Abstract
Rad54 protein is a key member of the RAD52 epistasis group required for homologous recombination in eukaryotes. Rad54 is a duplex DNA translocase that remodels both DNA and protein–DNA complexes, and functions at multiple steps in the recombination process. Here we use biochemical criteria to demonstrate the existence of this important protein in a prokaryotic organism. The Sulfolobus solfataricus Rad54 (SsoRad54) protein is a double-strand DNA-dependent ATPase that can alter the topology of duplex DNA. Like its eukaryotic homolog, it interacts directly with the S. solfataricus Rad51 homologue, SsoRadA, to stimulate DNA strand exchange. Confirmation of this protein as an authentic Rad54 homolog establishes an essential phylogenetic bridge for identifying Rad54 homologs in the archaeal and bacterial domains.
INTRODUCTION
Homologous recombination is necessary for accurate reproduction, DNA-damage repair, resistance to deleterious evolutionary change, and preservation of evolutionary innovation. Recombination is a universal process and has been documented for all three branches of the phylogenetic tree of life (1,2). Central to recombination is homologous pairing and DNA strand exchange, which is mediated by the RecA/Rad51/RadA/UvsX family of proteins (2). These proteins form a nucleoprotein filament with single-strand DNA (ssDNA) that searches for sequence homology and then promotes DNA strand exchange between the protein-bound ssDNA and a homologous double-strand DNA (dsDNA) partner. The Rad54 protein is an essential component of eukaryotic recombination processes (3). RAD54 was first identified in a genetic screen for mutations in Saccharomyces cerevisiae that conferred hypersensitivity to ionizing radiation (4). These mutations also had a dramatic effect on dsDNA break repair and recombination (5–9).
The Rad54 protein is a member of the Swi2/Snf2 family of DNA-binding proteins as defined by conserved amino-acid sequences which include helicase motifs (10). Despite the presence of helicase motifs, Rad54 protein does not display helicase activity (11,12). Rad54 protein does have a distinctive dsDNA-dependent ATPase activity that is important for its recombination function in vivo and in vitro (6,7,11–15). In vitro, Rad54 protein stimulates Rad51 protein-mediated DNA strand exchange by directly interacting with the Rad51 nucleoprotein filament (11,13,14,16–23). Additionally, the Rad54 protein can topologically remodel duplex DNA utilizing energy derived from ATP hydrolysis (13,24,25).
There has been significant interest in studying recombination and repair systems in extremophilic prokaryotes since they must contend with harsh environmental conditions that threaten genomic stability. Most prokaryotic extremophiles are members of the archaea, a distinct group of microbes distinguished from the eubacteria through 16S rDNA sequence analysis (26,27). Information from complete and partial genome sequences of these organisms indicates a definite evolutionary division between central metabolic and informational processes (28–30). The majority of intermediary metabolic processes are similar to those of eubacteria. Genomic informational processes, such as replication and recombination, are instead strikingly similar to activities observed in eukaryotes. This remarkable parallel makes archaeal microbes excellent model systems for understanding the generally more complex eukaryotic mechanisms.
Indeed, archaeal proteins with homology to several eukaryotic proteins involved in recombination have been identified. Single-stranded DNA-binding proteins with similarity to replication protein-A (RPA) have been extensively studied (1,31–36), as have archaeal homologues for eukaryotic Rad51, called RadA (37–44). Homologues for eukaryotic Rad50 and Mre11 have also been identified and characterized (1,45,46). Structures and activities of archaeal recombination proteins have yielded valuable clues regarding the function of the corresponding eukaryotic versions. For example, comparison of topoisomerase VI from Sulfolobus shibatae to the enigmatic eukaryotic Spo11 protein helped reveal the role of Spo11 in initiation of programmed meiotic dsDNA breaks (47,48). The crystal structure of Pyrococcus furiosus Mre11 protein and its interaction with Rad50 provided a framework to map macromolecular interaction sites for the eukaryotic equivalents (46). Critical insight for the role of Brca2 in formation of the Rad51 nucleoprotein filament was gained from studies with the P. furiosus RadA protein and BRC repeats (49).
While the existence of a putative Rad54 protein from the archaeaon Sulfolobus solfataricus has been previously noted (1,50), verification of this protein as a functional Rad54 protein has not been reported. A region of this protein was heterologously overexpressed, purified and crystallized (50). This core region comprises the ATPase domain and the Swi2/Snf2 family helicase domains, and displays ATPase activity. The structure shows that a central cleft within this region of the protein binds the minor groove of duplex DNA, suggesting the protein travels along the minor groove of dsDNA without strand separation. Indeed, it was shown that the Saccharomyces cerevisiae Rad54 protein and its meiotic homologue, Tid1/Rdh54, translocate rapidly and processively on dsDNA in an ATP-dependent manner (51–53).
Here we describe the purification and biochemical characterization of full-length Sulfolobus solfataricus Rad54 protein (SsoRad54). This protein is a dsDNA-dependent ATPase that lacks helicase activity. SsoRad54 directly interacts with Sulfolobus solfataricus RadA (SsoRadA) protein and it stimulates SsoRadA-mediated DNA strand exchange. Additionally, SsoRad54 can alter the topology of a covalently closed DNA substrate, implying that SsoRad54 can translocate along duplex DNA. By determining that SsoRad54 protein is an authentic member of the Rad54 protein group, we have established the presence of this activity in a prokaryote. Consequently, SsoRad54 protein provides a critical phylogenetic bridge between Rad54 homologues in bacteria and eukaryotes.
MATERIALS AND METHODS
Expression vector construction
The SsoRad54 gene was cloned into the pET28 expression vector (Novagen) in a two step process. A plasmid with the amino terminal region of the gene (SSO1653) generated by PCR amplification and ligated into the NheI-NotI sites of pET28 was the generous gift of John Tainer (Scripps). To add the carboxy terminus of the gene, the entire SsoRad54 open reading frame was first cloned into the BamHI site of pUC19 using PCR. Genomic DNA was prepared from Sulfolobus solfataricus P2 cells as previously described (54). PCR was performed using 10 mM potassium chloride, 10 mM ammonium sulfate, 2 mM magnesium chloride, 20 mM Tris–HCl (pH 8.75), 0.1% Triton X-100, 100 μM dNTPs, 100 pmol primers, 2 ng template DNA, 1 U of ExTaq DNA polymerase (TAKARA) and the following primers: 5′-CGGGATCCCTTAGCTCTTTGTGAAAATTTAACTAATCC-3′ (Rad54-F) and 5′-CGGGATCCATCATCAATTTCTTCTCTTATATTCTTTCC-3′ (Rad54-R). PCR was performed using a 55°C annealing temperature and the product was digested with BamHI and ligated into the BamHI site of pUC19. The amino terminal clone and the pUC19 clone were then digested with XhoI and BamHI, respectively. Blunt ends were produced in both digested products using Klenow from New England Biolabs (NEB) and 1 μM dNTPs using the manufacturer's recommendations. Blunt-ended products were digested with BsrGI and relevant restriction fragments were gel purified and ligated. Ligation products were transformed into DH5α and cells were cultivated at 37°C in Luria Broth (LB) with 50 μg/ml kanamycin.
The SsoRadA expression vector was constructed in pET3a (Novagen). The open reading frame was amplified using the same PCR conditions and the primers: 5′-GGGAATTCCATATGTCAAATGAAGTTGAACAGAAAAAG [RadA-F (start)] and 5′-CGCGGATCCTCTTCCGCATCCCTTAATTCCTTCTTCAG (RadA-R). The resulting PCR product and pET3a were digested with NdeI and BamHI (NEB) prior to ligation. Ligation products were transformed into DH5α and cells were grown at 37°C in LB with 100 μg/ml ampicillin. All sequences were verified at the Division of Biological Sciences Automated DNA sequencing facility at UC Davis.
Protein purification
For production of soluble protein, the SsoRad54 expression vector was transformed into XL1Blue (Stratagene) cells containing the pRARE plasmid (Novagen). Cells were grown in LB with 50 μg/ml kanamycin, 15 μg/ml tetracycline and 30 μg/ml chloramphenicol until mid-log phase at 25°C. M13-pKM2 (55) was added at a final concentration of 2 × 109 phages/ml for infection at mid-log phase. Protein production was induced by addition of isopropylthio-β-d-galactoside (IPTG) to a final concentration of 1 mM at mid-log phase and expression was for two hours at 25°C. Cells were harvested by centrifugation and stored at −20°C until processing.
Chromatography was performed at room temperature and all buffers contained 1 mM PMSF and 1 EDTA-free protease inhibitor cocktail tablet per liter (Roche Applied Science). Frozen cell pellets were resuspended in 20 mM Tris–HCl (pH 7.5), 1 mM EDTA, 1 M NaCl and 10% glycerol. Cells were disrupted by sonication and insoluble material was removed by centrifugation. Clarified sonicate was applied to a nickel charged HiTrap chelating sepharose column (GE Healthcare) equilibrated in 20 mM Tris–HCl buffer (pH 7.5), 1 M NaCl and 10% glycerol. Protein was eluted by the addition of 0.5 M imidazole in the same buffer. The eluate was diluted with buffer lacking salt to a final concentration of 0.1 M NaCl before application to a Resource Q column (GE Healthcare) that was developed with a linear gradient from 0.1 to 1 M NaCl in 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM DTT and 10% glycerol. The protein was then re-bound to a nickel charged HiTrap chelating sepharose column equilibrated in 20 mM Tris–HCl buffer (pH 7.5), 1 M NaCl and 10% glycerol. Following a 50 column volume wash of 1 M NaCl, the protein was eluted with 0.5 M imidazole. Protein was concentrated by dialysis against dry polyethylene glycol, dialyzed against 20 mM Tris–HCl (pH 7.5), 1 M NaCl, 1 mM EDTA, 1 mM DTT, 10% spectral grade glycerol, and stored at –80°C as a 6 μM stock.
