Abstract
FOXP2, the first gene causally linked to a human language disorder, is implicated in song acquisition, production and perception in oscine songbirds, the evolution of speech and language in hominids and the evolution of echolocation in bats. Despite the evident relevance of Foxp2 to vertebrate acoustic communication, a comprehensive description of neural expression patterns is currently lacking in mammals. Here we use immunocytochemistry to systematically describe the neural distribution of Foxp2 protein in four species of muroid rodents: Scotinomys teguina and S. xerampelinus (‘singing mice’), the deer mouse, Peromyscus maniculatus, and the lab mouse, Mus musculus. While expression patterns were generally highly conserved across brain regions, we identified subtle but consistent interspecific differences in Foxp2 distribution, most notably in the medial amygdala and nucleus accumbens, and in layer V cortex throughout the brain. Throughout the brain, Foxp2 was highly enriched in areas involved in modulation of fine motor output (striatum, mesolimbic dopamine circuit, olivocerebellar system), and in multimodal sensory processing and sensorimotor integration (thalamus, cortex). We propose a generalized model for Foxp2-modulated pathways in the adult brain including, but not limited to, fine motor production and auditory perception.
Keywords: acoustic communication, basal ganglia, language, limbic, sensorimotor integration, vocalization
Introduction
Since the identification of forkhead box transcription factor, FOXP21 as a causal factor in a severe speech and language disorder (Lai et al., 2001), it remains the only gene directly implicated in the genesis of language dysfunction (MacDermot et al., 2005; Shriberg et al., 2006; Zeesman et al., 2006). Likewise, a signature of positive selection on FOXP2 in the hominid lineage suggests a role in the evolution of verbal communication (Enard et al., 2002). Several lines of evidence, however, indicate a greater functional and taxonomic breadth for this gene. For example, mammalian Foxp2 is highly expressed in embryonic CNS, pulmonary, heart and gut tissue, and is essential to normal lung development (Shu et al., 2001; 2007). The recent identification of a large and functionally diverse array of FOXP2 transcriptional targets in human cell lines (Vernes et al., 2007) and fetal brain tissue (Spiteri et al., 2007) points to fundamental roles in neural patterning, development and connectivity. In vivo work in oscine songbirds demonstrates that the involvement of FoxP2 in the perception (Rochefort et al., 2007), acquisition (Haesler et al., 2007) and production (Teramitsu and White, 2006) of complex acoustic signals is not exclusive to humans.
Most recently, two independent studies in mice carrying the homolog of the best-characterized causative mutation in human FOXP2-mediated speech and language disorder (R553H; R552H in Mus; Groszer et al., 2008; Fujita et al., 2008) highlight the complex role of this gene and possible sensitivity to different genetic backgrounds. Whereas both studies found global motor deficits in mice homozygous for the R552H mutation, heterozygote animals in the study of Groszer et al. (2008) exhibited significant impairments in motor skill learning and synaptic plasticity but not in pup vocal behavior, while heterozygote pups studied by Fujita et al. (2008) produced fewer, less stable vocalizations than wild-type mice. However, neither study examined the vocal consequences of Foxp2 deficiency in adult mice.
While studies of vocal behavior in rodents have traditionally focused on the ultrasonic vocalizations produced by pups isolated from their dam (reviewed in Ehret, 2005), mounting evidence from diverse rodent taxa supports an integral role for acoustic communication in adult social behavior (Kalcounis-Rueppell et al., 2006; Kapusta et al., 2007; Yosida et al, 2007). Characterization of the diverse vocal repertoire of lab mice (Gourbal et al., 2004; Holy and Guo, 2005), and “laughter” in rats (reviewed in Panksepp, 2007) suggests that rodents are not only genetically tractable, but behaviorally relevant models for mammalian vocal communication.
To date, studies describing the neural distribution of Foxp2 mRNA and protein in rodents have been limited to Mus and Rattus (Ferland et al., 2003; Lai et al., 2003; Takahashi et al., 2003; Wijchers et al., 2006), and have not considered whether neural expression patterns may have functional relevance to vocal production and perception in these species, or in rodents in general. Likewise, Foxp2 expression studies in rodent models have focused primarily on embryonic and post-natal development, highlighting regions with relevance to human speech production, (e.g. striatum, cortex and cerebellum). However, the temporal distribution of Foxp2 is somewhat unusual among transcriptional regulators of neural development in that brain-wide expression persists in adults (e.g. Wang and Liu, 2001; Ohira et al., 2002); a functional role in regulating circuitry in the mature brain is supported by experimental studies in songbirds (Haesler et al., 2007) and mice (Groszer et al., 2008). While post-developmental expression has been described in some detail in the rat striatum (Takahashi et al., 2003), comprehensive characterization of Foxp2 distribution in the brain of any mammal is surprisingly lacking.
The unique acoustic structure and integral social function of vocalizations in ‘singing mice’, Scotinomys teguina and S. xerampelinus, motivated us to examine in detail the distribution of neural Foxp2 protein in these Central American muroid rodents. Scotinomys calls are highly stereotyped, comprising a temporally compact (1–16 s) but structurally complex series of notes (Miller and Engstrom, 2007). A repetition rate of up to 20 notes per second (S. M. Phelps, unpublished) requires precise coordination of fine orofacial movements. Calls span both audible and ultrasonic frequencies (8–50 kHz) and are produced by both sexes in social contexts (Fernandez, 2006; Miller and Engstrom, 2007). Call structure is sexually monomorphic but males call more than females and produce longer calls. Behavioral data on the elicitation of male call production (Fernandez, 2006) suggest that call functions include territorial advertisement and mate attraction.
Here, we systematically describe the brain-wide distribution of Foxp2 protein in S. teguina and S. xerampelinus, including multiple adults of both sexes to investigate the potential for individual or sex differences in expression patterns. To evaluate the extent to which neural Foxp2 is conserved in singing mice relative to other muroid rodents we compare expression patterns in the two Scotinomys species to those in the deer mouse, Peromyscus maniculatus, a representative of the family Sigmodontinae to which Scotinomys belongs, and the more distantly related lab mouse, Mus musculus. Phylogenetic relationships between the four species are represented in Fig. 1. Because call articulation in singing mice requires tight control of facial musculature and impaired FOXP2 function in humans is consistently associated with inability to coordinate fine orofacial movements (Lai et al., 2001; Watkins et al., 2002a; Shriberg et al., 2006), we predicted that expression patterns unique to Scotinomys should localize to brain regions subserving fine motor control.
Fig. 1.
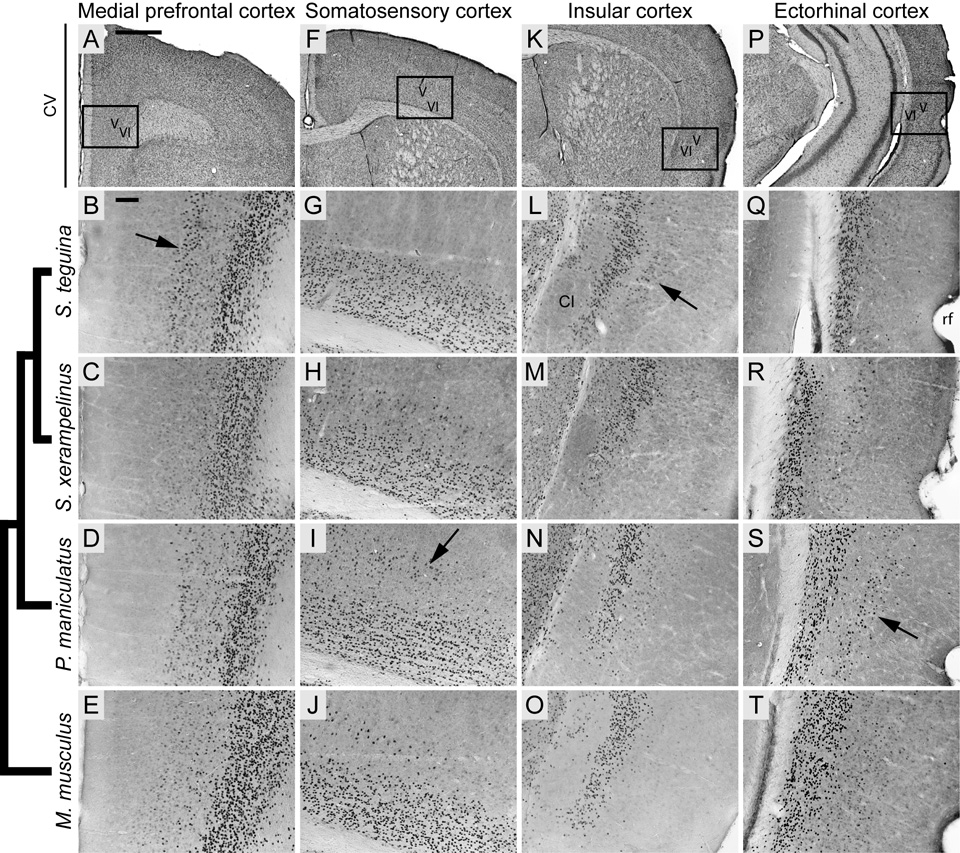
Cortical distribution of Foxp2 in four species of muroid rodents, showing high conservation in layer VI and variable expression in layer V. Phylogenetic relationships represented by cladogram on left after Steppan et al. (2004) and Reeder et al. (2006). A, F, K and P are coronal cresyl violet (CV) sections from S. teguina; boxed areas identify representative examples of Foxp2 expression from approximately the same levels in B–E, G–J, L–O and Q–T, respectively. B–E: Layer V Foxp2 expression in medial prefrontal cortex (ctx) is pronounced in S. teguina (arrow in B), lacking in S. xerampelinus and weak in P. maniculatus and M. musculus. G–J: Layer V Foxp2 expression in medial somatosensory ctx is lacking in S. teguina and most pronounced in P. maniculatus (arrow in I). L–O: Layer V Foxp2 expression in insular ctx at level of claustrum (CL) is pronounced in S. teguina (arrow in L), weak in S. xerampelinus and P. maniculatus and absent in M. musculus. Q–T: Layer V Foxp2 expression at ectorhinal/perirhinal ctx transition medial to the rhinal fissure (rf) is most pronounced in P. maniculatus (arrow in S). All sections are from adult males; females are indistinguishable from conspecific males. Scale bars = 500 µm in A (applies to A, F, K, P); 100 µm in B (applies to B–E, G–J, L–O, Q–T).
Taking advantage of the well-characterized neuronal connections in the rat brain (e.g. Paxinos, 2004) we asked whether the structures enriched for Foxp2 across all four species subserve related circuits. We used these data to develop a generalized model for Foxp2-modulated pathways in mammals. Given the strong association between FOXP2 mutations and speech and language deficits, we were particularly interested in defining the extent to which Foxp2 is preferentially expressed in neural circuits involved in fine motor production and auditory perception.
