Abstract
Background and aims
SCS is used to improve peripheral circulation in selected patients with ischemia of the extremities. However the mechanisms are not fully understood. The present study investigated whether blockade of ERK and AKT activation modulated SCS-induced vasodilation.
Methods
A unipolar ball electrode was placed on the left dorsal column at the lumbar 2-3 spinal segments in rats. Cutaneous blood flows from left and right hind foot pads were recorded with laser Doppler flow perfusion monitors. SCS was applied through a ball electrode at 60% or 90% of MT. U0126, an inhibitor of ERK kinase, or LY294002, an inhibitor of PI3K upstream of AKT, was applied to the lumbar 3-5 spinal segments (n=7, each group).
Results
U0126 (100 nM, 5 μM and 250 μM) significantly attenuated SCS-induced vasodilation at 60% (100 nM: P□0.05; 5 μM and 250 μM: P□0.01, respectively) and 90% of MT (100 nM and 5 μM: P□0.05; 250 μM: P□0.01, respectively). LY294002 at 100 μM also attenuated SCS-induced vasodilation at 60% and 90% of MT (P□0.05).
Conclusions
These data suggest that ERK and AKT pathways are involved in SCS-induced vasodilation.
Keywords: spinal cord stimulation, extracellular signal-regulated kinase, protein kinase B, vasodilation
1. Introduction
Spinal cord stimulation (SCS) delivers electrical impulses to different spinal segments via implanted electrodes. Currently SCS is used clinically to treat patients with pain related diseases, e.g., ischemic pain due to peripheral arterial diseases (PAD). Annually, at least 14,000 chronic implantations of electrodes for SCS are made worldwide (Linderoth and Foreman, 2006). SCS at caudal levels of the cervical spinal cord and rostral levels of the lumbar spinal cord has been shown to increase local blood flow in arms and hands, and in lower limbs and feet, respectively. SCS benefits include pain relief, increased claudication distance, and decreased ulcer size. (Cameron 2004). Although SCS usually is the last resort after vascular surgery and medications failed to prevent the development of a disease, the success rate is above 60% in the long term (Deer and Raso, 2006). However, mechanisms of SCS-induced vasodilation are still not fully understood.
Two theories have emerged to interpret SCS benefits. One theory is that SCS produces the release of vasodilators, e.g., calcitonin gene related peptide (CGRP) to vascular tissues in lower limbs and feet by antidromic activation of sensory fibers (Croom et al., 1996, 1997 a, b, 1998; Tanaka et al., 2001, 2003 a, b, 2004; Wu et al., 2006 a, 2007a c). An alternative theory is that SCS induces decreased sympathetic efferent activity and subsequently reduces vasoconstriction and enhances blood flow in lower limbs and feet (Linderoth et al., 1991 a, b, 1994). These two mechanisms are complementary and the balance between them are affected by tonic sympathetic activity, SCS intensity, and individual patients or animal strains (Tanaka et al., 2003 b; Wu et al., 2007 a).
Previous studies have indicated that depression of sympathetic activity may account for a part of the SCS effect, but antidromic activation of sensory fibers and subsequent release of vasodilators account for a major portion of the SCS response (Linderoth and Foreman, 2006). With respect to the antidromic mechanism, SCS-induced vasodilation is dependent on activation of central terminals of transient receptor potential vanilloid-1 (TRPV1) containing sensory fibers originating from L3-L5 spinal segments (Tanaka et al., 2003 a; Wu et al., 2006 a, 2007 a). However, it is still unclear how SCS of the dorsal column activates central terminals of sensory fibers at the spinal level. Since the synaptic integration of the spinal gray matter plays a pivotal role in SCS-induced vasodilation (Barron et al., 1999), there is a possibility that SCS activates sensory fibers via modulation of spinal neurons.
Activation of extracellular signal-regulated kinase (ERK) plays crucial roles in various cellular processes including cell growth, proliferation, differentiation, survival, innate immunity and development (Krens et al., 2006). Furthermore, activation of ERK in primary sensory neurons, epidermal nerve fibers, and dorsal horn neurons are associated with C-fiber stimulation and pain hypersensitivity (Rosen et al., 1994; Ji et al., 1999; Kawasaki et al., 2004; Lever et al., 2003; Wang et al., 2004; Zhuang et al., 2004; Xin et al., 2006; Walker et al., 2007). ERK is a useful marker for neuronal activation since its activation occurs after cytosolic calcium is increased and membrane is depolarized in cultured neurons (Agell et al., 2002, Lever et al., 2003). A membrane-associated second messenger protein, phosphatidylinositol 3-kinase (PI3K) and its downstream kinase, protein kinase B (AKT), are associated with neuronal survival (Markus et al., 2002) and plasticity (Izzo et al., 2002) via activation of transcription pathways and protein synthesis. Recent studies demonstrate that ERK and AKT pathways are associated with vasodilation (Armstead, 2003) and nociceptive transmission (Sun et al., 2006). Furthermore, our preliminary unpublished results showed that phosphorylation of ERK and AKT in neurons and axons in the superficial dorsal horn in L3-L5 spinal segments were enhanced after 5 minutes of SCS at 90% of MT.
