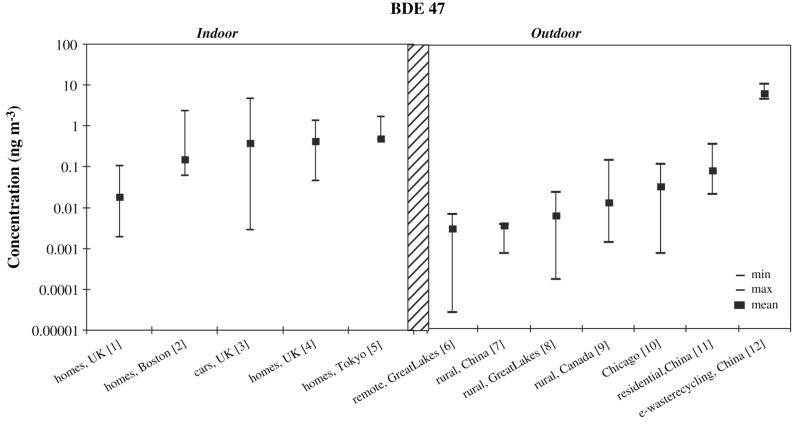
Indoor air concentrations of PBDEs are generally higher than outdoor air concentrations (see Figure 3) (Hazrati and Harrad, 2006). Levels of PBDEs in offices are generally higher than levels of PBDEs in homes (Harrad et al., 2006; Harrad et al., 2004; Saito et al., 2007). Outdoor concentrations vary regionally, with urban higher than rural, and both indoor and outdoor concentrations vary seasonally with higher concentrations in spring and summer ( Goel et al., 2006; Harrad et al., 2006; Hazrati and Harrad, 2006).
Figure 3.
[1] (Harrad et al, 2006; Harrad, personal communication), n = 31, gas phase only
[2] (Allen et al., 2007), n = 40
[3] (Harrad et al., 2006; Harrad, personal communication), n = 25, gas phase only
[4] (Harrad et al., 2004), n = 7
[5] (Saito et al., 2007), n = 18, median (not mean) plotted
[6] (Strandberg et al., 2001; Basu, personal communication), n = 12
[7] (Deng et al., 2007; Deng, personal communication), n = 30, TSP
[8] (Strandberg et al., 2001; Basu, personal communication), n = 24
[9] (Su et al., 2007), n = 32
[10] (Strandberg et al., 2001; Basu, personal communication), n = 12
[11] (Deng et al, 2007; Deng, personal communication), n = 30, TSP
[12] (Deng et al., 2007; Deng, personal communication), n = 30, TSP
Occupational exposures are also of concern. Workers at electronic waste recycling plants or automotive shredding/recycling facilities are often subject to substantial occupational exposures ( Cahill et al., 2007; Deng et al., 2007). Work environments where older computers are used may also lead to higher exposures ( Cahill et al., 2007; Harrad et al., 2006).
While some researchers conclude that indoor air is the primary source of exposure for the general population, others studies assert that dust is the more significant source, especially for toddlers, who have greater hand-to-mouth activity (Allen et al., 2007; Harrad et al., 2006). Diet is also considered to be a potentially important source of PBDE exposure (Harrad et al., 2004; Schecter et al., 2006). Darnerud et al (2006) estimated a daily total PBDE intake of 51 ng in Sweden, with the highest levels found in fish. Overall, although it is widely agreed that outdoor air is a minor source of PBDE exposure, the relative importance of indoor air, diet and dust exposures is not clear.
Though few studies have been conducted on health effects of PBDEs, they are similar to PCBs in their ability to interfere with thyroid hormone function, and so are likely to cause similar effects on neurodevelopment following in utero exposure (McDonald, 2005; Zhou et al., 2002).
c. Pesticides
Pesticides are generally classified according to the type of substance they are intended to repel or kill. Accordingly, the many varieties include insecticides, acaricides, fungicides, herbicides, rodenticides, avicides, larvicides, and germicides (Lewis, 2001). Pesticides can be composed of inorganic substances, organometallic compounds, volatile organic compounds, semivolatile organic compounds, and nonvolatile organic compounds (Lewis, 2001). Most conventional pesticides are semivolatile organic compounds or non volatile organic compounds (Lewis, 2001). Major classes of pesticides include organochlorines, such as DDT and chlordane; organophosphates such as chlorpyrifos; carbamates, such as carbaryl; and pyrethroids and pyrethrins such as permethrin. There are also many chemicals commonly introduced into products as antimicrobials, for example triclosan and triclocarban, and o-phenyl phenol. Semivolatile pesticides are often detected in both dust and indoor air, while non volatile pesticides are typically found only in dust (Lewis, 2001). Both have been frequently detected in indoor and outdoor environments in studies from 1992 to the present (Butte and Heinzow, 2002; Lewis, 2001; Rudel et al., 2003)
Pesticides are commonly used on pets, in the garden, and as repellant or disinfectant (Lewis, 2001). They may be added to carpets, paints, furnishings, and building materials during manufacturing and are often available “off the shelf” for use inside and outside homes and public places such as schools, hospitals, and office buildings (Lewis, 2001). In addition, pesticides applied outdoors can be tracked into the home (Lewis, 2001). Diet may also be an important source, especially for infants and children, because of their greater food and beverage consumption per kilogram body weight and their propensity to have less variety in their diets than adults (Clayton et al., 2003). Lu and Toepel et al. (2006) found that switching a particular population of children to an organic diet dramatically reduced concentrations of certain pesticides in urine, sometimes to a level below the detection limit. This suggests that dietary exposure predominates, at least for certain pesticides, such as malathion (Lu et al., 2006), while residential exposure is more important for other pesticides, such as pyrethroids (Lu et al., 2006).
Pesticide air concentrations are affected by a number of factors. Pesticide use is generally higher in warmer weather, which also increases pesticide volatility (Lewis, 2001). Thus pesticide concentrations vary seasonally (higher in the spring and summer months) and geographically (higher in southern verses northern US) (Lewis, 2001). Air concentrations of specific types of pesticides are also influenced by the varying volatility of the different chemical constituents (Lewis, 2001). Current use pesticides are less persistent than the now banned organochlorides which can still be detected in the environment (Lewis, 2001). However, degradation rates are typically much lower indoors, thus indoor air levels are typically higher than outdoor levels (Butte and Heinzow, 2002; Rudel et al., 2003). Pesticides tend to accumulate on toys, carpets, and dust, sources that can become major routes of exposure for infants and toddlers (Butte and Heinzow, 2002; Lewis, 2001).
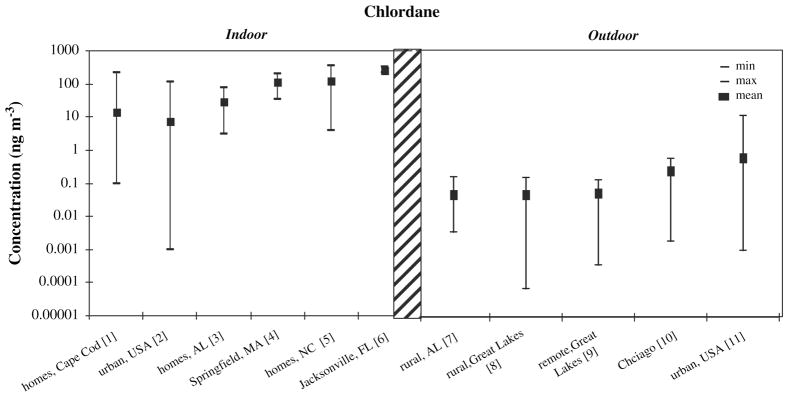
Figure 4 shows measured concentrations for the termiticide chlordane, a persistent organochlorine pesticide which was widely used in and around building foundations until its use was restricted in the mid 1980s (Kilburn and Thornton, 1995). Chlordane concentrations are widely variable indoors; and lower concentrations are seen outdoors compared with indoors, and in rural verses urban areas. Regional levels of pesticides in outdoor air have been shown to be correlated with concentrations in lichen, snow, and spruce needles (Daly et al., 2007; Hageman et al., 2006; Romanic and Krauthacker, 2007; Wang et al., 2006).