Materials and Methods
Animals and tissue preparation
Scotinomys teguina and S. xerampelinus (n = 12; 3 per sex, per species) used in this study were unrelated, lab-reared adults, derived from wild-caught individuals captured in Monteverde, Costa Rica (S. teguina) and Parque Internacional La Amistad, Panamá (S. xerampelinus). Lab-reared Peromyscus maniculatus (n = 2 males, 1 female) and Mus musculus (n = 1 male, 1 female; Jackson Laboratories B6 (C57BL) strain) were also adults. Subjects were euthanized by CO2 or isofluorane inhalation; brains were extracted immediately and drop fixed in 4% paraformaldehyde in PBS. Following a minimum of 24 hrs in fixative at 4°C, brains were cryoprotected for an additional 24–48 hrs in 30% sucrose made in 4% paraformaldehyde-PBS. Coronal sections from the olfactory bulbs through the cerebellum were cut to 40µm on a freezing microtome and collected into PBS. All animal protocols were approved by the IACUC committee at University of Florida and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Foxp2 Immunocytochemistry
Floating sections were incubated for 15 min in 3% H2O2 in 0.01M PBS (pH 7.2–7.4) to quench endogenous peroxidase activity, washed in PBS, blocked for 1 hr in 10% normal goat serum (NGS) with 3% Triton-X in PBS (PBSTX), and washed in PBS. Sections were incubated overnight at 4°C with rabbit polyclonal antibody directed against Foxp2 (Abcam, Cambridge, MA; ab16046), diluted 1:1000 in 5% NGS-PBSTX. The Foxp2 antibody used in this study was raised against a synthetic peptide conjugated to KLH and derived from residues 703–715 of exon 17 at the C-terminus of Human FOXP2. Control sections were incubated without the primary antibody, or with primary antibody that had been pre-incubated overnight at 4°C with 1µg/ml of the peptide used in antibody production (Abcam, ab16278). The next day sections were washed extensively in PBS, incubated for 1 hr with biotinylated goat anti-rabbit lgG, washed and incubated for 1 hr in avidin-biotin-horseradish peroxidase solution (Leinco, St. Louis, MO; R106 and A106, both diluted 1:200 in 5% NGS-PBSTX). Foxp2 protein was visualized using the peroxidase/diaminobenzidine (DAB) method with nickel intensification. Sections were immersed in 0.07% DAB (Sigma, St. Louis, MO) in PBS with 0.1% nickel chloride and 0.03% H2O2, and developed for 1–3 min until antibody-antigen binding sites were stained black. The reaction was stopped in PBS. Rinsed sections were mounted onto slides, air dried overnight, dehydrated in serial alcohol dilutions and cover slipped from xylene with Eukitt (Calibrated Instruments, Inc., Hawthorne, NY).
Analysis and figure preparation
Sections were visualized using a Zeiss Axiophot microscope interfaced with a CCD camera (Retiga 2000R; resolution 1,600 × 1,200 pixels; QImaging). Structures were identified using the mouse atlas (Paxinos and Franklin, 2001) for Mus, and a combination of the mouse and rat (Paxinos and Watson, 1998) atlases for the two Scotinomys species and P. maniculatus. For each species, a complete set of adjacent sections was stained with cresyl violet. To discriminate nuclei in the amygdala and thalamus, adjacent sections were stained for acetylcholinesterase. For regions with complex Foxp2 expression patterns (e.g. cortex, striatum, extended amygdala, hypothalamus, thalamus, periaqueductal grey), we chose 3–4 representative levels per region, digitized corresponding sections for each individual and constructed layouts in Adobe Illustrator CS3 (v. 13.0.0). This approach allowed direct visual comparison of Foxp2 distributions at three levels: among same-sex conspecifics, between conspecific males and females (S. teguina and S. xerampelinus only), and across all four species. Final images were imported into Adobe Photoshop CS3 (v10.0.1) and contrast and brightness adjusted to minimize among-individual differences in non-specific staining. Figures were assembled and labeled in Adobe Illustrator CS3 (v13.0.2).
Results
Overall, we found a highly conserved pattern of Foxp2 expression across the four muroid species examined in this study. With some notable exceptions (described below), Foxp2 was enriched in the same structures and between-structure differences in strength and density of expression were qualitatively comparable across species. We found no discernable inter-individual variation, or evidence of gross sex differences in any of the species. In the systematic description of Foxp2 expression below, expression patterns were consistent across all four species unless otherwise specified.
The specificity of the Foxp2 antibody used in this study was supported by the absence of Foxp2-positive cells in sections pre-adsorbed with FOXP2 peptide, and there was no Foxp2 expression in controls incubated without primary antibody (data not shown). We note that multiple FOXP2/FoxP2 splice variants have been identified in the nervous systems of humans (Lai et al., 2001; Bruce and Margolis, 2002) and zebra finch (Haesler et al., 2004). At least one variant does not contain the residues targeted by the antibody used in this study (FOXP2.S, Bruce and Margolis, 2002; alternately referred to as FOXP2.10+, Vernes et al., 2006). While the data presented here do not exclude the possibility that truncated splice variants have different neural distributions, the expression of multiple isoforms in the same tissue types (Lai et al., 2001; Bruce and Margolis, 2002) and the postulated role of the FOXP2.10+ variant in modulating other isoforms (Vernes et al., 2006), suggest that this is unlikely.
Cortex
The most striking feature of the cortical distribution of Foxp2 was strong localization to layer VI throughout the brain, an expression pattern previously reported for Mus (Ferland et al., 2003). However, Foxp2 was also enriched in layer V. While this distribution was diffuse relative to that in layer VI, localization to particular cortical areas was evident, with some clear differences among species (Fig. 1).
In the rostral forebrain, all species exhibited some degree of Foxp2 expression in dorsomedial layer V, particularly in premotor and medial motor cortices. In S. teguina, however, a thin but pronounced band of Foxp2-positive cells extended ventrally throughout cingulate and prelimbic cortices (Fig. 1B). Expression in these areas was weak and difuse in P. maniculatus and Mus and absent in S. xerampelinus (Fig. 1C–E) Progressing caudally, layer V expression in the hind- and forelimb compartments of primary somatosensory cortex was qualitatively denser in P. maniculatus relative to S. xerampelinus and Mus, and completely lacking in S. teguina (Fig. 1G–J).
In S. teguina, a small but distinct population of Foxp2-expressing cells was focused in layer V of granular and dorsal disgranular cortices (Fig. 1L), extending rostro-caudally from the level of the decussation of the corpus callosum to the beginning of the gray matter of the hippocampal formation. This distribution was weak and transient in S. xerampelinus and P. maniculatus and absent in Mus (Fig. 1M–O). All species exhibited moderate Foxp2 expression in layer V of the medial part of the posterior parietal association cortex. In P. maniculatus, however, expression extended laterally throughout parietal cortex and medially through visual and retrosplenial agranular cortices, being most pronounced in rostral visual areas. P. maniculatus was also distinguished by a distinct cluster of Foxp2-enriched cells in layer V at the transition between ectorhinal and perirhinal cortices (Fig. 1Q–T).
Olfactory system, septum and extended amygdala
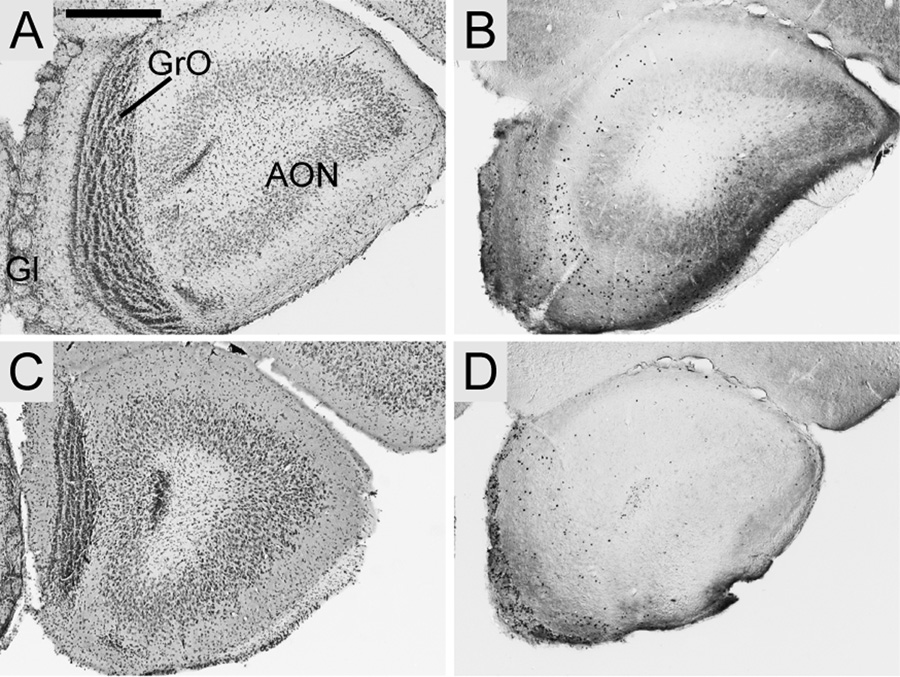
In the main olfactory bulb, Foxp2 expression was strong but diffuse in the glomerular layer, both in the core and surrounding shell of glomeruli. There was a minimal scattering of positive cells in the internal plexiform and granule cell layers and contrastingly dense expression in the accessory olfactory bulb. Contrary to an earlier study in Mus (Ferland et al., 2003) Foxp2 expression in the anterior olfactory nucleus was not observed in any species. Representative sections from S. teguina and Mus are shown in Fig. 2.
Fig. 2.
Foxp2 expression in the main olfactory bulb in coronal sections from S. teguina (A–B) and M. musculus (C–D). A and C are cresyl violet sections from each species; B and D are representative examples of Foxp2 expression from approximately the same level. Foxp2-enriched cells are present in the glomerular (Gl) and granular (GrO) cell layers, but not in the anterior olfactory nucleus (AON). Expression in S. teguina and M. musculus is representative of S. xerampelinus and P. maniculatus. Scale bar = 500 µm.
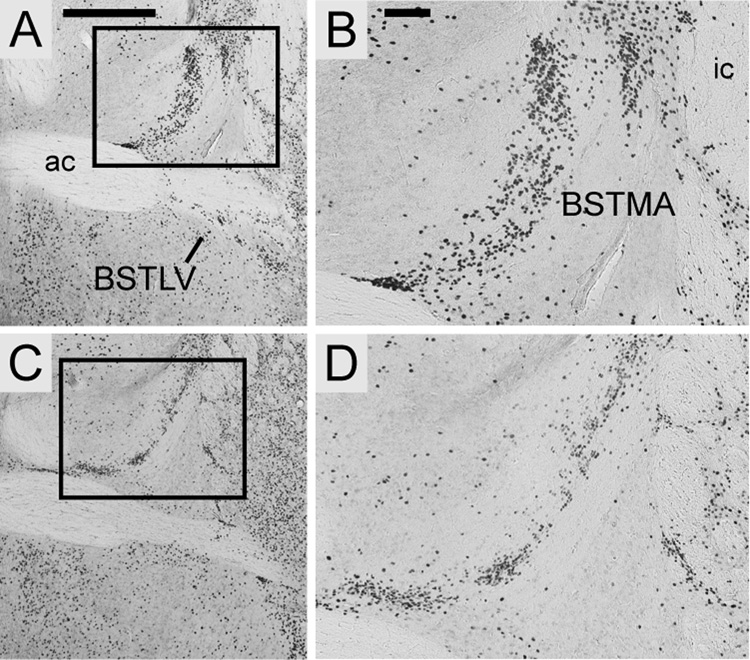
All species exhibited strong, punctate Foxp2 expression in the intermediate and ventral nuclei of the lateral septum, and in the triangular septal nucleus. Expression was most concentrated in the rostral intermediate nucleus, decreasing caudally. In the rostral portion of the bed nucleus of the stria terminalis (BST), Foxp2 protein was concentrated in the anterior nucleus of the medial division, with diffuse expression in the ventral nucleus of the lateral division (Fig. 3). More caudally in the BST, expression was diffuse in the intraamygdaloid division (BSTIA), with a more concentrated population of positive cells extending ventrolaterally along the medial margin of the BSTIA and the posterodorsal nucleus of the medial amygdala. Foxp2 was also enriched in the bed nucleus of accessory olfactory tract of all species. Expression was scattered in the central portion of the sublenticular extended amygdala (SLEA).
Fig. 3.
