Abstract
The Childhood Cancer Survivor Study (CCSS) has assembled the largest cohort to date for assessment of late mortality. Vital status and cause of death of all patients eligible for participation in CCSS was determined using the National Death Index and death certificates to characterize the mortality experience of 20,483 survivors, representing 337,334 person-years of observation. A total of 2,821 deaths have occurred as of December 31, 2002. The overall cumulative mortality is 18.1% (95% CI, 17.3 to 18.9) at 30 years from diagnosis. With time, while all-cause mortality rates have been stable, the pattern of late death is changing. Mortality attributable to recurrence or progression of primary disease is decreasing, with increases in rates of mortality attributable to subsequent neoplasms (standardized mortality ratios [SMR], 15.2; 95% CI, 13.9 to 16.6), cardiac death (SMR, 7.0; 95% CI, 5.9 to 8.2), and pulmonary death (SMR, 8.8; 95% CI, 6.8 to 11.2) largely due to treatment-related causes. In addition, the CCSS has identified specific treatment-related risk factors for late mortality. Radiotherapy (relative risk [RR], 2.9; 95% CI, 2.1 to 4.2), alkylating agents (RR, 2.2; 95% CI, 1.6 to 3.0), and epipodophyllotoxins (RR, 2.3; 95% CI, 1.2 to 4.5) increase the risk of death due to subsequent malignancy. Cardiac radiation exposure (RR, 3.3; 95% CI, 2.0 to 5.5) and high dose of anthracycline exposure (RR, 3.1; 95% CI, 1.6 to 5.8) are associated with late cardiac death. By continued longitudinal follow-up of the cohort and expansion of the cohort to include patients diagnosed between 1987 and 1999, the CCSS will remain a primary resource for assessment of late mortality of survivors of childhood cancers.
INTRODUCTION
Five-year survival rates for most childhood cancers have improved significantly over the past four decades. In the modern era of cancer treatment, more than 80% of children diagnosed with a pediatric malignancy will become 5-year survivors of their cancer, due largely to the use of advanced diagnostic modalities, and improvements in surgical and radiation therapy techniques, combination chemotherapy, and supportive care.1,2 This dramatic increase in survival has resulted in a new and growing population of long-term survivors of childhood cancer that did not exist just a few decades ago. Currently, it is estimated that one in every 640 young adults between the ages of 20 and 39 is a survivor of pediatric cancer.1
Unfortunately, this increased rate of survival does not come without a cost to the survivor. There is significant long-term morbidity and mortality associated with treatment of childhood cancer, the incidence of which continues to increase long after completion of therapy. The Childhood Cancer Survivor Study (CCSS) has assembled the largest cohort to date for assessment of late mortality and morbidity among 5-year survivors of childhood and adolescent cancer. The mortality experience of 20,483 survivors diagnosed between 1970 and 1986, representing 337,334 person-years of observation, including 2,821 deaths, has broadened our understanding of the true risk of death among 5-year survivors relative to background rates in the general population. In addition, cause-specific attribution of death has allowed identification of trends in mortality due to treatment-related causes separately from death due to recurrence or progression of primary disease. As such, these data have allowed identification of key risk factors for long-term treatment related mortality.
Only a limited number of studies exist that explore late mortality in patients treated before 1970. Many of the seminal findings from this earliest era of cancer care have been validated and markedly expanded on by the work of the CCSS on patients diagnosed between 1970 and 1986. These early reports established higher death rates in survivors compared to siblings, attribution of death primarily to recurrence of primary malignancy, an increasing risk of nonprimary malignancy related death with increasing time from diagnosis, and early associations of treatment modalities with late death.3–5 Li et al reported 83% overall survival at 20 years from diagnosis in a population of 1,807 5-year survivors diagnosed between 1950 and 1969.3 However, it is noteworthy that before 1970 the treatment exposures of 5-year survivors were substantially different as most treatment regimens were limited to surgical resection with or without radiotherapy, with minimal exposure to chemotherapeutic agents.5 As a result, certain primary diagnoses were associated with very high rates of late death, as in the case of acute lymphoblastic leukemia (ALL) where, before CNS-directed therapy, CNS relapse ultimately lead to an increase in late mortality rates, higher than that observed in CCSS. Ten-year survival probabilities were lower than 50% for ALL and only 65% for Hodgkin's lymphoma in this earlier era, as opposed to those with Wilms tumor, low-stage neuroblastoma, or retinoblastoma who responded well to surgery and radiotherapy.3 Regarding deaths attributable to treatment-related causes, Hawkins et al, in a population-based investigation of late mortality in 4,082 British patients diagnosed before 1971, first documented a five-fold increase in cardiovascular death largely due to early myocardial infarction and cerebrovascular accidents.4 In addition, they reported that more than 25% of 5-year survivors of Hodgkin's disease, ependymoma, medulloblastoma, and Ewing's sarcoma died of recurrent tumor, with a strong propensity for late relapse within these diagnostic groups. Overall, they observed three times the number of deaths expected due to non-neoplastic causes. Many questions remained, however, at the conclusion of these studies, largely regarding the emerging use of chemotherapy and increasing intensity of therapy in the late 1960s and early 1970s and its effect on late mortality. It was even hypothesized that late mortality may become more pronounced in future cohorts of survivors receiving these modern primary therapies, secondary to improved salvage rates.5
SPECIFIC METHODS FOR MORTALITY ASSESSMENT
Ascertainment of Cause of Death
Names of all patients eligible for participation in the CCSS were included in a search for deaths using the National Death Index (NDI) from 1979 to 2002. The NDI is an index of death record information compiled from individual state vital statistics offices through contractual agreements with the National Center for Health Statistics. The NDI also has available the underlying and multiple causes of death for deceased subjects using the International Classification of Disease (ICD) ninth revision. The underlying cause of death identifies the initiating cause of death, and the rules for selecting the underlying cause of death are standardized. In the US, death statistics are based on the underlying cause of death, and are therefore a useful means of standardizing classification of deaths in studies such as the CCSS.
For deaths that predated the NDI (ie, 1975 to 1978), death certificates from states where deaths occurred were requested. Cause of death was determined from information provided by the NDI in addition to the information provided on death certificates. Based on this information, and augmented by our knowledge of the original cancer diagnosis as well as telephone interviews with parents of deceased CCSS participants, the original CCSS mortality manuscript published in 20016 classified all deaths as either (1) a direct consequence of the original cancer diagnosis, including recurrence or progression of the primary disease, or death due to acute toxicity while on therapy for the original diagnosis; (2) cancer treatment-related death, defined as a death where nonacute treatment effects were considered to be the major contributing factor to death (eg, subsequent malignant neoplasm, cardiac, or pulmonary toxicity); or (3) non–treatment- related causes, such as deaths due to other conditions or external causes. The most recent mortality update from the CCSS, however, published in 2008, used a more restricted classification schema attributing death to either: (1) recurrence (or progression) of primary disease, (2) external causes, or (3) nonrecurrence, nonexternal causes.7 This review reports vital status as of December 31, 2002, congruent with the most recent report from CCSS.