SsoSSB was purified as described previously and stored as a 30 μM stock (35). Thermotoga maritima LDH was from laboratory stocks, purified as described, and stored as a 54 μM stock (56). Thermus thermophilus RecA protein was purchased from NEB and was a 69 μM stock. SsoRadA protein was purified as follows: the SsoRadA expression vector was transformed into the Rosetta strain (Novagen). Overexpression was as described for SsoSSB (35), in LB with 100 μg/ml ampicillin and 30 μg/ml chloramphenicol. Induced cells were lysed by sonication then heated at 80°C for 20 min. Precipitated protein was removed by centrifugation and the resulting clarified supernatant was applied to a HiTrap Blue column (GE Healthcare) equilibrated in 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM DTT, 100 mM NaCl and 10% glycerol. Protein was eluted using a NaCl step gradient, where the protein eluted in the 1 M NaCl step. Fractions containing SsoRadA protein were pooled and applied to a HiTrap Q column (GE Healthcare) equilibrated in the same buffer used for the HiTrap Blue column. With a step gradient, SsoRadA protein eluted at 200 mM NaCl. The protein was concentrated by dialysis against dry polyethylene glycol, dialyzed against 20 mM Tris–HCl (pH 7.5), 1 mM EDTA, 1 mM DTT, 100 mM NaCl and 10% spectral grade glycerol, and stored at −80°C as a 64 μM stock. All protein concentrations were determined by the Pierce BCA protein assay with BSA as a standard. The extinction coefficient for SsoRad54 is 118 400 M−1cm−1 and 17 420 M−1cm−1 for SsoRadA. Tricine SDS/PAGE gels to monitor protein purification were prepared with a 4% stacking gel and a 10% separating gel as described (57).
Nuclease assay
Nuclease activity was determined by incubating 0.3 μM of purified protein with end labeled single-stranded or annealed 48-mer oligonucleotides (5′-GTCGACGACGTCTGAGTACTCATCTAGTGTGACATCATCGCATCGAGA-3′ and 5′-CTCGATGCGATGATGTCACACTAGATGAGTACTCAGACGTCGTCGAC-3′). DNA was radiolabeled at the 5′-end with 32P using T4 polynucleotide kinase (NEB). Incubation was in 20 mM MES (pH 6.5), 15 mM Mg(OAc)2, 1 mM DTT, 2.5 mM ATP and 50 μg/ml BSA at 37 and 65°C for 90 min. The DNA was then subjected to electrophoresis using an 8% acrylamide gel run in TBE (0.1 M Tris–HCl, 0.1 M boric acid, 0.002 M EDTA and pH 7.5). The gel was dried and exposed to a phosphorimaging screen and analyzed with a Storm 840 PhosphorImager (Molecular Dynamics).
ATPase assay
Reaction mixtures contained 30 mM MES (pH 6.5), 6 mM MgCl2, 0.1 mM DTT, 1 mM ATP, 0.2 µCi [γ-32 P]ATP, protein concentrations as described, and 50 μM poly(dA), 50 μM poly(dT), 50 μM (base pairs) of annealed poly(dA)•poly(dT) or 50 μM (base pairs) of pUC19. Assay mixtures were incubated at 65°C for the times indicated, except for the temperature profile, where reactions were for 60 min at the indicated temperatures. The amount of ATP hydrolyzed was determined by thin layer chromatography using PEI cellulose sheets developed in 1 M formic acid and 0.5 M LiCl. The cellulose sheets were exposed to phosphorimaging screens and quantified using a Storm 840 PhosphorImager (Molecular Dynamics). Results are reported as standard error of the mean.
DNA topology assay
Relaxed pBluescript_SK+ was prepared by incubating 14.2 μM nucleotides pBS_SK+ in buffer containing 10 mM ATP, 20 mM MES (pH 6.5), 15 mM Mg(OAc)2, 1 mg/ml BSA and 1.5 U Escherichia coli Topoisomerase I (NEB) for 1 h at 37°C. Varying concentrations of purified SsoRad54 ranging from 0.01 μM to 0.12 μM were added to the relaxed DNA. Reactions were terminated after 1 h by addition of 0.6% SDS and 0.1 μg/ml Proteinase K (Boehringer Manheim) with incubation at 65°C for 20 min. Where noted, norfloxacin (Sigma) was dissolved in 10 mM NaOH, and added to a final concentration of 100 mM. Reactions were subjected to electrophoresis in 0.8% agarose with TBE buffer. DNA was visualized by staining with ethidium bromide.
Helicase assay
All oligonucleotides, am-55 (33-mer), am-55-3′T (43-mer) and am-55-5′T (43-mer), were purchased from OPERON and purified by polyacrylamide gel electrophoresis. The sequence of am-55 is complementary to the M13mp18 genome viral ssDNA sequence from 226 to 256, while am-55-3′T or am-55-5′T contain 10 thymidines at the 3′- or 5′-end of am-55. Substrates were prepared by mixing M13mp18 ssDNA with 5′-end labeled oligonucleotides am-55, am-55-5′T or am-55-3′T in 10 mM Tris–HCl, 1 mM EDTA, 50 mM NaCl (pH 8.0), heating to 100°C, followed by cooling to 25°C over a period of 1 h. Each substrate (10 μM) was incubated with either SsoRad54 (0.06 μM) or UvrD (2 nM) at 37°C for 1 h in 20 mM Tris–HCl (pH 7.5) with 10 mM Mg(OAc)2 or 20 mM MES (pH 6.5) with 10 mM Mg(OAc)2 and 10 mM ATP, respectively. Reactions were stopped by addition of SDS to a final concentration of 0.6% and Proteinase K (Boehringer Manheim) to a final concentration of 0.1 mg/ml and incubation at 37°C for 20 min. Reactions were loaded on a 10% non-denaturing polyacrylamide gel and subjected to electrophoresis. Polyacrylamide compositions were 19:1 (acrylamide:bis-acrylamide) for the 37°C experiments, and 14:1 for the 55°C and 60°C experiments. Gels were dried and exposed to a phosphorimaging screen. DNA bands were analyzed with a Storm 840 PhosphorImager (Molecular Dynamics).
Protein–protein interaction assay
Protein–protein interactions between 6×His-SsoRad54 and untagged proteins were studied through pull-down assays with Ni-NTA (Ni2+-charged nitriloacetic acid) magnetic beads (QIAGEN). Reaction mixtures contained interaction buffer [30 mM MES (pH 6.5), 200 mM NaCl, 15 mM Mg(OAc)2, 50 mM imidazole and 0.2% Triton X-100], 0.06 μM SsoRad54, and 0.2 μM SsoRadA, Thermus thermophilus RecA (NEB), Thermotoga maritima LDH, or SsoSSB as indicated and were incubated at 80°C for 1 h. Ni-NTA magnetic beads were then added to a final concentration of 1% and incubation was continued at 25°C for 1 h. Beads were separated from the solution phase by a QIAGEN ‘12-Tube Magnet’ and were washed with 2 × 500 μl of interaction buffer to remove free proteins. Unbound proteins from each assay mixture were concentrated using TCA precipitation. Proteins bound to the beads were eluted by addition of 0.5 M imidazole. Samples were then boiled 10 min in SDS–PAGE loading buffer prior to gel electrophoresis. Free and bound proteins were quantified using 8% or 16% SDS–PAGE gels, stained with Coomassie brilliant blue, and ImageQuant software. Intensities from the pull-down sample were compared to dilutions of the relevant protein that were run on the same gel (18).
DNA strand exchange reactions
SsoRadA (11 μM) and SsoRad54 (16 nM, where indicated) were incubated with φX174 ssDNA (NEB) at a concentration of 33 μM (nucleotides) in 30 mM MES (pH 6.5), 15 mM Mg(OAc)2, 2.5 mM ATP, 10 mM DTT, 5 μg/ml BSA at 80°C or 65°C for 10 min. After the addition of SsoSSB (7 μM), the reactions were incubated at 80°C or 65°C for another 5 min before introduction of φX174 dsDNA (NEB) at a concentration of 33 μM (nucleotides). Reaction mixtures were incubated for the times indicated and stopped by the addition of SDS to a final concentration of 0.6% and Proteinase K (Boehringer Manheim) to a final concentration of 0.1 mg/ml. Deproteinization of the reaction mixtures was at 65°C for 20 min. Agarose gels were prepared at 1% and run at approximately 30 V in TAE buffer (0.04 M TrisOAc and 0.002 M EDTA) for 15 h prior to ethidium bromide staining. Both utilization of the dsDNA substrate and production of the nicked circular dsDNA product were quantified by measuring the relative band intensities using the area quantification tool of ImageQuant software (Molecular Dynamics). Formation of the joint molecule intermediate was calculated as the difference between dsDNA substrate uptake and the amount of nicked circular dsDNA produced.
