Abstract
MDMA (3,4 methylenedioxymethamphetamine) has been used by millions of people worldwide as a recreational drug. MDMA and Ecstasy are often used synonymously but it is important to note that the purity of Ecstasy sold as MDMA is not certain. MDMA use is of public health concern, not so much because MDMA produces a common or severe dependence syndrome, but rather because rodent and non-human primate studies have indicated that MDMA (when administered at certain dosages and intervals) can cause long-lasting reductions in markers of brain serotonin (5-HT) that appear specific to fine diameter axons arising largely from the dorsal raphe nucleus (DR). Given the popularity of MDMA, the potential for the drug to produce long-lasting or permanent 5-HT axon damage or loss, and the widespread role of 5-HT function in the brain, there is a great need for a better understanding of brain function in human users of this drug. To this end, neuropsychological, neuroendocrine, and neuroimaging studies have all suggested that human MDMA users may have long-lasting changes in brain function consistent with 5-HT toxicity. Data from animal models leads to testable hypotheses regarding MDMA effects on the human brain. Because neuropsychological and neuroimaging findings have focused on the neocortex, a cortical model is developed to provide context for designing and interpreting neuroimaging studies in MDMA users. Aspects of the model are supported by the available neuroimaging data but there are controversial findings in some areas and most findings have not been replicated across different laboratories and using different modalities. This paper reviews existing findings in the context of a cortical model and suggests directions for future research.
Keywords: functional MRI, positron emission tomography, drug abuse, drug toxicity, magnetic resonance spectroscopy
Introduction
The widely used club drug, 3,4-methylenedioxymethamphetamine (MDMA) is commonly sold under the street name of Ecstasy. While MDMA is the principal ingredient in most Ecstasy sold in the U.S. and Europe, Ecstasy pills may vary in their content of MDMA and other additives.1,2,3,3 Human MDMA use is of concern because pre-clinical models have suggested that MDMA can produce chronic reductions in serotonergic function in animal models4,5 and studies of human MDMA users have found long-standing cognitive and neurophysiological alterations in MDMA-exposed humans.6,7 While pre-clinical animal models can not definitively answer the question of whether or not MDMA produces long-standing neurotoxicity in human users, these models can serve as a framework for organizing neuroimaging studies of humans self-exposed to MDMA. It is important to note that worldwide, almost all contemporary MDMA users are polydrug users, and this factor limits the ability of neuroimaging to directly address the presence or absence of MDMA effects.8,9,10,11,12 Finally, MDMA users may have differences in brain structure or function that pre-exist MDMA use or that pre-dispose to MDMA use.
An earlier detailed review of neuroimaging findings in MDMA users7 discussed imaging findings and suggested some alternatives for future study. This paper outlines a cortical model for MDMA effects that may be informative for organizing and interpreting neuroimaging findings and briefly reviews current findings in the context of the cortical model. Neuroimaging findings are grouped as structural, functional, and chemical according to their specific mode of investigating brain integrity.
A cortical model
While MDMA affects many neurotransmitter systems and has vascular consequences, long-lasting changes in the serotonergic system have been most prominently reported in animal models of MDMA administration. Early reports indicated that MDMA administration in most species led to destruction of fine diameter serotonergic axons (presumably arising from the dorsal raphe (DR) nucleus) with sparing of cell bodies.4,5 More contemporary studies have raised doubts that MDMA at dosages mimicking human recreational usage leads to actual destruction of serotonergic axons but evidence for long-lasting changes in serotonergic function persists.13,14 If one starts with the seminal event of actual or functional serotonergic axon loss then several brain consequences can be predicted and tested. Figure one outlines a cortical model of idealized and simplified regional brain anatomy illustrating predicted brain alterations arising from MDMA exposure. This model lends to hypotheses testable with neuroimaging methods and provides a provisional framework for organizing those findings into a cohesive understanding of MDMA effects.
Figure 1.