Localized Foxp2 expression in the bed nucleus of the stria terminalis, medial nucleus of the anterior division (BSTMA) and ventral nucleus of the lateral division (BSTLV). Expression in coronal sections from S. teguina (A–B) and M. musculus (C–D) is representative of S. xerampelinus and P. maniculatus. Boxed areas in A and C are magnified in B and D. Scale bars = 500 µm in A (applies to A, C); 100 µm in B (applies to B, D); ac, anterior commisure; ic, internal capsule.
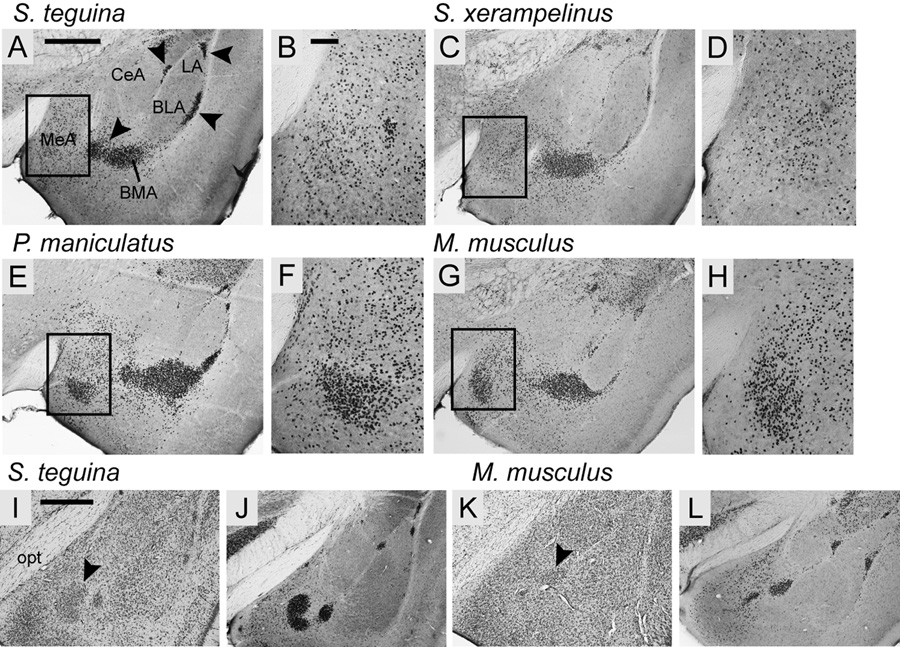
Foxp2 protein in the main amygdaloid nuclei was predominantly localized to the medial and basomedial amygdala, with scattered expression in the anterior cortical nuclei, and no expression in the central, lateral or basolateral nuclei (Fig. 4). Within the medial nuclei, all species exhibited strong, diffuse expression throughout the dorsal nucleus. However, a localized concentration of Foxp2 expressing cells in the anterior part of the ventral nucleus was observed in P. maniculatus and Mus (Fig. 4E–H), but not in either Scotinomys species (Fig. 4A–D).
Fig. 4.
The distribution of Foxp2 in the amygdala of four species of muroid rodents is conserved across nuclei but varies within the medial amygdala (MeA). A–H: In the anterior amygdala Foxp2 is mainly expressed in the MeA, basomedial (BMA) and the intercalated nuclei (arrowheads in A) of all four species, and is absent from the central (CeA), lateral (LA) and basolateral (BLA) nuclei. Boxed areas in A, C, E and G are magnified in B, D, F and H, showing a high concentration of Foxp2-positive cells in the ventral compartment of MeA in P. maniculatus (E–F) and M. musculus (G–H), but not in S. teguina (A–B) or S. xerampelinus (C–D). I–L: In the posterior amygdala Foxp2 is most concentrated in the intercalated nuclei. Arrowheads in cresyl violet sections for S. teguina (I) and M. musculus (K) identify the high density cell clusters in which Foxp2 is enriched. Differences between Foxp2 sections for S. teguina (J) and M. musculus (L) do not reflect species differences in expression; the representative section for S. teguina is slightly caudal to that for M. musculus. All sections are from adult males; females are indistinguishable from conspecific males. Scale bars = 500 µm in A (applies to A, C, E, G); 100 µm in B (applies to B, D, F, H); 500 µm in I (applies to I–L); opt, optic tract.
Most strikingly, Foxp2 protein was highly concentrated in the main intercalated nucleus (IM) and in the smaller intercalated cell masses along the medial margins of the basolateral anterior and the lateral nuclei, and along the lateral margin of basolateral posterior nucleus (Fig. 4). Foxp2 expression also extended medially from IM into the poorly characterized transitional region of the SLEA/BSTIA, which separates the medial and basomedial nuclei from the central and basolateral nuclei.
Basal ganglia and mesolimbic dopamine circuit
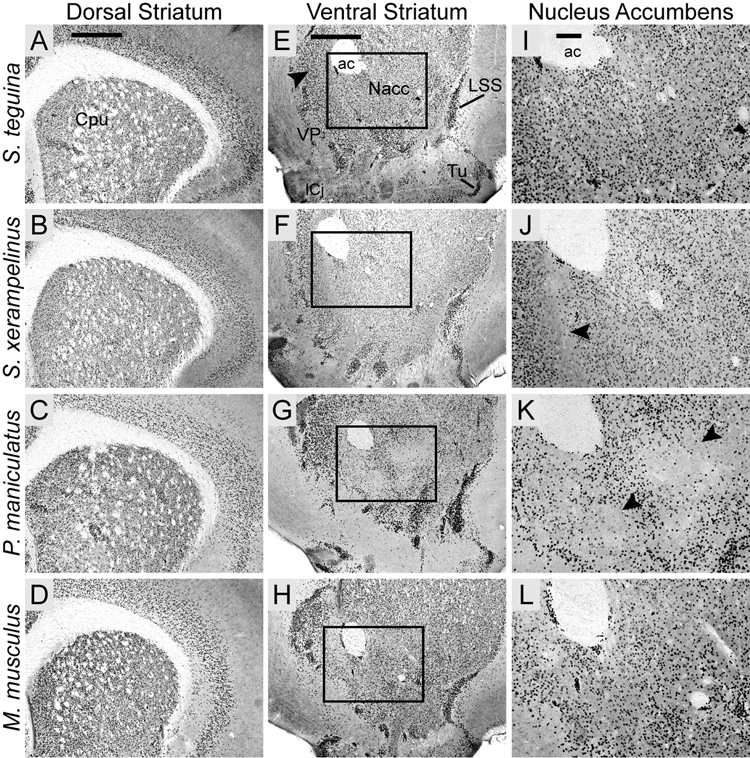
Consistent with studies in Mus and Rattus (Ferland et al., 2003; Takahashi et al., 2003), Foxp2 expression was strong but punctate and heterogeneously distributed throughout the dorsal striatum (caudate putamen) of all four species (Fig. 5A–D). Expression was similarly strong and heterogeneous in the ventral striatum, with a qualitatively higher density of Foxp2-expressing cells in the dorsomedial shell relative to the core of the nucleus accumbens (Nacc). Positive cells were distributed in clusters, particularly along the ventral margin of the shell (Fig. 5E–H). This punctate pattern persisted more caudally in the interstitial nucleus of the posterior limb of the accumbens. In P. maniculatus and S. xerampelinus, the Nacc distribution of Foxp2 included an area with minimal expression which extended dorsolaterally across the core-shell boundary. However, the Foxp2-poor area began ventromedial to the anterior commisure in S. xerampelinus (Fig. 5J) but ventrolateral to the commisure in P. maniculatus (Fig. 5K). These areas of low expression were evident in both species from the level of the decussation of the corpus callosum to the disappearance of the Nacc, corresponding to approximately 360 µm in S. xerampelinus and 480 µm in P. maniculatus. Even taking into account high cell densities, Foxp2 expression in all species was particularly pronounced in the lateral stripe of the striatum, the islands of Calleja and the olfactory tubercle. Small clusters of Foxp2-enriched cells were observed in anterior ventral pallidum (VP); expression was minimal in posterior VP and absent from the nuclei of the diagonal band (Fig. 5E–H).
Fig. 5.
The striatal distribution of Foxp2 in four species of muroid rodents is conserved in the caudate putamen but exhibits subtle interspecific variation in the nucleus accumbens. A–D: All four species exhibit a characteristically heterogeneous pattern of Foxp2 expression throughout the caudate putamen (CPu). E–H: A similarly heterogeneous distribution is observed in the ventral striatum with local concentrations of Foxp2 expression in the dorsomedial shell of the nucleus accumbens (Nacc; arrow in E). Foxp2 is highly concentrated in the lateral stripe of the striatum (LSS), the islands of Calleja (ICj) and the olfactory tubercle (Tu), and in some areas in the anteromedial ventral pallidum (VP). Boxed areas are magnified in I–L, showing species differences in Foxp2 distribution in lateral nucleus accumbens. Uninterrupted expression in S. teguina (I) and M. Musculus (L) contrasts with areas of minimal expression, located ventromedial to the anterior commisure (ac) in S. xerampelinus (arrow in J) and ventrolateral to the ac in P. maniculatus (arrows in K). Scale bars = 500 µm in A (applies to A–D); 500 µm in E (applies to E–H); 100 µm in I (applies to I–L).
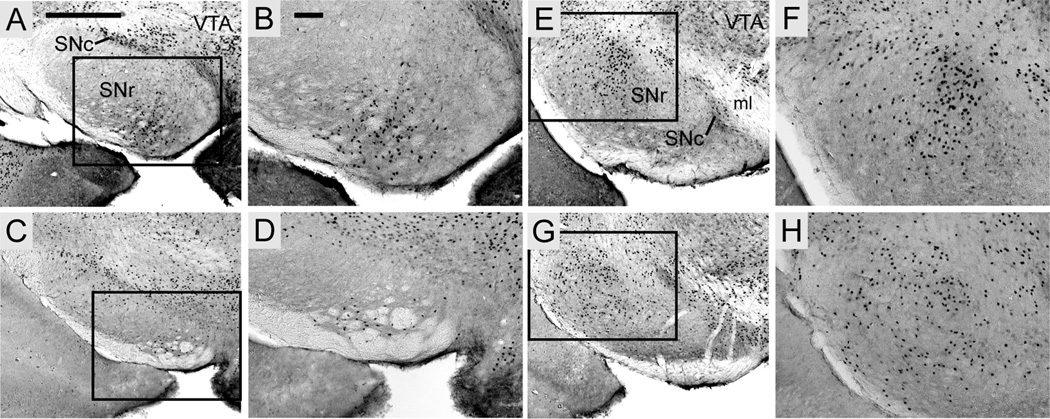
Foxp2 expression was scattered in both the lateral and medial compartments of the globus pallidus but was highly concentrated in the cell-dense subthalamic nucleus. Scattered positive cells in the rostral substantia nigra pars reticulata were mainly localized ventromedially (Fig. 6A–D); progressing caudally, expression shifted dorsolaterally (Fig. 6E–H). Expression was continuous throughout the substantia nigra pars compacta and the adjacent ventral tegmental area (Fig. 6).
Fig. 6.
Foxp2 expression in the substantia nigra pars reticulata (SNr) is localized ventromedially in the anterior compartment (A–D) and dorsolaterally in the posterior compartment (E–H). Foxp2 distribution is continuous in the pars compacta (SNc). Expression in S. teguina (A–B, E–F) and M. musculus (C–D, G–H) is representative of S. xerampelinus and P. maniculatus. Boxed areas in A, C, E and G are magnified in B, D, F and H, respectively. Scale bars = 500 µm in A (applies to A, C, E, G); 500 µm in B (applies to B, D, F, H); VTA, ventral tegmental area; ml, medial lemniscus.