Analytic Methods
Evaluation of late mortality for all CCSS studies began 5 years from the time of diagnosis and continued until the date of death or the date of censoring, December 31, 2002, if alive. Rates of deaths per 1,000 person-years were calculated for 5-year time intervals after cohort entry. Standardized mortality ratios (SMRs) were also used to quantify the relative rate of mortality in this cohort, relative to the age-, calendar year–, and sex-matched United States' general population. Details of methods used for calculation of mortality rates, SMRs, and survival functions have been previously reported.6,8 In addition to calculation of all-cause SMRs, cause-specific SMRs were calculated by excluding deaths attributable to recurrence or progression of the primary malignancy. Specific causes of death were grouped into six categories: secondary or subsequent cancer (ICD 140-239), cardiac (ICD 390- 398, 402, 404, 410- 429), pulmonary (ICD 460-519), external causes (ie, accidents, suicides, poisonings; ICD 800-999) and other causes (all other ICD codes). Multivariable Poisson regression was used to assess the impact of specific treatment modalities on the risk of mortality not attributable to recurrence or progression of primary disease, while adjusting for factors including sex, age at diagnosis, and time since diagnosis.
OVERALL MORTALITY
For decades, clinicians and patients alike have used 5-year survival from the time of diagnosis as a generalized benchmark for cure (5-year event-free survival) and for overall assessment of therapeutic regimens (5-year overall survival). However, the risk of cancer and cancer therapy-related death extends well beyond this arbitrary 5-year point. Unfortunately, most therapeutic protocols were not designed to monitor patients far beyond the time point for assessing clinical efficacy (eg, typically < 5 to 7 years from diagnosis). Moreover, most pediatric oncology programs are not structured to systematically follow patients as long-term survivors into adulthood. For these reasons, assessment of late mortality is generally dependent on epidemiologic studies to inform us of the existent risks and risk factors for late occurring death. The CCSS, as a retrospective cohort study that continues to monitor the cohort through longitudinal assessment, serves as an ideal platform to estimate the risk of late mortality in a well-characterized population.
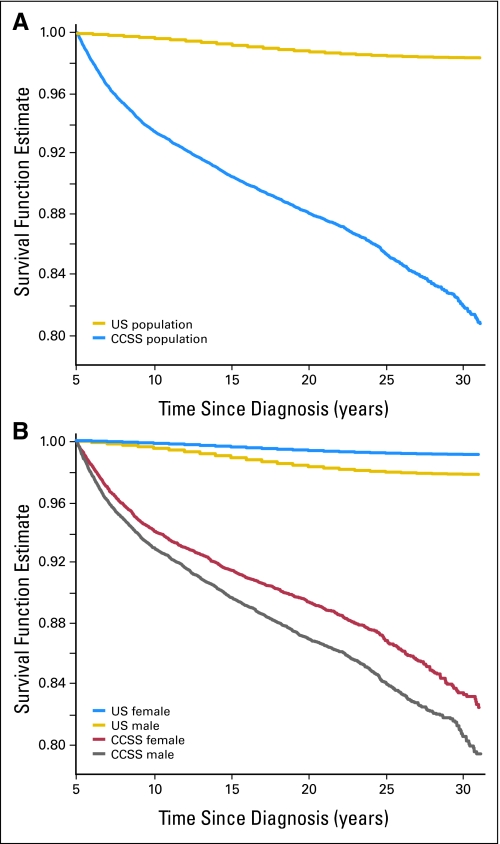
The most recent report of late mortality from the CCSS included a total of 2,821 deaths that have occurred among the 20,483 eligible 5-year survivors.7 With increasing time from primary cancer diagnosis, one would hope that the annual mortality rate of childhood cancer survivors may eventually return to a level that mirrors that of the background population in the United States. Unfortunately, this is not the case. When the all-cause mortality experience of 5-year survivors of childhood cancer is compared to the age-adjusted expected survival rates for the United States population (Fig 1A), at no point in the first 30 years after diagnosis is this estimate of survival for those with childhood cancer similar to the background population. The mortality rates from 5 to 9, 10 to 19, 20 to 29, and 30+ years from diagnosis are 13.57, 6.00, 6.52, and 14.22 deaths per 1,000 person-years, respectively, compared to expected rates of 0.66, 1.03, 1.44, and 2.07 deaths per 1,000 person-years for the United States population. In addition, the overall (cumulative) mortality increases significantly over time: 6.5% at 10 years (95% CI, 6.2 to 6.9), 11.9% at 20 years (95% CI, 11.5 to 12.4) increasing to 18.1% at 30 years from diagnosis (95% CI, 17.3 to 18.9). Considering that the risk of death due to primary malignancy decreases with increasing time from diagnosis, these elevated rates of mortality occurring 20 and 30 years after diagnosis suggest other causes for late mortality become increasingly important with time.
Fig 1.
(A) All-cause mortality (survival function estimate): entire cohort. (B) All-cause mortality (survival function estimate) by sex.
TEMPORAL CHANGES IN CAUSE-SPECIFIC MORTALITY
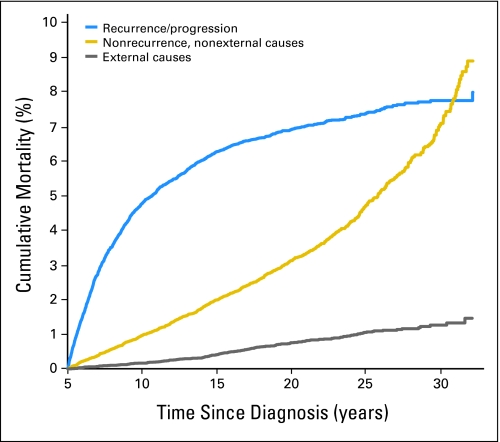
In the original report identifying deaths through December 31, 1996, recurrence, or progression of primary disease, was the cause of death in 67.4% of patients with nonrecurrence, nonexternal causes of death reported for 27.5%, and 5.1% of deaths attributable to external causes. With only 6 years of additional follow-up, however, these proportions changed dramatically to 58.0%, 34.7%, and 7.3%, respectively (Table 1). This change, with ongoing follow-up, in the proportion of deaths attributable to recurrence/progression compared to nonrecurrence, nonexternal death is confirmed by Figure 2. While the mortality rate for death due to the original malignancy begins to plateau beyond 20 years, the rate of death from all nonrecurrence, nonexternal causes increases. Between 15 and 30 years, the cumulative mortality attributable to primary disease increased from 6.3% to only 7.8%, while the cumulative mortality due to nonrecurrent, nonexternal causes increased from 2.0% to 7.0% over the same period. This sharp increase in late-mortality attributable to nonrecurrent, nonexternal causes exceeds what would be expected in the United States population and is largely due to an increase in treatment-related mortality as this population ages.
Table 1.