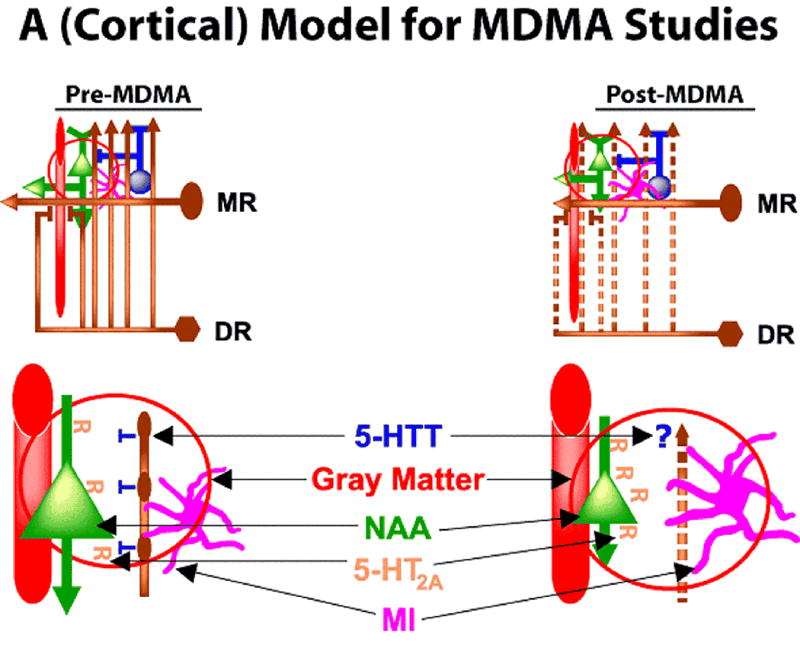
Figure one depicts a cortical model for designing and interpreting neuroimaging studies in MDMA users. Upper left panel indicates idealized cortical anatomy in Pre-MDMA condition. Serotonergic dorsal raphe (DR) and median raphe (MR) nuclei of the brainstem are depicted in brown. MR axons are larger to reflect wider diameter. Blood vessel is shown in red, pyramidal cell neuron in green, glial cell in magenta, inhibitory interneuron in blue. Red circle indicates region of enlargement (lower left and lower right). In Post-MDMA condition (upper right), DR axons are shown as dashed lines to represent either actual axotomy or functional loss of signaling and serotonin (5-HT) release. Lower panel (left) depicts normal anatomy42,43 and right depicts Post-MDMA model. Blood vessel is included in the enlargement because monoamines innervate brain arterioles that contribute to the fMRI response. Pyramidal cell (green) is shown with 5-HT2A receptors (R) on the surface. Pyramidal cell is shown smaller in Post-MDMA setting due to putative loss of neurotrophic factor coupling. N-acetylaspartate (NAA) is a neuronal marker found in pyramidal (and other) neurons. NAA levels would be predicted to decrease if pyramidal neurons shrink in the Post-MDMA condition. 5-HT2A receptors are shown as increasing in the Post-MDMA condition due to potential loss of agonist signaling from 5-HT axotomy or functional loss. MI (magenta) is depicted as contained in glial cells which may hypertrophy in the Post-MDMA condition (but, see text). Gray Matter includes elements assayed by structural neuroimaging studies as gray matter and includes blood vessel, neuronal, and glial elements. Gray Matter is depicted as reduced in Post-MDMA condition due to potential loss of neurotrophic factors or other conditions.
Structural
Structural neuroimaging in MDMA users can be broadly construed to include traditional morphometric measures of brain structure such as brain volume but can also be extended to include neuroimaging of molecular structural brain components. As depicted in figure 1, the known roles for serotonin (5-HT) and the demonstrated effects of MDMA in animal models offer several avenues via which MDMA could alter brain structure. These include the 5-HTtransporter, the 5-HT2A receptor, and regional brain volume.