Figure 4.
[1] (Rudel el al.. 2003), n = 120, sum of trans-chlordane, cis-chlordane. and heptachlor
[2] (Offenberg et al., 2004), n = 126, sum of trans-chlordane, cis-chlordane, trans-nonachlor, and cis-nonachlor
[3] (Jantunen et al., 2000; Jantunen, 2008), n = 5. trans-chlordane only
[4] (Whitmore et al., 1994), n = 85. min, max, and mean are from seasonal averages
[5] (Lewis et al., 1994), n = 8,
[6] (Whitmore et al.. 1994), n = 175. min, max, and mean are from seasonal averages
[7] (Jantunen et al., 2000), n = 25, trans-chlordane only
[8] (Strandberg et al., 2001; Basu, personal communication), n = 12
[9] (Strandberg et al, 2001; Basu, personal communication), n = 12
[10] (Strandberg et al., 2001; Basu, personal communication), n = 12
[11] (Offenberg et al., 2004), n = 95, sum of trans-chlordane, cis-chlordane, trans-nonachlor, and cis-nonachlor
Because pesticides include many types of chemicals, it is not possible to generalize about health effects. Many of the banned organochlorines such as DDT, chlordane, and methoxychlor have been identified as EDCs (Kelce et al., 1995; Soto et al., 1994; Soto et al., 1995), and health effect studies for these compounds suggest neurotoxicity, effects on developing reproductive system, effects on lactation, and cancers, including breast cancer (Brody et al., 2007; Cohn et al., 2007; Kilburn and Thornton, 1995; Rogan and Ragan, 2007). Some pesticides in current use appear to also have effects on the endocrine system, for example various pyrethroids have been demonstrated to have weak anti-androgenic, anti-estrogenic, or estrogenic activity; and chlorpyrifos has been shown to affect thyroid hormones in animal and human studies (Garey and Wolff, 1998; Go et al., 1999; Jeong et al., 2006; Kim et al., 2005; Kim et al., 2005; Meeker et al., 2006), and to affect infant development in human health studies (Rauh et al., 2006).
In general, more comprehensive toxicology and health information is available for current use pesticides than for most chemicals in commercial use, because tests are required for pesticide registrations. However, some important gaps persist in the data available on pesticide effects on neurodevelopment (an especially important endpoint since many pesticides affect neuroendocrine function) (Colborn, 2006).
d. Phthalates
Phthalates are common plasticizers in polyvinyl chloride (PVC) resins (European Union, 2004; European Union, 2007; Rudel, 2000). By weight, they contribute 10–60% of plastic products because of their ability to increase flexibility and transparency (Rakkestad et al., 2007). In 2004 world wide production of phthalates was estimated to be 6 million tons per year (Arbeitsgemeinschaft PVC und Umwelt e.V., 2006; Xie et al., 2007). Phthalates are found in a wide variety of products including vinyl upholstery, shower curtains, food containers and wrappers, toys, floor tiles, lubricants, sealers, and adhesives (Arbeitsgemeinschaft PVC und Umwelt e.V., 2006; European Union, 2007; Heudorf et al., 2007; Rakkestad et al., 2007; Rudel, 2000)}. Beyond their use in PVC resins, phthalates can be found in cosmetics such as perfume, eye shadow, moisturizer, nail polish, hair spray, and liquid soap, and as an inert ingredient in pesticides (European Union, 2004; European Union, 2007; Rudel, 2000; Schettler, 2006). Detection of phthalates in a variety of media began to be reported in the 1970s (Giam et al., 1978; Xie et al., 2007).
Because there is no covalent bond between phthalates and the plastics into which they are mixed, they are easily released from products into the environment (Fromme et al., 2004; Heudorf et al., 2007; Rakkestad et al., 2007). This process accelerates as plastic products age and break down (Rakkestad et al., 2007). Phthalates are subject to photo degradation, biodegradation, and anaerobic degradation and thus generally do not persist in the outdoor environment (Stales et al., 1997; Xie et al., 2007). Photo degradation half lives of common phthalates range from approximately 0.3 days to 15 days (Stales et al., 1997; Xie et al., 2007). In remote regions of the Norwegian Sea, where cold temperatures, low concentrations, and lack of nutrients can retard the degradation process, phthalates have been found despite the lack of a recognized source ( Xie et al., 2007). In this case, atmospheric transport and deposition is likely to be the major source, with adsorption of phthalates by snow and ice both slowing down the degradation process and contributing to an underestimation of the total phthalate load ( Xie et al., 2007).
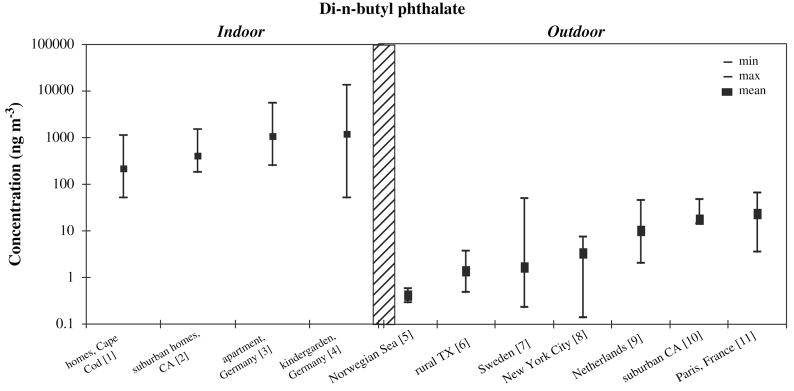
Due to the nature of phthalate sources, phthalates are ubiquitous in the indoor environment, with indoor air concentrations generally higher than outdoor concentrations (see Figure 5) (Rakkestad et al., 2007; Rudel, 2000). Outdoors, urban and suburban concentrations are higher than rural and remote concentrations (Figure 5). Generally, it is agreed that outdoor sources of phthalates, such as the wearing of tires, are secondary to indoor sources (Rakkestad et al., 2007).
Figure 5.
[1] (Rudel et al., 2003), n = 120, median (not mean) plotted
[2] (Sheldon et al., 1992), n = 104, range shown is 10th to 90lh percentile, median (not mean) plotted
[3] (Fromme et al., 2004), n = 59, median (not mean) plotted
[4] (Fromme et al., 2004), n = 74, median (not mean) plotted
[5] (Xie et al., 2007), n = 6
[6] (Atlas and Giam, 1988), n =13
[7] (Thuren and Larsson, 1990), n = 51, median (not mean) plotted
[8] (Bove et al., 1978), n = 138, particulate phase only, min and max from monthly averages, median (not mean) plotted
[9] (Peijnenburg and Struijs, 2006), n = 32, range shown is MQL to 95th percentile, gas phase only, median (not mean) plotted
[10] (Sheldon et al., 1992), n = 36, range shown is MQL to 90th percentile, median (not mean) plotted
[11] (Teil et al., 2006), n = 20
More volatile phthalates such as diethyl phthalate (DEP) and dimethyl phthalate (DMP) and dibutyl phthalate (DBP) are present at higher concentrations in air compared with heavier, less volatile phthalates such as di(2-ethylhexyl)phthalate (DEHP) and benzyl butyl phthalate (BBP), which are more prevalent in dust ( Heudorf et al., 2007; Rudel et al., 2003).