Alignment of protein sequences
In the complete genome of Sulfolobus solfataricus, two open reading frames comprise the entire coding sequence for SsoRad54. This sequence (SsoRad54) was identified using BLASTP at the Sulfolobus solfataricus genome website: http://niji.imb.nrc.ca/sulfolobus/as previously described (1) (SSO1653 is annotated as the amino terminus and SSO1655 is annotated as the carboxy terminus, which together comprise the entire gene; in this sequenced organism, the amino and carboxy termini are separated by an insertion element that disrupts the gene). Sequences for Methanosarcina acetivorans, strain C2A (Mac0189), Synechocystis sp. PCC 6803 (sll1366) and Methanospirillum hungatei, strain Jf-1 (Mhun1673) encoding putative Rad54 homologs were identified using BLAST at the NCBI website: http://www.ncbi.nlm.nih.gov. Sequences for Bacillus subtilis str. 168 HepA (Bs_ywqA) and Mycoplasma gallisepticum R HepA/SNF2 (MG018) were identified using COGs at http://www.ncbi.nlm.nih.gov/COG. Sequence alignments were performed using the ALIGN program at http://www.toulouse.inra.fr/multalin.html and additional features were highlighted by manual adjustment.
RESULTS
SsoRad54 protein is a dsDNA-dependent ATPase
A region of the Rad54 protein from Sulfolobus solfataricus, consisting of residues 430–906 (representing the C-terminal half), was previously examined biochemically (50). Named SsoRad54cd, this part of the protein includes a conserved ATPase domain as well as the seven helicase motifs typical of Swi2/Snf2 family proteins. We were interested in examining the activity of full length SsoRad54 protein and therefore constructed an expression clone that included the entire open reading frame, with the addition of an N-terminal 6-His tag to aid in purification. The full length SsoRad54 protein was heterologously expressed in E. coli. The protein was soluble and was purified to near homogeneity (98%, data not shown) using a multistep procedure.
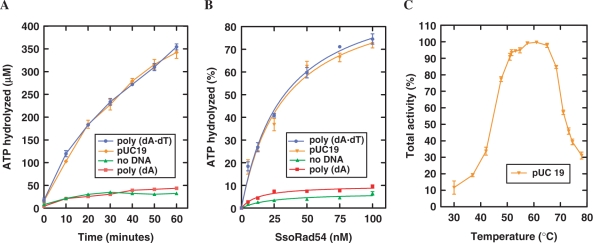
The presence of an ATPase domain and previous results with the C-terminal domain suggested that the protein should have ATPase activity (1,50). We therefore tested the full length SsoRad54 protein for DNA-dependent ATPase activity at an elevated temperature (65°C) that did not denature the dsDNA substrate (Figure 1A and B). SsoRad54 protein itself had a low level of ATP hydrolysis activity (1.2 ± 0.08 μM/min), which was not increased by the addition of the ssDNA, poly(dA) (1.0 ± 0.05 μM/min). However, stimulation of SsoRad54 ATPase activity (to 7.8 ± 0.2 μM/min) was obtained using dsDNA [poly(dA)•poly(dT)] as the substrate. This is precisely the same stimulatory behavior originally observed for the eukaryotic Rad54 protein (11,12). Figure 1B represents a protein titration, where slopes from the linear portion of the plot yield a turnover rate of 1.1 × 103 min−1 using dsDNA as the substrate. This rate is quite similar to the turnover rate of 1.3 × 103 min−1 observed with dsDNA for Saccharomyces cerevisiae Rad54 protein (11). Additionally, the SsoRad54 protein is active over a broad temperature range, with a temperature optimum at approximately 60 (±5)°C (Figure 1C). These data show that the SsoRad54 protein is a dsDNA-dependent ATPase.
Figure 1.
The SsoRad54 protein is a dsDNA-dependent ATPase. (A) ATP hydrolysis by SsoRad54 protein (12.5 nM) in the absence of DNA (green triangles), with poly(dA) (red squares), poly(dA)•poly(dT) (blue circles) or with pUC19 (orange inverted triangles) is shown as a function of time at 65°C. (B) Increasing concentrations of purified SsoRad54 were incubated for 30 min in the absence of DNA, with poly(dA) or with poly(dA)•poly(dT). Symbols are the same as for (A). (C) SsoRad54 is active over a broad temperature range. The dsDNA was pUC19; 100% activity represents the maximal activity obtained at the optimal temperature (60°C) and was equal to 7.8 ± 0.2 μM/min. Data for all panels are the result of three replicate experiments; error bars represent standard error of the mean between experiments.
SsoRad54 promotes changes in DNA topology
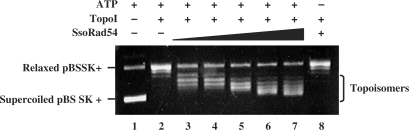
Both human and Saccharomyces cerevisiae Rad54 proteins alter the topology of dsDNA in an ATP-dependent manner (13,25). We used a topoisomerase I-linked assay to determine whether the action of SsoRad54, combined with E. coli topoisomerase I, can introduce net positive supercoils into relaxed covalently-closed circular dsDNA. In this assay, SsoRad54 protein was added to topologically relaxed DNA and topoisomerase I in the presence or absence of ATP. DNA domain trapping and translocation by SsoRad54 in the presence of ATP will produce equal amounts of positively and negatively supercoiled domains in the DNA duplex; the negatively supercoiled domains will be relaxed by topoisomerase I. Deproteinization of the reactions to eliminate bound SsoRad54 protein and the topoisomerase will then yield positively supercoiled DNA. Figure 2 shows that in the presence of SsoRad54 protein and ATP, supercoiled DNA topoisomers are formed (lanes 3–7). The formation of these supercoiled species is completely dependent on the presence of ATP; no supercoiled DNA is apparent when ATP was omitted from the reaction at the highest concentration of SsoRad54 protein (Figure 2, lane 8). No dsDNA topology alteration was observed when SsoRad54 and ATP were tested in the absence of topoisomerase I (data not shown). To eliminate the possibility that the observed activity was the result of a contaminating mesophilic type II topoisomerase, the experiment was conducted in the presence of the topoisomerase inhibitor norfloxacin, which did not eliminate SsoRad54-mediated dsDNA supercoiling (data not shown). These results show that SsoRad54 can alter dsDNA topology in an ATP-dependent manner and suggest that it is a dsDNA translocase.
Figure 2.
SsoRad54 remodels duplex DNA. Increasing concentrations of SsoRad54 protein (0.01, 0.03, 0.06, 0.09 and 0.12 μM in lanes 3 through 7, respectively) were incubated with relaxed dsDNA and E. coli topoisomerase I. In lane 2, DNA was incubated in buffer with topoisomerase I but without SsoRad54 protein. The novel topoisomers generated by the presence of both SsoRad54 and topoisomerase I are indicated at the right of the figure. Lane 8 represents a reaction containing both SsoRad54 and topoisomerase I and illustrates the requirement for ATP in the reaction, while lane 1 represents the reaction in the absence of added topoisomerase I and SsoRad54 protein.
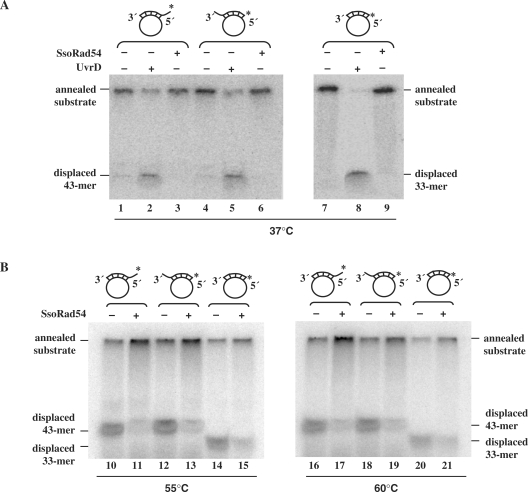
Many members of helicase superfamily II have DNA helicase activity, although none of the eukaryotic Rad54 proteins have been shown to be helicases. As the topological alterations observed in Figure 2 could be the result of helicase activity, we directly tested the SsoRad54 protein for this function (Figure 3A and B). We used three different DNA substrates that were generated by hybridizing oligonucleotides to circular single-stranded M13 DNA. The three oligonucleotides were either completely complementary over a 33 base region or contained an additional 10 nucleotide non-complementary region at either the 5′- or 3′-end. Each oligonucleotide was radio-labeled at the 5′-end. The UvrD protein from E. coli is a known helicase, and was used as a positive control in the experiment (Figure 3A). UvrD displaced ≥50% of the hybridized DNA fragment from all three substrates, whereas no unwinding of any substrate was observed in the presence of SsoRad54 protein under the same conditions where topological alterations were apparent. Various concentrations of SsoRad54 protein were tested for helicase activity, ranging from 0.01 μM to 0.6 μM, none of which resulted in oligonucleotide displacement (results not shown). As helicase activity of the protein might not be apparent at low temperature, we also tested for this activity at both 55°C and 60°C using the same substrates (Figure 3B). No unwinding of any substrate was observed at higher temperatures; instead, SsoRad54 protein slightly stabilized the duplex DNA (Figure 3B, lanes 11, 13, 15, 17, 19 and 21), likely due to its documented capacity to bind dsDNA. Thus, the topological alterations seen in Figure 2 are not caused by helicase action. Our results are consistent with those observed for the Saccharomyces cerevisiae and human Rad54 proteins, where helicase activity was not observed (11,12). Instead, ATPase activity is used by Rad54 and its homolog, Tid1, to translocate along duplex DNA (51–53).
Figure 3.