Therefore, it is very likely that ERK and AKT pathways are associated with SCS-induced vasodilation at the spinal level. In the present study, we determined whether the blockade of ERK or AKT activation modulates SCS-induced peripheral vasodilation. The results showed that U0126, a blocker of ERK pathways, and LY294002, a blocker of AKT pathways attenuated vasodilation induced by SCS at 60% and 90% of motor threshold (MT), respectively. These data suggest that activation of ERK and AKT signaling pathways is associated with SCS-induced vasodilation. The present study provides the basis to understand how SCS of the dorsal column activates sensory fibers at the spinal level.
2. Results
The experiments were performed in anesthesized, paralyzed and artificially ventilated rats. The detailed protocol was addressed in the methods.
A: Effects of ERK phosphorylation blocker, U0126, on SCS-induced vasodilation
Group 1: Effects on SCS-induced vasodilation at 60% of MT
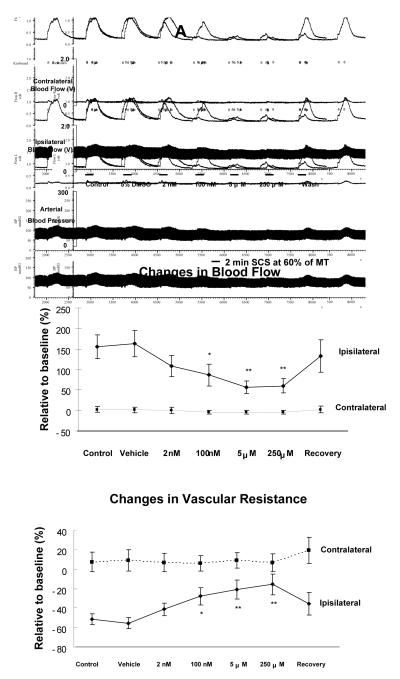
The average MT of the first group of rats was 467 ± 36 μA (n=7). SCS at 60% of MT increased the left (ipsilateral), but not the right (contralateral) blood flow (Fig 1A top panel). SCS still elicited a very similar increased blood flow after vehicle application for 10 minutes on L3-L5 segments compared to control responses. However, administration of U0126 for 10 minutes, from a dose of 100 nM to a dose of 250 μM, attenuated SCS-induced increases in blood flow. Recovery of SCS-induced increased blood flow was almost completed 10 minutes after flushing U0126 from spinal cord using a saline containing cotton ball. The changes in blood flow during SCS at 60% of MT before and after U0126 at different doses are summarized in Fig 1A middle panel, showing that the increased blood flow in the ipsilateral limb due to SCS at 60% of MT was significantly reduced after U0126 at 100 nM (P<0.05), and further attenuated after U0126 at 5 μM (P<0.01) and 250 μM (P<0.01). There were no alterations of contralateral blood flow during SCS before and after U0126. Changes in vascular resistance during SCS at 60% were calculated and summarized in Fig 1A (bottom panel). Ipsilateral vasodilation, assessed as decreased vascular resistance during SCS, was significantly attenuated after applications of U0126 (100 nM, P<0.05; 5 μM, P<0.01; 250 μM, P<0.01). There were no significant changes in contralateral vascular resistance during SCS before and after U0126.
Figure 1. Effects of U0126 on SCS-induced vasodilation.
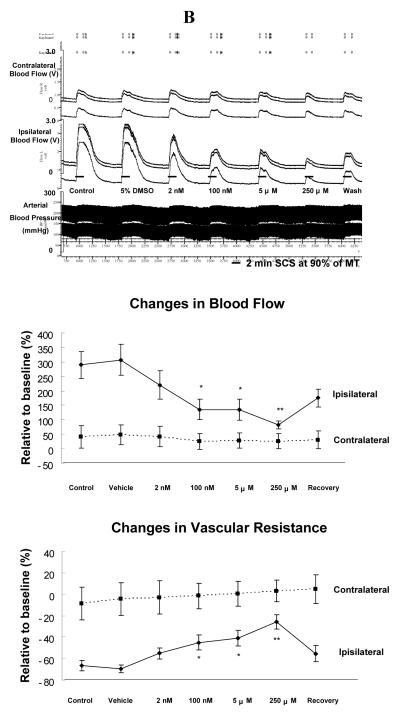
(A) Changes of blood flow and vascular resistance produced by SCS at 60% of MT before and after topical application of U0126 on the surface of L3-L5 spinal cord. Top panel: A representative example of changes in ipsilateral and contralateral blood flow and arterial blood pressure during SCS at 60% of MT, before and after administration of U0126 ( 2 nM, 100 nM, 5 μM and 250 μM; 10 minutes each ). Middle panel: A summary of the percent changes in blood flow in the ipsilateral and contralateral hindpaws in response to 2 minutes SCS at 60% of MT. Bottom panel: A summary of the percent changes in vascular resistance in the ipsilateral and contralateral hindpaws in response to 2 minutes SCS at 60% of MT. * P<0.05, ** P<0.01, compared to control group. (B) Changes of blood flow and vascular resistance produced by SCS at 90% of MT before and after topical application of U0126 on the surface of L3-L5 spinal cord. The figure legends are the same as those in Fig 1A, except that intensity of SCS is 90% of MT, instead of 60%.