It has been difficult to establish correlations between particular activities or objects and measured concentrations of phthalates (Fromme et al., 2004). Higher air temperatures lead to increased air concentrations of phthalates (Uhde et al., 2001), and PVC flooring has been associated with higher concentrations of BBP and DEHP in house dust, and may also influence indoor air concentrations (Bornehag et al., 2005; Bornehag et al., 2004).
Phthalate metabolites have been detected in virtually all human urine samples tested, indicating widespread exposure ( Heudorf et al., 2007). Most exposure studies have concluded that diet is the major route of exposure for phthalates, especially DEHP, (Fromme et al., 2007; Heudorf et al., 2007; National Toxicology Program (NTP), 2000; National Toxicology Program (NTP), 2000; National Toxicology Program (NTP), 2000). For example, phthalates are present in materials used in food packaging and processing, as well as gloves used in food handling (Tsumura et al., 2001). But other sources such as consumer products make a substantial contribution to overall exposure levels for some phthalates, such as DnBP and DiBP (Fromme et al., 2007); use of fragrance-containing personal care products is thought to be a considerable route of exposure for DEP (Duty et al., 2005). Some unique exposure pathways include medical equipment for medical staff and patients (IV bags and tubing are PVC made with DEHP) (European Union, 2008; U.S. Food and Drug Administration, 2001), some pharmaceutical products (Fromme et al., 2004; Hauser et al., 2004; Heudorf et al., 2007; National Toxicology Program (NTP), 2000) and ingestion of phthalates from teething rings, other toys, and house dust; for infants and young children (European Union, 2004; European Union, 2008; Heudorf et al., 2007; National Toxicology Program (NTP), 2000). Dermal absorption of phthalates in personal care products may be an important route of exposure, especially for individuals who use large quantities of these products (Janjua et al., 2008). Inhalation exposure has typically been considered to be relatively minor (European Union, 2004; European Union, 2008;Heudorf et al., 2007; National Toxicology Program (NTP), 2000; National Toxicology Program (NTP), 2000; National Toxicology Program (NTP), 2000). For example, the US NTP concluded that inhalation contributed less than 20% of total intake of DBP ( National Toxicology Program (NTP), 2000). However, correlations have been observed between levels of urinary metabolites of DEP, DBP, and BBP and indoor or personal air concentrations of the parent phthalate, suggesting that indoor air is a significant route of exposure or is a proxy for another route, namely dermal absorption (Adibi et al., 2008; Adibi et al., 2003; Rudel, 2008).
A number of the phthalates have been shown to interfere with androgen production, with the developing male fetus being the most sensitive to this effect. In animal studies, endpoints include effects on the developing male reproductive tract, including disrupted epididymal development, hypospadias, cryptorchidism, retained nipples, and reduced fertility (Henley and Korach, 2006; Mylchreest et al., 2000). Most animal studies show these effects occurring at higher exposure levels than are observed in the general human population, although certain medical procedures such as dialysis can result in much higher levels of exposure and adverse effects from these exposures are a serious concern (European Union, 2008; National Toxicology Program (NTP), 2000). However, one human study has shown an association between maternal levels of urinary phthalate metabolites and reproductive tract development in male offspring in the general population (Swan, 2006). Associations have also been observed between sperm quality and urinary phthalate metabolite levels in adult men (Duty et al., 2005; Duty et al., 2003; Duty et al., 2003). In addition, two studies have reported associations between phthalate levels in house dust and allergic symptoms in children (Bornehag et al., 2004; Kolarik et al., 2008). Thus, while animal studies suggest that current exposure levels in the general population are below levels of health concern, a small number of studies in humans with typical levels of exposure have demonstrated adverse health effects.
e. Alkylphenols
Alkylphenols, especially nonylphenol ethoxylates and octylphenol ethoxylates, are high production volume chemicals found throughout the world and are important indoor air contaminants (Brody and Rudel, 2003; Davis et al., 1994; Rudel et al., 2003; Warhurst, 1995; White, 1994). They are used as surfactants in common consumer products such as detergents, disinfectants, surface cleaners, and pesticides; and tris(nonylphenol) phosphite is also used as a plasticizer (Rudel, 2000). General use of alkylphenols began in the 1940s (Warhurst, 1995). Most of the nonylphenol used in commercial products is 4-t-nonylphenol, which is a mixture of the branched isomers, so it is important that measurement studies quantify the branched rather than the straight-chain isomers (4-n-nonylphenol).
Alkylphenols have been frequently detected in wastewater and aquatic environments (White, 1994). Sewage treatment biodegrades less than 40% of nonylphenol polyethoxylates and its metabolites (Warhurst, 1995). During biodegradation, alkylphenol polyethoxylates lose ethoxy groups to become alkylphenols (typically nonylphenol) which are more stable, persistent, and hydrophobic, leading to their accumulation in sewage and rivers (Davis et al., 1994; Warhurst, 1995; White, 1994), and their volatilization into ambient air. Nonylphenol is lipophilic and tends to bioaccumulate (Warhurst, 1995). Photochemical degradation of nonylphenol will occur, with a half life of about 10–15 hours in ideal conditions (Warhurst, 1995). The more soluble phenols (such as short chain alkylphenol polyethoxylate) have been found in drinking water and groundwater in the US (Rudel et al., 1998; Swartz et al., 2006; White, 1994).
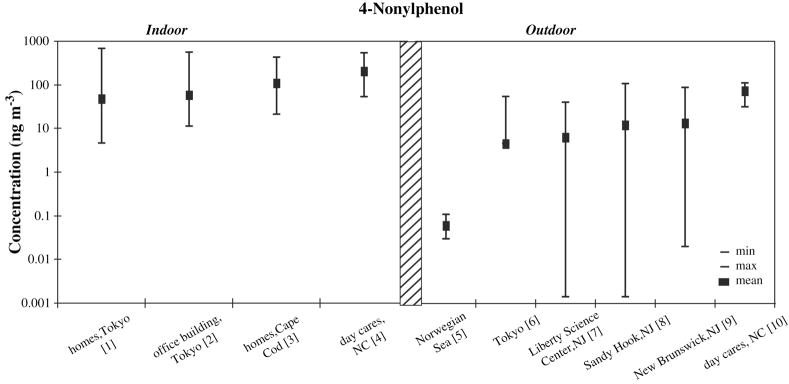
Air concentrations of alkylphenols are rarely reported in the literature. Existing studies show indoor concentrations are generally around 100 ng/m3, while outdoor concentrations are about ten times lower (see Figure 6). Weschler et al (1984) measured nonylphenol in the particulate phase of air samples and found mean values substantially lower than those in other studies, indicating that alkylphenols might exist primarily in the gas phase (Weschler, 1984). Studies in outdoor air have demonstrated elevated concentrations above wastewater-impacted surface waters, for example in the lower Hudson River estuary (Dachs et al., 1999).
Figure 6.
[1] (Saito et al., 2007), n = 90, median (not mean) plotted
[2] (Saito et al., 2007), n = 38, median (not mean) plotted
[3] (Rudel et al., 2003), n = 120, median (not mean) plotted
[4] (Wilson et al., 2001), n = 9
[5] (Xie et al., 2006), n = 6, gas phase only, sum of branched 4-nonylphenol isomers
[6] (Saito et al., 2004), n = 33, median (not mean) plotted
[7] (Van Ry et al., 2000), n = 23, sum of branched 4-nonylphenol isomers
[8] (Van Ry et al., 2000), n =38, sum of branched 4-nonylphenol isomers
[9] (Van Ry et al., 2000), n = 27, sum of branched 4-nonylphenol isomers
[10] (Wilson et al., 2001), n = 9
Major pathways of exposure to these compounds have not been described, and may include dietary and non dietary ingestion, dermal absorption from product use, and inhalation. A day care study concluded that dietary ingestion was the primary exposure pathway for toddlers to a group of phenolic compounds that included nonylphenol, though inhalation was found to be a secondary route of exposure (Wilson et al., 2001).
