Abstract
These studies were undertaken to determine the effect, if any, of treatment for cancer diagnosed during childhood or adolescence on ovarian function and reproductive outcomes. We reviewed the frequency of acute ovarian failure, premature menopause, live birth, stillbirth, spontaneous and therapeutic abortion and birth defects in the participants in the Childhood Cancer Survivor Study (CCSS). Acute ovarian failure (AOF) occurred in 6.3% of eligible survivors. Exposure of the ovaries to high-dose radiation (especially over 10 Gy), alkylating agents and procarbazine, at older ages, were significant risk factors for AOF. Premature nonsurgical menopause (PM) occurred in 8% of participants versus 0.8% of siblings (rate ratio = 13.21; 95% CI, 3.26 to 53.51; P < .001). Risk factors for PM included attained age, exposure to increasing doses of radiation to the ovaries, increasing alkylating agent score, and a diagnosis of Hodgkin's lymphoma. One thousand two hundred twenty-seven male survivors reported they sired 2,323 pregnancies, and 1,915 female survivors reported 4,029 pregnancies. Offspring of women who received uterine radiation doses of more than 5 Gy were more likely to be small for gestational age (birthweight < 10 percentile for gestational age; 18.2% v 7.8%; odds ratio = 4.0; 95% CI, 1.6 to 9.8; P = .003). There were no differences in the proportion of offspring with simple malformations, cytogenetic syndromes, or single-gene defects. These studies demonstrated that women treated with pelvic irradiation and/or increasing alkylating agent doses were at risk for acute ovarian failure, premature menopause, and small-for-gestational-age offspring. There was no evidence for an increased risk of congenital malformations. Survivors should be generally reassured although some women have to consider their potentially shortened fertile life span in making educational and career choices.
INTRODUCTION
The treatment of children and adolescents with cancer has become increasingly successful. Approximately 78% of all patients diagnosed younger than 15 years of age will survive for 5 years. The majority is expected to survive for many years after diagnosis.1
The treatment these patients receive may adversely affect their fertility and pregnancy outcomes. Testicular damage can result in either sterilization alone, as is frequently observed after chemotherapy that includes an alkylating agent and/or procarbazine,2–13 or both sterilization and loss of hormone production, as is observed after direct testicular irradiation.14 It is common to see preservation of Leydig function, as testicular hormonal production is more resistant to treatment-induced damage and is independent of the presence of spermatogonia in the testes. Loss of ovarian function after chemotherapy that includes an alkylating agent and/or procarbazine -12,15–22 or ovarian irradiation23 results in both sterilization and loss of hormone production because ovarian hormonal production is closely related to the presence of ova and maturation of the primary follicle. While fertility may be preserved in some women who have received abdominal irradiation, such women may have an increased risk for premature labor and low birthweight24,25 as the result of damage to the uterine vasculature and myometrium.26
We review the research of the Childhood Cancer Survivor Study (CCSS) conducted regarding ovarian failure, pregnancy outcome, and offspring health.
OVARIAN FAIURE
Premature Ovarian Failure
Depending on the extent of damage to the ovaries, two forms of premature ovarian failure can be distinguished.27 Survivors who lose ovarian function during cancer therapy or shortly after its completion are classified as having acute ovarian failure (AOF). Some survivors who retain ovarian function after the completion of cancer treatment will experience menopause younger than age 40 years and are classified as having premature menopause.27,28 In general, older age at treatment, exposure to abdominal, pelvic and spinal radiotherapy and certain chemotherapeutic drugs, especially alkylating agents, have been shown to increase the rate of ovarian failure in female cancer survivors.27,29
AOF
Subjects at high risk of developing AOF may benefit from the newer techniques of fertility preservation (eg, ovarian tissue cryopreservation) and need to be counseled accordingly.30 However, the data on the incidence of and risk factors for AOF are limited. Furthermore, previous studies are often based on small cohorts of patients with inadequate or incomplete assessment of therapeutic exposures.29,31 Previous data indicate that radiation affects the ovaries in a dose-dependent fashion.32 Doses in the range of 10 to 30 Gy have been noted to cause AOF in the majority of patients treated during childhood and adolescence.29,31,33 Among chemotherapeutic agents, alkylating agents are known to be associated with ovarian failure.29,31 Myeloablative chemotherapy regimens, such as high-dose cyclophosphamide combined with busulfan, are being used increasingly as preparation for stem-cell transplantation and are associated with a high risk of AOF.31,34
Female participants from the CCSS who were ≥ 18 years of age were considered for inclusion in a study of AOF.27 We excluded survivors who received cranial irradiation at doses of more than 30 Gy and those with hypothalamic/pituitary tumors (due to their risk of developing gonadotropin deficiency). Survivors who underwent bilateral oophorectomy were also excluded. Survivors who reported never menstruating or who had ceased having menses within 5 years after their cancer diagnosis were considered to have AOF.
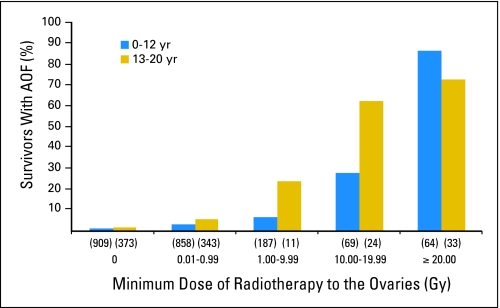
Of a total of 3,390 eligible survivors, 215 women (6.3%) developed AOF. Survivors with AOF were older at cancer diagnosis, more likely to have been diagnosed with Hodgkin's lymphoma, or more likely to have received abdominal or pelvic radiotherapy than survivors without AOF.27 Of survivors who developed AOF, 75% had received abdominal-pelvic irradiation. Radiation doses to the ovary ≥ 20 Gy were associated with the highest rate of AOF, with over 70% of such patients developing AOF (Fig 1).27 In a multivariable logistic regression model, increasing doses of ovarian irradiation, exposure to procarbazine at any age, and exposure to cyclophosphamide at age 13 to 20 years were independent risk factors for AOF (Table 1). As the only criterion used to diagnose AOF was self-reported amenorrhea, it is possible that cases of amenorrhea due to conditions other than primary ovarian failure (eg, stress-related amenorrhea) may have been included.
