Abstract
Objective
The Wnt family may contribute to hematopoietic stem cell (HSC) maintenance in bone marrow, but many questions remain concerning mechanisms. Vascular cell adhesion molecule-1 (VCAM-1) is expressed in cellular compartments of the bone marrow and might contribute to the HSC niche, but mechanisms concerning its constitutive expression are largely unknown. We now explore the influence of Wnt signaling on cellular adhesion molecule (CAM) expression by bone marrow stromal and hematopoietic cells.
Methods
Recombinant Wnt ligands, retroviral Wnt transductions and co-cultures with Wnt secreting cells were used to analyze the effect of Wnt on adhesion molecule expression by stromal and hematopoietic cells. In vivo experiments were also done to assess the ability of Wnt3a induced, VCAM-1 deficient hematopoietic cells to engraft bone marrow.
Results
We now report that the beta-catenin dependent canonical Wnt signaling pathway negatively regulates VCAM-1 expression on two types of bone marrow cells. Wnt pathway inhibitors, Axin (intracellular) or Dkk1 (extracellular) blocked the regulation of VCAM-1 by diffusible Wnt3a. Interestingly, lipopolysaccharide (LPS) restored a substantial degree of VCAM-1 expression, suggesting functional cross-talk between Wnt and TLR4 signaling pathways. Decreasing VCAM-1 on HSC enriched Lin- Sca-1+ c-KitHi Thy1.1Lo cells by exposure to Wnt3a did not prevent their successful transplantation.
Conclusions
Our results suggest that cells comprising and residing in the HSC niche can respond to Wnt ligands and extinguish VCAM-1. This response may be important for export of hematopoietic cells. Given the known contribution of VCAM-1 to inflammation, this may represent a new avenue for therapeutic intervention.
Keywords: Wnt, beta-catenin, VCAM-1, Stromal Cells, Hematopoietic Stem Cells
Introduction
Wnt is a family of secreted glycoproteins that interact with secreted and cell membrane associated proteins. Many Wnt ligands, receptors and function modifying molecules are expressed in bone marrow, and their potential contribution to hematopoiesis has been extensively studied (1-6). However, the results have often been conflicting, and the complexity of this family of molecules has made definitive interpretations difficult. This is because Wnt knock out mice are embryonic lethal, and functional redundancy exists among the 19 Wnt ligands, 8 Fzl receptors, 2 LRP co-receptors and an assortment of Wnt mediators.
Frizzleds (Fzd's), low-density lipoprotein receptor- related proteins (LRP5/6) and Kremen are membrane associated Wnt receptors that can initiate downstream Wnt signaling. Extracellular proteins such as Dickkopf (Dkk), Wnt-inhibitory factor (WIF), secreted Fzds (SFRP) and Norrin can also associate with Wnt ligands to modulate Wnt-receptor binding activities (http://www.stanford.edu/∼rnusse/wntwindow.html) (7,8). Further, depending on the expression pattern of surface receptors and presence of intracellular Wnt pathway components, the 19 known Wnt ligands can activate canonical or non-canonical signaling pathways in a responding cell. Canonical Wnt signaling stabilizes intracellular β-catenin which can then translocate to the nucleus and interact with transcription factors. Non-canonical Wnt signaling pathways do not (normally) stabilize β-catenin, and can increase intracellular Ca2+ levels (Wnt-Ca2+) through G-protein activation or they can activate Rho/Rac GTPases to induce the JNK pathway (Wnt-JNK) (9).
In hematopoiesis, this family is implicated in stem cell maintenance, development of hematopoietic cells and even in immune responses (3,4,10). Particular emphasis has been placed on the canonical signaling pathway, where β-catenin is stabilized as a result of surface Wnt receptor engagement and glycogen synthase kinase 3 beta (GSK3β) inhibition (10). For example, conditional deletion of β-catenin in one study, and both β- and γ-catenin in two additional reports, did not compromise hematopoietic stem cell (HSC) functions (11-14). However, there were indications that canonical Wnt signaling was not totally ablated by these protocols (14). Furthermore, HSC integrity was diminished by another means of conditional β-catenin targeting (15). Recombinant or feeder cell produced Wnts, such as Wnt3a or Wnt5a, have been used to support HSC expansion in culture (1-3), but other studies concluded that the influence is more inhibition of differentiation than growth promotion (16,17). Introduction of artificially stable β-catenin inhibited lineage progression of HSC in culture, and even reversed early steps in hematopoiesis (18,19). Furthermore, strong β-catenin transgenes caused marrow failure in mice (20,21). While further investigating these issues, we discovered that Wnt signaling altered the morphology of cultured stromal cells, and we now report that it negatively regulates expression of VCAM-1.
VCAM-1 is a member of the Ig-superfamily of transmembrane proteins that functions as an adhesion ligand for integrins such as VLA-4 (α4 β1), α4 β7 and α9 β1 (22,23). While VCAM-1 levels are markedly elevated on inflamed endothelial cells, it is constitutively made by stromal, endothelial and other cells in bone marrow (24-26). Antibodies to VCAM-1 or VLA-4 detached hematopoietic cells from stromal cells in long-term bone marrow cultures, suggesting this ligand/cell adhesion molecule (CAM) pair is also responsible for retention of immature lymphoid cells in bone marrow (27). However, knock out experiments indicate that only the later stages of B lymphopoiesis are VCAM-1 dependent. That is, pre-B and immature B cell numbers are reduced in the marrow, and elevated in the circulation (28). Antibody blocking experiments implicated VCAM-1 in the homing or engraftment of transplanted HSC (29). VCAM-1 also mediates rolling of hematopoietic progenitor cells on marrow endothelial cells (30). Studies with gene targeted mice indicated that VCAM-1 on endothelial cells is particularly important for homing of hematopoietic cells to the bone marrow (26,31).
VCAM-1 is expressed on long-term repopulating HSC in bone marrow of mice, and this protein is eventually lost as they become hematopoietic progenitors (32,33). However, little is known about how VCAM-1 levels are controlled in the bone marrow. The VCAM-1 promoter contains binding sites for NF-κB that may control up-regulation in response of endothelial cells to inflammatory cytokines (34-36). Furthermore, it is interesting that lithium treated patients also have increased numbers of CD34+ hematopoietic cells in the circulation (37). Lithium and other GSK3β inhibitors function like agonists of canonical Wnt signaling, and there is a report that GSK3β inhibitors blocked the induction of VCAM-1 on endothelial cells (38). We now show that Wnt3a, a prototypic canonical Wnt pathway ligand, and not Wnt5a, a prototypic non-canonical Wnt ligand, acts to extinguish VCAM-1 on two categories of marrow cells. Furthermore, this suppression in VCAM-1 is mediated through activated β-catenin in the corresponding cell types.
Materials and Methods
Mice
Mice, C57BL6 (B6; CD45.2 and CD45.1 alloantigen) and B6-Thy1.1 (PL), were bred and maintained in the OMRF Laboratory Animal Resource Center (LARC).
Isolation of stem cell enriched hematopoietic cells
Bone marrows from adult, 3-5 months old PL or C57BL6 mice were flushed with 3% fetal calf serum (FCS) from femurs, tibias and humeri. Stem cell enriched hematopoietic cells were isolated as described before (39). The Lineage negative, c-Kit antigen High, Sca-1 antigen positive and Thy1.1 antigen low fraction was sorted from bone marrow of PL mice. LSK from C57BL6 mice were isolated from the Lin- c-KitHi Sca-1+ gated population. Unless otherwise mentioned, all antibodies were purchased from BD Biosciences and were titrated to obtain optimal results. Propidium Iodide (PI) was used in all isolations to stain dead cells. Cells were sorted on either FACSAria (BD Biosciences, San Jose, CA) or MoFlo (DakoCytomation, Glostrup, Denmark) flow cytometers. Post-sort analyses confirmed isolation purities, and typically >93% purity were obtained after double sorting.