5-HT transporter (5-HTT)
Multiple research groups have addressed the status of the 5-HT transporter (5-HTT) in MDMA users.15,16,17,18
As depicted in Figure 1, the 5-HTT is expressed on the serotonergic axons arising from median and dorsal raphe (DR) neurons. If DR axons are selectively lost in neocortex following MDMA exposure, levels of the 5-HTT would be expected to be lower after MDMA use. As predicted by this model, MDMA users consistently show reduced 5-HTT radionuclide ligand binding across multiple brain regions.15,16,17,18 There has been some evidence to suggest that these levels began to recover with increased periods of MDMA abstinence. However, the sensitivity of this method for detecting serotonergic transporter loss is unclear.18 The finding of lower levels of 5-HTT does not confirm that serotonergic axons are lost in human MDMA users. Other explanations, such as long-lasting reduction in expression of the 5-HTT by MDMA are plausible. However, long-lasting or potentially permanent reductions in 5-HTT levels would be expected to have consequences related to the neurophysiological and other roles of serotonin, independently of actual axon loss.
5-HT2A receptor
An additional molecular structural component of the serotonergic signaling pathway is the 5-HT2A receptor. 5-HT2A receptors are densely distributed in human cortex.19 As depicted in figure 1, the 5-HT2A receptor is postsynaptic to 5-HT axons and therefore, levels of exposure of the 5-HT2A receptor to agonist (5-HT) could be influenced by MDMA-associated 5-HT release (with the caveat that if the axon is actually lost, MDMA would no longer increase 5-HT release), or as shown in figure 1, as a consequence of loss of the 5-HT axon. Here the 5-HT2A receptor is depicted as demonstrating behavior classic of g-protein coupled receptors, with increased receptor number (relative to baseline) in the presence of loss of agonist signaling due to 5-HT axon dropout (or functional loss of 5-HT release). To date, this aspect of the putative model has been confirmed by a single report using single photon emission computed tomography (SPECT). SPECT binding was compared across 3 groups, controls, ongoing/acute MDMA users, and MDMA users abstinent for at least x weeks. 5-HT2A receptor levels were reduced in multiple brain regions in the ongoing/acute MDMA user group possibly due to ongoing MDMA-mediated increases in 5-HT release. Abstinent MDMA users showed the reverse pattern, with greater 5-HT2A receptor binding in the abstinent MDMA user group.
Interpreting these results in the context of the proposed model is complicated. Under the simple assumption that 5-HT axons are lost due to MDMA toxicity, ongoing MDMA exposure would lose the ability to cause agonist-induced reductions in 5-HT2A receptors because there would be no 5-HT axon present to release 5-HT. However, there is the possibility for overlap of DR and MR innervations in many brain regions, suggesting that some brain regions could show decreased 5-HT2A receptors in recent MDMA users due to MDMA-mediated 5-HT efflux while other brain regions might have increased 5-HT2A receptors due to actual or functional loss of 5-HT release. To begin to address this problem, we plan to conduct positron emission tomography (PET) studies in long-abstinent MDMA users using both a 5-HT2A receptor and 5-HTT ligand. An additional complication in the interpretation of 5-HT2A receptor binding studies is that the receptor may show non-classical regulation under some circumstances.20,21
Brain gray matter
Cowan et al.. 2003 previously hypothesized that loss of serotonergic coupling to brain neurotrophic factors, such as brain derived nerve growth factor22 and S-100 beta23 might lead to secondary changes in neuronal volume. As shown in figure 1, this somewhat simplistic hypothesis predicts that individual neurons will shrink in the face of reduced growth factor signaling and that regional changes in neuronal size will be detectable as reduced regional brain volume. To test this hypothesis, Cowan et al.. 200324 used the voxel-based morphometry method (VBM) to compare regional brain gray matter concentration in a group of MDMA users and controls. As is typical of Ecstasy users worldwide, the MDMA users showed greater levels of polydrug use than did control subjects. In the overall VBM analysis, MDMA polydrug users showed multiple regions of reduced brain gray matter concentration when compared to the control group. These regions of reduced gray matter were found most prominently in left neocortex, and included left Brodmann Area (BA) 45, 21, and 18. Area 18 was affected bilaterally. Additional analyses, controlling for the effects of other drugs of abuse, suggested that these findings were not accounted for by polydrug use, but were associated with MDMA use per se. However, this study could not address the potential for pre-existing brain structural differences in this group, nor could the study address the origin of the finding. Studies using complementary methods have not reported overlapping or consistent results from studies of brain volume.7