4-Nonylphenol and 4-octylphenol have been shown to be estrogen mimics -- that is they are able to bind with the estrogen receptor and initiate transcription of estrogen-responsive genes (Bonefeld-Jorgensen et al., 2007; Soto et al., 1991). They are much less potent than the endogenous estrogen 17β-estradiol, however it has also been shown that endogenous estrogen mimics can act in combination, additively, to induce estrogen-receptor mediated effects, so it is expected that these exposures may add to endogenous background levels to induce effects (Silva et al., 2002). A recent study has also reported that these chemicals can act by additional endocrine mechanisms (Bonefeld-Jorgensen et al., 2007), so assessment of potential health effects is complex. In vivo studies in animals show effects at doses between 3 and 10 mg/kg-day (Lee et al., 1999; Lee and Lee, 1996). Though some information is available for these compounds, limited data on human exposure via dietary ingestion, non-dietary ingestion, and dermal absorption make it difficult to compare human exposures with doses associated with effects in animal studies.
f. Parabens
Parabens, characterized by a para-substituted hydroxybenzoate, are commonly used as preservatives and antimicrobial agents (Soni et al., 2001). They can be found in food such as jams, jellies, syrups, beverages, and baked goods (Soni et al., 2001). Parabens are also used in commercial products including shampoo, cream, deodorant, hairspray, and cosmetics, as well as pharmaceuticals (Shen et al., 2007; Soni et al., 2001). In a 1995 study, they were found in 77% of rinse-off and 99% of leave-on cosmetic products (Rastogi et al., 1995). Parabens are absorbed into the body through the skin and gastrointestinal tract (Soni et al., 2001). Though it is generally accepted that they do not bioaccumulate, parabens have been detected in human tissue, including human breast tumors (Darbre et al., 2004; Soni et al., 2001).
Few studies have measured for parabens in air. In a 2003 study of 120 homes in Cape Cod, Rudel et al. (2003) detected three parabens (butyl paraben, ethyl paraben, and methyl paraben) in indoor air and house dust (Rudel et al., 2003). Methyl paraben was most frequently detected, with air concentrations in 67% of homes above the reporting limit of 1 ng/m3 (Rudel et al., 2003). The median indoor air concentration of methyl paraben was 2.9 ng/m3 and the maximum was 21 ng/m3 (Rudel et al., 2003). Outdoor concentrations have not been reported in the literature. Because of their widespread use in lotions and other products applied to the skin, there has been interest in the likelihood of dermal absorption of parabens. In a study of human breast tumors, methyl paraben was found in the highest concentration of the six parabens tested (Darbre et al., 2004). This may be due to more common use of methyl paraben in products, or a greater tendency for methyl paraben to be absorbed (Darbre et al., 2004).
Parabens have been shown to be weak estrogen mimics in vitro and in vivo (Kang et al., 2002; Routledge et al., 1998). They appear to be rapidly metabolized when given orally, however little is known about absorption or toxicity by dermal or inhalation routes. It is expected that most paraben exposure may be associated with dermal application of paraben-containing products, however the relative importance of dermal, ingestion, and inhalation exposure routes remains unknown.
3. Conclusion
Ongoing research shows that indoor environments provide important opportunities for exposure to a wide variety of chemicals in commercial use, including pesticides (Rudel et al., 2003). Indoor environments are well documented as important opportunities for exposure due to the fact that so many chemicals have indoor sources, indoor concentrations of many compounds are much higher than outdoor, and a majority of time is spent indoors (US General Accounting Office, 1999). Therefore, future research on EDCs in air should focus on understanding factors influencing indoor air concentrations and exposure, such as sources, ventilation, and chemical degradation processes to be expected indoors.
In this review, we focused on six chemical families that have been detected in indoor environments and shown to be EDCs. Exposures to these and other EDCs are ubiquitous, although they have not been well characterized. National exposure monitoring programs to track indoor and outdoor chemical concentrations over space and time are a priority – such programs would provide important context for ongoing biomonitoring programs and facilitate design and implementation of health studies.
With approximately 80,000 chemicals registered by US EPA in commercial use, it is clear that there is little information available on exposure and health effects for many of these compounds (US Environmental Protection Agency, 1998; US General Accounting Office, 2005). In particular, endocrine-mediated effects of these chemicals are of concern, and no program has been implemented to screen chemicals for effects on the endocrine system. New initiatives to develop and use bioassays as integrated measures of estrogenic or other hormonal activity offer great promise for advancing the feasibility of health studies of EDC exposure, since integrated measures circumvent the limitations of a chemical-by-chemical analytical approach. This is a priority research area for both biomonitoring and exposure monitoring EDC research (DeCastro et al., 2006; Kortenkamp, 2006). Beyond chemical screening programs to identify chemicals with hormonal activity, many questions remain about the best way to assess implications of endocrine disruption for health effects.
While in some cases the portion of total exposure attributable to inhalation is relatively small, toxicity studies using an inhalation route of exposure are lacking and studies based on oral exposure may not provide relevant information due to differences in absorption, metabolism, and distribution. For example, some chemicals are rapidly metabolized in the liver following ingestion, but are more rapidly distributed with limited metabolism following inhalation. In addition, direct effects on respiratory endpoints such as asthma have been reported in several human and animal studies of inhaled phthalate exposure (Jaakkola and Knight, 2008), and these endpoints may not be observed in oral dosing studies.
Priorities for research include national exposure monitoring programs to document indoor and outdoor air concentrations and characterize trends over space and time, as well as development and application of novel integrated measurement methods, such as measurement of total estrogenic activity in an environmental sample. While research is underway to try to better understand exposures and health effects of the many chemicals in use, an emphasis on preventing adverse effects dictates that exposure to these compounds should be limited where possible.
Acknowledgments
This work was supported by grants from the US National Institute of Environmental Health Sciences (NIEHS), NIH grant 5R25ES13258-4 and from appropriations of the Massachusetts Legislature administered by the University of Massachusetts-Lowell and the Massachusetts Department of Public Health. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibi J, Whyatt R, et al. Characterization of Phthalate Exposure among Pregnant Women assessed by Repeat Air and Urine Samples. Environmental Health Perspectives. 2008 doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Perera FP, et al. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environmental Health Perspectives. 2003;111(14):1719–1722. doi: 10.1289/ehp.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polychlorinated Biphenyls (PCBs) Atlanta: US Department of Health and Human Services; 2000. [PubMed] [Google Scholar]
- Allen JG, McClean MD, et al. Personal exposure to polybrominated diphenyl ethers (PBDEs) in residential indoor air. Environmental Science & Technology. 2007;41(13):4574–9. doi: 10.1021/es0703170. [DOI] [PubMed] [Google Scholar]
- Arbeitsgemeinschaft PVC und Umwelt e.V. Plasticizers market data. 2006 from http://www.agpu.de.