Hypothalamus
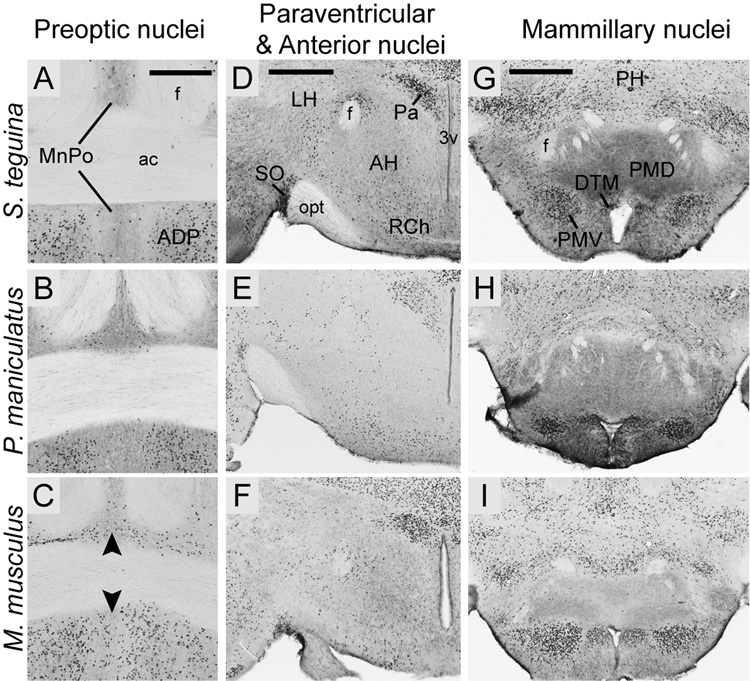
In the anterior hypothalamus, Foxp2 was selectively enriched in some but not all preoptic nuclei. Expression was strong but diffuse in the medial preoptic area and the circular nucleus, more concentrated in anterodorsal (Fig. 7 A–C) and strial nuclei, widely scattered in the lateral preoptic area and absent from the medial nucleus. Expression in the midline median preoptic nucleus was pronounced in Mus relative to P. maniculatus and Scotinomys (Fig. 7A–C).
Fig. 7.
Foxp2 expression in selected hypothalamic nuclei in coronal sections from S. teguina, P. maniculatus and M. musculus. A–C: Expression in the midline median preoptic nucleus (MnPO) is pronounced in M. musculus (arrows in C) and minimal in P. maniculatus (B) and S. teguina (A). Expression in the anterodorsal nucleus (ADP) of the preoptic area is conserved across species. D–F: In all species, expression is concentrated in the paraventricular nuclei (Pa) and the supraoptic nucleus (SO), diffuse in the retrochiasmatic area (RCh) and absent from the anterior hypothalamic nuclei (AH). D–F: Foxp2 in mammillary nuclei, showing concentrated expression in dorsal tuberomammillary (DTM) and ventral premammillary (PMV) nuclei, and lack of expression in dorsal premammillary nucleus (PMD). Expression in S. teguina (A, D, G) is representative of S. xerampelinus. Scale bars = 300 µm in A (applies to A–C); 500 µm in D (applies to D–F); 500 µm in G (applies to G–I). f, fornix; ac, anterior commisure; 3V, third ventricle; opt, optic tract; PH, posterior hypothalamus.
Progressing caudally, Foxp2 was concentrated in the paraventricular nuclei, particularly in the parvocellular nuclei, in the supraoptic nuclei (Fig. 7D–F), in the lateral part of the arctuate nucleus, and in the dorsomedial nuclei. There was strong but diffuse expression in medial tuberal nucleus and in the lateral and posterior hypothalamus. No Foxp2-positive cells were observed in the anterior or ventromedial nuclei of any species. In the mammillary nuclei, expression was strong in the dorsal tuberomammillary and ventral premammillary nuclei, and in supramammillary nuclei, but was minimal in the lateral nucleus and absent from the medial nuclei (Fig. 7G–I).
Thalamus and collicular nuclei
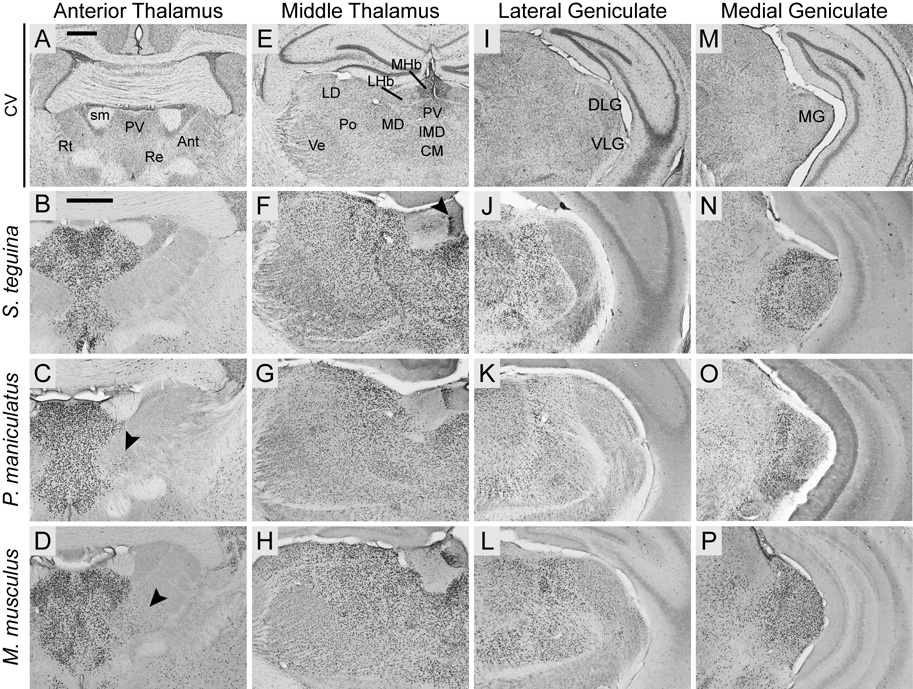
Thalamic Foxp2 expression was most highly concentrated in the midline and intralaminar nuclei (Fig. 8B–D), particularly the paraventricular, paracentral, paratenial, central medial, interanteromedial, rhomboid, reuniens and xiphoid nuclei. Foxp2 was completely absent from the reticular nucleus in all species and was minimally expressed in the anteromedial nucleus in Peromuscus and Mus, but not in either Scotinomys species (Fig. 8B–D).
Fig. 8.
Foxp2 is widely expressed throughout the thalamus in Scotinomys (represented by S. teguina), P. maniculatus and M. musculus. A, E, I andM are coronal cresyl violet (CV) sections from S. teguina from approximately the same levels as representative examples of Foxp2 expression in B–D, F–H, J–L and N–P, respectively. B–D: Foxp2 is concentrated in midline and intralaminar nuclei, including paraventricular (PV) and reuniens (Re), and absent from reticular nucleus (Rt). Expression in anterior nuclei (Ant) is observed only in the anteromedial nucleus in P. maniculatus (arrow in C) and M. musculus (arrow in D). F–H: Uninterrupted expression in PV, intermediodorsal (IMD), central medial (CM), mediodorsal (MD), posterior (Po), laterodorsal (LD) and ventral nuclei (Ve), strong localized expression in the medial habenular nucleus (MHb; arrow in F), and diffuse expression in the lateral nucleus (LHb). J–L: Expression in lateral geniculate is concentrated in dorsal (DLG) relative to ventral (VLG) nuclei. N–P: Foxp2 is highly enriched in medial geniculate (MG). Scale bars = 500 µm in A (applies to A, E, I, M); 500 µm in B (applies to B–D, F–H, J–L, N–P); sm, stria medullaris of thalamus.
Expression was strong in mediodorsal and most lateral nuclei (Fig. 8F–H), and comparatively weaker in ventromedial and pretectal nuclei. There was strong localized expression along the lateral margin of medial habenular nucleus, with contrastingly diffuse expression in the lateral nucleus (Fig. 8F–H). Foxp2 was also expressed throughout the parafascicular, posterior and the ventral posterior nuclei, with the exception of the gustatory nucleus.
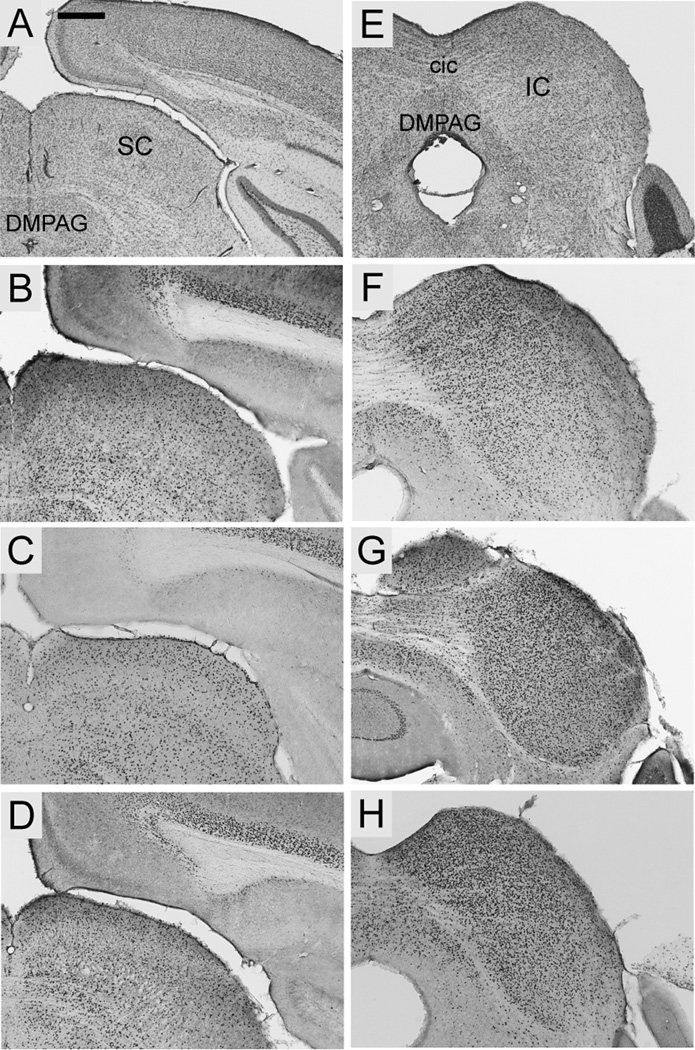
Foxp2 was highly enriched in both the lateral and medial geniculate, but was qualitatively stronger throughout the medial geniculate (Fig. 8J–L, N–P). Expression in the lateral geniculate was concentrated in the dorsal compartment and scattered in the ventral nuclei (Fig. 8 J–L). The superior colliculus exhibited strong Foxp2 expression with no evident differences among layers (Fig. 9B–D). Expression was, however, comparatively more dense in the inferior colliculus, being particularly pronounced in dorsal and central nuclei (Fig. 9F–H).
Fig. 9.
Foxp2 expression in the collicular nuclei in coronal sections from S. teguina (B, F), P. maniculatus (C, G) and M. musculus (D, H). A and E are coronal cresyl violet sections from S. teguina from approximately the same levels as representative examples of Foxp2 expression in B–D and F–H, respectively. B–D: Foxp2 is expressed throughout the superior colliculus (SC). F–H: Expression is qualitatively more concentrated in inferior colliculus (IC). Expression in S. teguina is representative of S. xerampelinus. Scale bars = 16 500 µm in A (applies to A, E); 500 µm in B (applies to B–D, F–H); DMPAG, dorsomedial periaqueductal grey; cic, commisure of inferior colliculus.
Pons, cerebellum and medulla oblongata
Foxp2 expression was minimal in the anterior region of the periaqueductal grey (PAG) with a scattering of positive cells along the midline. In posterior PAG, expression was relatively dense in the dorsal and ventrolateral nuclei. Foxp2 was not expressed in the lateral nucleus. Expression in raphe nuclei was limited to dorsal and ventrolateral parts of the dorsal nuclei (Fig. 10B–C).