Frequency of Deaths by Different Causes in the Childhood Cancer Survivor Study
| Specific Cause of Death | ICD-9 Code | % |
|||||
|---|---|---|---|---|---|---|---|
| Total Deaths |
Male |
Female |
|||||
| No. | % | No. | % | No. | % | ||
| Total known causes in each column | 2,534 | 1,511 | 1,023 | ||||
| Recurrence/progressive disease | 1,469 | 58.0 | 882 | 58.4 | 587 | 57.4 | |
| Medical causes of death | 879 | 34.7 | 477 | 31.6 | 402 | 39.3 | |
| Infectious and parasitic diseases | 001-139 | 48 | 1.9 | 23 | 1.5 | 25 | 2.4 |
| Subsequent neoplasm | 140-239 | 470 | 18.5 | 242 | 16.0 | 228 | 22.3 |
| Lip, oral cavity, pharynx | 140-149 | 7 | 0.3 | 4 | 0.3 | 3 | 0.3 |
| Digestive organs and peritoneum | 150-159 | 38 | 1.5 | 16 | 1.1 | 22 | 2.2 |
| Respiratory and intrathoracic organs | 160-165 | 23 | 0.9 | 10 | 0.7 | 13 | 1.3 |
| Bone, connective tissue, skin | 170-173 | 86 | 3.4 | 47 | 3.1 | 39 | 3.8 |
| Breast | 174-175 | 38 | 1.5 | 0 | 0.0 | 38 | 3.7 |
| Genitourinary organs | 179-189 | 23 | 0.9 | 8 | 0.5 | 15 | 1.5 |
| Brain and nervous system | 191-192 | 73 | 2.9 | 52 | 3.4 | 21 | 2.1 |
| Lymphatic and hematopoietic | 200-208 | 129 | 5.1 | 75 | 5.0 | 54 | 5.3 |
| Other subsequent cancer | 53 | 2.1 | 30 | 2.0 | 23 | 2.2 | |
| Endocrine, nutritional, and metabolic diseases and immunity disorders | 240-279 | 14 | 0.6 | 7 | 0.5 | 7 | 0.7 |
| Disease of blood and blood-forming organs | 280-289 | 9 | 0.4 | 5 | 0.3 | 4 | 0.4 |
| Mental disorders | 290-319 | 7 | 0.3 | 5 | 0.3 | 2 | 0.2 |
| Diseases of the nervous system and sense organs | 320-389 | 21 | 0.8 | 9 | 0.6 | 12 | 1.2 |
| Diseases of the circulatory system | 390-459 | 176 | 6.9 | 110 | 7.3 | 66 | 6.5 |
| Ischemic heart disease | 410-414 | 44 | 1.7 | 32 | 2.1 | 12 | 1.2 |
| Cardiomyopathy | 425 | 46 | 1.8 | 28 | 1.9 | 18 | 1.8 |
| Heart failure | 428 | 6 | 0.2 | 4 | 0.3 | 2 | 0.2 |
| Cerebrovascular diseases | 430-438 | 19 | 0.7 | 11 | 0.7 | 8 | 0.8 |
| Other cardiac | 61 | 2.4 | 35 | 2.3 | 26 | 2.5 | |
| Diseases of the respiratory system | 460-519 | 67 | 2.6 | 39 | 2.6 | 28 | 2.7 |
| Pneumonia | 480-486 | 24 | 0.9 | 14 | 0.9 | 10 | 1.0 |
| Pulmonary fibrosis | 515 | 13 | 0.5 | 8 | 0.5 | 5 | 0.5 |
| Other pulmonary | 30 | 1.2 | 17 | 1.1 | 13 | 1.3 | |
| Diseases of the digestive system | 520-579 | 28 | 1.1 | 15 | 1.0 | 13 | 1.3 |
| Diseases of the genitourinary system | 580-629 | 8 | 0.3 | 5 | 0.3 | 3 | 0.3 |
| Complications of the puerperium | 670-676 | 1 | 0.0 | 0 | 0.0 | 1 | 0.1 |
| Diseases of the musculoskeletal system and connective tissue | 710-739 | 8 | 0.3 | 3 | 0.2 | 5 | 0.5 |
| Congenital anomalies | 740-759 | 8 | 0.3 | 4 | 0.3 | 4 | 0.4 |
| Symptoms, signs, and ill-defined conditions | 780-799 | 14 | 0.6 | 10 | 0.7 | 4 | 0.4 |
| External causes of injury and poisoning | E800-E999 | 186 | 7.3 | 152 | 10.1 | 34 | 3.3 |
| Motor vehicle accidents | E810-E825 | 80 | 3.2 | 67 | 4.4 | 13 | 1.3 |
| Other accidents | E826-E929 | 44 | 1.7 | 37 | 2.4 | 7 | 0.7 |
| Suicide | E950-E959 | 39 | 1.5 | 35 | 2.3 | 4 | 0.4 |
| Homicide | E960-E978 | 17 | 0.7 | 10 | 0.7 | 7 | 0.7 |
| Other injury | E980-E999 | 6 | 0.2 | 3 | 0.2 | 3 | 0.2 |
| Unknown cause of death | 287 | 175 | 112 | ||||
NOTE. Bold font indicates four major categories of death.
Abbreviation: ICD-9, International Classification of Disease, 9th revision.
Fig 2.
Cumulative cause-specific mortality.
Assessment of mortality rates within 5-year time blocks from diagnosis (Table 2) demonstrates that the death rate from nonrecurrence, nonexternal causes eventually exceeds, with time, death from primary disease. By 15 years from diagnosis, the mortality rate from nonrecurrent and nonexternal causes exceed the rate from primary disease. By 20 years, death attributable to subsequent malignant neoplasm exceeds death from primary disease and at 30 years, recurrence of primary disease is the least likely cause of late mortality. These findings demonstrate that the pattern of late mortality changes over time and that death from sequelae of treatment received 20 to 30 years earlier is a considerable threat to this population now in young adulthood. It is essential, therefore, to assess which populations are at greatest risk and what treatment modalities lead to this increased risk for late death.
Table 2.
Mortality Rates (deaths/1,000 person-years) by Cause of Death in 5-Year Time Blocks From Diagnosis
| Time From Diagnosis (years) | Recurrence |
Nonrecurrence, Nonexternal Causes |
SMN |
Cardiac |
Pulmonary |
Other* |
External |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | |
| 5-9 | 9.9 | 9.3 to 10.6 | 2.0 | 1.7 to 2.3 | 1.3 | 1.1 to 1.6 | 0.2 | 0.1 to 0.3 | 0.1 | 0.1 to 0.2 | 0.3 | 0.2 to 0.4 | 0.3 | 0.2 to 0.5 |
| 10-14 | 3.3 | 2.9 to 3.7 | 2.2 | 2.0 to 2.6 | 1.2 | 1.0 to 1.4 | 0.3 | 0.2 to 0.4 | 0.2 | 0.1 to 0.3 | 0.6 | 0.4 to 0.7 | 0.5 | 0.4 to 0.7 |
| 15-19 | 1.5 | 1.2 to 1.8 | 2.5 | 2.2 to 2.9 | 1.3 | 1.0 to 1.5 | 0.5 | 0.4 to 0.7 | 0.2 | 0.1 to 0.3 | 0.5 | 0.4 to 0.7 | 0.8 | 0.6 to 1.0 |
| 20-24 | 1.1 | 0.8 to 1.4 | 3.4 | 2.9 to 4.0 | 1.7 | 1.3 to 2.1 | 0.8 | 0.5 to 1.1 | 0.2 | 0.1 to 0.3 | 0.8 | 0.6 to 1.2 | 0.6 | 0.4 to 0.9 |
| 25-29 | 1.0 | 0.6 to 1.6 | 5.2 | 4.2 to 6.4 | 2.3 | 1.7 to 3.1 | 0.8 | 0.5 to 1.4 | 0.5 | 0.2 to 0.9 | 1.6 | 1.1 to 2.3 | 0.5 | 0.2 to 0.9 |
| 30-34 | 0.5 | 0.0 to 2.6 | 10.1 | 6.3 to 15.3 | 4.6 | 2.2 to 8.4 | 1.4 | 0.3 to 4.0 | 0.9 | 0.1 to 3.3 | 3.2 | 1.3 to 6.6 | 0.9 | 0.1 to 3.3 |
Abbreviation: SMN, subsequent malignant neoplasm.
Other causes include deaths not due to recurrence, subsequent malignancy, cardiac, pulmonary, or external causes.