Group 2: Effects on SCS-induced vasodilation at 90% of MT
The average MT of the second group of rats was 479 ± 41 μA (n=7). Vehicle application for 10 minutes on L3-L5 did not affect SCS-induced ipisilateral increases in blood flow (Fig 1B top panel). Administration of U0126 for 10 minutes, from 100 nM to 250 μM, reduced SCS-induced increases in blood flow (Fig 1B top panel). SCS-induced increases in blood flow partially recovered after flushing U0126 from spinal cord (Fig 1B top panel). A summary of alterations of blood flow during SCS at 90% of MT before and after U0126 at different doses (Fig 1B middle panel) showed that U0126 significantly attenuated the increase in blood flow with SCS at 90% of MT (100 nM and 5 μM, P<0.05; 250 μM, P<0.01). The response of contralateral blood flow to SCS at 90% of MT was not altered before or after U0126. Changes of vascular resistance during SCS at 90% were calculated and summarized in Fig 1B (bottom panel), demonstrating that SCS-induced decreases in ipsilateral vascular resistance were attenuated after applications of U0126 (100 nM and 5 μM, P<0.05; 250 μM, P<0.01). Contralateral vascular resistance during SCS did not change before and after U0126.
B: Effects of blocker for AKT phosphorylation with LY294002 on SCS-induced vasodilation
Group 3: Effects on SCS-induced vasodilation at 60% of MT
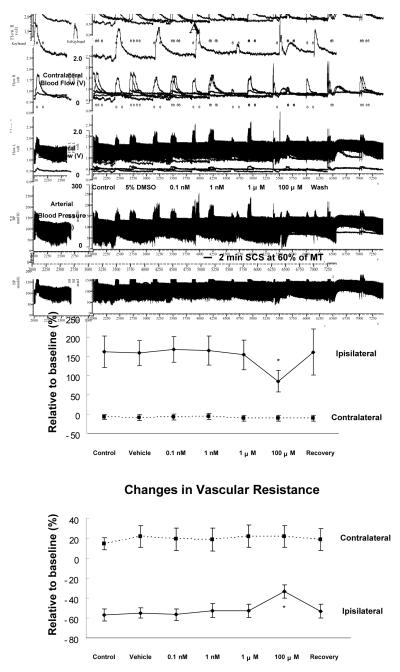
The average MT of the third group of rats was 442 ± 31 μA (n=7). At 60% of MT, SCS-induced ipsilateral increases in blood flow did not change after vehicle application on L3-L5 for 10 minutes (Fig 2A top panel). Also, administrations of LY294002, from 0.1 nM to 1 μM did not alter SCS-induced increases in blood flow (Fig 2A top panel). However, LY294002 at 100 μM (the highest dose in this study) significantly attenuated SCS-induced ipsilateral increases in blood flow at 60% of MT. SCS effects on blood flow partially recovered 10 minutes after flushing LY294002 from spinal cord. A summary of the changes of blood flow during SCS at 60% of MT before and after LY294002 (Fig 2A middle panel) showed that SCS-induced increased blood flow was only significantly attenuated by LY294002 at the highest dose (100 μM: P<0.05). There were no statistical alterations of contralateral blood flow during SCS before and after LY294002. Changes in vascular resistance in response to SCS at 60% were calculated and summarized in Fig 2A (bottom panel); SCS-induced decreases in ipsilateral vascular resistance during SCS were attenuated only after an application of the highest dose, 1 μM, of LY294002 (100 μM, P<0.05). Also, no significant changes of contralateral vascular resistance during SCS were detected before and after LY294002.
Figure 2. Effects of LY294002 on SCS-induced vasodilation.
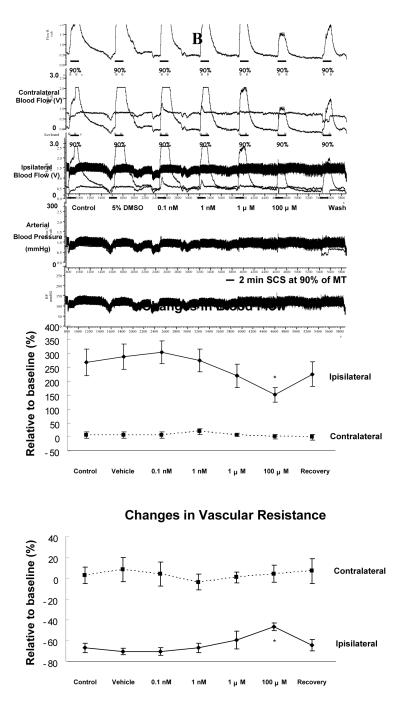
(A) Changes of blood flow and vascular resistance produced by SCS at 60% of MT before and after topical application of LY294002 on the surface of L3-L5 spinal cord. Top panel: A representative example of changes in ipsilateral and contralateral blood flow and arterial blood pressure during SCS at 60% of MT, before and after administration of LY294002 ( 0.1 nM, 1 nM, 1 μM and 100 μM) for 10 minutes. Middle panel: A summary of the percent changes in blood flow in the ipsilateral and contralateral hindpaws in response to 2 minutes SCS at 60% of MT. Bottom panel: A summary of the percent changes in vascular resistance in the ipsilateral and contralateral hindpaws in response to 2 minutes SCS at 60% of MT. The abbreviations are same as described in Fig 1. *P<0.05, compared to the control group. (B) Changes of blood flow and vascular resistance produced by SCS at 90% of MT before and after topical application of LY294002 on the surface of L3-L5 spinal cord. The figure legends are the same as those in Fig 2A, except that intensity of SCS is 90% of MT, instead of 60%.