- Arisawa K, Takeda H, et al. Background exposure to PCDDs/PCDFs/PCBs and its potential health effects: a review of epidemiologic studies. The Journal of Medical Investigation. 2005;52(1–2):10–21. doi: 10.2152/jmi.52.10. [DOI] [PubMed] [Google Scholar]
- Atlas E, Giam CS. Ambient concentration and precipitation scavenging of atmospheric organic pollutants. Water, Air, and Soil Pollution. 1988;38:19–36. [Google Scholar]
- Ballschmite K, Hackenberg R, et al. Man-made chemicals found in remote areas of the world: the experimental definition for POPs. Environmental science and pollution research international. 2002;9(4):274–88. doi: 10.1007/BF02987503. [DOI] [PubMed] [Google Scholar]
- Basu I. data request. L. Perovich; Newton, MA: 2008. e-mail. [Google Scholar]
- Bonefeld-Jorgensen EC, Long M, et al. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environmental Health Perspectives. 2007;115(Suppl 1):69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Lundgren B, et al. Phthalates in indoor dust and their association with building characteristics. Environmental Health Perspectectives. 2005;113(10):1399–404. doi: 10.1289/ehp.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, et al. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environmental Health Perspectives. 2004;112(14):1393–7. doi: 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove JL, Dalven P, et al. Airborne di-butyl and di-(2-ethylhexyl)-phthalate at three New York City air sampling stations. International Journal of Environmental Analytical Chemistry. 1978;5:189–194. [Google Scholar]
- Brody JG, Moysich KB, et al. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667–711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- Brody JG, Rudel RA. Environmental pollutants and breast cancer. Environmental Health Perspectives. 2003;111(8):1007–1019. doi: 10.1289/ehp.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte W, Heinzow B. Pollutants in house dust as indicators of indoor contamination. Reviews of Environmental Contamination and Toxicology. 2002;175:1–46. [PubMed] [Google Scholar]
- Cahill TM, Groskova D, et al. Atmospheric concentrations of polybrominated diphenyl ethers at near-source sites. Environmental Science & Technology. 2007;41(18):6370–7. doi: 10.1021/es070844j. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Third national report on human exposure to environmental chemicals. 2005 Retrieved May 22, 2006, from http://www.cdc.gov/exposurereport/3rd/default.htm.
- Clayton CA, Pellizzari ED, et al. Distributions, associations, and partial aggregate exposure of pesticides and polynuclear aromatic hydrocarbons in the Minnesota Children’s Pesticide Exposure Study (MNCPES) Journal of Exposure Analysis and Environmental Epidemiology. 2003;2003(13):100–111. doi: 10.1038/sj.jea.7500261. [DOI] [PubMed] [Google Scholar]
- Cohn BA, Wolff MS, et al. DDT and Breast Cancer in Young Women: New Data on the Significance of Age at Exposure. Environmental Health Perspectives. 2007;115(10):1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T. A case for revisiting the safety of pesticides: a closer look at neurodevelopment. Environmental Health Perspectectives. 2006;114(1):10–7. doi: 10.1289/ehp.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T, vomSaal F, et al. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environmental Health Perspectives. 1993;101(5):378–385. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachs J, van Ry DA, et al. Occurence of estrogenic nonylphenols in the urban and coastal atmosphere of the lower Hudson River estuary. Environmental Science & Technology. 1999;33(15):2676–2679. [Google Scholar]
- Daly GL, Lei YD, et al. Pesticides in western Canadian mountain air and soil. Environmental Science & Technology. 2007;41(17):6020–5. doi: 10.1021/es070848o. [DOI] [PubMed] [Google Scholar]
- Darbre PD, Aljarrah A, et al. Concentrations of parabens in human breast tumours. Journal of Applied Toxicology. 2004;24(1):5–13. doi: 10.1002/jat.958. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Atuma S, et al. Dietary intake estimations of organohalogen contaminants (dioxins, PCB, PBDE and chlorinated pesticides, e.g. DDT) based on Swedish market basket data. Food and Chemical Toxicology. 2006;44(9):1597–606. doi: 10.1016/j.fct.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Davis DL, Dinse GE, et al. Decreasing cardiovascular disease and increasing cancer among whites in the United States from 1973 through 1987. Journal of the American Medical Association. 1994;271(6):431–437. [PubMed] [Google Scholar]
- DeCastro BR, Korrick SA, et al. Estrogenic activity of polychlorinated biphenyls present in human tissue and the environment. Environmental Science & Technology. 2006;40(8):2819–25. doi: 10.1021/es051667u. [DOI] [PubMed] [Google Scholar]
- Deng WJ. L Perovich. Newton, MA: e-mail; 2008. data request. [Google Scholar]
- Deng WJ, Zheng JS, et al. Distribution of PBDEs in air particles from an electronic waste recycling site compared with Guangzhou and Hong Kong, South China. Environment International. 2007;33(8):1063–9. doi: 10.1016/j.envint.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Duty SM, Ackerman RM, et al. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environmental Health Perspectectives. 2005;113(11):1530–5. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, et al. Phthalate exposure and reproductive hormones in adult men. Human Reproduction. 2005;20(3):604–10. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- Duty SM, Silva MJ, et al. Phthalate exposure and human semen parameters. Epidemiology. 2003;14(3):269–77. [PubMed] [Google Scholar]
- Duty SM, Singh NP, et al. The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environmental Health Perspectives. 2003;111(9):1164–9. doi: 10.1289/ehp.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency (2008). Toxic Substances Control Act Title 40--Protection of the Environment, Electronic Code of Federal Regulations. Chapter I, Subchapter R, Part 761.
- European Union. Risk Assessment Report: dibutyl phthalate. European Chemicals Bureau; The Netherlands: 2004. [Google Scholar]
- European Union. Risk Assessment Report: benzylbutyl phthalate. European Chemicals Bureau; Norway: 2007. [Google Scholar]
- European Union. Risk Assessment Report: bis(2-ethylhexyl)phthalate (DEHP) European Chemicals Bureau; Sweden: 2008. [Google Scholar]
- Fromme H, Gruber L, et al. Intake of phthalates and di(2-ethylhexyl)adipate: results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environment International. 2007;33(8):1012–20. doi: 10.1016/j.envint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fromme H, Lahrz T, et al. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany) Indoor Air. 2004;14(3):188–95. doi: 10.1111/j.1600-0668.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- Gabrio T, Piechotowski I, et al. PCB-blood levels in teachers, working in PCB-contaminated schools. Chemosphere. 2000;40(9–11):1055–62. doi: 10.1016/s0045-6535(99)00353-7. [DOI] [PubMed] [Google Scholar]
- Garey J, Wolff MS. Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochemical and Biophysical Research Communications. 1998;251(3):855–9. doi: 10.1006/bbrc.1998.9569. [DOI] [PubMed] [Google Scholar]
- Giam CS, Chan HS, et al. Phthalate ester plasticizers: a new class of marine pollutant. Science (New York, NY) 1978;199(4327):419–21. [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Critical reviews in toxicology. 1998;28(6):511–69. doi: 10.1080/10408449891344263. [DOI] [PubMed] [Google Scholar]
- Go V, Garey J, et al. Estrogenic potential of certain pyrethroid compounds in the MCF-7 human breast carcinoma cell line. Environmental Health Perspectectives. 1999;107(3):173–7. doi: 10.1289/ehp.99107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, McConnell LL, et al. Spray irrigation of treated municipal wastewater as a potential source of atmospheric PBDEs. Environmental Science & Technology. 2006;40(7):2142–8. doi: 10.1021/es051931j. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby JS, et al. Dibutyl phthalate induces anti-androgenic but not estrogenic in vivo effects in LE hooded rats. Toxicological Sciences. 1998;42(1S):176. [Google Scholar]