POPULATIONS AT RISK
The CCSS population includes most major pediatric oncology diagnostic groups (ie, leukemia, CNS tumors, Hodgkin's disease, non-Hodgkin's lymphoma, malignant renal tumors [Wilms], neuroblastoma, soft tissue sarcoma, and bone tumors), diagnosed across a broad time interval (1970 to 1986), from multiple institutions, receiving a wide array of therapies and more than 20 years of mean follow-up. Performing a mortality analysis in such a large, heterogeneous population as the CCSS provides opportunity to identify specific subpopulations that may be at higher risk for late mortality. Furthermore, standardization of mortality data (Table 3) to the background rates of death in the United States population allows not only a relative assessment of the differences in rate of death compared to this established background rate, but also provides a meaningful way to compare the mortality experience of two populations within the CCSS cohort.
Table 3.
Life Status and SMRs in 5-Year Survivors of the Childhood Cancer Survivor Study
| Parameter | No. of Patients |
SMR* | 95% CI | |
|---|---|---|---|---|
| Alive | Dead | |||
| All patients | 17,662 | 2,821 | 8.4 | 8.0 to 8.7 |
| Sex | ||||
| Male | 9,636 | 1,686 | 6.7 | 6.4 to 7.0 |
| Female | 8,026 | 1,135 | 13.2 | 12.5 to 14.0 |
| Age at diagnosis, years | ||||
| 0-4 | 7,361 | 820 | 9.1 | 8.5 to 9.8 |
| 5-9 | 3,984 | 616 | 8.4 | 7.7 to 9.1 |
| 10-14 | 3,475 | 667 | 7.9 | 7.3 to 8.5 |
| 15-20 | 2,842 | 718 | 7.9 | 7.4 to 8.5 |
| Years of diagnosis | ||||
| 1970-1973 | 2,267 | 664 | 8.2 | 7.6 to 8.9 |
| 1974-1977 | 3,562 | 735 | 8.0 | 7.4 to 8.6 |
| 1978-1981 | 4,693 | 671 | 7.9 | 7.3 to 8.5 |
| 1982-1986 | 7,140 | 751 | 11.1 | 10.4 to 12.0 |
| Survival after diagnosis, years | ||||
| 5-9 | — | 1,336 | 20.7 | 19.6 to 21.8 |
| 10-14 | — | 611 | 7.2 | 6.6 to 7.7 |
| 15-19 | — | 431 | 4.7 | 4.2 to 5.1 |
| 20-24 | — | 268 | 4.3 | 3.8 to 4.9 |
| 25-29 | — | 144 | 5.0 | 4.2 to 5.9 |
| 30-34 | — | 31 | 6.9 | 4.7 to 9.8 |
| Diagnosis | ||||
| Leukemia | 5,482 | 913 | 10.0 | 9.4 to 10.7 |
| Acute lymphoblastic | 5,030 | 730 | 9.5 | 8.8 to 10.2 |
| Acute myeloid | 421 | 80 | 11.5 | 9.1 to 14.3 |
| Other | 391 | 103 | 14.7 | 12.0 to 17.8 |
| CNS tumors | 2,275 | 546 | 12.9 | 11.8 to 14.0 |
| Astrocytomas | 1,489 | 318 | 11.3 | 10.1 to 12.6 |
| Medulloblastoma, PNET | 424 | 128 | 17.7 | 14.8 to 21.1 |
| Other CNS malignancy | 362 | 100 | 14.1 | 11.5 to 17.1 |
| Hodgkin's disease | 2,207 | 510 | 7.8 | 7.1 to 8.5 |
| Non-Hodgkin's lymphoma | 1,381 | 143 | 4.4 | 3.7 to 5.2 |
| Kidney tumors | 1,638 | 97 | 4.6 | 3.8 to 5.6 |
| Neuroblastoma | 1,274 | 84 | 5.6 | 4.4 to 6.9 |
| Soft tissue sarcoma | 1,594 | 244 | 7.1 | 6.2 to 8.1 |
| Bone tumors | 1,451 | 284 | 7.8 | 7.0 to 8.8 |
| Ewings sarcoma | 432 | 136 | 13.3 | 11.2 to 15.8 |
| Osteosarcoma | 930 | 138 | 5.9 | 4.9 to 6.9 |
| Other bone tumors | 89 | 10 | 4.1 | 2.0 to 7.5 |
NOTE. SMRs are age-, sex, and calendar year–standardized according to National Center for Health Statistics criteria.
Abbreviations: SMR, standardized mortality ratios; PNET, primitive neuroectodermal tumor.
All SMRs are statistically significant at P < .01.
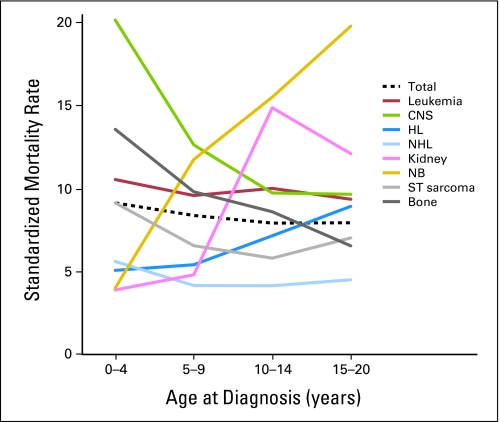
It is not surprising that long-term survivors have higher rates of late mortality in the 5- to 9-year period (SMR, 20.7; 95% CI, 19.6 to 21.8) from diagnosis with decreasing rates of death with ongoing time from diagnosis (Table 3). This corresponds with increased rates of death due to recurrence or progression of primary disease seen during this time period. In addition, young patients (those diagnosed and treated in the first 4 years of life) have higher SMRs (SMR, 9.1; 95% CI, 8.5 to 9.8) than those who were older at diagnosis. This may be driven by the fact that certain diagnostic groups, such as ALL and CNS tumors, the two most commonly diagnosed pediatric malignancies, young age is itself a poor prognostic factor (Fig 3).9 In addition, important therapeutic modalities are often limited in young patients, such as the use of CNS directed radiotherapy, in an attempt to prevent long-term injury. Avoidance of such therapies may also lower the risk of long-term survival.10
Fig 3.
Standardized mortality rates among 5-year survivors by age at diagnosis for all major childhood cancer diagnoses. HL, Hodgkin's lymphoma; NHL, non-Hodgkin's lymphoma; NB, neuroblastoma; ST, soft tissue.
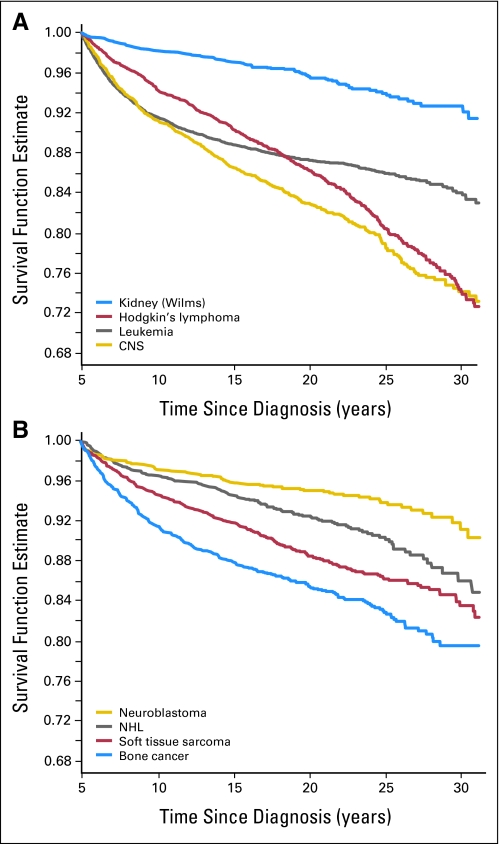
Certain primary diagnoses carry greater risk of poor long-term survival. As a group, CNS tumors have the highest SMR of any population (SMR, 12.9; 95% CI, 11.8 to 14.0) largely driven by the high long-term risk for death for patients with medulloblastoma (SMR, 17.7; 95% CI, 14.8 to 21.1). Thus, even after surviving to the 5-year time point, subjects with medulloblastoma or PNET had an almost 18-fold increased risk of death relative to a comparable age- and sex-matched United States population. It is unclear whether this high rate is attributable to the use of CNS directed radiotherapy without adjuvant chemotherapy in this era (1970 to 1986) or to inadequate salvage therapies for relapsed patients. In addition, long-term survivors of acute myelogenous leukemia (SMR, 11.5; 95% CI, 9.1 to 14.3) and Ewing's sarcoma (SMR, 13.3; 95% CI, 11.2 to 15.8) have increased SMRs relative to other diagnostic groups. At the opposite extreme, patients with renal tumors (predominately Wilms' tumor) and neuroblastoma had the best overall survival with cumulative incidence of death of 7.3% and 8.6%, respectively, at 30 years from diagnosis (Fig 4).