Fig 1.
Percentage of subjects with acute ovarian failure (AOF) by age at diagnosis of cancer of 0 to 12 years, 13 to 20 years, and radiation dose to the ovary.
Table 1.
Multiple Poisson Regression Model for Risk of Nonsurgical Premature Menopause Among Survivors of HL and Other Childhood Cancers
| Variable | Rate Ratio | 95% CI | P* |
|---|---|---|---|
| Attained age | 1.15 | 1.09 to 1.21 | < .001 |
| Minimum ovarian RT, Gy | |||
| Not HL | |||
| No RT | 1.00 | ||
| 0.01-0.99 | 4.30 | 1.20 to 15.47 | .03 |
| 1.00-9.99 | 5.70 | 1.12 to 28.99 | .04 |
| ≥ 10.00 | 109.59 | 28.15 to 426.70 | < .001 |
| HL | |||
| No RT | 9.18 | 1.52 to 55.24 | .02 |
| 0.01-0.99 | 12.26 | 3.41 to 44.14 | < .001 |
| 1.00-9.99 | 11.41 | 2.75 to 47.26 | < .001 |
| ≥ 10.00 | 6.74 | 0.63 to 71.74 | .11 |
| Summed alkylating agent dose score tertile | |||
| 0 | 1.00 | ||
| 1-2 | 2.30 | 1.08 to 4.90 | .03 |
| 3 | 5.78 | 2.90 to 11.55 | < .001 |
Abbreviations: RT, radiation therapy; HL, Hodgkin's lymphoma; Not HL, childhood cancers other than HL.
P values calculated with multiple Poisson regression likelihood ratio test (two sided).
In summary, AOF appears in a relatively small number of childhood cancer survivors. Exposure of the ovaries to high-dose radiation (especially more than 10 Gy), and exposure to alkylating agents and procarbazine, at older ages, were significant risk factors for AOF. These data will assist clinicians in counseling patients and their families at the time of diagnosis and before cancer therapy is initiated on possible risk of AOF.
Premature Menopause
While AOF occurs in a minority of females diagnosed with childhood cancer, survivors who retain ovarian function are known to be at increased risk of developing premature menopause, defined as cessation of menses younger than age 40 years.30,31,35 Premature menopause leads to the early and often unexpected loss of reproductive potential as well as the cessation of ovarian sex hormone production. Thus, survivors who experience premature menopause are at increased risk of developing a variety of adverse health outcomes, including osteoporosis,36,37 death from cardiovascular diseases,38 and psychosexual dysfunction39 compared with women who do not undergo premature menopause.
In order to counsel current survivors about their risk of experiencing an early menopause—information that would facilitate family planning and timing of future pregnancies—accurate risk estimates are needed. Data on the incidence of premature menopause and on the patient and treatment factors associated with the development of premature menopause in survivors of childhood cancer are limited30,31 but suggest that exposure to ovarian radiation and alkylating agents are major risk factors. Previously published studies suffer from a number of limitations, including small sample size, lack of detailed information on treatment exposures, failure to exclude individuals with a probable central cause for cessation of menses (eg, gonadotropin deficiency), and failure to separate surgical from nonsurgical cases of premature menopause.30,31
We assessed the incidence of and risk factors for premature menopause in 2,819 survivors of childhood cancer who were older than 18 years and were participants in the multicenter CCSS.40 The comparison group was 1,065 female siblings of participants in the CCSS. Subjects with AOF, those who had received more than 30 Gy to the hypothalamus/pituitary and those with tumors in the region of the hypothalamus/pituitary, were excluded. Subjects were considered to be menopausal if, based on their responses to a follow-up questionnaire (available at www.stjude.org/ccss) administered during 2000 and 2001, they had not experienced a spontaneous menses for at least 6 months and other causes (eg, pregnancy, use of agents such as injectable progesterone and gonadotropin-releasing hormone analogs) had been excluded.
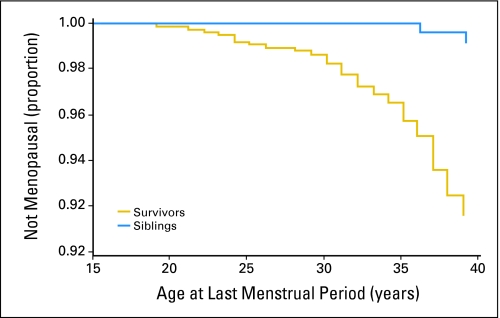
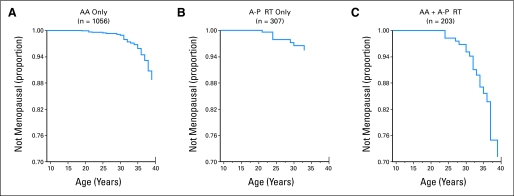
A total of 126 childhood cancer survivors and 33 control siblings developed premature menopause. Of these women, 61 survivors (48%) and 31 siblings (94%) had surgically-induced menopause (rate ratio [RR] = 0.8; 95% CI, 0.52 to 1.23). However, the cumulative incidence of nonsurgical premature menopause was substantially higher for survivors than for siblings (8% v 0.8%; RR = 13.21; 95% CI, 3.26 to 53.51; P < .001; Fig 2).40 A multiple Poisson regression model showed that risk factors for nonsurgical premature menopause included attained age, exposure to increasing doses of radiation to the ovaries, increasing alkylating agent score (based on number of alkylating agents and cumulative dose), and a diagnosis of Hodgkin's lymphoma (Table 1). For survivors who were treated with alkylating agents plus abdominal-pelvic radiation, the cumulative incidence of nonsurgical premature menopause approached 30% (Fig 3).40
Fig 2.