Group 4: Effects on SCS-induced vasodilation at 90% of MT
The average MT of the fourth group of rats was 471 ± 43 μA (n=7). The results are similar to the observations made in the third group. In brief, LY294002, only at 100 μM decreased SCS-induced increases in blood flow at 90% of MT (Fig 2B top panel). A partial recovery of SCS-induced increased blood flow was detected after flushing LY294002 from spinal cord (Fig 2B top panel). There was a significant attenuation of SCS-induced increased blood flow after LY294002 at the highest dose (100 μM: P<0.05), compared to control and vehicle groups (Fig 2B middle panel). SCS-induced decreases in ipsilateral vascular resistance were also reduced with LY294002 at 100 μM (P<0.05) as shown in Fig 2B (bottom panel).
In group 5, control studies showed that repeated applications of vehicles onto L3-L5 spinal segments did not produce obvious changes of vasodilation when SCS was administered at 90% of MT (n=3, data not shown). These studies were performed in addition to the vehicle controls when drugs were applied.
3. Discussion
In this study, blockade of ERK and AKT activation in spinal neurons using U0126 and LY294002, respectively, attenuated SCS-induced vasodilation. The data indicated that these two molecular signaling pathways are involved in SCS-induced vasodilation.
Roles of ERK and AKT in SCS-induced vasodilation
In the present study, U0126, an inhibitor of ERK kinase, was applied on the surface of L3-L5 spinal segments for 10 minutes to attenuate the activation of ERK. Vasodilation produced by SCS at 60% or 90% of MT was significantly attenuated after U0126 administration at a dose of 100 nM, and the maximal attenuation was observed after the dose of 250 μM, the highest dose used in this study. Therefore ERK activation at the spinal level appears to be related to SCS-induced vasodilation. Since ERK is expressed in the dorsal horn neurons, the data suggested that SCS-induced vasodilation was associated with ERK expressing neurons in the superficial lamiae of dorsal horn. LY294002 at a high dose (100 μM) also decreased SCS-induced vasodilation. Thus, activation of AKT pathways in the spinal neurons also appears to be involved in SCS-induced vasodilation. However we do not know whether the two pathways play the same or different roles in SCS-induced vasodilation and how they are interconnected with SCS-activated spinal terminals of sensory fibers. That question will be further investigated in future studies. Overall the results indicated that ERK and AKT signaling pathways in the spinal cord are associated with SCS-induced vasodilation.
Previous studies from this laboratory indicated that Aδ fibers are activated during SCS at 60% and 90% of MT. In contrast, C-fibers are antidromically activated when the intenisity of SCS is applied at 90% of MT or higher (Tanaka et al., 2003 a). In this present study U0126 or LY294002 showed similar effects on vasodilation when stimulation was at 60% and 90% of MT. Thus it is very likely that ERK and AKT may have similar functions in SCS-induced vasodilation at 60% and 90%.
Besides cell bodies, ERK and AKT are also expressed in the central terminals in the dorsal horn. They were also expressed in the fibers that were activated after 5 minutes SCS (90 % MT) of the L3-L5 segments (our preliminary data). Thus, it is possible that ERK and AKT pathways in central terminals of sensory fibers were also activated by SCS. Activation of ERK and AKT pathways can sensitize TRPV1 and other key channels (Zhuang et al., 2004) and finally antidromically activate central terminals of TRPV1 containing sensory fibers. Therefore, it is likely that U0126 and LY294002 attenuated the activation of ERK and AKT in the sensory terminals, in addition to spinal neurons.
U0126 and LY294002 on neuronal activities
In previous studies to block neuronal activities, the duration of administration of U0126 and LY294002 on the spinal cord varied from minutes to hours (Yang et al., 2006; Komatsu et al., 2007; Lu et al., 2007; Toborek et al., 2007). Administration of U0126 and LY294002 has been shown to block phosphorylation of ERK and AKT, respectively (Dolcet et al., 1999; Wu et al., 2005). A recent study showed that 5 minutes after co-administration with morphine, U0126 reduced spinal ERK activation produced by morphine and also caused a significant inhibition of morphine-induced nociception (Komatsu et al., 2007). Also, pretreating with LY294002 for 10 minutes exacerbated the morphine withdrawal response resulting from inhibition of spinal phosphoinositide 3-kinase (Yang et al., 2006). In the present study, SCS-induced vasodilation was attenuated 10 minutes after the administration of U0126 or LY294002. Therefore, a short time, e.g. 5-10 minutes, is long enough for U0126 and LY294002 to block the ERK and AKT phosphorylation or other relevant neuronal activities.