SsoRad54 protein shows no DNA helicase activity. (A) Either E. coli UvrD (lanes 2, 5 and 8) or SsoRad54 (lanes 3, 6 and 9) was incubated with one of the three radiolabeled helicase substrates at 37°C. The three control reactions shown (lanes 1, 4 and 7) lacked protein. (B) SsoRad54 was incubated with one of the three radiolabeled helicase substrates at 55°C (lanes 11, 13 and 15) or 60°C (lanes 17, 19 and 21). The control reactions shown (lanes 10, 12, 14, 16, 18 and 20) lacked protein. For both A and B, the positions of the substrate and the radiolabeled reaction products are indicated. The asterisk shows the position of the 32P label on the oligonucleotide.
SsoRad54 interacts directly with SsoRadA
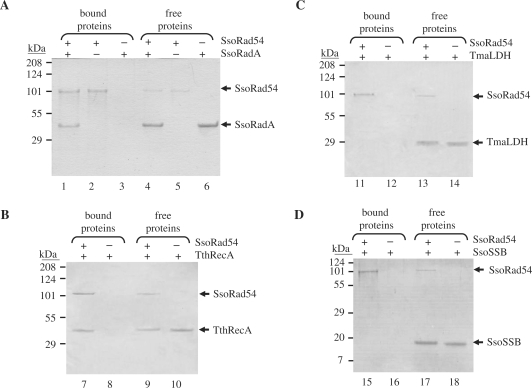
The Rad54 protein from Saccharomyces cerevisiae interacts with Rad51 (11,14,22). Therefore, we tested for an interaction between the archaeal Rad51 homolog, SsoRadA (38,44,58), and SsoRad54 by a pull-down assay using Ni-NTA beads (Figure 4A). An excess of both 6-histidine tagged SsoRad54 protein and untagged SsoRadA protein, relative to the bead capacity, was used (Figure 4A, lanes 4–6), as determined through trial experiments. After extensive washing to remove unbound material, protein retained on the beads was eluted with imidazole and analyzed by SDS-PAGE (Figure 4A, lanes 1–3). In cases where both SsoRad54 and SsoRadA were present (Figure 4A, lane 1), the SsoRad54 pulled-down ∼2 molecules of SsoRadA per SsoRad54 molecule. When SsoRad54 was omitted, no SsoRadA was retained by non-specific binding to the beads (Figure 4A, lane 3). These results imply a direct interaction between SsoRad54 and SsoRadA. To examine the specificity of this interaction, we also tested other thermophilic proteins under identical binding conditions (Figure 4B, C and D). Neither the SSB homologue, SsoSSB nor Thermotoga maritima lactate dehydrogenase (LDH) interacted appreciably with SsoRad54. RecA from Thermus thermophilus did show an interaction with SsoRad54, although somewhat less was bound (1.6 RecA/SsoRad54). This somewhat weaker interaction may be a reflection of evolutionary conservation between members of the RecA/RadA/Rad51 family of proteins, and is similar to the interactions observed between RecA/Rad51 and the RecA-loading domain of RecBCD enzyme (59). In this latter case, conserved interactions were manifest whose affinities decreased with phylogenetic distance.
Figure 4.
SsoRad54 protein interacts directly with SsoRadA protein. (A) Purified SsoRad54 protein was incubated with or without SsoRadA protein prior to the addition of Ni-NTA magnetic beads. The beads were magnetically sequestered and subjected to extensive washes to remove unbound proteins. Control reactions in lanes 2 and 3 represent the binding of SsoRad54 or SsoRadA, respectively, to the magnetic beads; lanes 5 and 6 show SsoRad54 and SsoRadA present in the supernatant following magnetic sequestration of the beads. Lane 1 shows the interaction between SsoRad54 and SsoRadA as revealed by binding of the protein complex to the beads, while lane 4 shows the protein that remains unbound and free in the supernatant. (B) SsoRad54 also interacts with Thermus thermophilus RecA but not with Thermotoga maritima LDH (C) or SsoSSB (D). Migration position for each protein is indicated to the right of each gel.
Stimulation of SsoRadA-mediated DNA strand exchange
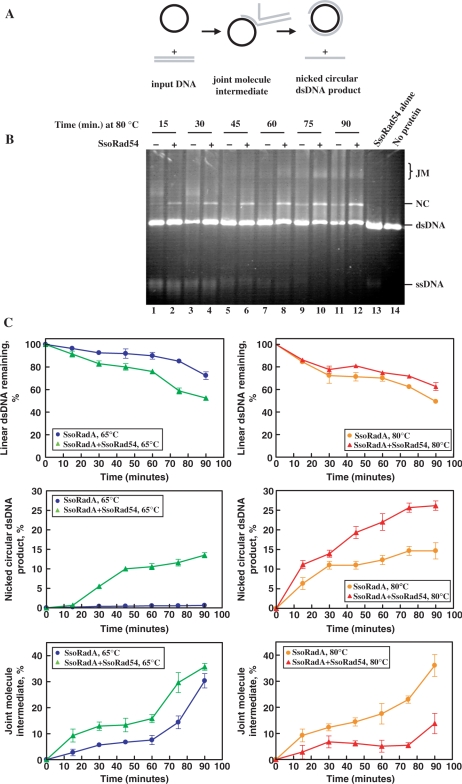
In both yeast and human systems, Rad54 protein stimulates DNA strand exchange mediated by Rad51 protein in vitro (13,19,20,60–63). We suspected that the SsoRad54 protein might stimulate SsoRadA-mediated DNA strand exchange, and tested this possibility with an in vitro assay using homologous circular ssDNA and linear dsDNA as substrates (Figure 5A). During the DNA strand exchange reaction, these substrates are converted in a time-dependent manner to homologously-paired joint molecule intermediates, and then to nicked circular dsDNA and the displaced ssDNA products. SsoRad54 protein significantly stimulated DNA strand exchange as measured by nicked circular dsDNA product formation at 80°C (Figure 5B and C). This effect is even more pronounced at conditions (65°C) that are sub-optimal for SsoRadA activity (Figure 5C), but where the SsoRad54 protein activity is near optimal (see Figure 1C). The SsoRad54 protein itself does not have DNA strand exchange activity, as neither joint molecules nor nicked circular dsDNA is formed in the absence of SsoRadA (Figure 5, lane 13). There is a time-dependent loss of ssDNA during the reaction that is not the result of nuclease activity, since no nuclease activity was detected in any of the protein preparations (data not shown). Instead, this loss is due to hydrolysis of ssDNA by the elevated temperatures and the slightly acidic pH, as is apparent in the control reaction that was incubated for 90 min in the absence of protein (Figure 5, lane 14).
Figure 5.
SsoRad54 protein stimulates SsoRadA-mediated DNA strand exchange. (A) Schematic of the DNA strand exchange reaction. (B) A time course of DNA strand exchange in the presence and absence of SsoRad54 protein at 80°C. Basal SsoRadA DNA strand exchange is shown over time in lanes 1, 3, 5, 7, 9 and 11. In lanes 2, 4, 6, 8, 10 and 12, SsoRad54 is added at the same time as SsoRadA, prior to the addition of linear φX174 dsDNA to the reaction. Lane 13 shows a reaction with SsoRad54 alone incubated for 60 min, while lane 14 shows a reaction lacking protein incubated for 90 min. JM indicates the position of joint molecules; NC represents the final nicked circular product, while dsDNA and ssDNA indicate the input DNA substrates. (C) A graphical representation of intermediate and product formation for data such as shown in (B), as well as results obtained from DNA strand exchange reactions conducted at 65°C. Reactions at 65°C that include SsoRad54 are shown with green triangles while those lacking SsoRad54 are represented by blue circles. Reactions at 80°C that include SsoRad54 are shown with red triangles while those lacking SsoRad54 are represented by orange circles. Utilization of the dsDNA substrate, and the formation of joint molecule intermediates and nicked circular dsDNA product are expressed as the percentage of dsDNA in each experiment. Error bars represent standard deviation between three replicate experiments.
SsoRad54 has homology to bacterial HepA protein
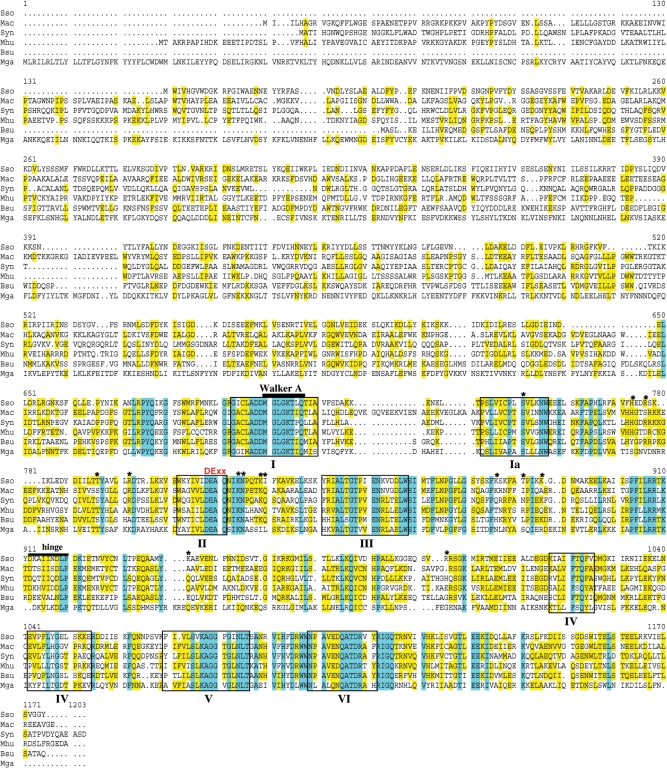
The existence of Rad54 protein in an archaeal organism suggests this protein is not exclusive to eukaryotes, but is perhaps unidentified in bacteria and other archaea due to sequence divergence. We undertook an examination of completely sequenced archaeal genomes to search for coding sequences that have similarity to proteins of the Swi2/Snf2 family. These proteins are characterized by the presence of ATPase motifs (Walker A and B) as well as the seven conserved helicase motifs. To identify other putative prokaryotic Rad54 proteins, we aligned the SsoRad54 sequence with similar protein sequences (Figure 6). BLAST searches using the SsoRad54 protein as a query sequence showed that two archaeal methanogens, Methanosarcina acetivorans and Methanospirillum hungatei, have open reading frames encoding proteins which are very similar to the SsoRad54 protein (40% and 43% identity, respectively, and 60% similarity for both sequences). Among bacterial sequences, the one with the most similarity to SsoRad54 is found in the model cyanobacterium, Synechocystis, with 44% identity and 61% similarity. Closely related sequences are found in other bacteria; both B. subtilis and Mycoplasma gallisepticum encode a protein annotated as HepA that has notable similarity to the SsoRad54 protein (39% and 33% identity, 59 and 53% similarity, respectively). First identified in E. coli (64), HepA-related SNF2 proteins were subsequently identified in both mouse and human (65). Also known as RapA, HepA is an RNA polymerase interacting protein that has ATPase and DNA-binding activity but lacks helicase activity, and has been implicated in RNA remodeling during transcription (66–69). While the involvement of this protein in recombination processes in bacteria is currently unknown, disruption of the coding sequence results in a UV sensitive phenotype (67).