Retrovirus production and transduction
Wnt3a, Wnt5a (40) (gift from Dr. S. Jones, Univ. of Massachusetts, USA), Dkk1 (gift from Dr. F. J.T. Staal, Erasmus Medical Center, Rotterdam, Netherlands), axin, wild-type βcatenin (WTβcat) or constitutively active βcatenin (mmβcat) plasmids were individually cloned into the multiple cloning site of LZRS-IRES-GFP retroviral vector (18) by restriction digestion and ligation reactions. These inserted and empty (control) vectors were transduced into Plat-E (41) virus packaging cell line (gift from Dr. Mark Coggeshall's laboratory, OMRF, USA) with the FuGENE 6 (Roche, Indianapolis, IN) method, and transduced cells were selected by 1μg/ml Puromycin, 10μg/ml Blasticidin, and by sorting for GFP+ transduced cells. Virus containing supernatants were harvested 20 hrs after changing to fresh media [X-VIVO 15 medium (Lonza, Walkersville, MD) containing 1% detoxified BSA (StemCell Technologies, Vancouver, BC), 2 mM L-glutamine, 5 × 10-5 β-ME, 100 U/ml Penicillin and 100 mg/ml Streptomycin] and used immediately for transduction, or stored at -70°C for later use.
Transduction of the stem cell enriched LSK fraction (isolated from B6 mice), and OP9 stromal cell was similar to as described before (19). Following transduction GFP+ OP9 stromal cells were sorted, cultured and then resorted for 4 cycles, and stable GFP+ OP9-Ctrl (empty vector transduced), OP9-W3A (Wnt3a transduced), OP9-W5A (Wnt5a transduced), OP9-Dkk1 (Dkk1 transduced), OP9-Axin/W3A (Wnt3a and axin double transduced), OP9-WTβcat (wild-type βcatenin transduced) or OP9-mmβcat (constitutively active βcatenin transduced) cell lines were generated. Transduced stromal cells made the expected proteins as determined by western blotting (data not shown).
Cell lines, cultures and flow cytometry
OP9 stromal cells have been described before and were used in transductions and co-cultures (42). Stromal cells were maintained and passaged in OP9 medium [α-MEM medium (Invitrogen, Grand Island, NY), 10% FCS, 2 mM L-glutamine, 100 U/ml Penicillin and 100 mg/ml Streptomycin]. Co-cultures with other transduced stromal cells were also carried out in regular OP9 medium, typically for 18 - 48hrs.
For stem cell co-culture, two thousand HSC enriched hematopoietic cells were double sorted and seeded, in triplicates, onto monolayers of OP9-Control or OP9-W3A cells in wells of 24-well plates as described before, for 5 to 7 days (19). Fluorescent labeled anti-CD45.2 (clone 104, eBioscience) antibody was used to positively distinguish hematopoietic cells from stromal cells.
For stroma free cultures of Wnt3a or control transduced stem cells (LSK), retrovirally transduced LSK cells were sorted for GFP+ expression and cultured in lymphoid cell culture, as described before (39)
The same VCAM-1 antibody (MK-2 clone) was used for all fluorescence analyses (24) and is available from Southern Biotech (Alabama, USA).
In all cases, where flow cytometry was used, PI was used to exclude dead cells. Flow cytometry was performed on a BD FACS LSR-II (BD Biosciences), and data analysis was done with BD FACSDiva (BD Biosciences). All histograms were made using FlowJo software (Tree Star). These analyses present results from independent experiments, where flow cytometer gains were set differently over a two year period. Therefore, histograms and representative bar graphs should not be compared between different experiments. Normalized MFI values in figures were calculated relative to control vector transduced or un-transduced OP9 cells (set at 100%).
Reagents
Lithium Chloride (Sigma Aldrich, St. Louis, MO), recombinant Wnt3a/ rWnt3a (R&D Systems, Inc., Minneapolis, MN), recombinant Wnt5a/ rWnt5a (R&D Systems, Inc.), LPS (Sigma Aldrich), CpG ODN (InvivoGen, San Diego, CA) were suspended in PBS+ BSA (0.1%) and used at the indicated concentrations.
Microscopy
Co-cultures were analyzed with a Zeiss Axioplan 2i microscope with a 64×/1.4 NA Plan Achromat objective. Photomicrographs were made using an AxioCam MRc color camera (Zeiss), and AxioVision 3.1 software (Zeiss). A Zeiss Axiovert 200m Inverted Fluorescent Microscope was used to take pictomicrographs and analyze cell size of OP9-Ctrl or OP9-W3A cells.
Immunofluorescence
Stromal cells were grown in chamber slides for two days. Briefly, cells were fixed in 2% PFA for 1 hr at 4°C, washed and transferred to cold (-20°C) methanol for 20 min on ice and then washed again with PBS. Blocking was done with 1% BSA in PBS (staining buffer) at room temperature for 20 min, followed by gentle washing. Staining with VCAM-1 (MK-2, Biotin conjugate, 1:100) antibody was carried out for one hour on ice, followed by Cy-5 (Streptavidin) labeling for 30 min. Cells were then washed, and DAPI was included as a nuclear stain. Stained cells were finally mounted using Slow-fade anti-fade kit (Molecular Probes, Eugene, OR). Cells were imaged in the OMRF imaging facility on a Zeiss LSM-510META Laser Scanning Confocal Microscope using C-Apochromat 40×/1.2 W corr objective. Zeiss LSM Image Examiner (Ver.#3.2.0.70) software was used to evaluate and prepare the photomicrographs.
Irradiation procedure
Recipient mice were given a single 6.5 Gy (sub-lethal) dose of radiation from a 137Cs source (Mark I gamma irradiator; J. L. Shepard and Associates, Glendale, CA).
Bone Marrow Transplantation
Mice were anesthetized with isofluorane (Isosol; Vedco, St Joseph, MO), and cell populations were infused intravenously through eye vein injections. Hosts were 6- to 12-week-old B6-CD45.1 mice. Donor cells were pre-cultured on OP9-W3A or OP9-control (empty vector transduced) cells, and were from 4- to 10-week-old B6- Thy1.1 mice expressing the CD45.2 alloantigen. Spleen, thymus, and marrow from two femurs were collected separately from recipient mice, and donor-derived cells were assayed by flow cytometry as described earlier. Antibodies used for staining cellular populations were as described in the section for isolation of HSC enriched cell fractions.
Analysis of gene expression
Semi-quantitative and real-time RT-PCR was used to assess mRNA expression of VCAM-1 on HSC enriched cell fractions and has been described before (19). Primer sequences and amplification conditions are available from the authors on request.