Fig 4.
(A and B) All-cause mortality, survival by original cancer diagnosis in the Childhood Cancer Survivor Study. CNS, central nervous system; NHL, non-Hodgkin's lymphoma.
Finally, while males in the CCSS cohort have a greater absolute risk for death than females (Fig 1B), when standardized to the background rate of death in the United States population, by sex, it is females who have a higher SMR (13.2; 95% CI, 12.5 to 14.0) compared to males (SMR, 6.7; 95% CI, 6.4 to 7.0). Young males in the general population are known to have a higher rate of death due to accidents and injuries; therefore, the use of these data as the denominator for standardization partially explains the lower SMR in males. However, female survivors are at significantly greater risk of second cancers, specifically breast cancer, than their male counterparts which likely increases the gap in the SMRs between the sexes.
CAUSE-SPECIFIC MORTALITY
Death Due to Recurrence
The overall mortality rate of death due to recurrence or progression of the primary malignancy is 4.4/1,000 person-years (annual risk of death, 0.44%/year). Males have a small, but statistically increased risk of recurring and then dying from their recurrence as compared to females (annual mortality rate 0.48 v 0.38; P < .05). Death due to recurrence varied across diagnostic groups with medulloblastoma (1.14%/year), other CNS tumors, largely consisting of ependymomas (0.95%/year) and Ewing's sarcoma (0.95%/year) having the highest annual rate of late death due to recurrence while non-Hodgkin's lymphoma (0.15%/year) and neuroblastoma (0.18%/year) had the lowest rates. In addition, patients diagnosed between 1970 and 1973 (earliest treatment era in this cohort) had the highest rate of death from recurrence (0.48%/year) as well as those who were diagnosed in late childhood. Participants diagnosed between the ages of 15 to 20 years (0.59%/year) were at increased risk compared to all other age groups while the youngest group, age 0 to 4 years had the lowest risk for death due to recurrence (0.33%/year).
Death Due to Nonrecurrent, Nonexternal Causes
Across all categories (ie, subsequent malignancy, cardiac, pulmonary, and other medical causes of death), survivors of childhood cancer are at increased risk of death compared to age-, calendar year–, and sex-specific rates in the United States population (Table 4). Survivors were 15.2 times more likely to die of a subsequent cancer, 7 times more likely to die from cardiac-related events, 8.8 times more likely to die of a pulmonary event, and 2.6 times more likely to die from other medical causes. There was no difference in death from external causes (SMR, 0.9; 95% CI, 0.8 to 1.1). These elevated rates draw important attention to the fact that therapy essential for cure of primary malignancies may well have long-term consequences many years after the risk of recurrence of a primary tumor has past. Such knowledge should not only be communicated to patients and their families at the time of primary diagnosis during the discussion of the risks and benefits of therapy, but also should be used to guide current care of long-term survivors by specifically targeting groups at high risk for late mortality. It is essential, therefore, to better understand who is at risk for these treatment-specific causes of death.
Table 4.
Cause-Specific SMRs and 95% CIs, Excluding Deaths Due to Recurrence
| Parameter | Subsequent Malignancy |
Cardiac |
Pulmonary |
Other Causes |
External Causes |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMR | No. of Deaths | 95% CI | SMR | No. of Deaths | 95% CI | SMR | No. of Deaths | 95% CI | SMR | No. of Deaths | 95% CI | SMR | No. of Deaths | 95% CI | |
| All patients | 15.2 | 470 | 13.9 to 16.6* | 7.0 | 142 | 5.9 to 8.2* | 8.8 | 67 | 6.8 to 11.2* | 2.6 | 200 | 2.3 to 3.0* | 0.9 | 186 | 0.8 to 1.1 |
| Sex‡ | |||||||||||||||
| Male | 14.2 | 242 | 12.4 to 16.1* | 6.2 | 89 | 5.0 to 7.6* | 8.5 | 39 | 6.1 to 11.7* | 2.1 | 107 | 1.7 to 2.5* | 0.9 | 152 | 0.8 to 1.1 |
| Female | 16.4 | 228 | 14.4 to 18.7* | 8.9 | 53 | 6.6 to 11.6* | 9.2 | 28 | 6.1 to 13.2* | 3.8 | 93 | 3.1 to 4.7* | 0.9 | 34 | 0.6 to 1.2 |
| Diagnosis | |||||||||||||||
| Acute lymphoblastic leukemia | 14.7 | 89 | 11.8 to 18.1* | 4.2 | 15 | 2.3 to 6.9* | 4.2 | 7 | 1.7 to 8.6* | 2.5 | 37 | 1.8 to 3.4* | 0.7 | 35 | 0.5 to 1.0† |
| Acute myeloid leukemia | 11.1 | 7 | 4.4 to 22.9* | 5.0 | 2 | 0.6 to 18.1 | 24.9 | 4 | 6.7 to 63.8* | 3.2 | 5 | 1.0 to 7.4† | 1.9 | 8 | 0.8 to 3.8 |
| Other leukemia | 19.0 | 11 | 9.5 to 33.9* | 5.4 | 2 | 0.6 to 19.4 | 32.6 | 5 | 10.5 to 76.1* | 0.7 | 1 | 0.0 to 3.8 | 0.4 | 2 | 0.1 to 1.6 |
| Astrocytomas | 12.4 | 31 | 8.4 to 17.6* | 6.2 | 10 | 3.0 to 11.4* | 20.7 | 13 | 11.0 to 35.4* | 4.0 | 25 | 2.6 to 6.0* | 1.1 | 19 | 0.7 to 1.7 |
| Medulloblastoma, PNET | 23.4 | 13 | 12.4 to 40.0* | 0.0 | 0 | 0.0 to 10.8 | 6.6 | 1 | 0.1 to 36.7 | 2.1 | 3 | 0.4 to 6.2 | 0.6 | 3 | 0.1 to 1.9 |