Fig. 10.
Foxp2 expression in periaqueductal grey and selected raphe, parabrachial and tegmental nuclei in coronal sections from S. teguina (B, E) and M. musculus (C, F). A and D are cresyl violet sections from S. teguina from approximately the same levels as representative examples of Foxp2 expression in B–C and E–F, respectively. B–C: Periaqueductal grey expression is concentrated in dorsomedial (DMPAG) and dorsolateral (DLPAG) nuclei. In raphe nuclei expression is locally concentrated in dorsal nuclei (DR). E–F: Parabrachial, lemniscal and tegmental nuclei, showing expression in lateral (LPB) and medial parabrachial nuclei (MPB), dorsal nucleus of the lateral lemniscus (DLL) and dorsal (DTg), but not lateral (LTg) tegmental nuclei. Differences between S. teguina (E) and M. musculus (F) in parabrachial nuclei do not reflect species differences in expression; the representative section for S. teguina is slightly caudal to that for M. musculus. Expression in S. teguina and M. musculus is representative of S. xerampelinus and P. maniculatus. Scale bar = 500 µm; Su, supraoculomotor nuclei; On, oculomotor nucleus; scp, superior cerebellar peduncle; LL, lateral lemniscus.
Expression in the paralemniscal area was strong but diffuse. Foxp2 was similarly enriched in the dorsal nucleus of the lateral lemniscus, in several parabrachial nuclei (dorsal, lateral, ventral and medial), with concentrated expression in the external compartment of the lateral nucleus (Fig. 10E–F). In tegmental nuclei, expression was concentrated in ventral and dorsal nuclei and scattered in the dorsomedial tegmental area (Fig. 10E–F).
In the cerebellum, most expression was localized to the Purkinje cell layer, as previously described in Mus and Rattus (Ferland et al., 2003; Takahashi et al., 2003). Positive cells in the deep cerebellar nuclei (interposed and medial compartments) were widely scattered (Fig. 11B). In the pons and medulla, Foxp2 expression was strong but diffuse in locus coeruleus and in the oral part of the pontine reticular nucleus. In posterior medulla, expression was highly concentrated throughout the inferior olivary complex, scattered in the prepositus nucleus, and lacking or minimal in all other nuclei (Fig. 11D).
Fig. 11.
Localized Foxp2 expression in cerebellum and hindbrain, shown in sagittal sections from S. teguina. A and C are cresyl violet; B and D are Foxp2. Boxed area in A indicates region magnified in B, showing localization of Foxp2 to the Purkinje cell layer (Pc) and the interposed nucleus (Int) in the cerebellum. In the hindbrain (C–D), Foxp2 is highly concentrated in the inferior olivary nuclei (IO), scattered in the prepositus nucleus (Pr) and absent from most other nuclei in the medulla, including the caudal part of the pontine reticular nucleus (PnC) and the nucleus of the solitary tract (Sol). Expression in S. teguina is representative of S. xerampelinus, P. maniculatus and M. musculus. Scale bars = 500 µm in A (applies to A, C, D); 100 µm in B; scp, superior cerebellar peduncle.
Discussion
In this study we demonstrate that the distribution of neural Foxp2 is highly conserved across four species of muroid rodents. The sheer breadth of Foxp2 expression indicates that the locus is unlikely to selectively regulate circuits governing verbal and vocal functions. Specific influences on vocal production and perception are more likely to emerge from Foxp2 interactions with genes whose expression is localized to relevant circuits (Vernes et al., 2007; for related arguments, see Fisher, 2006). If, as molecular data suggest, FOXP2 was a factor in the evolution of speech in hominids (Enard et al., 2002; Krause et al., 2007) and echolocation in bats (Li et al., 2007), it is in an as yet unidentified subset of downstream genes that we might expect to see major neural expression differences in humans and other species with complex vocal communication.
Given these observations, is the brain-wide distribution of Foxp2 relevant to hypotheses for this gene’s function in the adult brain? Do expression patterns suggest involvement in integrated or disparate circuitry? While these questions can only be answered experimentally, the anatomical and functional connectivity of the circuits in which the distribution of Foxp2 is abundant and contiguous is consistent with the suggestion that this gene plays a phylogenetically conserved role in sensorimotor pathways (e.g. Scharff and Haesler, 2005; Fisher and Marcus, 2006). The comprehensive expression data presented in this study suggest that Foxp2 is integrally, although not exclusively, involved in pathways that subserve modulation of fine motor output, multimodal sensory processing and sensorimotor integration. We discuss evidence supporting these hypotheses and consider the evolutionary and functional implications of the interspecific expression differences detected in this study. In closing, we propose a generalized schema for Foxp2-modulated circuitry in the adult brain and suggest ways in which experimental manipulations in rodents could provide insight into the role of Foxp2 in complex vocal communication in humans and other mammals.
Foxp2 in the basal ganglia: limbic modulation of motor output?
Current models of the neural circuitry of speech production and processing recognize that this mechanically and cognitively highly complex behavior relies extensively on sub-cortical regions whose functions are not language-specific (Ullman, 2001; Friederici, 2006). In the basal ganglia circuit, the caudate and putamen have been repeatedly implicated in speech planning, timing and articulation in normal and language-impaired subjects (Jernigan et al., 1991; Pickett et al., 1998; Riecker et al., 2006). Language-impaired members of an intensively studied three generational family (KE family) share a functional mutation in the FOXP2 DNA-binding domain (Lai et al., 2001; Vernes et al., 2006) and exhibit subtle volumetric abnormalities in striatal nuclei including the caudate, in which volume is correlated with impaired performance on word and non-word repetition tests (Watkins et al., 2002a; 2002b), and the putamen, which is significantly under-activated in affected family members during both silent and spoken language tasks (Liégeois et al., 2003). While these data suggest a relationship between FOXP2 dysfunction, abnormal striatal development, and impaired orofacial motor function and linguistic processing, Foxp2 expression patterns throughout basal ganglia suggest an even stronger association with striatal limbic functions in the adult brain.
Within the dorsal striatum Foxp2 is preferentially expressed in the striosomal compartment (Takahashi et al., 2003), which plays an integral role in reward-related processes driving motivation, attention and learning (reviewed in Canales, 2005). Corticostriatal afferents from Foxp2-enriched layer VI preferentially target striosomes (Kincaid and Wilson, 1996), with most projections originating in the limbic cortices (e.g. prelimbic, infralimbic, orbital and anterior cingulate; Donoghue and Herkenham, 1986; Bayer, 1990; Wang and Pickel, 1998). A reciprocal connection exists between the striosome compartment and the dopamine-rich substantia nigra pars compacta (SNc; Gerfen, 1984; Jimenez-Castellanos and Graybiel, 1987; Canales and Graybiel, 2000) in which Foxp2 was concentrated. Likewise, Foxp2 expression was strong in the ventral tegmental area (VTA) which, together with SNc, is the major source of striatal and limbic forebrain dopamine. The dopaminergic subthalamic nucleus (STh) was similarly highly enriched for Foxp2. The STh receives input from pallidal areas, SNc, intralaminar thalamus and prefrontal and motor cortices, and serves an integrative role in motor, cognitive and emotional processing (Heimer et al., 1995; Kolomiets et al., 2001; Lanciego et al., 2004).
In the ventral striatum, Foxp2 was expressed throughout the nucleus accumbens (Nacc) but was more concentrated in the shell (species differences in these regions are discussed below). While both shell and core are involved in reward-based learning, the shell is particularly dopamine-rich (Voorn et al., 2004) and is implicated in the expression of affective responses to external stimuli (Kelley, 2004). Notably, injection of dopamine agonists into the Nacc of adult rats elicits vocalizations in a frequency range associated with normal appetitive behaviors (Burgdorf and Panksepp, 1999; Thompson et al., 2006). Given that the Nacc does not directly effect motor responses, this suggests that dopaminergic activity in the Nacc enhances motivation to vocalize.
In combination, the strong localization of Foxp2 to deep limbic cortex, striosomes, Nacc shell, SNc, VTA and STh, and comparative scarcity in the major output nuclei of the basal ganglia (e.g. medial globus pallidus and substantia nigra pars reticulata), suggest a predominant role in motivational and integrative circuits, rather than in the direct regulation of motor output. Given that speech in humans and social vocalizations in other species are voluntary motor responses to external stimuli whose production requires both assignment of valence to sensory input and motivational regulation, this hypothesis is broadly compatible with current views on the speech-related function of FOXP2 in the caudate (Vargha-Khadem et al., 2005). Likewise, a striatal function in motor skill acquisition but not in motor output is indicated by the finding that impaired synaptic plasticity in the dorsolateral striatum of heterozygous R552H mutant mice is coupled with motor deficits that are exclusive to learned tasks (Groszer et al., 2008).
Foxp2 in the olivocerebellar system: a role in motor timing?
Like the caudate, the cerebellum is critical to normal speech production and is implicated in motor preparation preceding speech (Gordon, 1996; Riecker et al., 2005); motor output from the cerebellum is relayed to the motor cortices via the thalamus (Rouiller et al., 1994). In the cerebellum Foxp2 expression was mainly restricted to the Purkinje cells in the cerebellar cortex, which are fundamental to information transfer and processing within the cerebellum, and are the primary targets of climbing fibers from the Foxp2-enriched inferior olivary complex (IO) (Voogd, 2004).
The importance of Foxp2 to cerebellar development has been demonstrated in humans and lab mice. KE family members carrying the R553H mutation exhibit anomalies in gray matter volume in the cerebellum (Watkins et al., 2002a) and mice lacking both functional copies of Foxp2 are characterized by gross reduction in cerebellar volume (Shu et al., 2005; French et al., 2007; Fujita et al., 2008;; Groszer et al., 2008). Whereas heterozygote cerebellar abnormalities are subtle (Shu et al., 2005; Fujita et al., 2008), or undetectable (Groszer et al., 2008), detailed histochemical analysis of R552H knockin mice revealed weaker dendritic arbors and a reduction in synapses in the Purkinje cell layer (Fujita et al., 2008), suggesting reduced input from the IO.
Given the proposed role of the IO in temporal modulation of cerebellar motor output (Xu et al., 2006), including acoustic rhythmic processing and production (Ackermann et al., 1999; Penhune et al., 1998), it is notable that language-impaired KE family members exhibit deficits in the reproduction of both vocal and manual rhythm, but not in gross motor function (Alcock et al. 2000; Lai et al., 2003). Similarly, while baseline motor ability in heterozygous R552H mutant mice is normal, abnormal synaptic plasticity in the Purkinje cells may contribute to the deficits in motor learning described above (Groszer et al., 2008). Since gait ataxia is a common indicator of global cerebellar damage (Morton and Bastian, 2007), preservation of gross motor function suggests that, in both humans and mice, the olivocerebellar system is specifically compromised by impaired FOXP2 function.
Foxp2 in descending vocal motor circuits
Given the strong correlation between FOXP2 dysfunction and deficits in fine orofacial movements and speech production (Lai et al., 2001; Watkins et al., 2002b), one important finding of this study was the lack of expression in brainstem nuclei responsible for the control of facial musculature (e.g. facial nucleus, trigeminal nucleus, nucleus of solitary tract, nucleus retroambiguous), and laryngeal output (e.g. nucleus ambiguous; Jürgens, 2002). The absence of Foxp2 from the trigeminal circuit has been previously noted in birds (Haesler et al., 2004), but not in mammals.