Future studies are necessary to confirm the results from this study. Other inhibitors for ERK phosphorylation, e.g. PD98059 and AKT phosphorylation, e.g. KP372-1, should be used to confirm the specific roles of ERK and AKT in SCS-induced vasodilation. LY294002, at 100 μM, attenuated SCS-induced vasodilation. 100 μM is the highest dose that can be dissolved and this dose possibly affects other pathways, such as voltage-gated potassium (Kv) channels that regulate transmitter release and neuronal excitability (El-kholy et al., 2003; Brooke et al., 2004). Therefore, the exact mechanisms of LY294002 effects on SCS-induced vasodilation need to be investigated using molecular techniques, such as western blots to measure of p-AKT level after LY294002. The use of more specific inhibitors will also improve the ability to determine the roles of ERK and AKT.
Potential mechanisms how SCS activates central terminals of sensory fibers
The spinal segmental mechanisms of SCS-induced peripheral vasodilation are likely to be complex. Intra- and/or intersegmental pathways, as well as various neurotransmitters and neuromodulators in the spinal cord may be involved (Linderoth and Foreman 1999; 2006). Since SCS at a low intensity mainly activates large nerve fibers in dorsal column, an antidromic descending volley transmitted by collaterals of dorsal column fibers seems to be responsible for the action at the segmental level. Furthermore, several potential intraspinal segmental pathways might relate to spinal segmental mechanisms underlying effects of SCS on peripheral vasodilation observed in this study. With the method of intracellular recordings, it has been demonstrated that dorsal column stimulation can produce postsynaptic effects through inhibitory interneurons synapsing on spinothalamic tract neurons (STT) in spinal dorsal horn. These effects suppress the responses of STT neurons to electrical stimulation of peripheral nerves and mechanical stimulation of somatic fields and reduce the transmission of nociceptive information to the brain (Foreman et al. 1976). Therefore, this mechanism is more likely to be involved in pain relief by SCS rather than that for SCS-induced vasodilation. On the other hand, dorsal column stimulation has been shown to induce primary afferent depolarization at the central terminals of large primary afferents via excitatory interneurons that elicit presynaptic inhibition (Foreman et al. 1976). Also, primary afferent depolarization involving GABA interneurons normally inhibit noxious peripheral afferent barrages, but also can trigger dorsal root reflexes by greater peripheral afferent volleys in some pathological conditions such as inflammation (Willis 1999). Therefore, it may be reasonable to assume that primary afferent depolarization evoked by SCS could be intense enough to excite or activate central terminals of primary afferent neurons with smaller diameter fiber (Aδ and C fibers). This activation causes antidromic firing of sensory neurons and CGRP mediated vasodilation at peripheral terminals (Fig 3 A and B) (Tanaka et al., 2003). In support this, SCS has been shown to decrease the release of excitatory amino acids such as glutamate and increase the release of GABA in the dorsal horn of rat spinal cord (Cui et al. 1997, 1998 a, b; Stiller et al. 1996). Recently, central terminals of sensory afferent neurons containing TRPV1 have been demonstrated to be involved in SCS-induced vasodilation in rat paws (Wu et al. 2006 a). The present study examined the effects of inhibitors of ERK and AKT on SCS-induced vasodilation, and the results suggested that ERK and AKT pathways in spinal dorsal horn were involved in SCS-induced vasodilation. However, the exact pathways and downstream actions of AKT and ERK phosphorylation are not clear. GABA and N-methyl-D-aspartate (NMDA) receptors are associated with antidromic activity in acute neurogenic inflammation evoked by intradermal injection of capsaicin (Lin et al., 1999, 2004). Activation of ERK and AKT pathways are also associated with NMDA receptor activation in spinal cord (Daulhac et al., 2006; Sun et al., 2006), and the release of nitric oxide (Sung et al., 2005). Therefore, multiple molecular pathways are most likely involved in SCS-activated central terminals of sensory fibers in the spinal cord. Further physiological and molecular studies are necessary to elucidate the interactions of the related molecular pathways in the spinal cord and how they stimulate central terminals of sensory fibers.
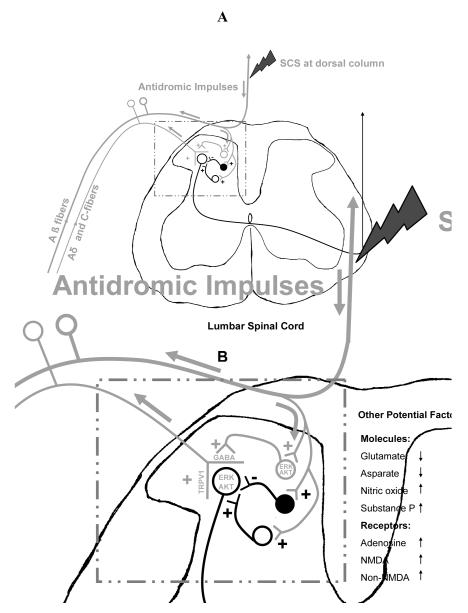
Figure 3. Potential mechanism how SCS activates central terminals of sensory fibers.
(A) Potential pathways. SCS at dorsal column antidromically activates large fibers, mainly Aβ fibers. A branch of Aβ fibers that connects spinal neurons in the superficial laminae of dorsal horn is also activated. Since these spinal neurons also presynaptically contact the central terminals of primary sensory fibers, activation of these spinal neurons subsequently produces primary afferent depolarization and further activate the central terminals of sensory fibers. (B) Potential factors. Based on the present study, ERK and AKT that localize in spinal neurons and central terminals of sensory fibers are involved in the activation of central terminals of sensory fibers produced by SCS at dorsal column. Other literature suggests that TRPV1 at the central terminals of sensory fibers is also activated.