Figure 6.
SsoRad54 protein shows sequence similarity to putative prokaryotic Rad54 homologues. Abbreviations are: Mac for Methanosarcina acetivorans strain C2A, Syn for Synechocystis sp. PCC 6803, Mhu for Methanospirillum hungatei, strain Jf-1, Bsu for B. subtilis str. 168 (HepA protein), and Mga for Mycoplasma gallisepticum R (HepA/SNF2 protein). Blue shading indicates sequence identity while yellow shading represents sequence similarity. The helicase motifs typical of Swi2/Snf2 family proteins are represented by boxes. Additionally, the Walker A ATPase motif is represented by a bar and a DExx motif is shown in red. Information from the SsoRad54cd crystal structure suggests the presence of a hinge region, represented by a bar in the figure, while an asterisk indicates putative and identified DNA contact residues (50).
DISCUSSION
Here we have biochemically established the existence of an archaeal Rad54 protein. Though predicted based on primary sequence and structural alignments (1,70,71), it remained unclear whether this archaeal protein was indeed a bona fide functional homolog of the eukaryotic Rad54 proteins. We purified full-length SsoRad54 protein and showed that it has biochemical activities which mirror those observed for eukaryotic Rad54 proteins. SsoRad54 protein has dsDNA-dependent ATPase activity, with properties that are similar to those of Saccharomyces cerevisiae Rad54. The protein displays no detectable helicase activity, but can alter the topology of covalently closed circular duplex DNA. SsoRad54 interacts directly with SsoRadA and stimulates SsoRadA-mediated DNA strand exchange. All of these activities have been described for eukaryotic Rad54 proteins, leading us to suggest that SsoRad54 represents an authentic prokaryotic Rad54 protein.
DNA unwinding has been implicated in the stimulation of Rad51-mediated DNA strand exchange (25,62,72). It was correctly inferred that the changes in DNA topology induced by Rad54 were the consequences of protein tracking along DNA, generating positively and negatively supercoiled domains (25,72). Direct single-molecule visualization established that Rad54 and its meiotic homolog, Rdh54/Tid1, translocate on duplex DNA at ∼100–300 bp/s (51–53). Consequently, it was speculated that the association of Rad54 with the Rad51 nucleoprotein filament might enhance the rate of homology sampling, while transient strand opening within the supercoiled domains could facilitate formation of nascent DNA joints (62,63). Alternatively, we have suggested that Rad54/Tid1 could stimulate DNA pairing by using its translocation capacity to convert short, nascent unstable DNA joints into longer, more stable heteroduplex regions (51). A specific interaction between Rad51 and Rad54 is required for efficient DNA remodeling and strand invasion (11,14,22). Association between Rad51 (or Dmc1) and Rad54 (or Rdh54/Tid1) prior to and at the DNA pairing site may permit directional extension of short regions, resulting in stimulation of DNA strand exchange. Indeed, the translocase activity of Rad54, and Tid1, was shown to be involved in both the stabilization and destabilization of the nascent heteroduplex DNA (51,73), in DNA heteroduplex extension (19,20,60,74), chromatin-remodeling (24,71,75–81), and also in the clearance of Rad51 from the DNA heteroduplex product (21,82). This complex array of behaviors can be understood as different manifestations of the translocation capacity of Rad54. Furthermore, we suggested that, in the absence of an interaction with Rad51, the binding of Rad54 to dsDNA is random (50) and, hence, translocation can be in either direction (51–53), resulting in either disruption or elongation of joint molecules. However, when Rad54 is targeted to the dsDNA by Rad51, the initial direction of translocation may be determined by the interaction with the Rad51 nucleoprotein filament, which has a polarity of pairing defined by the ssDNA.
Eukaryotic Rad54 protein can also alter the positioning of nucleosomes (24,71,75–81). Such translocation-dependent repositioning likely increases DNA accessibility for basal processes including replication, recombination, and repair (83–85). There are homologues for eukaryotic chromatin proteins in the archaea (86). Exclusive to euryarchaea, these proteins are generally shorter than their eukaryotic cousins and essentially comprise the histone-core without defined tails. Archaeal histones can compact DNA and form structures analogous to eukaryotic histones. Little is known about the role of archaeal histones in vivo, but changes in chromosome compaction or the distribution of histone subtypes could be used to modulate DNA access (87). While here we have identified two euryarchaeal sequences with homology to the SsoRad54 protein, whether archaeal Rad54 homologs are involved in archaeal nucleosome remodeling is unknown.
The SsoRad54 protein we described here is in a member of the crenarchaeal domain, where histones appear to be largely absent (88). Instead of classical histones, crenarchaea use highly abundant DNA-binding proteins to compact DNA. The Sul7d protein from the Sulfolobus genus binds DNA non-cooperatively and can compact relaxed or positively supercoiled DNA (89–91). Thermoproteus tenax, Pyrobaculum aerophilum and Aeropyrum pernix use the physically similar CC1 protein that can bind both ssDNA and dsDNA with high cooperativity (92). Archaea also have the Alba protein, which binds DNA non-specifically and may be involved in organizing higher-order folding of DNA-histone or DNA-Sul7d nucleoprotein assemblies (93,94). A second archaeal Alba protein (Alba2) was identified that forms obligate heterodimers with Alba and alters higher-order Alba-mediated DNA packing (95). Differential expression of the Alba proteins could modulate the nucleoid structure in Sulfolobus. DNA accessibility in these prokaryotes might be tightly controlled through packaging, much as it is in eukaryotes. SsoRad54 could affect distribution of these crenarchaeal DNA packing proteins, much as eukaryotic Rad54 alters nucleosome positioning.
The N-terminal domain of Rad54 proteins was implicated in direct interactions with Rad51 protein and histone H3 (80,96). While a number of eukaryotic Rad54 proteins maintain high homology at the N-terminus (15,97,98), the recently identified Rad54 protein of Arabidopsis thaliana, is highly divergent in this region (99). Divergence in this part of the protein may be the result of species-specific interactions with cognate protein partners. We find no apparent N-terminal homology between SsoRad54 protein and any of the eukaryotic Rad54 proteins identified to date. This divergence may be the consequence of the interactions with cellular protein that are necessary and specific to Sulfolobus solfataricus. We searched for other prokaryotic Rad54 proteins using the archaeal Rad54 sequence as a query in BLAST analyses. Homologous sequences are apparent in archaeal methanogens, as well as in bacterial systems including cyanobacterium, B. subtilis, and Mycoplasma gallisepticum. Relatively recently, there was an effort to categorize the Snf2 family of proteins (100). Snf2-related sequences are found in over two thirds of complete microbial genomes and can be divided into two groups: the SSO1653 subfamily, which is anchored by the Sulfolobus solfataricus SsoRad54 sequence, and the RapA/HepA group. The broad distribution of Snf2 sequences in microbial organisms suggests that these proteins perform non-essential functions that are sufficiently advantageous to maintain their prevalence. The sequences that we find through BLAST searches can be categorized within the SSO1653 subfamily of Snf2 proteins. Proteins identified within the RapA/HepA group are, by strict definition, outside the Snf2 family of proteins because they lack several features of Snf2 family proteins (100). Our BLAST analyses found homology between the SsoRad54 protein sequence and bacterial sequences annotated as HepA. The bacterial HepA sequence is of particular interest since disruption of this gene in E. coli results in a clear DNA-damage sensitivity phenotype (67). Biochemical analyses of bacterial HepA protein have not, however, further addressed the potential involvement of the protein in recombination or repair processes. Characterization of the SsoRad54 protein will aid in identification of proteins with similar function in other prokaryotes. The SsoRad54 protein is a significant phylogenetic link in the evolution of recombination mechanisms and its further characterization will serve to define the steps of this important process in prokaryotic organisms.
FUNDING
National Institute of Health (GM-62653 to S.C.K.); a postdoctoral fellowship (PF-03-043-01-GMC) from the American Cancer Society (to C.A.H.); start up funds from Washington State University School of Molecular Biosciences. Funding for open access charge: National Institutes of Health (GM-62653).