- Hageman KJ, Simonich SL, et al. Atmospheric deposition of current-use and historic-use pesticides in snow at national parks in the western United States. Environmental Science & Technology. 2006;40(10):3174–80. doi: 10.1021/es060157c. [DOI] [PubMed] [Google Scholar]
- Harner T, Shoeib M, et al. Using passive air samplers to assess urban-rural trends for persistent organic pollutants. 1. Polychlorinated biphenyls and organochlorine pesticides. Environmental Science & Technology. 2004;38(17):4474–83. doi: 10.1021/es040302r. [DOI] [PubMed] [Google Scholar]
- Harrad S. L Perovich. Newton, MA: e-mail; 2008. data request. [Google Scholar]
- Harrad S, Hazrati S, et al. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: implications for human exposure. Environmental Science & Technology. 2006;40(15):4633–8. doi: 10.1021/es0609147. [DOI] [PubMed] [Google Scholar]
- Harrad S, Wijesekera R, et al. Preliminary assessment of U.K. human dietary and inhalation exposure to polybrominated diphenyl ethers. Environmental Science & Technology. 2004;38(8):2345–50. doi: 10.1021/es0301121. [DOI] [PubMed] [Google Scholar]
- Hauser R, Duty S, et al. Medications as a source of human exposure to phthalates. Environmental Health Perspectives. 2004;112(6):751–3. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati S, Harrad S. Causes of variability in concentrations of polychlorinated biphenyls and polybrominated diphenyl ethers in indoor air. Environmental Science & Technology. 2006;40(24):7584–9. doi: 10.1021/es0617082. [DOI] [PubMed] [Google Scholar]
- Henley DV, Korach KS. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology. 2006;147(6 Suppl):S25–32. doi: 10.1210/en.2005-1117. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, et al. Phthalates: toxicology and exposure. International journal of hygiene and environmental health. 2007;210(5):623–34. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environmental Science & Technology. 2004;38(4):945–56. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Jaakkola JJ, Knight TL. The Role of Exposure to Phthalates from Polyvinyl Chloride Products in the Development of Asthma and Allergies: A Systematic Review and Meta-analysis. Environmental health perspectives. 2008;116(7):845–53. doi: 10.1289/ehp.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi A, Hunter S, et al. Concentrations and chiral signatures of polychlorinated biphenyls in outdoor and indoor air and soil in a major U.K. conurbation. Environmental Science & Technology. 2007;41(7):2153–8. doi: 10.1021/es062218c. [DOI] [PubMed] [Google Scholar]
- Janjua NR, Frederiksen H, et al. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. International Journal of Andrology. 2008;31(2):118–30. doi: 10.1111/j.1365-2605.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- Jantunen L. L Perovich. Newton, MA: e-mail; 2008. data request. [Google Scholar]
- Jantunen LMM, Bidleman TF, et al. Toxaphene, chlordane, and other organochlorine pesticides in Alabama air. Environmental Science & Technology. 2000;34(24):5097–5105. [Google Scholar]
- Jeong SH, Kim BY, et al. Effect of chlorpyrifos-methyl on steroid and thyroid hormones in rat F0- and F1-generations. Toxicology. 2006;220(2–3):189–202. doi: 10.1016/j.tox.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kang KS, Che JH, et al. Decreased sperm number and motile activity on the F1 offspring maternally exposed to butyl p-hydroxybenzoic acid (butyl paraben) The Journal of Veterinary Medical Science. 2002;64(3):227–35. doi: 10.1292/jvms.64.227. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Stone CR, et al. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Thornton JC. Protracted neurotoxicity from chlordane sprayed to kill termites. Environmental Health Perspectectives. 1995;103(7–8):690–4. doi: 10.1289/ehp.95103690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Kwack SJ, et al. Assessment of estrogenic and androgenic activities of tetramethrin in vitro and in vivo assays. Journal of Toxicology and Environmental Health Part A. 2005;68(23–24):2277–89. doi: 10.1080/15287390500182453. [DOI] [PubMed] [Google Scholar]
- Kim SS, Lee RD, et al. Potential estrogenic and antiandrogenic effects of permethrin in rats. The Journal of Reproduction and Development. 2005;51(2):201–10. doi: 10.1262/jrd.16060. [DOI] [PubMed] [Google Scholar]
- Klepeis NE, Nelson WC, et al. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. Journal of Exposure Science and Environmental Epidemiology. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- Kohler M, Tremp J, et al. Joint sealants: an overlooked diffuse source of polychlorinated biphenyls in buildings. Environmental Science & Technology. 2005;39(7):1967–73. doi: 10.1021/es048632z. [DOI] [PubMed] [Google Scholar]
- Kohler M, Zennegg M, et al. Coplanar polychlorinated biphenyls (PCB) in indoor air. Environmental Science & Technology. 2002;36(22):4735–40. doi: 10.1021/es025622u. [DOI] [PubMed] [Google Scholar]
- Kolarik B, Naydenov K, et al. The association between phthalates in dust and allergic diseases among Bulgarian children. Environmental Health Perspectives. 2008;116(1):98–103. doi: 10.1289/ehp.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A. Breast cancer, oestrogens and environmental pollutants: a re-evaluation from a mixture perspective. International Journal of Andrology. 2006;29(1):193–8. doi: 10.1111/j.1365-2605.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- La Rocca C, Mantovani A. From environment to food: the case of PCB. Annali dell’Istituto superiore di sanita. 2006;42(4):410–6. [PubMed] [Google Scholar]
- Lahl U, Hawxwell KA. REACH: The New European Chemicals Law. Environmental Science & Technology. 2006;40(23):7115–7121. doi: 10.1021/es062984j. [DOI] [PubMed] [Google Scholar]
- Lee KY, Shibutani M, et al. Diverse developmental toxicity of di-n-butyl phthalate in both sexes of rat offspring after maternal exposure during the period from late gestation through lactation. Toxicology. 2004;203(1–3):221–38. doi: 10.1016/j.tox.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Lee PC, Arndt P, et al. Testicular abnormalities in male rats after lactational exposure to nonylphenols. Endocrine. 1999;11(1):61–8. doi: 10.1385/ENDO:11:1:61. [DOI] [PubMed] [Google Scholar]
- Lee PC, Lee W. In vivo estrogenic action of nonylphenol in immature female rats. Bulletin of Environmental Contamination and Toxicology. 1996;57(3):341–8. doi: 10.1007/s001289900196. [DOI] [PubMed] [Google Scholar]
- Lehmann KP, Phillips S, et al. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol Sci. 2004;81(1):60–8. doi: 10.1093/toxsci/kfh169. [DOI] [PubMed] [Google Scholar]
- Lewis RG. Pesticides. In: Spengler JFM John D, Samet Jonathan M., editors. Indoor Air Quality Handbook. New York: McGraw-Hill; 2001. pp. 35.1–35.17. [Google Scholar]
- Lewis RG, Fortmann RC, et al. Evaluation of methods for monitoring the potential exposure of small children to pesticides in the residential environment. Archives of Environmental Contamination and Toxicology. 1994;26:37–46. doi: 10.1007/BF00212792. [DOI] [PubMed] [Google Scholar]
- Liebl B, Schettgen T, et al. Evidence for increased internal exposure to lower chlorinated polychlorinated biphenyls (PCB) in pupils attending a contaminated school. International Journal of Hygiene and Environmental Health. 2004;207(4):315–24. doi: 10.1078/1438-4639-00296. [DOI] [PubMed] [Google Scholar]