Functional
Functional magnetic resonance imaging (fMRI) blood oxygen level dependent (BOLD) studies have generally revealed task-associated differences in regional brain activation in MDMA users and controls.25,26,27 As multiple lines of evidence have suggested that visual cortex may be altered in MDMA users24,28 investigating visual function may be a promising area of research in this subgroup. We investigated29 visual cortical function in single slice surface coil studies of MDMA users. The brain regions included primary visual cortex, and most likely portions of secondary visual cortex as well. This study revealed that overall visual cortical activation was similar in MDMA users and controls. However, within the MDMA user group, there was a positively correlated dose-response association between the degree of prior MDMA use and the degree of spatial activation in visual cortex.
Using improved methods, including higher field fMRI at 3.0 Tesla and head coil methods to acquire functional signal across a broader area of cortical and subcortical regions, we have begun to study characteristics of visual cortical activation in an additional cohort of MDMA users and controls. This approach permits the assessment of regional brain activation to photic stimulation at all levels along the visual pathway from the lateral geniculate nucleus (LGN) of the thalamus, to primary and secondary visual cortices, the latter regions approximately corresponding to BA 17 and 18. While we are not aware of evidence that MDMA users have clinically abnormal vision, converging evidence from multiple modalities, such as transcranial magnetic stimulation 28, fMRI 29 and visual psychophysical studies 30 suggests a potential change in excitatory thresholds in this group.
Spectroscopic
Our group 31 and others 32,33 ,33 ,34 have used proton magnetic resonance spectroscopy (MRS) to assay for MDMA-induced effects in human MDMA-users. To date, MRS has been used mainly to measure N-acetylaspartate (NAA) and myoinositol (MI) concentrations in MDMA users. NAA, as a putative neuronal marker, may have utility in assaying MDMA effects if MDMA produces changes in neurona25 l size or metabolic viability 35 ,36 . As depicted in figure 1 , lower left, NAA (in green) is present in pyramidal and other cortical neurons and might be expected to decrease in the post-MDMA state if there is neuronal damage or neuronal shrinkage. Other potential causes for reduced NAA, including possible loss of fine diameter 5-HTaxons or scattered neuronal necrosis 37 do not appear likely to contribute to NAA changes detected by MRS because current methods have employed fairly large volumes of interest and thus might not detect loss of small numbers of neurons or the small amount of NAA contained in fine-diameter serotonergic axons. Alternatively, it is possible that NAA metabolic/catabolic processes may be modulated by MDMA in an unknown manner. MI, as a putative glial marker 38 ,39 (Figure 1, magenta glial cell), seems less likely to emerge as a specific marker of brain change in MDMA users. MI is depicted in figure 1 as a glial marker that might increase if there is glial proliferation or hypertrophy following MDMA exposure. MI is widespread in brain glia and does show alterations with gross brain damage such as in demyelinating disorders 38 , but there is little evidence to support gliosis following MDMA exposure 14 .
As reviewed in detail elsewhere 7, findings to date from MRS have not revealed a consistent pattern of findings. Assays of prefrontal, temporal, and parieto-occipital NAA and MI have revealed conflicting findings 32,33. These groups studied subjects having relatively high levels of MDMA and polydrug exposure, which may have contributed to disparate findings. Using high field MRS at 4.0 Tesla (which offers increased SNR), we recently examined NAA and MI concentrations in the occipital cortex of MDMA users having fairly low levels of MDMA and polydrug use 31 and found no differences, or even a clear trend for an association of MDMA use with metabolite levels. However, if subtle effects exist, this study would have been underpowered in detecting those changes.