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We are grateful to Dr Wolf Heyer, and to members of the Kowalczykowski laboratory, Ichiro Amitani, Aura Carreira, Petr Cejka, Anthony Forget, Ryan Jensen, Taeho Kim, Amitabh Nimonkar, Jody Plank, Behzad Rad, Edgar Valencia-Morales and Jason Wong, for their critical reading of the manuscript.
REFERENCES
- 1.Seitz EM, Haseltine CA, Kowalczykowski SC. DNA recombination and repair in the archaea. Adv. Appl. Microbiol. 2001;50:101–169. doi: 10.1016/s0065-2164(01)50005-2. [DOI] [PubMed] [Google Scholar]
- 2.Bianco PR, Tracy RB, Kowalczykowski SC. DNA strand exchange proteins: A biochemical and physical comparison. Front. Biosci. 1998;3:D570–D603. doi: 10.2741/a304. [DOI] [PubMed] [Google Scholar]
- 3.Heyer WD, Li X, Rolfsmeier M, Zhang XP. Rad54: the Swiss Army knife of homologous recombination? Nucleic Acids Res. 2006;34:4115–4125. doi: 10.1093/nar/gkl481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Game JC, Mortimer RK. A genetic study of X-ray sensitive mutants in yeast. Mutat. Res. 1974;24:281. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- 5.Game JC. DNA double-strand breaks and the RAD50-RAD57 genes in Saccharomyces. Semin. Cancer Biol. 1993;4:73–83. [PubMed] [Google Scholar]
- 6.Clever B, Schmuckli-Maurer J, Sigrist M, Glassner BJ, Heyer WD. Specific negative effects resulting from elevated levels of the recombinational repair protein Rad54p in Saccharomyces cerevisiae. Yeast. 1999;15:721–740. doi: 10.1002/(SICI)1097-0061(19990630)15:9<721::AID-YEA414>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Schmuckli-Maurer J, Heyer WD. The Saccharomyces cerevisiae RAD54 gene is important but not essential for natural homothallic mating-type switching. Mol. Gen. Genet. 1999;260:551–558. doi: 10.1007/s004380050928. [DOI] [PubMed] [Google Scholar]
- 8.Kasinova GV. Effect of Saccharomyces cerevisiae RAD54 mutation on gamma ray-induced reciprocal mitotic recombination. Genetika. 1980;16:2058–2060. [PubMed] [Google Scholar]
- 9.Glassner BJ, Mortimer RK. Synergistic interactions between RAD5, RAD16 and RAD54, three partially homologous yeast DNA repair genes each in a different repair pathway. Radiat. Res. 1994;139:24–33. [PubMed] [Google Scholar]
- 10.Gorbalenya AE, Koonin EV. Helicases: amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 1993;3:419–429. [Google Scholar]
- 11.Petukhova G, Stratton S, Sung P. Catalysis of homologous DNA pairing by yeast Rad51 and Rad54 proteins. Nature. 1998;393:91–94. doi: 10.1038/30037. [DOI] [PubMed] [Google Scholar]
- 12.Swagemakers SM, Essers J, de Wit J, Hoeijmakers JH, Kanaar R. The human RAD54 recombinational DNA repair protein is a double-stranded DNA-dependent ATPase. J. Biol. Chem. 1998;273:28292–28297. doi: 10.1074/jbc.273.43.28292. [DOI] [PubMed] [Google Scholar]
- 13.Petukhova G, Van Komen S, Vergano S, Klein H, Sung P. Yeast Rad54 promotes Rad51-dependent homologous DNA pairing via ATP hydrolysis-driven change in DNA double helix conformation. J. Biol. Chem. 1999;274:29453–29462. doi: 10.1074/jbc.274.41.29453. [DOI] [PubMed] [Google Scholar]
- 14.Clever B, Interthal H, Schmuckli-Maurer J, King J, Sigrist M, Heyer WD. Recombinational repair in yeast: functional interactions between Rad51 and Rad54 proteins. EMBO J. 1997;16:2535–2544. doi: 10.1093/emboj/16.9.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanaar R, Troelstra C, Swagemakers SM, Essers J, Smit B, Franssen JH, Pastink A, Bezzubova OY, Buerstedde JM, Clever B, et al. Human and mouse homologs of the Saccharomyces cerevisiae RAD54 DNA repair gene: evidence for functional conservation. Curr. Biol. 1996;6:828–838. doi: 10.1016/s0960-9822(02)00606-1. [DOI] [PubMed] [Google Scholar]
- 16.Mazin AV, Bornarth CJ, Solinger JA, Heyer WD, Kowalczykowski SC. Rad54 protein is targeted to pairing loci by the Rad51 nucleoprotein filament. Mol. Cell. 2000;6:583–592. doi: 10.1016/s1097-2765(00)00057-5. [DOI] [PubMed] [Google Scholar]
- 17.Wolner B, van Komen S, Sung P, Peterson CL. Recruitment of the recombinational repair machinery to a DNA double-strand break in yeast. Mol. Cell. 2003;12:221–232. doi: 10.1016/s1097-2765(03)00242-9. [DOI] [PubMed] [Google Scholar]
- 18.Mazin AV, Alexeev AA, Kowalczykowski SC. A novel function of Rad54 protein. Stabilization of the Rad51 nucleoprotein filament. J. Biol. Chem. 2003;278:14029–14036. doi: 10.1074/jbc.M212779200. [DOI] [PubMed] [Google Scholar]
- 19.Solinger JA, Heyer WD. Rad54 protein stimulates the postsynaptic phase of Rad51 protein-mediated DNA strand exchange. Proc. Natl Acad. Sci. USA. 2001;98:8447–8453. doi: 10.1073/pnas.121009898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solinger JA, Lutz G, Sugiyama T, Kowalczykowski SC, Heyer WD. Rad54 protein stimulates heteroduplex DNA formation in the synaptic phase of DNA strand exchange via specific interactions with the presynaptic Rad51 nucleoprotein filament. J. Mol. Biol. 2001;307:1207–1221. doi: 10.1006/jmbi.2001.4555. [DOI] [PubMed] [Google Scholar]
- 21.Solinger JA, Kiianitsa K, Heyer WD. Rad54, a Swi2/Snf2-like recombinational repair protein, disassembles Rad51:dsDNA filaments. Mol. Cell. 2002;10:1175–1188. doi: 10.1016/s1097-2765(02)00743-8. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H, Xie Y, Houston P, Stemke-Hale K, Mortensen UH, Rothstein R, Kodadek T. Direct association between the yeast Rad51 and Rad54 recombination proteins. J. Biol. Chem. 1996;271:33181–33186. doi: 10.1074/jbc.271.52.33181. [DOI] [PubMed] [Google Scholar]
- 23.Golub EI, Kovalenko OV, Gupta RC, Ward DC, Radding CM. Interaction of human recombination proteins Rad51 and Rad54. Nucleic Acids Res. 1997;25:4106–4110. doi: 10.1093/nar/25.20.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexiadis V, Kadonaga JT. Strand pairing by Rad54 and Rad51 is enhanced by chromatin. Genes Dev. 2002;16:2767–2771. doi: 10.1101/gad.1032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan TL, Essers J, Citterio E, Swagemakers SM, de Wit J, Benson FE, Hoeijmakers JH, Kanaar R. Mouse Rad54 affects DNA conformation and DNA-damage-induced Rad51 foci formation. Curr. Biol. 1999;9:325–328. doi: 10.1016/s0960-9822(99)80142-0. [DOI] [PubMed] [Google Scholar]
- 26.Woese CR, Fox GE. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc. Natl Acad. Sci. USA. 1977;74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl Acad. Sci. USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koonin EV, Mushegian AR, Galperin MY, Walker DR. Comparison of archaeal and bacterial genomes: computer analysis of protein sequences predicts novel functions and suggests a chimeric origin for the archaea. Mol. Microbiol. 1997;25:619–637. doi: 10.1046/j.1365-2958.1997.4821861.x. [DOI] [PubMed] [Google Scholar]
- 29.Makarova KS, Aravind L, Galperin MY, Grishin NV, Tatusov RL, Wolf YI, Koonin EV. Comparative genomics of the Archaea (Euryarchaeota): evolution of conserved protein families, the stable core, and the variable shell. Genome Res. 1999;9:608–628. [PubMed] [Google Scholar]
- 30.Rivera MC, Jain R, Moore JE, Lake JA. Genomic evidence for two functionally distinct gene classes. Proc. Natl Acad. Sci. USA. 1998;95:6239–6244. doi: 10.1073/pnas.95.11.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chédin F, Seitz EM, Kowalczykowski SC. Novel homologs of replication protein A in archaea: implications for the evolution of ssDNA-binding proteins. Trends Biochem. Sci. 1998;23:273–277. doi: 10.1016/s0968-0004(98)01243-2. [DOI] [PubMed] [Google Scholar]
- 32.Kelly TJ, Simancek P, Brush GS. Identification and characterization of a single-stranded DNA-binding protein from the archaeon Methanococcus jannaschii. Proc. Natl Acad. Sci. USA. 1998;95:14634–14639. doi: 10.1073/pnas.95.25.14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komori K, Ishino Y. Replication protein A in Pyrococcus furiosus is involved in homologous DNA recombination. J. Biol. Chem. 2001;276:25654–25660. doi: 10.1074/jbc.M102423200. [DOI] [PubMed] [Google Scholar]
- 34.Wadsworth RI, White MF. Identification and properties of the crenarchaeal single-stranded DNA binding protein from Sulfolobus solfataricus. Nucleic Acids Res. 2001;29:914–920. doi: 10.1093/nar/29.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haseltine CA, Kowalczykowski SC. A distinctive single-strand DNA-binding protein from the Archaeon Sulfolobus solfataricus. Mol. Microbiol. 2002;43:1505–1515. doi: 10.1046/j.1365-2958.2002.02807.x. [DOI] [PubMed] [Google Scholar]