Cumulative incidence curves of nonsurgical premature menopause in survivors compared with siblings.
Fig 3.
Cumulative incidence curves of nonsurgical premature menopause in survivors according to treatment exposures. (A) Survivors treated with alkylating agents (AA) but not with abdominal-pelvic radiation therapy (A-P RT). (B) Survivors treated with A-P RT but not AA. (C) Survivors treated with AA and A-P RT.
When interpreting the results of this study, certain limitations should be kept in mind. We relied on self-reports of menopausal status, which could have resulted in some misclassification of cases, particularly for women with surgically-induced menopause. Both the relatively young age of our cohort and the fact that a sizable percentage of participants who were classified as not menopausal were taking oral contraceptives, likely resulted in an underestimate of the incidence of nonsurgical premature menopause among study participants. Nonetheless, the results of this study facilitate counseling current survivors about their risk of experiencing an early menopause and will assist researchers in the design of new therapeutic protocols that aim to reduce late ovarian toxicity.
PREGNANCY OUTCOME
Several studies demonstrated that the offspring of women who received flank irradiation for Wilms tumor were more likely to have a birthweight of less than 2,500 g than were those born to women whose protocol treatment for Wilms tumor did not include flank irradiation.41–43 Chiarelli et al44 reported an increased relative risk of low-birthweight offspring among women treated for childhood cancer with greater than 25 Gy of abdominal-pelvic radiation. Hawkins et al45 reported that the mean birthweight of the offspring of women who received abdominal radiation for Wilms tumor, but not of those who received abdominal radiation for a malignancy other than Wilms tumor, was less than that of the offspring of unirradiated women or men. None of these studies reported the effect of the different radiation doses received by the musculoskeletal structures, uterus, or ovaries from the various treatment volumes included in their analyses of low birthweight.
Green et al25 reported that malposition of the fetus and early or threatened labor were more frequent among female Wilms tumor survivors who received abdominal irradiation than among those who did not, with the frequency increasing with increasing abdominal radiation dose. In addition, the frequency of both low birthweight (lower than 2,500 g) and early gestational age (younger than 36 weeks) increased with increasing abdominal radiation dose. No effect of abdominal irradiation on pregnancy outcome was observed in the partners of irradiated male Wilms tumor survivors or in their offspring.25
The mechanism responsible for low birthweight in these studies is unclear. The research of Critchley et al26,46 suggested that damage to both the uterine vasculature and myometrium contributed to restricted fetal growth and early birth. They demonstrated that uterine length was significantly less in 10 women with ovarian failure who had been treated with whole abdomen irradiation. Endometrial thickness, based on weekly ultrasound examinations, did not increase in response to hormone replacement therapy in three women. No blood flow was detectable with Doppler ultrasound through either uterine artery of five women and through only one uterine artery in three additional women.26,46 Others have confirmed the finding of reduced uterine volume despite sex steroid replacement therapy. In addition uterine blood flow did not normalize in three of nine women, despite treatment with sex steroid replacement therapy.47
We evaluated pregnancy outcome in male and female CCSS participants. A baseline questionnaire was returned by 7,514 men and 6,494 women. One thousand two hundred twenty-seven male survivors reported they sired 2,323 pregnancies and 1,915 female survivors reported 4,029 pregnancies.
Pregnancy Outcome of the Partners of the Male CCSS Participants
The proportion of pregnancies that resulted in a live birth was significantly lower for the partners of the male survivors than for the partners of the male siblings (RR = 0.79; 95% CI, 0.65 to 0.96, P = .016).48 The RR for a live birth was not significantly decreased overall for treatment with any combination of modalities when compared with treatment with surgery only (Table 2).48 The rate of live births was significantly lower among the partners of male survivors treated with dactinomycin (RR = 0.68; 95% CI, 0.49 to 0.94; P = .02). The rates of live birth and of stillbirth were not different for offspring of the partners of male survivors treated with any other particular chemotherapeutic agent. The rates of live birth and miscarriage were not different for offspring of the partners of male survivors when analyzed according to the tertile of the cumulative dose received of each chemotherapeutic agent.49
Table 2.
Pregnancy Outcomes of Partners of Male Cancer Survivors by Treatment
| Treatment | All Pregnancies (No.) | Live Births |
Stillbirths |
Miscarriages |
Abortions |
Unknown (No.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | |||
| S only | 194 | 139 | 1.00 | — | 2 | 1.00 | — | 26 | 1.00 | — | 21 | 1.00 | — | 6 |
| C only | 59 | 44 | 1.33 | 0.57 to 3.11 | 0 | — | — | 5 | 0.67 | 0.26 to 1.76 | 9 | 1.15 | 0.42 to 3.15 | 1 |
| RT only | 12 | 11 | 3.8 | 0.74 to 19.5 | 0 | — | — | 1 | 0.59 | 0.12 to 2.92 | 0 | — | — | — |
| C + S | 430 | 279 | 0.74 | 0.49 to 1.13 | 3 | 0.66 | 0.11 to 3.85 | 66 | 1.19 | 0.73 to 1.93 | 66 | 1.35 | 0.75 to 2.45 | 16 |
| C + RT | 103 | 73 | 0.99 | 0.57 to 1.7 | 0 | — | — | 13 | 0.95 | 0.48 to 1.88 | 13 | 1.16 | 0.5 to 2.67 | 4 |
| S + RT | 364 | 265 | 1.08 | 0.69 to 1.68 | 5 | 1.28 | 0.26 to 6.39 | 39 | 0.77 | 0.45 to 1.34 | 45 | 1.05 | 0.56 to 1.97 | 10 |
| C + S + RT | 759 | 519 | 0.96 | 0.65 to 1.42 | 5 | 0.64 | 0.13 to 3.19 | 98 | 1.02 | 0.64 to 1.62 | 95 | 0.94 | 0.53 to 1.67 | 42 |
| Unknown | 402 | 279 | 1.11 | 0.7 to 1.76 | 1 | 0.24 | 0.02 to 2.62 | 52 | 0.95 | 0.53 to 1.71 | 44 | 0.98 | 0.53 to 1.81 | 26 |
Abbreviations: RR, rate ratio; S, surgery; C, chemotherapy; RT, radiation therapy.