One possibility for negative or divergent results from MRS studies is that MDMA use may produce regional brain changes that are not detected if MRS volumes of interest are placed in regions unaffected my MDMA exposure. Based on this concern, we have begun studying NAA and MI using volumes of interest based upon our earlier findings (Cowan et al.. 43) of reduced brain gray matter concentration in MDMA users. We reasoned that areas having reduced brain gray matter concentration would be the most likely regions to yield evidence for altered NAA. Applying this approach to a group of MDMA users not overlapping with those studied in the original VBM report, we used proton MRS at 3.0 Tesla to examine NAA and MI using volumes of interest placed in left Brodmann Areas 45, 21, and 18 in MDMA users abstinent from all drug or alcohol for at least 2 weeks. These regions of interest were not only based on our earlier VBM findings 24 but also overlap with brain regions potentially associated with verbal memory deficits reported in cognitive studies of MDMA users 40. Our preliminary findings in a small sample subset do not suggest an association between MDMA use and metabolite ratios, nor for use of alcohol, cannabis, or methamphetamine and metabolite ratios in this group. This early negative finding may be due to the absence of drug effects, mixed effects from different drugs, or limitations of the sample size or methodology.
Conclusions
Data from rodent and non-human primate models of MDMA administration suggest a framework or a model for designing and interpreting neuroimaging studies examining MDMA effects in human cerebral cortex. A similar framework could easily be developed for interpreting subcortical findings but would require modification to reflect region-specific cellular organization. Some predictions deriving from this model, including reductions in levels of the serotonergic transporter, changes in the 5-HT2A receptor, and reductions in regional brain gray matter are consistent with neuroimaging findings in human MDMA users. However, reduced 5-HTtransporter ligand binding in MDMA users is the only result that has been replicated across multiple laboratories, modalities and cohorts. With regard to fMRI BOLD studies, the proposed cortical model has low utility in predicting the direction or location of task-evoked regional BOLD signal effects when considered in the absence of a detailed local neural circuit. The fMRI BOLD signal is not fully understood but it appears that increases in the BOLD signal most strongly correlate with increased local blood flow coupled to the release of glutamate from synaptic terminals 41. The proposed model of cortical effects of MDMA exposure suggests that MDMA use might alter regional brain activation in fMRI BOLD studies via multiple mechanisms, including alterations in neuronal structure, blood flow, and synaptic signaling. As such, meaningful interpretation of fMRI BOLD studies in MDMA users requires that results be considered in the context of the known input/output relationships of a brain region and with regard to specific interactions of inhibitory and excitatory neurotransmission. MRS studies in MDMA users have been negative or contradictory and additional work is needed to continue to explore the utility of this approach in MDMA users, particularly with regard to NAA and MI levels. Quantification of other metabolites, such as gamma-amino-butyric acid (GABA) or glutamate, may offer insight into the regulation of inhibitory and excitatory processes that may be altered in the presence of cognitive deficits. Additional studies employing prospective methods to address the possibility of pre-existing brain differences in MDMA users, coupled to multi-modal imaging approaches combining serotonin-specific assays with structural, spectroscopic, and functional neuroimaging are in order.
Acknowledgments
Supported by grants RO1 DA15137 and DA020149 from NIDA to RLC and M01 RR-00095 from the National Center for Research Resources, NIH, to the Vanderbilt General Clinical Research Center.