| Other CNS tumors | 11.4 | 7 | 4.6 to 23.5* | 0.0 | 0 | 0.0 to 8.6 | 12.8 | 2 | 1.4 to 46.3† | 4.9 | 8 | 2.1 to 9.7* | 2.1 | 9 | 1.0 to 4.0 |
| Hodgkin's disease | 20.0 | 149 | 16.9 to 23.5* | 11.9 | 62 | 9.1 to 15.3* | 10.8 | 17 | 6.3 to 17.2* | 2.6 | 46 | 1.9 to 3.5* | 1.0 | 33 | 0.7 to 1.4 |
| Non-Hodgkin's lymphoma | 14.0 | 37 | 9.8 to 19.3* | 6.5 | 13 | 3.5 to 11.1* | 9.1 | 6 | 3.3 to 19.7* | 2.1 | 15 | 1.2 to 3.4† | 1.0 | 19 | 0.6 to 1.5 |
| Kidney tumors | 16.4 | 27 | 10.8 to 23.8* | 12.7 | 11 | 6.3 to 22.8* | 4.2 | 2 | 0.5 to 15.1 | 3.1 | 12 | 1.6 to 5.5* | 0.8 | 11 | 0.4 to 1.4 |
| Neuroblastoma | 10.9 | 13 | 5.8 to 18.7* | 5.0 | 3 | 1.0 to 14.6† | 11.4 | 4 | 3.1 to 29.3* | 2.2 | 6 | 0.8 to 4.8 | 1.1 | 11 | 0.5 to 1.9 |
| Soft tissue sarcoma | 13.8 | 46 | 10.1 to 18.4* | 4.0 | 9 | 1.8 to 7.6* | 3.8 | 3 | 0.8 to 11.1 | 2.8 | 23 | 1.8 to 4.2* | 0.8 | 16 | 0.5 to 1.3 |
| Ewings sarcoma | 20.0 | 19 | 12.0 to 31.3* | 12.0 | 8 | 5.2 to 23.6* | 4.4 | 1 | 0.1 to 24.3 | 4.0 | 10 | 1.9 to 7.3* | 0.9 | 5 | 0.3 to 2.0 |
| Osteosarcoma | 8.1 | 20 | 4.9 to 12.4* | 3.9 | 7 | 1.6 to 8.1* | 3.6 | 2 | 0.4 to 12.9 | 1.4 | 9 | 0.6 to 2.7 | 1.1 | 14 | 0.6 to 1.9 |
| Other bone tumors | 3.4 | 1 | 0.0 to 18.9 | 0.0 | 0 | 0.0 to 16.8 | 0.0 | 0 | 0.0 to 60.0 | 0.0 | 0 | 0.0 to 5.3 | 0.9 | 1 | 0.0 to 4.8 |
NOTE. Subsequent malignancy reflects a new neoplasm. Cancer deaths resulting from progression of the original cancer are not included in the observed number of events. Other causes include deaths not due to recurrence, subsequent malignancy, cardiac, pulmonary, or external causes. External causes include non-medical deaths (ie, accidents, homicides, and suicides).
Abbreviations: SMR, standardized mortality ratio; PNET, primitive neuroectodermal tumor.
P < .01 for SMR.
P < .05 for SMR.
As previously mentioned, females have higher rates than males for all causes of death (SMR 13.2 v 6.7; P < .001), as well as higher rates of death from subsequent malignancy (SMR 16.4 v 14.2; P = .10), cardiac death (SMR 8.9 v 6.2; P = .04), and death from other causes (SMR 3.8 v 2.1; P < .001). This increased rate of long-term complications in females is not surprising as it had been demonstrated that there is an increased propensity for poor long-term outcomes among females across several major outcomes.11 In addition to their increased risk for breast cancer after exposure to chest wall radiation,12,13 females have disproportionate rates of poor cardiac outcomes after exposure to anthracycline chemotherapy,14,15 and higher rates of obesity after exposure to CNS directed radiotherapy,16–18 both of which may increase the rate of cardiac-related death.
SMRs were also evaluated based on primary diagnosis. Patients diagnosed originally with medulloblastoma were at highest risk of death due to a subsequent malignancy (SMR, 23.4; 95% CI, 12.4 to 40.0). These patients generally received craniospinal radiation, and in this era, few patients received chemotherapy. This radiation pattern placed these patients at increased risk of development of subsequent neoplasms, not only in the CNS, which can be rapidly fatal, but for an increased number of soft-tissue sarcomas. Patients treated for Hodgkin's disease (SMR, 20.0; 95% CI, 16.9 to 23.5) and Ewing's sarcoma (SMR, 20.0; 95% CI, 12.0 to 31.3) also had very high SMRs for death due to second malignancy relative to other populations. Patients treated for osteosarcoma had the lowest SMR (8.1; 95% CI, 4.9 to 12.4) perhaps because of the limited exposure to radiotherapy in this population.
Standardized rates for cardiac-related deaths were most elevated for survivors of renal tumors (SMR, 12.7; 95% CI, 6.3 to 22.8) and Hodgkin's disease (SMR, 11.9; 95% CI, 9.1 to 15.3), two populations in which both anthracycline chemotherapy and chest/pulmonary radiotherapy, with its subsequent exposure to the heart were routinely used. Survivors of acute myeloid leukemia (SMR, 24.9; 95% CI, 6.7 to 63.8) and neuroblastoma (SMR, 11.4; 95% CI, 3.1 to 29.3) were at highest risk for pulmonary death. No statistically significantly elevated rates were seen due to external causes including motor vehicle accidents (SMR, 1.0; 95% CI, 0.8 to 1.3), other accidents (SMR, 1.3; 95% CI, 1.0 to 1.8), and suicide (SMR, 1.0; 95% CI, 0.7 to 1.4).
TREATMENT-RELATED RISK FACTORS FOR LATE MORTALITY
The large size of the cohort and the detailed medical abstraction used to obtain treatment information affords CCSS the opportunity to investigate treatment-related risk factors for death while controlling for the effects of other known associations with death including: sex, time from diagnosis, and age at diagnosis (Table 5). For each cause of death the relative risk decreases with time from diagnosis. Relative risk was higher for females than males for death due to subsequent malignancy (RR, 1.4; 95% CI, 1.1 to 1.8) and other deaths (RR, 1.9; 95% CI, 1.5 to 2.5).
Table 5.