Overall, the hypothesis is that SCS on the dorsal column antidromically activates large fibers, mainly Aβ fibers (Foreman et al. 1976). Branches of Aβ fibers synapse with and activate spinal neurons in the superficial laminae of dorsal horn that express ERK, AKT and GABA. Since these spinal neurons also presynaptically contact central terminals of sensory fibers, it is likely that activation of these spinal neurons subsequently produces primary afferent depolarization and finally activate the central terminals of TRPV1 containing sensory fibers at the spinal level (Fig 3 A and B). The release of CGRP by sensory fibers decreases vascular resistance and increases local blood flow during SCS (Wu et al., 2007 a, b).
In conclusion, we identified two molecular pathways in the spinal cord, ERK and AKT that are involved in SCS-induced vasodilation. Further studies are required to fully explain how SCS at dorsal column activates central terminals of sensory fibers and subsequently induces vasodilation.
4. Methods
4.1 Experimental protocol
The protocol for this study was approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee. Experimental methods of this study were similar to those used in our previous studies (Tanaka et al., 2001, 2003 a, b, 2004; Wu et al., 2006 a, b). Male Sprague-Dawley rats (300-400g; Charles Rivers, MA) were anesthesized by sodium pentobarbital (60 mg/kg, i.p.). Body temperature was maintained between 36 and 38 °C with a heating pad throughout the experiment. One cannula (PE-50) was inserted into the left common jugular vein for a constant infusion of supplemental pentobarbital (19-25 mg/Kg/h). Pancuronium (boluses 0.2 mg/h) was also injected via this cannula for muscle paralysis. Another cannula (PE-50) was inserted into the left common carotid artery to monitor arterial blood pressure. The rats were tracheotomized and artificially ventilated with room air (CWE ventilator model SAR-830). A laminectomy was performed to expose the dorsal surface of lumbar spinal segments 2-5 (L2-L5). A spring-loaded unipolar ball electrode was placed on the left side of the subdural surface at L2 spinal segment.
The motor threshold (MT) stimulus intensity was determined in each animal at 50 Hz, 0.2 ms duration by slowly increasing the SCS current from zero until a clear skeletal muscle contraction of the left hindlimb was observed. Subsequently, pancuronium was injected for muscle paralysis. Before paralysis, animals were monitored for appropriate anesthesia by using withdrawal and corneal reflexes and stability of arterial blood pressure (ABP). After paralysis, stability of arterial blood pressure level was used to monitor anesthesia. Experimental SCS was repeated for 2 minutes at 60, or 90% of MT with at least 10 minutes between consecutive stimuli. The parameters are similar to those in clinical use (Linderoth et al., 1991 b).
Cutaneous blood flow in the hindlimb footpads of the rats was measured with laser Doppler flow perfusion monitors (Model 403A; Vasomedic, St. Paul, MN). Responses to SCS were determined as percent changes from the baseline of blood flow and vascular resistance. Five groups were used to address the aims of this study. 1) Rats (n=7) were used to determine the role of U0126, a blocker of ERK pathways, in vasodilation produced by SCS at 60% of MT. Agar (3-4% in saline) was used to make a well on the surface of spinal cord to prevent U0126 from diffusing to other spinal cord segments. SCS at 60% of MT on L2 for 2 minutes was repeated with at least 10 minutes between SCS applications to produced increases in blood flow. Sponges (□3 × 3 × 7 mm) containing 200 μl solution of vehicle (5% DMSO) or U0126 at 2nM, 100 nM, 5 μM and 250 μM were sequentially applied to the L3-L5 spinal segments, starting immediately after SCS and remaining for 10 minutes until the next SCS. After all the doses were administered the spinal segments were then cleaned up by using a cotton ball containing saline. SCS at 60% of MT was repeated again to determine the recovery of SCS-induced vasodilation. 2) Rats (n=7) were used to investigate the roles of U0126 with vasodilation produced by SCS at 90% of MT. The protocol was the same as that in group 1, except that the SCS was applied at 90% instead of 60% of MT to produce increases in blood flow. 3) Rats (n=7) were used to test the roles of LY294002 in vasodilation produced by SCS at 60% of MT. The protocol was the same as group 1, except that the tested chemical agent was LY294002, a blocker of AKT pathways at doses of 0.1 nM, 1 nM, 1μM and 100 μM. 4) Rats (n=7) were used to determine the roles of LY 294002 in vasodilation produced by SCS at 90% of MT. The protocol was the same as group 3, except that the SCS was applied at 90% instead of 60% of MT to produce increased blood flow. 5) Multiple applications of vehicle onto the surface of L3-L5 were also performed in rats (n=3) to determine its effects on vasodilation produced by SCS at 60% or 90% of MT. U0126 and LY 294002 were purchase from Sigma.
4.2 Statistical Analysis
Changes in peripheral blood flow were presented as the percentage of changes of the peak amplitude from baseline and reported as mean ± S.E.M. Mean blood pressure (MBP) was calculated using the formula 2/3 × diastolic blood pressure (BP) + 1/3 × Systolic BP. Vascular resistance was calculated by dividing mean arterial blood pressure by blood flow. Differences in blood flow and vascular resistance before and after applications of U0126 or LY294002 at different doses were examined with a one-way ANOVA with repeated measures followed by Tukey’s multiple comparisons test. The level of significance was set at P < 0.05.