Real time PCR to assess expression of extracellular matrix and adhesion molecule genes on OP9-Control (empty vector transduced) and OP9-W3A cellular populations was done using pre-optimized SYBR-Green 96 well plate pathway specific RT2 Profiler™ primer-array (Extracellular Matrix and Adhesion Molecule Array, Superarray, Frederick, MD). mRNAs were isolated from OP9-Control or OP9-W3A transduced cells and converted to cDNA, as described above. RT-PCR was done using reagents supplied with the primer-array and as described in the array user manual using ABI PRISM 7500 (ABI). A melting curve program was run immediately after the PCR for the entire 96 well plate, and only wells that showed a single peak (indicates specific amplification) following melting were included in analysis. All wells were also visually inspected for signs of evaporation. Data analysis for gene expression was calculated as 2-ΔΔCt (threshold cycles) values with β-actin as endogenous house keeping control.
Statistical analyses
Statistical significances of differences between groups were assessed with the Students t-test or ANOVA followed by Bonferroni post-test to compare replicate means. The statistical calculations were performed using GraphPad Prism software (Ver 5.01, GraphPad, San Diego, CA). A result was considered significant if the p-value was <0.05.
Results
Wnt3a alters cellular morphology and adhesion molecule expression
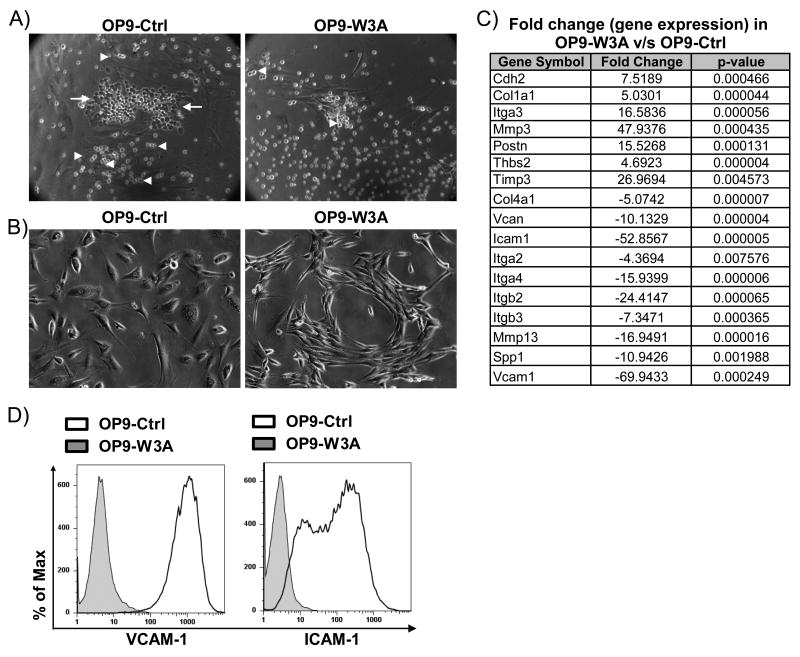
During co-culture with stromal cells, hematopoietic stem cells (HSC) crawl beneath the stromal cells to form “cobblestone areas”. While exploring the contribution of Wnt molecules to lymphopoiesis, we observed that hematopoietic cells formed very few cobblestones when cultured on Wnt3a transduced rather than wild-type stromal cells that do not make Wnt3a (Figure 1A, and data not shown). Furthermore, the hematopoietic cells seemed to be less self-adhesive and tended not to make clusters on the surfaces of Wnt3a secreting cells (Figure 1A). The morphology of the Wnt3a transduced stromal cells was also substantially altered; most had a spindly appearance and did not spread well on the culture dishes (Figure 1B). Wnt3a transduced OP9 cells were considerably more narrow than vector control OP9 cells when measured across the smallest axis (average of 22.4 microns versus 60 microns). Furthermore, slightly higher cell yields (average of 23% more cells) were consistently obtained when stromal cells were transduced with the Wnt3a vector as compared to the vector control.
Figure 1.
Wnt3a alters stromal cell morphology and expression of adhesion molecule genes. (A) The phase contrast photomicrographs illustrate hematopoietic cells in co-culture with Wnt3a secreting or vector control OP9 stromal cells. White arrows indicate hematopoietic cells crawling underneath stromal cells, white arrowheads indicate hematopoietic cells clustered together.(B) Phase contrast photomicrographs illustrate that transduction with Wnt3a reduces spreading and causes OP9 cells to become spindle shaped. (C) Real-time RT-PCR (SYBR) showing fold change in gene expression patterns of extracellular matrix and cell adhesion molecules in OP9-W3A relative to OP9-Ctrl cells. Gene symbols are indicated for molecules that were more than 3 fold different (and statistically significant, n=3) on OP9-W3A compared to OP9-Control (empty vector transduced). A negative value in fold change indicates lower gene expression after Wnt3a transduction. (D) Flow cytometry histograms show VCAM-1 or ICAM-1 protein levels on OP9-Ctrl (Black open line) or OP9-W3A (Grey shaded overlay) cells. Shown is one representative of four (A,B, D) or three (C) experiments.
These findings suggested that Wnt3a might alter the expression and/or function of cell adhesion molecules (CAMS) in one or both of these cell types. Therefore, cDNA was prepared from OP9-W3A and OP9-control stromal cells and compared using a real-time PCR array for 84 CAMS and extracellular matrix molecules. Significant (>3 fold and p<0.05) differences in gene expression were found for at least 17 genes (Figure 1C). VCAM-1 was of particular interest, and was down-regulated more than 60 fold in Wnt3a transduced stromal cells (Figure 1). This result was confirmed by RT-PCR (data not shown) and extended to surface protein levels by FACS (Figure 1D). ICAM-1, MMP13, integrins alpha 2, beta 2 and beta 3 were also down-regulated in Wnt3a transduced stromal cells (Figure 1C). These results suggest that Wnt3a has the potential to significantly alter expression of molecules assocated with adhesion and migration.
Soluble Wnt3a inhibits VCAM-1 expression
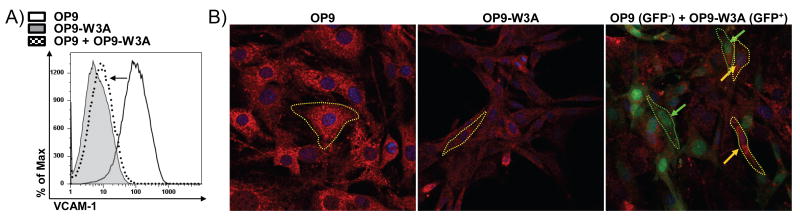
Wnt molecules normally act short-range, and we asked whether this phenenomenon could be attributed to secreted Wnt3a ligand. Accordingly, non-transduced OP9 (GFP-) cells that were VCAM-1Hi were grown together with VCAM-1Low/- Wnt3a secreting OP9 (GFP+) cells for two days. Flow cytometry revealed that surface VCAM-1 was greatly reduced on the GFP- OP9 cells as compared to OP9 cells that were not co-cultured (Figure 2A). Further, confocal imaging showed that VCAM-1 intensities were low on OP9-W3A cells (compare OP9 to OP9-W3A in Figure 2B), and during co-culture with Wnt3a producing stroma, GFP- OP9 cells also decrease their VCAM-1 staining (Figure 2B). The morphologies of OP9 cells also changed when they were exposed to secreted Wnt3a in these co-cultures. That is, they were not flattened on the plate, and assumed spindle shapes similar to OP9-W3A cells (Figure 2B). Therefore, Wnt3a produced by transduced stromal cells caused two, possibly unrelated changes in non-transduced cells. Their morphology was altered and VCAM-1 expression was extinguished.
Figure 2.