- Link B, Gabrio T, et al. Biomonitoring of persistent organochlorine pesticides, PCDD/PCDFs and dioxin-like PCBs in blood of children from South West Germany (Baden-Wuerttemberg) from 1993 to 2003. Chemosphere. 2005;58(9):1185–201. doi: 10.1016/j.chemosphere.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Wolff MS, et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environmental Health Perspectives. 2003;111(1):65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Barr DB, et al. A longitudinal approach to assessing urban and suburban children’s exposure to pyrethroid pesticides. Environmental Health Perspectives. 2006;114(9):1419–23. doi: 10.1289/ehp.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Toepel K, et al. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environmental Health Perspectives. 2006;114(2):260–3. doi: 10.1289/ehp.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey C, Coombs M, et al. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evolution & Development. 2003;5(1):67–75. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- Marsman D. NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice. Toxicity report series. 1995;30:1–G5. [PubMed] [Google Scholar]
- McDonald TA. Polybrominated diphenylether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integrated Environmental Assessment and Management. 2005;1(4):343–54. [PubMed] [Google Scholar]
- Meeker JD, Barr DB, et al. Thyroid hormones in relation to urinary metabolites of non-persistent insecticides in men of reproductive age. Reproductive Toxicology. 2006;22(3):437–42. doi: 10.1016/j.reprotox.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Morse DC, Wehler EK, et al. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254) Toxicology and Applied Pharmacology. 1996;136:269–279. doi: 10.1006/taap.1996.0034. [DOI] [PubMed] [Google Scholar]
- Motelay-Massei A, Harner T, et al. Using passive air samplers to assess urban-rural trends for persistent organic pollutants and polycyclic aromatic hydrocarbons. 2. Seasonal trends for PAHs, PCBs, and organochlorine pesticides. Environmental Science & Technology. 2005;39(15):5763–73. doi: 10.1021/es0504183. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Wallace DG, et al. Dose-Dependent Alterations in Androgen-Regulated Male Reproductive Development in Rats Exposed to Di(n-butyl) Phthalate during Late Gestation. Toxicological sciences. 2000;55(1):143–151. doi: 10.1093/toxsci/55.1.143. [DOI] [PubMed] [Google Scholar]
- National Research Council. Hormonally Active Agents in the Environment. Washington DC: National Academy Press; 1999. [Google Scholar]
- National Toxicology Program (NTP) NTP-CERHR Expert Panel Report on butyl benzyl phthalate. Alexandria, VA: US Department of Health and Human Services, National Toxicology Program (NTP), Center for the Evaluation of Risks to Human Reproduction (CERHR): 42; 2000. [Google Scholar]
- National Toxicology Program (NTP) NTP-CERHR Expert Panel Report on di-n-butyl phthalate. Alexandria, VA: US Department of Health and Human Services, National Toxicology Program (NTP), Center for the Evaluation of Risks to Human Reproduction (CERHR): 60; 2000. [Google Scholar]
- National Toxicology Program (NTP) NTP-CERHR Expert Panel Report on di(2-ethylhexyl) phthalate. Alexandria, VA: US Department of Health and Human Services, National Toxicology Program (NTP), Center for the Evaluation of Risks to Human Reproduction (CERHR): 150; 2000. [Google Scholar]
- Offenberg JH, Naumova YY, et al. Chlordanes in the indoor and outdoor air of three U.S. cities. Environmental Science & Technology. 2004;38(10):2760–8. doi: 10.1021/es035404g. [DOI] [PubMed] [Google Scholar]
- Peijnenburg WJ, Struijs J. Occurrence of phthalate esters in the environment of The Netherlands. Ecotoxicology and environmental safety. 2006;63(2):204–15. doi: 10.1016/j.ecoenv.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Rakkestad KE, Dye CJ, et al. Phthalate levels in Norwegian indoor air related to particle size fraction. Journal of environmental monitoring. 2007;9(12):1419–25. doi: 10.1039/b709947a. [DOI] [PubMed] [Google Scholar]
- Rastogi SC, Schouten A, et al. Contents of methyl-, ethyl-, propyl-, butyl- and benzylparaben in cosmetic products. Contact dermatitis. 1995;32(1):28–30. doi: 10.1111/j.1600-0536.1995.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan WJ, Ragan NB. Some evidence of effects of environmental chemicals on the endocrine system in children. International Journal of Hygiene and Environmental Health. 2007;210(5):659–67. doi: 10.1016/j.ijheh.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic SH, Krauthacker B. Are pine needles bioindicators of air pollution? Comparison of organochlorine compound levels in pine needles and ambient air. Arhiv za higijenu rada i toksikologiju. 2007;58(2):195–9. doi: 10.2478/v10004-007-0012-8. [DOI] [PubMed] [Google Scholar]
- Routledge EJ, Parker J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicology and Applied Pharmacology. 1998;153(1):12–9. doi: 10.1006/taap.1998.8544. [DOI] [PubMed] [Google Scholar]
- Rudel R. Polycyclic Aromatic Hydrocarbons, Phthalates, and Phenols. In: Samet J, Spengler J, McCarthy J, editors. Indoor Air Quality Handbook. New York: McGraw-Hill; 2000. [Google Scholar]
- Rudel RA. Correlations Between Urinary Phthalate Metabolites and Phthalates, Estrogenic Compounds 4-Butyl phenol and o-Phenyl phenol, and Some Pesticides in Home Indoor Air and House Dust. Joint Annual Conference for the International Society of Environmental Epidemiology and International Society of Exposure Analysis; Pasadena, California. 2008. [Google Scholar]
- Rudel RA. L Perovich. Newton, MA: e-mail; 2008. data request. [Google Scholar]
- Rudel RA, Camann DE, et al. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environmental Science & Technology. 2003;37(20):4543–53. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Geno P, et al. Identification of alkylphenols and other estrogenic phenolic compounds in wastewater, septage, and groundwater on Cape Cod, Massachusetts. Environmental Science & Technology. 1998;32(7):861–869. [Google Scholar]
- Rudel RA, Seryak LM, et al. PCB-containing wood floor finish is a likely source of elevated PCBs in residents’ blood, household air and dust: a case study of exposure. Environmental Health. 2008;7(1):2. doi: 10.1186/1476-069X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I, Onuki A, et al. Indoor air pollution by alkylphenols in Tokyo. Indoor Air. 2004;14(5):325–32. doi: 10.1111/j.1600-0668.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- Saito I, Onuki A, et al. Indoor organophosphate and polybrominated flame retardants in Tokyo. Indoor Air. 2007;17(1):28–36. doi: 10.1111/j.1600-0668.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, et al. Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environmental Health Perspectives. 2006;114(10):1515–20. doi: 10.1289/ehp.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Stanley J, et al. Polychlorinated biphenyl levels in the tissues of exposed and nonexposed humans. Environmental Health Perspectives 102 Suppl. 1994;1:149–58. doi: 10.1289/ehp.94102s1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettler T. Human exposure to phthalates via consumer products. International journal of andrology. 2006;29(1):134–9. doi: 10.1111/j.1365-2605.2005.00567.x. discussion 181–5. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Edwards RD, et al. Indoor time-microenvironment-activity patterns in seven regions of Europe. Journal of exposure science & environmental epidemiology. 2007;17(2):170–81. doi: 10.1038/sj.jes.7500490. [DOI] [PubMed] [Google Scholar]
- Sheldon L, Clayton A, et al. PTEAM: Monitoring of Phthalates and PAHS in Indoor and Outdoor Air Samples in Riverside. California: Research Triangle Park, California Environmental Protection Agency, Air Resources Board Research Division; 1992. [Google Scholar]
- Shen HY, Jiang HL, et al. Simultaneous determination of seven phthalates and four parabens in cosmetic products using HPLC-DAD and GC-MS methods. Journal of Separation Science. 2007;30(1):48–54. doi: 10.1002/jssc.200600215. [DOI] [PubMed] [Google Scholar]