There was no statistically significant difference in the distribution of birthweights of offspring of the partners of male survivors who had versus had not been treated with an alkylating agent (RR = 1.62; 95% CI, 0.84 to 3.11; P = .15), whose partner smoked during pregnancy (RR = 1.64, 95% CI, 0.76 to 3.53, P = .21), or who had versus had not received pelvic irradiation (RR = 1.51; 95% CI, 0.61 to 3.74; P = .38; Fig 2). The offspring of male survivors who were treated with non–alkylating agent chemotherapy (RR = 3.03; 95% CI, 1.15 to 7.98; P = .025) were more likely to weigh less than 2,500 g.48
In summary, pregnancy outcome of the partners of male survivors in general was not affected by their prior treatment exposures. Only treatment with non–alkylating agent chemotherapy increased the risk of low birthweight (birthweight < 2500 g) of offspring of the partners of the male survivors, a finding that needs to be reproduced in other studies.
Pregnancy Outcome of Female CCSS Participants
The distribution of pregnancy outcome by age at the start of pregnancy is presented in Table 1, in comparison to the pregnancy outcome of the female siblings. Women age 15 to 20 years, 21 to 25 years, and 26 to 30 years were significantly less likely than the female siblings of the same age to have a live birth. Those age 21 to 25 years were significantly more likely to have a medical abortion. Although not statistically significant, the RR of miscarriage was increased among women age 21 to 25 years and 26 to 30 years.24 All treatment groups of female survivors had lower RRs of live birth than did the female siblings (Table 3).24 The RR of miscarriage was increased among those treated with craniospinal irradiation (RR = 2.22; 95% CI, 1.36 to 3.64) or cranial irradiation (RR = 1.4; 95% CI, 1.02 to 1.94) compared with those who received no radiation therapy (Table 4). The RR of miscarriage at less than 12 weeks of gestation was not increased in any of the radiation therapy subgroups evaluated (Table 5).24 The RR of live birth was not affected by radiation that included the ovaries or was near the ovaries compared with no radiation therapy (Table 6). The rate of live birth was not lower and the rate of stillbirth was not higher49 for the patients treated with any particular chemotherapeutic agent in comparison to those who had not been treated with the agent. The cumulative doses of several chemotherapeutic agents were divided into tertiles. There was no significant difference in the rate of live birth, miscarriage, or medical abortion by tertile.24
Table 3.
Pregnancy Outcomes of Female Cancer Survivors by Treatment
| Treatment | All Pregnancies (No.) | Live Births |
Stillbirths |
Miscarriages |
Abortions |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | ||
| S only | 332 | 207 | 0.65 | 0.49 to 0.86 | 4 | 1.82 | 0.59 to 5.64 | 55 | 1.26 | 0.87 to 1.83 | 57 | 1.60 | 1.09 to 2.33 |
| C only | 154 | 91 | 0.52 | 0.36 to 0.76 | 1 | 1.00 | 0.13 to 7.71 | 23 | 1.07 | 0.61 to 1.85 | 37 | 2.47 | 1.58 to 3.88 |
| RT only | 5 | 3 | 0.52 | 0.47 to 0.59 | 0 | — | — | 1 | 1.73 | 1.49 to 2.00 | 1 | 1.81 | 1.53 to 2.13 |
| C + S | 661 | 417 | 0.66 | 0.53 to 0.81 | 7 | 1.53 | 0.50 to 4.71 | 82 | 0.90 | 0.67 to 1.21 | 141 | 2.04 | 1.56 to 2.68 |
| C + RT | 370 | 230 | 0.61 | 0.48 to 0.79 | 3 | 1.19 | 0.34 to 4.22 | 63 | 1.42 | 1.03 to 1.97 | 64 | 1.63 | 1.14 to 2.31 |
| S + RT | 593 | 366 | 0.67 | 0.53 to 0.84 | 6 | 1.48 | 0.55 to 3.95 | 103 | 1.32 | 0.98 to 1.78 | 97 | 1.48 | 1.09 to 2.02 |
| C + S + RT | 1381 | 873 | 0.71 | 0.59 to 0.84 | 14 | 1.49 | 0.60 to 3.66 | 217 | 1.19 | 0.96 to 1.49 | 229 | 1.49 | 1.18 to 1.89 |
| Unknown | 533 | 361 | 0.87 | 0.68 to 1.10 | 2 | 0.59 | 0.13 to 2.65 | 79 | 1.15 | 0.85 to 1.56 | 69 | 1.15 | 0.82 to 1.61 |
Abbreviations: RR, rate ratio; S, surgery; C, chemotherapy; RT, radiation therapy.
Table 4.
Frequency of Miscarriage by Cranial or Spinal Irradiation Among Female Cancer Survivors
| Treatment | Female Participants (n = 1,915) |
|||
|---|---|---|---|---|
| Total No. | Miscarriage | Rate Ratio | 95% CI | |
| No RT | 1,147 | 160 | 1.00 | — |
| Cranial + spinal RT | 110 | 28 | 2.22 | 1.36 to 3.64 |
| Cranial RT only | 499 | 91 | 1.4 | 1.02 to 1.94 |
| Spinal RT only | 5 | 1 | 2.1 | 0.18 to 24.5 |
| No cranio-spinal RT | 1,483 | 215 | 1.06 | 0.82 to 1.36 |
| Unknown | 785 | 128 | 1.23 | 0.92 to 1.64 |
Abbreviation: RT, radiation therapy.
Table 5.