- 36.Robbins JB, Murphy MC, White BA, Mackie RI, Ha T, Cann IK. Functional analysis of multiple single-stranded DNA-binding proteins from Methanosarcina acetivorans and their effects on DNA synthesis by DNA polymerase BI. J. Biol. Chem. 2004;279:6315–6326. doi: 10.1074/jbc.M304491200. [DOI] [PubMed] [Google Scholar]
- 37.Rashid N, Morikawa M, Imanaka T. A RecA/RAD51 homologue from a hyperthermophilic archaeon retains the major RecA domain only. Mol. Gen. Genet. 1996;253:397–400. doi: 10.1007/s004380050337. [DOI] [PubMed] [Google Scholar]
- 38.Seitz EM, Brockman JP, Sandler SJ, Clark AJ, Kowalczykowski SC. RadA protein is an archaeal RecA protein homolog that catalyzes DNA strand exchange. Genes Dev. 1998;12:1248–1253. doi: 10.1101/gad.12.9.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kil YV, Baitin DM, Masui R, Bonch-Osmolovskaya EA, Kuramitsu S, Lanzov VA. Efficient strand transfer by the RadA recombinase from the hyperthermophilic archaeon Desulfurococcus amylolyticus. J. Bacteriol. 2000;182:130–134. doi: 10.1128/jb.182.1.130-134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komori K, Miyata T, DiRuggiero J, Holley-Shanks R, Hayashi I, Cann IK, Mayanagi K, Shinagawa H, Ishino Y. Both RadA and RadB Are Involved in Homologous Recombination in Pyrococcus furiosus. J. Biol. Chem. 2000;275:33782–33790. doi: 10.1074/jbc.M004557200. [DOI] [PubMed] [Google Scholar]
- 41.Spies M, Kil Y, Masui R, Kato R, Kujo C, Ohshima T, Kuramitsu S, Lanzov V. The RadA protein from a hyperthermophilic archaeon Pyrobaculum islandicum is a DNA-dependent ATPase that exhibits two disparate catalytic modes, with a transition temperature at 75 degrees C. Eur. J. Biochem. 2000;267:1125–1137. doi: 10.1046/j.1432-1327.2000.01108.x. [DOI] [PubMed] [Google Scholar]
- 42.McIlwraith MJ, Hall DR, Stasiak AZ, Stasiak A, Wigley DB, West SC. RadA protein from Archaeoglobus fulgidus forms rings, nucleoprotein filaments and catalyses homologous recombination. Nucleic Acids Res. 2001;29:4509–4517. doi: 10.1093/nar/29.22.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Qian X, He Y, Moya IA, Luo Y. Crystal structure of an ATPase-active form of Rad51 homolog from Methanococcus voltae: insights into potassium dependence. J. Biol. Chem. 2005;280:722–728. doi: 10.1074/jbc.M411093200. [DOI] [PubMed] [Google Scholar]
- 44.Yang S, Yu X, Seitz EM, Kowalczykowski SC, Egelman EH. Archaeal RadA protein binds DNA as both helical filaments and octameric rings. J. Mol. Biol. 2001;314:1077–1085. doi: 10.1006/jmbi.2000.5213. [DOI] [PubMed] [Google Scholar]
- 45.Hopfner KP, Karcher A, Shin D, Fairley C, Tainer JA, Carney JP. Mre11 and Rad50 from Pyrococcus furiosus: cloning and biochemical characterization reveal an evolutionarily conserved multiprotein machine. J. Bacteriol. 2000;182:6036–6041. doi: 10.1128/jb.182.21.6036-6041.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hopfner KP, Karcher A, Craig L, Woo TT, Carney JP, Tainer JA. Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase. Cell. 2001;105:473–485. doi: 10.1016/s0092-8674(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 47.Bergerat A, de Massy B, Gadelle D, Varoutas PC, Nicolas A, Forterre P. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 48.Nichols MD, DeAngelis K, Keck JL, Berger JM. Structure and function of an archaeal topoisomerase VI subunit with homology to the meiotic recombination factor Spo11. EMBO J. 1999;18:6177–6188. doi: 10.1093/emboj/18.21.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin DS, Pellegrini L, Daniels DS, Yelent B, Craig L, Bates D, Yu DS, Shivji MK, Hitomi C, Arvai AS, et al. Full-length archaeal Rad51 structure and mutants: mechanisms for RAD51 assembly and control by BRCA2. EMBO J. 2003;22:4566–4576. doi: 10.1093/emboj/cdg429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durr H, Korner C, Muller M, Hickmann V, Hopfner KP. X-ray structures of the Sulfolobus solfataricus SWI2/SNF2 ATPase core and its complex with DNA. Cell. 2005;121:363–373. doi: 10.1016/j.cell.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 51.Nimonkar AV, Amitani I, Baskin RJ, Kowalczykowski SC. Single molecule imaging of Tid1/Rdh54, a Rad54 homolog that translocates on duplex DNA and can disrupt joint molecules. J. Biol. Chem. 2007;282:30776–30784. doi: 10.1074/jbc.M704767200. [DOI] [PubMed] [Google Scholar]
- 52.Amitani I, Baskin RJ, Kowalczykowski SC. Visualization of Rad54, a chromatin remodeling protein, translocating on single DNA molecules. Mol. Cell. 2006;23:143–148. doi: 10.1016/j.molcel.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Prasad TK, Robertson RB, Visnapuu ML, Chi P, Sung P, Greene EC. A DNA-translocating Snf2 Molecular Motor: Saccharomyces cerevisiae Rdh54 Displays Processive Translocation and Extrudes DNA Loops. J. Mol. Biol. 2007;369:940–953. doi: 10.1016/j.jmb.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rolfsmeier M, Blum P. Purification and characterization of a maltase from the extremely thermophilic crenarchaeote Sulfolobus solfataricus. J. Bacteriol. 1995;177:482–485. doi: 10.1128/jb.177.2.482-485.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morimatsu K, Horii T. Analysis of the DNA binding site of Escherichia coli RecA protein. Adv. Biophys. 1995;31:23–48. doi: 10.1016/0065-227x(95)99381-x. [DOI] [PubMed] [Google Scholar]
- 56.Seybert A, Scott DJ, Scaife S, Singleton MR, Wigley DB. Biochemical characterisation of the clamp/clamp loader proteins from the euryarchaeon Archaeoglobus fulgidus. Nucleic Acids Res. 2002;30:4329–4338. doi: 10.1093/nar/gkf584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price LB, Shand RF. Halocin S8: a 36-amino-acid microhalocin from the haloarchaeal strain S8a. J. Bacteriol. 2000;182:4951–4958. doi: 10.1128/jb.182.17.4951-4958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandler SJ, Satin LH, Samra HS, Clark AJ. recA-like genes from three archaean species with putative protein products similar to Rad51 and Dmc1 proteins of the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1996;24:2125–2132. doi: 10.1093/nar/24.11.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spies M, Kowalczykowski SC. The RecA binding locus of RecBCD is a general domain for recruitment of DNA strand exchange proteins. Mol. Cell. 2006;21:573–580. doi: 10.1016/j.molcel.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Mazina OM, Mazin AV. Human Rad54 protein stimulates DNA strand exchange activity of hRad51 protein in the presence of Ca2+ J. Biol. Chem. 2004;279:52042–52051. doi: 10.1074/jbc.M410244200. [DOI] [PubMed] [Google Scholar]
- 61.Sarai N, Kagawa W, Kinebuchi T, Kagawa A, Tanaka K, Miyagawa K, Ikawa S, Shibata T, Kurumizaka H, Yokoyama S. Stimulation of Dmc1-mediated DNA strand exchange by the human Rad54B protein. Nucleic Acids Res. 2006;34:4429–4437. doi: 10.1093/nar/gkl562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Komen S, Petukhova G, Sigurdsson S, Stratton S, Sung P. Superhelicity-driven homologous DNA pairing by yeast recombination factors Rad51 and Rad54. Mol. Cell. 2000;6:563–572. doi: 10.1016/s1097-2765(00)00055-1. [DOI] [PubMed] [Google Scholar]
- 63.Van Komen S, Petukhova G, Sigurdsson S, Sung P. Functional cross-talk among Rad51, Rad54, and replication protein A in heteroduplex DNA joint formation. J. Biol. Chem. 2002;277:43578–43587. doi: 10.1074/jbc.M205864200. [DOI] [PubMed] [Google Scholar]
- 64.Lewis LK, Jenkins ME, Mount DW. Isolation of DNA damage-inducible promoters in Escherichia coli: regulation of polB (dinA), dinG, and dinH by LexA repressor. J. Bacteriol. 1992;174:3377–3385. doi: 10.1128/jb.174.10.3377-3385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coleman MA, Eisen JA, Mohrenweiser HW. Cloning and characterization of HARP/SMARCAL1: a prokaryotic HepA-related SNF2 helicase protein from human and mouse. Genomics. 2000;65:274–282. doi: 10.1006/geno.2000.6174. [DOI] [PubMed] [Google Scholar]
- 66.Sukhodolets MV, Jin DJ. RapA, a novel RNA polymerase-associated protein, is a bacterial homolog of SWI2/SNF2. J. Biol. Chem. 1998;273:7018–7023. doi: 10.1074/jbc.273.12.7018. [DOI] [PubMed] [Google Scholar]