Acknowledgments
We thank Dr. S. Benyajati for helpful comments as well as D. Holston for her expert technical assistance. This study was supported by NIH grant HL075524 and NS35471 (R.D.F), 2005 University of Oklahoma Health Sciences Center Graduate Student Association Research Grant (M.W), American Heart Association (AHA) Predoctoral Fellowship 0615642Z (M.W), and Presbyterian Health Foundation grant 1401 (N.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca (2+), and calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Armstead WM. PTK, ERK and p38 MAPK contribute to impaired NMDA-induced vasodilation after brain injury. Eur J Pharmacol. 2003;474:249–254. doi: 10.1016/s0014-2999(03)02012-0. [DOI] [PubMed] [Google Scholar]
- Barron KW, Croom JE, Ray CA, Chandler MJ, Foreman RD. Spinal integration of antidromic mediated cutaneous vasodilation during dorsal spinal cord stimulation in the rat. Neurosci Lett. 1999;260:173–176. doi: 10.1016/s0304-3940(98)00972-0. [DOI] [PubMed] [Google Scholar]
- Brooke RE, Atkinson L, Batten TF, Deuchars SA, Deuchars J. Association of potassium channel Kv3.4 subunits with pre- and post-synaptic structures in brainstem and spinal cord. Neuroscience. 2004;126:1001–1010. doi: 10.1016/j.neuroscience.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100:254–267. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- Croom JE, Barron KW, Chandler MJ, Foreman RD. Cutaneous blood flow increases in the rat hindpaw during dorsal column stimulation. 1996;728:281–286. doi: 10.1016/0006-8993(96)00554-9. [DOI] [PubMed] [Google Scholar]
- Croom JE, Foreman RD, Chandler MJ, Barron KW. Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am J Physiol. 1997 a;272:950–957. doi: 10.1152/ajpheart.1997.272.2.H950. [DOI] [PubMed] [Google Scholar]
- Croom JE, Foreman RD, Chandler MJ, Koss MC, Barron KW. Role of nitric oxide in cutaneous blood flow increases in the rat hindpaw during dorsal column stimulation. Neurosurgery. 1997 b;40:565–70. doi: 10.1097/00006123-199703000-00027. discussion 571. [DOI] [PubMed] [Google Scholar]
- Croom JE, Foreman RD, Chandler MJ, Barron KW. Reevaluation of the role of sympathetic nervous system in cutaneous vasodilation during dorsal spinal cord stimulation: Are multiple mechanisms active? Neuromodulation. 1998;1:91–101. doi: 10.1111/j.1525-1403.1998.tb00022.x. [DOI] [PubMed] [Google Scholar]
- Cui JC, Linderoth B, Meyerson BA. Incidence of mononeuropathy in rats is influenced by pre-emptive alteration of spinal excitability. Eur J Pain. 1997;1:53–59. doi: 10.1016/s1090-3801(97)90053-7. [DOI] [PubMed] [Google Scholar]
- Cui JG, Meyerson BA, Sollevi A, Linderoth B. Effect of spinal cord stimulation on tactile hypersensitivity in mononeuropathic rats is potentiated by simultaneous GABA(B) and adenosine receptor activation. Neurosci Lett. 1998 a;247:183–186. doi: 10.1016/s0304-3940(98)00324-3. [DOI] [PubMed] [Google Scholar]
- Cui JG, O’Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1998 b;73:87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246–1254. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- Deer TR, Raso LJ. Spinal cord stimulation for refractory angina pectoris and peripheral vascular disease. Pain Physician. 2006;9:347–52. [PubMed] [Google Scholar]
- Dolcet X, Egea J, Soler RM, Martin-Zanca D, Comella JX. Activation of phosphatidylinositol 3-kinase, but not extracellular-regulated kinases, is necessary to mediate brain-derived neurotrophic factor-induced motoneuron survival. J Neurochem. 1999;73:521–531. doi: 10.1046/j.1471-4159.1999.0730521.x. [DOI] [PubMed] [Google Scholar]
- El-Kholy W, Macdonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB. The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J. 2003;17:720–722. doi: 10.1096/fj.02-0802fje. [DOI] [PubMed] [Google Scholar]
- Foreman RD, Beall JE, Coulter JD, Willis WD. Effects of dorsal column stimulation on primate spinothalamic tract neurons. J Neurophysiol. 1976;39:534–546. doi: 10.1152/jn.1976.39.3.534. [DOI] [PubMed] [Google Scholar]
- Izzo E, Martin-Fardon R, Koob GF, Weiss F, Sanna PP. Neural plasticity and addiction: PI3-kinase and cocaine behavioral sensitization. Nat Neurosci. 2002;5:1263–1264. doi: 10.1038/nn977. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van-Der-Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krens SF, Spaink HP, Snaar-Jagalska BE. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006;580:4984–4990. doi: 10.1016/j.febslet.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Pezet S, McMahon SB, Malcangio M. The signaling components of sensory fiber transmission involved in the activation of ERK MAP kinase in the mouse dorsal horn. Mol Cell Neurosci. 2003;24:259–270. doi: 10.1016/s1044-7431(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- Lin Q, Zou X, Ren Y, Wang J, Fang L, Willis WD. Involvement of peripheral neuropeptide Y receptors in sympathetic modulation of acute cutaneous flare induced by intradermal capsaicin. Neuroscience. 2004;123:337–347. doi: 10.1016/j.neuroscience.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation. 1999;2:150–164. doi: 10.1046/j.1525-1403.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Fedorcsak I, Meyerson BA. Peripheral vasodilatation after spinal cord stimulation: animal studies of putative effector mechanisms. Neurosurgery. 1991 a;28:187–195. [PubMed] [Google Scholar]