Wnt3a producers alter morphology and surface VCAM-1 levels of neighboring cells in co-cultures. (A) Flow cytometry histogram of OP9 (GFP-, VCAM1Hi, solid line), OP9-W3A (GFP+, VCAM1Lo/-, grey filled) and OP9 (GFP-, dotted line) co-cultured with OP9-W3A (GFP+) after two days. The arrow emphasizes the decrease in VCAM1 surface intensity. (B) OP9 (GFP-, left), OP9-W3A (GFP+, center) or OP9 (GFP-) cells were grown with OP9-W3A (GFP+, right). The cells were stained and confocal images were prepared to show VCAM1 (Red, all three panels), DAPI (Blue, all three panels) or GFP (Green, right panel only). The yellow arrows indicate OP9 (GFP-) cells and the green arrows indicate OP9-W3A (GFP+) cells (right panel only). The borders of typical OP9-W3A (green) or OP9 (yellow) cells were outlined with dotted lines.
The canonical Wnt signaling pathway inhibits VCAM-1 expression
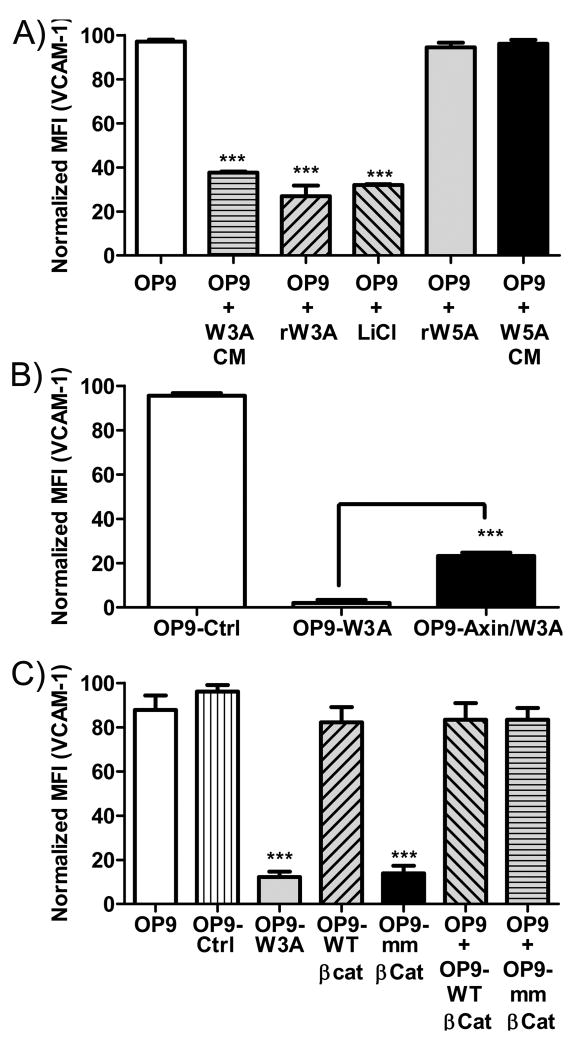
We then explored which of three known Wnt signaling pathways were responsible for regulating CAM expression. Lithium Chloride (LiCl) inhibits intracellular GSK3β and consequently mimicks canonical Wnt signaling (43). Therefore, OP9 cells were treated for two days with LiCl (5×10-2 mol/L), conditioned medium from Wnt3a producing OP9 cells or recominant Wnt ligands (5ng/ml) (Figure 3A). While LiCl and Wnt3a reduced surface densities of VCAM-1, the non-canonical Wnt5a ligand had no influence (Figure 3A). Note that recombinant or soluble stromal cell released Wnt3a was much less effective than seen in the co-culture experiments described above. That is consistent with the idea that these matrix binding factors act short-range and may be labile. Axin functions as an inhibitor of canonical Wnt signaling because it facilitates phosphorylation of intracellular β-catenin by GSK3β or caesin kinase I (CKI) resulting in its degradation (44,45). Transduction of OP9-W3A cells with an axin construct partially rescued the block on VCAM-1 expression (Figure 3B).
Figure 3.
Canonical Wnt signaling inhibits VCAM-1 expression. OP9 or vector transduced OP9 cells were cultured in medium alone, medium with the indicated molecules or in co-culture with the indicated transduced OP9 cells. The three panels indicate averaged and normalized (to control) VCAM-1 mean fluorescence intensities (MFI) determined by flow cytometry. (A) Normalized MFIs on OP9 cells were measured by FACS. CM indicates conditioned media from indicated cell lines, while rW3A and rW5A represent recombinant forms of Wnt3a or Wnt5a respectively. (B) Normalized MFIs are shown for control vector transduced OP9 (OP9-Ctrl), OP9-W3A or OP9-W3A cells transduced with an axin construct (OP9-Axin/Wnt3a). (C) Normalized surface VCAM-1 MFI from flow cytometry analysis are given for OP9 cells transduced with a wild type (WT)-β-catenin construct, a constitutively active (mm)-β-catenin construct or control vector (OP9-Ctrl). Surface VCAM-1 expression on OP9 (GFP-) cells after co-culture with OP9-WT-β-Cat (GFP+) or OP9-mm-β-Cat (GFP+) are also given. Shown is one representative experiment of four that produced similar results. Error bars indicate standard errors from replicate wells set-up at the same time and asterisks indicate statistical significances, *** (p<0.0001).
Canonical Wnt signaling involves the stabilization of intracellular β–catenin (7,8), so we transduced cells with wild-type or constitutively active β-catenin constructs. VCAM-1 levels on the wild-type β–catenin transduced cells were similar to those given the control retrovirus (Figure 3C). In contrast, VCAM-1 surface expression was considerably reduced in cells transduced with the constitutively active β-catenin construct (Figure 3C). Diffusible molecules were not involved in this case, because there were no changes in VCAM-1 when OP9 cells were cultured together with OP9-WT (wild-type)-β-catenin or OP9-mm (constitutively active)-β-catenin transduced cells (Figure 3C). These results indicate that signaling via the canonical Wnt pathway inhibits VCAM-1 expression.