Reference List
- 1.Cole JC, et al. The content of ecstasy tablets: implications for the study of their long-term effects. Addiction. 2002;97:1531–1536. doi: 10.1046/j.1360-0443.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- 2.Parrott AC. Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity. Psychopharmacology (Berl) 2004;173:234–241. doi: 10.1007/s00213-003-1712-7. [DOI] [PubMed] [Google Scholar]
- 3.Tanner-Smith EE. Pharmacological content of tablets sold as “ecstasy”: Results from an online testing service. Drug Alcohol Depend. 2006;83:247–254. doi: 10.1016/j.drugalcdep.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Green AR, et al. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 5.Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Rev. 2003;42:155–168. doi: 10.1016/s0165-0173(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 6.Morton J. Ecstasy: pharmacology and neurotoxicity. Curr Opin Pharmacol. 2005;5:79–86. doi: 10.1016/j.coph.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology (Berl) 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- 8.Schifano F, et al. MDMA (‘ecstasy’) consumption in the context of polydrug abuse: a report on 150 patients. Drug Alchohol Depend. 1998;52:85–90. doi: 10.1016/s0376-8716(98)00051-9. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen W, Skrondal A. Ecstasy and new patterns of drug use: a normal population study. Addiction. 1999;94:1695–1706. doi: 10.1046/j.1360-0443.1999.941116957.x. [DOI] [PubMed] [Google Scholar]
- 10.Gross SR, et al. Ecstasy and drug consumption patterns: a Canadian rave population study. Can J Psychiatry. 2002;47:546–551. doi: 10.1177/070674370204700606. [DOI] [PubMed] [Google Scholar]
- 11.de Almeida SP, Silva MT. Ecstasy (MDMA): effects and patterns of use reported by users in Sao Paulo. Rev Bras Psiquiatr. 2003;25:11–17. doi: 10.1590/s1516-44462003000100004. [DOI] [PubMed] [Google Scholar]
- 12.Scholey AB, et al. Increased intensity of Ecstasy and polydrug usage in the more experienced recreational Ecstasy/MDMA users: a WWW study. Addict Behav. 2004;29:743–752. doi: 10.1016/j.addbeh.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Fantegrossi WE, et al. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- 14.Baumann MH, et al. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semple DM, et al. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy’) users. Br J Psychiatry. 1999;175:63–69. doi: 10.1192/bjp.175.1.63. [DOI] [PubMed] [Google Scholar]
- 16.Reneman L, et al. Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet. 2001;358:1864–1869. doi: 10.1016/S0140-6736(01)06888-X. [DOI] [PubMed] [Google Scholar]
- 17.McCann UD, et al. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- 18.McCann UD, et al. Quantitative PET Studies of the Serotonin Transporter in MDMA Users and Controls Using [(11)C]McN5652 and [(11)C]DASB. Neuropsychopharmacology. 2005;30:1740–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varnas K, et al. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Human Brain Mapping. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 21.Van Oekelen D, et al. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003;72:2429–2449. doi: 10.1016/s0024-3205(03)00141-3. [DOI] [PubMed] [Google Scholar]
- 22.Duman RS, et al. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 23.Azmitia EC. Cajal's hypotheses on neurobiones and neurotropic factor match properties of microtubules and S-100 beta. Prog Brain Res. 2002;136:87–100. doi: 10.1016/s0079-6123(02)36010-2. [DOI] [PubMed] [Google Scholar]