Foxp2 was, however, expressed in three areas implicated in descending vocal control: periaqueductal gray (PAG), parabrachial nuclei (PB) and, to a lesser extent, in the pontine reticular nucleus (PRn). A large body of work in non-human primates and cats suggests that PAG serves a critical gating function in the production of innate vocalizations (Zhang et al., 1994; Jürgens, 2002; Dujardin and Jürgens, 2006). Parabrachial nuclei modulate the relation between respiration and vocalization in cats and primates (Farley et al., 1992; Simonyan and Jürgens, 2003), and the capacity to make minute adjustments in echolocation frequency in bats (Smotherman et al., 2003). A subpopulation of neurons in caudal PRn is thought to function as a vocal pattern generator for frequency-modulated call types in primates (Hage and Jürgens, 2006). While intriguing, we suggest that the presence of Foxp2 in these three areas should not, in itself, be taken as evidence for involvement in vocal-specific functions at this level. The PAG in particular mediates a range of behavioral and autonomic responses, including vocalization, lordosis, defensive rage, pain transmission and cardiovascular control (Behbehani, 1995; Jürgens, 2002); identifying which functions are influenced by Foxp2 awaits experimental manipulation of gene expression in PAG.
Foxp2 in the thalamus and cortex: sensory processing and sensorimotor integration
As the central relay for ascending auditory, visual and somatosensory input to sensory cortices, the thalamus plays an essential role in the integration of sensory input with cortical feedback and motor output from the cerebellum and basal ganglia (Nakano et al., 2000; Rouiller and Welker, 2000; Alloway et al., 2006). The high level of Foxp2 expression in thalamic nuclei was one of the most striking features of the neural distribution of this protein.
Foxp2 was highly enriched both in the ascending auditory relay nuclei, (lateral lemniscus, inferior colliculus, medial geniculate) and the parallel visual relay (superior colliculus, dorsal lateral geniculate, pretectal nuclei). However, no expression was detected lower in the auditory pathway (superior olivary and cochlear nuclei). In somatosensory circuits, Foxp2 was not found in sub-thalamic relays but was expressed throughout thalamic somatosensory areas in the ventral and posterior nuclei.
Expression was particularly pronounced in midline and intralaminar nuclei, which mediate both cortical arousal and sensorimotor integration via connections with the basal ganglia, motor and limbic cortices (Berendse and Groenewegen, 1991; Levesque and Parent, 1998; Nakamo et al., 2000). Foxp2 was also highly concentrated in mediodorsal (MD) nuclei and moderately enriched in ventral motor nuclei. MD thalamus receives both limbic and motor input and, while widely implicated in aspects of memory (reviewed in Van Der Werf et al., 2003), also participates in limbic control of voluntary vocalization in non-human primates (Dujardin and Jürgens, 2005). Similarly, the ventrolateral nucleus is critical to the transmission of cerebellar input to motor cortices and subserves a range of motor functions, including aspects of vocal production in cats and humans (Farley, 1997; Jürgens, 2002; Crosson et al., 2003).
Cortical Foxp2 expression in layer VI was pervasive across functionally differentiated compartments of the neocortex, whereas expression in layer V was localized and disjunct. Layer VI pyramidal neurons in limbic, motor, somatosensory, visual and auditory areas are the main source of excitatory cortical feedback to corresponding thalamic nuclei (Rouiller and Welker, 2000 and references therein; Gabbott et al., 2005). While layer V sends efferents to many of the same targets as layer VI (e.g. thalamic nuclei, striatum), it is also a major source of cortico-cortical projections and is therefore important to the transfer of sensory and limbic information, and integration with motor output. Notably, layer V Foxp2 expression was most consistently localized to association (prefrontal and posterior parietal) and premotor areas, supporting a potential role in the transformation of sensory and limbic input into planned movement. If, as seems likely based on its thalamic distribution, Foxp2 plays a functional role in parallel auditory, visual and somatosensory circuits, then it may influence cortico-thalamic feedback and participate in convergent sensory processing in the neocortex.
Together, these data suggest that Foxp2 is involved in subcortical and cortical sensory processing, and higher order motor planning, but not in the initial acquisition and transmission of sensory input from the periphery. Qualitatively stronger expression in subcortical auditory relative to visual circuits, and enrichment in thalamic nuclei involved in vocal production are certainly noteworthy. However, expression throughout auditory, visual, somatosensory and motor compartments of the thalamus suggests that, rather than modulating particular sensory or motor pathways, Foxp2 is critical to sensorimotor integration at the level of the thalamus and cortex. Thus, experimental manipulations targeting specific thalamic nuclei and corresponding cortical regions may prove particularly fruitful in deciphering the functions of this gene.
Foxp2 in the rodent olfactory system
In macrosmatic mammals such as rodents, the olfactory system is integral to social interactions and emotional response (Shipley et al., 2004). The muroid distribution of Foxp2 suggests involvement in valuation and emotional processing of olfactory input. Foxp2 was scattered in the glomerular layer in main olfactory bulb (MOB), and was highly concentrated in the accessory olfactory bulb (AOB) and the olfactory tubercle (Tu). MOB afferents to Tu are thought to influence reward-motivated motor response to olfactory input via connections between Tu and Nacc (Newman and Winans, 1980). In contrast, the medial amygdala (MeA) receives extensive input from AOB (Shipley et al., 2004; Pro-Sistiaga et al., 2007) and modulates emotional response via output to the hypothalamus and BST. It was notable that Foxp2 in the main amygdaloid nuclei of all four species was strongly localized to MeA, suggesting that local function may be linked to olfactory-driven input.
Species differences are localized to the limbic forebrain and cortex
Given the complex acoustic structure of singing mouse calls and the precise orofacial coordination required for their production, we expected to find expression differences in the two Scotinomys species relative to Peromyscus and Mus in circuits regulating vocal production. Instead, expression in subcortical motor and sensory circuits was highly conserved; detectable interspecific differences appeared random with respect to phylogeny and were mainly localized to cortex and subcortical limbic forebrain (nucleus accumbens, medial hypothalamus, medial amygdala).
In Mus and P. maniculatus Foxp2 was highly concentrated in the anteroventral medial amygdala (MeAV), an area with reciprocal projections to AOB that also receives convergent chemosensory input from MOB (von Campenhausen and Mori, 2000; Shipley et al., 2004). Given the well-established function of the rodent vomeronasal-AOB-MeA circuit in discriminating chemical cues (reviewed in Dulac and Wagner, 2006) and the integral role of the MeA in olfactory-mediated social memory (Ferguson et al., 2001), strong localization of Foxp2 to the MeAV in Mus and P. maniculatus suggests involvement in processing olfactory social input. One possible explanation for reduced expression in MeAV in Scotinomys is that singing mice are highly vocal and are among the few muroid species that are fully diurnal (Hooper and Carleton, 1976), factors which may increase reliance on audition and vision relative to olfaction.
In the ventral striatum, qualitative interspecific differences in Foxp2 expression were loosely defined by the topography of cortical projections to the shell and core divisions of the Nacc (reviewed in Voorn et al., 2004). Expression in P. maniculatus was discontinuous in lateral Nacc, a region that receives most projections from agranular insular cortex. In S. xerampelinus, an area with minimal expression was observed more medially in a region highly innervated by afferents from prelimbic cortices. Interestingly, Foxp2 is completely absent from Nacc core in Rattus (Takahashi et al., 2003).
In the cortex, the distribution of Foxp2 in layer V was an unexpected source of interspecific variation, particularly in S. teguina and P. maniculatus. While interpretation of these data depends on whether Foxp2 is localized to projection or interneurons, we note that layer V in the perirhinal cortex, in which a unique Foxp2-positive cell population was observed in P. maniculatus, sends extensive projections to posterior parietal, agranular, visual and infralimbic cortices (McIntyre et al., 1996), all areas with additional Foxp2 enrichment in P. maniculatus. This pattern of expression across spatially disjunct but anatomically connected regions suggests involvement in cortico-cortical transmission.
Whether Foxp2 in these variable regions directly influences species differences in behavior remains to be determined. However, the contrast between high conservation across subcortical sensorimotor circuits and localized variation in cortex and subcortical limbic forebrain has two important implications. 1) The function of Foxp2 in auditory and motor aspects of vocal communication may be fundamentally conserved and, at least in the case of muroid rodents, does not extend to species differences in articulatory and acoustic complexity. A comparable pattern of conserved expression in motor circuits is observed across song-learning and non-learning birds (Haesler et al., 2004). 2) Interspecific variation in limbic regions may reflect evolutionary lability in the role of Foxp2 in higher level sensory processing and emotional and motivational fine-tuning of motor responses, including vocalization. It is important to note, however, that a quantitative approach might detect more subtle interspecifc differences across other brain regions.
Can rodent models elucidate the role of Foxp2 in vocal production and perception?
While the importance of FOXP2 in learned forms of vocal communication is well-supported (Lai et al., 2001; Haesler et al., 2007), the role of Foxp2 in innate vocal production in rodents is currently contentious. Experimental studies in lab mice have yielded mixed results in relation to the nature of vocal deficits in Foxp2-compromised heterozygote pups: whereas Foxp2 knockouts (Shu et al., 2005) and R552H knockins (Fujita et al., 2008) produced fewer ultrasonic vocalizations (USVs) than wild-type controls, R552H mutants generated via ENU mutagenesis did not (Groszer et al., 2008). This disparity is further complicated by the global nature of these manipulations. When vocal deficits are found, it is impossible to determine whether they are due to Foxp2 dysfunction in vocal circuitry, or more general deficits in lung and motor development (Shu et al., 2001; Groszer et al., 2008).
While inconsistencies across studies may be explained by differences in genetic background (to which USVs are highly sensitive; Brunelli, 2005) and molecular approaches, a larger question is whether pup USVs are an informative measure of Foxp2-mediated vocal dysfunction in rodent models. USVs, which are emitted by pre-endothermic pups isolated from their nest, are not modulated by auditory feedback and have been interpreted as an acoustic byproduct of arousal due to the perception of cold or loss of social contact (Blumberg and Sokoloff, 2001; Ehret, 2005).
Given the functional role of FOXP2 in voluntary, socially motivated communication such as speech and birdsong, analysis of both production and perception of acoustic signals in adult R552H knockin/mutant mice should be more relevant to the neural mechanisms of speech and language dysfunction in humans carrying the equivalent FOXP2 substitution. Likewise, targeted gene silencing in appropriate rodent models may prove particularly useful in defining the role of Foxp2 in vocal production and perception relative to other sensorimotor, limbic and developmental functions. For example, the hypothesis that Foxp2 in the olivocerebellar system modulates motor timing of speech production could be tested by silencing expression in the inferior olive in species such as S. teguina and S. xerampelinus. The rapidly articulated and highly stereotyped calls of adult singing mice should be sensitive indicators of temporal deficits in vocal production. The proposed role of Foxp2 in motivational control of speech could be tested in the lab rat, a system which has already provided considerable insight into the involvement of the mesolimbic dopamine circuit in vocalizations of positive affect (Panksepp, 2007). Finally, the parallel distribution of Foxp2 in auditory and visual relays suggests a function in audio-visual integration, a process whose neural architecture is well-defined in rats (Wallace et al., 2004), and is characteristic of speech perception in humans (McGurk and MacDonald, 1976; Colin and Radeau, 2003). Testing this hypothesis using a modified McGurk paradigm (presentation of incongruent auditory and visual signals) in rats with selective Foxp2 knockdown in thalamic audio-visual relays could elucidate the contribution of Foxp2 to multimodal sensory processing.