Dkk1 prevents Wnt3a mediated inhibition of VCAM-1
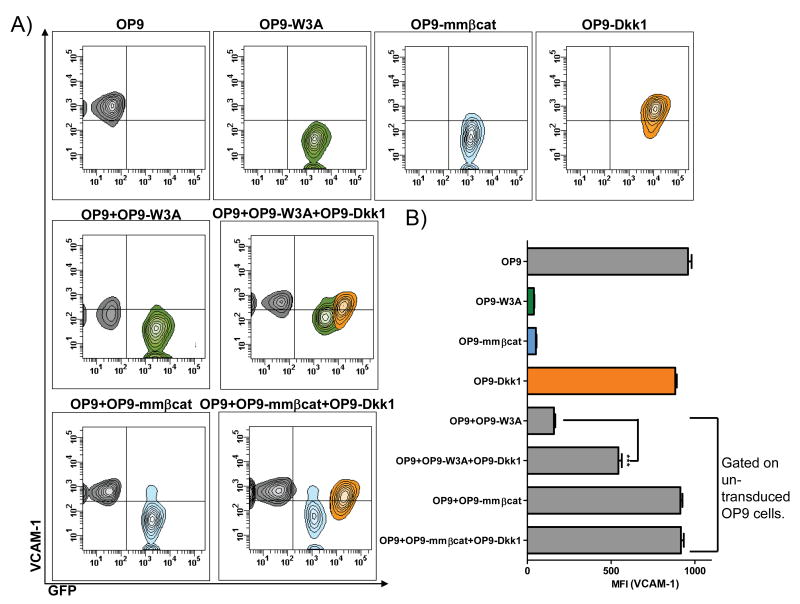
Dkk1 is a soluble protein that blocks Wnt ligand interactions with LRP5/6 co-receptors and thus prevents downstream signaling (46). Therefore OP9 stromal cells were transduced with a Dkk1 construct and used as another means to assess diffusible versus intracellular Wnt related events. VCAM-1 surface levels on OP9-Dkk1 cells were similar to control OP9 cells (Figure 4A and B), indicating that these stromal cells do not normally make significant quantities of Wnt. We next grew Dkk1 secreting stroma together with either Wnt3a secreting stroma or with stroma that was transduced with constitutively active -β-catenin. Fortunately, Dkk-1 transduced cells had substantially more GFP than Wnt3a transduced cells, allowing the two to be distinguished by flow cytometry (Figure 4A and B). Soluble Dkk1 significantly reversed the Wnt3a mediated inhibition of surface VCAM1 (quantified in Figure 4B). As expected, Dkk1 did not increase the low VCAM-1 present on stromal cells transduced with constitutively active β-catenin. Further, un-transduced (GFP-) OP9 cells were also co-cultured together with Wnt3a secreting OP9-W3A (GFP+) cells along with Dkk1 secreting OP9-Dkk1 (GFP+) cells (Figure 4A and B). In this circumstance, soluble Dkk1 prevented Wnt3a mediated inhibition of VCAM-1 on the GFP‐ cell population. In fact, Dkk1 also increased the basal surface VCAM-1 levels on GFP+ Wnt3a secreting OP9-W3A cells. These results demonstrate that Dkk1 can modulate VCAM-1 levels by interfering with Wnt3a-receptor interactions, but has no influence on intracellular signaling events.
Figure 4.
Dkk1 reverses Wnt3a mediated inhibition of VCAM-1. (A) Pseudo-colored contour plots show levels of VCAM-1 and GFP protein in the indicated OP9 or transduced OP9 cells held alone or after co-culture. (B) Averaged MFI of VCAM-1 are shown for the stromal cells cultured alone (top four bars), or when gated on un-transduced OP9 cells recovered from co-cultures (bottom four bars). The bars are color coded to indicate the populations gated in the contour plots for quantification of VCAM-1 levels. Shown is one representative experiment of four that produced similar results. Error bars indicate standard errors from replicate wells set-up at the same time and asterisks indicate statistical significances, *** (p<0.0001).
Wnt5a can in some instances inhibit canonical signaling by preventing nuclear β-catenin from interacting with key transcription factors (47,48). In other circumstances, it can function as an agonist of the same signaling pathway (47). However, we found that VCAM-1 levels and the appearance of OP9 cells did not change when they were transduced to secrete Wnt5a. Furthermore, these cells did not reverse the suppression of VCAM-1 when co-cultured with either Wnt3a secreting or constitutively active β-catenin transduced stromal cells (data not shown). In another study, we found that the Wnt5a produced by these transduced cells was biologically active when present in HSC co-cultures (manuscript submitted). The OP9 stromal cells may lack a functional Wnt5a receptor. Alternatively, non-canonical Wnt signaling may not be relevant to VCAM-1 expression.
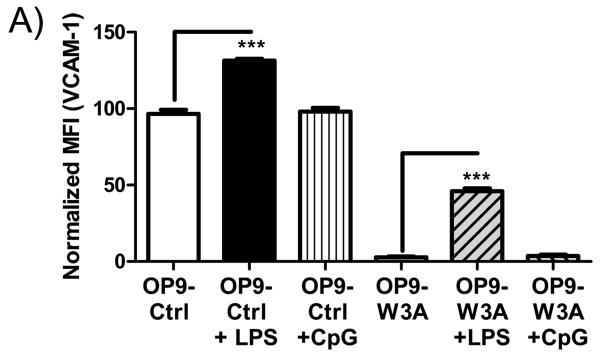
Wnt3a mediated down-regulation of VCAM-1 is reversed by exposure to a TLR4 ligand
Toll-like receptors (TLR) are important mediators of inflammation, and the TLR4 ligand LPS is known to stimulate VCAM-1 expression in endothelial cells via an NF-κB dependent signaling pathway (49). Furthermore, a GSK3β inhibitor that would simulate Wnt signaling prevented IκB-α degradation and decreased NF-κB interactions with DNA (38). Consistent with those reports, LPS (1μg/ml) restored a substantial degree of VCAM-1 expression to Wnt3a transduced OP9 cells (Figure 5). The TLR9 ligand CpG ODN (1μg/ml) had no influence. These findings would be consistent with cross competition between NF-κB mediated signaling elicited by TLR4 and canonical Wnt signaling stimulated by Wnt3a.
Figure 5.
LPS reverses Wnt3a mediated inhibition of VCAM-1. (A) Normalized MFI values from flow cytometry were averaged and shown when OP9-control (empty vector transduced) or OP9-W3A cells were cultured in medium alone, with 1μg/ml LPS or 1μg/ml CpG for 18hrs. Shown is one representative experiment of four that produced similar results. Error bars indicate standard error from replicate wells set-up at the same time and asterisks indicate statistical significance, *** (p<0.0001).
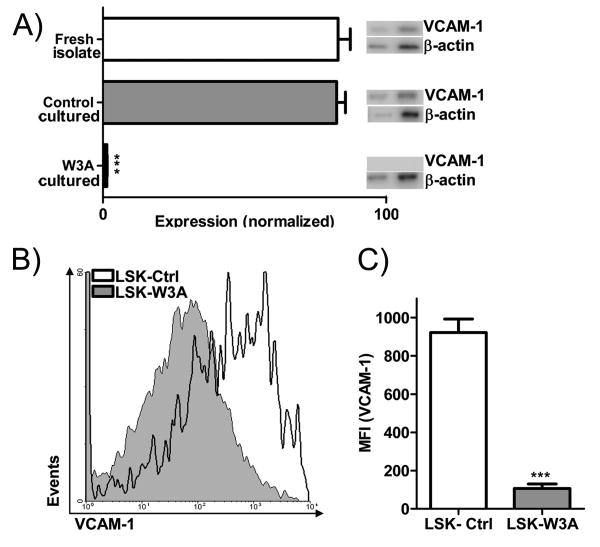
Wnt3a can inhibit VCAM-1 expression in hematopoietic cells
Stromal cells contribute to a specialized niche for HSC in the bone marrow, and both of these cell types express VCAM-1 (24,32). Therefore, we asked if this adhesion molecule was also subject to Wnt mediated regulation in hematopoietic cells. The HSC enriched fraction was cultured on Wnt3a secreting or control stroma overnight and sorted to purity before further analysis. Semi-quantitative RT-PCR showed that VCAM-1 expression by these hematopoietic cells was completely inhibited on Wnt3a secreting stroma (Figure 6A). Further, we transduced the stem cell enriched LSK fraction with Wnt3a-IRES-GFP or control IRES-GFP retroviral constructs, sorted GFP+ cells and cultured them in serum-free, stromal cell-free conditions. Flow cytometry revealed lower surface VCAM-1 protein levels in Wnt3a transduced, compared to controls (Figure 6B and 6C). One additional experiment involved co-culture of a VCAM-1 positive endothelioma cell line together with Wnt3a producing OP9 cells. Again, VCAM-1 levels were significantly reduced (data not shown). These results show that canonical Wnt signaling can alter adhesion molecule expression in multiple cell types.