Frequency of Miscarriage at Less Than 12 Weeks Gestational Age by Cranial and/or Spinal Radiation Among Female Cancer Survivors
| Treatment | Female Participants (n = 1,915) |
||||
|---|---|---|---|---|---|
| Total No. | Miscarriage | Rate Ratio | 95% CI | P | |
| No RT | 1,147 | 120 | 1.00 | — | — |
| Cranial + spinal RT | 110 | 9 | 0.87 | 0.43 to 1.76 | .7032 |
| Cranial RT only | 499 | 61 | 1.27 | 0.89 to 1.82 | .1922 |
| Spinal RT only | 5 | 0 | — | — | — |
| No cranio-spinal RT | 1,483 | 117 | 0.75 | 0.56 to 1.02 | .0684 |
| Unknown | 785 | 86 | 1.09 | 0.78 to 1.53 | .6031 |
Abbreviation: RT, radiation therapy.
Table 6.
Pregnancy Outcomes by Ovarian Irradiation Among Female Cancer Survivors
| Treatment | All Pregnancies (No.) | Live Births |
Stillbirths |
Miscarriages |
Abortions |
Unknown (No.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | |||
| No RT | 1,147 | 715 | 1.00 | 12 | 1.00 | 160 | 1.00 | 235 | 1.00 | 25 | ||||
| Ovaries in RT field | 79 | 39 | 0.76 | 0.39 to 1.48 | 1 | 1.05 | 0.14 to 7.91 | 18 | 1.86 | 0.82 to 4.18 | 13 | 0.82 | 0.36 to 1.88 | 8 |
| Ovaries near RT field | 131 | 78 | 0.89 | 0.60 to 1.33 | 0 | 25 | 1.64 | 0.97 to 2.78 | 23 | 0.84 | 0.49 to 1.44 | 5 | ||
| Ovaries shielded | 22 | 14 | 1.13 | 0.44 to 2.89 | 1 | 5.82 | 0.73 to 46.4 | 3 | 0.9 | 0.23 to 3.49 | 3 | 0.69 | 0.21 to 2.32 | 1 |
| No ovarian RT | 1,937 | 1,241 | 1.14 | 0.96 to 1.35 | 17 | 0.79 | 0.32 to 1.91 | 308 | 1.17 | 0.92 to 1.48 | 314 | 0.74 | 0.59 to 0.92 | 57 |
| Ovarian RT unknown | 713 | 461 | 1.18 | 0.95 to 1.47 | 6 | 0.87 | 0.31 to 2.46 | 109 | 1.17 | 0.87 to 1.57 | 107 | 0.68 | 0.51 to 0.92 | 30 |
Abbreviation: RR, rate ratio; RT, radiation therapy.
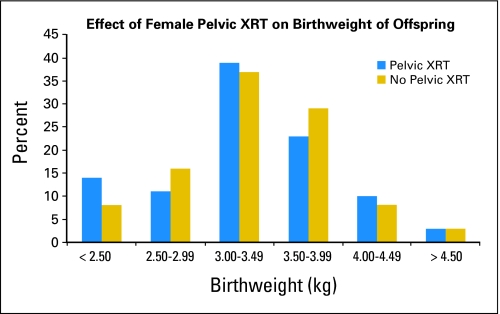
The offspring of survivors were more likely to weigh less than 2,500 g at birth than were the offspring of the female siblings (RR = 2.05; 95% CI, 1.42 to 2.95; P < .001). The offspring gender, maternal smoking history, and alcohol consumption history had no significant effect on offspring birthweight. The offspring of the survivors who received pelvic irradiation were more likely to weigh less than 2,500 g at birth than offspring of those who did not receive radiation to the pelvis (RR = 1.85; 95% CI, 1.07 to 3.18; P = .03; Fig 4).24
Fig 4.
Distribution of birthweight of the offspring of female cancer survivors by abdominal-pelvic radiation (A-P RT).
In a multivariate model that included daunorubicin or doxorubicin, live birth number, maternal age, maternal smoking, maternal drinking, and maternal educational level, pelvic irradiation (RR = 2.55; 95% CI, 1.45 to 4.47; P = .001), daunorubicin or doxorubicin (RR = 2.14; 95% CI, 1.43 to 3.21; P = .0002), and maternal educational level (did not complete high school; RR = 1.97; 95% CI, 1.04 to 3.72; P = .04) were all significant variables. There was no evidence of a dose-response relationship when doxorubicin or daunorubicin cumulative dose tertile was substituted for the dichotomous drug exposure variable.24
The offspring of female survivors were more likely to be born before 37 weeks of gestation compared with those of female siblings of survivors (21.1% v 12.6%, odds ratio [OR] = 1.9; 95% CI, 1.4 to 2.4; P < .001). Preterm birth was more likely for offspring of those who received uterine radiation doses ≥ 0.05 Gy compared with those who received no radiation therapy (0.05 to 2.50 Gy, 26.1% v 19.6%; OR = 1.8; 95% CI, 1.1 to 3.0; P = .03; 2.5 to 5.0 Gy, 39.6% v 19.6%; OR = 2.3; 95% CI, 1.0 to 5.1; P = .04; > 5 Gy, 50.0% v 19.6%; OR = 3.5; 95% CI, 1.5 to 8.0; P = .003). Offspring of women who received uterine radiation doses more than 5 Gy were more likely to be small for gestational age (birthweight < 10 percentile for gestational age; 18.2% v 7.8%, OR = 4.0; 95% CI, 1.6 to 9.8; P = .003). The frequency of premature birth was not increased by prior maternal exposure to increasing doses of alkylating agent chemotherapy.50
In summary, the offspring of women whose treatment included pelvic irradiation are more likely to be premature, have a low birthweight, and be small for gestational age. Prior treatment with doxorubicin or daunorubicin increased the risk of low birthweight independent of pelvic irradiation. The risk of miscarriage was increased among women whose treatment included high-dose cranial or craniospinal irradiation.