- Linderoth B, Gunasekera L, Meyerson BA. Effects of sympathectomy on skin and musclemicroculation during dorsal column stimulation: animal studies. Neurosurgery. 1991 b;29:874–879. doi: 10.1097/00006123-199112000-00012. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Herregodts P, Meyerson BA. Sympathetic mediation of peripheral mediation of peripheral vasodilation induced by spinal cord stimulation: animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery. 1994;35:711–719. doi: 10.1227/00006123-199410000-00018. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med. 2006;7:S14–26. [Google Scholar]
- Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- Rosen LB, Ginty DD, Weber MJ, Greenberg ME. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994;12:1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Stiller CO, Cui JG, O’Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma-aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39:367–374. doi: 10.1097/00006123-199608000-00026. discussion 374-375. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Tu YJ, Yan JY, Willis WD. Activation of protein kinase B/Akt signaling pathway contributes to mechanical hypersensitivity induced by capsaicin. Pain. 2006;120:86–96. doi: 10.1016/j.pain.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Sung CS, Wen ZH, Chang WK, Chan KH, Ho ST, Tsai SK, Chang YC, Wong CS. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem. 2005;94:742–52. doi: 10.1111/j.1471-4159.2005.03226.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Crane AM, Jehle J, Cook M, Kennedy C, Sokoloff L. Role of the cerebellar fastigial nucleus in the physiological regulation of cerebral blood flow. J Cereb Blood Flow Metab. 1995;15:128–142. doi: 10.1038/jcbfm.1995.15. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Low intensity spinal cord stimulation may induce cutaneous vasodilation via CGRP release. Brain Res. 2001;896:183–187. doi: 10.1016/s0006-8993(01)02144-8. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Role of primary afferents in spinal cord stimulation-induced vasodilation: characterization of fiber types. Brain Res. 2003 a;959:191–198. doi: 10.1016/s0006-8993(02)03740-x. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Local cooling alters neural mechanisms producing changes in peripheral blood flow by spinal cord stimulation. Auton Neurosci. 2003 b;28:117–127. doi: 10.1016/S1566-0702(03)00017-1. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Komori N, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Mechanisms of sustained cutaneous vasodilation induced by spinal cord stimulation. Auton Neurosci. 2004;114:55–60. doi: 10.1016/j.autneu.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Lickiss T, Moss A, Fitzgerald M. Primary and secondary hyperalgesia can be differentiated by postnatal age and ERK activation in the spinal dorsal horn of the rat pup. Pain. 2007;128:157–168. doi: 10.1016/j.pain.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Wang H, Dai Y, Fukuoka T, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Enhancement of stimulation-induced ERK activation in the spinal dorsal horn and gracile nucleus neurons in rats with peripheral nerve injury. Eur J Neurosci. 2004;19:884–890. doi: 10.1111/j.0953-816x.2004.03203.x. [DOI] [PubMed] [Google Scholar]
- Willis WD., Jr. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- Wu J, Su G, Ma L, Zhang X, Lei Y, Li J, Lin Q, Fang L. Protein kinases mediate increment of the phosphorylation of cyclic AMP-responsive element binding protein in spinal cord of rats following capsaicin injection. Mol Pain. 2005;1:1–26. doi: 10.1186/1744-8069-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Sensory fibers containing vanilloid receptor-1(VR-1) mediate spinal cord stimulation-induced vasodilation. Brain Res. 2006 a;1107:177–184. doi: 10.1016/j.brainres.2006.05.087. [DOI] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Extracellular signal-regulated kinase (ERK) and protein kinase B (AKT) pathways involved in spinal cord stimulation (SCS)-induced vasodilation. FASEB J. 2006 b;20:A1402. doi: 10.1016/j.brainres.2007.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanilloid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res. 2007a;1156:80–92. doi: 10.1016/j.brainres.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Autonomic Neuroscience: Basic and Clinical. 2007b doi: 10.1016/j.autneu.2007.11.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Thorkilsen M, Qin C, Farber JP, Linderoth B, Foreman RD. Effects of spinal cord stimulation on peripheral circulation in stretozotocin-induced diabetic rats. Neuromodulation. 2007c;10:217–223. doi: 10.1111/j.1525-1403.2007.00111.x. [DOI] [PubMed] [Google Scholar]
- Xin WJ, Gong QJ, Xu JT, Yang HW, Zang Y, Zhang T, Li YY, Liu XG. Role of phosphorylation of ERK in induction and maintenance of LTP of the C-fiber evoked field potentials in spinal dorsal horn. J Neurosci Res. 2006;84:934–943. doi: 10.1002/jnr.21013. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]