Figure 6.
Wnt3a blocks VCAM-1 expression on a HSC enriched fraction. (A) VCAM-1 gene expression (by semi-quantitative RT-PCR) is shown normalized to actin for freshly isolated HSC enriched cells from bone marrow, the same hematopoietic cells cultured overnight on vector control stroma or on Wnt3a secreting stroma. Also shown are the corresponding PCR products at 28 and 31 cycles for β-actin and 35 and 38 cycles for VCAM-1. (B) Stem cell enriched LSK were transduced with vector control or Wnt3a containing IRES-GFP retroviruses and cultured stromal cell-free with cytokines. Shown are the flow cytometry analyses of VCAM-1 staining in cells gated for HSC rich Lin- Sca-1Hi c-Kit Hi fraction after 5 days of culture. The grey filled curve illustrates LSK transduced with Wnt3a, while the black line shows LSK transduced with the control vector. (C) Averaged MFI (VCAM-1) are shown for the LSK represented in (B). Shown is one representative experiment of three that produced similar results. Error bars indicate standard error and asterisks indicate statistical significance, *** (p<0.0001).
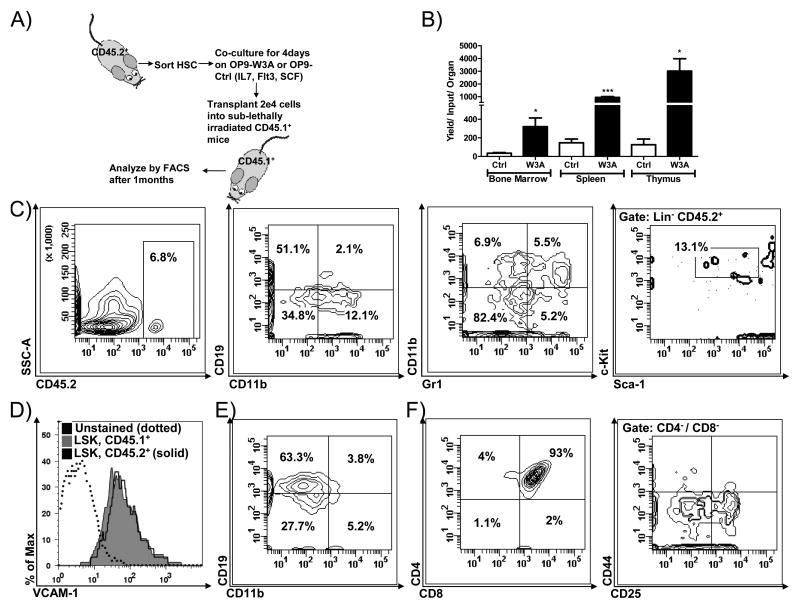
Loss of VCAM-1 does not compromise hematopoietic cell transplantation
Previous studies have implicated VCAM-1 in the homing and/or engraftment of transplanted stem cells (26,29,31). Furthermore, it has also been reported that recombinant Wnt3a presented along with cyclodextrin allowed functional stem cells from Bcl-2 transgenic mice to be maintained in long term cultures containing only one growth factor (3). Therefore, we sorted HSC enriched Th1.1Lo LSK from CD45.2+ mice and seeded them on OP9-Control or OP9-W3A stromal cells along with supporting cytokines for 5 days (Figure 7). These conditions lead to complete extinction of VCAM-1 (Figure 6A/B above, and data not shown), and we assessed if they were still competent to engraft sub-lethally irradiated CD45.1+ mice. FACS analysis one month later revealed that Th1.1Lo LSK exposed to stromal cell-presented Wnt3a retained the ability to generate hematopoietic cells following transplantation. In fact, yields of CD45.2+ donor type cells were much higher than when they were cultured on control OP9 stromal cells (Figure 7B). Chimerism was very high in the thymus, and particularly in the CD4+ CD8+ subset. The spleen and marrow contained donor-type CD19+ B lineage lymphocytes and CD11b+ myeloid cells as well as CD4+ and CD8+ T cells. While cultured HSC enriched LSK lacked VCAM-1 at the time of transplantation, we recovered small numbers of donor-type cells that expressed this CAM among the HSC rich LSK bone marrow fraction (Figure 7D). The VCAM-1 levels appeared to be similar to those on the corresponding CD45.1+ cells of the transplant recipients (Figure 7D). These conditions did not support retention of transplantable cells after longer-term (8 days) co-culture (data not shown). We conclude that temporary Wnt mediated down-regulation of VCAM-1 on hematopoietic cells does not prevent them from engrafting and differentiating following transplantation.
Figure 7.
Wnt3a preserves the ability of hematopoietic cells to engraft and differentiate even after 5 days of co-culture. (A) A schematic of the experimental design is given. (B) CD45.2+ cells were recovered from the engrafted tissues and identified by gating one month after transplantation. Yields per transplanted cell per organs (two femurs) were calculated. (C) Flow cytometry contour plots show engraftment and differentiation from Wnt3a co-culture cells in bone marrow. CD19 and CD11b versus Gr-1 discern B and myeloid lineages (two middle panels), while the right panel shows cells with stem cell-like LSK characteristics. (D) The VCAM-1 levels on donor (CD45.2+) and host (CD45.1+) cells recovered from bone marrow and gated on the stem cell rich LSK fraction are shown. (E) Donor type B lineage and myeloid cells in spleen and (F) lymphocytes in the thymus were characterized according to expression of CD4 and CD8 surface proteins. The far right panel in (F) was gated on CD4- CD8- cells and shows progression through CD25+ CD44- DN3 and CD25- CD44- DN4 thymic subsets. Shown is one representative experiment of two that produced similar results, using 3 mice per transplant group. Error bars indicate standard error and asterisks indicate statistical significance, * (p<0.05), *** (p<0.0001).
Discussion
We discovered that the morphology of cultured stromal cells markedly changed when they were exposed to Wnt3a, and real-time PCR analyses identified several adhesion molecules that appeared to be either positively or negatively regulated. VCAM-1 was of particular interest because of its known roles in hematopoiesis and inflammation. A series of observations suggested that VCAM-1 expression is negatively regulated by the canonical Wnt signaling pathway, and this was reversed by exposure to a TLR4 ligand. Stromal cells contribute to niches that support hematopoietic cells in bone marrow, and it was therefore interesting to find that VCAM-1 on a HSC enriched cell fraction was also subject to Wnt regulation. However, transient loss of VCAM-1 did not compromise the ability of hematopoietic cells to be transplanted. The findings suggest new roles for Wnt in regulating adhesion molecule expression on cells important for hematopoiesis and inflammation.
Antibody blocking and conditional knock out mouse experiments indicated that VCAM-1 might be important for positioning stem and progenitor cells within the bone marrow (29,31,33). Further investigation revealed that efficient engraftment of transplanted HSC and retention of newly formed lymphocytes in bone marrow are VCAM-1 dependent processes (28,50). The integrins that recognize VCAM-1 are dynamically regulated, and this might cause hematopoietic cell movement within and from the bone marrow (51-53). VEGF, LPS, thrombin and inflammatory cytokines such as TNFα and IL-13 all increase VCAM-1 expression on endothelial cells (36,54-57). However, little information was available about control of the normally high VCAM-1 levels in the marrow. Our culture studies show that Wnt3a signals can diminish VCAM-1 expression by stromal and hematopoietic cells. It will now be important to determine if this has a role in normal hematopoiesis.