A synthetic hypothesis for Foxp2-modulated circuitry in adult mammals
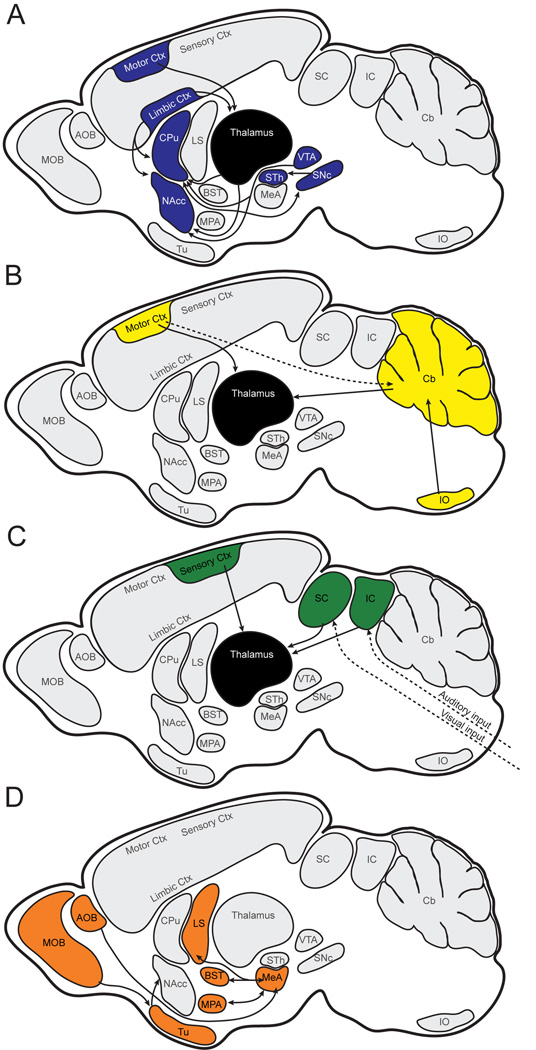
Vargha-Khadem and colleagues developed a model for FOXP2-dependent circuitry in speech production, in which output from Broca’s and premotor areas in frontal cortex is transduced and returned to motor cortex via two parallel loops: basal ganglia-thalamus and pontine grey-cerebellum-thalamus (Vargha-Khadem et al., 2005). Here, we propose a functionally and taxonomically broader hypothesis for circuits influenced by adult expression of Foxp2 (Fig. 12). The brain-wide distribution of Foxp2 suggests involvement in at least three major pathways, all of which are dependent on thalamic processing and transmission of subcortical and cortical inputs. 1) Striatal modulation of fine motor response based on dopamine-mediated valuation of input from the thalamus and limbic cortex (Fig. 12a). 2) Olivocerebellar modulation of motor timing (Fig. 12b). 3) Thalamic integration of sensory input with an emphasis on auditory and visual circuits (Fig. 12c). We include a fourth circuit, in which olfactory social cues are processed and assigned emotional valence in the limbic forebrain (Fig. 12d). This pathway is integral to social communication in rodents, but may be de-emphasized in mammals with reduced olfactory investment (e.g. primates).
Fig. 12.
Schematic hypothesis for circuitry modulated by Foxp2 in the adult brain. A: Striatal modulation of fine motor response. B: Olivocerebellar modulation of motor timing. C: Thalamic integration of auditory and visual inputs. D: Limbic processing of olfactory input. Solid arrows indicate connectivity between regions in which Foxp2 expression is strong and continuous; broken arrows indicate pathways with discontinuous or weak expression. Connectivity between regions in which Foxp2 is not reciprocally expressed is not shown. AOB, accessory olfactory bulb; BST, bed nucleus of stria terminalis; Cb, cerebellum; CPu, caudate putamen; Ctx, cortex; IO, inferior olivary complex; LS, lateral septum; MeA, medial amygdala; MOB, main olfactory bulb; MPA, medial preoptic area; NAcc, nucleus accumbens; SNc, substantia nigra pars compacta; STh, subthalamic nucleus; Tu, olfactory tubercle; VTA, ventral tegmental area.
While none of the pathways outlined in Fig. 12 are exclusive to vocal production or processing, each has the potential to influence vocal communication through distinct mechanisms. By understanding how Foxp2 functions in this broader network of circuits we may gain a more complete appreciation for the nature and specificity of its contributions to adult behaviors. Such work would deepen our understanding of vertebrate vocal communication and its relationship to human language.
Acknowledgments
This manuscript was significantly improved by the comments of two anonymous reviewers. The Mus and Peromyscus brains used in this study were generously donated by Dr S. Paul Oh and Dr Mark H. Lewis, respectively. We thank Harumi Kamashina for assistance in the lab.
Grant sponsor: NSF; Grant number: IOS 0548404 (to S. M. P.); Grant sponsor: NIDCD; NRSA fellowship F32 DC008269 (to P.C.).
Footnotes
Taxon-specific forkhead gene family nomenclature follows Kaestner et al. (2000).
References
- Ackermann H, Graber S, Hertrich I, Daum I. Cerebellar contributions to the perception of temporal cues within the speech and nonspeech domain. Brain and Language. 1999;67:228–241. doi: 10.1006/brln.1999.2056. [DOI] [PubMed] [Google Scholar]
- Alcock KJ, Passingham RE, Watkins K, Vargha-Khadem F. Pitch and timing abilities in inherited speech and language impairment. Brain and Language. 2000;75:34–46. doi: 10.1006/brln.2000.2323. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. Journal of Comparative Neurology. 2006;499:33–48. doi: 10.1002/cne.21039. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Neurogenetic patterns in the medial limbic cortex of the rat related to anatomical connections with the thalamus and striatum. Experimental Neurology. 1990;107:132–142. doi: 10.1016/0014-4886(90)90151-h. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Progress in Neurobiology. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Sokoloff G. Do infant rats cry? Psychological Review. 2001;108:83–95. doi: 10.1037/0033-295x.108.1.83. [DOI] [PubMed] [Google Scholar]
- Bruce HA, Margolis RL. FOXP2: novel exons, splice variants, and CAG repeat length stability. Human Genetics. 2002;111:136–144. doi: 10.1007/s00439-002-0768-5. [DOI] [PubMed] [Google Scholar]
- Brunelli SA. Selective breeding for an infant phenotype: rat pup ultrasonic vocalization (USV) Behavior Genetics. 2005;35:53–65. doi: 10.1007/s10519-004-0855-6. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. Evidence that rat ultrasonic calls can index both positive and negative affective states. Soc Neurosci Abstr. 1999;25:875. [Google Scholar]
- Canales JJ. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transmitting from instrumental responding to habitual behavior in drug addiction. Neurobiology of Learning and Memory. 2005;83:93–103. doi: 10.1016/j.nlm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nature Neuroscience. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Colin C, Radeau M. The McGurk illusions in speech: 25 years of research. Annee Psychologique. 2003;103:497–542. [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Moore AB, Wierenga CE, Gopinath K, Soltysik D, Bauer RM, Auerbach EJ, Gokcay D, Leonard CM, Briggs RW. Left and right basal ganglia and frontal activity during language generation: contributions to lexical, semantic, and phonological processes. Journal of the International Neuropsychological Society. 2003;9:1061–1077. doi: 10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Herkenham M. Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Research. 1986;365:397–403. doi: 10.1016/0006-8993(86)91658-6. [DOI] [PubMed] [Google Scholar]
- Dujardin E, Jürgens U. Afferents of vocalization-controlling periaqueductal regions in the squirrel monkey. Brain Research. 2005;1034:114–131. doi: 10.1016/j.brainres.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu. Rev. Genet. 2006;40:449–467. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds--a gate to the understanding of sound communication. Behavior genetics. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Enard W, Przeworski M, Fisher SE, Lai CSL, Wiebe V, Kitano T, Monaco AP, Pääbo S. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- Farley GR. Neural firing in ventrolateral thalamic nucleus during conditioned vocal behavior in cats. Experimental Brain Research. 1997;115:493–506. doi: 10.1007/pl00005719. [DOI] [PubMed] [Google Scholar]
- Farley GR, Barlow SM, Netsell R. Factors influencing neural activity of parabrachial regions during cat vocalizations. Experimental Brain Research. 1992;89:341–351. doi: 10.1007/BF00228250. [DOI] [PubMed] [Google Scholar]
- Fendt M, Schwienbacher I, Schnitzler HU. Carbachol injections into the nucleus accumbens induce 50 kHz calls in rats. Neuroscience Letters. 2006;401:10–15. doi: 10.1016/j.neulet.2006.02.069. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. The Journal of Neuroscience. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460:266–279. doi: 10.1002/cne.10654. [DOI] [PubMed] [Google Scholar]
- Fernandez M. M. S. thesis. St. Louis, MS: University of Missouri; 2006. Olfactory responses of Neotropical singing mice (Scotinomys teguina) to the odors of the mid-ventral sebaceous gland. [Google Scholar]
- Fisher SE. Tangled webs: tracing the connections between genes and cognition. Cognition. 2006;101:270–297. doi: 10.1016/j.cognition.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Marcus GF. The eloquent ape: genes, brains and the evolution of language. Nature Reviews Genetics. 2006;7:9–20. doi: 10.1038/nrg1747. [DOI] [PubMed] [Google Scholar]
- Friederici AD. What’s in control of language? Nature Neuroscience. 2006;9:991–992. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- French CA, Groszer M, Preece C, Coupe A-M, Rajewsky K, Fisher SE. Generation of mice with a conditional Foxp2 null allele. Genesis. 2007;45:440–446. doi: 10.1002/dvg.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Tanabe Y, Shiota A, Ueda M, Suwa K, Momoi MY, Momoi T. Ultrasonic vocalizaiton impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. PNAS. 2008;105:3117–3122. doi: 10.1073/pnas.0712298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output system. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gordon N. Speech, language, and the cerebellum. European Journal of Disorders of Communication. 1996;31:359–367. doi: 10.3109/13682829609031327. [DOI] [PubMed] [Google Scholar]
- Gourbal BEF, Barthelemy M, Petit G, Gabrion C. Spectrographic analysis of the ultrasonic vocalizations of adult male and female BALB/c mice. Naturwissenschaften. 2004;91:381–385. doi: 10.1007/s00114-004-0543-7. [DOI] [PubMed] [Google Scholar]
- Groszer M, Keays DA, Deacon RMJ, de Bono JP, Prasad-Mulcare S, Gaub S, Baum MG, French CA, Nicod J, Coventry JA, Enard W, Fray M, Brown SDM, Nolan PM, Pääbo S, Channon KM, Costa RM, Eilers J, Ehret G, Rawlins JNP, Fisher SE. Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Current Biology. 2008;18:354–362. doi: 10.1016/j.cub.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C. FoxP2 expression in avian vocal learners and non-learners. Journal of Neuroscience. 2004;24:3164–3175. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus area X. PLoS Biology. 2007;5:e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage SR, Jürgens U. Localization of a vocal pattern generator in the pontine brainstem of the squirrel monkey. European Journal of Neuroscience. 2006;23:840–844. doi: 10.1111/j.1460-9568.2006.04595.x. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Alheid GF. Basal Ganglia. In: Paxinos G, editor. The Rat Nervous System. 2nd Ed. San Diego, CA: Academic Press; 1995. [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper ET, Carleton MD. Vol. 151. Miscellaneous Publications of the Museum of Zoology, University of Michigan; 1976. Reproduction, growth and development in two contiguously allopatric rodent species, genus Scotinomys; pp. 1–52. [Google Scholar]
- Jernigan TL, Hesselink JR, Sowell E, Tallal PA. Cerebral structure on magnetic resonance imaging in language- and learning-impaired children. Arch Neurol. 1991;48:539–545. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- Jiminez-Castellanos J, Graybiel AM. Subdivisions of the dopamine containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and the extrastriosomal matrix. Neuroscience. 1987;23:223–242. doi: 10.1016/0306-4522(87)90285-5. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neuroscience and Biobehavioral Reviews. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes and Development. 2000;14:142–146. [PubMed] [Google Scholar]
- Kalcounis-Rueppell MC, Metheny JD, Vonhof MJ. Production of ultrasonic vocalizations by Peromyscus mice in the wild. Frontiers in Zoology. 2006;3:3. doi: 10.1186/1742-9994-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusta J, Sales GD, Czuchnowski R. Aggression and vocalization behaviour of three sympatric vole species during conspecific and heterospecific same-sex encounters. Behaviour. 2007;144:283–305. [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and Biobehavioral Reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Wilson CJ. Corticostriatal innervation of the patch and matrix in the rat neostriatum. Journal of Comparative Neurology. 1996;374:578–592. doi: 10.1002/(SICI)1096-9861(19961028)374:4<578::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kolomiets BP, Deniau JM, Mailly P, Menetrey A, Glowinski J, Thierry AM. Segregation and convergence of information flow through the cortico-subthalamic pathways. Journal of Neuroscience. 2001;21:5764–5772. doi: 10.1523/JNEUROSCI.21-15-05764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Lalueza-Fox C, Orlando L, Enard W, Green RE, Burbano HA, Hublin J-J, Hänni C, Fortea J, de la Rasilla M, Bertranpetit J, Rosas A, Pääbo S. The derived FOXP2 variant of modern humans was shared with Neandertals. Current Biology. 2007;17:1–5. doi: 10.1016/j.cub.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Lai CSL, Fischer SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Lai CSL, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126:2455–2462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Gonzalo N, Castle M, Sanchez-Escobar C, Aymerich MS, Obeso JA. Thalamic innervation of striatal and subthalamic neurons projecting to the rat entopeduncular nucleus. European Journal of Neuroscience. 2004;19:1267–1277. doi: 10.1111/j.1460-9568.2004.03244.x. [DOI] [PubMed] [Google Scholar]