GENETIC DISEASE
The intense radiotherapy and chemotherapy received by cancer survivors is known to cause somatic mutations in humans and germline mutations in animals.51 Studies of the offspring of survivors of childhood cancer offer a unique opportunity to evaluate whether preconception radiation or mutagenic chemotherapy can result in a detectable increase in heritable genetic effects.52 Because of improved survival, the numbers of childhood cancer survivors and their offspring are now sufficient to test genetic hypotheses, and the radiotherapy doses can be determined with precision.53 For survivors of childhood and adolescent cancer who are able to become pregnant, there is concern as to the possible risk of fetal deaths, birth defects, or genetic disease. To study adverse pregnancy outcomes and possible germline mutagenesis, we evaluated self-reported genetic and congenital diseases among approximately 6,100 offspring of survivors and 3,100 offspring of sibling controls.
Genetic diseases in patients, families, and offspring were ascertained by self-administered questionnaires that included questions on birth defects and hereditary conditions. Genetic disease included cytogenetic abnormalities, single-gene birth defects, and simple malformations; verification was by medical records and consensus rules for inclusion were by a three-person panel. Radiation doses to the gonads were calculated from original records and phantoms to estimate dose-response and doubling dose; mean doses were 1.26 Gy to ovaries and 0.46 Gy to testes. The self-administered questionnaire analyses based on the self-reported genetic diseases were reassuring.54
The self-reported conditions were then validated as described in Leisenring et al.55 Preliminary results of the validated genetic and congenital diseases were again reassuring in that 157 (2.6%) occurred among the children of survivors, compared with 111 (3.6%) among the children of sibling controls (Table 7).56
Table 7.
Genetic Disease in Offspring of Cancer Survivors and Sibling Controls
| Genetic Disease | Survivor Offspring (n = 6,129) |
Sibling Offspring (n = 3,101) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Cytogenetic abnormality | 7 | 0.1 | 6 | 0.2 |
| Single-gene (Mendelian) disorder | 14 | 0.2 | 8 | 0.3 |
| Simple malformation | 136 | 2.2 | 97 | 3.1 |
| Total | 157 | 2.6 | 111 | 3.6 |
There were no apparent differences in the proportion of offspring with simple malformations (136 in case offspring and 97 in sibling offspring).56 Similar to a parallel study being conducted in Denmark, malformations of the heart occurred most often.51 There were no apparent differences in the proportion of offspring with cytogenetic syndromes (seven in survivor offspring and six in sibling offspring). Most of the chromosomal syndromes were trisomy 21, but there were also reports of trisomy 18, trisomy 13, and a few others. Results were similar to those reported in a parallel study in Denmark.57
There were no apparent differences in the proportion of offspring with single-gene defects (14 in survivor offspring and eight in sibling offspring). Single gene disorders included neurofibromatosis, Angelman syndrome, and Rubinstein-Taybi syndrome, among others.55 Radiation dose response relationships are being evaluated.
These preliminary results provide reassurance that cancer treatment using modern protocols does not carry a large risk of genetic disease in offspring conceived after treatment.56 Ongoing work includes final validation of the self-reported outcomes and analyses taking into account chemotherapy exposures, based on a DNA-damaging agent score, as well as individual radiation doses to the gonads (ovaries and testes), pituitary, and uterus.
SUMMARY AND FUTURE RESEARCH DIRECTION
Women whose treatment for childhood cancer includes direct ovarian radiation or administration of higher cumulative doses of alkylating agents are at risk for acute ovarian failure and premature nonsurgical menopause. The offspring of those treated with radiation therapy that included the uterus but remained fertile are at more likely to be premature and small for gestational age. The reproductive outcomes of the partners of men treated for childhood cancer are not significantly affected by their prior treatment. Future research will focus on defining the reproductive risks associated with newer chemotherapeutic agents, such as ifosfamide; refining risk estimates for adverse outcomes associated with increasing doses of gonadal or uterine irradiation; and continued evaluation of the offspring for evidence of increased risk for new genetic disease.
Footnotes
Support provided by the National Cancer Institute Grants No. U24 CA55727 (Principal investigator, L.L.R.) and R01CA104666 (Principal investigator, C.A.S.) for the National Institutes of Health and the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Manuscript writing: Daniel Green, Charles Sklar, John Boice, John J. Mulvihill, John Whitton, Marilyn Stovall, Yutaka Yasui
REFERENCES
- 1.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 2.da Cunha MF, Meistrich ML, Fuller LM, et al. Recovery of spermatogenesis after treatment for Hodgkin's disease:Limiting dose of MOPP chemotherapy. J Clin Oncol. 1984;2:571–577. doi: 10.1200/JCO.1984.2.6.571. [DOI] [PubMed] [Google Scholar]
- 3.Heikens J, Behrendt H, Adriaansse R, et al. Irreversible gonadal damage in male survivors of pediatric Hodgkin's disease. Cancer. 1996;78:2020–2024. doi: 10.1002/(sici)1097-0142(19961101)78:9<2020::aid-cncr25>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Charak BS, Gupta R, Mandrekar P, et al. Testicular dysfunction after cyclophosphamide-vincristine-procarbazine-prednisolone chemotherapy for advanced Hodgkin's disease: A long-term follow-up study. Cancer. 1990;65:1903–1906. doi: 10.1002/1097-0142(19900501)65:9<1903::aid-cncr2820650905>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Viviani S, Santoro A, Ragni G, et al. Gonadal toxicity after combination chemotherapy for Hodgkin's disease: Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol. 1985;21:601–605. doi: 10.1016/0277-5379(85)90088-4. [DOI] [PubMed] [Google Scholar]