At least three intracellular signaling pathways can be utilized by the 19 known Wnt ligands (7). All of our findings are consistent with the canonical pathway that is both axin and GSK3β dependent and culminates in stabilization of β -catenin. That is, VCAM-1 was down-regulated by the canonical Wnt3a ligand but not Wnt5a. Transducing the same cells with axin blocked this response, and VCAM-1 was extinguished by introduction of constitutively active β-catenin. Finally, LiCl that is known to inhibit GSK3β and thus mimic canonical signaling also decreased surface VCAM-1 levels.
Wnts are known to bind matrix molecules and function short-range (58-61). Individual Wnt3a expressing stromal cells, but not cells with constitutively active β -catenin, were able to influence neighboring stromal and hematopoietic cells, diminishing their ability to express VCAM-1. Dkk1 functions as a soluble Wnt inhibitor by associating with Wnt co-receptors LRP5/6 or Kremen (46). While Dkk1 reversed the effects of secreted Wnt3a in our cultures, it had as expected no influence on constitutively active β -catenin transduced cells. VCAM-1 was unchanged on Dkk1 transduced OP9 cells. This indicates that these cultured stromal cells either make maximum amounts of VCAM-1 or do not produce sufficient Wnts to constitute an autocrine signaling pathway.
The promoter region of the VCAM-1 gene contains several NF-κB binding sites, and the NF-κB signaling pathway has been very well characterized in endothelial cells (34,35,62). Of particular relevance to our findings, the GSK3β inhibitor TDZD-8 prevented IκB-α degradation, decreased NF-κB interactions with DNA and inhibited the TNF-α mediated induction of VCAM-1 in endothelial cells (38). Thus, stimulating normally VCAM-1 negative cells to express this adhesion molecule was blocked by artificial Wnt signaling. We now report that VCAM-1 positive cells down-regulate this molecule in response to Wnt3a or introduction of constitutively active β-catenin. Moreover, this extinction of VCAM-1 expression was reversed by exposure to LPS, a TLR4 ligand that is known to induce NF-κB. OP9 cells are similar to mesenchymal stem cells which lack TLR9, accounting for their non-responsiveness to CpG ODN (63,64). As another possible coupling of TLRs and Wnt, LPS has been reported to induce production of the non-canonical Wnt5a ligand in macrophages (65). However, we found no role for Wnt5a in regulating VCAM-1.
Stem cells also express VCAM-1, and rapidly lose it as they differentiate to become lymphoid progenitors (33). Thus, this molecule might be involved in determining HSC residence in, or migration from discrete regions of the marrow. Tie2-Cre-mediated VCAM-1 deletion in adult murine fibroblast or endothelial like cells resulted in increased circulating progenitor cells (26). We show here that VCAM-1 gene expression on a HSC enriched fraction decreased dramatically within 18hrs of co-culture on stroma producing Wnt3a. Further, autocrine production of this ligand by transduced LSK also had decreased surface VCAM-1 expression. Exposure to Wnt3a producing stromal cells enhanced rather than compromised the ability of hematopoietic cells to be transplanted, and we recovered donor-type VCAM-1+ LSK from recipients. These findings are consistent with reports that recombinant Wnt3a retained stem cell competence for extended periods in culture (3). Furthermore, conditional loss of VCAM-1 in the hematopoietic system did not alter numbers of circulating progenitors or homing to hematopoietic tissues (31).
VCAM-1 is thought to contribute to pathological changes in diseases as disparate as diabetes, allergy, arthritis, inflammatory bowel disease, lupus nephritis and malignancies (66-72). For example, blocking antibodies to VCAM-1 or ICAM-1 decreased experimentally induced intestinal inflammation in mice (73). VCAM-1 might also play a role in inflammatory joint disease by recruiting leukocytes and activating lymphocytes in affected tissues (74,75). The CAM also participates in transplant rejection (76,77). Methods for blocking VCAM-1 expression are also being clinically tested with a view to treating atherosclerosis (78). Our new findings indicate that canonical Wnt signaling can regulate CAM expression, raising the possibility that Wnt pathway manipulation might be used to diminish inflammation in some circumstances.
Acknowledgments
This work was supported in part by grant AI 058162 from the National Institutes of Health as well as private institutional funds. We very much appreciate the expert assistance of Karla Garrett.
Reference List
- 1.Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W. A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood. 1997;89:3624–3635. [PubMed] [Google Scholar]
- 2.Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood. 1998;92:3189–3202. [PubMed] [Google Scholar]
- 3.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, III, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, O'Riordan M, Okamura R, Devaney E, Willert K, Nusse R, Grosschedl R. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity. 2000;13:15–24. doi: 10.1016/s1074-7613(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 6.Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22:849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- 7.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 8.Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 9.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 10.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 12.Cobas M, Wilson A, Ernst B, Mancini SJ, Macdonald HR, Kemler R, Radtke F. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 14.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, Huelsken J, Held W. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamane T, Kunisada T, Tsukamoto H, Yamazaki H, Niwa H, Takada S, Hayashi SI. Wnt signaling regulates hemopoiesis through stromal cells. J Immunol. 2001;167:765–772. doi: 10.4049/jimmunol.167.2.765. [DOI] [PubMed] [Google Scholar]
- 17.Dosen G, Tenstad E, Nygren MK, Stubberud H, Funderud S, Rian E. Wnt expression and canonical Wnt signaling in human bone marrow B lymphopoiesis. BMC Immunol. 2006;7:13. doi: 10.1186/1471-2172-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baba Y, Yokota T, Spits H, Garrett KP, Hayashi S, Kincade PW. Constitutively active beta-catenin promotes expansion of multipotent hematopoietic progenitors in culture. J Immunol. 2006;177:2294–2303. doi: 10.4049/jimmunol.177.4.2294. [DOI] [PubMed] [Google Scholar]
- 19.Baba Y, Garrett KP, Kincade PW. Constitutively active beta-catenin confers multilineage differentiation potential on lymphoid and myeloid progenitors. Immunity. 2005;23:599–609. doi: 10.1016/j.immuni.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 21.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 22.Petruzzelli L, Takami M, Humes HD. Structure and function of cell adhesion molecules. Am J Med. 1999;106:467–476. doi: 10.1016/s0002-9343(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 23.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyake K, Medina K, Ishihara K, Kimoto M, Auerbach R, Kincade PW. A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. J Cell Biol. 1991;114:557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leuker CE, Labow M, Muller W, Wagner N. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J Exp Med. 2001;193:755–768. doi: 10.1084/jem.193.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulyanova T, Scott LM, Priestley GV, Jiang Y, Nakamoto B, Koni PA, Papayannopoulou T. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106:86–94. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oostendorp RA, Reisbach G, Spitzer E, Thalmeier K, Dienemann H, Mergenthaler HG, Dormer P. VLA-4 and VCAM-1 are the principal adhesion molecules involved in the interaction between blast colony-forming cells and bone marrow stromal cells. Br J Haematol. 1995;91:275–284. doi: 10.1111/j.1365-2141.1995.tb05290.x. [DOI] [PubMed] [Google Scholar]