- Levesque M, Parent A. Axonal arborization of corticostriatal and corticothalamic fibers arising from prelimbic cortex in the rat. Cerebral Cortex. 1998;8:602–613. doi: 10.1093/cercor/8.7.602. [DOI] [PubMed] [Google Scholar]
- Li G, Wang J, Rossiter SJ, Jones G, Zhang S. Accelerated FoxP2 evolution in echolocating bats. PLoS one. 2007 doi: 10.1371/journal.pone.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liégeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Khadem F. Language fMRI abnormalities associated with FOXP2 gene mutation. Nature Neuroscience. 2003;6:1230–1237. doi: 10.1038/nn1138. [DOI] [PubMed] [Google Scholar]
- MacDermot KD, Bonora E, Sykes N, Coupe A-M, Lai CSL, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, Fisher SE. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. American Journal of Human Genetics. 2005;76:1074–1080. doi: 10.1086/430841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. The Journal of Neuroscience. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264:746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Kelly ME, Staines WA. Efferent projections of the anterior perirhinal cortex in the rat. Journal of Comparative Neurology. 1996;369:302–318. doi: 10.1002/(SICI)1096-9861(19960527)369:2<302::AID-CNE10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Miller JR, Engstrom MD. Vocal stereotypy and singing behavior in baiomyine mice. Journal of Mammalogy. 2007;88:1447–1465. [Google Scholar]
- Morton SM, Bastian AJ. Mechanisms of cerebellar gait ataxia. Cerebellum. 2007;6:79–86. doi: 10.1080/14734220601187741. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. Journal of Neurology. 2000;247 Suppl 5:1–15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Ohira R, Zhang YH, Guo W, Dipple K, Shih SL, Doerr J, Huang BL, Fu LJ, Abu-Khalil A, Geschwind D, McCabe ERB. Human ARX gene: genomic characterization and expression. Molecular Genetics and Metabolism. 2002;77:179–188. doi: 10.1016/s1096-7192(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Neuroevolutionary sources of laughter and social joy: modeling primal human laughter in laboratory rats. Behavioural Brain Research. 2007;182:231–244. doi: 10.1016/j.bbr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Paxinos G, editor. The Rat Nervous System. 3ird Ed. New York: Academic Press; 2004. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. New York: Academic Press; 1998. [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd Ed. New York: Academic Press; 2001. [Google Scholar]
- Penhune VB, Zatorre RJ, Evans AC. Cerebellar contributions to motor timing: a PET study of auditory and visual rhythm reproduction. Journal of Cognitive Neuroscience. 1998;10:752–765. doi: 10.1162/089892998563149. [DOI] [PubMed] [Google Scholar]
- Pickett ER, Kuniholm E, Protopapas A, Friedman J, Lieberman P. Selective speech motor, syntax and cognitive deficits associated with bilateral damage to the putamen and the head of the caudate nucleus: a case study. Neuropsychologia. 1998;36:173–188. doi: 10.1016/s0028-3932(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Arroyo-Jimenez MDM, Marcos P, Artacho-Krula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. Journal of Comparative Neurology. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Reeder SA, Carroll DS, Edwards CW, Kilpatrick CW, Bradley RD. Neotomine-peromyscine rodent systematics based on combined analyses of nuclear and mitochondrial DNA sequences. Molecular Phylogenetics and Evolution. 2006;40:251–258. doi: 10.1016/j.ympev.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64:700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Rochefort C, He X, Scotto-Lomassese S, Scharff C. Recruitment of FoxP2-expression neurons to Area X varies during song development. Developmental Neurobiology. 2007;67:809–817. doi: 10.1002/dneu.20393. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Research Bulletin. 2000;53:727–741. doi: 10.1016/s0361-9230(00)00364-6. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Liang F, Babalian A, Moret V, Wiesndenger M. Cerebellothalamocortical and pallidothalamocortical projections to the primary and supplementary motor cortical areas - a multiple tracing study in macaque monkeys. Journal of Comparative Neurology. 1994;345:185–213. doi: 10.1002/cne.903450204. [DOI] [PubMed] [Google Scholar]
- Scharff C, Haesler S. An evolutionary perspective on FoxP2: strictly for the birds? Current Opinion in Neurobiology. 2005;15:694–703. doi: 10.1016/j.conb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Ennis M, Puche AC. Olfactory system. In: Paxinos G, editor. The Rat Nervous System. 3ird Ed. San Diego, CA: Elsevier Academic Press; 2004. [Google Scholar]
- Shriberg LD, Ballard KJ, Tomblin JB, Duffy JR, Odell KH, Williams CA. Speech, prosody, and voice characteristics of a mother and daughter with a 7;13 translocation affecting FOXP2. Journal of Speech, Language, and Hearing Research. 2006;49:500–525. doi: 10.1044/1092-4388(2006/038). [DOI] [PubMed] [Google Scholar]
- Shu W, Yang H, Zhang L, Lu MM, Morrisey EE. Characterization of a new subfamily of wing-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. Journal of Biological Chemistry. 2001;276:27488–27497. doi: 10.1074/jbc.M100636200. [DOI] [PubMed] [Google Scholar]
- Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Gama Sosa MA, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Research. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- Smotherman M, Zhang S, Metzner W. A neural basis for auditory feedback control of vocal pitch. Journal of Neuroscience. 2003;23:1464–1477. doi: 10.1523/JNEUROSCI.23-04-01464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri E, Konopka G, Coppola G, Bomar J, Oldham M, Ou J, Vernes SC, Fisher SE, Ren B, Geschwind DH. Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. American Journal of Human Genetics. 2007;81:1144–1157. doi: 10.1086/522237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan SJ, Adkins RM, Anderson J. Phylogeny and divergence-date estimates of rapid radiations of muroid rodents based on multiple nuclear genes. Systematic Biology. 2004;53:533–553. doi: 10.1080/10635150490468701. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Liu F-C, Hirokawa K, Takahashi H. Expression of Foxp2, a gene involved in speech and language, in the developing and adult striatum. Journal of Neuroscience Research. 2003;73:61–72. doi: 10.1002/jnr.10638. [DOI] [PubMed] [Google Scholar]
- Teramitsu I, White SA. FoxP2 regulation during undirected singing in adult songbirds. Journal of Neuroscience. 2006;26:7390–7394. doi: 10.1523/JNEUROSCI.1662-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behavioural Brain Research. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: the declarative/procedural model. Nature Reviews Neuroscience. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Jolles J, Witter MP, Uylings HBM. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39:1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nature Reviews Neuroscience. 2005;6:131–138. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Nicod J, Elahi FM, Coventry JA, Kenny N, Coupe A-M, Bird LE, Davies KE, Fisher SE. Functional genetic analysis of mutations implicated in a human speech and language disorder. Human Molecular Genetics. 2006;15:3154–3167. doi: 10.1093/hmg/ddl392. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Spiteri E, Nicod J, Groszer M, Taylor JM, Davies KE, Geschwind DH, Fisher SE. High-throughput analysis of promoter occupancy reveal direct neural targets of FOXP2, a gene mutated in speech and language disorders. American Journal of Human Genetics. 2007;81:1232–1250. doi: 10.1086/522238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Campenhausen H, Mori K. Convergence of segregated pheromonal pathways from the accessory olfactory bulb to the cortex in the mouse. European Journal of Neuroscience. 2000;12:33–46. doi: 10.1046/j.1460-9568.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- Voogd J. Cerebellum. In: Paxinos G, editor. The Rat Nervous System. 3ird Ed. San Diego, CA: Elsevier Academic Press; 2004. [Google Scholar]
- Voorn P, Vanderschuren LJMJ, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral striatum. Trends in Neurosciences. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Ramachandran R, Stein BE. A revised view of sensory cortical parcellation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2167–2172. doi: 10.1073/pnas.0305697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Lin D, Li C, Tucker P. Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. The Journal of Biological Chemistry. 2003;278:24259–24268. doi: 10.1074/jbc.M207174200. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dendritic spines containing mu-opioid receptors in rat striatal patches receive asymmetric synapses from prefrontal corticostriatal afferents. Journal of Comparative Neurology. 1998;396:223–237. [PubMed] [Google Scholar]
- Wang H-F, Liu F-C. Developmental restriction of the LIM homeodomain transcription factor Isl-1 expression to cholinergic neurons in the striatum. Neuroscience. 2001;103:999–1016. doi: 10.1016/s0306-4522(00)00590-x. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Dronkers NF, Vargha-Khadem F. Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain. 2002a;125:452–464. doi: 10.1093/brain/awf058. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Vargha-Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, Frackowiak RSJ, Mishkin M, Gadian DG. MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain. 2002b;125:465–478. doi: 10.1093/brain/awf057. [DOI] [PubMed] [Google Scholar]
- Wijchers PJEC, Hoekman MFM, Burbach PH, Smidt MP. Identification of forkhead transcription factors in cortical and dopaminergic areas of the adult murine brain. Brain Research. 2006;1068:23–33. doi: 10.1016/j.brainres.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Xu D, Liu T, Ashe J, Bushara KO. Role of the olivo-cerebellar system in timing. Journal of Neuroscience. 2006;26:5990–5995. doi: 10.1523/JNEUROSCI.0038-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosida S, Kobayasi KI, Ikebuchi M, Ozaki R, Okanoya K. Antiphonal vocalization of a subterranean rodent, the naked mole-rat (Heterocephalus glaber) Ethology. 2007;113:703–710. [Google Scholar]
- Zeesman S, Nowaczyk MJ, Teshima I, Roberts W, Cardy JO, Brian J, Senman I, Feuk L, Osborne LR, Scherer SW. Speech and language impairment and oromotor dyspraxia due to deletion of 7q31 that involves FOXP2. American Journal of Medical Genetics A. 2006;140:509–514. doi: 10.1002/ajmg.a.31110. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Davis PJ, Bandler R, Carrive P. Brain-stem integration of vocalization – role of the midbrain periaqueductal gray. Journal of Neurophysiology. 1994;72:1337–1356. doi: 10.1152/jn.1994.72.3.1337. [DOI] [PubMed] [Google Scholar]