- 6.Chapman RM, Sutcliffe SB, Malpas JS. Male gonadal dysfunction in Hodgkin's disease. JAMA. 1981;245:1323–1328. [PubMed] [Google Scholar]
- 7.Chapman RM, Rees LH, Sutcliffe SB, et al. Cyclical combination chemotherapy and gonadal function. Lancet. 1979;1:285–289. doi: 10.1016/s0140-6736(79)90701-3. [DOI] [PubMed] [Google Scholar]
- 8.Sherins RJ, Olweny CLM, Ziegler JL. Gynecomastia and gonadal dysfunction in adolescent boys treated with combination chemotherapy for Hodgkin's disease. N Engl J Med. 1978;299:12–16. doi: 10.1056/NEJM197807062990103. [DOI] [PubMed] [Google Scholar]
- 9.Asbjornsen G, Molne K, Klepp O, et al. Testicular function after combination chemotherapy for Hodgkin's disease. Scand J Haematol. 1976;16:66–69. doi: 10.1111/j.1600-0609.1976.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 10.DeVita VT, Arseneau JC, Sherins RJ, et al. Intensive chemotherapy for Hodgkin's disease: Long-term complications. Natl Cancer Inst Monogr. 1973;36:447–454. [PubMed] [Google Scholar]
- 11.Shafford EA, Kingston JE, Malpas JS, et al. Testicular function following the treatment of Hodgkin's disease in childhood. Br J Cancer. 1993;68:1199–1204. doi: 10.1038/bjc.1993.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackie EJ, Radford M, Shalet SM. Gonadal function following chemotherapy for childhood Hodgkin's disease. Med Pediatr Oncol. 1996;27:74–78. doi: 10.1002/(SICI)1096-911X(199608)27:2<74::AID-MPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Clark ST, Radford JA, Crowther D, et al. Gonadal function following chemotherapy for Hodgkin's disease: A comparative study of MVPP and a seven-drug hybrid regimen. J Clin Oncol. 1995;13:134–139. doi: 10.1200/JCO.1995.13.1.134. [DOI] [PubMed] [Google Scholar]
- 14.Sklar CA, Robison LL, Nesbit ME, et al. Effects of radiation on testicular function in long-term survivors of childhood acute lymphoblastic leukemia: A report from the Childrens Cancer Study Group. J Clin Oncol. 1990;8:1981–1987. doi: 10.1200/JCO.1990.8.12.1981. [DOI] [PubMed] [Google Scholar]
- 15.Andrieu JM, Ochoa-Molina ME. Menstrual cycle, pregnancies and offspring before and after MOPP therapy for Hodgkin's disease. Cancer. 1983;52:435–438. doi: 10.1002/1097-0142(19830801)52:3<435::aid-cncr2820520308>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Santoro A, Bonadonna G, Valagussa P, et al. Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin's disease: Superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987;5:27–37. doi: 10.1200/JCO.1987.5.1.27. [DOI] [PubMed] [Google Scholar]
- 17.Waxman JHX, Terry Y, Wrigley PFM, et al. Gonadal function in Hodgkin's disease:long-term follow-up of chemotherapy. BMJ. 1982;285:1612–1613. doi: 10.1136/bmj.285.6355.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schilsky RL, Sherins RJ, Hubbard SM, et al. Long-term follow-up of ovarian function in women treated with MOPP chemotherapy for Hodgkin's disease. Am J Med. 1981;71:552–556. doi: 10.1016/0002-9343(81)90205-9. [DOI] [PubMed] [Google Scholar]
- 19.Chapman RM, Sutcliffe SB, Malpas JS. Cytotoxic-induced ovarian failure in women with Hodgkin's disease: I. Hormone function. JAMA. 1979;242:1877–1881. [PubMed] [Google Scholar]
- 20.Hudson MM, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin's disease. J Clin Oncol. 1993;11:100–108. doi: 10.1200/JCO.1993.11.1.100. [DOI] [PubMed] [Google Scholar]
- 21.Papadakis V, Vlachopapadopoulou E, Van Cyckle K, et al. Gonadal function in young patients successfully treated for Hodgkin disease. Med Pediatr Oncol. 1999;32:366–372. doi: 10.1002/(sici)1096-911x(199905)32:5<366::aid-mpo10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Ortin TT, Shostak CA, Donaldson SS. Gonadal status and reproductive function following treatment for Hodgkin's disease in childhood: The Stanford experience. Int J Radiat Oncol Biol Phys. 1990;19:873–880. doi: 10.1016/0360-3016(90)90007-7. [DOI] [PubMed] [Google Scholar]
- 23.Wallace WHB, Shalet SM, Crowne EC, et al. Ovarian failure following abdominal irradiation in childhood: Natural history and prognosis. Clin Oncol (R Coll Radiol) 1989;1:75–79. doi: 10.1016/s0936-6555(89)80039-1. [DOI] [PubMed] [Google Scholar]
- 24.Green DM, Whitton JA, Stovall M, et al. Pregnancy outcome of female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187:1070–1080. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 25.Green DM, Peabody EM, Nan B, et al. Pregnancy outcome after treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20:2506–2513. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 26.Critchley HOD. Factors of importance for implantation and problems after treatment for childhood cancer. Med Pediatr Oncol. 1999;33:9–14. doi: 10.1002/(sici)1096-911x(199907)33:1<9::aid-mpo3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2006;91:1723–1728. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 28.Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol. 2005;6:209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 29.Sklar C. Reproductive physiology and treatment-related loss of sex hormone production. Med Pediatr Oncol. 1999;33:2–8. doi: 10.1002/(sici)1096-911x(199907)33:1<2::aid-mpo2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Chiarelli AM, Marrett LD, Darlington GA. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964-1988 in Ontario, Canada. Am J Epidemiol. 1999;150:245–254. doi: 10.1093/oxfordjournals.aje.a009995. [DOI] [PubMed] [Google Scholar]