- 24.Cowan RL, et al. Reduced cortical gray matter density in human MDMA (Ecstasy) users: a voxel-based morphometry study. Drug Alchohol Depend. 2003;72:225–235. doi: 10.1016/j.drugalcdep.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Daumann J, et al. Neural correlates of working memory in pure and polyvalent ecstasy (MDMA) users. Neuroreport. 2003;14:1983–1987. doi: 10.1097/00001756-200310270-00021. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsen LK, et al. Preliminary evidence of hippocampal dysfunction in adolescent MDMA (“ecstasy”) users: possible relationship to neurotoxic effects. Psychopharmacology (Berl) 2004;173:383–390. doi: 10.1007/s00213-003-1679-4. [DOI] [PubMed] [Google Scholar]
- 27.Moeller FG, et al. Functional MRI study of working memory in MDMA users. Psychopharmacology (Berl) 2004;177:185–194. doi: 10.1007/s00213-004-1908-5. [DOI] [PubMed] [Google Scholar]
- 28.Oliveri M, Calvo G. Increased visual cortical excitability in ecstasy users: a transcranial magnetic stimulation study. J Neurol Neurosurg Psychiatry. 2003;74:1136–1138. doi: 10.1136/jnnp.74.8.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan RL, et al. MDMA use is associated with increased spatial BOLD fMRI visual cortex activation in human MDMA users. Pharmacol Biochem Behav. 2006;84:219–228. doi: 10.1016/j.pbb.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 30.Brown J, et al. A long-term ecstasy-related change in visual perception. Psychopharmacology (Berl) 2007;193:437–446. doi: 10.1007/s00213-007-0785-0. [DOI] [PubMed] [Google Scholar]
- 31.Cowan RL, et al. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007;155:179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang L, et al. Cerebral (1)H MRS alterations in recreational 3, 4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users. J Magn Reson Imaging. 1999;10:521–526. doi: 10.1002/(sici)1522-2586(199910)10:4<521::aid-jmri4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Reneman L, et al. Reduced N-acetylaspartate levels in the frontal cortex of 3,4-methylenedioxymethamphetamine (Ecstasy) users: preliminary results. Am J Neuroradiol. 2002;23:231–237. [PMC free article] [PubMed] [Google Scholar]
- 34.Obergriesser T, et al. Hippocampal 1H-MRSI in ecstasy users. Eur Arch Psychiatry Clin Neurosci. 2001;251:114–116. doi: 10.1007/s004060170044. [DOI] [PubMed] [Google Scholar]
- 35.Gujar SK, et al. Magnetic resonance spectroscopy. J Neuroopthalmol. 2005;25:217–226. doi: 10.1097/01.wno.0000177307.21081.81. [DOI] [PubMed] [Google Scholar]
- 36.Kalra S, et al. Recovery of N-acetylaspartate in corticomotor neurons of patients with ALS after riluzole therapy. Neuroreport. 1998;9:1757–1761. doi: 10.1097/00001756-199806010-00016. [DOI] [PubMed] [Google Scholar]
- 37.Schmued LC. Demonstration and localization of neuronal degeneration in the rat forebrain following a single exposure to MDMA. Brain Res. 2003;974:127–133. doi: 10.1016/s0006-8993(03)02563-0. [DOI] [PubMed] [Google Scholar]
- 38.Bitsch A, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. Am J Neuroradiol. 1999;20:1619–1627. [PMC free article] [PubMed] [Google Scholar]
- 39.Rumpel H, et al. Is myo-inositol a measure of glial swelling after stroke? A magnetic resonance study. J Magn Reson Imaging. 2003;17:11–19. doi: 10.1002/jmri.10233. [DOI] [PubMed] [Google Scholar]
- 40.Laws KR, Kokkalis J. Ecstasy (MDMA) and memory function: a meta-analytic update. Hum Psychopharmacol. 2007;22:381–388. doi: 10.1002/hup.857. [DOI] [PubMed] [Google Scholar]
- 41.Logothetis NK. The ins and outs of fMRI signals. Nat Neurosci. 2007;13:1230–1232. doi: 10.1038/nn1007-1230. [DOI] [PubMed] [Google Scholar]
- 42.Morrison JH, et al. Noradrenergic and serotonergic fibers innervate complementary layers in monkey primary visual cortex: an immunohistochemical study. Proc Natl Acad Sci. 1982;7:2401–2405. doi: 10.1073/pnas.79.7.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhard JF, Jr, et al. Serotonin neurons project to small blood vessels in the brain. Science. 1979;206:85–87. doi: 10.1126/science.482930. [DOI] [PubMed] [Google Scholar]