Risk of Mortality Due to Subsequent Malignancy, Cardiac Disease, and Other Causes
| Independent Risk Factor | Subsequent Malignancy |
Cardiac |
Other Causes |
|||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Sex | ||||||
| Male | 1.0 | 1.0 | 1.0 | |||
| Female | 1.3 | 1.1 to 1.5* | 1.4 | 1.0 to 1.9† | 1.9 | 1.5 to 2.4* |
| Age at diagnosis, years | ||||||
| 0-4 | 1.5 | 1.1 to 1.9* | 2.1 | 1.3 to 3.6* | 1.3 | 0.9 to 1.9 |
| 5-9 | 1.1 | 0.8 to 1.4 | 1.4 | 0.9 to 2.3 | 1.5 | 1.1 to 2.2† |
| 10-14 | 0.9 | 0.7 to 1.2 | 0.8 | 0.5 to 1.3 | 1.1 | 0.8 to 1.6 |
| 15-20 | 1.0 | 1.0 | 1.0 | |||
| Years of diagnosis | ||||||
| 1970-1973 | 1.3 | 1.0 to 1.8 | 1.7 | 0.9 to 3.4 | 1.2 | 0.7 to 1.8 |
| 1974-1977 | 1.1 | 0.8 to 1.4 | 2.1 | 1.2 to 3.7† | 0.9 | 0.6 to 1.3 |
| 1978-1981 | 0.8 | 0.6 to 1.1 | 1.8 | 1.0 to 3.1 | 0.8 | 0.5 to 1.2 |
| 1982-1986 | 1.0 | 1.0 | 1.0 | |||
| Years since diagnosis | ||||||
| 5-9 | 2.7 | 2.1 to 3.5* | 2.2 | 1.3 to 3.6* | 1.6 | 1.1 to 2.3† |
| 10-14 | 1.9 | 1.5 to 2.5* | 1.7 | 1.0 to 2.7† | 1.4 | 1.0 to 2.0 |
| 15-19 | 1.4 | 1.1 to 1.8† | 1.5 | 1.0 to 2.3† | 0.9 | 0.6 to 1.3 |
| 20+ | 1.0 | 1.0 | 1.0 | |||
| Radiation‡ | ||||||
| Yes | 2.9 | 2.1 to 4.2* | 3.3 | 2.0 to 5.5* | 2.0 | 1.3 to 3.1* |
| No | 1.0 | 1.0 | 1.0 | |||
| Alkylating agent score | ||||||
| Not exposed | 1.0 | 1.0 | 1.0 | |||
| 1-2 | 1.4 | 1.0 to 2.0† | 0.6 | 0.3 to 1.2 | 1.0 | 0.7 to 1.7 |
| 3-4 | 1.8 | 1.1 to 2.8† | 0.6 | 0.3 to 1.4 | 1.2 | 0.8 to 1.9 |
| 5+ | 2.2 | 1.6 to 3.0* | 1.7 | 1.0 to 3.0 | 1.7 | 1.0 to 2.9† |
| Anthracycline, mg/m2 | ||||||
| Not exposed | 1.0 | 1.0 | 1.0 | |||
| 1-100 | 1.1 | 0.5 to 2.1 | 2.5 | 0.7 to 9.2 | 1.7 | 0.7 to 4.1 |
| 101-250 | 1.1 | 0.6 to 1.9 | 2.3 | 0.9 to 6.0 | 1.6 | 0.9 to 3.1 |
| 251-400 | 1.2 | 0.8 to 1.7 | 2.2 | 1.3 to 4.0* | 1.4 | 0.9 to 2.2 |
| 401+ | 1.4 | 0.9 to 2.1 | 3.1 | 1.6 to 5.8* | 1.7 | 0.9 to 3.0 |
| Epipodophyllotoxin, mg/m2 | ||||||
| Not exposed | 1.0 | 1.0 | 1.0 | |||
| 1-982 | 1.1 | 0.4 to 2.7 | 0.7 | 0.1 to 5.1 | 1.2 | 0.3 to 4.5 |
| 983-4,108 | 1.6 | 0.6 to 4.0 | 0.8 | 0.1 to 6.0 | 0.9 | 0.2 to 3.8 |
| 4,109+ | 2.3 | 1.2 to 4.5† | 1.9 | 0.4 to 8.5 | 0.8 | 0.2 to 3.9 |
| Bleomycin, mg/m2 | ||||||
| Not exposed | 1.0 | 1.0 | 1.0 | |||
| 1-59 | 1.2 | 0.7 to 2.1 | 1.7 | 0.8 to 3.9 | 0.8 | 0.3 to 2.3 |
| 60-119 | 1.2 | 0.5 to 2.7 | 0.9 | 0.3 to 2.8 | 1.0 | 0.3 to 3.0 |
| 119+ | 1.9 | 0.8 to 4.5 | 1.3 | 0.3 to 5.7 | 0.6 | 0.1 to 3.3 |
NOTE. Other causes include deaths not due to recurrence, subsequent malignancy, cardiac, pulmonary or external causes.
Abbreviation: RR, relative risk.
P < .01.
P < .05.
Radiation is overall radiation for subsequent malignancy; chest/spine/total-body irradiation for cardiac; chest/total-body irradiation for pulmonary.
Higher rates of death due to subsequent malignancy were seen in patients diagnosed between 0 and 4 years, those who received any radiotherapy exposure, and those who received alkylator or the highest dose level of epipodophyllotoxin exposure. Cardiac radiation and higher doses of anthracyline exposure, both previously established risk factors for cardiac death, were associated with cardiac related death. Finally, in addition to being female, exposure to radiotherapy was associated with death from other causes.
COMPARISON OF CCSS WITH OTHER STUDIES
Several studies, in addition to the CCSS, have analyzed late mortality in the modern era of cancer treatment, beyond 1970. Robertson et al19 followed their assessment of the British population diagnosed before 1971 with an analysis of 5-year survivors diagnosed between 1971 and 1985. While all childhood cancer diagnoses were eligible, this population was limited to those younger than 15 years of age at diagnosis. Like the CCSS, they reported an increase in non-neoplastic death over time (ie, nonrecurrence and nonsubsequent neoplasm) with an observed to expected ratio of 4. The proportion of deaths due to causes other than recurrent tumor increased from 23% during the 5- to 9-year time period from diagnosis to 53% in the 15- to 19-year time period. Perhaps the most important contribution of this publication,19 however, is in its comparison to patients diagnosed before 1970. First, initial 5-year survival improved dramatically between theses two cohorts (25.9% v 50.4%); an improvement that changes entirely the make-up of the subsequent surviving population. As a result, there was a reduction in death from recurrence (11.5% v 8.3%) and a slight increase in death from treatment related causes (0.8% v 1.9%), perhaps suggesting that improvement in 5-year survival was also resulting in durable remissions, but this may be at the cost of an increase in late treatment-related death due to more intense, multimodal therapeutic regimens. Unfortunately, however, this analysis was limited by its absence of information regarding specific treatments and was unable to estimate the risks of specific causes of death in relation to type of treatment received.
A single institution study from St Jude Children's Research Hospital that included 2,053 5-year survivors also documented an improvement in long-term mortality across treatment eras (patients diagnosed 1962 to 1970 compared to those diagnosed 1971 to 1983). In addition to a reduction in the cumulative incidence of death at 20 years (23.6% v 13.4%; P < .0001), the cumulative incidence of death from recurrence at 20 years improved as well (17.6% v 6.7%; P < .0001). The incidence of death from second malignancies increased (1.7% v 3.7%), but this did not reach statistical significance, emphasizing the important need for larger studies that have the power to determine differences for such important evaluations. While a number of other small, institutionally based studies and late mortality studies of specific diagnostic groups exist, none approach the size of the CCSS population.20–33
One important exception, however, is a population-based study from five Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden) that identified 13,711 survivors diagnosed between 1960 and 1989 before the age of 20.34 Many of their findings confirm what the CCSS has identified among survivors in the United States including an overall SMR of 10.8, overall mortality at 25 years of 14%, and higher absolute mortality rates in males (Table 6). The CCSS, as an institutionally based study, may be at risk of not representing the broader population of childhood cancer survivors (poor generalizability), however, such strong correlation of findings with a population-based study suggests this is not the case. In addition, however, the Nordic study also reported only 7.1% of their population deceased due to subsequent malignancy for an SMR of 4.9, very much lower than that reported by the CCSS (SMR, 15.2). Possible explanations for this discrepancy include inclusion of patients from the 10-year period before 1970 that were not included in the CCSS study as these patients may have lower rates of SMN. In addition, due to the rules of the official mortality statistics as applied the Nordic countries, this study is at risk for misclassification of outcome by overestimating death attributable to primary disease and underestimating death attributable to subsequent neoplasms and treatment-related death.
Table 6.