- 31.Sklar C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J Natl Cancer Inst Monogr. 2005;34:25–27. doi: 10.1093/jncimonographs/lgi018. [DOI] [PubMed] [Google Scholar]
- 32.Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–744. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 33.Sarafoglou K, Boulad F, Gillio A, et al. Gonadal function after bone marrow transplantation for acute leukemia during childhood. J Pediatr. 1997;130:210–216. doi: 10.1016/s0022-3476(97)70345-7. [DOI] [PubMed] [Google Scholar]
- 34.Thibaud E, Ramirez M, Brauner R, et al. Preservation of ovarian function by ovarian transposition performed before pelvic irradiation during childhood. J Pediatr. 1992;121:880–884. doi: 10.1016/s0022-3476(05)80332-4. [DOI] [PubMed] [Google Scholar]
- 35.Byrne J. Infertility and premature menopause in childhood cancer survivors. Med Pediatr Oncol. 1999;33:24–28. doi: 10.1002/(sici)1096-911x(199907)33:1<24::aid-mpo5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 36.Pouillès JM, Tremollieres F, Bonneu M, et al. Influence of early age at menopause on vertebral bone mass. J Bone Miner Res. 1994;9:311–315. doi: 10.1002/jbmr.5650090304. [DOI] [PubMed] [Google Scholar]
- 37.Vega EM, Egea MA, Mautalen CA. Influence of the menopausal age on the severity of osteoporosis in women with vertebral fractures. Maturitas. 1994;19:117–124. doi: 10.1016/0378-5122(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 38.de Kleijn MJ, van der Schouw YT, Verbeek AL, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339–345. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 39.Schover LR. Sexuality and body image in younger women treated for breast cancer. J Natl Cancer Inst Monogr. 1994;16:177–182. [PubMed] [Google Scholar]
- 40.Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890–896. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 41.Green DM, Fine WE, Li FP. Offspring of patients treated for unilateral Wilms' tumor in childhood. Cancer. 1982;49:2285–2288. doi: 10.1002/1097-0142(19820601)49:11<2285::aid-cncr2820491114>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 42.Li FP, Gimbrere K, Gelber RD, et al. Outcome of pregnancy in survivors of Wilms' tumor. JAMA. 1987;257(2):216–219. [PubMed] [Google Scholar]
- 43.Byrne J, Mulvihill JJ, Connelly RR, et al. Reproductive problems and birth defects in survivors of Wilms' tumor and their relatives. Med Pediatr Oncol. 1988;16:233–240. doi: 10.1002/mpo.2950160403. [DOI] [PubMed] [Google Scholar]
- 44.Chiarelli AM, Marrett LD, Darlington GA. Pregnancy outcomes in females after treatment for childhood cancer. Epidemiology. 2000;11:161–166. doi: 10.1097/00001648-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Hawkins MM, Smith RA. Pregnancy outcomes in childhood cancer survivors: Probable effects of abdominal irradiation. Int J Cancer. 1989;43:399–402. doi: 10.1002/ijc.2910430309. [DOI] [PubMed] [Google Scholar]
- 46.Critchley HO, Wallace WH, Shalet SM, et al. Abdominal irradiation in childhood: The potential for pregnancy. Br J Obstet Gynaecol. 1992;99:392–394. doi: 10.1111/j.1471-0528.1992.tb13755.x. [DOI] [PubMed] [Google Scholar]
- 47.Holm K, Nysom K, Brocks V, et al. Ultrasound B-mode changes in the uterus and ovaries and Doppler changes in the uterus after total body irradiation and allogeneic bone marrow transplantation in childhood. Bone Marrow Transplant. 1999;23:259–263. doi: 10.1038/sj.bmt.1701569. [DOI] [PubMed] [Google Scholar]
- 48.Green DM, Whitton JA, Stovall M, et al. Pregnancy outcome of partners of male survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:716–721. doi: 10.1200/JCO.2003.04.085. [DOI] [PubMed] [Google Scholar]
- 49.Childhood Cancer Survivor Study: New Public Access Data Tables. 2008 http://www.stjude.org/stjude/v/index.jsp?vgnextoid=0d5dd3ce38e70110VgnVCM1000001e0215acRCRD&vgnextchannel=448d0817933fe010VgnVCM1000005f2015acRCRD.
- 50.Signorello LB, Cohen SS, Bosetti C, et al. Female survivors of childhood cancer: Preterm birth and low birth weight among their children. J Natl Cancer Inst. 2006;98:1453–1461. doi: 10.1093/jnci/djj394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boice JDJ, Tawn EJ, Winther JF, et al. Genetic effects of radiotherapy for childhood cancer. Health Phys. 2003;85:65–80. doi: 10.1097/00004032-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Boice JDJ, Robison LL, Mertens A, et al. Stillbirths and male irradiation. J Radiol Prot. 2000;20:321–322. doi: 10.1088/0952-4746/20/3/101. [DOI] [PubMed] [Google Scholar]
- 53.Stovall M, Donaldson SS, Weathers RE, et al. Genetic effects of radiotherapy for childhood cancer: Gonadal dose reconstruction. Int J Radiat Oncol Biol Phys. 2004;60:542–552. doi: 10.1016/j.ijrobp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Mulvihill JJ, Strong LC, Robison LL. Genetic disease in offspring of survivors of childhood and adolescent cancer. Presented at the annual meeting of the American Society of Human Genetics; October 12-16, 2001; San Diego, CA. abstr 1219. [Google Scholar]
- 55.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric Cancer Survivorship Research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulvihill JJ, Munro H, Whitton JA, et al. Genetic disease in offspring of survivors of childhood and adolescent cancer. Presented at the annual meeting of the American Society of Human Genetics; October 23-27, 2007; San Diego, CA. abstr 2002. [Google Scholar]
- 57.Winther J, Boice JDJ, Mulvihill JJ, et al. Chromosomal abnormalities among offspring of childhood cancer survivors in Denmark: Population-based study. Am J Hum Genet. 2004;74:1282–1285. doi: 10.1086/421473. [DOI] [PMC free article] [PubMed] [Google Scholar]