- 67.Muzzin O, Campbell EA, Xia L, Severinova E, Darst SA, Severinov K. Disruption of Escherichia coli hepA, an RNA polymerase-associated protein, causes UV sensitivity. J. Biol. Chem. 1998;273:15157–15161. doi: 10.1074/jbc.273.24.15157. [DOI] [PubMed] [Google Scholar]
- 68.Sukhodolets MV, Cabrera JE, Zhi H, Jin DJ. RapA, a bacterial homolog of SWI2/SNF2, stimulates RNA polymerase recycling in transcription. Genes Dev. 2001;15:3330–3341. doi: 10.1101/gad.936701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McKinley BA, Sukhodolets MV. Escherichia coli RNA polymerase-associated SWI/SNF protein RapA: evidence for RNA-directed binding and remodeling activity. Nucleic Acids Res. 2007;35:7044–7060. doi: 10.1093/nar/gkm747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Durr H, Flaus A, Owen-Hughes T, Hopfner KP. Snf2 family ATPases and DExx box helicases: differences and unifying concepts from high-resolution crystal structures. Nucleic Acids Res. 2006;34:4160–4167. doi: 10.1093/nar/gkl540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thoma NH, Czyzewski BK, Alexeev AA, Mazin AV, Kowalczykowski SC, Pavletich NP. Structure of the SWI2/SNF2 chromatin-remodeling domain of eukaryotic Rad54. Nat. Struct. Mol. Biol. 2005;12:350–356. doi: 10.1038/nsmb919. [DOI] [PubMed] [Google Scholar]
- 72.Ristic D, Wyman C, Paulusma C, Kanaar R. The architecture of the human Rad54-DNA complex provides evidence for protein translocation along DNA. Proc. Natl Acad. Sci. USA. 2001;98:8454–8460. doi: 10.1073/pnas.151056798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bugreev DV, Hanaoka F, Mazin AV. Rad54 dissociates homologous recombination intermediates by branch migration. Nat. Struct. Mol. Biol. 2007;14:746–753. doi: 10.1038/nsmb1268. [DOI] [PubMed] [Google Scholar]
- 74.Bugreev DV, Mazina OM, Mazin AV. Rad54 protein promotes branch migration of Holliday junctions. Nature. 2006;442:590–593. doi: 10.1038/nature04889. [DOI] [PubMed] [Google Scholar]
- 75.Alexeev A, Mazin A, Kowalczykowski SC. Rad54 protein possesses chromatin-remodeling activity stimulated by the Rad51-ssDNA nucleoprotein filament. Nat. Struct. Biol. 2003;10:182–186. doi: 10.1038/nsb901. [DOI] [PubMed] [Google Scholar]
- 76.Jaskelioff M, Van Komen S, Krebs JE, Sung P, Peterson CL. Rad54p is a chromatin remodeling enzyme required for heteroduplex DNA joint formation with chromatin. J. Biol. Chem. 2003;278:9212–9218. doi: 10.1074/jbc.M211545200. [DOI] [PubMed] [Google Scholar]
- 77.Sugawara N, Wang X, Haber JE. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol. Cell. 2003;12:209–219. doi: 10.1016/s1097-2765(03)00269-7. [DOI] [PubMed] [Google Scholar]
- 78.Alexiadis V, Lusser A, Kadonaga JT. A conserved N-terminal motif in Rad54 is important for chromatin remodeling and homologous strand pairing. J. Biol. Chem. 2004;279:27824–27829. doi: 10.1074/jbc.M402648200. [DOI] [PubMed] [Google Scholar]
- 79.Wolner B, Peterson CL. ATP-dependent and ATP-independent roles for the Rad54 chromatin remodeling enzyme during recombinational repair of a DNA double strand break. J. Biol. Chem. 2005;280:10855–10860. doi: 10.1074/jbc.M414388200. [DOI] [PubMed] [Google Scholar]
- 80.Kwon Y, Chi P, Roh DH, Klein H, Sung P. Synergistic action of the Saccharomyces cerevisiae homologous recombination factors Rad54 and Rad51 in chromatin remodeling. DNA Repair. 2007;6:1496–1506. doi: 10.1016/j.dnarep.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Z, Fan HY, Goldman JA, Kingston RE. Homology-driven chromatin remodeling by human RAD54. Nat. Struct. Mol. Biol. 2007;14:397–405. doi: 10.1038/nsmb1223. [DOI] [PubMed] [Google Scholar]
- 82.Li X, Zhang XP, Solinger JA, Kiianitsa K, Yu X, Egelman EH, Heyer WD. Rad51 and Rad54 ATPase activities are both required to modulate Rad51-dsDNA filament dynamics. Nucleic Acids Res. 2007;35:4124–4140. doi: 10.1093/nar/gkm412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pâques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fyodorov DV, Kadonaga JT. The many faces of chromatin remodeling: SWItching beyond transcription. Cell. 2001;106:523–525. doi: 10.1016/s0092-8674(01)00478-0. [DOI] [PubMed] [Google Scholar]
- 85.Langst G, Becker PB. Nucleosome mobilization and positioning by ISWI-containing chromatin-remodeling factors. J. Cell Sci. 2001;114:2561–2568. doi: 10.1242/jcs.114.14.2561. [DOI] [PubMed] [Google Scholar]
- 86.Sandman K, Reeve JN. Chromosome packaging by archaeal histones. Adv. Appl. Microbiol. 2001;50:75–99. doi: 10.1016/s0065-2164(01)50004-0. [DOI] [PubMed] [Google Scholar]
- 87.Sandman K, Grayling RA, Dobrinski B, Lurz R, Reeve JN. Growth-phase-dependent synthesis of histones in the archaeon Methanothermus fervidus. Proc. Natl Acad. Sci. USA. 1994;91:12624–12628. doi: 10.1073/pnas.91.26.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cubonova L, Sandman K, Hallam SJ, Delong EF, Reeve JN. Histones in crenarchaea. J. Bacteriol. 2005;187:5482–5485. doi: 10.1128/JB.187.15.5482-5485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McAfee JG, Edmondson SP, Zegar I, Shriver JW. Equilibrium DNA binding of Sac7d protein from the hyperthermophile Sulfolobus acidocaldarius: fluorescence and circular dichroism studies. Biochemistry. 1996;35:4034–4045. doi: 10.1021/bi952555q. [DOI] [PubMed] [Google Scholar]
- 90.Gao YG, Su SY, Robinson H, Padmanabhan S, Lim L, McCrary BS, Edmondson SP, Shriver JW, Wang AH. The crystal structure of the hyperthermophile chromosomal protein Sso7d bound to DNA. Nat. Struct. Biol. 1998;5:782–786. doi: 10.1038/1822. [DOI] [PubMed] [Google Scholar]
- 91.Napoli A, Zivanovic Y, Bocs C, Buhler C, Rossi M, Forterre P, Ciaramella M. DNA bending, compaction and negative supercoiling by the architectural protein Sso7d of Sulfolobus solfataricus. Nucleic Acids Res. 2002;30:2656–2662. doi: 10.1093/nar/gkf377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo X, Schwarz-Linek U, Botting CH, Hensel R, Siebers B, White MF. CC1, a novel crenarchaeal DNA binding protein. J. Bacteriol. 2007;189:403–409. doi: 10.1128/JB.01246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lurz R, Grote M, Dijk J, Reinhardt R, Dobrinski B. Electron microscopic study of DNA complexes with proteins from the Archaebacterium Sulfolobus acidocaldarius. EMBO J. 1986;5:3715–3721. doi: 10.1002/j.1460-2075.1986.tb04705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bell SD, Botting CH, Wardleworth BN, Jackson SP, White MF. The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science. 2002;296:148–151. doi: 10.1126/science.1070506. [DOI] [PubMed] [Google Scholar]
- 95.Jelinska C, Conroy MJ, Craven CJ, Hounslow AM, Bullough PA, Waltho JP, Taylor GL, White MF. Obligate heterodimerization of the archaeal Alba2 protein with Alba1 provides a mechanism for control of DNA packaging. Structure. 2005;13:963–971. doi: 10.1016/j.str.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 96.Raschle M, Van Komen S, Chi P, Ellenberger T, Sung P. Multiple interactions with the Rad51 recombinase govern the homologous recombination function of Rad54. J. Biol. Chem. 2004;279:51973–51980. doi: 10.1074/jbc.M410101200. [DOI] [PubMed] [Google Scholar]
- 97.Kooistra R, Vreeken K, Zonneveld JB, de Jong A, Eeken JC, Osgood CJ, Buerstedde JM, Lohman PH, Pastink A. The Drosophila melanogaster RAD54 homolog, DmRAD54, is involved in the repair of radiation damage and recombination. Mol. Cell Biol. 1997;17:6097–6104. doi: 10.1128/mcb.17.10.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muris DF, Vreeken K, Carr AM, Murray JM, Smit C, Lohman PH, Pastink A. Isolation of the Schizosaccharomyces pombe RAD54 homologue, rhp54+, a gene involved in the repair of radiation damage and replication fidelity. J. Cell Sci. 1996;109:73–81. doi: 10.1242/jcs.109.1.73. [DOI] [PubMed] [Google Scholar]
- 99.Osakabe K, Abe K, Yoshioka T, Osakabe Y, Todoriki S, Ichikawa H, Hohn B, Toki S. Isolation and characterization of the RAD54 gene from Arabidopsis thaliana. Plant J. 2006;48:827–842. doi: 10.1111/j.1365-313X.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 100.Flaus A, Martin DM, Barton GJ, Owen-Hughes T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;34:2887–2905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]