- Shen L, Wania F, et al. Polychlorinated biphenyls and polybrominated diphenyl ethers in the North American atmosphere. Environmental Pollution. 2006;144(2):434–44. doi: 10.1016/j.envpol.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, et al. Something from “nothing” -- Eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environmental Science & Technology. 2002;36(8):1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, et al. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environmental health perspectives. 2004;112(6):654–8. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni MG, Burdock GA, et al. Safety assessment of propyl paraben: a review of the published literature. Food and Chemical Toxicology. 2001;39(6):513–32. doi: 10.1016/s0278-6915(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Soni MG, Carabin IG, et al. Safety assessment of esters of p-hydroxybenzoic acid (parabens) Food and Chemical Toxicology. 2005;43(7):985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Soto AM, Chung KL, et al. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environmental Health Perspectives. 1994;102(4):380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AM, Justicia H, et al. P-Nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environmental Health Perspectives. 1991;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AM, Sonnenschein C, et al. The E-SCREEN assay as a tool to identify estrogens: An update on estrogenic environmental pollutants. Environmental Health Perspectives. 1995;103(Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stales CA, Peterson DR, et al. The environmental fate of phthalate esters: A literature review. Chemosphere. 1997;35(4):667–749. [Google Scholar]
- Strandberg B, Bodder NG, et al. Concentrations and spatial variations of polybrominated diphenyl ethers and other organohalogen compounds in Great Lakes air. Environmental Science & Technology. 2001;35(6):1078–1083. doi: 10.1021/es001819f. [DOI] [PubMed] [Google Scholar]
- Su Y, Wania F, et al. Temperature dependence of the air concentrations of polychlorinated biphenyls and polybrominated diphenyl ethers in a forest and a clearing. Environmental Science & Technology. 2007;41(13):4655–61. doi: 10.1021/es070334p. [DOI] [PubMed] [Google Scholar]
- Swan SH. Prenatal phthalate exposure and anogenital distance in male infants. Environmental Health Perspectives. 2006;114(2):A88–9. doi: 10.1289/ehp.114-a88b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz CH, Reddy S, et al. Steroid estrogens, nonylphenol ethoxylate metabolites, and other wastewater contaminants in groundwater affected by a residential septic system on Cape Cod, MA. Environmental Science and Technology. 2006;40(16):4894–4902. doi: 10.1021/es052595+. [DOI] [PubMed] [Google Scholar]
- Teil MJ, Blanchard M, et al. Atmospheric fate of phthalate esters in an urban area (Paris-France) The Science of the Total Environment. 2006;354(2–3):212–23. doi: 10.1016/j.scitotenv.2004.12.083. [DOI] [PubMed] [Google Scholar]
- Thuren A, Larsson P. Phthalate esters in the Swedish atmosphere. Environmental Science & Technology. 1990;24:554–559. [Google Scholar]
- Tsumura Y, Ishimitsu S, et al. Di(2-ethylhexyl) phthalate contamination of retail packed lunches caused by PVC gloves used in the preparation of foods. Food additives and contaminants. 2001;18(6):569–79. doi: 10.1080/02652030120071. [DOI] [PubMed] [Google Scholar]
- Turyk ME, Anderson HA, et al. Relationships of thyroid hormones with polychlorinated biphenyls, dioxins, furans, and DDE in adults. Environmental Health Perspectives. 2007;115(8):1197–203. doi: 10.1289/ehp.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency Region 10. PCBs in Fluorescent Light Fixtures. 2007 Retrieved July 18, 2008, from http://yosemite.epa.gov/R10/OWCM.NSF/88fa11a23f885ef3882565000062d635/d053fb2a8fcba715882569ed00782e8a!OpenDocument#cap.
- U.S. Food and Drug Administration. Safety Assessment of Di (2-ethylhexyl) phthalate (DEHP) Released from PVC Medical Devices. Rockville, MD: Center for Devices and Radiological Health; 2001. [Google Scholar]
- Uhde E, Bednarek M, et al. Phthalic esters in the indoor environment--test chamber studies on PVC-coated wallcoverings. Indoor Air. 2001;11(3):150–5. doi: 10.1034/j.1600-0668.2001.011003150.x. [DOI] [PubMed] [Google Scholar]
- United States Government Accountability Office. Chemical Regulation: Options Exist to Improve EPA’s Ability to Assess Health Risks and Manage Its Chemical Review Program. 2005 Retrieved January 21, 2008, from http://www.gao.gov/cgi-bin/getrpt?GAO-05-458.
- US Environmental Protection Agency. Chemical Hazard Data Availability Study. 1998 http://www.epa.gov/hpv/pubs/general/hazchem.pdf.
- US General Accounting Office. Indoor Pollution: Status of Federal Research Activities. Washington DC: 1999. [Google Scholar]
- US General Accounting Office. Chemical Regulation: Options Exist to Improve EPA’s Ability to Assess Health Risks and Manage Its Chemical Review Program 2005 [Google Scholar]
- Van Ry DA, Dachs J, et al. Atmospheric Seasonal Trends and Environmental Fate of Alkylphenols in the Lower Hudson River Estuary. Environmental Science & Technology. 2000;34(12):2410–2417. [Google Scholar]
- Vives I, Canuti E, et al. Occurrence of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs), polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in Lake Maggiore (Italy and Switzerland) Journal of Environmental Monitoring. 2007;9(6):589–98. doi: 10.1039/b700919d. [DOI] [PubMed] [Google Scholar]
- Vorhees DJ. Polychlorinated Biphenyls. In: Spengler JMS John D, McCarthy John F., editors. Indoor Air Quality Handbook. New York: McGraw-Hill; 2001. pp. 36.1–36.24. [Google Scholar]
- Wang XM, Ding X, et al. Polybrominated diphenyl ethers in airborne particulates collected during a research expedition from the Bohai Sea to the Arctic. Environmental Science & Technology. 2005;39(20):7803–9. doi: 10.1021/es051088p. [DOI] [PubMed] [Google Scholar]
- Wang XP, Yao TD, et al. Gradient distribution of persistent organic contaminants along northern slope of central-Himalayas, China. The Science of the Total Environment. 2006;372(1):193–202. doi: 10.1016/j.scitotenv.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Warhurst AM. An Environmental Assessment of Alkylphenol Ethoxylates and Alkylphenols. Edinburgh: Friends of the Earth Scotland; 1995. [Google Scholar]
- Weschler C. Major changes in the indoor environment and indoor pollutants since the 1950s. Atmospheric Environment Under review. [Google Scholar]
- Weschler CJ. Indoor-outdoor relationships for non-polar organic constituents of aerosol particles. Environmental Science & Technology. 1984;18:648–652. [Google Scholar]
- White R, et al. Environmentally Persistent Alkylphenolic Compounds Are Estrogenic. Endocrinology. 1994;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Whitmore RW, Immerman FW, et al. Non-occupational exposures to pesticides for residents of two US cities. Archives of Environmental Contamination Toxicology. 1994;26:47–59. doi: 10.1007/BF00212793. [DOI] [PubMed] [Google Scholar]
- Wilson NK, Chuang JC, et al. Levels of persistent organic pollutants in several child day care centers. Journal of Exposure Analysis and Environmental Epidemiology. 2001;11(6):449–58. doi: 10.1038/sj.jea.7500190. [DOI] [PubMed] [Google Scholar]
- Winneke G, Walkowiak J, et al. PCB-induced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology. 2002;181–182:161–5. doi: 10.1016/s0300-483x(02)00274-3. [DOI] [PubMed] [Google Scholar]
- Xie Z, Ebinghaus R, et al. Occurrence and air-sea exchange of phthalates in the Arctic. Environmental Science & Technology. 2007;41(13):4555–60. doi: 10.1021/es0630240. [DOI] [PubMed] [Google Scholar]
- Xie Z, Lakaschus S, et al. Atmospheric concentrations and air-sea exchanges of nonylphenol, tertiary octylphenol and nonylphenol monoethoxylate in the North Sea. Environmental Pollution. 2006;142(1):170–80. doi: 10.1016/j.envpol.2005.08.073. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, et al. Developmental exposure to brominated diphenyl ehters results in thyroid hormone disruption. Toxicological Sciences. 2002;66(1):105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]