Comparison of Demographics and Data of 5-Year Survivors of Childhood Cancer
| Parameter | Childhood Cancer Survivor Study6 |
Nordic Study34 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Country | United States | Denmark, Finland, Norway, Iceland, Sweden | ||
| Type of study | Hospital based | Population based | ||
| Age at diagnosis | < 21 | < 20 | ||
| Years of diagnosis | 1970-1986 | 1960-1989 | ||
| No. of 5-year survivors | 20,227 | 13,711 | ||
| Alive | 18,197 | 90.0 | 12,289 | 89.6 |
| Dead | 2,030 | 10.0 | 1,422 | 10.4 |
| Death due to recurrent tumor | 1,246 | 67.4 | 976 | 69.6 |
| Death due to second cancer | 235 | 12.7 | 99 | 7.1 |
| Noncancer treatment-related deaths | 159 | 7.8 | 160 | 11.3 |
| Unrelated deaths | 208 | 10.2 | 167 | 11.9 |
| Standardized mortality ratio | 10.8 | 10.8 | ||
CONCLUSION
For children diagnosed with pediatric malignancies, 5-year survival has served not only as a benchmark for survival but as a goal for patients and families alike. However, significant risk for late mortality is now well-established, with higher risk for late recurrence in the early decades followed by increasing rates of subsequent malignancy and treatment-related deaths in later decades. Additional associations with sex, primary diagnosis, and therapeutic modalities should now inform future treatment regimens in an attempt to minimize long-term treatment related mortality while maintaining durable remissions. The CCSS investigations of late mortality provide the largest published population assessed to date with detailed treatment information, allowing assessment of potential associations between therapeutic modalities and late-mortality that could not be examined previously. Limitations include the absence of patients with retinoblastoma and the inability to draw conclusions regarding the genetic associations that may predispose to subsequent neoplasms. However, the ongoing, prospective follow-up of this population as they age into their fourth and fifth decades of life will serve as an essential early warning system for the detection of new trends and developments in late mortality. In addition, with the CCSS expansion to include survivors diagnosed between 1987 and 1999, it will be possible to determine whether our most modern primary therapies are improving 5-year survival, as well as producing durable, long-term remissions, with minimal risk for treatment-related mortality.
Beyond continued observation of the mortality experience of this unique cohort, the CCSS will be able to provide new information regarding the potential role of health-related behaviors on late mortality. Health behaviors such as tobacco use, alcohol consumption, diet, exercise, and health screening practices all influence mortality among the general population. It is likely that all or some of these health behaviors may have a greater impact on the mortality experience of adult survivors of childhood cancer. It is only through continued follow-up and characterization of this survivor population that it will be possible to identify and quantify the influence of the multitude of factors that will determine their ultimate longevity.
Footnotes
Support by American Lebanese-Syrian Associated Charities and grant No. U24-CA55727 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Manuscript writing: Gregory T. Armstrong, Qi Liu, Yutaka Yasui, Joseph P. Neglia, Wendy Leisenring, Leslie L. Robison, Ann C. Mertens
REFERENCES
- 1.Hewitt M, Weiner SL, Simone JV, editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 2.Ries LA, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2003. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 3.Li FP, Cassady JR, Jaffe N. Risk of second tumors in survivors of childhood cancer. Cancer. 1975;35:1230–1235. doi: 10.1002/1097-0142(197504)35:4<1230::aid-cncr2820350430>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins MM, Kingston JE, Kinnier Wilson LM. Late deaths after treatment for childhood cancer. Arch Dis Child. 1990;65:1356–1363. doi: 10.1136/adc.65.12.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson HS, Fears TR, Byrne J. Death during adulthood in survivors of childhood and adolescent cancer. Cancer. 1994;73:3094–3102. doi: 10.1002/1097-0142(19940615)73:12<3094::aid-cncr2820731231>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 7.Reference deleted
- 8.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reaman G, Zeltzer P, Bleyer WA, et al. Acute lymphoblastic leukemia in infants less than one year of age: A cumulative experience of the Children's Cancer Study Group. J Clin Oncol. 1985;3:1513–1521. doi: 10.1200/JCO.1985.3.11.1513. [DOI] [PubMed] [Google Scholar]
- 10.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong GT, Sklar CA, Hudson MM, et al. Long-term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol. 2007;25:4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 12.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 14.Silber JH, Jakacki RI, Larsen RL, et al. Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol. 1993;21:477–479. doi: 10.1002/mpo.2950210704. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 16.Didi M, Didcock E, Davies HA, et al. High incidence of obesity in young adults after treatment of acute lymphoblastic leukemia in childhood. J Pediatr. 1995;127:63–67. doi: 10.1016/s0022-3476(95)70258-x. [DOI] [PubMed] [Google Scholar]
- 17.Odame I, Reilly JJ, Gibson BE, et al. Patterns of obesity in boys and girls after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1994;71:147–149. doi: 10.1136/adc.71.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 19.Robertson CM, Hawkins MM, Kingston JE. Late deaths and survival after childhood cancer: Implications for cure. Bmj. 1994;309:162–166. doi: 10.1136/bmj.309.6948.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardous-Ubbink MC, Heinen RC, Langeveld NE, et al. Long-term cause-specific mortality among five-year survivors of childhood cancer. Pediatr Blood Cancer. 2004;42:563–573. doi: 10.1002/pbc.20028. [DOI] [PubMed] [Google Scholar]
- 21.Dama E, Pastore G, Mosso ML, et al. Late deaths among five-year survivors of childhood cancer: A population-based study in Piedmont Region, Italy. Haematologica. 2006;91:1084–1091. [PubMed] [Google Scholar]
- 22.MacArthur AC, Spinelli JJ, Rogers PC, et al. Mortality among 5-year survivors of cancer diagnosed during childhood or adolescence in British Columbia, Canada. Pediatr Blood Cancer. 2007;48:460–467. doi: 10.1002/pbc.20922. [DOI] [PubMed] [Google Scholar]
- 23.Eng C, Li FP, Abramson DH, et al. Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993;85:1121–1128. doi: 10.1093/jnci/85.14.1121. [DOI] [PubMed] [Google Scholar]
- 24.Cotterill SJ, Pearson AD, Pritchard J, et al. Late relapse and prognosis for neuroblastoma patients surviving 5 years or more: A report from the European Neuroblastoma Study Group “Survey”. Med Pediatr Oncol. 2001;36:235–238. doi: 10.1002/1096-911X(20010101)36:1<235::AID-MPO1057>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: Report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DM, Hyland A, Chung CS, et al. Cancer and cardiac mortality among 15-year survivors of cancer diagnosed during childhood or adolescence. J Clin Oncol. 1999;17:3207–3215. doi: 10.1200/JCO.1999.17.10.3207. [DOI] [PubMed] [Google Scholar]
- 28.Lawless SC, Verma P, Green DM, et al. Mortality experiences among 15+ year survivors of childhood and adolescent cancers. Pediatr Blood Cancer. 2007;48:333–338. doi: 10.1002/pbc.20723. [DOI] [PubMed] [Google Scholar]
- 29.Morris EB, Gajjar A, Okuma JO, et al. Survival and late mortality in long-term survivors of pediatric CNS tumors. J Clin Oncol. 2007;25:1532–1538. doi: 10.1200/JCO.2006.09.8194. [DOI] [PubMed] [Google Scholar]
- 30.Pui CH, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 31.Bluhm EC, Ronckers C, Hayashi RJ, et al. Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood. 2008;111:4014–4021. doi: 10.1182/blood-2007-08-106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Blood. 2008;111:5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulrooney DA, Dover DC, Li S, et al. Twenty years of follow-up among survivors of childhood and young adult acute myeloid leukemia: A report from the Childhood Cancer Survivor Study. Cancer. 2008;112:2071–2079. doi: 10.1002/cncr.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries. J Clin Oncol. 2001;19:3173–3181. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]