Abstract
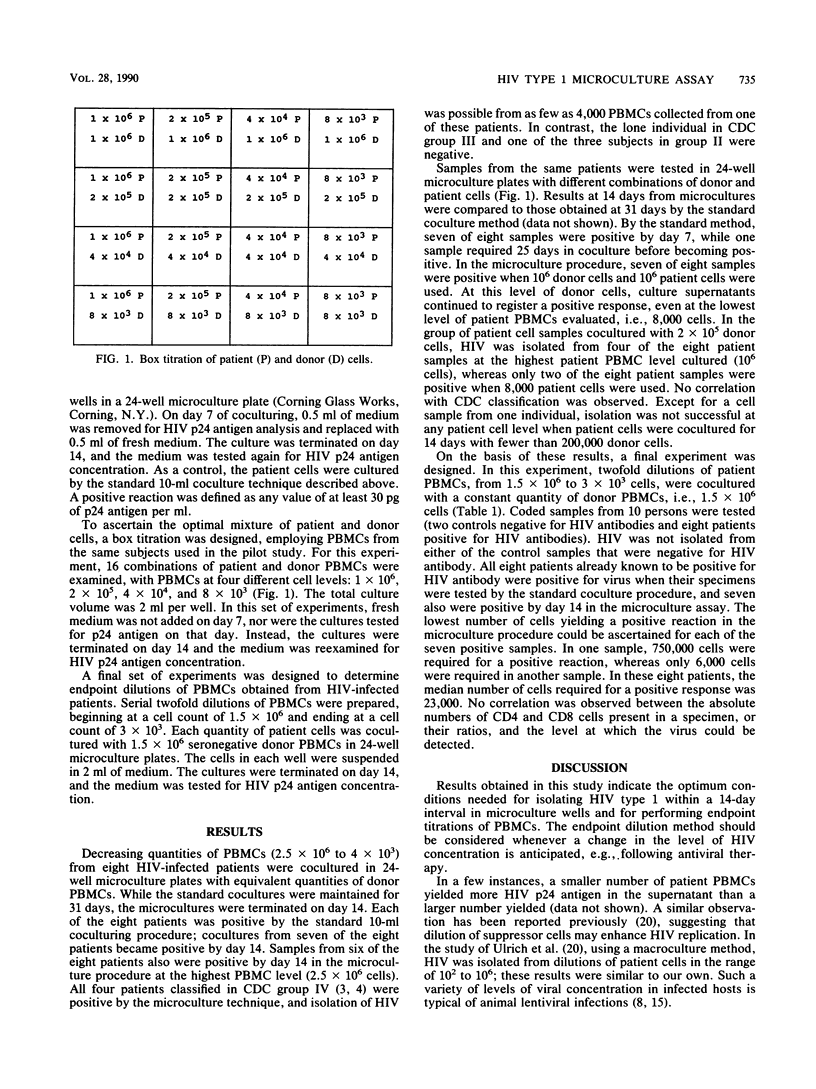
To define the optimal conditions for human immunodeficiency virus (HIV) detection in microcultures, experiments were conducted with different ratios of patient and donor peripheral blood mononuclear cells (PBMCs). Donor/patient PBMC ratios ranged from 1:1 to 1:125. Optimal results were obtained when 1,500,000 donor cells were cocultured with equal or smaller quantities of patient PBMCs. Thus, virologic endpoints could be achieved by diluting patient cells. Smaller numbers of donor cells, with or without larger numbers of patients cells, resulted in lower rates of HIV isolation. Similarly, the direct stimulation of patient PBMCs with phytohemagglutinin without the addition of normal donor cells lowered the sensitivity of the assay significantly. We suggest that a microculture procedure using a fixed quantity of donor cells with different dilutions of patient cells may be useful for monitoring changing HIV levels during antiviral therapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Castro B. A., Weiss C. D., Wiviott L. D., Levy J. A. Optimal conditions for recovery of the human immunodeficiency virus from peripheral blood mononuclear cells. J Clin Microbiol. 1988 Nov;26(11):2371–2376. doi: 10.1128/jcm.26.11.2371-2376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feorino P., Forrester B., Schable C., Warfield D., Schochetman G. Comparison of antigen assay and reverse transcriptase assay for detecting human immunodeficiency virus in culture. J Clin Microbiol. 1987 Dec;25(12):2344–2346. doi: 10.1128/jcm.25.12.2344-2346.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T., Kelly J., Benn S., Kinter A., Justement J., Gold J., Redfield R., Sell K. W., Fauci A. S. Susceptibility of normal human lymphocytes to infection with HTLV-III/LAV. J Immunol. 1986 Jun 1;136(11):4049–4053. [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Coombs R. W., Sannerud K., Rhame F. S., Balfour H. H., Jr Rapid and sensitive viral culture method for human immunodeficiency virus type 1. J Clin Microbiol. 1988 Jul;26(7):1416–1418. doi: 10.1128/jcm.26.7.1416-1418.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky L. S., McHugh T., Stites D., Volberding P., Henle G., Henle W., Levy J. A. High prevalence of antibodies to acquired immune deficiency syndrome (AIDS)-associated retrovirus (ARV) in AIDS and related conditions but not in other disease states. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5535–5539. doi: 10.1073/pnas.82.16.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Mack D. H., Mullis K. B., Poiesz B., Ehrlich G., Blair D., Friedman-Kien A., Sninsky J. J. Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol. 1987 May;61(5):1690–1694. doi: 10.1128/jvi.61.5.1690-1694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985 Oct;152(4):734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Newell A. L., Malkovsky M., Orr D., Taylor-Robinson D., Dalgleish A. G. Antigen test versus reverse transcriptase assay for detecting HIV. Lancet. 1987 Nov 14;2(8568):1146–1147. doi: 10.1016/s0140-6736(87)91570-4. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Pan L. Z., Cheng-Mayer C., Levy J. A. Patterns of antibody response in individuals infected with the human immunodeficiency virus. J Infect Dis. 1987 Apr;155(4):626–632. doi: 10.1093/infdis/155.4.626. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Ulrich P. P., Busch M. P., el-Beik T., Shiota J., Vennari J., Shriver K., Vyas G. N. Assessment of human immunodeficiency virus expression in cocultures of peripheral blood mononuclear cells from healthy seropositive subjects. J Med Virol. 1988 May;25(1):1–10. doi: 10.1002/jmv.1890250102. [DOI] [PubMed] [Google Scholar]