- 28.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulyanova T, Priestley GV, Nakamoto B, Jiang Y, Papayannopoulou T. VCAM-1 ablation in nonhematopoietic cells in MxCre+ VCAM-1f/f mice is variable and dictates their phenotype. Exp Hematol. 2007;35:565–571. doi: 10.1016/j.exphem.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 33.Welner RS, Pelayo R, Kincade PW. Evolving views on the genealogy of B cells. Nat Rev Immunol. 2008;8:95–106. doi: 10.1038/nri2234. [DOI] [PubMed] [Google Scholar]
- 34.Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1) J Biol Chem. 1992;267:16323–16329. [PubMed] [Google Scholar]
- 35.Cybulsky MI, lan-Motamed M, Collins T. Structure of the murine VCAM1 gene. Genomics. 1993;18:387–391. doi: 10.1006/geno.1993.1480. [DOI] [PubMed] [Google Scholar]
- 36.Minami T, Abid MR, Zhang J, King G, Kodama T, Aird WC. Thrombin stimulation of vascular adhesion molecule-1 in endothelial cells is mediated by protein kinase C (PKC)-delta-NF-kappa B and PKC-zeta-GATA signaling pathways. J Biol Chem. 2003;278:6976–6984. doi: 10.1074/jbc.M208974200. [DOI] [PubMed] [Google Scholar]
- 37.Ballin A, Lehman D, Sirota P, Litvinjuk U, Meytes D. Increased number of peripheral blood CD34+ cells in lithium-treated patients. Br J Haematol. 1998;100:219–221. doi: 10.1046/j.1365-2141.1998.00537.x. [DOI] [PubMed] [Google Scholar]
- 38.Eto M, Kouroedov A, Cosentino F, Luscher TF. Glycogen synthase kinase-3 mediates endothelial cell activation by tumor necrosis factor-alpha. Circulation. 2005;112:1316–1322. doi: 10.1161/CIRCULATIONAHA.105.564112. [DOI] [PubMed] [Google Scholar]
- 39.Welner RS, Pelayo R, Nagai Y, Garrett KP, Wuest TR, Carr DJ, Borghesi LA, Farrar MA, Kincade PW. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008 doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–360. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 41.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 42.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 43.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 47.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovach NL, Lin N, Yednock T, Harlan JM, Broudy VC. Stem cell factor modulates avidity of alpha 4 beta 1 and alpha 5 beta 1 integrins expressed on hematopoietic cell lines. Blood. 1995;85:159–167. [PubMed] [Google Scholar]
- 52.Levesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J Exp Med. 1995;181:1805–1815. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solanilla A, Grosset C, Duchez P, Legembre P, Pitard V, Dupouy M, Belloc F, Viallard JF, Reiffers J, Boiron JM, Coulombel L, Ripoche J. Flt3-ligand induces adhesion of haematopoietic progenitor cells via a very late antigen (VLA)-4- and VLA-5-dependent mechanism. Br J Haematol. 2003;120:782–786. doi: 10.1046/j.1365-2141.2003.04155.x. [DOI] [PubMed] [Google Scholar]
- 54.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 55.Sironi M, Sciacca FL, Matteucci C, Conni M, Vecchi A, Bernasconi S, Minty A, Caput D, Ferrara P, Colotta F. Regulation of endothelial and mesothelial cell function by interleukin-13: selective induction of vascular cell adhesion molecule-1 and amplification of interleukin-6 production. Blood. 1994;84:1913–1921. [PubMed] [Google Scholar]
- 56.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 57.Sawa Y, Ueki T, Hata M, Iwasawa K, Tsuruga E, Kojima H, Ishikawa H, Yoshida S. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J Histochem Cytochem. 2008;56:97–109. doi: 10.1369/jhc.7A7299.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bradley RS, Brown AM. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990;9:1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papkoff J, Schryver B. Secreted int-1 protein is associated with the cell surface. Mol Cell Biol. 1990;10:2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blasband A, Schryver B, Papkoff J. The biochemical properties and transforming potential of human Wnt-2 are similar to Wnt-1. Oncogene. 1992;7:153–161. [PubMed] [Google Scholar]
- 61.Lin X, Perrimon N. Role of heparan sulfate proteoglycans in cell-cell signaling in Drosophila. Matrix Biol. 2000;19:303–307. doi: 10.1016/s0945-053x(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 62.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 63.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 64.Hwa CH, Bae YC, Jung JS. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006;24:2744–2752. doi: 10.1634/stemcells.2006-0189. [DOI] [PubMed] [Google Scholar]
- 65.Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 66.Soriano A, Salas A, Salas A, Sans M, Gironella M, Elena M, Anderson DC, Pique JM, Panes J. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000;80:1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 67.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 68.Koizumi M, King N, Lobb R, Benjamin C, Podolsky DK. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992;103:840–847. doi: 10.1016/0016-5085(92)90015-q. [DOI] [PubMed] [Google Scholar]
- 69.Mori Y, Shimizu N, Dallas M, Niewolna M, Story B, Williams PJ, Mundy GR, Yoneda T. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104:2149–2154. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- 70.Nakatani K, Fujii H, Hasegawa H, Terada M, Arita N, Ito MR, Ono M, Takahashi S, Saiga K, Yoshimoto S, Iwano M, Shiiki H, Saito Y, Nose M. Endothelial adhesion molecules in glomerular lesions: association with their severity and diversity in lupus models. Kidney Int. 2004;65:1290–1300. doi: 10.1111/j.1523-1755.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 71.Abonia JP, Hallgren J, Jones T, Shi T, Xu Y, Koni P, Flavell RA, Boyce JA, Austen KF, Gurish MF. Alpha-4 integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood. 2006;108:1588–1594. doi: 10.1182/blood-2005-12-012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diab Vasc Dis Res. 2007;4:84–88. doi: 10.3132/dvdr.2007.025. [DOI] [PubMed] [Google Scholar]
- 73.Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology. 2001;121:1428–1436. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 74.van Dinther-Janssen AC, Horst E, Koopman G, Newmann W, Scheper RJ, Meijer CJ, Pals ST. The VLA-4/VCAM-1 pathway is involved in lymphocyte adhesion to endothelium in rheumatoid synovium. J Immunol. 1991;147:4207–4210. [PubMed] [Google Scholar]
- 75.Reparon-Schuijt CC, van Esch WJ, van K C, Rozier BC, Levarht EW, Breedveld FC, Verweij CL. Regulation of synovial B cell survival in rheumatoid arthritis by vascular cell adhesion molecule 1 (CD106) expressed on fibroblast-like synoviocytes. Arthritis Rheum. 2000;43:1115–1121. doi: 10.1002/1529-0131(200005)43:5<1115::AID-ANR22>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 76.Pelletier RP, Morgan CJ, Sedmak DD, Miyake K, Kincade PW, Ferguson RM, Orosz CG. Analysis of inflammatory endothelial changes, including VCAM-1 expression, in murine cardiac grafts. Transplantation. 1993;55:315–320. doi: 10.1097/00007890-199302000-00017. [DOI] [PubMed] [Google Scholar]
- 77.Brockmeyer C, Ulbrecht M, Schendel DJ, Weiss EH, Hillebrand G, Burkhardt K, Land W, Gokel MJ, Riethmuller G, Feucht HE. Distribution of cell adhesion molecules (ICAM-1, VCAM-1, ELAM-1) in renal tissue during allograft rejection. Transplantation. 1993;55:610–615. doi: 10.1097/00007890-199303000-00027. [DOI] [PubMed] [Google Scholar]
- 78.Preiss DJ, Sattar N. Vascular cell adhesion molecule-1: a viable therapeutic target for atherosclerosis? Int J Clin Pract. 2007;61:697–701. doi: 10.1111/j.1742-1241.2007.01330.x. [DOI] [PubMed] [Google Scholar]